Abstract
A microfluidic device with unidirectional perfusion has been developed to observe the effect of human embryonic germ (hEG) cells on SKOV3 cells. The hEG and SKOV3 cells were seeded in the inlet and the outlet reservoirs separately, and co-cultured for 2 days. The medium was perfused unidirectionally from the inlet to the outlet. The growth inhibition of SKOV3 cells was monitored online and the apoptosis signals in SKOV3 culture area decreased along the flow of the medium. In conclusion, microfluidic chip is a potentially useful tool to investigate the effect of stem cells on cancer cells with intuitionistic cell-based screens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pluripotent stem cells, when displaying normal proliferation and differentiation, share numerous similarities with cancer cells (Postovit et al. 2007). This comparison led us to investigate the question: do human pluripotent stem cells, that follow this normal developmental process, have the potential to keep the growth of tumor cells under control? There is some evidence that embryonic stem (ES) cells may inhibit the growth of cancer cells or revert their malignance (Lightfoot et al. 2004; Postovit et al. 2006). Therefore, the interactions between cells, which consist of mixed signals, are important in determining numerous biological effects, such as proliferation, migration.
Co-culture is a common approach utilized to investigate the relationship between two kinds of cells. However, conventional co-culture assaying has a number of limitations. Direct co-culture is limited in separating two kinds of adherent cells objectively. The use of transwell is the most popular method for indirect co-culture, but using this approach it is not possible to undertake real-time observation of cell growth and this must be undertaken by a series of examinations. Moreover, the transwell plate approach is very expensive. Both in the direct and in the indirect co-culture approach, the change of microenvironment which is important in the communication between two cells varies uncontrollably. There exists a bidirectional effect between the two kinds of cells, which could inevitably influence the results (Sia and Whitesides 2003; Rosenthal et al. 2007).
Recently, perfused microfluidic environments have gained more interest in cell culture due to a number of advantages that this system offers including design flexibility and reduced use of reagents (Chung et al. 2005). Compared with traditional culture tools, microfluidic systems allow several experiment groups to be assembled on one small chip and offer the potential for monitoring of living cells online. Because of the transparence of poly-dimethylsiloxane (PDMS) microfluidic devices, we can directly and dynamically observe the development of living cells (Nie et al. 2007; Chung et al. 2005). Moreover, compared with transwells, PDMS microfluidic devices are very cheap. Thus PDMS microfluidic devices are increasingly applied in immunoassays, cell manipulation, cell separation and cell patterning systems and experimental approaches (Hatch et al. 2001).
The human embryonic germ cell (hEGC) is a pluripotent stem cell. It is derived from primordial germ cells and has similar characteristics to ES cells (Shamblott et al. 2001; Mueller et al. 2005). In the present study we investigated the effect of hEGCs on a human ovarian cancer cell line (SKOV3) on the chip device. A chip was designed to co-culture the two cells separately and permit the medium to flow from the hEG cell culture area to the SKOV3 cell culture area. Co-culture on 6-well plates was used as a parallel approach to compare the differences between the two cell culture methods.
Materials and methods
The isolation, culture and identification of hEG cells
HEG cells were derived from gonad ridges of human embryos at 5–9 weeks post-conception at the termination of pregnancy and were collected within the guidelines of the Chinese National Ethics Committee. The isolation and culture were undertaken using previously published procedures (Shamblott et al. 1998; Turnpenny et al. 2003). In brief, dissociated primordial germ cells (PGCs) were plated onto the feeder layer in DMEM/F-12 (Gibco) containing 20% (v/v) ES-cell-tested fetal bovine serum (HyClone), 1 mM l-glutamine (Gibco), 0.1 mM β-mercaptoethanol (Sigma), 0.1 mM nonessential amino acids (Gibco), 1 mM sodium pyruvate (Sigma), 10 μM forskolin (Upstate), 4 ng human recombinant basic fibroblast growth factor/ml (bFGF, Peprotech), 100 U penicillin/ml, and 100 μg streptomycin/ml. The cultures were maintained at 37°C, 5% CO2, 95% humidity. Alkaline phosphatase (AP) activity and antibodies for stage-specific embryonic antigens (SSEA-1, 3, 4), OCT-4, TRA-1-60, TRA-1-81 (CHEMICON) were used to detected for, and characterize, hEG cells. Secondary antibodies conjugated with fluorescein isothiocyanate (FITC; Beijing ZhongShan Golden Bridge Biotechnology Co. Ltd.) were used for detection purposes.
Plate co-culture of hEG cells with ovarian cancer cells
The human epithelial ovarian cancer cell line, SKOV3, was obtained from the Basic Medicine Research Institute, Qilu Hospital, Shandong University, P.R. China. Cells were cultured in RPMI-1640 (Gibco) with 10% (v/v) heat-inactivated newborn calf serum and incubated in 37°C, 5% CO2, 95% humidity. SKOV3 cells were plated onto the 6-well culture plates at 2 × 104 cells/well. After 12 h, EG clones (10 clones/well) were placed on half of each 6-well culture plate with EG culture medium as the experiment groups. The other wells were the control groups and only the EG culture medium was changed. After co-culturing for 48 h, in some plates, hEG clones in the experiment groups were taken out and correspondingly the cells of the control groups in the same area were also scraped. SKOV3 cells of the two groups in these plates were collected respectively for cell counting. The cells in residual plates were fixed in 4% polyformaldehyde for further examination.
Fabrication and modification of the microfluidic device
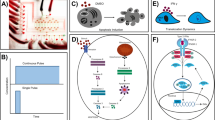
The microfluidic culture device was fabricated on a PDMS (Sylgard 184, Dow Corning, Midland, MI)/glass microchip using photolithographic patterning and wet chemical etching following previously published procedures (Ju et al. 2008). The glass formed the bottom of the channels and provided a surface for cellular adhesion (Fig. 1). The dimensions of the microfluidic channel system used in the testing were as follows: inlet and outlet reservoirs were 5 mm diameter, barrier channels were 40 μm wide, cell culture areas were 5 mm wide, 6 mm long and 100 μm deep.
Chip sterilization and SKOV3 cell seeding
Prior to cell seeding, microchips were sterilized by a microwave oven (power output 100%, 10 min). To avoid damage to the chips, the temperature inside the oven was kept below 70°C using an ice-box. SKOV3 cells were harvested, re-suspended in RPMI-1640 with 10% (v/v) heat-inactivated newborn calf serum (5 × 105 cells/ml). Firstly the culture medium was injected into the inlet reservoirs, and then SKOV3 cells were introduced into the chip through the outlet reservoir. The hydraulic pressure from the inlet reservoirs prevented the diffusion of cell suspension. The devices were placed in a humidified incubator at 37°C and 5% CO2 and the SKOV3 cells were allowed to attach in the static culture of the outlet reservoir. After the SKOV3 cells had attached, the medium in the inlet reservoir was aspirated off. From now on, the medium could only be changed from the outlet reservoir everyday and the waste medium in the inlet reservoirs was aspirated at the same time. When cells just grew out of the edge of outlet reservoir, they were subsequently used for co-culturing.
Co-culture of hEG cells with ovarian cancer cells on the chip
In the co-culture system, three hEG clones (around 104 hEG cells/clone) with hEG cells culture medium were seeded onto the inlet reservoirs after the full perfusion of the outlet reservoir. After the attachment of hEG clones, the medium in the outlet reservoir was aspired. From this time on, the medium was changed through the inlet reservoirs with hEG cells culture medium. The medium between the inlet reservoir and the outlet reservoir could achieve a balance after 12 h and was changed every 24 h. The control group was only changed with hEG cells culture medium through the inlet reservoir every day. The waste medium in the outlet reservoirs was aspirated everyday. After co-culturing for 48 h, the cells on the chip were fixed in 4% polyformaldehyde for further examination.
Immunocytochemistry of apoptosis assay
The apoptosis assay was undertaken on the fixed cells both in the plate and on the chip. The Tunnel assay (KeyGEN Inc.) was used to detect apoptosis according to the manufacturer’s instructions. Briefly, the fixed cells were incubated with blocking solution consisting of formaldehyde and 3% H2O2 at room temperature for 10 min, then permeabilized with 0.1% Triton X-100 for 2 min at 4°C. The cells were then incubated with Terminal deoxynucleotidyl Transferase (TdT) enzyme reacting solution and kept in the dark for 60 min at 37°C, sequentially followed by streptavidin-HRP for 30 min at 37°C. Staining was visualized using diaminobezidin (DAB) system (BeiJing ZhongShan Golden Bridge Biotechnology Co. Ltd.). Finally, counterstaining with haematoxylin was performed. For immunohistochemistry on the chip, all reagents were added through the inlet reservoirs.
Statistical analysis
All data are presented as the mean ± SD in the text and figures. Differences on the proliferation of SKOV3 cells were evaluated statistically using a two-sided t-test. A P-value of 0.05 was adopted for determining statistically significant differences.
Results and discussion
Derivation and characterization of hEG cells
We successfully derived human EG cells from the primary gonad ridges of abortion fetus about 5–7 weeks of age. The cell clones grew onto the feeder layer like a bird’s nest with a distinct border (Fig. 2a). The hEG clones showed high levels of AP activity (Fig. 2b) and were characterized by a range of cell surface markers including: SSEA-1, SSEA-3, SSEA-4, OCT-4, TRA-1-60, TRA-1-81 (Fig. 2d–i). When the medium was changed with DMEM/F-12 and 10% (v/v) heat-inactivated fetal calf serum, hEG cells could spontaneous differentiate into nerve-sphere liked clumps (Fig. 2c). These observations are consistent with the published characteristics of EG cells (Shamblott et al. 1998; Turnpenny et al. 2003). We therefore investigated hEG cells further and the effect these have on SKOV3 cells.
(a) HEG clones grew on the feeder. Human EG cells are positive for the enzyme alkaline phosphatase (b), the stage-specific embryonic antigens SSEA-1 (d), SSEA-3 (e) and SSEA-4 (f), the tumor rejection antigens TRA-1-60 (g) and TRA-1-81 (h), as well as OCT-4 (I). (c) HEG cells can spontaneously differentiate to nerve-sphere liked clumps. Scale bar = 200 μm
HEG cells inhibited proliferation and induce apoptosis of SKOV3 cells
Plate co-culture showed that the growth of SKOV3 cells was inhibited by hEG clones. Further details are shown in Fig. 3a. Through use of the tunnel assay, more positive staining signals in the experiment group were observed than in the control group, especially near the EG clones (Fig. 3b).
(a) Cell counting of the two groups in the plate co-culture. It showed there was an average of (7.8 ± 2.4) × 104 SKOV3 cells in the experiment group versus (11.5 ± 2.3) × 104 SKOV3 in the control group. The experiment was performed three times (*P = 0.0258). (b) Staining of tunnel apoptosis assay (brown) of the two groups in the plate co-culture. Scale bar = 200 μm
Upon chip co-culture, the growth inhibition and apoptosis in the experiment group was also detected. Figure 4a showed the proliferation of SKOV3 cells in the chamber was inhibited by hEG cells compared with the control groups. The experiment was performed six times. The distances of cell growth are showed in Fig. 4c. More positive tunnel staining signals were observed in the experiment group than in the control group (Fig. 4d). It is apparent that the positive signals gradually decreased along the perfused medium flow (Fig. 4b).
(a) The online observation of SKOV3 cells development on the chip. (b) The gradual decrease of the positive tunnel signals along the medium flowing on the chip. The arrow indicated the flowing of medium. (c) The growth distances of SKOV3 cells. The distance (μm) was measured from the out edge of cell growth to the out edge of outlet reservior. (d) Staining of tunnel apoptosis assay (brown) of the two groups on the chip co-culture. Scale bar = 200 μm
Comparison between the two culture methods
In the plate culture, hEG clones in the experiment groups were taken out after 2 days. Meantime, the cells of the control groups in the same area were also correspondingly scraped. SKOV3 cells of the two groups in these plates were collected, respectively, for cell counting. Thus a certain amount of variation and error is inevitable. The PDMS based microfluidic devices can be designed optionally, so we designed two culture areas to box off two cells and some channels between the areas to promote the flowing of medium. Further, because the PDMS based microfluidic devices are transparent, the growth of SKOV3 cells could be observed with an inverted microscope and the two groups could be compared directly. Thus, the chip incorporating a microfluidic device provides us with design flexibility and monitoring online.
Our results show that hEG cells induced the apoptosis of SKOV3 cells. In the plate culture, more tunnel positive signals of SKOV3 cells in the co-culture group were observed in the area around the EG clones whilst others were distributed and scatted throughout the plate. However, on the chip culture, the gradual change of tunnel positive signals could be seen. As the media containing factors secreted by hEG cells flowed from the hEG culture area to the SKOV3 culture area, the tunnel positive signals of SKOV3 cells dropped off. The results showed that the perfused microfluidic system could control the change of culture microenvironment better.
The microfluidic flow equalization system assures uniform perfusion of the cell culture media throughout the cell culture chamber. Under the influence of gravity, the medium can flow only from one side to the other without returning. Thus we can control the change of the microenvironment and investigate how the secretion of one cells effects the biological activity of the lower cells. The barrier channels limited the velocity of medium flowing. This microfluidic system offers a slow, continuous and single-line flow of reagents, which is a better platform to investigate the unilaterally effect of one cell to the other cell.
In conclusion, our research has demonstrated that hEG cells can induce the apoptosis of SKOV3 cells, and consequently inhibit the growth of SKOV3 cells in vivo. The microfluidic implementation enabled culture in flowing perfusion and provided us with the ability to undertake the online inspection of the proliferation of cells. Therefore, the microfluidic device serves as a powerful tool, enabling us to create novel cell-pattern technology in the stem cell research field.
References
Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL (2005) Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 5:401–406
Hatch A, Kamholz AE, Hawkins KR, Munson MS, Schilling EA, Weigl BH, Yager P (2001) A rapid diffusion immunoassay in a T-sensor. Nat Biotechnol 19:461–465
Ju XL, Li D, Gao N, Shi Q, Hou HS (2008) Hepatogenic differentiation of mesenchymal stem cells using microfluidic chips. Biotechnol J doi:10.1002/biot.200707152
Lightfoot HM, Cairns BA, Lapaglia M B, Pleasant BA, Meyer AA, Fair J (2004) Murine Embryonic Stem (ES) cells inhibit human pancreatic carcinoma cell growth in vitro. J Surg Res 121:342
Mueller D, Shamblott MJ, Fox HE, Gearhart JD, Martin LJ (2005) Transplanted human embryonic germ cell-derived neural stem cells replace neurons and oligodendrocytes in the forebrain of neonatal mice with excitotoxic brain damage. J Neurosci Res 82:592–608
Nie FQ, Yamada M, Kobayashi J, Yamato M, Kikuchi A, Okano T (2007) On-chip cell migration assay using microfluidic channels. Biomaterials 27:4017–4022
Postovit LM, Seftor EA, Seftor RE Hendrix MJ (2006) A three-dimensional model to study the epigenetic effects induced by the microenvironment of human embryonic stem cells. Stem Cells 24:501–505
Postovit LM, Costa FF, Bischof JM, Seftor EA, Wen B, Seftor RE, Feinberg AP, Soares MB, Hendrix MJ (2007) The commonality of plasticity underlying multipotent tumor cells and embryonic stem cells. J Cell Biochem 101:908–917
Rosenthal A, Macdonald A, Voldman J (2007) Cell patterning chip for controlling the stem cell microenvironment. Biomaterials 21:3208–3216
Shamblott MJ, Axelman J, Wang S Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD (1998) Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA 95:13726–13731
Shamblott MJ, Axelman J, Littlefield JW, Blumenthal PD, Huggins GR, Cui Y, Cheng L, Gearhart JD (2001) Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc Natl Acad Sci USA 98:113–118
Sia SK, Whitesides GM (2003) Microfluidic devices based on poly (dimethylsiloxane) for biological studies. Electrophoresis 24:3563–3576
Turnpenny L, Brickwood S, Spalluto CM, Piper K, Cameron IT, Wilson DI, Hanley NA (2003) Derivation of human embryonic germ cells: an alternative source of pluripotent stem cells. Stem Cells 21:598–609
Acknowledgment
This work was supported by grants from National Natural Science Foundation of China (No. 30571953 & 30700163 & 30700897).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, X., Kong, B. & Li, D. A new tool for probing of cell–cell communication: human embryonic germ cells inducing apoptosis of SKOV3 ovarian cancer cells on a microfluidic chip. Biotechnol Lett 30, 1537–1543 (2008). https://doi.org/10.1007/s10529-008-9725-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9725-2