Abstract
Plant sterols lower serum cholesterol concentration. Available data have confirmed the lipid-lowering efficacy in adults, while there is a relative dearth of data in children and almost exclusively restricted to subjects with familial hypercholesterolemia (FH). Aim of the present study was to evaluate the efficacy, tolerability and safety of plant sterol supplementation in children with different forms of primary hyperlipidemias. The effect of plant sterol consumption on plasma lipids was evaluated in 32 children with heterozygous FH, 13 children with Familial Combined Hyperlipidemia (FCH) and 13 children with Undefined Hypercholesterolemia (UH) in a 12-week open-label intervention study using plant sterol–enriched yoghurt. Plasma lipids and apolipoproteins were measured by routine methods. Markers of cholesterol synthesis (lathosterol) and absorption (campesterol and sitosterol) were measured by GC–MS. Tolerability and adherence to recommended regimen was very high. A significant reduction was observed in LDL-cholesterol in the three groups (10.7, 14.2 and 16.0% in FH, FCH and UH, respectively). Lathosterol concentrations were unchanged, reflecting a lack of increased synthesis of cholesterol. Of the two absorption markers, only sitosterol showed a slight but significant increase. Daily consumption of plant sterol dairy products favorably changes lipid profile by reducing LDL-cholesterol. To our knowledge, this is the first report of the use of plant sterols–enriched foods in treating children with primary hyperlipidemia such as FCH and UH, likely to be the most frequent form also in the young age in the western populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impact of coronary heart disease risk factors on mortality is evident in all ages and is becoming especially strong in young persons [1]. Familial hyperlipidemia represents a major cardiovascular (CV) risk factor often occurring since pediatric age, when early atherosclerotic changes can already be demonstrated [2]. The relationship between hyperlipidemia and vascular events is unquestioned [3], and it is now clear that dietary and eventually drug treatment needs to be started as soon as possible [4]. Lowering of low-density lipoprotein (LDL) cholesterol below 160, 130 and 100 mg/dl, in low, medium and high risk subjects, respectively, is the target of the therapy in adults. In patients with diabetes, elevated triglyceride levels and low HDL-cholesterol levels become targets of therapy as well [5]. In children and adolescents, total plasma and LDL-cholesterol concentration are strongly influenced by age, gender, ethnicity and pubertal status. Acceptable levels are usually set below the 75th percentile, corresponding to <170 and <110 mg/dl for total and LDL-cholesterol, respectively [6]. Habitual dietary advice yields a 10–15% cholesterol reduction, not sufficient to reach the above reported goals in a large proportion of patients. However, there is still some reluctance to start a lipid lowering drug therapy in pediatric age. As a consequence, many young hyperlipidemic patients who would need a substantial reduction of plasma cholesterol actually do not receive the appropriate treatment.
Phytosterols are plant components presenting a chemical structure similar to that of cholesterol [7–9]. They include mainly sitosterol and campesterol which are introduced with diet vegetable oils in amount corresponding to that of cholesterol (200–400 mg/die). Phytosterols compete with cholesterol for intestinal absorption and display cholesterol from micelles. Both cholesterol and phytosterols require the Niemann-Pick C1 Like 1 Protein (NPC1L1) to obtain entry in enterocytes. Non-esterified cholesterol and phytosterols are pumped back in the intestine lumen through the ABCG5 complex. Eventually, about 50% of cholesterol, but less than 5% of plant sterols, is absorbed [10–12].
It has been demonstrated that phytosterol supplementation is able to reduce LDL-C levels in adults with familial hypercholesterolemia (FH) by 10%, and the consumption of 2 g/day of phytosterols is part of the recommendation of NCEP [13]. Trials performed in FH children have confirmed their usefulness [14].
The clinical experience with phytosterols in pediatric subjects is virtually restricted to FH patients, a genetic disorder characterized by elevated cholesterol from birth. A number of children with hypercholesterolemia, however, have different disorders, like familial combined hyperlipidemia (FCH) or polygenic hypercholesterolemia (PH). Particularly FCH is a condition with increased risk of CV outcome in which LDL-C levels monitoring is mandatory.
Aim of the present study was to evaluate the efficacy, tolerability and safety of plant sterol supplementation in children with different forms of primary hyperlipidemias.
Methods
Patients
Fifty-eight outpatients affected by primary hyperlipidemia including 32 heterozygous FH (M/F,15/17), 13 FCH (M/F,6/7) and 13 children (M/F,6/7) with Undefined Hypercholesterolemia (UH) were recruited. The family tree was examined for two generations to clearly detect the disorder heritage. The diagnosis of FH was made according to the following criteria: LDL-cholesterol levels n >95th age- and sex-specific percentile [males and females (5–9 years) exceeding 129 and 140 mg/dl, respectively; males and females (10–14 years) exceeding 132 and 136 mg/dl, respectively]; dominant inherited hypercholesterolemia; family history of precocious cardiovascular events in parent or grandparent, tendon xantomata in parent or grandparent [15].
FCH was diagnosed in subjects with total cholesterol (TC) and/or triglyceride (TG) serum levels greater than 90th percentile of the reference population, with hypercholesterolemia and/or hypertriglyceridemia in at least one first-degree relative and intrafamilial variability [16]. Children were diagnosed as UH when showing LDL-C exceeding 90th percentile, with or without family history of dyslipidemia, and did not fulfill criteria for inclusion in FH or FCH group. Secondary forms of dyslipidemia were excluded, i.e. renal disease, liver disease, endocrinopathies, overweight and obesity, diabetes, immune-hematological disorders as well patients submitted to drug therapy such as anticonvulsants known to affect lipid metabolism.
Children in the age range 8–16 years were enrolled. They had to be on stable recommended Step 1 diet since at least 6 months and none of them was smoker or on a lipid lowering treatment in at least the past 3 months.
Study design
Enrolled children underwent a full clinical and biochemical examination (visit 0). Clinical and biochemical examination were repeated after a 12-week treatment with a yoghurt supplemented with phytosterols (visit 1). Yoghurt (100 ml) was monthly provided to children. The sterol content was 1.6 or 2.0 g per day, the higher content delivered to patients weighing n >40 kg.
To assess the adherence to diet, children were asked to provide a weekly diary at visit 0 and at visit 1 from which the nutrient intake was calculated. Tolerability was assessed by asking the patients and/or their guardians to daily record the assumption of the yoghurt, to record any intolerance and/or possible side effect.
Efficacy was evaluated through the modifications in blood lipids and apoproteins. Patients were arbitrarily classified in two groups (A and B) on the basis of LDL-C decrease at Visit 1 >5 or ≤5%, respectively. Safety was evaluated mainly through the variation in campesterol and sitosterol levels standardized for cholesterol concentration.
Ethics committee approved the trial and written informed consent was obtained from the legal guardians of the children and from the proband child. The study was conducted according to the declaration of Helsinki.
Analytical procedures
Blood samples for lipoprotein analysis were obtained after an overnight fast. Plasma cholesterol and triglycerides were measured by routine methods on a MODULAR ANALYTICS Serum Work Area (Roche Diagnostics GmbH, Germany). All other plasma sterols were measured by gas chromatography coupled to mass spectrometry (GC–MS) with multiple selected ion monitoring (SIM) according to a method recently optimized in our laboratory [17]. Briefly, lipids were extracted from 200 μl of plasma from peripheral blood samples, dried under a stream of N2 and derivatized with 200 μl of pyridine-Bis(Trimethylsilyl)-Trifluoroacetamide with 1% trimethylchlorosilane. Samples were then incubated at 70°C for 60 min and analyzed by GC–MS on an AutoSystem XL (Perkin Elmer Instruments, Norwalk, CT, USA) gas chromatograph (injector temperature 270°C; transfer line temperature 230°C). Separation was performed on a Rtx®-1701 (RESTEK, Bellefonte, PA, USA) 60-m capillary column (0.25 mm ID, 0.25-μm film thickness; 14% cyanopropylphenyl- 86% dimethylpolysiloxane), using helium as the carrier gas under the following conditions: 3 min 90°C, from 90 to 260°C at 25°C/min, 28 min 260°C, from 260 to 275°C at 1°C/min, 13 min 275°C (total run time 65.80 min). Clarus 500 (Perkin Elmer Instruments, Norwalk, CT, USA) mass spectrometer operated in the electron impact mode with an ion source temperature of 200°C. SIM was carried out by monitoring m/z 443 and 458 for lathosterol (synthesis marker), m/z 382 and 472 for campesterol (absorption marker), m/z 129 and 486 for beta-sitosterol (absorption marker), m/z 217 and 357 for 5alpha-cholestane. Peak identification was based on comparison with standards of retention times and mass spectra fragmentation. Quantitative determination was performed using 5alpha-cholestane as an internal standard. Results were standardized to cholesterol levels. Ratios are defined as the concentration of the particular sterol (in mmol × 100) to the concentration of cholesterol in mol. Plasma apolipoprotein B (apoB) and apolipoprotein AI (apoA) concentrations were measured by immunoturbidimetry (Olympus AU 2700, Japan). LDL-C was estimated using the Friedewald formula, when appropriate.
Statistical analysis
The ANOVA one-way was employed to compare baseline lipid variables between different groups. The average change in lipid variables in the 3 patient groups was assessed with a Student’s paired t test. The Wilcoxon rank test was used to evaluate the changes within-group of phytosterols measurements. Spearman’s correlation coefficient was applied to assess correlations between skewed variables.
Statistical analysis was performed using the SPSS 12.0 software (SPSS Inc, Chicago, IL). A P value <0.05 was considered significant.
Results
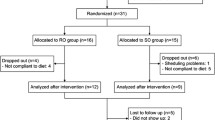
Fifty-eight dyslipidemic children were enrolled in the study. Six of them discontinued the trial before Visit 1: two had difficulties in drinking the yoghurt; two had poor adherence to the diet program; one was unable to attend visits and only one reported recurrent abdominal discomfort. No other patient presented symptoms or other intolerance signs. Among the 52/58 (89%) patients who completed the trial, the compliance was very high, with 98% of yoghurt preparation regularly assumed. At the end of the study treatment, 71% (37/52) of subjects (Group A) and 29% (15/52) (Group B) experienced a LDL-cholesterol reduction greater or lower than 5%, respectively.
The three groups of children with different forms of primary hyperlipidemia were comparable in terms of age, gender and BMI. Children with FH had significantly higher levels of TC, LDL-cholesterol and apoB, compared with FCH and UH. Children with FCH had higher levels of triglycerides compared to the other two groups. The ratios of non-cholesterol sterols to cholesterol were not significantly different among the three groups (Table 1). As demonstrated in Table 2, no dietary variations occurred throughout the study.
Phytosterol supplementation caused a significant decrease in total, LDL-cholesterol and apoB. The LDL-cholesterol reduction observed in FCH and UH patients was on average about 15%, resulting higher than in FH children who showed a decrease around 10% (Table 3).
Triglycerides, HDL-cholesterol and apoAI were unmodified. Basal lipid and apoB levels did not seem to predict the variation following phytosterol supplementation (data not shown).
Taking in account Group A and Group B, we did not observe any significant difference between lipid profiles and sterol non-cholesterol levels (Table 4).
The ratios of lathosterol, campesterol and sitosterol were not significantly different between the three groups of patients and were not associated with therapeutic efficacy when considering the patients as a whole or divided according to hyperlipidemia.
As expected, synthesis marker (lathosterol ratio) was directly correlated, and absorption markers (campesterol and sistosterol ratios) were inversely correlated to total and LDL-cholesterol (data not shown) in the whole group, although there was a non-significant tendency toward higher levels of synthesis markers and lower levels of absorption markers in subjects with FCH when compared with subjects with FH or other forms of hypercholesterolemia. At baseline, campesterol ratio was also associated with HDL-cholesterol (R = 0.420, P = 0.005) in the whole group. This result was explained for by the association in FCH and UH patients, while it was absent in FH patients.
Ratio of lathosterol and campesterol to cholesterol was substantially unchanged in the three groups following supplementation, while sitosterol ratio consistently increased in the three groups (Table 3), reaching statistical significance in the population considered as a whole (P < 0.05).
Discussion
In the present study, we have evaluated the short-term efficacy of plant sterols in the treatment of children with different forms of primary hyperlipidemias. These disorders represent a major cardiovascular risk factor, and the outcome of patients is improved by decreasing LDL-C levels. We focused our attention on primary dyslipidemias in children. Overweight and obesity are conditions possibly related to dyslipidemias but since our aim was to exclude any possible misunderstanding and misdiagnosis in children we restricted our analysis to children showing only lipid changes and typical familial segregation.
A small number of studies have been so far produced in pediatric age, mainly in FH children [14, 15]. This is a particular condition, characterized by a defect in the clearance of LDL particles and with evidence of dominant autosomic inheritance. Other forms of hypercholesterolemia are probably more frequent and show more complex traits, with multiple genes and environmental factors involved in the pathogenesis. FCH is phenotipically characterized by an increase in serum cholesterol and/or triglycerides with variability of the lipid profile within the affected individuals and their first degree relatives. In the present study, children not fulfilling criteria for inclusion in FH or FCH were classified as with undefined hypercholesterolemia (UH). It is possible that some of them might be considered affected by other forms of Familial Autosomal Dominant Hypercholesterolemia or by polygenic hypercholesterolemia. However, no sterol- or stanol-controlled study has been so far performed in these patients. Therefore, this is the first study, to our knowledge, to report on the effect of phytosterol supplementation in children with different forms of hyperlipidemias.
The use of phytosterols as lipid lowering agents has been well documented in the past fifty years both in animal models and in humans [18]. More recently, new formulations with increased lipid solubility have allowed their incorporation in various foods, like bread, cereals, salad dressing or yoghurt [19]. The latter is widely known and commercialized with different flavors and good palatability. These factors are likely to increase acceptability and favor compliance. As a matter of fact in the present investigation, in the 52 pediatric patients who completed the 12-week study, we were able to register an adherence to recommended intake of some 98%.
In the published trials in which stanols or plant sterols have been used to lower serum cholesterol, a dose–response relation up to 2 g/day has usually been reported, with LDL-cholesterol reduction ranging from 8 to 15% [20]. Trials performed in subjects of pediatric age with primary hyperlipidemia are scanty and restricted almost exclusively to FH patients. While our results regarding FH subjects of pediatric age are comparable and in line with other similar studies conducted in children [14, 15, 21, 22], our finding in subjects with FCH and UH are probably unique and therefore not comparable with previous reports. The results observed in the present study demonstrate a greater reduction in FCH and UH children than in FH. The follow-up of the dietary regimen provides the evidence that no variation occurred across the three groups with respect to baseline; therefore, the hypolipidemic effect was likely to be attributed uniquely to phytosterol supplementation. Furthermore, the observation that a 29% of children showed a low response (LDL-C reduction <5%) provides a further explanation of the complex cholesterol homeostasis and differences between inherited lipid disorders.
FH, FCH and UH have different genetic background and recognize different pathophysiologic mechanisms. However, in our study, markers of cholesterol synthesis and absorption in these different forms of pediatric hyperlipidemia show similar pictures. These results confirm previous data by Ketomaki et al. [23] who have investigated hypercholesterolemic children with or without FH, while they are at variance with those published by Garcia-Otin et al. [24]. In this paper, the authors report significantly higher levels of synthesis markers and lower levels of absorption markers in subjects with FCH when compared with subjects with non-FH Autosomal Dominant Hypercholesterolemia, who showed an opposite profile. Although we could observe a similar tendency in our subjects with FCH, the difference was not statistically significant. The likely explanation for these different findings might lie in the fact that Garcia et al. have looked at adults and not at subjects of pediatric age.
An unexpected finding of the present study was the positive association between the absorption marker campesterol ratio and HDL-cholesterol in children with FCH and UH, but not FH. One explanation is that absorbed sterols are probably transported back to the liver through the HDL-mediated cholesterol transport, sharing the common pathways including transfer proteins. This is in agreement with Ketomaki et al. [23] who have investigated the ratios of the non-cholesterol sterols to cholesterol among different lipoproteins and found high HDL ratios of the absorption marker sterols. The lack of this association in FH subjects may be due to the described higher cholesterol ester transfer protein activity and thus enhanced sterol transfer from HDL in this form of hyperlipidemia [25].
Since 2001, NCEP Guidelines have included plant sterol–enriched food as part of a dietary strategy aimed at reducing LDL-cholesterol levels [3]. Since then also other societies in Europe and other countries of the world have agreed in proposing phytosterols as a dietary option. However, some controversies have recently emerged on the role of non-cholesterol sterol levels as risk factor for cardiovascular disease [26]. The role of beta-sitosterol as a causative agent of premature atherosclerosis in the autosomal recessive familial form of sitosterolemia has been described in detail [27]. On the other hand, patients homozygous for beta-sitosterolemia have plant sterol levels ten to fifty times more elevated than normal subjects and presence of sitosterol in the atherosclerotic plaques is certainly due to the excessive level of plant sterols in this genetic disorder. Furthermore, few observational studies have shown evidence that even slightly elevated levels of plant sterols might be associated with an increased cardiovascular risk [28]. In the large epidemiological cohort of the PROCAM Study, Assmann et al. showed that men in the highest tertile of plasma sitosterol had a threefold increase of risk for coronary event [29]. In the prospective Study MONICA/KORA campesterol correlated directly with incidence of acute myocardial infarction [30]. On the other hand, some other reports seem to contradict these findings (Dallas Heart Study, LASA) [31, 32]. The latter one does even attribute a protective role to moderately elevated sterol levels. More recently, it has been suggested that phytosterols might act as risk factors in carriers of common variants in ABCG8 and ABO loci [33]. With regard to our study, overall there was a moderate but significant increase in the ratio of sitosterol to cholesterol, while the campesterol ratio was unaltered. Whether this moderate increase in sitosterol ratio is relevant to cardiovascular risk remains a matter of debate. In our opinion, long-term trials with plant sterol–enriched foods are mandatory for solving the question. The ratio of lathosterol, which is a marker of cholesterol synthesis, was unmodified reflecting that inhibition of cholesterol absorption with phytosterols did not cause an increase in cholesterol synthesis in a 12-week period.
One limitation of the study was the open-label nature of the intervention without a control group assuming the dairy product devoid of phytosterols. The limited number of pediatric subjects affected by primary hyperlipidemia and possible ethical issues might have created problems.
In conclusion, medical handling of pediatric primary hyperlipidemias is particularly difficult, especially because of a reluctance in prescribing a statin at prepubertal age particularly in patients who are not affected by FH. Our study, together with previous reports, demonstrates the efficacy and excellent tolerability of plant sterol–enriched food in a 12-week period. To our knowledge, this is the first report of the use of plant sterols–enriched foods in treating children with primary hyperlipidemia other than FH, such as FCH and UH, likely to be the most frequent form also in the young age in the Western populations.
References
Pontiroli AE (2004) Type 2 diabetes mellitus is becoming the most common type of diabetes in school children. Acta Diabetol 41:85–90
Berenson GS, Srinivasan SR, Bao W, Newman WP III, Tracy RE, Wattigney WA, For the Bogalusa heart study (1998) Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med 338:1650–1656
Third report of the national cholesterol education program (NCEP) (2002) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Final report. Circulation 106:3143–3421
American academy of pediatrics (1998) Committee on nutrition. Cholesterol in childhood. Pediatrics 101:141–147
Taskinen MR (2002) Controlling lipid levels in diabetes. Acta Diabetol 39(2):S29–S34
Daniels SR, Greer FR, The Committee on Nutrition (2008) Lipid screening and cardiovascular health in childhood. Pediatrics 122:198–208
Grundy SM, Ahrens EH Jr, Davignon J (1969) The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res 10:304–315
Heinemann T, Kullak-Ublick GA, Pietruck B, von Bergmann K (1991) Mechanisms of action of plant sterols on inhibition of cholesterol absorption: comparison of sitosterol and sitostanol. Eur J Clin Pharmacol 40(1):S59–S63
Nissinen M, Gylling H, Vuoristo M, Miettinen TA (2002) Micellar distribution of cholesterol and phytosterols after duodenal plant stanol ester infusion. Am J Physiol Gastrointest Liver Physiol 282:G1009–G1015
Tammi A, Rönnemaa T, Gylling H, Rask-Nissilä L, Viikari J, Tuominen J et al (2000) Plant stanol ester margarine lowers serum total and low-density lipoprotein cholesterol concentrations of healthy children: the STRIP project. J Pediatr 136:503–510
Berge KE, von Bergmann K, Lutjohann D, Guerra R, Grundy SM, Hobbs HH et al (2002) Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res 43:486–494
Gylling H, Miettinen TA (2002) Inheritance of cholesterol metabolism of probands with high or low cholesterol absorption. J Lipid Res 43:1472–1476
Stone NJ, Van Horn L (2002) Therapeutic lifestyle change and Adult Treatment Panel III: evidence then and now. Curr Atheroscler Rep 4:433–443
Ketomäki AM, Gylling H, Antikainen M, Siimes MA, Miettinen TA (2003) Red cell and plasma plant stanol and sterol ester spreads in children with hypercholesterolemia. J Pediatr 142:524–531
Nicholls DP, Cather M, Byrne C, Graham CA, Young IS (2008) Diagnosis of heterozygous familial hypercholesterolemia in children. Int J Clin Pract 62:990–994
Veerkamp MJ, de Graaf J, Bredie SJH, Hendriks JCM, Demacker PNM, Stalenhoef AFH (2002) Diagnosis of combined familial hyperlipidemia based on lipid phenotype expression in 32 families. Results of a 5-year follow-up study. Arterioscler Thromb Vasc Biol 22:274–282
Ahmida HSM, Bertucci P, Franzò L, Massoud R, Cortese C, Lala A et al (2006) Simultaneous determination of plasmatic phytosterols and cholesterol precursors using gas cromatography-mass spectrometry (GS-MS) with selective ion monitoring (SIM). J Chromatogr B Analyt Technol Biomed Life Sci 842:43–47
Peterson DW (1951) Effect of soybean sterols in the diet on plasma and liver cholesterol in chicks. Proc Soc Exp Biol Med 78:143–147
Thompson G, Grundy MS (2005) History and development of plant sterols and stanol esters for cholesterol lowering purposes. Am J Cardiol 96(1):3D–9D
Brynes AE, Thomson GR (2003) Functional foods in lipid-lowering and coronary prevention. In: Gaw A, Shepherd J (eds) Lipids and Atherosclerosis Annual. Martin Dunitz, London, pp 119–138
Hedman M, Miettinen TA, Gylling H, Ketomaki A, Antikainen M (2006) Serum noncholesterol sterols in children with heterozygous familial hypercholesterolemia undergoing pravastatin therapy. J Pediatr 148:241–246
Jakulj L, Vissers MN, Rodenburg J, Wiegman A, Trip MD, Kastelein JJP (2006) Plant stanols do not restore endothelial function in prepubertal children with familial hypercholesterolemia despite reduction of low-density lipoprotein cholesterol levels. J Pediatr 148:495–500
Ketomaki A, Gylling H, Siimes NA, Vuorio A, Miettinen TA (2003) Squalene and non cholesterol sterols in serum and lipoproteins of children with and without familial hypercholesterolemia. Pediatr Res 53:648–653
García-Otín AL, Cofán M, Junyent M, Recalde D, Cenarro A, Pocoví M, Ros E, Civeira F (2007) Increased intestinal cholesterol absorption in autosomal dominant hypercholesterolemia and no mutations in the low-density lipoprotein receptor or apolipoprotein B genes. J Clin Endocrinol Metab 92(9):3667–3673
Inazu A, Koizumi J, Mabuchi H, Kajinami K, Takeda R (1992) Enhanced cholesteryl ester transfer protein activities and abnormalities of high density lipoproteins in familial hypercholesterolemia. Horm Metab Res 24:284–288
Weingartner O, Bohm M, Laufs U (2009) Controversial role of plant sterol ester in the management of hypercholesterolemia. Eur Heart J 30:404–409
Mannucci L, Guardamagna O, Bertucci P, Pisciotta L, Liberatoscioli L, Bertolini S, Irace C, Gnasso A, Federici G, Cortese C (2007) Beta-sitosterolaemia: a new nonsense mutation in the ABCG5 gene. Eur J Clin Invest 37(12):997–1000
Glueck CJ, Speirs J, Tracy T, Streicher P, Illig E, Vandegrift J (1991) Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol, and premature coronary heart disease in hyperphytosterolemic probands and their first-degree relatives. Metabolism 40:842–848
Assmann G, Cullen P, Erbey J, Ramey DR, Kannenberg F, Schulte H (2006) Plasma sistosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the prospective cardiovascular munster (PROCAM) study. Nutr Metab Cardiovasc Dis 16:13–21
Thiery J, Ceglarek U, Fiedler GM, Leichtle A, Baumann S, Teupser D, Lang O, Baumert J, Meisinger M, Loewell H, Doering A (2006) Elevated campesterol serum levels—a significant predictor of incident myocardial infarction: results of the population-based MONICA/KORA follow-up study 1994–2005. Circulation 114:II_884
Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH (2004) No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler Thromb Vasc Biol 24(12):2326–2332
Fassbender K, Lütjohann D, Dik MG, Bremmer M, König J, Walter S, Liu Y, Letièmbre M, von Bergmann K, Jonker C (2008) Moderately elevated plant sterol levels are associated with reduced cardiovascular risk–the LASA study. Atherosclerosis 196(1):283–288
Saleheen D, Soranzo N, Rasheed A et al (2010) Genetic determinants of major blood lipids in Pakistanis compared with Europeans. Circ Cardiovasc Genet 3:348–357
Acknowledgments
This investigation was partly supported by COFIN_MIUR 2008 and by Turin University funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guardamagna, O., Abello, F., Baracco, V. et al. Primary hyperlipidemias in children: effect of plant sterol supplementation on plasma lipids and markers of cholesterol synthesis and absorption. Acta Diabetol 48, 127–133 (2011). https://doi.org/10.1007/s00592-010-0233-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-010-0233-1