Abstract
Stem cell niches are discrete anatomical microenvironments that present a rich collection of extrinsic factors to govern stem cell behavior. In particular, these niche signals—including soluble cues, extracellular matrix (ECM)-associated signals, and cues from neighboring cell types—play key roles in balancing stem cell self-renewal and differentiation. Recapitulating fundamental features of the stem cell niche in vitro to investigate regulatory mechanisms has proved to be a challenging, yet necessary, task for dissecting the regulatory role of stem cell niches and for developing stem cell-based therapies. However, the recent emergence of innovative engineering strategies has enabled the development of model platforms that have yielded key insights into niche-directed stem cell behavior. As such control mechanisms of the stem cell niche are elucidated, engineering strategies have evolved concurrently to facilitate the study of more complex phenomena. In this review, we highlight the progression of these emerging engineering strategies, focusing on the growing emphases on spatiotemporal control and single-cell studies for examining stem cell-ECM interactions and the effects of heterotypic cellular interactions, respectively.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Stem cells have drawn great attention from the biomedical community as diverse players that assume central roles in development, tissue homeostasis, and tissue regeneration [1]. Defined by their ability to self-renew and differentiate into mature cell lineages, stem cells can be generally categorized into three main subtypes: embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs). ESCs and iPSCs share similarities in their morphology, proliferation, and ability to differentiate into cell types from any of the three germ layers: endoderm, ectoderm, and mesoderm. However, ESCs and iPSCs differ in their point of origin. While ESCs are derived from the inner cell mass of mammalian blastocysts, iPSCs are generated via reprogramming of somatic cells through the retroviral introduction of key factors, such as the four Yamanaka factors Oct3/4, Sox2, c-Myc, Klf4 [2]. ASCs, in contrast, generate a more limited or restricted number of cell lineages that help mediate cell turnover within adult tissues. ASCs populations, which by convention and contrary to their name can be derived from adult or fetal tissue, include, but are not limited to, hematopoietic stem cells (HSCs), neural stem cells (NSCs), satellite muscle stem cells, epidermal stem cells, and intestinal stem cells (ISCs).
Collectively, stem cells offer exciting therapeutic potential for replacing diseased and injured cell populations through regenerative medicine and tissue engineering strategies . These approaches include transplantation of stem cells and their differentiated progeny as well as stimulation of endogenous stem cell populations (i.e. ASCs). The clinical success of both these approaches hinges on the ability to control stem cell behavior, in particular through precise regulation of stem cell expansion and differentiation. For ex vivo stem cell therapies, a major challenge is producing cells of high purity, yield, and quality. In the case of endogenous cell stimulation, the ability to target specific stem cell niches to support endogenous repair represents another major hurdle [3, 4]. To date, considerable progress has been made in developing therapies based on stem and progenitor cells in the hematopoietic system. The use of HSCs has found encouraging success in treating conditions such as autoimmune diseases and blood defects [5, 6]. The primary challenge in stem cell research is to extend this clinical success to other stem cell systems. Therefore, it has become clear that, before stem cells can become a viable therapeutic agent, the complex mechanisms regulating their behavior must be deconstructed.
2 Stem Cells and Their Niches
Efforts within the past few decades have demonstrated that stem cells localize within physiological domains referred to as “niches”—a concept that Schofield first formulated in 1978 to describe the bone-marrow microenvironment of HSCs [7–9]. Since this time, a multitude of studies have confirmed the existence of a variety of microenvironments that house stem cells. For instance, NSCs have been found within the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone of the hippocampal dentate gyrus of the adult mammalian brain [10–12]. Epidermal stem cells have been shown to reside in a distinct anatomical location called the hair follicle bulge [13–16], muscle stem cells localize between basal lamina and the periphery of myofiber plasma membrane [17–19], and ISCs have been suggested to reside at the +4 position of the crypt base as well as the crypt base itself [20–22]. In addition to being described by their anatomical locations, stem cell niches are also defined by their functional properties [7, 23]. In response to physiological or pathological circumstances or demands, niches play an integral role in coordinating stem cell behavior to maintain homeostasis and stimulate repair [23].
The niche’s regulatory role is the result of a dynamic interplay of signaling components that include soluble cues, surrounding extracellular matrix (ECM)-associated cues, and neighboring niche constituent cells [4]. These signals manifest in various ways, including biophysical signals in the form of the stiffness and topography of imposing ECM in addition to biochemical cues, such as secreted paracrine factors as well as ECM-sequestered growth factors and cytokines [24–27]. Understanding the mechanisms by which these signals modulate stem cell behavior is an essential step in clinically translating stem cell therapies. Specifically, exploring the length and time scales over which individual signals and combinations of signals modulate stem cell behavior has increasingly become a research thrust within the field. In vitro models that mimic aspects of in vivo niche microenvironments have facilitated this investigation and have been made possible through an extensive breadth of novel engineering strategies . In this review, we examine the various strategies employed for recapitulating stem cell-ECM and stem cell-niche cell interactions, with a particular focus on more recent engineering strategies that have progressed in parallel with the field’s growing knowledge of stem cell behavior.
3 Stem Cell-ECM Interactions
The ECM is an intrinsically complex, heterogeneous physical structure that plays key roles within stem cell niches . In addition to supporting cellular adhesion, the ECM presents biophysical cues related to the material’s physical properties as well as biochemical cues in the form of insoluble ligands. Stem cells actively and dynamically probe this matrix by applying traction forces to “sense” these instructive inputs and subsequently respond by altering their cytoskeleton, adjusting focal adhesions, and remodeling the ECM via degradation and deformation [28–30]. This bidirectional communication is a major topic of interest, as studies have collectively demonstrated that the niche’s ECM directly and indirectly regulates key stem cell behaviors, such as adhesion, proliferation, differentiation, and migration [28, 31–33].
3.1 Stem Cell Adhesion to Niche ECM via Integrins
The ECM is an intricate three-dimensional (3D) architecture comprised of diverse biomolecules, including proteins, polysaccharides, proteoglycans, morphogens, cytokines, and growth factors [34]. The composition of this ECM is unique to a given stem cell niche but, despite their considerable structural diversity, similarities among niches have been noted. One common feature is stem cell localization adjacent to basal lamina or basement membranes, which have specialized ECM structures rich in laminins, collagens, proteoglycans, and other important adhesive proteins (tenascin, fibronectin, nidogen, etc.) [35, 36]. For example, NSCs within the SVZ contact finger-like extensions of basal lamina (termed “fractones”), which extend from surrounding vasculature [37, 38]. Similarly, ISCs inhabit the crypts of intestinal villi, where they share an interaction with the gut epithelial basement membrane [35], a physical fusion of basal and reticular laminas. Likewise, muscle satellite stem cells reside under the basal lamina of myofibers, and interfollicular epidermal stem cells lie adjunct to the encasing basal lamina in the hair follicle bulge [15, 18].
Integrins are a well-characterized family of heterodimeric cell surface receptors that mediate stem cell adhesion to this common interface [28, 39]. These receptors consist of two transmembrane chains (18 α- and 8 β-subunits), which combine to form more than 24 different integrins (excluding splice variants) [40]. Examples of integrins in stem cell niches include α5β1 integrin, a laminin receptor expressed by some NSCs, and α8β1, which mediates hair follicle stem cell binding to the ECM protein nephronectin. Many integrins also possess the capability to recognize the Arg-Gly-Asp (RGD) tripeptide motif within their ligands. Stem cells assemble these nanoscale integrin complexes into macroscale focal adhesions [41]. These adhesions are proposed to play a large role in translating extracellular ECM protein stimuli into intracellular biochemical signals (a process referred to as mechanotransduction), ultimately leading to global changes in cell morphology as well as regulating gene expression to modulate cellular behavior [42]. This complex cascade of signaling events, initiated from the binding of ECM ligands to focal adhesions, exerts tension onto the cell’s cytoskeleton and induces stress on the nucleus, as the cytoskeleton is connected to the nuclear envelope [43]. As a result, nuclear remodeling occurs, which asserts force back onto the cytoskeleton and alters focal adhesions. The subsequent “inside-out” signaling allows cells to manipulate the clustering of integrins to their membrane, increasing or decreasing binding of their integrin receptors [44]. Therefore, focal adhesions represent a key mediator of dynamic spatial and temporal interactions between the environment and intracellular signaling [42]. Disruption to this integrin-based interaction can result in stem cells exiting their niche via differentiation or apoptosis [45]. Some integrin signaling pathways under investigation are the Ras/MAPK, RhoA/ROCK, and P13K/Akt pathways. YAP and TAZ have also recently been identified as key downstream transcription factors sensitive to mechanical cues [28, 43, 46].
Integrin signaling has also been shown to interface with growth factor-initiated pathways [39]. In neural progenitor cells (NPCs), for example, the addition of fibroblast growth factor upregulated the expression of β1 integrins, which is believed to enhance cell responsiveness to its ECM [39, 44]. Another example of growth factor-integrin interplay was suggested for mesenchymal stem cells (MSCs)—multipotent adult stromal cells of a mesodermal lineage. The activation of MSC α5β1 integrins on stretched fibronectin fibers promoted osteogenesis; however, inhibition of the epidermal growth factor receptor on the same stretched fibers decreased osteogenesis from 41 to 27 % [35]. As an example in ESCs, it is hypothesized that platelet-derived growth factor receptor coordinates with collagen IV-integrin α1/β1/αv to induce differentiation toward smooth muscle cells [40].
3.2 Cadherins, Another Class of Adhesion Receptors
While adhesion via integrins is a recurring theme in a majority of the stem cell niches , HSCs and likely other stem cells rely on another adhesion protein to interface indirectly with their physical microenvironment. Specifically, HSCs interact with an intermediate cell type, osteoblasts, to anchor themselves to the inner surface of the trabecular bone [47]. This physical cell coupling relies on the recruitment of cadherins and catenins, proteins that assemble to form intercellular adheren-junction complexes [38, 48]. Cadherins have been demonstrated to regulate stem cell behavior in a manner similar to that of integrins. For instance, in the testis stem cell niche of Drosophila melanogaster, N-cadherin assists in orienting stem cells for asymmetric division within the niche [49]. In the Drosophila ovary niche, loss of N-cadherin results in the retreat of stem cells from the niche [49]. In mammalian systems, N-cadherin-mediated anchoring of NSCs to ependymal cells lining the ventricle has been implicated in regulating the quiescence of NSCs within the SVZ niche. Upon the degradation of this cell-cell adhesion, NSCs translocate from the ependymal cells towards the blood vessels, enhancing their interaction with ECM and initiating their activation [49].
3.3 Molecular Sequestering of Growth Factors and Cytokines by ECM
In addition to mediating stem cell adhesion, the ECM acts as a reservoir for growth factors and cytokines [50]. Immobilization is achieved through non-covalent binding to ECM proteins, proteoglycans, and glycosaminoglycans [51, 52]. Specifically, ECM proteins possess intrinsic binding domains that facilitate the spatial localization of these regulatory factors [52, 53]. Collagen II binds through its von Willebrand domain to transforming growth factor β1 and bone morphogenetic protein 2 [54]. Similarly, fibronectin harbors a heparin II domain that binds molecules such as vascular endothelial growth factor and platelet-derived growth factor [54]. These factors can either be released to establish local morphogen gradients or instigate signaling from a bound state [50]. Liberation of these molecules occurs by either proteolytic degradation of the ECM or cell-generated forces.
While some growth factors directly bind ECM proteins, many others harbor domains that bind to heparan sulfate, a glycosaminoglycan consisting of a linear polysaccharide that attaches to core proteins to form heparan sulfate proteoglycans (HSPGs) [55, 56]. In addition to organizing the presentation of these ligands, HSPGs play a functional role in modulating signaling. They assist in bridging growth factors with their receptors and can serve as co-receptors, influencing growth factor activity by biasing activation thresholds and binding specificities [53, 55, 57]. HSPGs also assist in extending signaling duration through the inhibition of receptor-mediated endocytosis [54, 58, 59].
4 Seminal Engineering Strategies—Establishing a Foundation
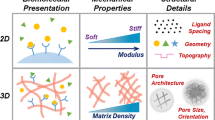
An increased understanding of the regulatory role that native ECM plays within stem cell niches has been achieved through the synergistic efforts of biologists, materials scientists, engineers, chemists, and physicists [34]. Early investigations clearly established the importance and the associated mechanisms by which ECM composition, matrix rigidity, topography (both nano- and micro-), porosity, ligand presentation, and control of cell geometry regulate stem cell behavior [51, 60]. These findings were realized with the aid of engineering techniques that re-created static representations of stem cell-ECM interfaces. Materials with pre-defined topographies, patterned peptide sequences, and fixed mechanical properties represent only a few of these early approaches, and these initial studies were critical advances that stimulated interest in dissecting the surrounding physical microenvironment within the stem cell niche . The following sections highlight a variety of early, landmark engineering strategies pursued for studying the role that ECM elements play within the niche.
4.1 Micro/Nanofabrication Techniques for Generating Pre-Printed Topographies
Topography is an inherent characteristic of ECM that has been investigated as an instructive cue that guides the formation of focal adhesions and cytoskeletal tension [41]. The complex, heterogeneous composition of the niche’s ECM contributes to an intricate blend of structural features, including pores, protrusions, ridges, and grooves [61]. Deconstructing the biophysical responses to these physiological topographies has required a reductionist approach due to the complexity of the dynamic bi-directional interactions between stem cells and ECM. Thus, many efforts have focused on recapitulating single-feature architectures in vitro and observing how these static systems affect stem cell behavior.
Studying the effects of static topographies requires a platform that must be precise and reproducible on the micro- and nanoscale. A wide spectrum of fabrication methods—including photolithography, soft lithography, dip-pen nanolithography, and electron-beam lithography—have been used in these platforms [61, 62]. Posts and grooves are two examples of structures that have been heavily investigated. Studies have not only manipulated the overall scale of these features (macro vs. micro vs. nano) but also varied the physical aspect ratios of these structures. Ahn and colleagues, for instance, employed ultraviolet (UV)-assisted capillary-force lithography to generate polyurethane nanoposts (Fig. 1a) [63]. They then investigated how varying post-to-post distances (i.e. post densities) at the micron scale influenced human mesenchymal stem cell (hMSC) fate and subsequently discovered that certain topographies biased the process of hMSC differentiation. In particular, a greater nanopost separation (i.e. a post-to-post distance of 5.6 μm) favored osteogenic differentiation, whereas adipogenesis was maximized at a smaller post-to-post separation (2.4 μm) [63]. Motemani et al. [64] also investigated the effect of nano-columnar surfaces, created using glancing angle deposition, on hMSCs. Nanoscale columns were fabricated in vertical, slanted, and chevron geometries from titanium dioxide (TiO2), a common implant material, by sputtering titanium at an oblique angle and using substrate rotation to bias the columnar growth direction before annealing to oxidize the films. Following plating of MSCs on these surfaces, unique nano-sized pseudopodia extensions were observed and suggested to cause cytoskeletal tension and trigger mechanotransduction, though additional studies would be required to confirm these assumptions [64]. While the focus was not on hMSC differentiation but rather on cell morphology and cytocompatibility, this work does yield a promising technique for future studies in exploring the effects of nanoscale topographies on stem cell behavior [64]. In contrast, Fu et al. engineered elastomeric micropost arrays of varying post heights (0.97, 6.1, and 12.9 μm) for generating different mechanical substrate rigidities (1556 nN/μm, 18.16 nM/μm, 1.90 nN/μm) (Fig. 1b) [65]. Single hMSCs were adhered to islands of different post heights, and cell traction forces were tracked over a 7-day period. A strong correlation between osteogenic and adipogenic lineage commitment and traction forces suggested that MSC contractile state could be used as a noninvasive predictor of hMSC differentiation [65].
Engineering strategies for generating static, pre-printed topographies. Panel a polyurethane nanoposts of varying densities fabricated using UV-assisted capillary force lithography [63]. Panel b SEM images of hMSCs cultured on islands of different PDMS micropost height arrays (top); brightfield micrographs and traction force maps of hMSCs exposed to osteogenic or adipogenic medium (bottom) [65]. Panel c Micropatterned PDMS grooves applied towards influencing NSC differentiation; cells stained for neuronal marker Tuj-1 (red) and nuclei (blue) [66]
In addition to posts, considerable work has explored the effects of grooves on stem cell behavior, and in particular the effects of groove depth, groove pitch, and terrace widths. For example, Béduer and colleagues used conventional soft-lithography techniques to assess how adult NSCs responded to imposed micro-patterned polydimethylsiloxane (PDMS) surfaces with varying terrace and groove widths (5–5, 10–10, 20–20, 10–60 μm, respectively) (Fig. 1c) [66]. They found that smaller groove separations lowered differentiation rates and hindered the number of neurite extensions from differentiated neurons, despite promoting a high degree of cellular alignment [66]. Recknor et al. [67] also examined the effects of a micro-patterned polystyrene groove topography as a guidance cue for NPCs. Rather than modulating the physical dimensions of the grooves, however, Recknor et al. [67] studied the synergistic effects of a 16 × 13 × 4 μm (width/mesa width/groove) groove depth pattern in conjunction with a chemical and a biological cue. Specifically, NPCs were co-cultured on a confluent monolayer of cortical astrocytes, which resided on top of a laminin-coated, micro-patterned polystyrene substrate. The resulting microenvironment was found to enhance NPC neuronal differentiation selectively [67].
Many other creative approaches, including techniques for constructing 3D structures, have also been pursued in engineering models of ECM topology. To start, Christopherson et al. [68] revealed that modulations to nanofiber diameters were sufficient for biasing NSC proliferation and differentiation (Fig. 2a). Specifically, they fabricated laminin-coated polyethersulfone fiber mesh matrices exhibiting a range of average fiber diameters (283 ± 45 nm, 749 ± 153 nm, and 1452 ± 312 nm). An increase in fiber diameter in the presence of fibroblast growth factor-2, a mitogen that promotes stem cell maintenance, induced a decrease in NSC proliferation rate and migratory activity [68]. When cultured in differentiation conditions, on the other hand, NSCs tended toward a glial lineage on the 283-nm fibers as cells displayed a better ability to spread randomly along the nanofiber matrix. For the larger fiber diameters, NSCs were restricted to extending along single fibers, promoting a neuronal lineage [68]. Though correlations have been observed between topographies and cell behavior, the mechanisms of shape regulation remain elusive. Another such innovative study involves preparing porous honeycomb polystyrene scaffolds by casting the polymer under humid conditions to form hexagonally arranged pores [69]. Kawano et al. [69] used this system to dissect the influence that cellular- and subcellular-scaled pore sizes have on hMSC behavior. For pore sizes smaller than the cell (1.6 μm), osteospecific differentiation was prominent. In contrast, myospecific differentiation was associated with larger pore sizes (3.8 μm) [69]. Along the same lines, hMSCs were cultured on TiO2 nanotubes of different pore diameters—30, 50, 70, and 100 nm [70]. The self-assembled, highly-ordered nanotube arrays were created by anodization, where different diameters were a result of manipulating anodizing potentials (5–20 V) (Fig. 2b). With this platform, Oh et al. [70] demonstrated that hMSC elongation increased with nanotube diameter and correlated with differentiation into an osteogenic lineage. Moreover, a saturation effect of hMSC differentiation was observed as diameters approach 100 nm. Finally, Das et al. [71] drew inspiration from collagen by engineering helical, silica nanoribbons covalently modified with RGD to mimic collagen fibril structures (Fig. 2c). They probed the role that different periodicities (63.5 ± 5 vs. 110 ± 15 nm) had in directing the lineage commitment of hMSCs and found that helical nanoribbons with smaller periodicity induced a strong commitment to the osteoblast lineage [71].
Engineering strategies for generating 3D static topographies. Panel a SEM images of NPCs cultured on nanofibers of varying diameter [68]. Panel b SEM images of TiO2 nanotubes of different pore diameters and hMSCs cultured on nanotube surfaces [71]. Panel c SEM images of silica-RGD nanoribbons with twisted and helical morphologies (top); SEM images of hMSCs cultured on grafted helical nanoribbon substrate, exhibiting extended filopodia-like structures (bottom) [70]
To increase the throughput of topographical investigations, novel on-chip systems that encompass various dimensions and architectural complexities within a single platform have been developed. Yim et al. [72] fabricated one such system, which they termed the Multi-ARChitecture (MARC) chip (Fig. 3). By utilizing nanoimprinting lithography, they generated not only a variety of isotropic (1 μm pillars, 2 μm holes, 1.8 μm concave and convex lenses) and anisotropic (2 μm and 250 nm gratings) features but also hierarchical, composite structures of 2 μm lines and 250 nm dimples on top of 2 μm gratings [72]. Neuronal differentiation of human embryonic stem cells (hESCs) was studied with this system. When hESCs grew on laminin-coated PDMS replicas of these MARC chips, grating topographies favored neuronal differentiation, whereas isotopic patterns favored the glial lineage [72].
MARC chip for high-throughput topographical investigation of hESC neural differentiation. Panel a Schematic overview of chip design [72]. Panel b SEM images of single and multi-architectural PDMS patterns [72]. Panel c Immunostaining of hESCs for neuronal (Tuj-1 green) and astrocytic (GFAP, red) lineages on the different topographies [72]
4.2 Micropatterning Techniques to Relate Stem Cell Shape to Behavior
Micropatterning techniques have been developed to control cell shape on a single-cell level to understand better how cytoskeletal state orchestrates stem cell behavior. The pioneering works of Ingber and Whitesides paved the way for the development of a multitude of chemical patterning techniques, important tools for dissecting the relationship between stem cell shape and response [73–75]. These two groups demonstrated the ability to engineer cellular geometry through microcontact printing, a technique in which an elastomeric stamp is used to transfer, for example, square or rectangular patterns (2–80 μm) of self-assembled monolayers of alkanethiols onto a gold substrate [75]. An ECM component, such as laminin, can then be deposited onto the alkanethiol micro-islands and thereby be selectively adsorbed onto the printed regions, while the gold substrate remains adhesion-resistant. Though this platform was initially explored with hepatocytes, analogous efforts have extended into the stem cell field. A seminal effort by McBeath and colleagues helped elucidate the molecular basis of cell shape-mediated effects on hMSC commitment to an adipogenic or osteogenic fate [76]. Microcontact-printed fibronectin islands of 1024 and 10,000 μm2 areas were used to control cell shape. The smaller islands promoted more rounded morphologies in contrast to the larger islands, which stimulated well-spread morphologies. Using this system, they discovered that hMSC differentiation was mediated by RhoA signaling with lineage specification occurring through the RhoA effector, ROCK [76]. RhoA activity, though capable of displacing soluble factor signaling, was found to be dependent on cell shape. A rounded morphology was necessary for adipogenesis and, similarly, a spread-out morphology was needed for osteogenesis. ROCK, on the other hand, was found to be downstream of these instructive signals. hMSCs with constitutively-active ROCK become osteoblasts, regardless of cell shape [76]. This landmark study highlights the importance of cell mechanics as an inductive cue for stem cell differentiation.
More recent efforts have focused on further dissecting the relationship between stem cell shape and behavior, resulting in the development of additional innovative materials. For example, Peng et al. [77] patterned a polyethylene glycol (PEG) hydrogel with gold micro-islands conjugated with RGD peptides. They investigated the effect that different anisotropic patterns (circle, square, triangle, and star) and rectangles of varying aspect ratios (1, 1.5, 2, 4, 8, and 16) had on single rat MSC differentiation (Fig. 4a). They found that cell-shape perimeter could be used as a simple parameter for predicting stem cell differentiation in the case of anisotropic patterns; however, isotropic patterns exhibited a non-monotonic osteospecific differentiation as a function of aspect ratio [77]. A similar study investigating the influence of cell shape on lineage commitment was conducted by Kilian et al., who also harnessed microcontact printing [78]. MSCs were cultured on three shapes with pentagonal symmetry but different curvatures: (1) flower shape with large convex curves; (2) pentagon with straight edge lines; and (3) star shape with concave edges and sharp vertices (Fig. 4b) [78]. The subtle geometric differences were sufficient to generate strikingly different differentiation profiles through varying degrees of actin-myosin contractility [78]. In general, pointed features between concave regions resulted in enhanced stress filaments and increased myosin contractility. Additionally, these local shape cues were associated with pathways promoting osteogenesis [78].
Strategies for engineering stem cell shape. Panel a RGD-conjugated gold microislands of different anisotropic geometries patterned onto PEG hydrogels (left); immunostaining of single rat MSCs under different geometrical shape constraints [77]. Panel b Immunofluorescent images of single MSCs stained for F-actin (green), vinculin (red), and nuclei (blue) on flower and star shape patterns created by microcontact printing [78]. Panel c Microcontact printing schema for generating circular collagen microislands of different diameters applied towards studying single primary human keratinocytes [79]
Connelly et al. [79] also utilized microcontact printing in their system to generate patterned, polymer-brush surfaces for investigating the role of cell-ECM interactions in regulating human epidermal stem cell differentiation. Circular micro-islands of collagen were prepared with diameters ranging from 20 to 50 μm, thereby enabling the capture of single epidermal stem cells and control over cell spreading (Fig. 4c) [79]. More importantly, this platform enabled the researchers to dissect how changes to cytoskeletal organization influenced differentiation. This was achieved by altering individual parameters of the microenvironment systematically through the addition of actin-disrupting agents, such as latrunculin A, ROCK inhibitor Y27632, blebbistatin, and cytochalasin D. Connelly and colleagues thereby demonstrated that cell shape guides the initiation of differentiation more strongly than other factors, such as adhesive area, ECM composition, or ECM density [79].
4.3 Soft Matter Hydrogel Systems with Predefined Characteristics
Great strides have also been made in the development of biomimetic hydrogel systems—both naturally-derived and synthetic—that recapitulate biofunctionality as well as key mechanical properties of the stem cell niche [80–83]. Hydrogel matrices have been utilized as a platform for presenting specific biological moieties to stem cells in vitro, such as cell adhesion ligands and growth factors (in both soluble and tethered fashions) [81, 84–86]. Strategies to explore the effects of tethered ligand type, ligand density, ligand flexibility, and ligand spatial patterns have been at the forefront of these recent studies. The RGD peptide motif (arginine-glycine-aspartic acid), a major binding site of fibronectin and other ECM proteins, is one integrin-binding ligand that has been frequently studied, tethered to many hydrogel matrices, and applied to a wide spectrum of stem cell systems. For example, Salinas and Anseth investigated hMSC attachment and viability when RGD peptides conjugated to PEG hydrogels were presented via two covalent mobilization schemas: pendant tethering with a spacer arm sequence (aka mono-functionalization) or dually attached with a loop-like structure (i.e. di-functionalization) (Fig. 5a) [87]. In short, they found that hMSCs demonstrated lower viability in the dually-tethered gel in addition to a lower expression level of αvβ3 integrins, most likely due to steric hindrance from the two links that prevented hMSCs from binding to the RGD motif through their integrins [87]. The use of a spacer arm sequence for immobilizing RGD was offered as a solution for overcoming integrin inaccessibility.
Engineering ligand presentation in hydrogel systems. Panel a Investigating the effects of RGD tethering via two mobilization schemas, i.e. mono- versus di-functionalization, on hMSC attachment. hMSCs stained for nuclei (blue) and αvβ3 cell surface integrin (green) [87]. Panel b Schematic of RGD clustering within hyaluronic acid hydrogels [89]. Panel c The effect of small vs. large RGD nanospacing on MSC differentiation [90]. Panel d ESCs cultured on an ECM microarray platform consisting of varying ECM compositions [92]
Building on earlier work with fibroblasts [88], Lam and Segura [89] investigated another mode of RGD presentation by exploring the effects of RGD clustering on guiding the behavior of encapsulated mouse MHCs within 3D hyaluronic acid hydrogels (Fig. 5b). While it did not play a significant role in altering MHC proliferation, varying the distribution of the bioactive signals did have an effect on cell spreading and integrin expression. Homogenous gels (i.e. gels that display the lowest level of RGD clustering) induced a low degree of spreading. As signal clustering increased, so did the degree of MHC spreading. Furthermore, the expression of cell integrins also varied. For example, the number of cells that expressed α2 and β1 integrins was significantly higher in gels with the lowest amount of clustering and, conversely, α3 integrins were more prominent in the highly-clustered gels [89]. Along similar lines, Wang and colleagues explored the effect of five RGD nanospacings from 37 to 124 nm on PEG hydrogels on MSCs lineage commitment (Fig. 5c) [90]. These underlying nanopatterns were obtained by grafting RGD peptides onto patterned gold nanodots, enabling single nanodot-integrin interactions. With this platform, the authors observed that cell circularity (i.e. area multiplied by 4π and divided by square of perimeter) increased in response to increases in RGD nanospacing [90]. Furthermore, under solely osteogenic or adipogenic differentiation conditions, increases in RGD nanospacings translated to an increase in the extent of respective osteogenic and adipogenic differentiation of MSCs. In the case of co-induction conditions, however, osteogenesis was found to be more sensitive to RGD nanospacings, as more MSCs pursued an osteogenic fate as nanospacings increased [90].
Elucidating the effect of ECM composition has also been a recent interest in the field. Battista et al. [91] dissected the role that material structure and molecular-binding domain density have in controlling embryoid body growth, cavitation, and differentiation of mESCs. Semi-interpenetrating polymer networks consisting of collagen type I fibers, fibronectin, and laminin were modulated to produce scaffolds of varying physical properties and compositions. Cellular adhesion cues from laminin in the 3D scaffold were found to guide EB differentiation into cardiac-tissue lineages, while the addition of fibronectin cues induced dose-dependent differentiation into epithelial lineage without the addition of soluble factors [91]. In addition, high-throughput microarray systems have been developed to allow for the simultaneous screening of ECM factors, both individually and combinatorially, to better investigate the complexity of the stem cell niche’s ECM. Jongpaiboonkit et al. generated 3D PEG hydrogel arrays to screen for both individual and combinatorial effects of various ECM features: cell-adhesion ligand type, ligand density, and ECM degradability [85]. This group focused primarily on the fibronectin-derived Arg-Gly-Asp-Ser-Pro (RGDSP) and laminin-derived Ile-Lys-Val-Ala-Val (IKVAV) sequences. Additionally, degradability was induced by photocrosslinking PEG-diacrylate chains with varying concentrations of dithiothreitol (DTT), resulting in “DTT bridge” with ester bonds prone to hydrolytic degradation [85]. Other high-throughput techniques have involved adopting robotic spotting printing technologies. For example, Flaim et al. presented an ECM microarray platform that deposits an array of ECM molecule mixtures [92]. 32 combinations were investigated with varying collagen I, collagen III, collagen IV, laminin, and fibronectin compositions (Fig. 5d) [92]. This method can be expanded to include a vast range of insoluble and soluble ECM cues.
Biochemical information within the ECM has thus been a focus of numerous studies. However, hydrogels have also enabled major strides in the field’s understanding of how mechanical properties regulate and affect stem cell function. In particular, the elastic modulus (or stiffness) of the substrate has been widely explored. The initial landmark study utilized a collagen-coated polyacrylamide gel with tunable cross-linking properties, correlating to varying matrix stiffnesses as low as 0.1–40 kPa [33]. With this system, the physiological stiffnesses characteristic of brain, muscle, and bone were recapitulated in vitro and presented to naïve MSCs. The resulting differentiation of MSCs into tissue-specific cell types along with corresponding altered gene expression patterns demonstrated the significance that matrix mechanical properties have in the stem cell niche [33].
Gilbert and colleagues extended this initial strategy to illustrate the potency that substrate elasticity has on muscle stem cell self-renewal and cell fate [93]. In doing so, they engineered a tunable PEG hydrogel system covalently cross-linked with laminin in which stiffness could be controlled by varying the PEG polymer percentage in the precursor solution. Muscle stem cells cultured on soft PEG gels with an elastic modulus that mimicked adult murine skeletal muscle (~12 kPa) was found to enhance muscle stem cell survival when compared to cultures on traditional, stiff polystyrene surfaces (~106 kPa) [93]. Substrate rigidity also influenced Myogenin expression (a transcription factor expressed by differentiated muscle stem cells). Soft substrates demonstrated a 3-fold decrease in Myogenin-positive cells. Additionally, muscle stem cells cultured on PEG substrates most closely tuned to their native muscle niche stiffness (as opposed to brain or cartilage) were found to retain the greatest stemness [93].
While many studies that investigate the effects of substrate stiffness on stem cell behavior (including the aforementioned studies) employ model systems that yield thin layers of tunable hydrogels coated on a rigid substrate, Saha et al. [94] highlighted one potential problem with this approach. Soft polyacrylamide hydrogels are prone to equi-biaxial compressive stress when exposed to an aqueous environment due to osmotic pressure difference. The ensuing instability causes the formation of sharp folds (i.e. creases) as a result of induced buckling of the polyacrylamide surfaces. The authors emphasized that these surface creases must be characterized and controlled as they influence stem cell behavior [94]. NSCs were demonstrated to migrate towards the folds and adopted mature neuronal and astrocytic phenotypes when compared to NSCs that were uniformly attached and differentiated when cultured on smooth and stable polyacrylamide surfaces [94]. Therefore, instable surface creasing of polyacrylamide substrates (and potentially other soft hydrogel systems) may bias stem cell mechanotransduction studies [94]. This highlights the need for well-characterized and tightly controlled synthesis of soft-matter substrates. An overview of other key studies investigating the importance of matrix elasticity in stem cell biology are described in a number of extensive reviews [45, 51, 60, 95].
5 Second Generation Engineering Strategies—Increased Complexity with a Focus on Spatiotemporal Control
Engineered microenvironments are thus clearly valuable tools for dissecting how the ECM affects stem cell fate decisions, and there have been increasing advances in elucidating how these extrinsic cues modulate core transcriptional networks [79]. As demonstrated in the above section, initial engineering strategies in the stem cell field focused primarily on recapitulating static representations of the niche ECM. More recent engineering strategies, however, have evolved to emulate the dynamic interaction between stem cells and their physical environment. The creation of platforms with increasingly sophisticated structural and functional complexity is helping to bridge a gap between in vitro systems and what are likely highly dynamic in vivo physiological environments. In particular, the ability to engineer and incorporate tightly-coupled spatial and temporal control into these platforms has become a key objective of the field. The following section provides an overview of these emerging second-generation engineering strategies .
5.1 Biomaterials with Tunable Properties
An increased interest in mimicking the dynamic properties of the stem cell niche’s ECM has spurred the development of smart biomaterials—ones whose properties can be manipulated by external stimuli [96]. Light, temperature, pH, electric fields, small molecules, and shear stress represent a variety of “triggers” that have been employed to induce changes in stiffness, topography, and adhesion [96]. These in situ perturbations are powerful tools because they allow for the investigation of spatial and temporal ECM cues, providing a deeper insight into stem cell behavior.
5.2 Spatiotemporal Control over Topography
To complement landmark studies with pre-printed substrates, in recent years, topographic presentation has evolved toward materials with active and tunable topographies. Shape-memory polymers represent one class of active materials that have been employed for probing stem cell response to localized topographical changes, and studies involved with such polymer systems have provided insights into the dynamics of cytoskeletal organization and mechanotransductive signaling events [97]. These systems have relied primarily on the use of temperature as a temporal control for switching topography from a primary temporary pattern to a secondary permanent pattern. Davis et al. was one of the first groups to harness this effect [98]. They utilized a thermally-responsive polyurethane polymer substrate with end-linked thiol-ene crosslinks that was programmed to change from a lamellar surface to a flat surface upon a temperature transition from 30 to 37 °C [98]. More recent techniques have extended this strategy a step further by demonstrating the capability to switch between two distinct patterns. Le et al. [97] established this dual-shape capability by developing a poly(ε-caprolactone) surface in which the primary pattern was formed with replica molding, while the secondary pattern was generated by mechanically deforming the substrate at 130 °C using a second replica mold and, subsequently, cooling it to 78 °C. With this technique, a combination of pattern transformations was introduced to hMSCs: micron-sized cube arrays to hexnuts, cylinders to boomerangs, and channels to planar surface (Fig. 6a) [97]. Though pattern versatility was evident, there were significant challenges, including a lack of pattern reversibility and a high transition temperature of 40 °C (resulting in cell toxicity).
Engineering dynamic topographies with spatiotemporal control. Panel a Schematic for fabricating thermally-responsive poly(ε-caprolactone) topographies [97]. Panel b Four-stage shape memory platform with tunable microgrooves applied towards studying MSC behavior; cells immunostained for F-actin (red) and nuclei (blue) [99]. Panel c Dynamic switching from fiber-aligned state to random fiber orientation via a cytocompatible temperature increase (top); cells stained with phalloidin (green) to visualize actin (bottom) [100]. Panel d Spatial control of lamellar patterns dictated by mask applied during UVO treatment (right); illustration of quadruple topographical switching from flat to lamellar patterns at 90° to lamellar at 180° to zigzag patterns (right); live hMSCs labeled with CellTracker red, and fixed cells stained for F-actin (green) and nuclei (blue) [101]
Gong et al. [99] illustrated another approach for utilizing shape-memory systems. They engineered a four-stage shape memory platform with tunable microgrooves (Fig. 6b). To start, poly(ε-caprolactone) was modified with A allyl alcohol as a plasticizer, shifting the shape-memory recovery function to within the physiological range of 32–41 °C. Two different dynamic surfaces were then pursued. The first modulated microgroove depth, increasing from 0 to 1.7, 3.5, and 4.9 μm at 32, 35, 38, and 41 °C, respectively. The other surface transitioned from a temporal microgroove with a width of 9 μm at 32 °C to 7, 4.5, and 3.1 μm at the same increasing temperature set-points. The changes in the first surface induced parallel upward forces, which had little to no effect on cultured rat bone marrow MSCs [99]. The latter, convergent force from the second surface, however, greatly affected cytoskeletal arrangement and biased differentiation fate towards a myogenic lineage [99]. In a final example, Tseng et al. [100] translated shape-memory polymers into 3D by utilizing an electrospun scaffold whose fibrous architecture transitioned from a strain-aligned state to its original random fiber arrangement upon thermal activation (Fig. 6c). This controllable change in scaffold architecture exhibited desirable shape recovery properties as well as cytocompatibility for human adipose-derived stem cells. Moreover, the recovery rate of the scaffold could be controlled by modulating the chemical composition of the polyurethane scaffold, which comprised of hard segments of polyhedral oligomeric silsesquioxane and soft segments of polylactide/caprolactone copolymer (resulting in an increase in the glass transition temperature or decrease in hydrophilicity) [100]. These shape-memory-actuated materials, while still in the early stages of development, offer exciting potential for supporting further in-depth studies of stem cell regulation.
While thermally-induced shape-memory polymers offer considerable advances, another means of creating quasi-static topography was demonstrated through a technique that combined strain-induced buckling of PDMS substrates with plasma oxidation. Guvendiren and Burdick [101] introduced a strategy for fabricating versatile, high-fidelity, and reversible lamellar wrinkling patterns (Fig. 6d). To obtain this, PDMS sheets were stretched uniaxially, followed by exposure to ultraviolet/ozone (UVO). This exposure created stiff regions that resulted in perpendicular buckling when the strain was released. With this system, hMSCs were exposed to four changing patterns, starting with a flat, unpatterned surface to lamellar with 90-degree patterns to lamellar with 180-degree patterns and, finally, to zigzag patterns. hMSCs responded to these in situ dynamic patterning switches through changes in cell orientation angle [101]. Key advantages of this system include the ability to modulate pattern amplitude and periodicity by altering the degree of strain release. Moreover, spatial control of topographies could be regulated by selectively exposing the surface to UVO with different shadow-mask patterns. One disadvantage, however, is that high hMSC proliferation could lead to “masking” of the triggered topographical change. In other words, as culture time and cell division increases, cellular alignment to induced topographies diminishes [101].
Photo-induced manipulation of surface topography is another powerful approach that enables high spatial and temporal control. In comparison to its shape-memory polymer counterparts, light-responsive materials can be operated at standard physiological temperature (37 °C) as well as undergo countless sequential alterations that are not pre-determined, as long as phototoxicity does not occur. Kirschner and Anseth [102] engineered one such system—a photodegradable PEG-based hydrogel platform in which topographical cues can be formed in situ by user-controlled spatial erosion. Specifically, photolithographic techniques were used to pattern features (such as anisotropic channels and isotropic square patterns) on a photolabile gel, where pattern depths could be controlled by modulating the time of UV exposure (10 mW/cm2). Moreover, sequential patterning steps could be applied to alter surface topography concurrently during cell culture. hMSCs were cultured on this tunable surface and demonstrated reversible changes in cell morphology and alignment [102]. Similar to the shape-memory materials, only initial studies have been conducted with this system. Future perspectives involve using this system for better understanding how stem cells respond to real-time changes of ECM topographical cues within their niches.
5.3 Spatiotemporal Control over Matrix Stiffness
In addition to modulating topography, light has also been used as a tool for creating dynamic cultures of switchable substrate stiffnesses. Yang et al. [103] synthesized a phototunable hydrogel that incorporates a poly(ethylene glycol) di-photodegradable acrylate crosslinker. Upon controlled exposure to UV light, the initially stiff hydrogel (Young’s modulus of 10 kPa) transitioned into a soft hydrogel with a modulus of 2 kPa (Fig. 7a). With this system, they investigated the effects of mechanical dosing and mechanical memory on hMSCs [103]. In statically soft gels, hMSCs retained the capability to differentiate into both adipogenic and osteogenic lineages. However, upon mechanical dosing (i.e. culturing the cells on stiff substrates at variable time frames before in situ softening of the hydrogel), differentiation became biased towards osteogenic lineages. Specifically, cells were cultured from 1 to 10 days on stiff substrates prior to transitioning to soft hydrogels. The longer hMSCs were cultured on the stiff substrate, the more biased the cells became towards osteogenesis [103]. Transcriptional coactivators that play a key role in mechanotransduction, YAP and TAZ, were found also to persist in the nucleus (i.e. mechanical memory) even after cells were transitioned to soft substrates, suggesting that hMSCs retain information about past ECM states [103]. This system helped uncover a temporal aspect of stem cell mechanotransduction, where brief periods of mechanical dosing resulted in reversible activation of YAP and longer periods resulted in constitutive YAP nuclear localization [103].
Engineering strategies for dynamic control over substrate stiffness. Panel a Illustration of photodegradable hydrogel system (top); immunostaining of hMSCs for YAP (green) and RUNX (blue) localization (bottom) [103]. Panel b Crosslinking schematic (left) and traction stress maps of single hMSCs during in situ stiffening (right) [104]. Panel c Crosslinking schematic for generating porous hydrogel architectures; changes in bulk compressive moduli in responsive to UV crosslinking exposure times; hMSCs stained for actin (red) and nuclei (blue); porous hydrogels stained with FITC (green) [86]
In an analogous fashion, Guvendiren and Burdick [104] engineered a complementary strategy for in situ hydrogel stiffening in the presence of hMSCs—a system characterized by fast kinetics, long-term stability, and structural uniformity (Fig. 7b). This approach is potentially biologically relevant since matrix stiffening has been generally associated with key biological phenomena, such as disease and tissue development. To develop this dynamic substrate, hyaluronic acid macromers were functionalized with methacrylates, which react with thiols and radicals for crosslinking. Gelation was obtained through the addition of DTT, providing an initial stiffness of 3 kPa. Further, secondary crosslinking was achieved through a photoinitiator and subsequent UV light exposure for 2 min at 10 mW/cm2, increasing the matrix modulus to 30 kPa. This temporal stiffening not only can be tuned by exposure time but also can be achieved via sequential exposures during cell culture [104]. The use of DTT, however, poses a potential caveat for this hydrogel system as it may impact hMSC redox state.
Marklein et al. [86] extended this photoactivated crosslinking approach to study hMSC behavior in 3D porous hydrogels, investigating the importance of the magnitude, context, and timing of presented stiffness stimuli. In their work, Marklein et al. generated a macroporous architecture by initially crosslinking methylated hyaluronic acid around a hexagonally-organized template of microspheres (Fig. 7c). These hydrogels were triggered to stiffen from 2.6 to 12.4 kPa, either on Day 2 or 7 of a 14-day culture. These variable mechanics were controlled by UV exposure (10 mW/cm2) and found to affect the secretion profiles of cytokine and angiogenic factors [86]. In particular, hMSCs cultured on hydrogels that were stiffened on Day 2 (i.e. transitioned to the stiffer substrate sooner) displayed a greater reduction in key angiogenic factors and cytokine molecules compared to samples stiffened on Day 7. In contrast, morphology, proliferation, and differentiation did not exhibit significant dependence on stiffness dynamics [86].
Yoshikawa et al. [105] explored a different approach to achieving a dynamically tunable hydrogel platform. In lieu of using light as a stimulus, changes in viscoelastic properties were achieved through subtle pH changes and subsequently manipulating hydrophobic and interchain interactions. In this study, the pH-responsive polymer films consisted of a triblock ABA-type hydrogel, where A represented poly-(2-(diisopropylamino)ethyl methacrylate) and B represented poly(2-(methacryloyloxy)ethyl phosphorylcholine) [105]. By narrowly adjusting the pH range between 7 and 8—a range that does have the potential to affect cellular function—the stiffness of the copolymer could be reversibly transitioned between 1.4 and 40 kPa. Mouse myoblasts were used as a model system for this study, where morphological changes and cell adhesion strength were evaluated in relation to dynamic modulations of substrate stiffness [105]. While recent efforts have demonstrated the capability of either dynamic stiffening or softening of gels, a significant advance within the field would be a system that allows for reversible switching with cues that are inert to cells. This level of control would enable more complex investigations of the effects of stiffness pulses at different temporal onsets and durations.
5.4 Dynamic Control of Integrin-Based Focal Adhesions
Achieving precise control over the spatiotemporal presentation of ECM bioactive ligands has warranted the development of additional sophisticated engineering strategies . As illustrated in the previous sections, cell-adhesive ligands are key mediators of cell-matrix interactions and, thus, stem cell function. While previous strategies investigated the influence of pre-patterned peptides that mimic the active domains of key ECM components in a static fashion, several groups have recently fabricated smart biointerfaces that control the activation and de-activation of these integrin-based signals.
Photolabile protecting groups are an attractive approach for achieving dynamic control over the formation of stem cell focal adhesions, which activate downstream signaling cascades. Weis et al. pursued this approach by anchoring “caged” RGD peptides to self-assembled monolayers of alkanethiols on a gold substrate (Fig. 8a) [106]. To ensure only specific cell attachment to RGD-anchored SAMs, oligo(ethylene glycol) groups were conjugated to the SAMs lacking tethered peptides, providing a non-biofouling background. This system was applied to study how RGD peptide density influenced the differentiation of myoblasts (myofiber precursors) [106]. With an initial surface RGD density ~17 %, few cells attached to the substrate. However, upon a 3-min light exposure, the maximum surface RGD density was unmasked, and integrin-mediated myoblast interaction with the substrate was thus enabled. Light exposure for 3 min was applied at different time points during the culture timeframe: 1, 6, 24, and 48 h. Myogenic differentiation—analyzed via sarcomeric myosin expression and the formation of multi-nucleated myotubes—was more prominent when cells were exposed to high-density RGD peptides during earlier culture times [106]. This discovery highlights the importance of temporal presentation of ECM ligands, motivating additional exploration of their relationship to dynamic mechanical cues.
Engineering strategies for in situ modulation of ligand presentation and hydrogel degradation. Panel a Schematic illustrating ligand tethering and UV irradiation to release caged RGD molecules; myoblasts stained for actin (red), vinculin (green), and nuclei (blue) [106]. Panel b 3D photopatterning of surface features, such as various sizes of microwells and a bifurcation channel, within a photodegradable hydrogel [107]. Panel c SEM images of paramagnetic beads attached to MSC β1 integrin subunit [108]
Another approach for achieving dynamic ligand manipulation during stem cell culture was demonstrated by Kloxin et al. [107]. Photolabile tethers consisting of a photodegradable acrylate monomer were conjugated to the fibronectin epitope Arg-Gly-Asp-Ser (RGDS) within a nondegradable PEG-based hydrogel. Upon irradiation, the photolytic removal of RGDS moieties locally modified peptide presentation within the 3D microenvironment (Fig. 8b) [107]. The importance of persistent RGDS signaling on hMSC viability and differentiation was investigated by photolytically removing RGDS on Day 10 of a 21-day culture. In response to the temporal changes, hMSCs were found to downregulate the expression of αvβ3 integrins, while increasing the production of glycosaminoglycans as well as type II collagen, both of which are key markers of chondrogenic differentiation [107].
While photoresponsive materials have proved very effective for achieving spatiotemporal control over ligand presentation, Kasten et al. [108] demonstrated an alternate technique for probing stem cell mechanotransduction: the use of magnetic forces to induce integrin response. This strategy drew inspiration from earlier efforts, which utilized ferromagnetic microbeads coated with synthetic RGD peptides. These materials were employed for applying controlled mechanical loads to fibronectin receptors without inducing global changes to cell shape [109]. In this particular application, however, Kasten et al. [108] coupled paramagnetic microbeads to hMSC integrins by coating beads with an antibody specific for the β1 integrin subunit (Fig. 8c). A custom magnetic device with an average magnetic field strength of 0.015 T was then applied to the culture system, thereby inducing the displacement of the magnetic beads, which subsequently applied a drag force on stem cell integrin receptors, created mechanical stress, and temporarily distorted the cell membrane. This study was also tested in conjunction with three different types of substrates: polystyrene, RGD-functionalized, and fibronectin-coated surfaces [108]. Differentiation markers associated with adipogenic (i.e. PPARγ), osteogenic (i.e. ALP), and chondrogenic lineages (i.e. Sox9) were investigated in addition to released soluble factors relating to angiogenesis (i.e. VEGF) and osteogenesis (i.e. collagen I). Kasten et al. [108] observed that VEGF expression increased in response to short-term integrin stress stimulated by the magnetic field when hMSCs were cultured on RGD peptides and fibronectin but not on polystyrene. Collagen I expression, in contrast, was upregulated when hMSCs were cultured on polystyrene but not the other two surfaces [108]. These initial results not only highlighted the dynamic ability to control integrin stress through a magnetic field but also emphasize the importance of multifactor interactions of ECM-niche components.
6 Dissecting Cell-Cell Interactions within the Stem Cell Niche
Cellular components within the stem cell niche serve as another key source of instructive inputs for regulating stem cell quiescence, proliferation, and cell-fate determination [4, 48, 110, 111]. The spectrum of intercellular communication that takes place within these niches encompasses a stem cell’s interactions with other stem cells, stem cell progeny, and neighboring niche cells. Cell-cell signaling among these parties is achieved through various means: release of secreted soluble factors between neighboring cells (paracrine signaling), release of factors back to the same cell (autocrine signaling), cell-surface ligand-receptor binding between cells in direct contact (juxtacrine signaling), the transmembrane flux of signals through intimate gap junctions, and potentially mechanical interactions between cells.
The importance of cellular interactions and organization within stem cell niches was first demonstrated in early studies involving Drosophila germline stem cells (GSCs). Investigations of the ovary and testes niches showed that stemness and differentiation are balanced by critical communication between stem cells and their non-stem cell niche neighbors [112–114]. In the female fly, for instance, GSCs populate the anterior end of the ovariole and interact with three somatic cell types. GCSs indirectly adhere to the niche by intimately associating with cap cells via adherens junctions, cell-cell connections that form via homotypic cadherin binding [26]. During asymmetric division, the daughter cell that maintains this adhesion also retains its stem cell identity, whereas the daughter cell lacking adhesion differentiates into a cystoblast [115]. Terminal filament cells and inner germarium sheath cells (also referred to as escort cells) augment this maintenance of stem cell phenotype by repressing the key differentiation gene bag-of-marbles (bam). This repression is achieved through the secretion of cytokines by terminal filament cells, which signal the cap and escort cells to produce bone morphogenic protein (Bmp) ligands that bind with receptors that act to downregulate bam in GSCs [115]. The Drosophila testis, though a more complex microenvironment, shares similar hallmarks with the ovary niche. Only GSCs that contact adjacent hub cells within the apex of the testis self-renew. Hub cells also secrete Upd, which stimulates GSC adhesiveness and prevents surrounding cells from outcompeting GSCs for niche contact [115]. Moreover, somatic cyst progenitor cells indirectly activate the Bmp pathway by secreting Gbb and Dpp, repressing differentiation yet again. These examples illustrate the balance of communication between stem cells and non-stem cell niche neighbors.
The degree of interaction between stem cells and other cellular players is particular to the stem cell niche under investigation. For instance, muscle satellite stem cells remain relatively isolated and quiescent as they reside near basal lamina of muscle fibers [48]. Not until activation do they proliferate and fuse with one other to form differentiated myotubes. HSCs, on the other hand, tightly associate with not only osteoblasts that line the endosteal surface of the trabecular bone but also endothelial cells that line blood vessels [26]. Similarly, NSCs closely associate with endothelial cells of surrounding vasculature, neighboring astrocytes, microglia, and in some cases ependymal cells [11, 110]. Epithelial stem cells that reside in a specialized “bulge” structure within hair follicles, in contrast, encounter periodic stimuli from specialized mesenchymal cells, referred to as dermal papilla (DP). Specifically, the regeneration of hair follicles exposes resident stem cells to dynamic, perpetual cycles of growth (anagen), regression (catagen), and rest (telogen). During the anagen stage, massive cell death occurs below the bulge area for all cells except DP. The basement membrane then shrinks and draws DP into close contact with stem cells within the bulge. This close association is believed to be necessary for re-activating hair follicle regeneration, thereby initiating a brief telogen phase followed by rapid anagen phase [116]. As a final example, intestinal stem cells populate the crypt base of intestinal villi and drive rapid cell turnover of the epithelial lining of the small intestine and colon [21]. Within this niche, stem cells receive a complex array of signals from neighboring epithelial and stromal cells—paneth cells, goblet cells, and transit-amplifying cells, to name but a few. Renewal of the epithelium is orchestrated by a complex array of cellular signals, which ultimately drive budding transit-amplifying cells to differentiate into mature lineages, such as enteroendocrine cells, tuft cells, and absorptive enterocytes. These committed cells migrate out of the crypt and up to the base of the villi [21].
While the well-studied Drosophila niches are not as complex as vertebrate niches, the insights obtained from these lower organism counterparts were essential in stimulating more rigorous investigations of key regulatory cellular signals. These efforts have exposed a sophisticated interplay of signaling factors. Diffusible growth factors represent one class of secreted soluble signals that can positively or negatively regulate stem cell behavior within the niche—the effects of which are under strict spatial and temporal constraints [4]. For example, in the SVZ of the lateral ventricles, endothelial cells from surrounding vasculature produce a variety of paracrine factors that modulate key aspects of neurogenesis. The production of vascular endothelial growth factor, for instance, has been found to promote NSC self-renewal within the adult rat brain [117–119]. Also, the secretion of brain-derived neurotrophic factor (BDNF) has been suggested to direct NSC proliferation and balance the rates of neuroblast migration and differentiation in adult neurogenic niches [119–121]. In addition to these growth factor examples, endothelial cells are capable of secreting other types of short-range signals. For example, the chemokine stromal cell-derived factor-1 (SDF-1) is believed to regulate the migration and survival of SVZ NPCs. Additionally, the secreted glycoprotein pigment epithelium-derived factor (PEDF) promotes NSC self-renewal within the murine SVZ [119, 122]. These secreted factors have complex but essential functions in regulating stem cell behavior. Thus, engineering strategies for identifying and dissecting these paracrine signals is a key objective within the field.
Integral membrane proteins that mediate juxtacrine (i.e. cell-cell contact dependent) signaling are another important class of molecules. For example, Ephrin receptor tyrosine kinases (Ephs) and their membrane-bound ephrin ligands allow for bidirectional communication between ligand-expressing and ligand-receiving cells [123]. Several studies have investigated Eph-ephrin signaling within adult NSC and intestinal stem cell niches . A and B subclass ephrins and Eph receptors have, for example, been suggested to regulate proliferation negatively within the adult SVZ of the lateral ventricles [124]. In the adult hippocampal niche, the presentation of ephrin-B2 by hippocampal astrocytes induces neuronal differentiation of NSCs [125]. Eph-ephrin has also been implicated in coordinating migration and proliferation of stem cells within the intestinal epithelium [123]. Notch receptors and their Delta-like or Jagged family ligands represent another key signaling pathway active between juxtaposed cells in adult stem cell niches [126]. For instance, niche ependymal cells and astrocytes in the early postnatal SVZ express Jagged1, which activate Notch1 and inhibit differentiation of neural progenitors [126]. Specifically, forced Notch1 activation was found to increase NSC proliferation, whereas Notch1 repression promoted cell cycle exit [127]. Additionally, inactivation of the Notch/RBPJκ signaling pathway in adult hippocampal stem cells resulted in the depletion of Sox2-positive neural precursors and long-term suppression of hippocampal neurogenesis [128]. Therefore, Notch is viewed as a regulator of cell cycle progression that also prevents premature NSC depletion [129]. Recent in vivo studies also revealed that Notch also plays an instructive role in biasing NSCs towards an astrocytic fate within the hippocampus [130]. While Notch signaling has been demonstrated to play a crucial role in NSC maintenance in the adult dentate gyrus, it also been shown to participate in regeneration of muscle. Notch is active in quiescent muscle satellite cells; however, upon injury, muscle stem cells experience a downregulation of Notch signaling and accordingly exit their quiescent state [126, 131].
7 Early Approaches for Studying Stem Cell-Niche Cell Interactions In Vitro
A diverse spectrum of engineering strategies has emerged in the stem cell field for modeling and dissecting heterotypic cellular interactions within stem cell niches . Early efforts focused primarily on the use of bulk co-culture studies for elucidating the effects of cell-cell juxtacrine signaling and soluble paracrine factors. To study juxtacrine signaling, co-culture systems have seeded two or more cell types onto the same monolayer culture, yielding random heterotypic interactions. To study soluble paracrine factors, permeable transwell inserts have often been employed to separate two cell populations while allowing for the diffusion of soluble factors between cells. Additionally, applying conditioned media—i.e. medium that has been cultured with one cell type that contains paracrine factors—to stem cell cultures can achieve a similar result to the transwell system, with the caveat that particularly labile factors can undergo decay in conditioned medium. In either case, the degree of cell-cell signaling can be controlled by adjusting the cell numbers for each population [132]. Often, both direct co-cultures and transwell co-cultures are conducted in parallel to isolate the paracrine from juxtacrine effects.
This two-pronged strategy has proved useful in a variety of studies. Ottone et al., for instance, employed this approach for investigating how cell-cell contact-dependent signaling of vascular epithelium governs NSC behavior [133]. In doing so, they pursued both co-cultures and transwell cultures of NSCs with three types of murine endothelial cells: primary brain microvascular endothelial cells, brain microvascular endothelial cell line, and conditionally immortalized pulmonary endothelial cells. Direct cell contact between NSCs and all three cell types through bulk co-culture studies was found to induce cell-cycle arrest in the G0–G1 phase and thereby promote quiescence [133]. To assess whether this outcome resulted from contact-dependent signaling, cell-cycle profiles of transwell cultures were conducted in parallel and compared with NSC monocultures. Similar results between these two culture systems indicated that the observed quiescence was, indeed, a result of juxtacrine signaling from endothelial cells [133]. In addition, this study showed that NPCs cultured in contact with epithelial cells as opposed to cultured in transwells failed to produce differentiated progeny, instead maintaining multipotent GFAP+Sox2+ markers [133]. Song et al. [134] also exploited the advantages of the two co-culture systems to study how niche cell types within the hippocampus affect neurogenesis. When NSCs were plated in primary neuron-enriched cultures, they observed an increase in oligodendrocyte production and a lack of neurogenesis. In contrast, NSCs cultured on a feeder layer of primary hippocampal astrocytes displayed a 10-fold increase in the percentage of differentiated neurons compared to control laminin-coated surfaces. To elucidate whether hippocampal astrocytes instructed neuronal fate commitment via paracrine or membrane-bound factors, NSCs were cultured in medium conditioned by astrocytes and found to result in a lower level of neurons [134]. These parallel cultures indicated that hippocampal neurogenesis stems from a mixture of soluble and contact-dependent cues. Later work by Ashton et al. [125] revealed that the juxtacrine signal responsible for neurogenesis was ephrin-B2.
Dual co-culture approaches have also played an integral role in helping dissect the contributions of neighboring niche cell types in influencing the behavior of other adult stem cell types. For example, Loibl et al. [135] utilized this strategy for studying whether endothelial progenitor cells (EPCs) promoted angiogenesis through the induction of a pericyte-like phenotype in MSCs, which can be identified by an upregulation of CD146, NG2, αSMA, and PDGFR-β. In a method analogous to that of Ottone et al., cell-cell crosstalk was investigated by comparing direct co-cultures to transwell cultures and single-cell type control cultures. After 3 days in the different cultures, they reported an approximate 15-fold increase of CD146 expression for the direct co-culture versus only a three-fold and two-fold increase for single and transwell cultures, respectively [135]. A similar but less pronounced trend in gene expression was observed for NG2. Additionally, for αSMA and PDGFR-β, MSCs in direct co-cultures were better able to maintain expression while the other cultures demonstrated decreases in expression [135]. These findings suggest that EPCs play a key role in mediating differentiation of MSCs into pericytes through cell-cell juxtacrine interactions [135]. Moreover, these findings (along with those of Ottone and Song) highlight the major role that direct co-cultures and transwell co-cultures have in elucidating the effects of cellular interactions within stem cell niches .
7.1 Patterned Bulk Stem Cell Co-Cultures
While random bulk co-cultures are useful tools for studying cellular interactions that may occur within the stem cell niche, there has been significant work in developing patterned co-culture systems. These platforms are motivated by two key advantages. The first is the enhanced spatial control for more precise manipulation of heterotypic cellular interactions. The second is the high reproducibility of patterning techniques, which ensures consistent cellular localization across multiple experiments for statistical analysis [136]. These spatially-defined in vitro culture systems are also deemed by some as more accurate predictors of heterotypic cell-cell effects as they better mimic the inherently structured cellular organization of in vivo microenvironments [137].
Soft-lithography techniques are broadly utilized for fabricating such platforms, where success depends upon one cell type preferentially attaching to patterned regions comprised of a particular type of ECM and a second cell type preferring the unpatterned regions [136, 138]. Rodriguez et al. [138] demonstrated this strategy by combining microcontact printing with avidin-biotin chemistry to generate hMSC and human umbilical vein endothelial cell (HUVEC) co-cultures of various geometrical interfaces at both the multicellular and single-cell level (Fig. 9a). This specific strategy relied on the patterning of three distinct regions: adhesive, nonadhesive, and dynamically adhesive. Microcontact printing was first utilized to pattern regions of fibronectin, a cell-adhesive material, followed by the printing of neutravidin, an initially non-adhesive material. Pluronic F127 was physisorbed onto the remaining non-patterned regions to produce a nonbiofouling background. For cell patterning, the first population was seeded onto the substrate and attached to the fibronectin areas. Neutravidin was then dynamically switched from non-adhesive to adhesive upon addition of biotinylated fibronectin, which allowed for the selective patterning of the second cell type [138]. Fukuda et al. [139] employed an analogous strategy by utilizing capillary force lithography and layer-by-layer assembly of polyelectrolytes to demonstrate the capacity to establish patterned co-cultures of ESCs and NIH-3T3 fibroblasts (Fig. 9b). Specifically, glass substrates were patterned with cell-resistive hyaluronan (HA) utilizing capillary force lithography. This was achieved by placing a PDMS mold on top of a spin-coated thin film of HA and subsequently allowing capillary action to create a positive replica of the PDMS mold. Fibronectin was then deposited onto the HA-patterned substrate and adsorbed to the bare glass-exposed regions. ES cells then selectively adhered to the fibronectin patterns. In order to accommodate the secondary cell type, fibroblasts, collagen was deposited onto the surface, adhered to the HA regions, and switched the regions to cell-adhesive [139]. Such patterned co-cultures offer useful platforms for studying fundamental stem cell biology and even exploring various tissue engineering strategies , though they rely upon selectivity of ECM proteins that may, in many other cases, be somewhat promiscuous in their cell adhesive properties.
Patterned bulk co-culture strategies. Panel a Patterning schematic for generating bulk and single-cell patterned co-culture systems; two MSC populations labeled with either CellTracker red or CellTracker green [138]. Panel b Schematic illustrating the use of capillary force lithography and layer-by-layer deposition for generating ESC (green) and NIH-3T3 (red) co-culture on a patterned HA/collagen surface [139]. Panel c Schematic for patterning static and dynamic co-cultures of mESCs (red), AML12 cells (green), and NIH-3T3 cells (blue) [140]
Another engineering approach for controlling heterotypic cellular interactions involves the utilization of microfabricated elastomer stencils, which are advantageous because they do not rely on patterning of ECM components. In this approach, stencils with a distinct pattern are coupled to a substrate, thereby physically blocking cellular adhesion to specific regions upon seeding of the first cell type. The stencil is removed to expose the previously covered underlying substrate, and the second cell type is seeded. Wright et al. [140] employed this strategy for creating static and dynamic co-cultures of mouse ES cells with fibroblasts and/or hepatocytes. The static co-culture was achieved by attaching a reversibly sealed parylene-C stencil with hole patterns of diameters ranging from 40 to 200 μm to a fibronectin-coated PDMS substrate. Upon attachment of ES cells to the exposed hole regions, the stencil was gently peeled off. AML12 hepatocyte cells were subsequently seeded on the cell micropatterned surface, filling in the unpatterned regions. In the case of the dynamic co-culture, the authors demonstrated the capacity for temporal regulation of cell-cell interactions, though efficiencies of the process were not noted. Specifically, ES cells were cultured with fibroblasts and hepatocytes in a sequential manner, thereby exposing ES cells to two different cell types (Fig. 9c) [140]. Unlike the static platform that accommodated only two cell types, the dynamic platform utilized a parylene-C stencil initially treated with hyaluronic acid. ES cells were then seeded within the open hole patterns of the micro-stencil. To support the second cell type, collagen was absorbed onto the HA-coated stencil, switching the non-patterned regions from cell repulsive to adhesive. Finally, ES cells were exposed to a secondary support cell by completely removing the stencil and seeding the third cell type [140]. This dynamic strategy has potential not only to elucidate how cues from other niche cells act independently but also for dissecting how these disparate cues may act in a combinatorial and hierarchical manner. Additionally, Wright et al. [140] claim that hole patterns on the parylene-C stencils could be fabricated down to a 3 μm diameter and can easily be adapted to support single-cell studies. These methods make elastomer stencils a powerful and unique engineering strategy for controlling heterotypic cellular interactions beyond two cell types.
7.2 Patterned 3D Stem Cell Co-Cultures
The push toward 3D patterned co-cultures has also been of recent interest within the stem cell field as they better emulate native cellular microenvironments within in vivo tissue niches. The drive from 2D to 3D has led to the development of many new engineering strategies . While micropatterning techniques generally manipulate cell-surface adhesion to obtain cellular patterns, this strategy cannot be applied for the formation of cell spheroids. Thus, additional approaches are required. Microfluidic methods encompass one such approach for generating patterned 3D co-cultures. Torisawa et al. [141] for instance, illustrated the ability to generate co-culture spheroids with various compositions and geometries (Fig. 10a). Their technique involved the fabrication of a two-layered PDMS device with two microchannels separated by a semi-porous membrane of polycarbonate. The top channel was dedicated to guiding the relative positions of the two cell types via laminar streams, thereby hydrodynamically focusing the cell populations into the bottom layer and ultimately controlling the geometry of the multicellular spheroids. Spatial control of these 3D co-cultures was achieved by changing the geometry of the bottom microchannel. With this system, Torisawa and colleagues patterned spheroids within a straight 200 μm channel, juxtaposing mouse ES cells with hepatocarcinoma HepG2 cells [141]. They demonstrated that ESC differentiation within the patterned co-culture spheroids revealed regional differentiation dependent upon initial cell-cell positioning. This microfluidic system was shown also to be compatible with other cell types and generated a variety of 3D co-culture spheroid patterns of breast cancer cells with HUVECs and monkey kidney cells. The capability of recapitulating more complex co-cultures was presented by patterning up to five distinct groups of cells (i.e. five alternating lines of cells with a total width of 1 mm that formed contacting spheroids after 3 days of culture) through the use of a five-inlet top channel [141].
Microfluidic strategies for generating patterned bulk co-cultures. Panel a Encapsulation of fluorescently labeled populations of mESCs into agarose microgels and formation of embryoid bodies after 4.5 days of culture [142]. Panel b Fluorescent images comparing 3D mixed dish versus patterned mESC spheroid co-cultures generated from a two-layered microfluidic device [141]. Panel c Patterning of mESCs (red) and polystyrene beads (green) using spiral electrodes [143]
Droplet microfluidics is another promising technique for generating high-throughput 3D cell co-cultures. Tumarkin et al. [142] utilized this technology to synthesize microgel emulsions that served as “micro-reactors”, in which discrete numbers of cells were compartmentalized to enhance heterotypic cellular interactions (Fig. 10b). The encapsulation of two different cell populations in agarose droplets was achieved using a T-junction microfluidic device. Co-encapsulation was tested on two populations of mESCs, where one was fluorescently labeled with a green cell tracker and the other labeled with a red cell tracker. Cells were suspended in agarose solution and supplied to the microfluidic device. Despite relying on random Poisson seeding, the relative cell numbers encapsulated from each population could be roughly controlled by tuning the ratio of flow rates for the cell suspensions. To generate droplets, a carrier phase of mineral oil containing 3 %(wt) of Span 80 surfactant was introduced perpendicular to the cell streams. Downstream of the junction, droplets were collected and cooled to induce gelation of the microgels, and analysis was conducted using optical microscopy and flow cytometry. Encapsulated cells not only demonstrated the ability to form embryoid bodies but also demonstrated viability approaching 80 % at the end of a 4.5-day culture [142]. These results are useful first steps, showing the viability of the technique for precisely encapsulating two different cell populations. Limitations of this strategy, however, include a practical restriction to two cell types due to Poisson statistics, an inability to control cell stoichiometry directly, and potential difficulties in extending the approach to adhesion-dependent cells.
The ability to control the assembly of heterotypic cellular interactions in 3D has also been demonstrated by Bajaj et al. utilizing a different microfluidic technique [143]. Dielectrophoresis (DEP), in combination with stereolithography and custom-made electrodes, was used to pattern and encapsulate two distinct populations of mouse ESCs within poly(ethylene glycol) diacrylate hydrogels of tunable stiffnesses (Fig. 10c) [143]. DEP refers to the induced motion of electrically polarizable entities (such as cells) when exposed to an electric field gradient [144]. Without dielectrophoretic forces, the two different cell populations exhibited minimal cell contact. However, upon inducing DEP by energizing the electrodes with an AC voltage, cell-cell contacts were stimulated and led to pearl chain geometries. In addition to patterning cells, Bajaj et al. extended this strategy to organize spheroids of cells spatially within hydrogels [143]. This method holds potential for enabling more robust investigations of stem cell-niche cell communication.
While microfluidics has been a key technology for generating in vitro platforms for studying juxtacrine signaling within stem cell niches , it has also played a pivotal role in elucidating the effects of paracrine signaling. Unlike standard cell-culture platforms, which are prone to unequal distributions of secreted factors, microfluidic devices utilize laminar flow to impose precise control of soluble factor profiles [145]. Microfluidic gradient generators, for example, have been employed for exogenous delivery of soluble factors (i.e. growth factors and cytokines) to stem cell cultures [146, 147]. Flow has also been used to modulate the distribution of secreted factors from niche cells to stem cells [148–150]. Moreover, another advantage of using microfluidics is the ability to isolate soluble factors for downstream analysis [145].
8 Shifting Focus to Single-Cell Resolution and Artificial Niches
The aforementioned bulk co-culture systems (both random and patterned) have yielded valuable insight into the effects of cellular signaling within stem cell niches . However, there are a number of additional features that would be advantageous to address. Micropatterned surfaces enable spatial control of cellular interactions yet can restrict cell motility and proliferation to chemically patterned regions [132]. Additionally, the ability to pattern more than two cell types remains a challenge. Microfluidic platforms, on the other hand, introduce shear forces, which may affect and bias stem cell behavior. Another significant concern with bulk co-culture systems is the difficulty in discerning each cell type’s relative contribution to overall behavior [151]. In an attempt to address the latter issue, there is a growing focus within the stem cell field on developing engineering strategies that operate at the single-cell level. These types of systems allow for more focused and robust analyses of the effects of juxtacrine and paracrine signaling. Moreover, they hold potential for shedding insight onto the heterogeneity of intercellular interactions [145].
8.1 Microfluidic Approaches for Single-Cell Co-Cultures
The microfluidic field has fostered the development of a multitude of strategies to capture and pair different cell types at a single-cell resolution. Skelley et al. [152] presented a technique for individually pairing thousands of mouse ESCs with mouse embryonic fibroblasts at an efficiency approaching 70 %. Their microfluidic device consisted of a dense array of passive hydrodynamic traps, referred to as weirs, that operated via a three-step loading protocol (Fig. 11a) [152]. Each weir was comprised of a larger front-side cup optimized to accommodate two cells and a smaller back-side capture cup for temporary capture. mESCs were first flown toward the smaller back-side cups. Once cells fully occupied these cups, the flow direction was switched, and the captured mESCs were rapidly transferred to the large front-side cup. Fibroblasts were then flown in the same direction, trapped, and loaded adjacent to the captured mESCs [152]. Though these authors focused on applying the system to enhance cellular fusion, this platform also holds potential for elucidating the effects of heterotypic cellular interactions on dictating stem cell behavior, though attachment-dependent cells may pose a challenge.
Single-cell co-cultures using microfluidics. Panel a Cell-loading schematic for capturing cell tracker-labeled mouse 3T3s (red and green) (left); fusion of a paired green fluorescent protein-expressing mESC (green) and Hoerchst-stained mouse embryonic fibroblast (blue) (right) [152]. Panel b Overview of single-cell pairing protocol in which sequential trapping of mouse embryonic fibroblasts (red) and mESCs (green) is achieved [153]
Hong et al. [153] developed another microfluidic device that performed heterotypic cell pairing at a single-cell level and supported the culture and tracking of cell pairs over multiple generations. Rather than employing weir-based hydrodynamic traps, they relied on trapping junctions that implemented self-variable fluidic resistance to generate high-efficiency cell groupings (Fig. 11b) [153]. The basic principle of this approach is that, once cells enter the individual culture chamber and are trapped by small junctions located at the bottom of these chambers, fluidic flow resistance increases and blocks additional cells from infiltrating the chamber. Following capture of the first cell, cells are incubated to allow for migration away from the junction, resetting the traps to an “active” state and allowing for the capture of a second cell type [153]. Advantages of this device include high-throughput and minimized physical constraint to cell growth, allowing for multiple cell divisions and migration. Hong et al. [153] applied this system for the single-cell co-cultures of mouse embryonic fibroblasts and mESCs as a proof-of-concept.
Other microfluidic-based tools with considerable spatial control over sequential trapping and pairing of heterotypic single-cell pairs have been developed but not yet implemented within the stem cell field. The adoption of these emerging technologies offers potential for shedding light on the role of specific cellular interactions within stem cell niches . Dura et al. presented a deformability-based, cell-pairing device which utilized weir-based traps, similar to Skelley and colleagues [154]. However, upon capturing the first cell type, a transient increase in flow rate squeezed the arrested cells into the larger double-cell traps through constriction by flow-induced deformation (Fig. 12a) [154]. The second cell type was captured consecutively in a similar fashion. An advantage of this system is that paired cells were secured within the traps, allowing for the device to be disconnected and applied for other off-chip applications, while retaining cell pairing integrity. Dura et al. [154] also developed methods for pairing heterotypic cells of different sizes by tuning the geometry of the trapping structures. Finally, the ability to pair triplets of cells was illustrated, where one red fluorescently-labeled NIH3T3 fibroblast was sandwiched between two green fluorescently-labeled fibroblasts [154].
Examples of additional microfluidic platforms with potential applications for studying stem cell-niche cell interactions. Panel a Loading protocol for pairing cells into traps possessing lock-in features (top); two-component and three-component pairings demonstrated (bottom) [154]. Panel b Heterotypic single-cell co-culture arrays, pairing one unlabeled SW480 cell with one fluorescently labeled with calcein AM [155]
Frimat et al. [155] demonstrated another microfluidic approach for inducing single-cell co-culture contacts for studying the formation of gap junctions (Fig. 12b). To start, a microfluidic circuit based on differential fluidic resistance directed single cells into an array of trap structures within a superimposed serpentine channel. To capture a second single cell adjunct to the first, a second trapping structure was designed using a mirrored configuration. Despite the heterogeneous size characteristics of the cells employed (HT29 colon carcinoma cells, MCF-7 epithelial-like breast cancer cells, and SW480 epithelial cells), these cells were captured at an efficiency approaching 81 % with 96 % of cells retained within these traps during the first two days [155].
8.2 Artificial Stem Cell-Niche Cell Signaling Approaches
The precise manipulation of different cell types remains an ongoing challenge within the field. While micropatterning and microfluidics enable more precise spatial control over the design of in vitro platforms, other approaches for dissecting cellular communication within the niche have been pursued. These approaches involve analyzing the natural complexity of cellular interactions and re-engineering more simplified versions in vitro. One notably powerful approach involves the immobilization of key cell surface ligands (cadherins, EpCAM, Delta-1, Jagged-1, and ephrins) to biomaterials as a means of mimicking communication from a secondary cell type.
Roccio et al. [156] demonstrated the fabrication of a microarrayed artificial niche platform dedicated to better understanding the role that the Notch ligand, Jagged-1, has on regulating single NSC behavior (Fig. 13a). A robotic spotter was utilized to immobilize the protein of interest to the bottom of PEG-based hydrogel microwells. Tethered Jagged-1 was found to increase survival and neurosphere-forming efficiency of single NSCs. They also assessed the potential synergistic effects of Jagged-1 in combination with Laminin-1 (though no additive effect was observed) [156]. In another system developed by the same group, a 3D-niche microarray system was presented that expanded the cell ligand repertoire to include E-cadherin and EpCAM—not to mention a plethora of other key niche factors, including control over ECM stiffness, ECM components, soluble factors, cell density, and ECM degradability (Fig. 13b) [157].
Strategies for engineering artificial niche microenvironments that mimic cell-cell interactions. Panel a Overview of steps for fabricating microarrayed artificial niches (left); representative images of NSC cultures immunostained for Nestin (red) and βIII-tubulin (green) on hydrogels co-functionalized with Laminin-1 alone or Jagged-1 and Laminin-1 (right) [156]. Panel b Cell-cell interaction components incorporated into 3D microarray platform in combination with other factors (i.e. matrix elasticity, proteolytic degradability, cell density, ECM components, and soluble factors) for studying mESC behavior [157]. Panel c Illustration of DLL4-coated microbead interacting with Notch receptor on HSCs [158]
An additional approach for developing functionalized biomaterials for mimicking cellular interactions was demonstrated by Taqvi et al. [158]. Magnetic microbeads were functionalized with the notch ligand, Delta-like ligand 4 (DLL4), thus creating a synthetic alternative to niche stromal cells that communicate with HSCs. Functionalization was achieved by first coating magnetic polystyrene microbeads with streptavidin. These beads were then washed and incubated with a biotinylated histidine tag antibody and, again, with the histidine-tagged DLL4 protein (schematic illustrated in Fig. 13c) [158]. This biomaterial-based artificial Notch-signaling system was utilized for investigating the induction of T-cell differentiation in HSCs [158]. This approach offers a simplified alternative to modifying niche stromal cells genetically to express Notch ligands followed by co-culture. More importantly, this system enables more thorough investigation of the effects of Notch ligand-receptor interaction. Quantitative and temporal studies are enabled by, respectively, tuning the ligand-cell ratio and duration of signaling. For instance, Taqvi et al. [158] found that a 1:1 bead-to-cell ratio generated a significantly higher T-cell differentiation efficiency when compared to a 5:1 functionalized bead-to-cell ratio.
9 Conclusions and Future Directions
Understanding the complexity of stem cell behavioral regulation remains a formidable challenge, and insights into the underlying mechanisms will greatly enable the development of stem cell-based therapies. The successful control of stem cell expansion and differentiation ex vivo in addition to the targeted activation of endogenous stem cell populations demands a comprehensive understanding of the regulatory role of environmental (i.e. niche) signals. Accordingly, the development of innovative engineering strategies for recapitulating key facets of stem cell-ECM interactions and stem cell-niche interactions has been instrumental in providing deeper insights into how stem cells respond to extrinsic cues at a molecular level.
Within the past few decades alone, the stem cell field has made tremendous progress in understanding these niche principles through initial strategies that focused primarily on fabricating static representations of niche ECM features (i.e. topography, matrix elasticity, ligand presentation, etc.) and bulk co-culture studies of heterotypic cellular interactions (i.e. paracrine and juxtacrine signaling). However, the desire to mimic dynamic in vivo niche phenomena has spurred the evolution of more sophisticated second-generation engineering tools. The push to incorporate spatiotemporal control into biomaterial systems has enabled an unprecedented ability for probing stem cell response to dynamic changes in the duration or intensity of presented ECM cues. In the case of studying niche cellular interactions, the robust isolation of single-cell co-cultures and the development of artificial cell-signaling platforms allows for more controlled and reproducible study of cell-cell interactions.
As our knowledge of stem cell biology continues to expand, we anticipate that engineering strategies will also progress. Biomaterials with not only tunable but also reversible properties will be key for dissecting how stem cells respond to ECM-related signaling dynamics. For instance, biomaterials engineered to allow reversible stiffening and softening will be a significant advancement within the field. Additionally, platforms that allow for the ability to investigate combinations of ECM cues simultaneously and at different temporal onsets will be valuable for obtaining a more comprehensive understanding of stem cell niches that can ultimately be applied to accelerate the development of clinical applications. For studying the role of intercellular communication within stem cell niches, high-throughput strategies for creating precise cellular communities of more than two cell types at a single-cell resolution will reveal potential juxtacrine/paracrine signaling hierarchies. Another important advance would include engineering strategies that control the timing of cellular interactions to understand the duration of contact that is necessary to bias stem cell behavior towards a desired fate. With these advanced strategies in hand, the stem cell field will be better positioned to make stem cell therapies a clinical reality.
Abbreviations
- 3D:
-
Three-dimensional
- ASC:
-
Adult stem cell
- BDNF:
-
Brain-derived neurotrophic factor
- Bmp:
-
Bone morphogenic protein
- DEP:
-
Dielectrophoresis
- DLL4:
-
Delta-like ligand 4
- DP:
-
Dermal papilla
- DTT:
-
Dithiothreitol
- ECM:
-
Extracellular matrix
- EPC:
-
Endothelial progenitor cell
- Ephs:
-
Ephrin receptor tyrosine kinases
- ESC:
-
Embryonic stem cell
- GSC:
-
Germline stem cell
- hMSC:
-
Human mesenchymal stem cell
- HA:
-
Hyaluronan
- HSC:
-
Hematopoietic stem cell
- HSPG:
-
Heparan sulphate proteoglycan
- HUVEC:
-
Human umbilical vein endothelial cell
- IKVAV:
-
Ile-Lys-Val-Ala-Val
- iPSC:
-
Induced pluripotent stem cell
- ISC:
-
Intestinal stem cell
- MARC:
-
Multi-ARChitecture
- mESC:
-
Mouse embryonic stem cell
- MSC:
-
Mesenchymal stem cell
- NPC:
-
Neural progenitor cell
- NSC:
-
Neural stem cell
- PEDF:
-
Pigment epithelium-derived factor
- PEG:
-
Polyethylene glycol
- PDMS:
-
Polydimethylsiloxane
- RGD:
-
Arg-Gly-Asp
- RGDS:
-
Arg-Gly-Asp-Ser
- RGDSP:
-
Arg-Gly-Asp-Ser-Pro
- SDF-1:
-
Stromal cell-derived factor-1
- SVZ:
-
Subventricular zone
- TiO2 :
-
Titanium dioxide
- UV:
-
Ultraviolet
- UVO:
-
Ultraviolet/ozone
References
Sun Y, Chen CS, Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys. 2012;41:519–42. doi:10.1146/annurev-biophys-042910-155306.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi:10.1016/j.cell.2006.07.024.
Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32(8):795–803. doi:10.1038/nbt.2978.
Vazin T, Schaffer DV. Engineering strategies to emulate the stem cell niche. Trends Biotechnol. 2010;28(3):117–24. doi:10.1016/j.tibtech.2009.11.008.
Liu B, Shu S, Kenny TP, Chang C, Leung PSC. Stem cell therapy in autoimmune rheumatic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2014;47(2):244–57. doi:10.1007/s12016-014-8445-8.
Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10(6):740–9. doi:10.1016/j.stem.2012.05.010.
Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6(2):103–15. doi:10.1016/j.stem.2010.01.011.
Iwasaki H, Suda T. Hematopoietic stem cell biology. In: Kondo M, editor. Stem cell biology and regenerative medicine. Totowa: Humana Press; 2010. p. 37–56.
Scholfield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25.
Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10(6):698–708. doi:10.1016/j.stem.2012.05.012.
Miller FD, Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell. 2009;4(6):507–10. doi:10.1016/j.stem.2009.05.008.
Gage FH. Mammalian neural stem cells. Science. 2000;25(287):1433–8. doi:10.1126/science.287.5457.1433.
Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102(4):451–61. doi:10.1016/S0092-8674(00)00050-7.
Gambardella L, Barrandon Y. The multifaceted adult epidermal stem cell. Curr Opin Cell Biol. 2003;15(6):771–7. doi:10.1016/j.ceb.2003.10.011.
Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–63. doi:10.1126/science.1092436.
Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180(2):273–84. doi:10.1083/jcb.200708185.
Boonen KJM, Post MJ. The muscle stem cell niche: regulation of satellite cells during regeneration. Tissue Eng Part B Rev. 2008;14(4):419–31. doi:10.1089/ten.teb.2008.0045.
Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2(1):22–31. doi:10.1016/j.stem.2007.12.012.
Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23–67. doi:10.1152/physrev.00043.2011.
Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334(6061):1420–4. doi:10.1126/science.1213214.
Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi:10.1038/nrm3721.
Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154(2):274–84. doi:10.1016/j.cell.2013.07.004.
Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–9. doi:10.1038/nature04957.
Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;1(414):98–104. doi:10.1038/35102160.
Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311(5769):1880–5. doi:10.1126/science.1110542.
Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9(1):11–21. doi:10.1038/nrm2319.
Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–7. doi:10.1126/science.1171643.
Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840(8):2506–19. doi:10.1016/j.bbagen.2014.01.010.
Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12(5):458–65. doi:10.1038/nmat3586.
Vincent LG, Engler AJ. Stem cell differentiation: post-degradation forces kick in. Nat Mater. 2013;12(5):384–6. doi:10.1038/nmat3636.
Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341(1):126–40. doi:10.1016/j.ydbio.2009.10.026.
Tibbitt MW, Anseth KS. Dynamic microenvironments: the fourth dimension. Sci Transl Med. 2012;4(160):160ps24. doi:10.1126/scitranslmed.3004804.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi:10.1016/j.cell.2006.06.044.
Tam RY, Owen SC, Shoichet MS. ECM-inspired chemical cues: biomimetic molecules and techniques of immobilization. In: Brennan AB, Kirschner CM, editors. Bio-inspired materials for biomedical engineering. Hoboken: Wiley; 2014. p. 7–24.
Li ACY, Thompson RPH. Basement membrane components. J Clin Pathol. 2003;56(12):885–7.
Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Path. 1997;78:219–43. doi:10.1046/j.1365-2613.1997.280362.x.
Kazanis I, Lathia J, Moss L, ffrench-Constant C. The neural stem cell microenvironment. In: StemBook. The Stem Cell Research Community, StemBook. 2008;1–26. http://www.stembook.org.
Marthiens V, Kazanis I, Moss L, Long K, Ffrench-Constant C. Adhesion molecules in the stem cell niche–more than just staying in shape? J Cell Sci. 2010;123:1613–22. doi:10.1242/jcs.054312.
Campos LS, Decker L, Taylor V, Skarnes W. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J Biol Chem. 2006;281(8):5300–9. doi:10.1074/jbc.M511886200.
Xiao Q, Zeng L, Zhang Z, Hu Y, Xu Q. Stem cell-derived Sca-1+ progenitors differentiate into smooth muscle cells, which is mediated by collagen IV-integrin alpha1/beta1/alphav and PDGF receptor pathways. Am J Physiol Cell Physiol. 2007;292(1):C342–52. doi:10.1152/ajpcell.00341.2006.
Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13(6):558–69. doi:10.1038/nmat3980.
Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10(1):21–33. doi:10.1038/nrm2593.
Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16(4):376–81. doi:10.1038/ncb2927.
Ross RS. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res. 2004;63(3):381–90. doi:10.1016/j.cardiores.2004.04.027.
Watt FM, Huck WTS. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol. 2013;14(8):467–73. doi:10.1038/nrm3620.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. doi:10.1038/nature10137.
Zhang J, Niu C, Ye L, Huang H, He X, Harris S, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;23(425):836–41. doi:10.1038/nature02041.
Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116(6):769–78. doi:10.1016/S0092-8674(04)00255-7.
Long KR, Ffrench-Constant C. Neural stem cell quiescence comes to an un-sticky end. Nat Cell Biol. 2014;16(7):625–7. doi:10.1038/ncb3003.
Brzoska E, Ciemerych MA, Przewozniak M, Zimowska M. Regulation of muscle stem cells activation: the role of growth factors and extracellular matrix. Vitam Horm. 2011;87:239–76. doi:10.1016/B978-0-12-386015-6.00031-7.
Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43(1):55–62. doi:10.1016/j.jbiomech.2009.09.009.
Rodda AE, Meagher L, Nisbet DR, Forsythe JS. Specific control of cell–material interactions: Targeting cell receptors using ligand-functionalized polymer substrates. Prog Polym Sci. 2014;39(7):1312–47. doi:10.1016/j.progpolymsci.2013.11.006.
Kim S-H, Turnbull J, Guimond S. Extracellular matrix and cell signaling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209(2):139–51. doi:10.1530/JOE-10-0377.
Zhu J, Clark RAF. Fibronectin at select sites binds multiple growth factors and enhances their activity: expansion of the collaborative ECM-GF paradigm. J Invest Dermatol. 2014;134(4):895–901. doi:10.1038/jid.2013.484.
Hudalla GA, Murphy WL. Biomaterials that regulate growth factor activity via bioinspired interactions. Adv Funct Mater. 2001;21(10):1754–68. doi:10.1002/adfm.201002468.
Turnbull J, Powell A, Guimond S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 2001;11(2):75–82. doi:10.1016/S0962-8924(00)01897-3.
Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3(7):1–33. doi:10.1101/cshperspect.a004952.
Conway A, Schaff DV. Biophysical regulation of stem cell behavior within the niche. Stem Cell Res Ther. 2012;3(6):50. doi:10.1186/scrt141.
Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446(7139):1030–7. doi:10.1038/nature05817.
Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi:10.1016/j.stem.2009.06.016.
Mendes PM. Cellular nanotechnology: making biological interfaces smarter. Chem Soc Rev. 2013;42(24):9207–18. doi:10.1039/c3cs60198f.
Tsimbouri P, Gadegaard N, Burgess K, White K, Reynolds P, Herzyk P, et al. Nanotopographical effects on mesenchymal stem cell morphology and phenotype. J Cell Biochem. 2014;115(2):380–90. doi:10.1002/jcb.24673.
Ahn EH, Kim Y, Kshitiz An SS, Afzal J, Lee S, et al. Spatial control of adult stem cell fate using nanotopographic cues. Biomaterials. 2014;35(8):2401–10. doi:10.1016/j.biomaterials.2013.11.037.
Motemani Y, Greulich C, Khare C, Lopian M, Buenconsejo PJS, Schildhauer TA, et al. Adherence of human mesenchymal stem cells on Ti and TiO2 nano-columnar surfaces fabricated by glancing angle sputter deposition. Appl Surf Sci. 2014;15(292):626–31. doi:10.1016/j.apsusc.2013.12.022.
Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7(9):733–9. doi:10.1038/nmeth.1487.
Béduer A, Vieu C, Arnauduc F, Sol J-C, Loubinoux I, Vaysse L. Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials. 2012;33(2):504–14. doi:10.1016/j.biomaterials.2011.09.073.
Recknor JB, Sakaguchi DS, Mallapragada SK. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials. 2006;27(22):4098–108. doi:10.1016/j.biomaterials.2006.03.029.
Christopherson GT, Song H, Mao H-Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30(4):556–64. doi:10.1016/j.biomaterials.2008.10.004.
Kawano T, Sato M, Yabu H, Shimomura M. Honeycomb-shaped surface topography induces differentiation of human mesenchymal stem cells (hMSCs): uniform porous polymer scaffolds prepared by the breath figure technique. Biomater Sci. 2014;2(1):52. doi:10.1039/C3BM60195A.
Oh S, Brammer KS, Li YSJ, Teng D, Engler AJ, Chien S, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci USA. 2009;106(7):2130–5. doi:10.1073/pnas.0813200106.
Das RK, Zouani OF, Labrugère C, Oda R, Durrieu MC. Influence of nanohelical shape and periodicity on stem cell fate. ACS Nano. 2013;7(4):3351–61. doi:10.1021/nn4001325.
Yim EKF, Ankam S, Moe AAK, Chan LYT. Effect of spatial arrangement of substrate topography on neuronal differentiation of stem cells. In: Goh J, editor. The 15th international conference on biomedical engineering IFMBE proceedings. Cham: Springer International Publishing. 2014;43:60–3. doi:10.1007/978-3-319-02913-9_16.
Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, et al. Engineering cell shape and function. Science. 1994;264(5159):696–8. doi:10.1126/science.8171320.
Xia Y, Whitesides GM. Soft lithography. Annu Rev Mater Sci. 1998;28:153–84. doi:10.1146/annurev.matsci.28.1.153.
Whitesides GM, Ostuni E, Takayama S, Jian X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–73. doi:10.1146/annurev.bioeng.3.1.335.
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and rhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–95. doi:10.1016/S1534-5807(04)00075-9.
Peng R, Yao X, Ding J. Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. Biomaterials. 2011;32(32):8048–57. doi:10.1016/j.biomaterials.2011.07.035.
Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA. 2010;107(11):4872–7. doi:10.1073/pnas.0903269107.
Connelly JT, Gautrot JE, Trappmann B, Tan DW-M, Donati G, Huck WTS, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol. 2010;12(7):711–8. doi:10.1038/ncb2074.
DeForest CA, Anseth KS. Advances in bioactive hydrogels to probe and direct cell fate. Annu Rev Chem Biomol Eng. 2012;3:421–44. doi:10.1146/annurev-chembioeng-062011-080945.
Saha K, Irwin EF, Kozhukuh J, Schaffer D, Healy KE. Biomimetic interfacial interpenetrating polymer networks control neural stem cell behavior. J Biomed Mater Res A. 2007;81(1):240–9. doi:10.1002/jbm.a.30986.
Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi:10.1038/nbt1055.
Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater. 2013;2(1):57–71. doi:10.1002/adhm.201200197.
Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–41. doi:10.1038/nature08602.
Jongpaiboonkit L, King WJ, Murphy WL. Screening for 3D environments that support human mesenchymal stem cell viability using hydrogel arrays. Tissue Eng Part A. 2009;15(2):343–53. doi:10.1089/ten.tea.2008.0096.
Marklein RA, Soranno DE, Burdick JA. Magnitude and presentation of mechanical signals influence adult stem cell behavior in 3-dimensional macroporous hydrogels. Soft Matter. 2012;8(31):8113–20. doi:10.1039/C2SM25501D.
Salinas CN, Anseth KS. The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med. 2008;2(5):296–304. doi:10.1002/term.95.
Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113(Pt 10):1677–86.
Lam J, Segura T. The modulation of MSC integrin expression by RGD presentation. Biomaterials. 2013;34(16):3938–47. doi:10.1016/j.biomaterials.2013.01.091.
Wang X, Yan C, Ye K, He Y, Li Z, Ding J. Effect of RGD nanospacing on differentiation of stem cells. Biomaterials. 2013;34(12):2865–74. doi:10.1016/j.biomaterials.2013.01.021.
Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, et al. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26(31):6194–207. doi:10.1016/j.biomaterials.2005.04.003.
Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2(2):119–25. doi:10.1038/nmeth736.
Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–81. doi:10.1126/science.1191035.
Saha K, Kim J, Irwin E, Yoon J, Momin F, Trujillo V, et al. Surface creasing instability of soft polyacrylamide cell culture substrates. Biophy J. 2010;99(12):L94–6. doi:10.1016/j.bpj.2010.09.045.
Kshitiz, Park J, Kim P, Helen W, Engler AJ, Levchenko A, et al. Control of stem cell fate and function by engineering physical microenvironments. Integ Biol (Camb). 2012;4(9):1008–18.
Higuchi A, Ling QD, Kumar SS, Chang Y, Kao TC, Munusamy MA, et al. External stimulus-responsive biomaterials designed for the culture and differentiation of ES, iPS, and adult stem cells. Prog Polym Sci. 2014;39(9):1585–613. doi:10.1016/j.progpolymsci.2014.05.001.
Le DM, Kulangara K, Adler AF, Leong KW, Ashby VS. Dynamic topographical control of mesenchymal stem cells by culture on responsive poly(ε-caprolactone) surfaces. Adv Mater. 2011;23(29):3278–83. doi:10.1002/adma.201100821.
Davis KA, Burke KA, Mather PT, Henderson JH. Dynamic cell behavior on shape memory polymer substrates. Biomaterials. 2011;32(9):2285–93. doi:10.1016/j.biomaterials.2010.12.006.
Gong T, Zhao K, Yang G, Li J, Chen H, Chen Y, et al. The control of mesenchymal stem cell differentiation using dynamically tunable surface microgrooves. Adv Healthc Mater. 2014;20:1–12. doi:10.1002/adhm.201300692.
Tseng LF, Mather PT, Henderson JH. Shape-memory-actuated change in scaffold fiber alignment directs stem cell morphology. Acta Biomater. 2013;9(11):8790–801. doi:10.1016/j.actbio.2013.06.043.
Guvendiren M, Burdick JA. Stem cell response to spatially and temporally displayed and reversible surface topography. Adv Healthc Mater. 2013;2(1):155–64. doi:10.1002/adhm.201200105.
Kirschner CM, Anseth KS. In situ control of cell substrate microtopographies using photolabile hydrogels. Small. 2013;9(4):578–84. doi:10.1002/smll.201201841.
Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13(6):645–52. doi:10.1038/nmat3889.
Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun. 2012;3:792. doi:10.1038/ncomms1792.
Yoshikawa HY, Rossetti FF, Kaufmann S, Kaindl T, Madsen J, Engel U, et al. Quantitative evaluation of mechanosensing of cells on dynamically tunable hydrogels. J Am Chem Soc. 2011;133(5):1367–74. doi:10.1021/ja1060615.
Weis S, Lee TT, del Campo A, García AJ. Dynamic cell-adhesive microenvironments and their effect on myogenic differentiation. Acta Biomater. 2013;9(9):8059–66. doi:10.1016/j.actbio.2013.06.019.
Kloxin AM, Tibbitt MW, Anseth KS. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat Protoc. 2010;5(12):1867–87. doi:10.1038/nprot.2010.139.
Kasten A, Müller P, Bulnheim U, Groll J, Bruellhoff K, Beck U, et al. Mechanical integrin stress and magnetic forces induce biological responses in mesenchymal stem cells which depend on environmental factors. J Cell Biochem. 2010;111(6):1586–97. doi:10.1002/jcb.22890.
Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–7.
Rezza A, Sennett R, Rendl M. Adult stem cell niches: cellular and molecular components. Curr Top Dev Bio. 2014;107:333–72. doi:10.1016/B978-0-12-416022-4.00012-3.
Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Curr Opin Cell Biol. 2004;16(6):693–9. doi:10.1016/j.ceb.2004.09.003.
Xie T. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290(5490):328–30. doi:10.1126/science.290.5490.328.
Yamashita YM, Fuller MT, Jones DL. Signaling in stem cell niches: lessons from the Drosophila germline. J Cell Sci. 2005;118(Pt 4):665–72. doi:10.1242/jcs.01680.
Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316(5823):402–4. doi:10.1126/science.114086.
Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21(1):159–71. doi:10.1016/j.devcel.2011.06.018.
Tumbar T, Fuchs E. Epithelial hair follicle stem cells. In: Lanza R, Gearhart J, Hogan B, Melton D, Pedersen R, Thomas ED, et al., editors. Essentials of stem cell biology. 2nd ed. London: Academic; 2009. p. 189–97.
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99(18):11946–50. doi:10.1073/pnas.182296499.
Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi:10.1016/j.stem.2008.07.026.
Porlan E, Perez-Villalba A, Delgado AC, Ferrón SR. Paracrine regulation of neural stem cells in the subependymal zone. Arch Biochem Biophys. 2013;534(1–2):11–9. doi:10.1016/j.abb.2012.10.001.
Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;464:450–64. doi:10.1006/mcne.1999.0762.
Bath KG, Akins MR, Lee FS. BDNF control of adult SVZ neurogenesis. Dev Psychobiol. 2012;54(6):578–89. doi:10.1002/dev.20546.
Ramírez-Castillejo C, Sánchez-Sánchez F, Andreu-Agulló C, Ferrón SR, Aroca-Aguilar JD, Sánchez P, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9(3):331–9. doi:10.1038/nn1657.
Holmberg J, Genander M, Halford MM, Annerén C, Sondell M, Chumley MJ, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125(6):1151–63. doi:10.1016/j.cell.2006.04.030.
Genander M, Frisén J. Ephrins and Eph receptors in stem cells and cancer. Curr Opin Cell Biol. 2010;22(5):611–6. doi:10.1016/j.ceb.2010.08.005.
Ashton RS, Conway A, Pangarkar C, Bergen J, Lim K-I, Shah P, et al. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci. 2012;15(10):1399–406. doi:10.1038/nn.3212.
Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140(4):689–704. doi:10.1242/dev.080614.
Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci USA. 2007;104(51):20558–63. doi:10.1073/pnas.0710156104.
Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, et al. RBPJk-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30(41):13794–807. doi:10.1523/JNEUROSCI.1567-10.2010.
Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: Notch signaling in the adult brain. Nat Rev Neurosci. 2011;12(5):269–83. doi:10.1038/nrn3024.
Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–90. doi:10.1146/annurev.neuro.25.030702.130823.
Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30(2):232–42. doi:10.1002/stem.773.
Rosenthal A, Macdonald A, Voldman J. Cell patterning chip for controlling the stem cell microenvironment. Biomaterials. 2007;28(21):3208–16. doi:10.1016/j.biomaterials.2007.03.023.
Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, et al. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol. 2014;16(11):1045–56. doi:10.1038/ncb3045.
Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi:10.1038/417039a.
Loibl M, Binder A, Herrmann M, Duttenhoefer F, Richards RG, Nerlich M, et al. Direct cell-cell contact between mesenchymal stem cells and endothelial progenitor cells induces a pericyte-like phenotype in vitro. Biomed Res Int. 2014;20(2014):395781. doi:10.1155/2014/395781.
Kaji H, Camci-Unal G, Langer R, Khademhosseini A. Engineering systems for the generation of patterned co-cultures for controlling cell-cell interactions. Biochim Biophys Acta. 2011;1810(3):239–50. doi:10.1016/j.bbagen.2010.07.002.
Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, et al. Layer-by-layer deposition of hyaluronic acid and poly-L-lysine for patterned cell co-cultures. Biomaterials. 2004;25(17):3583–92. doi:10.1016/j.biomaterials.2003.10.033.
Rodriguez NM, Desai RA, Trappmann B, Baker BM, Chen CS. Micropatterned multicolor dynamically adhesive substrates to control cell adhesion and multicellular organization. Langmuir. 2014;30(5):1327–35. doi:10.1021/la404037s.
Fukuda J, Khademhosseini A, Yeh J, Eng G, Cheng J, Farokhzad OC, et al. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials. 2006;27(8):1479–86. doi:10.1016/j.biomaterials.2005.09.015.
Wright D, Rajalingam B, Selvarasah S, Dokmeci MR, Khademhosseini A. Generation of static and dynamic patterned co-cultures using microfabricated parylene-C stencils. Lab Chip. 2007;7(10):1272–9. doi:10.1039/B706081E.
Torisawa Y, Mosadegh B, Luker GD, Morell M. Microfluidic hydrodynamic cellular patterning for systematic formation of co-culture spheroids. Integr Biol (Camb). 2009;1(11–12):649–54. doi:10.1039/b915965g.
Tumarkin E, Tzadu L, Csaszar E, Seo M, Zhang H, Lee A, et al. High-throughput combinatorial cell co-culture using microfluidics. Integr Biol (Camb). 2011;3(6):653–62. doi:10.1039/c1ib00002k.
Bajaj P, Marchwiany D, Duarte C, Bashir R. Patterned three-dimensional encapsulation of embryonic stem cells using dielectrophoresis and stereolithography. Adv Healthc Mater. 2013;2(3):450–8. doi:10.1002/adhm.201200318.
Martinez-Duarte R. Microfabrication technologies in dielectrophoresis applications—a review. Electrophoresis. 2012;33(21):3110–32. doi:10.1002/elps.201200242.
Guo F, French JB, Li P, Zhao H, Chan CY, Fick JR, et al. Probing cell-cell communication with microfluidic devices. Lab Chip. 2013;13(16):3152–62. doi:10.1039/c3lc90067c.
Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, et al. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 2005;5(4):401–6. doi:10.1039/B417651K.
Park JY, Kim SK, Woo DH, Lee EJ, Kim JH, Lee SH. Differentiation of neural progenitor cells in a microfluidic chip-generated cytokine gradient. Stem Cells. 2009;27(11):2646–54. doi:10.1002/stem.202.
Blagovic K, Kim LY, Voldman J. Microfluidic perfusion for regulating diffusible signaling in stem cells. PLoS ONE. 2011;6(8):e22892. doi:10.1371/journal.pone.0022892.
Ellison D, Munden A, Levchenko A. Computational model and microfluidic platform for the investigation of paracrine and autocrine signaling in mouse embryonic stem cells. Mol BioSyst. 2009;5(9):1004–12. doi:10.1039/b905602e.
Song X, Kong B, Li D. A new tool for probing of cell-cell communication: human embryonic germ cells inducing apoptosis of SKOV3 ovarian cancer cells on a microfluidic chip. Biotechnol Lett. 2008;30(9):1537–43. doi:10.1007/s10529-008-9725-2.
Bogdanowicz DR, Lu HH. Multifunction co-culture model for evaluating cell-cell interactions. Methods Mol Biol. 2014;1202:29–36. doi:10.1007/7651_2013_62.
Skelley AM, Kirak O, Suh H, Jaenisch R, Voldman J. Microfluidic control of cell pairing and fusion. Nat Methods. 2009;6(2):147–52. doi:10.1038/nmeth.1290.
Hong S, Pan Q, Lee LP. Single-cell level co-culture platform for intercellular communication. Integr Biol (Camb). 2012;4(4):374–80. doi:10.1039/c2ib00166g.
Dura B, Liu Y, Voldman J. Deformability-based microfluidic cell pairing and fusion. Lab Chip. 2014;14(15):2783–90. doi:10.1039/c4lc00303a.
Frimat JP, Becker M, Chiang YY, Marggraf U, Janasek D, Hengstler JG, et al. A microfluidic array with cellular valving for single cell co-culture. Lab Chip. 2011;11(2):231–7. doi:10.1039/c0lc00172d.
Roccio M, Gobaa S, Lutolf MP. High-throughput clonal analysis of neural stem cells in microarrayed artificial niches. Integr Biol (Camb). 2012;4(4):391–400. doi:10.1039/c2ib00070a.
Ranga a, Gobaa S, Okawa Y, Mosiewicz K, Negro A, Lutolf MP. 3D niche microarrays for systems-level analyses of cell fate. Nat Commun. 2014;5:4324. doi: 10.1038/ncomms5324.
Taqvi S, Dixit L, Roy K. Biomaterial-based notch signaling for the differentiation of hematopoietic stem cells into T cells. J Biomed Mater Res A. 2006;79(3):689–97. doi:10.1002/jbm.a.30916.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Scheideler, O.J., Sohn, L.L., Schaffer, D.V. (2015). Emerging Engineering Strategies for Studying the Stem Cell Niche. In: Turksen, K. (eds) Biology in Stem Cell Niche. Stem Cell Biology and Regenerative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-21702-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-21702-4_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21701-7
Online ISBN: 978-3-319-21702-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)