Abstract
Several agronomic and horticultural crops, such as barley, cucumbers, oats, rice, sugarcane, and wheat, benefit from applications of silicon. Growth enhancements results, in part, from reductions in the intensities of plant diseases. For the rice-Pyricularia oryzae model pathosystem, the mechanical barrier formed from silicon polymerization below the cuticle and in the cell walls was the first proposed hypothesis to explain how this element reduced the number of blast lesions and the lesion sizes. However, new insights have revealed that silicon's effect on plant resistance to a number of diseases may also occur through mediated host plant resistance mechanisms against pathogen infection. Plants supplied with silicon exhibit potentiated activation of the phenylpropanoid pathway resulting in increases in total soluble phenolics and lignin. The activities of defense enzymes, such as chitinases and β-1,3-glucanases, are maintained at higher levels during infection and the transcription of defense related genes occur faster and with greater output. When plants are supplied with silicon and then challenged with a pathogen, there is an enhanced activation in antioxidant metabolism, which in turn, suppresses the damaging cytotoxic effect of the reactive oxygen species that causes lipid peroxidation in the cell membrane. At the physiological level, leaf gas exchange parameters of silicon-treated plants are higher upon pathogen infection for crops, such as common beans, rice, sorghum and wheat, indicating the ameliorating effect of this element on photosynthesis. Although our understanding of how silicon affects plants in response to infection has advanced, the exact mechanism(s) by which silicon modulates plant physiology through the potentiation of host defense mechanisms still requires further investigation at the genomics, proteomics, and metabolomics levels.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The beneficial effects of silicon in plants under biotic and/or abiotic stresses, whether direct or indirect, reportedly occur in a wide variety of crops such as barley, cucumbers, oats, rice, sugarcane and wheat. Although the most remarkable effect of silicon is the reduced intensity of a number of plant diseases in many crops of great economic importance, the hypothesis that was first proposed for this underlying phenomenon was a mechanical barrier resulting from silicon polymerization in plant tissue. However, in addition to this passive mechanical role played by silicon, a plethora of biological, physiological and molecular data now suggests that this element may act as a modulator of host resistance against pathogen infection . To gain further understanding of this subject, we will discuss the mechanisms involved in host resistance against pathogen infections as mediated by silicon as well as highlights of our current knowledge at the -omics level in this chapter.
The Physical Barrier Hypothesis
To establish a successful infection, plant pathogens must gain access to the host’s tissue by overcoming the physical barriers conferred primarily by wax, the cuticle and a thick cell wall (Freeman and Beattie 2008). The microscopic evidence used to explain how silicon may increase host resistance against plant diseases is based on the following: pre-formed defense barriers (a cuticle and cell wall) and post-formed defense barriers (papillae deposition and cell wall reinforcement at the infection sites) in an attempt to avoid or delay pathogen ingress.
The physical barrier was the first mechanism proposed to explain why silicon increased rice resistance to blast caused by Pyricularia oryzae. The great number of silicified bulliform cells in the epidermis of rice leaves was believed to act as a physical barrier that efficiently impeded or delayed penetration by P. oryzae (Ito and Hayashi 1931; Suzuki 1940; Hemmi et al. 1941). This physical barrier hypothesis gained more credence because a silica layer with a thickness of approximately 2.5 μm was observed beneath the cuticle of rice leaves and sheaths (Yoshida et al. 1962). This cuticle-silicon double layer was associated with a decrease in the number of blast lesions observed on the leaf blades and with a reduction in the number of infection pegs formed by the appressoria that pierced the underlying cell wall, allowing fungal access into the epidermal cell (Yoshida et al. 1962). Indeed, more detailed studies showed that the epidermal cell wall of plants that were supplied with silicon was made of an outer electron-dense silicon layer and an inner electron-translucent layer, which often had thin, electron-dense silicon layers embedded in cellulose microfibrils (Kim et al. 2002). Interestingly, the epidermal cell wall thickness was not significantly affected by silicon. However, the thickness ratios of silica layers to epidermal cell walls were much higher for plants from a resistant cultivar than for plants from a susceptible one. This finding supported the idea that silicified epidermal cell walls were closely associated with reduced blast severity for plants that were supplied with silicon (Kim et al. 2002). He and colleagues (2015) recently reported that most silicon was cross-linked with hemicellulose in the rice cell wall, which improved both the mechanical properties and the regeneration of the cell walls.
Seebold et al. (2001) noted that the reduced number of blast lesions (which were evaluated as the relative infection efficiency) on rice leaves from partially resistant and susceptible cultivars that were amended with silicon had fewer successful established infections per unit of inoculum, lending partial support to the physical barrier hypothesis. The reduction in the number of blast lesions as the silicon rates increased in the soil clearly indicated that silicon manifested its beneficial effects before the penetration peg from P. oryzae gained full access to the epidermal cell. Therefore, Seebold et al. (2001) proposed that silicon does more than just act as a physical barrier in rice resistance against blast. Based on light microscopy observations of the leaf adaxial surfaces from rice plants that were supplied with silicon, Hayasaka et al. (2008) noticed that the number of appressorial sites for P. oryzae with successful penetration was reduced in proportion to the amount of silicon deposited in the leaf epidermis. Although this fact does not necessarily support a cause-and-effect relation between the denser silicon layer and the reduced number of appressorial sites for P. oryzae with successful penetration, it is plausible that the denser silicon layer contributed to a longer incubation period. These studies emphasized the importance of the silicon deposition beneath the rice cuticle and in the cell wall to prevent or delay penetration by P. oryzae. Abed-Ashtiani et al. (2012) also observed that the blast severity dramatically decreased as the foliar silicon concentration increased when increasing silicon rates were added to the soil. In an oat-Blumeria graminis f. sp. avenae interaction, the fortification of the epidermal cell walls through silicification was also reported as a structural barrier against fungal penetration (Carver et al. 1998).
Although the silicification of the epidermal cell walls was believed to be the primary cause associated with the reduced number of leaf blast lesions, no direct supportive evidence was provided to show that the narrow fungal penetration peg did not actually overcome the cuticle-silicon double layer and the epidermal cell silicification. For many years, the density of silicified cells in the leaf epidermis of some rice cultivars was known for not being proportional to their level of blast resistance always (Hashioka 1942; Kawamura and Ono 1948). This effect may be related to the fact that the silicified cells, in which silicon was deposited and polymerized in the form of amorphous silica bodies, were not uniformly distributed throughout the leaf surfaces, thereby leaving unprotected areas of the leaf surface exposed to pathogen penetration (Kim et al. 2002; Motomura et al. 2004; Ma and Yamaji 2006; Cacique et al. 2013). Rodrigues and his colleagues (2005) noted a decrease in the number of leaf blast lesions in rice plants that were supplied with silicon, which was likely caused by the inability of the P. oryzae-formed appressoria to overcome the physical impediment created by the cuticle-silica double layer. However, the presence of silica cells and silica bodies were again observed to be not uniformly distributed in the adaxial epidermis of leaves, and, as a consequence, they may have allowed successful fungal penetration at some infection sites.
It is known that the resistance of epidermal cells against fungal penetration is not strictly related to the increased thickness of the cuticle-silicon double layer or the number of silica cells found in the leaf epidermis (Rodrigues et al. 2005). Studies measuring the puncture resistance of rice epidermal cells to a needle tip from beneath a torsion balance in leaves were collected from rice plants that had been supplied with different silicon rates, and the results suggest that the puncture resistance was not explained solely by leaf epidermis silicification (Ishiguro 2001). Rather, this resistance might also be attributed to the nature of the epidermal cell protoplasm (Ito and Sakamoto 1939). Schurt et al. (2012) also observed that the leaf sheaths of rice plants that were supplied with silicon had increased puncture resistance. Therefore, the high silicon deposition most likely contributed to a delay in leaf sheath colonization by Rhizoctonia solani.
Domiciano et al. (2013) noted that the time needed for Bipolaris sorokiniana ingress into wheat epidermal cells was lengthened, and the foliar tissue colonized by the fungus was reduced because of the physical barrier formed by the double cuticle-silicon layer. According to these authors, this physical barrier may have reduced the diffusion of the lytic enzymes and the non-host selective toxins released by the pathogen at the leaf surface as shown by the reduced degradation of the waxy layer. Sousa et al. (2013) investigated the effect of silicon on cytological aspects arising from the infection of wheat leaves by P. oryzae at the microscopic level. According to these authors, P. oryzae hyphae grew successfully and formed an extensively branched mycelium in the first-invaded epidermal cell and then invaded several neighboring leaf cells from plants that were not supplied with silicon. By contrast, the leaves of silicon-supplied plants contained fungal hyphae that were restricted to the first-invaded epidermal cell. The number of brown (necrotic) adaxial epidermal cells and their browning intensities were significantly lower for silicon-supplied plants than those that were not supplied with silicon. The frequency of appressorial sites that exhibited a type B reaction (infection hyphae within the epidermal cell and an absence of cytoplasm granulation) was lower for silicon-supplied plants than for those that were not supplied from 72 to 96 h after inoculation, and the frequency of appressorial sites showing a type A reaction (unsuccessful penetration) was much higher in comparison with the non-supplied plants as well. Schurt et al. (2015) used light microscopy and scanning electron microscopy to observe the reduced growth of R. solani on the leaf sheaths of rice plants that were supplied with silicon, which exhibited intense autofluorescence in tissues near necrotic areas because of fungal colonization.
In addition to the reinforcement of cell walls by silicon, the formation of papillae has also been greatly stimulated by this element. Carver et al. (1987) observed localized silicon deposition in host cells beneath the appressoria of B. graminis f. sp. hordei, which failed to penetrate the barley epidermal cells. Silicon accumulation was found to occur in the haustorial neck and collar area of the fungus as well as in the papillae, regardless of the outcome of attempted penetration. At 20 h after inoculation, when the successful and failed penetration attempts became evident, the silicon concentration was three to four times greater at the infection sites where fungal penetration failed in comparison with the infection sites where successful fungal penetration occurred. The absence of high background silicon levels in the barley epidermal cells that were distant and adjacent to the penetrated cells suggested that the cuticle-silicon double layer likely did not play a role in the increasing barley resistance to powdery mildew, unlike the findings reported earlier in rice epidermal cells against P. oryzae (Kim et al. 2002). This finding further suggests that silicon was deposited in response to the penetration of the barley epidermal cells by B. graminis f. sp. hordei (Carver et al. 1998). Zeyen et al. (1993) demonstrated that barley epidermal cells would produce papillae in response to infections by B. graminis f. sp. hordei in the presence of soluble silicon . This finding suggested that an active process occurs in the cytoplasmic aggregate that then presumably concentrated soluble silicon and prevented its polymerization before it was transported across the plasma membrane into the epidermal cell wall and mature papillae. Jiang (1993) experimentally interrupted the papilla deposition in barley leaf epidermal cells that were and were not supplied with silicon, and were inoculated with B. graminis f. sp. hordei. The researchers noted a delay in the fungal penetration of leaves from silicon-supplied plants before papilla formation. Because soluble silicon was abundant at that time, it is unlikely that the physical barrier that was formed by insoluble silicon was more important in increasing resistance to penetration. Furthermore, the deposition of silicon appears to have required the availability of phenolics and hydroxyproline-rich glycoproteins in the leaf epidermal cells to prevent haustorium formation by Uromyces vignae in the leaves of French beans (Perumalla and Heath 1991). According to these authors, although the callose and cell wall reinforcement contributed to preventing haustorium formation, silicon deposition played the most pivotal role in this process. Kauss et al. (2003) reported that a strongly cationic, proline-rich protein reinforced the cell wall at the infection sites, and silicon deposition was enhanced during the development of systemic acquired resistance in cucumber leaves in response to Colletotrichum lagenarium infection, thus preventing fungal infection. In roses, the quantity of papillae was greater in the leaf cells of plants that were supplied with silicon in response to Podosphaera pannosa infection (Shetty et al. 2012). In wheat, the epidermal cells of plants that were supplied with silicon reacted against B. graminis f. sp. tritici infection by massive papilla formation (Bélanger et al. 2003). Pozza et al. (2004) reported a thicker cuticle on the lower leaf surface of coffee seedlings that received silicon. This thickened cuticle helped to reduce the penetration of Cercospora coffeicola and subsequently reduced the number of leaf lesions that developed. Taken together, these observations indicate that the proposed physical silicon barrier enhances resistance by decreasing the intensity of a number of plant diseases, and the mechanism is likely very complex.
The contribution of the physical barrier to the mechanism of silicon-conferred resistance against plant diseases (as conferred by silicon polymerization beneath the cuticle and in the cell wall) is still not widely agreed on. According to Fauteux et al. (2005), a silicon accumulation as determined by scanning electron microscopy and X-ray microanalysis at the leaf infection sites of Arabidopsis thaliana by Erysiphe cichoracearum was attributed to higher transpiration rates caused by cuticle damage rather than active transport. According to Samuels et al. (1991), cucumber plants that were transferred from pots containing 100 ppm of silicon to pots without silicon had higher powdery mildew severities. According to these authors, the presence of polymerized silicon in the cucumber leaves before the inoculum arrival did not seem to be more important than the constant presence of soluble silicon during the time course of the fungal infection. Sun et al. (2010) showed that rice plants that were initially grown in the presence of silicon and were then switched to a nutrient solution without this element prior to inoculation with P. oryzae still exhibited lower blast severity. However, the disease severity levels were greater for this treatment when compared with those of inoculated plants that received a continuous supply of silicon. It is likely that insoluble silicon affected blast development by physically strengthening the cell wall and thus reduced fungal leaf colonization (Sun et al. 2010). However, the authors also highlighted that the deposited (insoluble) silicon may not be as important as the available silicon (soluble) that is found in the cells at the time of fungal infection for reducing disease severity (Sun et al. 2010). These findings help to support the concept that a reduction in disease intensities cannot solely be attributed to the presence of insoluble silicon in the papillae and cell wall as reported for cucumber epidermal cells. Furthermore, Chérif et al. (1992b) observed the deposition of silicon in needle-punctured leaf holes and the absence of these deposits when the plants were grown under saturated humidity. This finding again suggested that when silicon accumulated in areas in which the cuticle was damaged, this accumulation was caused by an increase in the transpiration rate. Menzies et al. (1991) reported a negative correlation between a high foliar silicon concentration in cucumber plants with the leaf area covered by Sphaerotheca fuliginea colonies, the number of colonies per leaf, the individual colony size and the germination of conidia. These authors believed that the increased resistance of cucumber leaves to powdery mildew was associated with the reinforcement of epidermal cell walls by silicon. Samuels et al. (1991) also noted that the accumulation of silicon around powdery mildew colonies on cucumber leaves affected the fungal growth and, consequently, the diameter of the colonies.
Taken together, these studies clearly showed that silicon is deposited below the cuticle and in the cell walls, and it contributed in part to increased physical resistance against pathogen penetration. However, insoluble silicon may not be more important than soluble silicon in enhancing resistance to infection by plant pathogens. The resistance of plants that were supplied with silicon to both soil-borne and foliar diseases is a very complex phenomenon, and the physical resistance barrier is likely only one small aspect of how silicon confers plant disease resistance.
Biochemical and Molecular Aspects of Silicon-Mediated Host Resistance to Pathogens
Wheat plants that were supplied with silicon produced fungitoxic aglycones in response to Blumeria graminis f. sp. tritici infection as demonstrated by TLC chromatogram analyses coupled with bioassays (Rémus-Borel et al. 2005). According to these authors, after the leaf fractions were analyzed by high-performance liquid chromatography and comparative analyses of the profiles were performed, at least three compounds were confirmed to occur in higher amounts for inoculated wheat plants that had been supplied with silicon (Rémus-Borel et al. 2005). Fresh transverse sections of leaves from wheat plants that were supplied with silicon and infected with B. graminis f. sp. tritici were analyzed by fluorescence microscopy, and intense autofluorescence was observed. This finding suggested that the presence of phenolics likely contributed to the collapse of conidial chains at the examined fungal infection sites (Rémus-Borel et al. 2005). Rémus-Borel et al. (2009) further investigated if trans-aconitate (TA) could act as a precursor of methylated TA forms in wheat, and they addressed the possible relations between the silicon supply, disease development, and TA and methyl TA concentrations in leaf tissues. According to these authors, the TA concentration in non-inoculated plants increased as the disease progressed, regardless of the presence of silicon. By contrast, the TA concentration remained fairly constant in the leaf tissue of inoculated plants regardless of whether silicon was present. However, for plants that were supplied with silicon, the TA concentration was significantly lower than it was for the plants that were not supplied with silicon. For the inoculated plants supplied with silicon, an increase in wheat resistance to powdery mildew was closely associated with the methyl TA concentration. Silicon apparently had an effect on the methyl TA concentration only for inoculated plants, suggesting that this element does not act directly on the TA concentration, but increases the production of methyl TA for infected plants. Based on the increase in methyl TA and the leveling off of the TA concentration, it appears that the latter, instead of accumulating, was used by the diseased plants to produce methylated forms of antifungal TA so that they would act as phytoalexins to decrease disease development. This observed phenomenon was more pronounced for silicon-supplied plants (Rémus-Borel et al. 2009).
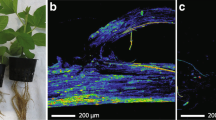
Rodrigues et al. (2003) provided the first cytological evidence that silicon-mediated resistance to P. oryzae in rice was correlated with a specific leaf cell reaction that interfered with pathogen development. Ultrastructural observations of samples from plants that were not supplied with silicon revealed that some host cells were devoid of organelles and that some host cell walls were no longer discernible in the massively colonized mesophyll and vascular bundle (Fig. 5.1a and b). A light deposition of osmiophilic material with a granular texture that occasionally interacted with fungal walls was observed in some epidermal cells (Fig. 5.1c, arrows). In plants that received silicon, empty fungal hyphae were evenly surrounded by a dense layer of granular osmiophilic material that partially occluded the epidermal cells (Fig. 5.1d, arrows), the vascular bundle (Fig. 5.1e, arrowheads) and the mesophyll cells (Fig. 5.1f, arrows). The cytochemical labeling of chitin revealed no difference in the pattern of chitin localization over fungal cell walls regardless of the presence of silicon at 96 h after inoculation, indicating a limited production of chitinases by the rice plant as a mechanism of defense. However, the occurrence of empty fungal hyphae that were surrounded or trapped in amorphous material, which were found in samples from plants that were supplied with silicon, suggested that phenolic-like compounds or phytoalexins played a primary role in rice defense response against P. oryzae infection. In a further study, Rodrigues et al. (2004) provided evidence that higher levels of momilactone phytoalexins were found in leaf extracts from plants that were inoculated with P. oryzae and supplied with silicon than in leaf extracts from inoculated plants that were not supplied with silicon or were not inoculated and supplied with silicon. On this basis, the more efficient terpenoid pathway stimulation in the plants receiving silicon and, consequently, the increase in the momilactone levels, appeared to be a factor that contributed to enhanced rice resistance to blast. Maekawa et al. (2002) observed a dramatic increase in superoxide generation in the rice leaves of plants that were supplied with silicon 15 min after P. oryzae inoculation. After this time, the superoxide generation rapidly decreased to levels observed for inoculated plants that had not been supplied with silicon. Fortunato et al. (2014) performed a study to investigate, at the histochemical level, whether silicon could enhance the production of phenolics in banana roots in response to F. oxysporum f. sp. cubense infection. According to these authors, intense orange-yellow autofluorescence was detected in the metaxylem and phloem vessels of the root sections of inoculated plants that were not supplied with silicon at 24 and 32 dai, respectively (Figs. 5.2a and 5.3a). Autofluorescence was also observed in the phloem vessels and the sclerenchyma cells in the root sections of the inoculated plants that received silicon at 24 and 32 dai (Figs. 5.2b and 5.3b). For non-inoculated plants, the autofluorescence in the medulla and in the cortex was weak regardless of whether silicon was provided. There was an absence of fluorescence in the root sections of the inoculated plants that were not supplied with silicon when they were treated with both Neu’s and Wilson’s reagents, which are used to stain flavonoid compounds, at 24 and 32 dai (Figs. 5.2c and e; 5.3c and e). At 24 dai, a strong yellow-orange fluorescence was observed in the phloem and a lemon-yellow fluorescence was observed in the sclerenchyma and metaxylem vessels in the root sections of the inoculated plants that had been supplied with silicon stained with Neu’s reagent (Figs. 5.2d and 5.3d). With Wilson’s reagent, an orange-yellow autofluorescence was more pronounced around the phloem vessels, and a yellow fluorescence was more pronounced around the metaxylem vessels in the root sections of the inoculated plants that received silicon at 24 and 32 dai (Figs. 5.2f and 5.3f). Lignin was densely deposited in the cortex of the root sections in the inoculated plants that received silicon (Figs. 5.2h and 5.3h) in comparison with the root sections of the inoculated plants that did not at 24 dai (Fig. 5.2g) and 32 dai (Fig. 5.3g). Dopamine was barely detected in the root sections of the inoculated plants that were not supplied with silicon at 24 and 32 dai according to lactic and glyoxylic acid stains (Figs. 5.2i and 5.3i). However, dopamine was strongly suspected to occur in the phloem and metaxylem vessels of the root sections in inoculated plants that were supplied with silicon, and it was confirmed by the intense orange-yellow fluorescence detected at 24 (Fig. 5.2j) and 32 dai (Fig. 5.3j). Da Silva et al. (2015) investigated whether silicon could enhance the production of flavonoids in wheat leaves in response to P. oryzae infection at the histochemical level. According to these authors, a high foliar silicon concentration was correlated with reduced fungal growth inside the epidermal cells. A strong fluorescence, which was an indication of the presence of flavonoids, was detected in the leaf cells of plants that received silicon. According to Carver et al. (1998), oat plants deprived of silicon showed increased phenylalanine ammonia-lyase activity and, consequently, there was increased accumulation of phenolic compounds in the epidermal cells colonized by B. graminis f. sp. avenae. In other words, the activation of the energy-expensive phenylpropanoid pathway likely replaced the fortification of epidermal cell walls by silicon. By contrast, Bélanger et al. (2003) found that the greatest cytochemical difference between wheat plants that were and were not supplied with silicon was the extensive deposition of glycosylated phenolics, as determined by cytochemical labeling, in the cell wall of infected epidermal cells in silicon-supplied plants as well as on the extra-haustorial membrane of B. graminis f. sp. tritici. The autofluorescence of barley epidermal cells upon B. gramini f. sp. hordei infection showed a hypersensitive response. This finding suggested that phenolics were present and likely occurred before silicon accumulated to neutralize the dead cell contents while providing the strength and integrity of the surrounding epidermal cells (Koga et al. 1988).
Transmission electron micrographs of leaf samples collected from rice plants non-supplied (−Si) and supplied (+Si) with silicon (Si) at 96 h after inoculation with Pyricularia oryzae. (a) Ultrastructurally normal fungal hyphae colonize both the epidermis and mesophyll. Host cell walls are no longer discernible in the mesophyll (−Si). Bar = 2 μm. (b) The vascular bundle is massively colonized by the fungal hyphae (−Si). Bar = 5 μm. (c) Some amorphous material (arrows) accumulates in an epidermal cell and irregularly interacts with a fungal cell wall (−Si). Bar = 1 μm. (d) A dense amorphous material (arrows) accumulates around an empty fungal hyphae in the epidermal cell and also is found in an epidermal cell neighboring the colonized one (+Si). Bar = 1 μm. (e) Fungal hyphae invading the vascular bundle are often surrounded by dense amorphous material and often reduced to empty shells (+Si; arrowheads). Bar = 2 μm. (f) Two fungal hyphae in a mesophyll cell are evenly coated by the amorphous material (+Si; arrows) Bar = 1 μm. AM Amorphous material, F fungal hyphae, E epidermis, M mesophyll, HCW host cell wall, and V vascular bundle (Reproduced from Datnoff and Rodrigues (2005))
Histochemical characterization of flavonoids, lignin and dopamine in the roots of banana plants cultivar Maçã supplied (+Si) (b, d, f, h and j) or non-supplied with silicon (−Si) (a, c, e, g and i) at 24 days after inoculation with Fusarium oxysporum f. sp. cubense. (a) Metaxylem vessels located in the vascular bundles of the roots of the −Si plants exhibit a yellow-orange autofluorescence (arrow). (b) The roots of +Si plants exhibit slight yellow-orange autofluorescence (arrow) Fig. 5.2 (continued) on the phloem and metaxylem vessels. (c) No fluorescence was observed in the roots of the−Si plants after staining with Neu’s reagent. (d) Strong yellow-orange fluorescence (arrow) observed in the phloem to lemon-yellow fluorescence in the sclerenchyma and metaxylem vessels in the roots of the +Si plants after staining with Neu’s reagent. (e) Metaxylem vessels on the roots of −Si plants stained with Wilson’s reagent exhibited slight orange fluorescence (arrow). (f) Intense orange yellow fluorescence (arrowheads) in the cells neighboring the vascular bundles of phloem and metaxylem vessels in the roots of +Si plants stained with Wilson’s reagent. (g) No evidence of lignin deposition in the roots of −Si plants after staining with phloroglucinol-HCl. (h) Strong lignin deposition in the cortex of roots of +Si plants stained with phloroglucinol-HCl (arrow). (i) Absence of dopamine in the roots of −Si plants. (j) Dopamine was strongly suspected to occur in the vascular bundles of phloem and metaxylem vessels (arrow) of roots of +Si plants as confirmed by orange-yellow fluorescence after staining with lactic acid + glyoxylic acid stain. c cortex, e endodermis, mx metaxylem, p phloem, and s sclerenchyma. Bars = 50 μm (Reproduced from Fortunato et al. (2014), with permission from the American Phytopathological Press)
Histochemical characterization of flavonoids, lignin and dopamine in the roots of banana plants cultivar Maçã supplied (+Si) (b, d, f, h and j) or non-supplied with silicon (−Si) (a, c, e, g and i) at 32 days after inoculation with Fusarium oxysporum f. sp. cubense. (a) Strong yellow-orange autofluorescence (arrow) in the vascular bundles and in the sclerenchyma cells in the roots of −Si plants. (b) Slight yellow-orange autofluorescence (arrow) observed near the phloem and metaxylem vessels in the roots of +Si plants. (c) Absence of fluorescence in the vascularFig. 5.3 (continued) bundles,sclerenchyma and endodermis cells in the roots of −Si plants stained with Neu’s reagent. (d) Strong yellow fluorescence in the roots of +Si plants stained with Neu’s reagent (arrow). (e) Slight orange-yellow fluorescence in the cells surrounding the phloem and metaxylem vessels and the sclerenchyma cells in the roots of −Si plants stained with Wilson’s reagent. (f) Strong orange-yellow fluorescence in the phloem vessels and in the sclerenchyma cells in the roots of +Si plants stained with Wilson’s reagent (arrow). (g) Absence of lignin deposition in the roots of −Si plants stained with phloroglucinol-HCl. (h) Strong lignin deposition in the cortex of the roots of +Si plants stained with phloroglucinol-HCl (arrow). (i) Absence of dopamine in the roots of −Si plants. (j) Dopamine was strongly suspected to occur in the phloem and metaxylem vessels (arrow) of the roots of +Si plants as confirmed by orange-yellow fluorescence after staining with lactic acid + glyoxylic acid stain. c cortex, e endodermis, mx metaxylem, p phloem, and s sclerenchyma. Bars = 50 μm (Reproduced from Fortunato et al. (2014), with permission from the American Phytopathological Press)
Defense responses have also been reported for dicots such as cucumbers when amended with silicon and then infected by fungal pathogens. Menzies et al. (1991) reported that a great number of leaf cells in cucumber plants that were supplied with silicon and inoculated with Podosphaera xanthii showed a rapid accumulation of phenolic-like compounds. Biochemical analyses of leaf extracts from cucumbers that received silicon and were inoculated with P. xanthii indicated the presence of flavonoids and phenolic acids that accumulated specifically and strongly in a manner typical of phytoalexins (Fawe et al. 1998). The root cells of cucumbers that were supplied with silicon showed a rapid and more extensive accumulation of electron-dense, phenolic-like material with antifungal activity against the root rot pathogen Pythium ultimum (Chérif et al. 1992a). Moreover, Chérif et al. (1994) noted an increase in the activities of chitinases, peroxidases and polyphenoloxidases in cucumber leaves from plants that received silicon and were inoculated with P. ultimum than in infected plants that did not receive silicon. Additionally, leaf extracts from plants that were supplied with silicon and inoculated with P. ultimum showed a marked increase in the concentration of antifungal phenolic compounds. Dann and Muir (2002) reported that pea seedlings that received silicon showed an increase in chitinase and β-1,3-glucanase activities prior to Mycosphaerella pinodes inoculation. In addition, few lesions were observed on pea leaf seedlings that were supplied with silicon than on the seedlings that were not supplied with this element. In an incompatible cowpea-Uromyces vignae interaction, Heath (1981) observed that silicon was associated with electron-opaque regions of the haustorium encasement, the necrotic host cytoplasm and adjacent host cell walls and wall deposits. Although silicon accumulation was not involved in haustorium formation, this element could have been associated with the phenolics found in many disorganized cowpea epidermal cells. Heath and Stumpf (1986) also observed that in cowpea plants supplied with silicon, fungal development apparently ceased while the penetration peg was traversing the haustorial mother cell wall, often before the peg reached the adjacent silicified plant cell wall. However, in plants that were not supplied with silicon, haustorial mother cells for three out of ten infection sites had already formed a haustorium. In the majority of the remaining infection sites, fungal growth appeared to have ceased before the initiation of a visible penetration peg as well as during a stage of development that was observed when the haustorial mother cell and the host cell wall were bridged by an electron-opaque material. The fact that most penetration pegs stopped their development earlier in plants that did not receive silicon than in plants that received silicon supports the previous suggestion that the higher levels of wall-associated phenolic compounds in the former resulted in a faster inhibition of the hydrolytic enzymes that were released by the fungus and were involved in the formation of the penetration peg. These and other ultrastructural observations suggested that the silicified cell walls in silicon-supplied plants may have reduced the interchange of materials between the host and the fungus. As a consequence, the resulting phenolic materials would restrict the flow of materials to the haustorial mother cell that normally prevents its premature senescence, or they would act as a physical barrier if the penetration peg reaches the host cell wall. This finding is in accordance with previous reports that silicon might form complexes with organic compounds in the epidermal cell walls, consequently increasing their resistance to degradation by the hydrolytic enzymes and non-host-selective toxins released by plant pathogens (Volk et al. 1958; Inanaga et al. 1995a, b). Li and Heath (1990) found an increase in the number of U. vignae haustoria, but they found a reduction in the concentration of silicon and in the intensity of the autofluorescence of mesophyll cell walls when injecting abscisic acid and gibberellic acid into bean leaves. Rose plants that were supplied with silicon showed an increase in the concentration of antimicrobial phenolic acids and flavonoids in response to infection by Podosphaera pannosa (Shetty et al. 2011). In addition, phenylpropanoid pathway genes such as those coding for phenylalanine ammonia-lyase, cinnamyl alcohol dehydrogenase and chalcone synthase were up-regulated for rose plants that were supplied with silicon (Shetty et al. 2011).
For incompatible and compatible rice-P. oryzae interactions, the differential accumulations of glucanase, peroxidase and PR-1 transcripts were associated with limited fungal colonization in the epidermal cells for a blast-susceptible cultivar (Rodrigues et al. 2005). However, the resistant cultivar responded against fungal penetration by developing a hypersensitive response that was associated with a strong induction of PR-1 and peroxidase transcripts regardless of whether silicon was supplied (Rodrigues et al. 2005). Cai et al. (2008) showed that the lower leaf blast disease severity in rice plants that received silicon was linked to higher activities in peroxidases, polyphenoloxidases and phenylalanine ammonia-lyases. Perennial ryegrass plants that were supplied with silicon and were infected by P. oryzae exhibited the increased production of several phenolic acids, including chlorogenic acid and flavonoids, greater peroxidase and polyphenoloxidase activities and higher relative expression levels of the genes encoding phenylalanine ammonia-lyase and lipoxygenase compared with the non-silicon-supplied plants (Rahman et al. 2015). For the pathosystems banana-Fusarium oxysporum f. sp. cubense, coffee-Meloidogyne exigua, cotton-Colletotrichum gossypii var. cephalosporioides, cotton-Ramularia areola, rice-Monographella albescens, rice-R. solani, soybean-Phakopsora pachyrhizi and tomato-Pseudomonas syringae pv. tomato, the activity of phenylalanine ammonia-lyase, the key enzyme in the phenylpropanoid pathway that is responsible for the production of different types of phenolics with antimicrobial properties, increased when plants were supplied with silicon (Silva et al. 2010; Fortunato et al. 2012; Andrade et al. 2013; Cruz et al. 2013; Curvelo et al. 2013b; Guerra et al., 2013a; Tatagiba et al. 2014; Schurt et al. 2014). The greater phenylalanine ammonia-lyase activity was linked to an increase in the concentrations of total soluble phenolics and lignin -thioglycolic acid derivatives in the leaves of banana, coffee and cotton plants that were supplied with silicon and to a decrease in disease development (Silva et al. 2010; Fortunato et al. 2012; Curvelo et al. 2013b; Guerra et al. 2013a, b). Moreover, the beneficial effect of silicon for suppressing infections in the banana-F. oxysporum f. sp. cubense, cotton-Colletotrichum gossypii var. cephalosporioides, cotton-Phakopsora gossypii, coffee-M . exigua, rice-Bipolaris oryzae, rice-M. albescens, rice-R. solani, sorghum-Colletotrichum sublineolum, soybean-Phakopsora pachyrhizi and tomato-Pseudomonas syringae pv. tomato pairings was in part explained by an increase in the activities of defense-related enzymes such as peroxidases, polyphenoloxidases, β-1,3-glucanases, and chitinases as well as by an increase in the anthocyanin concentrations for sorghum (Silva et al. 2010; Dallagnol et al. 2011; Fortunato et al. 2012; Tatagiba et al. 2014; Andrade et al. 2013; Cruz et al. 2013; Guerra et al. 2013a, b; Resende et al. 2013; Schurt et al. 2014). Silva et al. (2012) investigated the effects of silicon (0 and 2 mmol) and manganese (0.5, 2.5 and 10 μmol) rates on the activities of peroxidases, polyphenoloxidases and phenylalanine ammonia-lyases in rice that was infected by B. oryzae, and they observed that the activities of these three enzymes were not boosted by silicon at any manganese rate. In some rare cases, silicon may not contribute to increased host resistance to disease. Nascimento et al. (2014) examined the response of the soybean cultivars Bossier and Conquista that were or were not supplied with silicon to frogeye leaf spot (Cercospora sojina). These authors looked for defense enzyme activities, cell wall-degrading enzymes produced by the fungus (cellulases, xylanases, pectin methyl esterases and polygalacturonase) as well as concentrations of total soluble phenolics and lignin-thioglycolic acid derivatives. According to their findings, the severity of frogeye leaf spot was greater in the Bossier cultivar (susceptible) than in the Conquista cultivar (resistant) as well as in the plants receiving silicon compared with those that did not receive silicon. Except for the concentrations of total soluble phenolics and lignin-thioglycolic acid derivatives, the activities of the defense enzymes and the cell wall-degrading enzymes did not change for non-inoculated plants that were supplied with silicon regardless of the cultivar. The activities of lipoxygenases, phenylalanine ammonia-lyases, chitinases, and polyphenoloxidases as well as the activities of cell wall-degrading enzymes decreased for the inoculated plants that were supplied with silicon and likely compromised their resistance to frogeye leaf spot.
Schurt et al. (2013a) used analytical pyrolysis coupled with gas chromatography and mass spectrometry to investigate possible changes in the chemical composition of lignin in leaf sheaths for the BR-Irga 409 and Labelle rice cultivars that were not and were supplied with silicon and were infected with R. solani. Based on the resulting mass spectra, 33 compounds were identified, ten of which were products from the degradation of carbohydrates and 23 of which were derived from lignin. From the lignin derivatives, eight compounds were of the p-hydroxyphenyl type, 11 compounds were of the guaiacyl type and four compounds were of the syringyl type. From the leaf sheaths of both cultivars, the concentrations of lignin (p-hydroxyphenyl, syringyl (S) and guaiacyl (G)) were approximately 15 %, regardless of whether silicon was present. There was no increase in the S/G ratio except in the leaf sheaths of BR-Irga 409 that were supplied with silicon and infected with R. solani. The high silicon concentration in the leaf sheaths of both cultivars, which in turn resulted in an increase in the S/G ratio, most likely contributed to a reduction in the area under the progress curve for sheath blight. In another study, Schurt et al. (2013b) investigated the effect of silicon on the concentrations of soluble and insoluble lignin and sugars in rice leaf sheaths that were infected by R. solani. Based on their results, there was no effect from silicon or fungal inoculation on the concentrations of arabinans, galactans, glucans, mannans, sugars, and xylans for the BR-Irga-409 and Labelle cultivars. In addition, no variation was detected in the concentrations of insoluble, soluble and total lignin between the cultivars. The concentrations of total and insoluble lignin were higher for plants that were supplied with silicon regardless of whether they were inoculated. In conclusion, the authors hypothesized that the rice plants that were supplied with silicon were more resistant to sheath blight because of an increase in the lignifications of the leaf sheath tissues and the lower concentration in total sugars.
The silicon effect on the potentiation of host resistance that leads to an increased synthesis of antimicrobial compounds depends on whether this element is supplied via foliar application or via the roots. Foliar-applied silicon can successfully reduce infections of Podosphaera xanthii in cucumbers and melons, Hemileia vastatrix in coffee, Bipolaris oryzae in rice and Pseudomonas syringae pv. tomato in tomato by affecting pathogen penetration, but this application is never as effective as silicon root applications (Carré-Missio et al. 2009; Rezende et al. 2009; Liang et al. 2005; Andrade et al. 2013; Dallagnol et al. 2015). Because no silicon transporter genes have been identified to date for plant foliage, this foliar effect is likely related to the formation of a physical barrier after the deposition of the material on the leaf surface and/or by an osmotic or pH effect on germinating conidia. Rezende and colleagues (2009) partially demonstrated this finding for brown spot development in rice by showing that foliar applications of silicon to the adaxial leaf surface had practically the same x-ray microanalysis intensity for silicon as applying silicon to the roots. However, for the abaxial leaf surface, the foliar silicon x-ray microanalysis was identical to the non-amended control, whereas applying silicon to the roots expressed the same x-ray microanalysis intensity as the adaxial side of the same treatment. When these authors compared the silicon concentrations in rice tissue among the non-amended control, foliar-applied and applying silicon to the roots treatments, only root-supplied silicon showed significant plant tissue uptake of this element. Furthermore, both Liang et al. (2005) and Dallagnol et al. (2015) demonstrated that when comparing foliar with applying silicon to the roots, only the root applications and not the foliar applications of silicon potentiated plant defense responses such as the activities of peroxidases, polyphenoloxidases, β-1,3-glucanases and chitinases (Pereira et al. 2009a, b; Liang et al. 2005; Carré-Missio et al. 2009; Andrade et al. 2013; Dallagnol et al. 2015). Proposed models for the modes of action of potassium silicate, a source of soluble silicon , is shown in Fig. 5.4a and b for foliar or root applications.
Models proposed for the modes of action of potassium silicate (PS) when applied to the leaves (a) or via the roots (b). The numbers 1, 2 and 3 on top of the yellow circle represent the host defense mechanism s . (a) 1 – conidial germination is inhibited due to the deposition of PS on the leaf surface, 2 – the penetration peg is inhibited due to the deposition of PS on the leaf surface, 3 – a leaf region without the deposition of PS favor fungal penetration; (b) 1 – the fungal peg penetration is inhibited due to the presence of silica bodies inside the epidermal cells, 2 – a leafFig. 5.4 (continued) region without silica bodies. The host defense is potentiated by the presence of soluble silicon at the fungal infection site. Continuous blue lines indicate the stimulated route; dashed green lines indicate the probable stimulated route; continuous red lines indicate the repressed route; dashed red lines indicate the probable repressed route; gray lines indicate routes that are not directly affected. ap appressorium, CAT catalase, CHI chitinase, cn conidium, ct cuticle, cw cell wall, da direct action on pathogen, GLU β-1,3-glucanases, H 2 O 2 hydrogen peroxide, lp lipid peroxidation, O 2 − superoxide anion, OH − hydroxyl radical, ph penetration hyphae, pm plasma membrane, POX peroxidases, PPO polyphenoloxidases, Psp polymerized potassium silicate, Si soluble silicon, SOD superoxide dismutases and tm transition metal
Several studies have reported a link between the silicon supply and an improvement in the antioxidant metabolism of plants when they are infected by plant pathogens. The rapid production of reactive oxygen species (ROS) in the apoplast in response to infections by these pathogens has been proposed as one way in which a plant may orchestrate the establishment of defensive barriers, such as the strengthening of host cell walls via the cross-linking of glycoprotein, to delay host tissue colonization (Lamb and Dixon 1997; Torres et al. 2006). However, ROS are known to be toxic and can directly cause lipid peroxidation in the cell membrane, leading to a demand for increased capacity in the antioxidant system to scavenge them (Lamb and Dixon 1997). Lipid peroxidation was dramatically alleviated for the banana-F. oxysporum f. sp. cubense, cotton-Ramularia areola, rice-P. oryzae, sorghum-C . sublineolum and wheat-P. oryzae interactions, as indicated by the lower malonic aldehyde concentration in plants that were supplied with silicon (Fortunato et al. 2012; Resende et al. 2012; Curvelo et al. 2013a; Debona et al. 2014; Domiciano et al. 2015). An increase in the activities of ROS-scavenging enzymes, such as ascorbate peroxidases, glutathione reductases, superoxide dismutases and catalases in plants receiving silicon restricted the ROS-dependent cellular damage that was indirectly linked to the high concentration of malonic aldehyde (Mohaghegh et al. 2011; Sun et al. 2010; Li et al. 2012; Resende et al. 2012; Curvelo et al. 2013a; Polanco et al. 2014; Domiciano et al. 2015).
In a proteomic analysis, Liu et al. (2014) found that the quantities of ascorbate peroxidase, dehydroascorbate reductase and superoxide dismutase were reduced after P. oryzae infection, but they increased for rice plants that were supplied with silicon. Collectively, the findings of these authors clearly suggest the pivotal role that is played by silicon in managing the ROS generated in response to infection by plant pathogens through an efficient activation of the ROS-scavenging systems. By contrast, Debona et al. (2014) demonstrated that wheat plants that were supplied with silicon and infected by P. oryzae showed lower cellular damage and decreased superoxide dismutase, catalase, peroxidase, ascorbate peroxidase and glutathione-S-transferase activities, which was postulated to occur because of the activation of other mechanisms that limited leaf tissue colonization by the fungus, therefore reducing cellular oxidative stress.
A few transcriptomic studies have been conducted in an effort to better understand the molecular mechanisms of silicon-mediated resistance to infection by plant pathogens. Interestingly, the beneficial effect has so far only been manifested when plants received silicon via the roots and were then challenge-inoculated by these plant pathogens (Fauteux et al. 2006; Chain et al. 2009; Ghareeb et al. 2011; Van Bockhaven et al. 2015). Fauteux et al. (2006) conducted a microarray study to examine the effect of silicon on the increased resistance of Arabidopsis plants against B. graminis f. sp. tritici infection. According to these authors, the expression of all but two genes was unaffected by silicon for non-inoculated plants. By contrast, for inoculated plants that were not and were supplied with silicon, the expression of a set of nearly 4,000 genes was dramatically altered. After a functional categorization, many of the up-regulated genes were found to be defense-related, whereas a large proportion of down-regulated genes were involved in primary plant metabolism. The regulated defense genes included R genes, stress-related transcription factors, genes involved in signal transduction, the biosynthesis of stress hormones (salicylic acid, jasmonic acid and ethylene) and the metabolism of ROS. In inoculated plants that were supplied with silicon, the magnitude of down-regulation was attenuated by more than 25 % (Fauteux et al. 2006). Chain et al. (2009) performed a large transcriptomic analysis (55,000 unigenes) to compare the differential responses of wheat plants that were not and were supplied with silicon and were not or were inoculated with B. graminis f. sp. tritici. The response to the silicon supply in the non-inoculated plants was limited to 47 genes with diverse functions, and there was little evidence of the regulation of a specific metabolic process. For the inoculated plants, there was an up-regulation of many genes that were linked to stress and metabolic processes and a down-regulation of genes linked to photosynthesis . For plants that were supplied with silicon and infected by B. graminis f. sp. tritici, the disease symptoms were reduced and translated into a nearly perfect reversal of genes that were regulated in infected plants that had not received silicon. According to these authors, silicon played a limited role in the wheat transcriptome in the absence of fungal infection. However, the benefits of silicon in reducing disease symptoms were remarkably aligned with a counter-response to transcriptomic changes upon fungal infection. According to the microarray analysis of tomato plants that were infected with Ralstonia solanacearum as conducted by Ghareeb et al. (2011), there was an up-regulation in the expression of jasmonic acid/ethylene marker genes, oxidative stress marker genes and basal defense marker in the presence of silicon. These findings help to explain partly why the wilt symptoms caused by R. solanacearum were dramatically reduced.
Brunings and her colleagues (2009) described the effect of silicon on the molecular response of rice to P. oryzae infection on a genome-wide scale when using a 44 k rice microarray to compare gene expression levels between rice plants that were not or were supplied with silicon and were not or were inoculated with P. oryzae. The primary purpose of their study was to investigate the interaction between silicon and P. oryzae inoculation on the transcriptional profile of rice. They found that defense/stress-related genes were differentially up and down-regulated in P. oryzae comparisons with silicon + P. oryzae treatments. These comparisons were of particular interest because they highlighted how silicon changed plant reactions to fungal infections. Among these defense/stress-related genes were ethylene signaling pathway genes, a gene encoding a thaumatin/pathogenesis-related protein (Os12 g0568900), a class III peroxidase (Os07 g0677500) and a number of transcription factors and protein kinases. The authors further noted that in addition to simply attenuating the plant response to P. oryzae infection, a substantially different pattern of expression was noted in their experiment. Not only did the silicon comparison with the silicon + P. oryzae treatment reveal 440 differentially expressed genes less than the control comparison with the P. oryzae treatment, but the two comparisons had only 236 genes in common. Silicon therefore affected the interactions between the host and the pathogen at the molecular level by attenuating the rice response to the pathogen and by influencing the differential expression of a unique set of genes. Silicon was clearly responsible for preconditioning plants to react to stress, which was supported in this study by the fact that P. oryzae infection resulted in less than half the number of differentially expressed genes in plants that were supplied with silicon than those that were not supplied with silicon (298 compared with 738).
Pursuant to the fact that photorespiration is important in plants that are under biotic stress, diatom photorespiration has been shown to be dependent on silicon polymerization , and the activities of photorespiratory enzymes were higher in plants supplied with silicon under stress conditions. Von Bonkhaven et al. (2013) proposed a hypothesis in which photorespiration might play an important role in rice resistance to brown spot as caused by B. oryzae in the presence of silicon. A transcriptome study conducted by Van Bockhaven et al. (2015) showed that fungal infections repressed photosynthesis and lowered nitrate concentrations in plants that were not supplied with silicon, which resulted in greater brown spot symptom development. By contrast, for plants that were supplied with silicon, there was an up-regulation of several photorespiratory marker genes, leading to the hypothesis that increased photorespiration rates may be one of the driving forces behind the possible effects of silicon on rice photosynthesis (Van Bockhaven et al. 2015).
It has been suggested that silicon primes the plant immune response rather than constitutively inducing defense-related genes that result in an increase in host resistance (an increase in the magnitude of host defensive processes and/or the speed with which they are activated) after the plant has been challenged by a plant pathogen (Ghareeb et al. 2011; Van Bockhaven et al. 2013, 2015; Dallagnol et al. 2015; Vivancos et al. 2015). This primed state allows the plant to respond more quickly and effectively to challenges because of the accumulation of inactive cellular proteins that are involved in signal transduction such as mitogen-activated protein kinases, chromatin modifications and alterations in primary metabolism with a minimal metabolic cost (Van Hulten et al. 2006; Conrath 2011). According to Van Bockhaven et al. (2013), the broad-spectrum disease resistance found in rice plants that received silicon was related, at least in part, to the priming effect that resulted in a differential accumulation of defense-regulatory transcription factors, a process that was sufficient for priming defense genes but less effective at activating them directly. An additional mechanism underpinning the potentiation of host resistance to pathogen infection by silicon has been the involvement of this element in plant hormone responses. This response has been observed for host-pathogen interactions that are mediated by salicylic acid, jasmonate and ethylene in the Arabidopsis-Erysiphe cichoracearum interaction (Fauteux et al. 2006), the ethylene in rice-P. oryzae interaction (Brunings et al. 2009), the jasmonate and ethylene in tomato-R. solanacearum interaction (Ghareeb et al. 2011) and the ethylene in rice-B. oryzae interaction (Van Bockhaven et al. 2015). For the rice-B. oryzae interaction, the increased resistance of plants that were supplied with silicon to brown spot was attributed to the production and/or action of fungal ethylene that impaired the fungus’ ability to suppress the rice innate immune system and, as a consequence, resulted in a faster and stronger activation of the basal mechanisms of host defense (Van Bockhaven et al. 2015). Vivancos et al. (2015) engineered Arabidopsis plants with a silicon transporter from wheat (TaLsi1) and exploited mutants (pad4 and sid2) that were deficient in salicylic acid-dependent defense responses. The purpose of this transporter engineering and mutant exploitation was to study the phenotypic response and changes in defense expressions against Golovinomyces cichoracearum infection when plants were amended with silicon. According to these authors, the TaLsi1 plants exhibited significantly greater concentrations of silicon in plant tissue and were significantly more resistant to infection by G. cichoracearum than the non-inoculated control plants that were supplied with silicon. The resistant plants accumulated higher levels of salicylic acid and expressed higher levels of transcripts encoding for defense-related genes. However, TaLsi1 pad4 and TaLsi1 sid2 plants were also more resistant to G. cichoracearum infection than pad4 and sid2 plants in the presence of silicon. An analysis of the resistant phenotypes revealed a significant reduction in the production of salicylic acid and the expression of defense genes in comparison with those of the susceptible controls. The results obtained by these authors indicated that silicon contributed to Arabidopsis defense priming following G. cichoracearum infection, and they further highlighted that silicon could confer protection even when the priming was altered.
Silicon-Mediated Host Resistance to Pathogens Through Changes in the Primary Metabolism
Changes in the growth and development of plants are the results of the occurrence of and constant exposure to several abiotic and biotic stresses (Berger et al. 2007). Biotic stresses, particularly those caused by plant pathogens, lead to changes in primary plant metabolism. This response will in turn provide energy for the host’s defense responses that originate from secondary metabolism and are primarily based on activating the expression of hundreds of genes that are involved in many defense pathways (Berger et al. 2007; Rojas et al. 2014). During pathogen infection , the host’s physiology is negatively affected primarily because of changes in leaf gas exchange, once the amount of healthy leaf area is decreased and the efficiency of the photosynthetic process is dramatically lowered (Shtienberg 1992). The most notable negative effects that have resulted from pathogen infections of their hosts are the reduced concentration of pigments, damage to the chloroplasts, impairments in energy dissipation via chlorophyll a fluorescence and an increase in the foliar temperature (Petit et al. 2006; Zhao et al. 2011). For instance, genes involved in photosynthesis and chlorophyll biosynthesis have been found to be down-regulated in many different host-parasite interactions (Scholes and Rolfe 1996; Berger et al. 2004; Swarbrick et al. 2006; Bilgin et al. 2010). By contrast, several genes that are involved in energy production, such as glycolysis and the pentose phosphate pathways, the tricarboxylic acid cycle, mitochondrial electron transport and ATP biosynthesis become up-regulated during the infection time-course by a plant pathogen (Less et al. 2011; Rojas et al. 2014).
Although the aforementioned examples are now well-known, our current understanding of how silicon affects the physiology and biochemistry of plants that are infected by plant pathogens remains to be elucidated. For this reason, research efforts over the last few years have focused on examining the role played by this element in host physiology and primary metabolic pathways, especially for alterations in photosynthesis that occur during the infection process of several plant pathogens in crops of economic importance. For sorghum-Colletotrichum sublineolum and common bean-C. lindemuthianum interactions, in addition to the reduction of anthracnose symptoms in plants supplied with silicon, the values for the net carbon assimilation rate (A), stomatal conductance to water vapor (g s) and transpiration rate (E) were higher for infected plants that had received silicon than for infected plants that were not supplied with this element. These findings suggested that the physiology of sorghum and common bean plants was negatively impaired upon pathogen infection , but it was greatly reduced in the presence of silicon. There were no changes in the A, g s and E for the non-infected plants supplied with silicon. Furthermore, the impaired photosynthetic performance of plants that received silicon was deeply associated with stomatal limitations, whereas in the non-infected plants, those impairments likely reflected dysfunctions at the biochemical reaction level that were involved in CO2 fixation (Resende et al. 2012; Polanco et al. 2014). Under field conditions, Rodrigues et al. (2015) reported that no difference was detected between bean plants that were not and were sprayed with potassium silicate (KSi) with respect to the A, g s, E and internal CO2 concentration. However, the A significantly increased by 17 % in plants that were treated with the fungicide azoxystrobin. The A was not affected by KSi or sodium molybdate (NaMo); however, the A was significantly increased by 13 % after NaMo + KSi applications. In conclusion, bean plants that were sprayed with KSi and NaMo were associated with decreased anthracnose severity as well as enhanced photosynthesis.
For the rice- and wheat-P. oryzae interactions, higher A, g s and E values were obtained for infected plants that were supplied with silicon in contrast to the lower values of infected plants that were not supplied with this element (Aucique-Perez et al. 2014; Rios et al. 2014; Domiciano et al. 2015). Biochemical dysfunctions at the chloroplast level likely played a key role in limiting A upon P. oryzae infection for both rice and wheat plants instead of causing diffusive (stomatal) limitations to photosynthesis . Higher A, g s and E values and an increased concentration of leaf pigments were reported to occur in cotton plants that were supplied with silicon and infected by Ramularia areola (Curvelo et al. 2013a) and Colletotrichum gossypii var. cephalosporioides (Guerra et al. 2013a).
By measuring the emission of chlorophyll a fluorescence, which is considered to be a powerful tool and a very sensitive probe for the physiological status of plants (Baker 2008), the authors demonstrated that some photochemical parameters such as the quantum yield of photosystem II (PSII) photochemistry (F v/F m), photochemical quenching coefficient (q p) and electron transport rate (ETR), together with the quantification of chlorophylls and carotenoid concentrations, were greatly improved for rice and wheat plants that were supplied with silicon. By contrast, the heat dissipation of the chlorophyll excitation energy, which is measured on the basis of the non-photochemical quenching (NPQ) parameter, decreased for rice and wheat plants that were supplied with silicon and inoculated with P. oryzae. Therefore, the PSII electron transport at the chloroplast level was not impaired and the photoprotective processes were kept at the desired physiological level, thus contributing to the integrity of the photosynthetic apparatus. According to Gao et al. (2011), an increase in rice resistance to P. oryzae infection from silicon was associated with an enhancement in the photochemical efficiency, more specifically, an increase in the maximum/potential quantum efficiency (F v/F m) and the maximum primary yield (F v/F 0) of the photochemistry of PSII.
Microarray and proteome techniques have been exploited in an attempt to increase our current knowledge regarding the beneficial effects of silicon on the physiology of several plant species during pathogen infection at the molecular level. A transcriptome analysis of silicon’s effect on powdery mildew (Erysiphe cichoracearum) development in Arabidopsis thaliana plants indicated that several genes that were involved in primary metabolism such as photosynthesis and energy pathways as well as amino acid, carbohydrate and lipid metabolism were down-regulated as a direct result of fungal infection. However, many of these same genes, particularly those involved in photosynthesis and energy pathways, were less responsive for plants that were supplied with silicon (Fauteux et al. 2006). Chain et al. (2009) performed a transcriptomic analysis of the silicon effect on a B. graminis f. sp. tritici infection for wheat and found that many genes that were associated with stress and metabolic processes were up-regulated for infected plants, and genes related to photosynthesis were down-regulated. Conversely, when plants were supplied with silicon prior to fungal inoculation, the genes that were associated with stress and metabolic processes were down-regulated and the genes linked to photosynthesis were up-regulated. In conclusion, the authors noted that the stress imposed by fungal infection was greatly diminished in the presence of silicon. A proteomic study performed by Liu et al. (2014) to examine the effect of silicon on rice resistance to P. oryzae infection indicated that the pattern of protein spots was greatly affected by fungal infection regardless of the presence of silicon. Many proteins related to photosynthesis (the chlorophyll a/b-binding protein, chloroplast putative thylakoid lumenal 16.5 kDa protein, sedoheptulose-1,7-bisphosphatase and ribulose bisphosphate carboxylase large chain) were down-regulated upon fungal infection. In the presence of silicon and fungal infection, these proteins were all up-regulated. These photosynthesis-related proteins in silicon-mediated higher abundance as mediated by silicon may function as light receptors or they may play a role in protein biosynthesis at the chloroplast level, thus affecting rice photosynthesis. Moreover, the differential expression of energy metabolism-related proteins that are involved in the tricarboxylic acid or the pentose phosphate pathways may increase rice resistance against P. oryzae infection.
Because the activation of host defense responses requires a large amount of energy together with the induction of the sink metabolism, the photosynthetic capacity and carbohydrate metabolism can be negatively impacted in response to pathogen infection (Ehness et al. 1997). Dallagnol et al. (2013) investigated the effect of silicon uptake on the photosynthesis and leaf sugar concentration in rice plants from the Oochikara cultivar and the lsi1 mutant (low-silicon; defective in the active silicon uptake) that were not or were infected with B. oryzae. The inefficiency of the lsi1 mutant plants in actively taking up silicon negatively affected rice resistance against B. oryzae infection, and it reduced photosynthesis and the sugar concentration. However, the high foliar silicon concentration resulted in an increase in the soluble sugar concentration, photosynthesis, and, consequently, rice resistance to B. oryzae infection. The authors concluded that a minimum silicon concentration was needed in the leaf tissue of rice plants to avoid the negative impacts of B. oryzae infection on photosynthesis and the sugar concentration. Indeed, rice resistance to brown spot was independently and additively affected by the silicon and soluble sugar concentrations in the leaf tissue (Dallagnol et al. 2013).
Conclusions
In spite of recent advances linking silicon to host resistance via the -omics, i.e., genomics, proteomics and metabolomics, the exact mechanism(s) by which this element modulates plant physiology through an increase in host resistance still requires further investigation. The information generated to date has provided novel insights into silicon’s potential to interact with multiple pathways in the plant’s primary metabolism to cope better with infections caused by both soil-borne and foliar pathogens. In considering the current plant nutriomics scenario, it remains to be determined as to whether the involvement of silicon in plant-signaling pathways leads to the potentiation of host defense mechanism s and simultaneously makes it feasible to modify some key regulator(s) to enhance silicon uptake. In the near future, the real functions of silicon will be possible to elucidate at the molecular, cellular, organ and even whole plant levels. The function of silicon as linked through enzyme antioxidants and photosynthesis would be a few of the targeted focus areas, and thus the use of this quasi-essential element may be enhanced for sustainable plant health and plant performance.
References
Abed-Ashtiani F, Kadir JB, Selamat AB et al (2012) Effect of foliar and root application of silicon against rice blast fungus in MR219 rice variety. Plant Pathol J 28:164–171. doi:10.5423/PPJ.2012.28.2.164
Andrade CCL, Resende RS, Rodrigues FA et al (2013) Silicon reduces bacterial speck development on tomato leaves. Trop Plant Pathol 38:436–442. doi:10.1590/S1982-56762013005000021
Aucique-Perez CEA, Rodrigues FA, Moreira WR, DaMatta FM et al (2014) Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae. Phytopathology 104:143–149. doi:10.1094/PHYTO-06-13-0163-R
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. doi:10.1146/annurev.arplant.59.032607.092759
Bélanger RR, Benhamou N, Menzies JG (2003) Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp. tritici). Phytopathology 93:402–412. doi:10.1094/PHYTO.2003.93.4.402
Berger S, Papadopoulos M, Schreiber U et al (2004) Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol Plant 122:419–428. doi:10.1111/j.1399-3054.2004.00433.x
Berger S, Sinha AK, Roitsch T (2007) Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot 58:4019–4026. doi:10.1093/jxb/erm298
Bilgin DD, Zavala JA, Zhu J et al (2010) Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ 33:1597–1613. doi:10.1111/j.1365-3040.2010.02167.x
Brunings AM, Datnoff LE, Ma JF et al (2009) Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Ann Appl Biol 155:161–170. doi:10.1111/j.1744-7348.2009.00347.x
Cacique IS, Domiciano GP, Moreira WR et al (2013) Effect of root and leaf applications of soluble silicon on blast development in rice. Bragantia 72:304–309. doi:10.1590/brag.2013.032
Cai K, Gao D, Luo S et al (2008) Physiological and cytological mechanisms of silicon‐induced resistance in rice against blast disease. Physiol Plant 134:324–333. doi:10.1111/j.1399-3054.2008.01140.x
Carré-Missio V, Rodrigues FA, Schurt DA et al (2009) Ineficiência do silício no controle da ferrugem do cafeeiro em solução nutritiva. Trop Plant Pathol 34:416–421. doi:10.1590/s1982-56762009000600009
Carver TLW, Zeyen RJ, Ahlstrand GG (1987) The relationship between insoluble silicon and success or failure of attempted primary penetration by powdery mildew (Erysiphe graminis) germlings on barley. Physiol Mol Plant Pathol 31:133–148. doi:10.1016/0885-5765(87)90012-9
Carver TLW, Robbins MP, Thomas BJ et al (1998) Silicon deprivation enhances local autofluorescent responses and phenylalanine ammonia lyase activity in oat attacked by Blumeria graminis. Physiol Mol Plant Pathol 52:245–257. doi:10.1006/pmpp.1998.0149
Chain F, Côté-Beaulieu C, Belzile F et al (2009) A comprehensive transcriptomic analysis of the effect of silicon on wheat plants under control and pathogen stress conditions. Mol Plant Microbe Interact 22:1323–1330. doi:10.1094/MPMI-22-11-1323
Chérif M, Benhamou N, Menzies JG et al (1992a) Silicon induced resistance in cucumber plants against Pythium ultimum. Physiol Mol Plant Pathol 41:411–425. doi:10.1016/0885-5765(92)90053-X
Chérif M, Menzies JG, Benhamou N, Bélanger RR et al (1992b) Studies of silicon distribution in wounded and Pythium ultimum infected cucumber plants. Physiol Mol Plant Pathol 41:371–385. doi:10.1016/0885-5765(92)90022-N
Chérif M, Asselin A, Bélanger RR (1994) Defense responses induced by soluble silicon in cucumber roots infected by Pythium spp. Phytopathology 84:236–242. doi:10.1094/Phyto-84-236
Conrath U (2011) Molecular aspects of defence priming. Trends Plant Sci 16:524–531. doi:10.1016/j.tplants.2011.06.004
Cruz MFA, Rodrigues FA, Polanco LR et al (2013) Inducers of resistance and silicon on the activity of defense enzymes in the soybean-Phakopsora pachyrhizi interaction. Bragantia 72:162–172. doi:10.1016/1369-5266(88)80053-0
Curvelo CRS, Rodrigues FA, Pereira LF et al (2013a) Trocas gasosas e estresse oxidativo em plantas de algodoeiro supridas com silício e infectadas por Ramularia areola. Bragantia 72:346–359. doi:10.1590/brag.2013.053
Curvelo CRS, Rodrigues FA, Silva LC et al (2013b) Mecanismos bioquímicos da defesa do algodoeiro à mancha de ramularia mediados pelo silício. Bragantia 72:41–51
Da Silva WL, Cruz MFA, Fortunato AA, Rodrigues FA (2015) Histochemical aspects of wheat resistance to leaf blast mediated by silicon. Sci Agric 72(4):322–327
Dallagnol LJ, Rodrigues FA, DaMatta FM et al (2011) Deficiency in silicon uptake affects cytological, physiological, and biochemical events in the rice-Bipolaris oryzae interaction. Phytopathology 101:92–104. doi:10.1094/PHYTO-04-10-0105
Dallagnol LJ, Rodrigues FA, Chaves ARM, DaMatta FM (2013) Photosynthesis and sugar concentration are impaired by the defective active silicon uptake in rice plants infected with Bipolaris oryzae. Plant Pathol 62:120–129. doi:10.1111/j.1365-3059.2012.02606.x
Dallagnol LJ, Rodrigues FA, Pascholati SF et al (2015) Comparison of root versus foliar applied potassium silicate in potentiating postinfection defences of melon against powdery mildew. Plant Pathol. doi:10.1094/PHYTO.2003.93.4.402
Dann EK, Muir S (2002) Peas grown in media with elevated plant-available silicon levels have higher activities of chitinase and β-1,3-glucanase, are less susceptible to a fungal leaf spot pathogen and accumulate more foliar silicon. Australas Plant Pathol 31:9–13
Datnoff LE, Rodrigues FA (2005) The role of silicon in suppressing rice diseases. APSnet Features. doi:10.1094/APSnetFeature-2005-0205
Debona D, Rodrigues FA, Rios JA et al (2014) The effect of silicon on antioxidant metabolism of wheat leaves infected by Pyricularia oryzae. Plant Pathol 63:581–589. doi:10.1111/ppa.12119
Domiciano GP, Rodrigues FA, Guerra AMN et al (2013) Infection process of Bipolaris sorokiniana on wheat leaves is affected by silicon. Trop Plant Pathol 38:258–263. doi:10.1590/S1982-56762013005000006
Domiciano GP, Cacique IS, Freitas CC et al (2015) Alterations in gas exchange and oxidative metabolism in rice leaves infected by Pyricularia oryzae are attenuated by silicon. Phytopathology 105:738–747. doi:10.1094/PHYTO-10-14-0280-R
Ehness R, Ecker M, Godt DE et al (1997) Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. The Plant Cell Online 9:1825–1841. doi:10.1105/tpc.9.10.1825
Fauteux F, Rémus-Borel W, Menzies JG et al (2005) Silicon and plant disease resistance against pathogenic fungi. FEMS Microb Lett 249:1–6. doi:10.1016/j.femsle.2005.06.034
Fauteux F, Chain F, Belzile F et al (2006) The protective role of silicon in the Arabidopsis–powdery mildew pathosystem. Proc Natl Acad Sci U S A 103:17554–17559. doi:10.1073/pnas.0606330103
Fawe A, Abou-Zaid M, Menzies JG, Bélanger RR et al (1998) Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 88:396–401. doi:10.1094/PHYTO.1998.88.5.396
Fortunato AA, Rodrigues FA, Nascimento KJT (2012) Physiological and biochemical aspects of the resistance of banana plants to Fusarium wilt potentiated by silicon. Phytopathology 102:957–966. doi:10.1094/PHYTO-02-12-0037-R
Fortunato AA, da Silva WL, Rodrigues FA (2014) Phenylpropanoid pathway is potentiated by silicon in the roots of banana plants during the infection process of Fusarium oxysporum f. sp. cubense. Phytopathology 104:597–603. doi:10.1094/PHYTO-07-13-0203-R
Freeman BC, Beattie GA (2008) An overview of plant defenses against pathogens and herbivores. Plant Health Instruc. doi:10.1094/PHI-I-2008-0226-01
Gao D, Cai K, Chen J et al (2011) Silicon enhances photochemical efficiency and adjusts mineral nutrient absorption in Magnaporthe oryzae infected rice plants. Acta Physiol Plant 33:675–682. doi:10.1007/s11738-010-0588-5
Ghareeb H, Bozsó Z, Ott PG et al (2011) Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol Mol Plant Pathol 75:83–89. doi:10.1016/j.pmpp.2010.11.004
Guerra AMNM, Rodrigues FA, Berger PG et al (2013a) Aspectos bioquímicos da resistência do algodoeiro à ramulose potencializada pelo silício. Bragantia 72:292–303. doi:10.1590/brag.2013.036
Guerra AMNM, Rodrigues FA, Berger PG et al (2013b) Resistência do algodoeiro à ferrugem tropical potencializada pelo silício. Bragantia 72:279–291. doi:10.1590/brag.2013.037
Hashioka Y (1942) Studies on rice blast disease in the tropics. (I) Anatomical comparison of leaf epidermis of Formosan rice with that of Taiwan rice from plant pathological viewpoints. Agric Hortic 17:846–852
Hayasaka T, Fujii H, Ishiguro K (2008) The role of silicon in preventing appressorial penetration by the rice blast fungus. Phytopathology 98:1038–1044. doi:10.1094/PHYTO-98-9-1038
He C, Ma J, Wang L (2015) A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytol 206:1051–1062
Heath MC (1981) Insoluble silicon in necrotic cowpea cells following infection with an incompatible isolate of the cowpea rust fungus. Physiol Plant Pathol 19:273–276
Heath MC, Stumpf MA (1986) Ultrastructural observations of penetration sites of the cowpea rust fungus in untreated and silicon-depleted French bean cells. Physiol Mol Plant Pathol 29:27–39
Hemmi T, Abe T, Inoue Y (1941) Studies on the rice blast disease: relation of the environment to the development of blast disease and races of the blast fungus. Noji Kairyo Shiryo 157:1–232
Inanaga S, Okasaka A, Tanaka S (1995a) Calcium and silicon binding compounds in cell walls of rice shoots. Jpn J Soil Sci Plant Nutr 41:103–110
Inanaga S, Okasaka A, Tanaka S (1995b) Does silicon exist in association with organic compounds in rice plant? Jpn J Soil Sci Plant Nutr 11:111–117
Ishiguro K (2001) Review of research in Japan on the roles of silicon in conferring resistance against rice blast. In: Datnoff LE, Snyder GH, Korndörfer GH (eds) Silicon in agriculture, studies in Plant Science 8. Elsevier Science, Amsterdam, pp 277–291
Ito S, Hayashi H (1931) On the relation of silica supply to rice blast. J Sapporo Soc Agric Sci 103:460–461
Ito S, Sakamoto M (1939) Studies on rice blast. Hokkaido University, Hokkaido
Jiang D (1993) The role of silicon in barley resistance to powdery mildew (Erysiphe graminis f. sp. hordei). PhD thesis, University of Minnesota, St. Paul
Kauss H, Seehaus K, Franke R, Gilbert S et al (2003) Silica deposition by a strongly cationic proline-rich protein from systemically resistant cucumber plants. Plant J 33:87–95. doi:10.1046/j.1365-313X.2003.01606.x
Kawamura E, Ono K (1948) Study on the relation between the pre-infection behavior of rice blast fungus, Piricularia oryzae, and water droplets on rice plant leaves. J Natl Agr Expt Sta 4:1–12
Kim SG, Kim KW, Park EW et al (2002) Silicon-induced cell wall fortification of rice leaves: a possible cellular mechanism of enhanced host resistance to blast. Phytopathology 92:1095–1103. doi:10.1094/PHYTO.2002.92.10.1095
Koga H, Zeyen RJ, Bushnell WR et al (1988) Hypersensitive cell death, autofluorescence, and insoluble silicon accumulation in barley leaf epidermal cells under attack by Erysiphe graminis f. sp. hordei. Physiol Mol Plant Pathol 32:395–409
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Biol 48:251–275. doi:10.1146/annurev.arplant.48.1.251
Less H, Angelovici R, Tzin V et al (2011) Coordinated gene networks regulating Arabidopsis plant metabolism in response to various stresses and nutritional cues. Plant Cell 23:1264–1271. doi:10.1105/tpc.110.082867
Li A, Heath MC (1990) Effect of plant growth regulators on the interactions between bean plants and rust fungi non-pathogenic on beans. Physiol Mol Plant Pathol 37:245–254
Li W, Bi Y, Ge Y et al (2012) Effects of postharvest sodium silicate treatment on pink rot disease and oxidative stress-antioxidative system in muskmelon fruit. Eur Food Res Technol 234:137–145. doi:10.1007/s00217-011-1611-9
Liang YC, Sun WC, Si J, Römheld V (2005) Effects of foliar‐and root‐applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol 54:678–685. doi:10.1111/j.1365-3059.2005.01246.x
Liu M, Cai K, Chen Y et al (2014) Proteomic analysis of silicon-mediated resistance to Magnaporthe oryzae in rice (Oryza sativa L.). Eur J Plant Pathol 139:579–592. doi:10.1007/s10658-014-0414-9
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397. doi:10.1016/j.tplants.2006.06.007
Maekawa K, Watanabe K, Kanto T et al (2002) Accumulation of silicon around penetration sites of Magnaporthe grisea and silicon-dependent promotion of superoxide generation after inoculation on rice leaf. In: Matoh T (ed) Second silicon in agriculture conference. Press-Net, Kyoto, pp 34–38
Menzies JG, Ehret DL, Glass ADM, Samuels AL et al (1991) The influence of silicon on cytological interactions between Sphaerotheca fuliginea and Cucumis sativus. Physiol Mol Plant Pathol 39:403–414. doi:10.1016/0885-5765(91)90007-5
Mohaghegh P, Khoshgoftarmanesh AH, Shirvani M et al (2011) Effect of silicon nutrition on oxidative stress induced by Phytophthora melonis infection in cucumber. Plant Dis 95:455–460. doi:10.1094/PDIS-05-10-0379
Motomura H, Fujii T, Suzuki M (2004) Silica deposition in relation to ageing of leaf tissues in Sasa veitchii (Carrière) Rehder (Poaceae: Bambusoideae). Ann Bot 93:235–248. doi:10.1093/aob/mch034
Nascimento KJT, Debona D, França SKS et al (2014) Soybean resistance to Cercospora sojina infection is reduced by silicon. Phytopathology 104:1183–1191. doi:10.1094/PHYTO-02-14-0047-R
Pereira SC, Rodrigues FA, Carré-Missio V et al (2009a) Aplicação foliar de silício na resistência da soja à ferrugem e na atividade de enzimas de defesa. Trop Plant Pathol 34:164–170. doi:10.1590/S1982-56762009000300005
Pereira SC, Rodrigues FA, Carré-Missio V et al (2009b) Efeito da aplicação foliar de silício na resistência à ferrugem e na potencialização da atividade de enzimas de defesa em cafeeiro. Trop Plant Pathol 34:223–230. doi:10.1590/S1982-56762009000400004
Perumalla CJ, Heath MC (1991) The effect of inhibitors of various cellular processes on the wall modifications induced in bean leaves by the cowpea rust fungus. Physiol Mol Plant Pathol 38:293–300. doi:10.1016/S0885-5765(05)80120-1
Petit AN, Vaillant N, Boulay M et al (2006) Alteration of photosynthesis in grapevines affected by esca. Phytopathology 96:1060–1066. doi:10.1094/PHYTO-96-1060
Polanco LR, Rodrigues FA, Nascimento KJT et al (2014) Photosynthetic gas exchange and antioxidative system in common bean plants infected by Colletotrichum lindemuthianum and supplied with silicon. Trop Plant Pathol 39:35–42. doi:10.1590/S1982-56762014000100005
Pozza AAA, Alvez E, Pozza EA et al (2004) Efeito do silício no controle da cercosporiose em três variedades de cafeeiro. Fitopatol Bras 29:185–188. doi:10.1590/S0100-41582004000200010
Rahman A, Wallis C, Uddin W (2015) Silicon induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology 105:748–757. doi:10.1094/PHYTO-12-14-0378-R
Rémus-Borel W, Menzies JG, Bélanger RR (2005) Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiol Mol Plant Pathol 66:108–115. doi:10.1094/PDIS-04-11-0274
Rémus-Borel W, Menzies JG, Bélanger RR (2009) Aconitate and methyl aconitate are modulated by silicon in powdery mildew-infected wheat plants. J Plant Physiol 166:1413–1422. doi:10.1016/j.jplph.2009.02.011
Resende RS, Rodrigues FA, Cavatte PC et al (2012) Leaf gas exchange and oxidative stress in sorghum plants supplied with silicon and infected by Colletotrichum sublineolum. Phytopathology 102:892–898. doi:10.1094/PHYTO-01-12-0014-R
Resende RS, Rodrigues FA, Gomes RJ, Nascimento KJT et al (2013) Microscopic and biochemical aspects of sorghum resistance to anthracnose mediated by silicon. Ann Appl Biol 163:114–123. doi:10.1111/aab.12040
Rezende DC, Rodrigues FA, Carré-Missio V, Schurt DA, Kawamura K, Korndörfer GH (2009) Effect of root and foliar applications of silicon on brown spot development in rice. Australas Plant Pathol 38:67–73. doi:10.1071/ap08080
Rios JA, Rodrigues FA, Debona D et al (2014) Photosynthetic gas exchange in leaves of wheat plants supplied with silicon and infected with Pyricularia oryzae. Acta Physiol Plant 36:371–379. doi:10.1007/s11738-013-1418-3
Rodrigues FA, Benhamou N, Datnoff LE, Jones JB, Bélanger RR (2003) Ultrastructural and cytochemical aspects of silicon-mediated rice blast resistance. Phytopathology 93:535–546
Rodrigues FA, McNally DJ, Datnoff LE et al (2004) Silicon enhances the accumulation of diterpenoid phytoalexins in rice: a potential mechanism for blast resistance. Phytopathology 94:177–183
Rodrigues FA, Jurick WM, Datnoff LE et al (2005) Silicon influences cytological and molecular events in compatible and incompatible rice-Magnaporthe grisea interactions. Physiol Mol Plant Pathol 66:144–159
Rodrigues FA, Polanco LR, Duarte HSS et al (2015) Photosynthetic gas exchange in common bean submitted to foliar sprays of potassium silicate, sodium molybdate and fungicide and infected with Colletotrichum lindemuthianum. J Phytopathol 163:554–559. doi:10.1111/jph.12353
Rojas CM, Senthil-Kumar M, Tzin V, Kirankumar S et al (2014) Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front Plant Sci 5:17. doi:10.3389/fpls.2014.00017
Samuels AL, Glass ADM, Ehret DL et al (1991) Mobility and deposition of silicon in cucumber plants. Plant Cell Environ 41:485–492. doi:10.1111/j.1365-3040.1991.tb01518.x
Scholes JD, Rolfe SA (1996) Photosynthesis in localised regions of oat leaves infected with crown rust (Puccinia coronata): quantitative imaging of chlorophyll fluorescence. Planta 199:573–582
Schurt DA, Rodrigues FA, Reis RD et al (2012) Resistência física de bainhas de plantas de arroz supridas com silício e infectadas por Rhizoctonia solani. Trop Plant Pathol 37:281–285. doi:10.1590/S1982-56762012000400008
Schurt DA, Rodrigues FA, Carré-Missio V et al (2013a) Silício alterando compostos derivados da pirólise de bainhas foliares de plantas de arroz infectadas por Rhizoctonia solani. Bragantia 72:52–60
Schurt DA, Rodrigues FA, Colodette JL et al (2013b) Efeito do silício nas concentrações de lignina e de açúcares em bainhas de folhas de arroz infectadas por Rhizoctonia solani. Bragantia 72:360–366
Schurt DA, Cruz MF, Nascimento KJT et al (2014) Silicon potentiates the activities of defense enzymes in the leaf sheaths of rice plants infected by Rhizoctonia solani. Trop Plant Pathol 39:457–463. doi:10.1590/S1982-56762014000600007
Schurt DA, Reis RD, Araujo L, Carré-Missio V, Rodrigues FA (2015) Análise microscópica da resistência do arroz à queima das bainhas mediada pelo silício. Bragantia 74:93–101, doi: http://dx.doi.org/10.1590/1678-4499.0355
Seebold KW, Kucharek TA, Datnoff LE et al (2001) The influence of silicon on components of resistance to blast in susceptible, partially resistant, and resistant cultivars of rice. Phytopathology 91:63–69. doi:10.1094/PHYTO.2001.91.1.63
Shetty R, Fretté X, Jensen B et al (2011) Silicon-induced changes in antifungal phenolic acids, flavonoids, and key phenylpropanoid pathway genes during the interaction between miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiol 157:2194–2205. doi:10.1104/pp.111.185215
Shetty R, Jensen B, Shetty NP et al (2012) Silicon induced resistance against powdery mildew of roses caused by Podospahera pannosa. Plant Pathol 61:120–131. doi:10.1111/j.1365-3059.2011.02493.x
Shtienberg D (1992) Effects of foliar diseases on gas exchange processes: a comparative study. Phytopathology 82:760–765. doi:10.1094/Phyto-82-760
Silva RV, Oliveira RDL, Nascimento KJT, Rodrigues FA et al (2010) Biochemical responses of coffee resistance against Meloidogyne exigua mediated by silicon. Plant Pathol 59:586–593. doi:10.1111/j.1365-3059.2009.02228.x
Silva MRJ, Pereira SC, Rodrigues FA, Zanão Júnior KA et al (2012) Silicon and manganese on the activity of enzymes involved in rice resistance against brown spot. Trop Plant Pathol 37:339–345. doi:10.1590/S1982-56762012000500006
Sousa RS, Rodrigues FA, Schurt DA, Souza NFA, Cruz MFA (2013) Cytological aspects of the infection process of Pyricularia oryzae on leaves of wheat plants supplied with silicon. Trop Plant Pathol 38:472–477. doi:10.1590/S1982-56762013000600002
Sun W, Zhang J, Fan Q et al (2010) Silicon-enhanced resistance to rice blast is attributed to silicon-mediated defence resistance and its role as physical barrier. Eur J Plant Pathol 128:39–49. doi:10.1007/s10658-010-9625-x
Suzuki H (1940) On the relationship between rice susceptibility and penetration into host plants. Ngyo Oyobi Engei 10:1999–2010
Swarbrick PJ, Schulze‐Lefert P, Scholes JD (2006) Metabolic consequences of susceptibility and resistance (race‐specific and broad‐spectrum) in barley leaves challenged with powdery mildew. Plant Cell Environ 29:1061–1076
Tatagiba SD, Rodrigues FA, Filippi MCC et al (2014) Physiological responses of rice plants supplied with silicon to Monographella albescens infection. J Phytopathol 162:596–606. doi:10.1111/jph.12231
Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378. doi:10.1104/pp.106.079467
Van Bockhaven J, De Vleesschauwer D, Höfte M (2013) Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. J Exp Bot 64:1281–1293. doi:10.1093/jxb/ers329
Van Bockhaven J, Spíchal L, Novák O et al (2015) Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanus by preventing the pathogen from hijacking the rice ethylene pathway. New Phytol 206:761–773
Van Hulten M, Pelser M, Van Loon LC et al (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci U S A 103:5602–5607
Vivancos J, Labbé C, Menzies JG, Bélanger RR et al (2015) Silicon‐mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)‐dependent defence pathway. Mol Plant Pathol 16:572–582. doi:10.1111/mpp.12213
Volk RJ, Kahn RP, Weintraub RL (1958) Silicon content of the rice plant as a factor influencing its resistance to infection by the blast fungus, Piricularia oryzae. Phytopathology 48:179–184. doi:10.1590/S0100-41582005000500001
Yoshida S, Ohnishi Y, Kitagishi K (1962) Chemical forms, mobility, and deposition of silicon in the rice plant. Jpn JSoil Sci Plant Nutr 8:107–111
Zeyen RJ, Ahlstrand GG, Carver TLW (1993) X-ray microanalysis of frozen-hydrated, freeze-dried, and critical point dried leaf specimens: determination of soluble and insoluble chemical elements at Erysiphe graminis epidermal cell papilla sites in barley isolines containing Ml-o and ml-o alleles. Can J Bot 71:284–296
Zhao D, Glynn NC, Glaz B et al (2011) Orange rust effects on leaf photosynthesis and related characters of sugarcane. Plant Dis 95:640–647. doi:10.1094/PDIS-10-10-0762
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
A. Rodrigues, F., Resende, R.S., Dallagnol, L.J., E. Datnoff, L. (2015). Silicon Potentiates Host Defense Mechanisms Against Infection by Plant Pathogens. In: Rodrigues, F., Datnoff, L. (eds) Silicon and Plant Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-22930-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-22930-0_5
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22929-4
Online ISBN: 978-3-319-22930-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)