Abstract
To better appraise how the field is moving forward and where we are heading, a historical view introduces the spirit of the Baveno consensus workshops. In an era with introduction of effective treatments of the etiological factors, the clinical scenario is changing and the natural history along with it. Novel direct-acting antivirals in hepatitis C is probably just the beginning, and other effective treatments of hepatitis B, NASH, alcohol, and others may follow. This may affect the need of surveillance and clinical follow-up and change the focus. The complex symptomatology and involved organs in chronic liver diseases underline the systemic nature of the diseases. But there is a gap in appropriate care targeted to patients with cirrhosis, and care coordination should be expanded. Integrated care to enable early detection and prevention of decompensation beyond detection of varices and prevention of first variceal bleeding is needed. Thus, early ascites, malnutrition, minimal hepatic encephalopathy, and comorbidities should be within our approach. This introductory section outlines current status, changing scenarios and emerging therapies, and approaches to the patient with early-stage cirrhosis.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Portal Hypertension
- Hepatic Encephalopathy
- Sustained Virological Response
- Primary Biliary Cirrhosis
- Esophageal Varix
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction: Historical Perspective
The history of the treatment of portal hypertension is characterized by a progressive interest in treating earlier and earlier phases of this condition. Fifty years ago treatment of portal hypertension only included acute treatment of variceal bleeding or surgical prevention of recurrent bleeding. The seminal paper by Didier Lebrec [1] opened the era of medical treatment of portal hypertension, and initially all efforts were dedicated to the prevention of rebleeding in patients who survived an acute bleeding episode. Later prevention was progressively expanded to earlier and earlier phases. Indeed, few words were dedicated to the prevention of first bleeding in the Baveno 1 meeting (1990) [2], and in the Baveno II meeting (1995), prevention of bleeding was considered “A look into the future of pharmacological treatment of portal hypertension,” according to the title of the lecture by Didier Lebrec and Richard Moreau [3].
At the Baveno III meeting (2000), primary prophylaxis was the subject of a chapter by itself, and we started to evaluate the subject of an earlier phase of portal hypertension called preprimary prophylaxis. In this meeting a section was dedicated to “Can (and should) we prevent the formation and growth of varices?”[4] The problem was considered important and was mainly related to the problem of treating patients with small varices, since only few data were available on potential treatments of these subjects, previously considered as not requiring treatment. In this section, for the first time, the possible occurrence of regression of varices was considered, in particular as related to a possible improvement in liver status. At that time, the association of abstinence from alcohol in alcoholic cirrhosis with possible regression of varices was considered the paradigm of an etiological treatment of liver disease with beneficial consequences on liver disease and portal hypertension at the same time.
The preprimary prophylaxis was also the subject of a session of the Baveno IV meeting in 2005, but at this time, it was defined that the term preprimary prophylaxis should only include the prevention of the formation of varices [5]. Based on the results of the only trial that specifically addressed the usefulness of treatment with NSBB patients with cirrhosis without varices and with HVPG in the range 5–9 mmHg in order to prevent the formation of varices [6], it was stated that there was no indication to treat patients to prevent the formation of varices. The lack of effect of NSBB in these subjects was interpreted as the expression of the fact that in this phase the mechanisms provoking portal hypertension (formation of collaterals, hyperdynamic circulation) was not halted by NSBB.
At the 2010 meeting (Baveno V), the statements related to preprimary prophylaxis were updated [7], and among the recommendations, there was for the first time a statement that suggested that treatment of the underlying liver disease may have a beneficial influence on portal hypertension and reduce the risk of clinical complications. The impact of treating the underlying disease in the development of portal hypertensive-related complications, including varices, was also considered an area requiring further study.
In the present session we report available evidence about the possible treatment of patients in an evolving scenario which considers cirrhotic patients as treated successfully (or not successfully) in relation to their etiological factors. In this scenario there will be an overlap between prevention of the formation of varices (a traditional approach) and prevention of the progression of disease or even regression (a novel approach). In this approach varices appear as a part of the disease process, which has portal hypertension as a marker of disease stage and at the same time a pathophysiological mechanism involved in its progression.
Changing Scenario: Natural History of Chronic Liver Diseases from Compensated to Decompensated Stage in the Era of Direct-Acting Antivirals
The rate of progression in fibrogenic liver diseases depends on a number of factors and varies individually. Previously, fibrosis was considered an inactive tissue without regenerative potential for the organ affected. Within the last decade, this concept has changed, and fibrosis is no longer considered static or irreversible but the result of a continuous remodeling process and thereby susceptible to interventions. Presently, no treatments that specifically target the mechanism of fibrosis are available for clinical use. However, therapies that address or eliminate the cause of tissue damage (e.g., tenofovir in chronic hepatitis B virus infection) have the potential to lead to regression of fibrosis and even cirrhosis [8]. Thus, the risk of progression may be halted or even reversed with cure of the etiological factor, and consequently the prognosis may be altered. A similar pattern may occur in hepatitis C upon sustained virological response (SVR), though this has not been documented yet. However, one would expect that control of viral replication would prevent, slow down, or delay further accumulation of fibrosis and thus risk of decompensation and risk of HCC [9]. If this holds true, the indications and interval between surveillance for varices and HCC may change. In addition the populations of patients with liver diseases and portal hypertension are also changing. With the arrival of highly efficacious and well-tolerated treatments for HCV along with an increasing burden of obesity and alcohol overuse, the need and focus may change within the foreseeable future. Obesity is a global health challenge with prevalence of up to 30 % of the population at risk of nonalcoholic fatty liver disease (NAFLD), among whom approximately 4 % will develop or have nonalcoholic steatohepatitis (NASH) and a few will develop progressive fibrosis. NAFLD is considered the hepatic manifestation of the metabolic syndrome and has become a leading cause of liver disease worldwide. Currently NASH is the second leading cause of liver transplantation in the USA, and the numbers have tripled within the last decade [10]. Similarly, alcohol abuse is a leading risk factor for morbidity and death worldwide among the young, working population (15–49 years). Chronic alcoholic liver disease is a major cause of alcohol-related mortality, accounting for 570,000 annual deaths worldwide. In 2010, alcoholic liver fibrosis and subsequent cirrhosis led to nearly 500,000 deaths and cost 14.5 million disability-adjusted life years (DALYs) worldwide [11]. Compared to other common chronic diseases, mortality from alcoholic liver disease is on the rise [12]. Yet, there is a striking mismatch between burden of alcoholic liver disease and prioritization due to the socially stigmatized status of the disease [13].
Hidden Burden of Disease
To have an effective primary prevention, we need early diagnosis of fibrosis and cirrhosis. However, due to the lack of widely available tools for early diagnosis of liver fibrosis, the latter is mostly discovered at an advanced stage after reaching cirrhosis, with 5-year mortality up to 88 % in late cirrhosis compared to 1.5 % in the earliest stage of cirrhosis [14]. In a study with more than 1300 patients, 76 % of patients had their initial diagnosis of alcoholic cirrhosis during hospitalization with a decompensating event [15]. This underlines the huge hidden burden of disease but also the unmet need of early diagnosis and associated potential of applying primary prevention in a larger portion of patients. Viral hepatitis identifies a relevant population at risk and screening for viral hepatitis is cheap. In alcohol and NASH-induced liver fibrosis and cirrhosis, this is more difficult, as the population at risk is large and noninvasive markers with high diagnostic accuracy in early stages are not available or not integrated in clinical practice. In patients who discontinue alcohol overuse, lose weight, or control the metabolic syndrome, there is likely a recovery potential similar to that observed in viral hepatitis.
Risk Stratification
The risk and speed of progression from compensated to decompensated stage define the prognosis. Therefore, early detection and preventative strategies may affect outcomes. In the advanced stages of the disease, with portal hypertension and decompensation, prognostic tools like the MELD and the Child-Pugh scores are useful, but in the early stages of the disease, measures to predict risk of decompensation, morbidity, and mortality are less developed and not widely adopted in general care. In these stages, standard liver function tests can be normal even among patients with significant fibrosis and early cirrhosis. Imaging tools are powerful [16], but the static nature of imaging limits its prognostic power in early stages because it does not reflect tissue activity (inflammation, remodeling of extracellular matrix, and fibrogenesis). However, direct markers of the pathological processes are not yet ready for clinical use [16]. HVPG measurements have repeatedly and consistently been reported as a very strong tool to assess, prognosticate, and measure the efficacy of specific interventions [17]. Thus, HVPG measurements enable diagnosis (HVPG >6 mmHg) of portal hypertension and its severity, with a level of >10 mmHg being associated with varices formation and high risk of decompensation including ascites and HCC. Higher levels of >12 mmHg imply the risk of bleeding, and similarly a reduction of >20 % or below 12 mmHg suggests effective pharmacological interventions. Thus, HVPG measurements are unique as both a diagnostic and prognostic tool and a measure of efficacy of pharmacological interventions. Limitations include limited availability and expertise outside referral centers, time, cost, and patient acceptability.
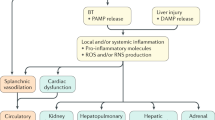
Changing Scenario: Cirrhosis and Portal Hypertension as a Systemic Disease, Need for Collaborative Care
A large proportion of patients emerge in the health-care systems with a decompensating event. A number of treatments have been developed to handle acute events and been successful to improve outcomes [18]. However, patients who develop decompensation and complications of cirrhosis have a poor prognosis which is associated to hospital admissions and frequent readmissions [19]. In addition, quality of life and working ability are negatively affected and thus associated with a significant economic burden. Thus, there is an urgent need to strengthen efforts to prevent decompensation and prevention of first variceal bleeding, and other key events are an essential part of care. The complex symptomatology and multiplicity of involved organs in chronic liver diseases underline their systemic nature. This calls for care coordination or “collaborative care” [20]. Most studies and guidelines focus on one event, i.e., varices, ascites, or hepatic encephalopathy, which increases the risk of fragmented and poorly coordinated care [21]. The overall goals are to improve clinical care by adaption and adherence to best clinical standards to prevent complications and decompensation. Currently, screening for esophageal varices and HCC are generally accepted standard of care, although the direct evidence from randomized trials supporting the benefit of screening is weak. In clinical practice patients often fail to receive the evidence and guideline-based treatments [21]. In one study with 774 patients, only 24.3 % had an upper endoscopy during the first year after cirrhosis was diagnosed, and only 60 % of those with varices received appropriate primary prophylaxis with beta-blockers or band ligation [22]. Similarly nonadherence to HCC screening is high. Thus, less than 20 % of patients with cirrhosis undergo surveillance for HCC, with the lowest adherence in nonspecialized centers [23]. Overall there is a mismatch between recommended standards and clinical practice in this field, which likely has an impact on outcomes and resource utilization. Thus, integrated care with adoption of all documented treatments together improves outcomes [20]. A recent study among outpatients with ascites documented how care coordination versus standard care improved 12-month mortality (45.7 % vs. 23.1 %, p < 0.025) and rate of 30-day readmission (42.4 % vs. 15.4 %, p < 0.01). In addition, the global cost attributable to the management per patient-month of life was lower [20]. General care in early-stage disease, to prevent decompensation, should probably go beyond surveillance of varices and HCC and include comorbidities, nutrition, physical training, hepatic encephalopathy, minimal hepatic encephalopathy, early ascites, and general symptoms like fatigue. However, evidence-based treatments at this stage of disease are limited. In addition at least 40 % of patients with cirrhosis have comorbidities such as diabetes, cancer, osteoporosis, pulmonary, and cardiac diseases that increase morbidity and mortality [24, 25]. Successful treatment of comorbid diseases in the first year after diagnosis may substantially reduce the mortality rate, and thus, the presence of comorbidities is an important issue in clinical hepatology that deserves more attention [24].
Detection of Esophageal Varices and Primary Prevention of Bleeding: New Insights into NSBB
Endoscopy is still the preferred standard to screen for the presence of varices. A number of other noninvasive methods have been investigated including spleen and liver stiffness [26, 27]. In the prevention first variceal bleeding NSBB and EVL are both valid first choices [28]. In approximately one third of the patients, there are contraindications or intolerance to NSBB and EVL can be applied. In patients who tolerate NSBB, these are the best choice, especially if long-term treatment is expected, as there may be a survival benefit of NSBB above EVL in the long run. On the other hand, EVL may offer better protection against bleeding in the short term [28]. NSBB including carvedilol have been and still are the cornerstone in primary prevention of bleeding from esophageal varices. Their clinical efficacy is covered in other chapters. However, our understanding of the pharmacodynamics and safety of NSBB has been changing in recent years. In particular NSBB seem to have an impact in patients with portal hypertension in a clinically significant way which goes beyond the hemodynamic effects. Gut bacterial translocation is believed to be a key driver in the pathogenesis, progression, and cause of decompensating events [29]. NSBB have been shown to reduce bacterial translocation [30]. This may translate into reduced risk of infections in general and spontaneous bacterial peritonitis in particular [31, 32]. Thus, both direct and indirect evidences suggest that NSBB reduce the risk of bacterial translocation. In addition NSBB may reduce risk of HCC [32]. The mechanism of action is incompletely understood but may include a weak antiangiogenic effects [33]. These non-hemodynamic effects may have a clinically significant impact, but more data are needed before NSBB can be recommended beyond the prevention of bleeding in patients with esophageal varices.
For decades, NSBB have been a cornerstone in clinical hepatology due to their very well-documented effects in terms of preventing variceal bleeding and improving survival. However, a serious concern about the safety of NSBB in advanced-stage disease has been raised in recent years [34, 35]. Thus, NSBB may only be beneficial during a certain window during disease progression, and at certain tipping points in advanced-stage disease, NSBB should be discontinued [36]. The available data are observational and prone to confounding factors that can be difficult to eliminate completely. Consequently the controversy if and when to stop NSBB in advanced-stage disease is ongoing, and there is currently no consensus on when to stop NSBB and, if they are stopped, when and if to reinstitute. In the most fragile patients with advanced-stage disease with refractory ascites, low blood pressure, acute kidney injury, or SBP, NSBB should be used with extreme caution and discontinued readily if the situation deteriorates. In patients without previous bleeding, EVL can substitute NSBB without the safety concern [28].
Emerging Interventions in the Primary Prevention of Decompensation
Prevention implies surveillance with early detection at subclinical or asymptomatic levels. Screening tests should be validated, cheap, and safe, and adherence is essential for the overall success. Treatment should be available for early stages, and early treatment should offer better outcomes than late treatment. The population at risk of decompensation is clear, but screening tools and relevant interventions are limited, and the demonstration of benefit if early detection is achieved is currently also limited; however, a number of interventions are emerging.
Cognitive dysfunction is an important event in cirrhosis that affects quality of life and the socioeconomic status [37]. Cognitive dysfunction is associated with minimal hepatic encephalopathy, which is a risk factor for overt hepatic encephalopathy [38]. Thus, minimal hepatic encephalopathy would be an important target for early detection. However, therapies to improve cognition and prevent progression to overt stages need better validation. Currently, a number of interventions including prebiotics, probiotics, antibiotics, and nutritional supplements are tested. However, current guidelines advise against routine treatment of minimal hepatic encephalopathy due to lack of evidence [39]. Consequently the drive to assess patients is limited.
The gut-liver axis in terms of translocation of bacteria and bacterial products from the gut is considered a key driver in the development and progression of liver disease [29]. This is true in particular in the more advanced stages with ascites and portal hypertension due to slower transit times, bacterial overgrowth, and increased permeability in the gut. However, in earlier stages this may also be important, in particular in alcoholics as alcohol itself induces a leaky gut. Rifaximin is a nonabsorbable antibiotic with a main effect in the small bowel, the most important area of bacterial translocation. Rifaximin is well established as a treatment that reduces the risk of recurrent episodes of hepatic encephalopathy and hospitalizations in patients with previous episodes of hepatic encephalopathy [40, 41]. In addition this treatment is associated with improved quality of life [42]. In advanced-stage disease, sarcopenia and malnutrition are very frequent and important prognostic indicators [43]. Recent evidence suggests that nutritional therapy may have beneficial effects on clinical outcomes in cirrhosis and alcoholic hepatitis [44]. The mechanisms of sarcopenia are incompletely understood, but muscle mass improves after liver transplantation and also after TIPS treatment, which suggests a relation to portal hypertension. In compensated disease, the nutritional status is also associated with prognosis [45]. Physical training can improve exercise capacity, muscle mass, and quality of life [46], and this effect may translate into an impact on portal pressure and risk of complications. Overall the concept of assessing nutritional status and muscle mass is intriguing, because interventions to modify these risk factors are at hand. However, more clinical trial data are needed to document the clinical efficacy and insight on when and how to intervene. Other pharmacological treatments with promising results include statins and low-molecular-weight heparin. Both of these may improve survival and prevent risk of decompensation and deterioration in liver function [47–49]. Obeticholic acid, a bile acid derivative which seems promising in patients with NASH and primary biliary cirrhosis, is described in detail elsewhere in this book.
Preventing Reinstitution of the Etiological Factor
The flip side of the coin in liver diseases after cure of the etiological factor is the risk of reinstitution of the same or another factor. In hepatitis C there is a risk of reinfection, and a number of patients have concomitant alcohol overuse, thus the risk of progressive disease and decompensation is not necessarily over after successful treatment with antivirals. Reactivation of hepatitis B is an important and rising clinical problem due to increasing use of immunosuppressive drugs including biologics and novel anticancer drugs. All patients undergoing chemotherapy, immunosuppressive therapy, hematopoietic stem cell transplantation, or solid-organ transplantation should be screened for active or prior hepatitis B viral infection [50]. Abstinence remains the most important therapeutic intervention in alcoholic liver disease, and in NASH lifestyle interventions with weight loss are key to success, but the success rate of lifestyle interventions is low and the rate of relapse high and better options to achieve these goals are warranted.
References
Lebrec D, Nouel O, Corbic M, Benhamou JP (1980) Propranolol – a medical treatment for portal hypertension? Lancet 2(8187):180–182
de Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W et al (1992) Definitions, methodology and therapeutic strategies in portal hypertension. A Consensus Development Workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. J Hepatol 15(1–2):256–261. PubMed Epub 1992/05/01. eng
Lebrec D, Moreau R (1992) A look into the future of the pharmacological treatment of portal hypertension. In: de Franchis R (ed) Portal hypertension II. Blackwell, Oxford, pp 100–113
Merkel C, Escorsell A, Sieber CC, Lee FY, Groszmann RJ (2001) Pre-primary prophylaxis: can (and should) we prevent the formation and growth of varices? In: de Franchis R (ed) Portal hypertension III. Blackwell, Oxford, pp 97–111
Groszmann RJ, Merkel C, Ywakiri Y, Shah V, Shneider BL, Zoli M, Berzigotti A, Vorobioff J, Morabito A (2006) Prevention of the formation of varices (pre-primary prophylaxis). In: di Franchis R (ed) Portal hypertension IV. Blackwell, Malden, pp 103–151
Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R et al (2005) Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 353(21):2254–2261
Groszmann R, Merkel C, Dell’Era A, Merli M, Ripoll C, Vorobioff J (2011) Pre-primary and primary prophylaxis. In: di Franchis R (ed) Portal hypertension V. Blackwell, Oxford, pp 73–74
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM et al (2013) Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 381(9865):468–475. PubMed Epub 2012/12/14. eng
Kimer N, Dahl EK, Gluud LL, Krag A (2012) Antiviral therapy for prevention of hepatocellular carcinoma in chronic hepatitis C: systematic review and meta-analysis of randomised controlled trials. BMJ Open 2(5). pii: e001313. doi:10.1136/bmjopen-2012-001313. Print 2012. PubMed PMID: 23089208; PubMed Central PMCID: PMC4400677
Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM et al (2015) Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148(3):547–555. PubMed Epub 2014/12/03. eng
Rehm J, Samokhvalov AV, Shield KD (2013) Global burden of alcoholic liver diseases. J Hepatol 59(1):160–168. PubMed
Williams R, Horton R (2013) Liver disease in the UK: a Lancet Commission. Lancet 382(9904):1537–1538. PubMed
Shah VH (2010) Alcoholic liver disease: the buzz may be gone, but the hangover remains. Hepatology 51(5):1483–1484
D’Amico G, Pasta L, Morabito A, D’Amico M, Caltagirone M, Malizia G et al (2014) Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 39(10):1180–1193. PubMed
Dam Fialla A, Schaffalitzky de Muckadell OB, Touborg Lassen A (2012) Incidence, etiology and mortality of cirrhosis: a population-based cohort study. Scand J Gastroenterol 47(6):702–709. PubMed Epub 2012/03/21. eng
Karsdal MA, Krarup H, Sand JM, Christensen PB, Gerstoft J, Leeming DJ et al (2014) Review article: the efficacy of biomarkers in chronic fibroproliferative diseases – early diagnosis and prognosis, with liver fibrosis as an exemplar. Aliment Pharmacol Ther 40(3):233–249. PubMed Epub 2014/06/10. eng
Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC (2009) The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 6(10):573–582. PubMed Epub 2009/09/03. eng
Schmidt M, Barritt AS, Orman ES, Hayashi PH (2015) Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology 148(5):967–977. PubMed Epub 2015/01/28. Eng
Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS (2012) Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 107(2):247–252. PubMed Pubmed Central PMCID: 3470789. Epub 2011/09/21. eng
Morando F, Maresio G, Piano S, Fasolato S, Cavallin M, Romano A et al (2013) How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol 59(2):257–264. PubMed Epub 2013/03/26. eng
Kanwal F, Kramer JR, Buchanan P, Asch SM, Assioun Y, Bacon BR et al (2012) The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology 143(1):70–77. PubMed Epub 2012/04/03. eng
Buchanan PM, Kramer JR, El-Serag HB, Asch SM, Assioun Y, Bacon BR et al (2014) The quality of care provided to patients with varices in the department of Veterans Affairs. Am J Gastroenterol 109(7):934–940. PubMed Epub 2014/07/06. eng
Singal AG, Yopp A, SSkinner C, Packer M, Lee WM, Tiro JA (2012) Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 27(7):861–867. PubMed Pubmed Central PMCID: 3378733. Epub 2012/01/05. eng
Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sorensen HT (2008) Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology 48(1):214–220. PubMed Epub 2008/06/10. eng
Jepsen P, Vilstrup H, Lash TL (2014) Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology 146(1):147–156; quiz e15-6. PubMed Epub 2013/09/24. eng
Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, Garcia-Pagan JC et al (2013) Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 144(1):102–111 e1. PubMed Epub 2012/10/13. eng
Procopet B, Cristea VM, Robic MA, Grigorescu M, Agachi PS, Metivier S et al (2015) Serum tests, liver stiffness and artificial neural networks for diagnosing cirrhosis and portal hypertension. Dig Liver Dis 47(5):411–416. PubMed Epub 2015/03/04. Eng
Gluud LL, Krag A (2012) Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev 8:CD004544. PubMed Epub 2012/08/17. eng
Wiest R, Lawson M, Geuking M (2014) Pathological bacterial translocation in liver cirrhosis. J Hepatol 60(1):197–209. PubMed Epub 2013/09/03. eng
Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H et al (2013) Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol 58(5):911–921. PubMed Epub 2012/12/25. eng
Merli M, Lucidi C, Di Gregorio V, Giannelli V, Giusto M, Ceccarelli G et al (2015) The chronic use of beta-blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver Int 35(2):362–369. PubMed Epub 2014/05/20. eng
Thiele M, Albillos A, Abazi R, Wiest R, Gluud LL, Krag A (2015) Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: a meta-analysis of randomized trials. Liver Int. 35(8):2009–16. doi: 10.1111/liv.12782. Epub 2015 Feb 6. PubMed PMID: 25581713
Thiele M, Wiest R, Gluud LL, Albillos A, Krag A (2013) Can non-selective beta-blockers prevent hepatocellular carcinoma in patients with cirrhosis? Med Hypotheses 81(5):871–874. PubMed Epub 2013/09/26. eng
Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M et al (2014) Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 146(7):1680–1690 e1. PubMed Epub 2014/03/19. eng
Serste T, Melot C, Francoz C, Durand F, Rautou PE, Valla D et al (2010) Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 52(3):1017–1022
Krag A, Wiest R, Albillos A, Gluud LL (2012) The window hypothesis: haemodynamic and non-haemodynamic effects of beta-blockers improve survival of patients with cirrhosis during a window in the disease. Gut 61(7):967–969. PubMed Epub 2012/01/12. eng
Bajaj JS, Riggio O, Allampati S, Prakash R, Gioia S, Onori E et al (2013) Cognitive dysfunction is associated with poor socioeconomic status in patients with cirrhosis: an international multicenter study. Clin Gastroenterol Hepatol 11(11):1511–1516. PubMed Pubmed Central PMCID: 3808846. Epub 2013/05/28. eng
Riggio O, Amodio P, Farcomeni A, Merli M, Nardelli S, Pasquale C et al (2015) A model for predicting development of overt hepatic encephalopathy in patients with cirrhosis. Clin Gastroenterol Hepatol 13(7):1346–1352. PubMed Epub 2015/01/13. Eng
American Association for the Study of Liver Diseases (2014) European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 61(3):642–659. doi:10.1016/j.jhep.2014.05.042. Epub 2014 Jul 8. Review. PubMed PMID: 25015420
Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB et al (2010) Rifaximin treatment in hepatic encephalopathy. N Engl J Med 362(12):1071–1081. PubMed Epub 2010/03/26. eng
Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT et al (2014) Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol 12(8):1390–1397 e2. PubMed Epub 2013/12/25. eng
Sanyal A, Younossi ZM, Bass NM, Mullen KD, Poordad F, Brown RS et al (2011) Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy – a double-blind placebo-controlled study. Aliment Pharmacol Ther 34(8):853–861. PubMed Epub 2011/08/19. eng
Durand F, Buyse S, Francoz C, Laouenan C, Bruno O, Belghiti J et al (2014) Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol 60(6):1151–1157. PubMed Epub 2014/03/13. eng
Fialla AD, Israelsen M, Hamberg O, Krag A, Gluud LL (2015) Nutritional therapy in cirrhosis or alcoholic hepatitis: a systematic review and meta-analysis. Liver Int. 35:2072–2078. PubMed Epub 2015/02/04. Eng
Ruiz-Margain A, Macias-Rodriguez RU, Duarte-Rojo A, Rios-Torres SL, Espinosa-Cuevas A, Torre A (2015) Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: a prospective cohort study. Dig Liver Dis 47(4):309–314. PubMed Epub 2015/01/27. Eng
Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M et al (2014) Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 12(11):1920–1926.e2. PubMed Epub 2014/04/29. eng
Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M et al (2010) Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol 53(4):702–712. PubMed Epub 2010/07/17. eng
Villa E, Camma C, Marietta M, Luongo M, Critelli R, Colopi S et al (2012) Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 143(5):1253–1260 e1–4. PubMed Epub 2012/07/24. eng
Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M et al (2007) Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology 46(1):242–253. PubMed Epub 2007/06/29. eng
Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH (2015) Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology 61(2):703–711. PubMed Epub 2014/11/22. eng
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Krag, A., Merkel, C. (2016). Introduction: Prevention of Decompensation Versus Prevention of First Bleeding. In: de Franchis, R. (eds) Portal Hypertension VI. Springer, Cham. https://doi.org/10.1007/978-3-319-23018-4_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-23018-4_21
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23017-7
Online ISBN: 978-3-319-23018-4
eBook Packages: MedicineMedicine (R0)