Abstract
Irruptive forest insects have a rich history of empirical study and as subjects of modeling in both theoretical and applied ecology, yet compared with other disturbance agents landscape-scale insect disturbance modeling is rare. We examine the history of spruce budworm (Choristoneura fumiferana) disturbance modeling to provide insight into landscape-scale insect disturbance modeling more generally. First, we outline the evolution of competing approaches to budworm population modeling, illustrating the interplay of models and data, and highlighting the roles of reciprocal feedbacks among trophic levels (i.e., budworm, its forest host and its natural enemies) and broader-scale processes (i.e., dispersal, synchronization, climatic variation and change). We then overview studies relating budworm defoliation to its effects on forests, culminating in spruce budworm decision support tools designed for forest operations planning. Modeling applications using landscape disturbance and succession models are a more recent addition, focused on long-term responses of forested landscapes to a given budworm disturbance regime. Cross-scale interactions—recognized within the budworm–forest system for over three decades—demand sophisticated analyses and modeling that will ultimately lead to a more robust synthesis of budworm response to forest conditions, particularly under different climatic contexts. We describe the budworm case study to illustrate how insights from divergent perspectives can be complementary and ultimately lead to more complete understanding of the system. We propose that the most fruitful modern avenue of research in forest–insect–climate interactions is in testing inclusive hypotheses that allow for multiple processes acting simultaneously using integrative, multiscale landscape models that embrace the possible existence of a range of dynamic behaviors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Introduction
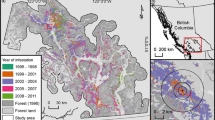
Insects are important disturbance agents affecting temperate and boreal biomes (Wermelinger 2004; Johnson et al. 2005; Cooke et al. 2007; Raffa et al. 2008). Defoliating insects in particular have historically affected a staggering area of North American forests, particularly across the boreal biome (Fig. 5.1). Principal among these boreal forest defoliators is the spruce budworm (Choristoneura fumiferana Clemens) that in the 1970s affected over 50 million hectares of fir (Abies spp.) and spruce (Picea spp.) forests at its peak in Eastern Canada and the Northeastern United States, making it among the most economically and ecologically important forest insects on the continent. Its significance is reflected in an extensive history of research to support modeling and management activities (e.g., Morris 1963a; Greenbank et al. 1980; Royama 1984; Sanders et al. 1985). The most recent review of spruce budworm modeling was by Régnière and Lysyk (1995, but see also Cooke et al. 2007). Since 1995 (when spruce budworm became primarily endemic), 1103 papers were published with the keyword C. fumiferana (Web of Science, accessed December 2014), indicating a strong need for new synthesis.
Comparative disturbance statistics for major insect species in the contiguous 48 states of the United States (inset) versus the Canadian provinces (Canadian Council of Forest Ministers 2013; http://nfdp.ccfm.org National forestry database program. Canadian Forest Service, Ottawa, Ontario, Canada)
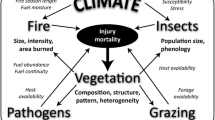
Modeling insect outbreak dynamics requires understanding of the insect’s population dynamics , phenology, host preferences (i.e., species, size), feeding dynamics, and factors affecting outbreak severity in time and space. Spruce budworm defoliates balsam fir (A. balsamea) and spruce species, emerging from winter hibernacula as tiny second instars that bore into emerging buds and then feed on the new foliage as shoots expand. Its population cycles are longer than most other defoliating species, both in terms of time between outbreaks and their duration (Cooke et al. 2007; Myers and Cory 2013). Mortality generally begins after 5 to 6 consecutive years of heavy defoliation (MacLean 1980) in balsam fir, followed by white spruce (P. glauca) and then red spruce (P. rubens) and black spruce (P. mariana) (Erdle and MacLean 1999). Adult budworm moths are strong fliers that actively use wind currents to facilitate long-distance dispersal (Greenbank et al. 1980; Anderson and Sturtevant 2011; Sturtevant et al. 2013).
The most commonly reported outbreak interval is on the order of 30–40 years (e.g. Jardon et al. 2003), and the species is best known for regionally synchronized outbreaks (Royama 1984; Peltonen et al. 2002) that cause widespread forest decline over broad areas (MacLean 1984). However, a wide range of outbreak frequencies and spatial scales of synchronization have been observed in different parts of the insect’s extensive range (e.g., Williams and Liebhold 2000; Robert et al. 2012). Despite its apparent “destructive” nature and economic impacts (Chang et al. 2012a, b), the spruce budworm is an integral part of boreal forest ecology, with extensive outbreaks observed over several centuries within the dendroecological record (Boulanger and Arseneault 2004; Boulanger et al. 2012) and over several millennia within the paleoecological record (Simard et al. 2006).
Current understanding of budworm disturbance ecology comes from two divergent areas of research, both with extensive histories and both involving modeling of budworm disturbance. The first group of researchers sought empirical solutions to assess risk and effects of defoliation, primarily by building defoliation-growth reduction and defoliation-mortality relationships into stand growth models, as a means of prioritizing individual stands for either aerial spraying or preemptive salvage logging or for estimating effects of budworm outbreaks on timber supply (e.g., Baskerville and Kleinschmidt 1981; Erdle and MacLean 1999; MacLean et al. 2001). The second group researched the details of the budworm’s population biology and dynamics to develop simulation models for evaluating feedback between forest conditions and budworm populations, and to inform population management (e.g., Morris 1963b; Jones 1977; Ludwig et al. 1978; Royama 1984). The two approaches have not been well integrated, in part because they derive from different disciplines, objectives, and traditions with respect to modeling uncertainty . However, an early and effective example of integration is reflected in the Holling–Baskerville efforts to use the Jones (1977) defoliation effects model in the Report of the Task Force for Budworm Control Alternatives (Baskerville 1976). This work also inspired defoliation impact field work (e.g., Erdle and MacLean 1999) by exposing key information gaps related to tree growth-defoliation and tree survival-defoliation relationships.
More recently, we have observed parallel developments in modeling of budworm disturbance at landscape scales. The first development involves applying budworm defoliation effects on forest stands at landscape-scale within a timber supply and scheduling framework (MacLean et al. 2001; Hennigar et al. 2007). This strategy is generally used as decision support for tactical planning of forest resources during a given outbreak. The second development is the integration of budworm defoliation disturbance within landscape disturbance and succession models (e.g., Sturtevant et al. 2004; James et al. 2011; Sturtevant et al. 2012). This strategy is applied for ecological insights, strategic planning, and development of broad-scale policy over longer time periods (e.g., centuries). As of the writing of this chapter, these divergent areas of research and parallel landscape modeling strategies have not been integrated. Recent advancements in the understanding of budworm population biology and ecology (Régnière and Nealis 2007; Eveleigh et al. 2007; Régnière et al. 2012, 2013), in combination with the recent increase in budworm outbreak activity in Eastern Canada (Canadian Council of Forest Ministers 2013) warrant a fresh synthesis of budworm science and modeling approaches to inform the next generation of budworm disturbance models.
In this chapter, we examine the history of spruce budworm disturbance modeling to provide insights into landscape insect disturbance modeling more generally. We do so by first outlining the evolution of competing approaches to budworm population modeling, illustrating the interplay of models and data, and highlighting key insights (including failures and advances) into the roles of reciprocal feedbacks among trophic levels (i.e., budworm, its forest host, and its natural enemies), and broader-scale processes (i.e., dispersal, synchronization, climatic variation and change). Second, we overview studies relating budworm defoliation to its effects on forests, culminating in spruce budworm decision support tools designed for forest operations planning. Third, we summarize more recent contributions using landscape disturbance and succession models focused on long-term responses of forested landscapes to a given budworm disturbance regime, and examine the opportunities for synthesis provided by this modeling framework. We conclude with our recommendations for a modern synthesis based on lessons learned from over five decades of research and modeling in the budworm-forest system, and its implications for the modeling of analogous defoliator-forest systems in North America and elsewhere.
5.2 Population Dynamics
Authoritative reviews have been written on the biology and dynamics of Choristoneura species (Volney 1985), including the comparative dynamics of the spruce budworm relative to other closely related defoliator species (Cooke et al. 2007). The spruce budworm is an early season herbivore whose dynamics are influenced by a large array of agents, operating at a range of spatial scales , including many species of vertebrate and invertebrate natural enemies, various host plant effects, a range of weather effects, and dispersal. With so many agents contributing to the system’s dynamics it is perhaps not surprising that no fewer than 15 budworm models have been published over the last five decades (Table 5.1).
5.2.1 Data Sources and Modeling Challenges
To understand the evolution in the thought behind model development it helps to first understand (1) how the data sources developed over time, and (2) how improvements in computational tools and technologies facilitated the development of ever more powerful methods of hypothesis testing. Over the course of the last century, three major phases in data acquisition, analysis, and modeling may be discerned (Fig. 5.2). The earliest studies of spruce budworm ecology and population dynamics indicated that researchers were well aware of the recurring nature of budworm outbreaks (Blackman 1919), and of the roles of multiple factors in influencing the rising and declining phases of population change (Swaine and Craighead 1924). Analytical tools at this time were limited to graphical and conceptual models . During a second phase of discovery through the 1950s to the early 1980s, studies became more comprehensive and methods became more quantitative. Intensive data collection from the Green River Watershed in New Brunswick, Canada (Morris 1963a; Fig. 5.2) led to the simple (yet formal and mathematical) multiple equilibrium model of Watt (1963), followed by the more complex budworm site model of Jones (1977) and its elegant mathematical simplification, which resulted in the cross-scale manifold model of Ludwig et al. (1978) (Table 5.1). A third phase of synthesis centers intellectually around the publication by Royama (1984), in which he offered an alternative interpretation of the Morris (1963a) database via time series analyses, and that by Royama (1992), in which he attempted to place the spruce budworm system in a broader ecological content by comparing it with other animal systems with cyclical population dynamics. Consequently, more intensive studies necessary to accurately distinguish among factors regulating budworm populations over time were established in the 1980s, while the availability of geographic information system (GIS) technology and spatial data sets in the 1990s provided opportunity to quantify factors affecting outbreak synchronization and dynamics in space (Fig. 5.2). Advanced statistical methods in both time series and spatial analysis methods are leading to increasingly nuanced characterizations of system behavior that require a revised understanding of the budworm–forest system.
Clearly, the time scale of observation influences one’s ability to infer cyclic behavior. The earliest quantitative models (listed in Table 5.1) were developed on the empirical basis of just one cycle from the 1950s. For example, Turchin (1990) concluded that budworm populations were nonstationary (i.e., insufficient data to declare the trend-like pattern cyclic) based on plot-level population data from across New Brunswick available from 1945 to 1972 (Fig. 5.3). Similarly, Williams and Liebhold (2000) concluded that budworm populations were not regulated by density-dependent feedback based on 1945–1988 aerial survey data from across Eastern North America. The later models benefitted from two cycles of observations and insights garnered through the 1970s and 1980s. As the length of the time series increases the evidence in favor of cyclic dynamics increases (Fig. 5.3). Data limitations help to explain in part some of the evolution in modeling behavior, i.e., the emphasis on eruptive behavior in the early phase of modeling and on cyclical behavior in the more recent phase.
The history of spruce budworm area defoliated in Quebec, Canada 1938–2001. The province-wide time series in the far right column has been divided to create two additional, shorter series, and all three subjected to autocorrelation analysis (bottom row; ACF = autocorrelation function). The shorter time frames were chosen to match those used in time series studies by Turchin (1990) and Williams and Liebhold (2000) (see text)
Authors of the earliest models were nonetheless aware of the recurrent nature of budworm outbreaks, due to numerous tree-ring studies by Blais (1954, 1961, 1965, 1968). As groundbreaking as these early studies were, Blais did not explicitly recognize the role of scale in interpreting the tree-ring data. The relevance of scale is exemplified by, for example, Boulanger et al. (2012) in a locally intensive study that illustrated the remarkable stability of the budworm outbreak cycle through multiple centuries, while Jardon et al. (2003), using dendroecological studies placed on a systematic spatial grid across much of Quebec, Canada, emphasized the complexity in patterns of recurrence and a lack of repetition in the spatial pattern of outbreak progression. Apparently, just as the temporal scale of observation may influence the perception of cyclicity, so may the spatial scale of observation influence the perception of outbreak cycle homogeneity and synchrony, as budworm populations do not behave identically everywhere.
5.2.2 Competing Hypotheses and Modeling Paradigms
Two major paradigms underlie budworm outbreak models (Table 5.1). The first paradigm, peaking in the 1970s, focused on the role of the forest in precipitating and terminating devastating budworm outbreaks (i.e., multiple equilibrium “eruptive” models). The second paradigm emphasized the role of natural enemies in generating periodic outbreaks that do not necessarily result in host forest collapse (i.e., cyclic predator–prey models). These paradigms differ in two primary areas. The first is the relative strength of top-down versus bottom-up effects (Box 5.1). The second is the relative significance of dispersal in generating complex dynamic behavior and spatial patterning. Both paradigms persist in the literature to the present day.
BOX 5.1
Two elegant mathematical models represent alternative paradigms of the fundamental processes underlying budworm population dynamics. In the Ludwig-Jones-Holling model (LJH; Ludwig et al. 1978) budworm dynamics were assumed to be fundamentally eruptive, owing to positive and nonlinear feedbacks between budworms and forest and the effect of predators on budworms, which was thought to vary as a function of tree size. Royama (1992), in contrast, emphasized the role of delayed feedback from natural enemies in a generic predator-prey model (which Royama (1984) and Fleming et al. (2002) implemented in a univariate autoregressive form, as in our Fig. 5.4) that induced a periodic harmonic oscillation. In each case, a factor viewed as critical in one model was downplayed in the other. Specifically, Ludwig et al. (1978) represented the effect of predation as being conditional on budworm populations and tree size, where predator density was not modeled explicitly, and used two nonlinear feedback equations to describe vegetation dynamics. In contrast, Royama (1992) largely ignored vegetation dynamics by treating food resource competition as a fixed effect, and represented predation as a delayed reciprocal feedback process.
Although Ludwig et al. (1978) contended that their model was an accurate abstraction of the dynamics of the original Jones budworm site model (Jones 1977), Hassell et al. (1999) showed that Ludwig et al. (1978) actually mischaracterized the Jones model by including the predation saturation effect (i.e., the second term in the budworm (B) equation; Fig. B5.1), which in addition to the nonlinear foliage equation (E) (Fig. B5.1) contributed to the relaxed oscillation dynamic that produces the same effect. In contrast, in the Jones model the oscillation dynamics are driven by nonlinear foliage dynamics alone. This finding only serves to strengthen the degree of contrast between eruptive and forest-dominated versus non-eruptive/cyclic and predator-dominated modeling paradigms.
Notably, Royama never actually suggested that the budworm could be adequately represented by any univariate, or even predator-prey, model. Indeed, his use of the second-order autoregressive model in his 1984 monograph was for ancillary demonstrative purposes only. Moreover, in the synthesis to his Chap. 9 on spruce budworm, Royama (1992) specifically referred to budworm oscillating about a single, conditional equilibrium state, which would vary as a function of forest conditions and natural enemy community composition. Rather than ignoring vegetation dynamics per se, it is more accurate to state that Royama (1992) chose not to attempt to summarize the effect of forest conditions in quantitative terms.
Tri-trophic interactions in the spruce budworm system as depicted by two simple models from the metastable eruptive (left) versus harmonic oscillation (right) paradigms, in two common modeling frameworks. The center panel highlights congruencies and differences in the two system models, color-coded to link variable or mathematical expressions to each of the three dominant trophic levels (predators, herbivores, foliage). Solid circles indicate trophic levels represented by dynamic state variables and double arrows indicate inter-trophic relationships that are characterized by reciprocal feedback. In the Ludwig-Jones-Holling model predation is a variable effect; there is no equation for predator population rate of change. In the Royama model the intensity of feeding competition for foliage is a fixed effect; there is no equation for forest foliage dynamics
Clearly, although the two models (and modeling paradigms) differ starkly in terms of which trophic level is emphasized, such a difference could be reconciled through the development of a hybrid model that allows for either fixed or dynamical effects of both trophic layers below and above the budworm (i.e., predators, forest). To examine the effect of ancillary factors, such as weather or dispersal, on budworm tritrophic interactions, a hybrid model (see Fig. 5.8 in text) might be considered that would allow for altering critical assumptions about the nature of the feedbacks occurring among trophic levels.
5.2.2.1 Multi-equilibrium Model
The suite of models produced under the eruptive outbreak paradigm, summarized previously (Cuff and Baskerville 1983; Fisher 1983), emphasized a critical role for tree size, and hence forest age, in shaping the budworm’s ability to acquire food and shelter and to evade natural enemies. Large amounts of host foliar biomass were assumed to be a necessary condition for budworm outbreaks, and sharp declines in available host foliar biomass were assumed to be a necessary condition for population collapse. Consequently, these models shared a “relaxed oscillation” dynamic characterized by alternating host depletion and regrowth. This dependence on bottom-up constraints was thought to explain why the duration between outbreaks was so long in relation to other periodic defoliators, which tend to erupt at roughly decadal intervals (Myers and Cory 2013). The primary differences between alternative models developed under the eruptive outbreak paradigm are reflected in different hypotheses about the relative importance of factors triggering population eruption (Fisher 1983): temperature (e.g., Watt 1964), sufficiently large quantities of host to overcome low-density predator regulation or a so-called predator pit (e.g., Jones 1977; Ludwig et al. 1978), and external invasion by adult moths (e.g., Stedinger 1984). All shared the assumption that forest collapse was responsible for outbreak decline.
The evolution of budworm models under the eruptive paradigm led to seminal insights into insect disturbance modeling. The first computer simulation model (Watt 1963) was characterized by “bistability,” referring to the simultaneous existence of two distinct alternative stable equilibrium states (i.e., endemic and epidemic), both of which are accessible at any moment in time, and neither of whose existence is “conditional” on varying environmental conditions. The Jones (1977) model and the Ludwig et al. (1978) abstraction were characterized by “metastability,” which refers to the temporary existence, and conditional stability, of multiple equilibrium states that arise through dynamic interplay among multiple regulatory processes operating at distinct timescales. The interaction between fast and slow consumptive and regenerative processes gives rise to a “manifold” characterized by a cusp, or critical bifurcation point, which separates two flexible domains of attraction, and a “hysteresis” effect of irreversibility, where return to a basal equilibrium state is inevitable, but requires the slow passage of time to precipitate a critical change in environmental circumstances. Berryman et al. (1984) used the term “metastable” to refer to the forest–insect limit cycle (i.e., also termed a “relaxation oscillation”) that results from the inevitability of a “slow” forest regeneration cycle after a fast process of insect population eruption and forest collapse.
Authors of early budworm models were also pioneers in the simulation of spatial dynamics of outbreaks (e.g., Clark et al. 1979). Spatial processes were recognized as important because budworm densities “were not solely determined by local factors, but remain at least partially synchronized with neighboring areas” (Fisher 1983, p. 107). Under the eruptive model paradigm, adult dispersal was a key factor underlying the radial expansion of outbreaks from so-called “epicenters” (Hardy et al. 1983), analogous to how we currently understand the spread of bark beetle epidemics (Powell et al. 1998). The primacy of wind-mediated dispersal of budworm moths underlying the spatial spread of outbreaks under the eruptive paradigm––and the implications for budworm suppression programs––was underscored by an unprecedented research program to investigate the aerobiology of spruce budworm dispersal (Greenbank et al. 1980) (Fig. 5.2).
5.2.2.2 Harmonic Oscillation Model
Despite the obviously destructive nature of budworm outbreaks, several widespread observations were inconsistent with the multi-equilibrium models (Royama 1992). First, budworm outbreaks frequently end before host foliar biomass is fully depleted. Second, these outbreaks occur at fairly regular intervals ranging between 20 and 40 years between peaks (Burleigh et al. 2002; Jardon et al. 2003; Boulanger et al. 2012) that are less than even the “pathological rotation age” of balsam fir (70 years; Burns and Honkala 1990). While the eruptive models of this era (Table 5.1) were not scale-specific, they were typically applied at the resolution of a “forest block” (e.g., Clark et al. 1979; 170 km2). If budworm outbreaks resulted in forest collapse at this scale every three to four decades then very rarely would forests have the opportunity to develop into the mature and overmature age classes that are frequently observed in the boreal forest. Likewise, immature and mixed species stands generally show only partial stand mortality (MacLean 1980; Su et al. 1996). Finally, cyclic budworm outbreaks are often synchronized at regional scales, despite high spatial variability in forest conditions, suggesting broad-scale population fluctuations are governed by more than just local forest conditions.
An alternative paradigm, elaborated most forcefully by Royama (1984), is that something other than forest age and abundance––such as natural enemies––restricts generation recruitment of budworm. By acting in a delayed density-dependent manner, these agents induce a harmonic (i.e., sinusoidal) oscillation, much like a predator–prey cycle (Box 5.1), resulting in a system that is statistically “stationary” in its cycling (i.e., autoregressive) properties, including time series mean and variance. According to Royama’s model no upper (or lower) stable state is ever realized; the unique equilibrium state is never achieved because of an unending regime of stochastic perturbations that continually force the system away from its globally stable single-point attractor. Royama (1984) noted that, according to this theory, some population cycles may not rise to a level where defoliation becomes observable. This, he suggested, might explain the occasional missing cycle in long time series records––defoliation events that could not easily be detected by aerial surveys and tree-ring analysis.
The paradigm is parsimoniously expressed in a model phase space diagram (Fig. 5.4a). Using a second-order density-dependent approach that is essentially a phenomenological predator–prey model, one can produce long cycle lengths by careful selection of an appropriate parameter space. Zone IV of the parameter space (Fig. 5.4a) produces slow-damping cyclical behavior consistent with the idea of “phase-forgetting quasi-cycles” (Nisbet and Gurney 1982). Royama (1984) illustrated parameter combinations in Zone IV that generate low-frequency, high-amplitude cycling, and Fleming et al. (2002) discussed these parameterizations in the context of the spruce budworm . Figure 5.4a illustrates two contrasting parameterizations that lead to robust 36- and 24-year cycling behavior (stochastic realizations illustrated in Fig. 5.4b). A 36-year outbreak cycle (i.e., time between peaks) is indicative of budworm outbreak dynamics in the northeastern boreal forest (e.g., Jardon et al. 2003), while a 24-year cycle is more common to the Appalachian region (Cooke et al. in prep.), to Western Canada (Burleigh et al. 2002), and also to western spruce budworm, C. occidentalis Freeman (Alfaro et al. 2014). Clearly, a 24-year outbreak cycle is not sufficiently long for forest regrowth, particularly in Northwestern Canada where balsam fir is absent, and the dominant conifer is the relatively long-lived white spruce. Notably, very small differences in parameter values may result in significantly different cycle lengths (Fig. 5.4).
a The parameter space of a delayed feedback time series model (Rt = Φ0Nt + Φ1Nt-1 + et) overlaid on a phase plane diagram representing different zones of model behavior (Royama 1992; see inset), where Zone IV parameter space generates low-frequency dampening oscillations. Tiny variations in the delayed feedback parameter (Φ0) are sufficient to generate large difference in cycle frequency, as indicated by the parameter sets (Φ0, Φ1) required to generate sustained oscillations of 36- and 24-year periodicity. b The effect of coupling via reciprocal dispersal on two populations cycling at differing frequencies. Even when the strength of dispersal coupling is low (exchange rate = 0.01 % amongst populations), the 36- and 24-year cycling populations (top left) converge on a phase-synchronized 29-year cycle (bottom left), as demonstrated by corresponding spectral peaks in the respective time series (right), with correlations (r) rising from ~ 0 to ~1
Trophic interactions affecting budworm populations were recently documented to show a complex food web involving at least 56 different species, including alternative parasitoid hosts, predators, and hyperparasitoids (Eveleigh et al. 2007). One might ask how a 56-dimensional trophic interaction could possibly be represented in a simple two-dimensional autoregressive model. The answer lies in Royama (1971, 2001). The competitive interaction between parasitoid species that occurs with multiparasitism may serve a compensatory mechanism whereby a reduction in one parasitoid species is readily offset by compensatory gains in another parasitoid species with a similar attack phenology. Trophic redundancy thus may result in a relatively stable predator–prey multispecies complex that behaves more or less as a pure two-species predator–prey system.
In theory, a more rapid response of predators translates into lower amplitude, higher frequency predator–prey cycles. With a very large food web, relatively small changes to just a few key budworm parasitoid species might produce such differences. In this way, interaction between forest composition and the spruce budworm natural enemy complex may help to explain the lower amplitude and higher frequency cycle more typical of the floristically diverse Laurentian forests relative to the higher amplitude, lower frequency cycle more typical of the less diverse boreal forest (Cooke, unpublished manuscript). Indeed, it is interesting to speculate on the potential role of the closely related hardwood defoliators in the Choristoneura genus (e.g., C. conflictana and C. rosaceana), whose presence or absence would serve to perturb the natural enemy communities that surround the spruce budworm (see Sect. 5.3 for discussion on reduced budworm defoliation in hardwood-rich stands and forests of New Brunswick).
Analyses of recent data that included high-frequency sampling of parasitoids (Nealis and Régnière 2004a, b; Régnière and Nealis 2007) support Royama’s assertion that “the primary oscillation is governed by lagged, negative feedbacks between budworm density and generational survival as influenced by the impact of natural enemies on late-feeding stages of the insect” (Régnière and Nealis 2007, p. 14). Yet host mortality was also clearly documented as contributing to the decline––just not consistently across study sites. Among the strongest sources of bottom-up feedbacks are dispersal mortality within young instars when seeking food resources in heavily defoliated stands (Nealis and Régnière 2004a). In short, substantial losses in host foliar biomass can certainly contribute to––but is not a requirement for––outbreak decline.
5.2.2.3 Upscaling Local Dynamics to Landscapes and Regions
As indicated previously, the champions of each model paradigm further differed in the relative significance of dispersal in generating complex dynamic behavior and spatial patterning of budworm outbreaks. While Royama (1980, 1984, 1992) acknowledged that dispersal by both larvae and adults was an integral part of budworm life history, he invoked Moran’s (1953) theorem to argue that synchronized patterns of outbreaks are primarily caused by weather-driven (i.e., density-independent) fluctuations in recruitment between the adult and egg stage, where egg-laden female moths are prone to dispersal within and between forest stands. Under Moran’s theorem, modest environmental perturbations that are regionally autocorrelated in space can synchronize independently oscillating populations, even when the environmental factor is independent of the cause of the population oscillation. This “Moran Effect” has since been identified as an important factor contributing to the regional outbreak synchrony for a wide range of Lepidopteran species (Ranta et al. 1997; Myers 1998; Bjørnstad et al. 1999; Myers and Cory 2013), including two species of lymantrids with females that cannot fly (Mason 1996; Bjørnstad et al. 2008).
The interaction between dispersal and spatiotemporal dynamics of outbreaks depends in part on how immigration affects local population dynamics , i.e., the underlying population model, or harmonic oscillation versus metastable eruption. For the latter, dispersal acts as successive triggering of eruptions through a spatial chain reaction, similar to a “domino-effect” (Clark et al. 1979) also known as a traveling wave (Bjørnstad et al. 2002). This so-called “epicenter hypothesis” fell out of favor with the advent of the harmonic oscillation paradigm that emphasized the Moran effect (Royama 1984). Régnière and Lysyk (1995) later proposed a high-resolution spatial model that illustrated how dispersal may act as a significantly more robust synchronization process that forces independently oscillating systems to converge to a common cycling frequency even if their intrinsic cycling frequencies differ (as illustrated in Fig. 5.4b). Régnière and Lysyk (1995) fundamentally changed the nature of the discussion of cycle synchronization to place equal emphasis on weather and dispersal as potential synchronizing forces (e.g., Peltonen et al. 2002). More recently, Régnière et al. (2013) empirically documented an “Allee effect” (Allee 1931) within low-density budworm populations––not in the form of a predator pit as proposed by Holling and colleagues (Ludwig et al. 1978), but due to low mate-finding success at very low densities. This Allee effect, i.e., the positive dependence of population growth rates on population densities when densities are low, suggests that a low endemic state can be overcome via immigration. If dispersal can produce both kinds of effects, i.e., synchronization and traveling waves, then hybrid models that include both effects may be necessary to fully capture the relevant dynamics.
The recent data and syntheses suggest that neither of the supposedly competing paradigms (i.e., host abundance vs. natural enemies, and Moran Effect vs dispersal) is sufficient to characterize the full range of budworm outbreak behavior in time and space. Insights from landscape ecology, including recognition of the critical role of neighborhood effects, spatial heterogeneity , and the appropriate scaling of ecological processes (Addicott et al. 1987) suggests that the discrepancy between paradigms might be related to the spatial and temporal scale of observation and empirical data. This perspective is supported by the multiscale modeling efforts of Fleming et al. (1999, 2002) who showed that finely resolved gridded data differ qualitatively in behavior from models parameterized using coarsely gridded data. More specifically, whereas local dynamics appear to conform to an eruptive hypothesis, the landscape scale dynamics appear to conform to a cyclic hypothesis. Although the models used were purely phenomenological and univariate, the results are consistent with Holling’s idea of “cross-scale drivers” (Holling 1973, 1986), with eruptive behavior emerging locally as the result of a fast process (in this case positively density-dependent mating success or predator escape), and cyclic behavior emerging at the landscape scale as the result of some slow process (in this case delayed density-dependent parasitism and dispersal-driven cycle synchronization, as illustrated in Fig. 5.4b). The spruce budworm thus appears to behave as hybrid cyclic-eruptive, with the characteristic oscillatory and eruptive relaxation behaviors emerging at distinct, well-separated spatial scales of landscapes versus stands.
5.2.3 Gradient Models and Climate Drivers
In his synthesis of the spruce budworm system, Royama (1992) emphasized the conditional nature of its equilibrium state, with cycle frequency, amplitude, and time series mean and variance all potentially varying depending on environmental factors, effectively serving as “gradients” in space and time. He suggested these environmental factors might include forest composition, food web composition, and climate. Recognition of gradients affecting insect population dynamics (Table 5.1) derives from the notion that population densities are controlled by environmental carrying capacities (Andrewartha and Birch 1954). Indeed, as early as the 1950s, spruce budworm “outbreaks” were occasionally referred to as “gradations” (Morris et al. 1958). Very early “hazard-rating” models were built on this same premise of the outbreak as a spatial gradient, substituting the forest resource for the environment (e.g., Webb et al. 1956). Hazard modeling has become increasingly sophisticated with time and is elaborated in Sect. 5.3. However, the concept of environmental gradients––and more specifically climate––as a key factor contributing to nonstationary budworm outbreak dynamics in time and space both transcends the two modeling paradigms summarized above and has emerged most recently as fundamental to the understanding of outbreak behavior (Fleming and Candau 1998).
There are two fundamental approaches to understanding the climate–budworm outbreak interaction. The first approach relates to processes underlying budworm outbreak response to climate: investigations of the underlying process and empirical pattern analysis of past outbreaks using climate variables as covariates. Results from models used to investigate the process suggest climate and weather interact with budworm on multiple levels, i.e., growth, survival, and movements of both the budworms and their natural enemy complex (Fleming 1996). Gray (2008) argued that it is challenging to understand the cumulative effects of these multiple interacting processes. Pattern analyses of broad-scale aerial surveys clearly indicate an overall climatic signal affecting the budworm outbreak dynamic, but to date these types of analyses have been limited to a single outbreak cycle (e.g., Candau and Fleming 2005; Gray 2008, 2013). Régnière et al. (2012) simplified the processes to two dominant temperature-dependent limitations on population dynamics : development rate (Régnière and You 1991) and consumption of energy reserves over winter during diapause (Han and Bauce 1997, 2000). Consequently, budworm outbreaks become limited by the budworm’s ability to complete its phenological life cycle at the northern (or altitudinal) extent of its range, and by the exhaustion of energy reserves due to higher metabolism at the southern extent of its range. The key insight from this combined body of modeling (i.e., process- and pattern-based) is that climate is an influential environmental factor. Climate change is anticipated to significantly affect future outbreak dynamics, with a northward shift in periodic outbreak behavior (much as predicted by Fleming and Volney 1995), a reduction in outbreak cycle amplitude in regions that have historically seen the most regular oscillations, and the elimination of detectable defoliation at the southern range limit (Régnière et al. 2012). Indeed evidence is mounting that northward range shift of spruce budworm may be attributable to climate warming, and that climate and forest composition both influence outbreak dynamics (Gray 2013).
5.2.4 Insights Following Five Decades of Research and Modeling
The evolution of thought and the interplay of data and modeling over time can be described by five phases of model development (observational, formal, digital, empirical, spatial), punctuated by the publication of four revolutionary papers (Fig. 5.5) (Watt 1963; Ludwig et al. 1978; Royama 1984; Régnière and Lysyk 1995). As the data accumulate over time, and as the methods evolve, we see that the prevailing paradigm oscillates from cyclic to eruptive to cyclic and is currently settling on a hybrid between these extremes. A unifying theme that emerged in the background of these opposing paradigms was the idea of population regulation by environmental factors such as forest and climate. One is led to conclude that this system may exhibit all three features of cyclic, gradient, and eruptive behavior (Table 5.1). Ideas that were once dismissed as unimportant or improbable (e.g., epicenter theory; anthropogenic forcing) come back into vogue as new data emerge, as the role of heterogeneity and scale become increasingly explicit, and as the demands of operational management force modelers to consider the full array of actual dynamic behavior across multiple spatial scales.
5.3 Budworm Risk, Impacts, and Decision Support
5.3.1 Empirical Understanding
The historical spruce budworm modeling of Holling and colleagues (Jones 1977; Clark et al. 1979), in collaboration with Baskerville, directly contributed to forest policy discussions (Baskerville 1976) and considerations of timber supply and effects of defoliation on provincial economy and employment (e.g., Baskerville 1982). It also contributed to broader development of timber supply modeling and forest management decision-making frameworks in New Brunswick (e.g., Baskerville and Kleinschmidt 1981; Hall 1981), and was the precursor of stand- and forest-level modeling inherent in the modern spruce budworm decision support system (SBWDSS) . Here we (1) review empirical relationships between defoliation levels and reductions in stand growth and survival, and the factors that influence them; (2) relate the population processes from Sect. 5.2 to the impacts measured at tree-, stand-, and neighborhood scales; and (3) describe the foundation and functioning of the SBWDSS.
Defoliation links insect budworm population factors to stand responses (MacLean 1980; Erdle and MacLean 1999). Current defoliation is directly correlated with late larval stage population density, so current and cumulative defoliation link budworm population dynamics to stand responses. From the standpoint of predicting or inferring effects of budworm outbreaks on growth and yield or timber supply, defoliation is easier to assess at the branch-, tree-, stand-, or landscape scale than are budworm population levels. Current defoliation sampling methods include manually assessing percentage defoliation by foliage age class, on shoots, branches, or trees (e.g., Fettes 1950; Sanders 1980; MacLean and Lidstone 1982) and also well-developed aerial survey techniques (e.g., Dorais and Kettela 1982; MacLean and MacKinnon 1996) that rely on the reddish coloration of foliage resulting from budworm larvae severing and webbing together needles as they feed. Repeated annual measurement of current and cumulative defoliation on individual trees in permanent sample plots, and relating cumulative defoliation to growth and survival of those trees over time, is the basis for much of our empirical understanding of budworm impacts.
So what do we know about budworm defoliation, impact relationships, and effects on trees and stands? First, budworm population density is the main driver of annual current defoliation (e.g., Figure 5.3), and several factors influence budworm population trends. But host tree species also influence current defoliation, with an extensive permanent sample plot data set (>27000 tree and 1117 stand measurements from 1984 to 1992) revealing a clear and consistent hierarchy of host species defoliation. Regardless of budworm population density (defoliation severity) and various stand variables tested, white, red, and black spruce had approximately 72, 41, and 28 % as much current defoliation as balsam fir, respectively. Phenology of host bud burst and budworm larval development may be the leading cause of reduced defoliation on red and black spruce compared with balsam fir and white spruce. Red and black spruce bud burst occurs on average 2 weeks later than on balsam fir (Greenbank 1963), causing lower early instar larvae survival on red-black spruce and lower percent defoliation relative to other host species (Lawrence et al. 1997; MacLean and MacKinnon 1997).
Second, host tree defoliation and tree growth reduction and mortality are consistently reduced when host trees are mixed with deciduous trees not just within stands, but also in relation to surrounding stands (Bergeron et al. 1995; Su et al. 1996; Campbell et al. 2008). Reduced budworm impacts within mixed forests may be attributed at least in part to the effect of hardwood species on the abundance and composition of budworm natural enemy communities. Tachinid parasitism of larvae and ichneumonid parasitism of pupae were elevated in stands mixed with or surrounded by deciduous species (Cappuccino et al. 1998), and a similar response was observed for egg parasitism by a hymenopteran species (Quayle et al. 2003). Importantly, stands with a hardwood tree species component contain many alternative host Lepidoptera species for multivoltine parasitoids that parasitize budworm. This can be critical as multivoltine parasitoids need to subsist on an alternate host in the late summer and autumn to continue their life cycle (Maltais et al. 1989). High levels of non-host deciduous species could also contribute to significant losses of first- and second-instar larvae during dispersal to other hosts (Kemp and Simmons 1978). The effects outlined here refer to factors affecting levels of defoliation at the plot to neighborhood scale (e.g., 1 km radius; Campbell et al. 2008). We speculate that the feedback between forest composition and budworm population dynamics may scale up to influence regional differences in outbreak frequency (Fig. 5.4) and intensity (Cooke, unpublished manuscript).
Third, growth reduction and mortality impacts at the tree- and stand scale are strongly related to cumulative defoliation over successive years (e.g., Erdle and MacLean 1999; Ostaff and MacLean 1995). Tree mortality rates are also a function of species and age class, whereas growth reduction has been observed to be similar, for a given level of 5-year cumulative defoliation among balsam fir, white spruce, and red–black spruce (Erdle and MacLean 1999). The relationships between growth, survival, and cumulative annual defoliation, by species, appear to be robust among differing outbreaks and studies, and can largely be considered deterministic. Outbreak severity and duration largely determines the amount of cumulative defoliation. However, real-world defoliation patterns are generated by the multiscaled interaction among forest conditions, nonlinear budworm population dynamics , complex trophic interactions, and the climatic and weather drivers affecting the predator–prey interaction (Sect. 5.2). For example, recent cluster analysis of aerial survey data for the last major outbreak in New Brunswick shows spatially aggregated regions with current defoliation ranging between 1 and 16 years (Zhao et al. 2014; Fig. 5.6). Other authors have identified analogous spatial heterogeneity in defoliation within an outbreak cycle across much of the boreal forest (Candau and Fleming 2005; Gray 2008). Such variability in current and cumulative defoliation underscores the importance of drawing together budworm population modeling and impact/decision support system modeling approaches.
Cluster analysis of spruce budworm defoliation in New Brunswick, Canada, from 1966 to 1993 resulted in 28 representative defoliation patterns, which were grouped into five categories (a–e) with divergent defoliation duration (ranging from 1 to 16 years; Yrs. defol.) and amounts (equivalent to removal of 2 to 10 age classes of foliage; Sum defol. %) (Zhao et al. 2014). Duration and severity of spruce budworm defoliation determines the resulting magnitude of impacts
Fourth, stand and site characteristics, and tree vigor, have been observed to influence current defoliation, growth reduction, and survival in some studies (e.g., Lynch and Witter 1985; Hix et al. 1987; Osawa 1989; Archambault et al. 1990; Dupont et al. 1991; MacKinnon and MacLean 2003) but not in others (Bergeron et al. 1995; MacLean and MacKinnon 1997). These appear to be weaker relationships, which break down during severe defoliation episodes.
5.3.2 Spruce Budworm Decision Support System (SBWDSS)
Advances in computer and information gathering technology have made the evaluation of alternative management practices through model simulation a feasible and valuable tool for forest managers (MacLean 1996). The SBWDSS, originally developed conceptually by Erdle (1989) and refined into a software application by Canadian Forest Service researchers (MacLean et al. 2001), was developed to project effects of budworm outbreaks on tree growth, mortality, and timber supply, and to incorporate potential management actions into a decision-making framework. It is built on the empirical impact relationships described in the previous subsection. Annual defoliation data obtained from aerial surveys and various user-defined defoliation scenarios are converted into cumulative 5-year defoliation. The model is deterministic and thus multiple defoliation scenarios are used but could eventually be coupled to population dynamic models as these improve. Estimates for the different scenarios are used to model tree growth reduction and stand mortality in a GIS forest inventory database (MacLean et al. 2001). Hennigar et al. (2007) improved the SBWDSS modeling framework by integrating stand-level budworm volume impacts into a forest estate model (Remsoft Spatial Planning System 2010), allowing pest management decisions such as foliage protection, harvest rescheduling, and salvage to be considered when maximizing timber flows during a budworm outbreak (MacLean et al. 2000, 2002).
The latest iteration of the SBWDSS allows integration between forest management planning and optimization models and underlying tree impact information derived from pest management decision support tools (McLeod et al. 2012). This tool can assist land managers in quantifying marginal benefits of protecting forest stands against insect defoliation (e.g., in terms of timber volume in m3 ha−1 or value as $ ha−1). Protection cost: benefit analyses can be conducted using existing forest inventory and insect monitoring data in combination with forest management planning models to project the effects of foliage protection strategies on forest development and forest values.
This decision support system (DSS) comprises several specialized tools that allow users to simulate insect impacts on trees, stands, and forests (McLeod et al. 2012). These tools leverage stand growth modeling capabilities (FORUS Research, Fredericton, NB) and allow forest impact analyses to be conducted with existing strategic forest management optimization models (Remsoft Spatial Planning System 2010). These capabilities permit efficient exploration of cost-effective foliage protection or wood salvage scenarios. The tools can be divided into those used in estimating stand effects for strategic forest impact analysis (nonspatial tools) and those used for optimal spatial design of operational spray blocks (spatial tools). Nonspatial tools can be used to calibrate and implement the SBWDSS.
Strengths of the SBWDSS approach include robust stand-scale relationships, integration with existing forestry modeling tools, and use in tactical decision-making. Limitations of empirical DSS are that outbreak severity (number of years of >30 % annual defoliation) varies considerably and is scale- and context-dependent; studies are underway to better understand spatial and temporal variability of historic defoliation patterns in New Brunswick and Maine, United States, and to relate variability to budworm sample numbers. Since the SBWDSS stand impact matrix includes effects of all possible combination of cumulative defoliation on volume production, analyses can be scaled to differing outbreak severities. Nonetheless, until population modeling can actually project outbreak severity in time and space several years into the future, the stand growth/DSS approach will likely continue to use “what if,” user-specified defoliation scenarios that are typically based on previous outbreak patterns, as well as monitoring and annual updating of stand-scale defoliation conditions during outbreaks using aerial survey or potentially remote sensing.
5.3.3 Transcending Traditional Disciplines
A commonality between the budworm population modeling and hazard modeling implied by Fig. 5.5 is the predominant absence of spatial context, particularly with respect to supporting data collected at plot (e.g., Royama 1984) and stand (e.g., MacLean 1980) scales. Analogous to insights described in the previous section (Fleming et al. 1999, 2002), landscape ecology left its mark on traditional impact studies starting with the recognition by Bergeron et al. (1995) that neighborhood and spatial context had implications for budworm damage, including the community composition and abundance of natural enemies (Cappuccino et al. 1998). The fact that both budworm and its natural enemy complex are mobile and therefore sensitive to spatial context could no longer be ignored.
An important consideration is that the vegetation equations underlying the eruptive class of models (Table 5.1) were not developed from data and have never been validated. The vegetation equations, which Hassell et al. (1999) showed to exhibit intrinsically explosive dynamics, were derived from Jones (1974, 1977), which in turn were supposedly inherited from an unpublished 1973 M.Sc thesis by a J.M. Stander at the University of British Columbia, Canada. Given the critical dependency of the eruptive models on vegetation dynamics , and the absence of vegetation dynamics in Royama’s framework (Box 5.1), any modern hybrid modeling approach should revisit the question of how foliar biomass changes as trees and forests grow and prune themselves, and how this variable responds to budworm defoliation. Forest empiricists may be well-positioned to address this question, and in so doing, greatly inform the scaling of budworm populations to stands and landscapes. Likewise, budworm disturbance scenarios projected by the SBWDSS generally operate on the premise that budworm population dynamics are not affected by slower dynamics such as changes in forest conditions and climate—in other words, past dynamics are reasonable indicators of future outbreak behavior. We suggest that SBWDSS projections could be improved by incorporating feedback between forest and budworm defoliation scenarios in a way that is consistent with a modern synthesis of past modeling paradigms.
To fully understand critical feedbacks between forests and budworm dynamics relevant to both populations and their effects, we need to understand both tree losses (growth reduction and tree mortality) and tree response, i.e., regeneration and infilling of small or large gaps by surrounding trees (e.g., Spence and MacLean 2012). The former represents short-term hazard analysis, that is, within the realm of the current DSS. The latter represents long-term risk to timber supply and implications for future forest conditions, and requires a process-based modeling approach such as is embedded in landscape disturbance and succession models.
5.4 Landscape Disturbance and Succession Models
Landscape disturbance and succession (LDSM) models form a subset of forest landscape simulation models, identified by their explicit simulation of dynamic interactions between vegetation and disturbances in both space and time, and thus address the need to evaluate long-term risk by modeling long-term forest dynamics (Scheller and Mladenoff 2007). These LDSMs share a set of common traits, albeit with differing levels of sophistication, process, and complexity. Each simulates vegetation change in response to one or more disturbances and over time in the absence of disturbance. Each includes some environmental representation of the biophysical template (sensu Urban 2005) that affects the growing environment. While these processes generally operate and manifest locally at the level of a grid cell or polygon, they may be affected by neighborhood processes (e.g., seed source), environmental change (e.g., climate change), or vegetation–environment interactions (e.g., nutrient cycling). Simulated disturbance processes also vary in their respective degree of sophistication and coupling with other model processes. Unlike the deterministic optimization approach characteristic of the coupled SBWDSS and timber scheduling software described in the previous section, LDSMs explicitly incorporate the stochastic nature of disturbance and vegetative processes that reflects their intended purpose—to project future landscape conditions over comparatively longer timescales (e.g., centuries to millennia compared with DSS projections of years to decades). Spatial interactions among processes are fundamental to the approach because they “produce emergent behavior that contributes to the evolution of landscape pattern and changes in spatial heterogeneity at multiple scales” (Scheller and Mladenoff 2007, p. 493–494).
Implementation of defoliator disturbance regimes within the LDSM framework requires methods to (1) define outbreak dynamics in time and space, (2) define the spatial distribution of resulting impacts, and (3) implement the vegetation response to those impacts (Sturtevant et al. 2004). As should be apparent from the previous sections, predicting outbreak severity and duration across a complex landscape cannot be done with any certainty (e.g., Figure 5.6). Uncertainty is factored into LDSMs by applying disturbance processes according to stochastic functions. To date these functions have been defined simply, where outbreak periodicity and extent may be derived empirically from either dendrochronological studies (e.g., Jardon et al. 2003; Bouchard et al. 2006) or aerial survey data (e.g., Peltonen et al. 2002; Gray and MacKinnon 2006, 2007).
Simulating the characteristic spatial patterns of budworm-related mortality within a given outbreak (i.e., aggregation, severity, etc.) requires scaling the plot- and stand-scale impact relationships defined in the previous section to heterogeneous landscapes. Section 5.3 describes how forest composition, age, and growing environments can contribute to heterogeneity in defoliation impacts. The consequence of these combined relationships is that, in pure fir stands, mortality can be stand replacing, i.e., coarse scale, large opening (e.g., Baskerville 1975; MacLean 1980) but in mixed species stands overstory mortality is partial, forming small canopy gaps (e.g., Kneeshaw and Bergeron 1998; D’Aoust et al. 2004; Kneeshaw et al. 2008). Sturtevant et al. (2012) therefore scaled plot-level relationships between host abundance and disturbance impacts to landscapes as disturbance probabilities, resulting in a gradient of patchy “gaps” (i.e., isolated disturbed cells) where hosts were rare to more continuous mortality where hosts were dominant (Fig. 5.7). Neighborhood effects, such as reduced impacts within forests dominated by hardwoods (Su et al. 1996; Cappuccino et al. 1998), may be similarly approximated by adjusting disturbance probabilities based on neighborhood context (James et al. 2011). Nonetheless, considerable variability remains in defoliation impacts that both overlay and interact with the landscape structure and composition (e.g., Belle-Isle and Kneeshaw 2007). Such methods can be further refined by quantifying spatial patterns of defoliation mapped using remote sensing (e.g., Foster 2011).
Simulated budworm disturbance within a landscape disturbance and succession model (Sturtevant et al. 2012), illustrating realistic simulation of budworm defoliation disturbance in both time (e.g., alternating moderate and severe outbreaks) and space (i.e., diffuse mortality moderated by spatial heterogeneity in host tree species). Large patches where budworm damage is absent reflects absence of host due to recent fires. Importantly, outbreaks were predefined to occur every 33.5 ± 10.6 years (mean ± SD) constrained to a 10-year time step. Alternating moderate and severe outbreaks emerged from the lagged response of regenerating host species
Forecasting forest dynamics following spruce budworm outbreaks requires that tree species recruitment is understood as a function of overstory mortality. One of the simplest conceptual models of budworm-related forest succession is the cyclical model of balsam fir replacing itself in fir-dominated stands (Baskerville 1975; MacLean 1980). This model is based on the high shade tolerance of balsam fir (Kneeshaw et al. 2006) and its ability to form dense seedling banks that are released following overstory mortality (Morin et al. 2009). As suggested by Baskerville (1975), this model becomes more complex as forest structure and composition increase in complexity. For example, Kneeshaw and Bergeron (1998) show that budworm-caused mortality can lead to both the maintenance of shade intolerant hardwoods and the increase of non-host shade tolerant species such as eastern white cedar (Thuja occidentalis) in mixed species stands. Others have shown that outbreak periods are associated with recruitment peaks of many companion species in addition to balsam fir (Bouchard et al. 2006). An increase in the presence of competing shrub species has also been linked to budworm outbreaks (Batzer and Popp 1985; Kneeshaw and Bergeron 1999; Kneeshaw and Prevost 2007).
Modeling of post-outbreak forest dynamics will be improved by a better understanding of understory seedling dynamics and their interactions with overstory composition and spruce budworm populations. For example, in some cases the forest may be too young or dominated by mixed species such that advance balsam fir regeneration banks are sparse. In other cases, as budworm populations increase and exhaust food resources in the overstory, the larvae may disperse downward (“down-spinning”) and defoliate the understory regeneration layer (Ruel and Huot 1993). This can then lead to a patchy forest structure and permit the recruitment of species other than balsam fir. However, knowledge of the effects of larvae down-spinning and defoliation of the understory seedling bank is lacking limiting our ability to establish the budworm population densities at which this process occurs.
The above elements are sufficient to examine budworm–forest interactions, with useful insights. For example, emergent behavior, such as alternating low severity and high severity outbreaks (Blais 1981; Bouchard et al. 2006), may be observed due to the inertia of host recovery following severe outbreaks (Fig. 5.7). Interactions between budworm, forest composition, and other disturbance regimes including fire and harvesting have also been investigated using LDSMs (James et al. 2011; Sturtevant et al. 2012). Nonetheless the current “state of the art” in insect modeling within LDSMs lags somewhat behind that of some other disturbances, such as fire. For example, nonstationary fire “regimes” may be derived from the emergent behavior of the underlying processes affecting fire ignition, spread and extinguishment, and the interactions of these processes with forest conditions, weather, and climate (McKenzie and Perera, in this volume). Coupled forest–budworm feedbacks have not yet been attempted within the LDSM framework, despite empirical evidence for such feedbacks at the landscape scale (as described above) (Candau and Fleming 2005; Bellier et al. 2007; Robert et al. 2012). Including such feedbacks could generate additional complexity in outbreak patterning, such as alternating patterns in per capita impact on host trees (i.e., high intensity in one outbreak, low intensity in the next), as reported by Bouchard et al. (2006). This alternating pattern in outbreak intensity would constitute a multiplicative effect over and above the far more intuitive pattern of outbreaks that vary in extent in proportion to host cover. Such scenarios can then serve as the basis for sensitivity analysis and scenario testing within the SBWDSS framework.
Landscape applications of the eruptive era models (i.e., Clark et al. 1979; Stedinger 1984; Fleming and Shoemaker 1992) used very coarse resolution forest compartments, with dispersal between them. Environmental factors have considerable influence on local dynamics (Gray 2013), such that spatial heterogeneity in landscapes is an important consideration. LDSMs can supply the more realistic forest responses that include spatial heterogeneity, although this opportunity for scaling has yet to be taken advantage of. Yet other processes—in particular the multiple scales and magnitudes of movements by both budworm and their natural enemies—remain unresolved. Computational demands of such multiscaled movements may also restrict their practical application within LDSMs, despite exponentially increasing computing technologies. Consequently, the same fundamental problem limiting SBWDSS also limits LDSMs, albeit at a different temporal scale (i.e., decade vs. century).
The development of such coupled modeling will also be critical for evaluating the effect of climate change on budworm–tree dynamics. LDSMs are already commonly applied to investigate the effects of climatic change on vegetation dynamics , typically by using climate–envelope relationships to define vegetation response to changing environments (e.g., Scheller and Mladenoff 2005; Keane et al. 2008; Ravenscroft et al. 2010). Among the many insights from these investigations is that spatial heterogeneity can influence vegetation response to environmental change (Scheller and Mladenoff 2008) and that indirect consequences of climate change , particularly consequent changes to disturbance regimes, can have larger consequences than the direct effects of environmental change on vegetation (Gustafson et al. 2010).
While LDSMs provide opportunity to investigate climate–budworm–landscape interactions, the science of climate–insect interactions has not yet been integrated into this framework. The regional empirical analyses of Gray (2008) and Candau and Fleming (2011) provide some guidance for how to parameterize future outbreak patterns under a warming climate. Alternatively, the processes underlying climatic constraints on population dynamics may include the ability of budworm to complete its life cycle based on cold temperature constraints on growth at high latitudes, and energetic constraints on survival of overwintering larvae at low latitudes (Régnière et al. 2012). Fleming (1996) suggested temperature-dependent predation or parasitism rates may also be involved, and as yet no evidence suggests these processes are not operating simultaneously. Other authors suggest that differences in weather variability across the same latitudinal gradient may also affect budworm population dynamics (Cooke et al. 2007; Dukes et al. 2009; Cooke, unpublished manuscript). To date, conceptual insights and advances have not been integrated into a single modeling framework.
The strength of the LDSM approach lies in its integration of processes occurring at different spatial and temporal scales , with both direct and indirect feedback between vegetation, multiple disturbances , and environment in time and space. The degree of complexity in the way these processes are represented can range from purely empirical functions based on past observations to theoretical relationships grounded in first principles. Stochasticity is fundamental to the approach to account for what we can and cannot project reliably. With respect to insect disturbance in general, and budworm disturbance in particular, applications have been weighted more toward empirically derived functions with associated probabilistic uncertainty.
The extensive research and modeling of budworm population and disturbance processes summarized in the previous two sections provides a rigorous basis for incorporating additional insect disturbance processes into the LDSM approach. In essence, the LDSM provides dynamics of critical vegetation and environmental gradients in time and space that could determine the “conditional equilibrium state” affecting harmonic oscillations of budworm populations (sensu Royama 1992). To simulate the disturbance process more precisely, empirical relationships underlying the SBWDSS could be adapted into impact functions. Insect movement could be simulated to interact with spatial heterogeneity of vegetation and environment to account for neighborhood and synchronization processes, while the flexible architecture of the LSDM may allow evaluation of competing hypotheses in a way that remains sensitive to scale. Integration of these processes with climate drivers affecting population dynamics provides opportunity for a “whole-system” approach to the question of climate change effects that may be compared directly for consistency with empirical study (e.g., Gray 2013).
Equally important is clear recognition of the appropriate domain of applicability for the LDSM approach. It is intended to capture spatial patterns at the landscape scale and temporal patterns on the order of a century or longer. This approach is therefore more amenable to strategic questions such as evaluation and consequences of alternative future scenarios at the above spatial and temporal time scales. It is not well-suited to address tactical decisions such as the specific placement of forest treatments in time and space, nor short-term projections of impacts and system response.
5.5 Opportunities for Synthesis
5.5.1 Model Integration
It is not surprising that multiple modeling paradigms have persisted over time: the data, as good as they are, have been insufficient to fully parameterize or refute any one model. Data uncertainties and model uncertainties were such that conjectures went unrefuted for long periods. Hypotheses that fell out of favor were revisited as new data, new methods, and new operational needs emerged (Figs. 5.2 and 5.5).
Consequences of the modeling paradigm for using insecticides for population control were particularly acute. For example, logical consequences of the cyclic model by Royama (1984) suggested local-scale foliage protection could be pursued without any threat of prolonging the region-wide cycle due to the role of natural enemies in promoting cyclic behavior (Royama et al. 2005), while the eruptive model by Ludwig et al. (1978) that minimized the role of natural enemies indicated foliage protection would necessarily prolong the outbreak. Similarly, Stedinger (1984) suggested that spruce budworm populations could be driven to local extinction if intense insecticide spraying occurred early enough in the growth phase of the outbreak cycle, while Royama (1984) contended that very little could be done to manage area-wide outbreaks, and that epicenter suppression would always end in futility when landscape-wide cycles proceeded to rapid synchronization via the Moran effect (Moran 1953; Royama 1984, 1997, 2005; Régnière and Lysyk 1995; Cooke et al. 2007). The most recent documentation of Allee effects (Régnière et al. 2013) and the renewed interest in dispersal as a synchronizing agent affecting the spatial scale of outbreaks (Régnière and Lysyk 1995; Sturtevant et al. 2013) has revitalized the debate about the efficacy of early intervention programs to manage outbreaks versus simply protecting foliage. Such divergent policy implications underscore the importance of clarifying model assumptions and of the role of science in reducing the most critical uncertainties. Meanwhile, recent warming of the North American boreal forest is changing the rules under which outbreaks have historically developed (Gray 2013).
As illustrated repeatedly by the spruce budworm modeling history, there is a natural tension between the inclusion of detail (model specificity) versus keeping models simple (model generality). This tension cuts across the contrasting paradigms outlined here, in that Ludwig et al. (1978) and Royama (1984) both advocated parsimonious models of minimal complexity (see Box 5.1), whereas Jones’s original (1974) site model is aptly described as a “big, ugly model” (sensu Logan 1994) with a high degree of complexity. We suggest that a middle ground is necessary to attain a qualitatively well-specified model of the feedback couplings that give rise to the major features of budworm disturbance regimes. The opportunity for a modern synthesis can be represented by a hybrid model involving two levels of reciprocal feedback, one for the herbivore and its host tree, and one for the herbivore and its parasites, each contained within the larger context of dynamic climate gradients (Fig. 5.8). While simple in concept, model behavior will be complicated by the movement of budworm and their natural enemies, by coupled nonlinear relationships among trophic levels, and by cross-scale interactions among processes occurring at different temporal rates and spatial scales . Such complexities were well-recognized by budworm modelers as far back as the 1970s (e.g., Ludwig et al. 1978). What was underappreciated by followers of either paradigm was the combination of major features of outbreak behavior necessary to minimally characterize its dynamics in time and space, i.e.:
-
Synchronizable (phase-forgetting), low-frequency oscillations that are more frequent (every 20–40 years) than the average rate of stand replacement
-
High-frequency sawtooth oscillations (every 4–7 years), associated with fluctuations in fecundity and dispersal propensity
-
Eruptive epicenters that serve as early sources for area-wide outbreaks
-
Outbreak cycles that tend to alternate between non-stand replacing and stand replacing (i.e., not all outbreaks rising to the same level of devastating impact)
-
Climatic gradients that track northward with climate warming
Multiple equilibrium models championed by Holling and colleagues (Ludwig et al. 1978; blue) included predation; however, strong reciprocal feedback centers on the budworm–forest interaction. In contrast, the harmonic oscillation model championed by Royama (1992; red) emphasized strong reciprocal feedback in the higher trophic levels, with budworm–forest interactions as a secondary consideration. A modern synthesis (black) would consider both levels jointly, including the potential for eruptive and cyclic behavior, as well as the broader-scale context of climate
Likewise, decades of empirical study of budworm impacts on forests (Sect. 5.3) suggest the following common response, in decreasing order of strength and consistency of relationships:
-
Host species composition and age determine mortality
-
Outbreak severity and duration determine the magnitude of impacts
-
Host species influences defoliation
-
Deciduous content within stands and neighborhoods reduces host defoliation
-
Stand and site characteristics and tree vigor show influence under some circumstances but are the weakest effect
Of these five drivers of budworm impacts, outbreak severity and duration are notoriously variable (Fig. 5.6) with major implications for projecting effects on forest. Accurate modeling of spruce budworm population trajectories may never be useful for precise predictions at an operational scale. Yet we expect consistent, high-resolution monitoring of forest conditions will improve the utility of the SBWDSS approach for decision-making. In addition, insights from complementary modeling (e.g., climate drivers, LSDMs) may improve the current approach (i.e., using past behavior to predict the future) by developing more plausible and informative alternative outbreak scenarios. For example, one might expect a light or moderate outbreak to follow a severe one, but what is the relative plausibility of such a scenario? Likewise, how might current climate affect the severity of the next outbreak cycle?
Integration of coupled landscape–climate–insect feedbacks—using insights and relationships from population and impact modeling approaches, respectively—within an LDSM framework can address many pressing issues facing land managers today. These include the cumulative effects of land management activities on insect disturbance frequency and intensity (Miller and Rusnock 1993), the direct influence of climate change on outbreak dynamics (Régnière et al. 2012; Gray 2013), and the interactions between these factors as they affect forest resilience to novel anthropogenic change. Among the key uncertainties are:
-
Multiscaled insect movement as mediated by heterogeneity in resources and weather patterns
-
Adaptive response of budworm to novel climates
-
Defoliation effects on advance fir regeneration and subsequent successional pathways
-
Responses of parasitoids to new habitats, landscape configuration, and climate
5.5.2 Transferability of Insights Among Defoliator Systems
Spruce budworm is arguably one of the most studied insects on the planet. Spruce budworm share many elements of system behavior with other important forest-defoliator systems (Cooke et al. 2007), and many of the lessons learned under the extensive budworm research programs may be transferable to modeling these systems. Features common among many forest defoliator systems include the near universal role of the Moran effect as a regional synchronization factor (Bjørnstad et al. 2008), prevalence of simultaneous top-down and bottom-up drivers affecting population cycling behavior (Myers and Cory 2013), and the importance of climate drivers affecting system behavior—including outbreak dynamics and range expansion (Jepsen et al. 2008; Bentz et al. 2010; Zografou et al. 2014) and the robust empirical relationships between defoliation and growth reduction and mortality (e.g., Osawa 1989; Erdle and MacLean 1999). Indeed, the generalized framework of the SBWDSS has also been applied to insects other than spruce budworm. It has been used to calibrate and predict timber supply impacts of balsam fir sawfly (Neodiprion abietis) and eastern hemlock looper (Lambdina fiscellaria) defoliation scenarios in Newfoundland, Canada (Iqbal et al. 2012). It also includes defoliation impact modeling data for jack pine budworm (C. pinus). Likewise, a generic insect disturbance approach for LDSMs used budworm as a demonstration (Sturtevant et al. 2004) and has since been applied to defoliation disturbance in China (Chen et al. 2011) and Siberia (Gustafson et al. 2010), and enhanced to simulate effects of defoliation on carbon cycling within eastern deciduous forests of North America (Foster 2011; Kretchun et al. 2014).
Berryman (1986) proposed that individual forest insect pest systems could be classified into discrete categories “eruptive,” “cyclical,” “gradient,” “pulse,” and “sustained” according to the dominant pattern of fluctuation in space and time. After five decades of research on a cross-scale system such as spruce budworm it is apparent that the system exhibits a broad array of complex dynamics, any facet of which may be observed, depending on the spatial and temporal scale of observation. There is little reason to suspect that other cross-scale systems, being regulated both from above and below and by weather and climate, should behave any more simply. For example, the forest tent caterpillar (Malacosoma disstria) system has also been approached from two divergent perspectives: the eruptive paradigm (Rose and Harmsen 1981) versus that of the harmonic oscillation (Cooke et al. 2012). Although evidence is mounting that the effect of specialist and generalist parasitism is strong enough to induce a predator–prey oscillation in this system (Roland and Taylor 1997; Roland 2005), there is also evidence of traveling waves of eruption (Cooke et al. 2009), which may be a result of the positive density dependence of mating success on population density at low population densities (Sjostrom and Roland, unpublished data), as reported for spruce budworm (Régnière et al. 2013). Indeed, it is increasingly clear that the forest tent caterpillar also responds strongly to spatial and temporal gradients in both climate conditions (Daniel and Myers 1995) and landscape structure (Roland et al. 1998; Cooke and Roland 2000, Wood et al. 2010), suggesting a remarkable parallel with spruce budworm. We suggest that the most fruitful modern avenue of research in forest–insect–climate interactions is in testing inclusive hypotheses that allow for multiple processes acting simultaneously using integrative, multiscale landscape models that embrace the possible existence of a range of dynamical behaviors.
Research and modeling of other defoliator systems can likewise inform modeling of spruce budworm . One of the major limiting factors restricting understanding of budworm cycling dynamics is its unusually long cycle length that exceeds most scientific careers. Other systems that cycle more frequently are more amenable to both statistical modeling and model validation , providing valuable insights into the processes underlying outbreak dynamics in time and space (e.g., larch bud moth (Zeiraphera diniana): Johnson et al. 2006, forest tent caterpillar: Cobbold et al. 2005; Hughes 2012; Robert 2014; European gypsy moth (Lymantria dispar): Haynes et al. 2009). Similarly, the detailed study of the invasion by the gypsy moth in North America offers unique insights into processes such as Allee effects in time and space, while multiscaled and multi-trophic level study of the forest tent caterpillar offers unique insights into scale-specific factors affecting host–parasitoid dynamics (Roland and Taylor 1997; Roth et al. 2006). Insights from these other systems suggest fruitful areas of research within the spruce budworm system.
5.6 Concluding Remarks
The spruce budworm case study is a dramatic illustration of the interplay between data and modeling, the broader challenge of simulating insect–forest interactions in time and space, and the conflicting management guidance that can emerge from such activities. Indeed, foundational concepts such as cross-scale theory (Holling 1992), adaptive cycles and ecological resilience (Gunderson and Holling 2002), spatial population dynamics (Bjørnstad et al. 1999), and aerobiology (Gage et al. 1999) can all trace pivotal roots to the collective spruce budworm research program. Such broad influence speaks to the power of modeling on the scientific enterprise and its interaction with land management and policy. While not emphasized here, there is an undeniable human dimension to the artistic and nonscientific aspects of model development that are apparent in the divergent perspectives applied to the problem (Table 5.1; Box 5.1; Fig. 5.5). The budworm case study illustrates how insights from each perspective can be complimentary, and how conflict and debate among these perspectives provided deeper insights that ultimately led to more complete understanding of the system. It also illustrates how aspatial perspectives can lead to conflict in data interpretation, and how appreciation of scale, spatial heterogeneity, and movement can help reconcile seemingly disparate schools of thought. Such lessons and insights have clear implications not only for budworm as the latest widespread outbreak to hit North America develops (i.e., 3.2 million ha of moderate-to-severe defoliation in Quebec in 2013; QMRNF 2013), but also insect disturbance modeling more generally.
References
Alfaro RI, Berg J, Axelson J (2014) Periodicity of western spruce budworm in southern British Columbia, Canada. For Ecol Manage 315:72–79
Allee WC (1931) Animal aggregations, a study in general sociology. University of Chicago Press, Chicago
Anderson DP, Sturtevant BR (2011) Pattern analysis of eastern spruce budworm Choristoneura fumiferana dispersal. Ecography 34:488–497
Addicott JF, Aho JM, Antolin MF, Padilla DK, Richardson JS, Soluk DA (1987) Ecological neighborhoods: scaling environmental patterns. Oikos 49:340–346
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. University of Chicago Press, Chicago
Archambault L, Gagnon RR, Pelletier G, Chabot M, Bélanger L (1990) Influence du drainage et de la texture du depot sur la vulnérabilité du Sapin baumier et de l’Épinette blanche aux attaques de la Tordeuse des bourgeons de l’épinette. Can J For Res 20:750–756
Baskerville GL (1975) Spruce budworm: super silviculturist. For Chron 51:138–140
Baskerville, GL (1976) Report of the task force for evaluation of budworm control alternatives. Prepared for New Brunswick Cabinet Committee on Economic Development. New Brunswick Department of Natural Resources, Fredericton, NB
Baskerville GL (1982) The spruce fir wood supply in New Brunswick. New Brunswick Department of Natural Resources, Forest Management Branch, Fredericton, NB. Report No. 0-88838-408-4
Baskerville G, Kleinschmidt S (1981) A dynamic model of growth in defoliated fir stands. Can J For Res 11:206–214
Batzer HO, Popp MP (1985) Forest succession following spruce budworm outbreak in Minnesota. For Chron 61:75–80
Bellier E, Monestiez, P Durbec JP, Candau JN (2007) Identifying spatial relationships at multiple scales: principal coordinates of neighbour matrices (PCNM) and geostatistical approaches. Ecography 30:385–399
Belle-Isle J, Kneeshaw D (2007) A stand and landscape comparison of the effects of a spruce budworm (Choristoneura fumiferana (Clem.)) outbreak to the combined effects of harvesting and thinning on forest structure. For Ecol Manage 246:163–174
Bentz BJ, Régnière J, Fettig CJ et al (2010) Climate change and bark beetles of the western United States and Canada: direct and indirect effects. Bioscience 60:602–613
Bergeron Y, Leduc A, Morin H, Joyal C (1995) Balsam fir mortality following the last spruce budworm outbreak in northwestern Quebec. Can J For Res 25:1375–1384
Berryman AA (1986) Forest insects. Plenum Press, Principles and practice of population management
Berryman AA, Stenseth NC, Wollkind DJ (1984) Metastability of forest ecosystems infested by bark beetles. Res Popul Ecol 26:13–29
Blackman MW (1919) Report on the spruce budworm. Maine Forestry Department
Blais JR (1954) The recurrence of spruce budworm infestations in the past century in the Lac Seul area of northwestern Ontario. Ecology 35:62–71
Blais JR (1961) Spruce budworm outbreaks in the lower St. Lawrence and Gaspé regions. For Chron 37:192–202
Blais JR (1964) History of spruce budworm outbreaks in the past two centuries in southeastern Quebec and northern Maine. Can For Entomol Pathol Branch Bi-mon Prog Rep 20:1–2
Blais JR (1965) Spruce budworm outbreaks in the past three centuries in the Laurentide Park, Quebec. For Sci 11:130–138
Blais JR (1968) Regional variation in susceptibility of eastern North American forests to budworm attack based on history of outbreaks. For Chron 44:17–23
Blais JR (1981) Mortality of balsam fir and white spruce following a spruce budworm outbreak in the Ottawa River watershed in Quebec. Can J For Res 11:620–629
Bjørnstad ON, Ims RA, Lambin X (1999) Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends Ecol Evol 14:427–432
Bjørnstad ON, Liebhold AM, Johnson DM (2008) Transient synchronization following invasion: revisiting Moran’s model and a case study. Popul Ecol 50:379–389
Bjørnstad ON, Peltonen M, Liebhold AM, Baltensweiler W (2002) Waves of larch budmoth outbreaks in the European Alps. Science 298:1020–1023
Bouchard M, Kneeshaw D, Bergeron Y (2006) Forest dynamics after successive spruce budworm outbreaks in mixedwood forests. Ecology 87:2319–2329
Boulanger Y, Arseneault D (2004) Spruce budworm outbreaks in eastern Quebec over the last 450 years. Can J For Res 34:1035–1043
Boulanger Y, Arseneault D, Morin H, Jardon Y, Bertrand P, Dagneau C (2012) Dendrochronological reconstruction of spruce budworm (Choristoneura fumiferana) outbreaks in southern Quebec for the last 400 years. Can J For Res 42:1264–1276
Burleigh JS, Alfaro RI, Borden JH, Taylor S (2002) Historical and spatial characteristics of spruce budworm Choristoneura fumiferana (Clem.) (Lepidoptera : Tortricidae) outbreaks in northeastern British Columbia. For Ecol Manage 168:301–309
Burns RM, Honkala BH (1990) Silvics of North America: 1. Conifers. USDA Forest Service, Agriculture Handbook 654
Campbell EM, MacLean DA, Bergeron Y (2008) The severity of budworm-caused growth reductions in balsam fir/spruce stands varies with the hardwood content of surrounding forest landscapes. For Sci 54:195–205
Canadian Council of Forest Ministers (2013) National Forestry Database Program. http://nfdp.ccfm.org. Canadian Forest Service, Ottawa, Ontario, Canada
Candau J-N, Fleming RA (2005) Landscape-scale spatial distribution of spruce budworm defoliation in relation to bioclimatic conditions. Can J For Res 35:2218–2232
Candau J-N, Fleming RA (2011) Forecasting the response of spruce budworm defoliation to climate change in Ontario. Can J For Res 41:1948–1960
Cappuccino N, Lavertu D, Bergeron Y, Régnière J (1998) Spruce budworm impact, abundance and parasitism rate in a patchy landscape. Oecologia 114:236–242
Chang W-Y, Lantz VA, Hennigar CR, MacLean DA (2012a) Economic impacts of forest pests: a case study of spruce budworm outbreaks and control in New Brunswick, Canada. Can J For Res 42:490–505
Chang W-Y, Lantz VA, Hennigar CR, MacLean DA (2012b) Benefit-cost analysis of spruce budworm (Choristoneura fumiferana Clem.) control: incorporating market and non-market values. J Env Manage 93:104–112
Chen HW, Hu YM, Chang Y, Bu RC, Li YH, Liu M (2011) Simulating impact of larch caterpillar (Dendrolimus superans) on fire regime and forest landscape in Da Hinggan Mountains, Northeast China. Chinese Geog Sci 21:575–586
Clark WC, Jones DD, Holling CS (1979) Lessons for ecological policy design: a case study of ecosystem management. Ecol Model 7:1–53
Cobbold CA, Lewis MA, Lutscher F, Roland J (2005) How parasitism affects critical patch-size in a host-parasitoid model: application to the forest tent caterpillar. Theor Popul Biol 67:109–125
Cooke BJ, MacQuarrie CJK, Lorenzetti F (2012) The dynamics of forest tent caterpillar outbreaks across east-central Canada. Ecography 35:422–435
Cooke BJ, Nealis VG, Régnière J (2007) Insect defoliators as periodic disturbances in northern forest ecosystems. In: Johnson EA, Miyanishi K (eds) Plant disturbance ecology: the process and the response. Academic Press, London, pp 487–526
Cooke BJ, Roland J (2000) Spatial analysis of large scale patterns of forest tent caterpillar outbreaks. Ecoscience 7:410–422
Cooke BJ, Lorenzetti F, Roland J (2009) On the duration and distribution of forest tent caterpillar outbreaks in eastcentral Canada. J Entomol Soc Ontario 140:3–18
Cuff W, Baskerville GL (1983) Ecological modelling and management of spruce budworm infested fir-spruce forests of New Brunswick, Canada. In: Lauenroth WK, Skogerboe GV, Flug M (eds) Analysis of ecological systems: state-of-the-art in ecological modelling: proceedings of a symposium. Developments in environmental modelling, Fort Collins, CO, vol 5, 24–28 May 1982. Elsevier Scientific Publishing Company, New York, pp 93–98
Daniel CJ, Myers JH (1995) Climate and outbreaks of the forest tent caterpillar. Ecography 18:353–362
D’Aoust V, Kneeshaw DD, Bergeron Y (2004) Characterisation of changes in canopy structure before and after a spruce budworm outbreak. Can J For Res 34:339–352
Dorais L, Kettela EG (eds) (1982) A review of entomological survey and assessment techniques used in regional spruce budworm, Choristoneura fumiferana (Clem.), surveys and in the assessment of operational spray programs. Report, Committee for Standardization of Survey and Assessment Techniques. Eastern Spruce Budworm Council, Quebec Department of Energy and Resources, Quebec
Dukes JS, Pontius J, Orwig D et al (2009) Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: what can we predict? Can J For Res 39:231–248
Dupont A, Bélanger L, Bousquet J (1991) Relationships between balsam fir vulnerability to spruce budworm and ecological site conditions of fir stands in central Québec. Can J For Res 21:1752–1759
Erdle TA (1989) Concept and practice of integrated harvest and protection design in the management of eastern spruce fir forests. PhD thesis, University of New Brunswick
Erdle TA, MacLean DA (1999) Stand growth model calibration for use in forest pest impact assessment. For Chron 75:141–152
Eveleigh ES, McCann KS, McCarthy PC et al (2007) Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc Nat Acad Sci 104:16976–16981
Fettes JJ (1950) Investigations of sampling techniques for population studies of the spruce budworm on balsam fir in Ontario. Canada Department of Agriculture, Forest Insect Laboratory. Sault Ste. Marie, ON. Annu Tech Rep 4:163–401
Fleming RA (1996) A mechanistic perspective of possible influences of climate change on defoliating insects in North America’s boreal forests. Silva Fenn 30:281–294
Fleming RA, Barclay HJ, Candau JN (2002) Scaling-up an autoregressive time-series model (of spruce budworm population dynamics) changes its qualitative behavior. Ecol Model 149:127–142
Fleming RA, Candau JN (1998) Influences of climatic change on some ecological processes of an insect outbreak system in Canada’s boreal forests and the implications for biodiversity. Environ Monitor Assess 49:235–249
Fleming RA, Lyons DB, Candau J-N (1999) Spatial transmutation and its consequences in spatially upscaling models of spruce budworm population dynamics. Can J Rem Sens 25:388–402
Fleming RA, Shoemaker CA (1992) Evaluating models for spruce budworm-forest management: Comparing output with regional field data. Ecol Appl 2:460–477
Fleming RA, Volney WJA (1995) Effects of climate change on insect defoliator population processes in Canada’s boreal forest: some plausible scenarios. Water Soil Air Poll 82:445–454
Fisher RA (1983) Two decades of budworm simulacra: a retrospective view of comprehensive simulation models. In: Lauenroth WK, Skogerboe GV, Flug M (eds) Analysis of ecological systems: state-of-the-art in ecological modelling. Developments in Environmental Modelling, vol 5. Elsevier Scientific Publishing Company, New York
Foster JR (2011) Forest insect defoliation patterns and carbon dynamics: Linking remote sensing with simulation models. Dissertation, University of Wisconsin-Madison
Gage HS, Isard AS, G-Colunga M (1999) Ecological scaling of aerobiological dispersal processes. Agr Forest Meteor 97:249–261
Greenbank DO (1963) Host species and the spruce budworm. Mem Entomol Soc Can 31:219–223
Greenbank DO, Schaefer GW, Rainey RC (1980) Spruce budworm (Lepidoptera: Tortricidae) moth flight and dispersal: new understanding from canopy observations, radar, and aircraft. Mem Entomol Soc Can 110:1–49
Gray DR (2008) The relationship between climate and outbreak characteristics of the spruce budworm in eastern Canada. Clim Change 87:361–383
Gray DR (2013) The influence of forest composition and climate on outbreak characteristics of the spruce budworm in eastern Canada. Can J For Res 43:1181–1195
Gray DR, MacKinnon WE (2006) Outbreak patterns of the spruce budworm and their impacts in Canada. For Chron 82:550–561
Gray DR, MacKinnon WE (2007) Historical spruce budworm defoliation records adjusted for insecticide protection in New Brunswick, 1965–1992. J Acad Entomol Soc 3:1–6
Gunderson LH, Holling CS (2002) Panarchy: Understanding transformations in human and natural systems. Island Press, Washington
Gustafson EJ, Shvidenko AZ, Sturtevant BR, Scheller RM (2010) Predicting global change effects on forest biomass and composition in south-central Siberia. Ecol Appl 20:700–715
Hall TH (1981) Forest management decision-making; art or science? For Chron 57:233–238
Han E-N, Bauce E (1997) Effects of early temperature exposure on diapause development of spruce budworm (Lepidoptera: Tortricidae). Environ Entomol 26:307–310
Han E-N, Bauce E (2000) Dormancy in the life cycle of the spruce budworm: physiological mechanisms and ecological implications. Rec Res Develop Entomol 3:43–64
Hardy YJ, Lafond A, Hamel L (1983) The epidemiology of the current spruce budworm outbreak in Quebec. For Sci 29:715–725
Hassell DC, Allwright DJ, Fowler AC (1999) A mathematical analysis of Jones’s site model for spruce budworm infestations. J Math Biol 38:377–421
Haynes KJ, Liebhold AM, Johnson DM (2009) Spatial analysis of harmonic oscillation of gypsy moth outbreak intensity. Oecologia 159:249–256
Hennigar CR, MacLean DA, Porter KB, Quiring DT (2007) Optimized harvest planning under alternative foliage-protection scenarios to reduce volume losses to spruce budworm. Can J For Res 37:1755–1769
Hix DM, Barnes BV, Lynch AM, Witter JA (1987) Relationships between spruce budworm damage and site factors in spruce-fir dominated ecosystems of western Upper Michigan. For Ecol Manage 21:129–140
Holling CS (1973) Resilience and stability of ecological systems. Ann Rev Ecol System 4:1–23
Holling CS (1986) The resilience of terrestrial ecosystems; local surprise and global change. In: Clark WC, Munn RE (eds) Sustainable development of the biosphere. Cambridge University Press, Cambridge
Holling CS (1992) Cross-scale morphology, geometry, and dynamics of ecosystems. Ecol Monogr 62:447–502
Hughes J (2012) Patterns and processes in forest insect population dynamics. Dissertation, University of Toronto
Iqbal J, Hennigar CR, MacLean DA (2012) Modeling insecticide protection versus forest management approaches to reducing balsam fir sawfly and hemlock looper damage. For Ecol Manage 265:150–160
James PMA, Fortin M-J, Sturtevant BR, Fall A, Kneeshaw D (2011) Modelling spatial interactions among fire, spruce budworm, and logging in the boreal forest. Ecosystems 14:60–75
Jardon Y, Morin H, Dutilleul P (2003) Periodicity and synchronism of spruce budworm outbreaks in Quebec. Can J For Res 33:1947–1961
Jepsen JU, Hagen SB, Ims RA, Yoccoz NG (2008) Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. J Anim Ecol 77:257–264
Johnson DM, Bjørnstad ON, Liebhold AM (2006) Landscape mosaic induces traveling waves of insect outbreaks. Oecologia 148:51–60
Johnson DM, Liebhold AM, Bjørnstad ON, McManus ML (2005) Circumpolar variation in periodicity and synchrony among gypsy moth populations. J Anim Ecol 74:882–892
Jones DD (1974) Biology of the budworm model. Int Inst Appl Syst Anal 12:1–42
Jones DD (1977) The budworm site model. In: Proceedings of a conference on pest management, 25–29 Oct 1976. Int Inst Appl Syst Anal CP-77-6, pp 91–155
Keane RE, Holsinger LM, Parsons RA, Gray K (2008) Climate change effects on historical range and variability of two large landscapes in western Montana, USA. For Ecol Manage 254:375–389
Kemp WP, Simmons GA (1978) The influence of stand factors on parasitism of spruce budworm eggs by Trichogramma minutum. Environ Entomol 7:685–688
Kneeshaw DD, Lauzon E, de Römer A et al (2008) Appliquer des connaissances sur les régimes de perturbations naturelles pour développer une foresterie qui s’inspire de la nature dans le sud de la péninsule Gaspésienne. In: Gauthier S et al (eds) Aménagement forestier écosystémique en forêt. University of Quebec Press, Quebec
Kneeshaw DD, Bergeron Y (1999) Spatial and temporal patterns of seedling and sapling recruitment within canopy gaps caused by spruce budworm. Écoscience 6:214–222
Kneeshaw DD, Bergeron Y (1998) Canopy gap characteristics and tree replacement in the southeastern boreal forest. Ecology 79:783–794
Kneeshaw DD, Kobe RK, Coates KD, Messier C (2006) Sapling size influences shade tolerance ranking among southern boreal tree species. J Ecol 94:471–480
Kneeshaw DD, Prévost M (2007) Natural canopy gap disturbances and their role in maintaining mixed species forests of central Québec. Can J For Res 37:1534–1544
Kretchun AM, Scheller RM, Lucash MS, Clark KL, Hom J, Van Tuyl S (2014) The effects of multiple disturbances on carbon sequestration in the forests of the New Jersey Pine Barrens under different climate scenarios. PLoS ONE 9:e102531
Lawrence RK, Mattson WJ, Haack RA (1997) White spruce and the spruce budworm: Defining the phenological window of susceptibility. Can Entomol 129:291–318
Logan JA (1994) In defense of big ugly models. Am Entomol 40:202–207
Ludwig D, Jones DD, Holling CS (1978) Qualitative analysis of insect outbreak systems: the spruce budworm and the forest. J Anim Ecol 47:315–332
Lynch AM, Witter JA (1985) Relationships between balsam fir mortality caused by the spruce budworm and stand, site, and soils variables in Michigan’s Upper Peninsula. Can J For Res 15:141–147
MacKinnon WE, MacLean DA (2003) The influence of forest and stand conditions on spruce budworm defoliation in New Brunswick, Canada. For Sci 49:657–667
MacLean DA (1980) Vulnerability of fir-spruce stands during uncontrolled spruce budworm outbreaks: a review and discussion. For Chron 56:213–221
MacLean DA (1984) Effects of spruce budworm outbreaks on the productivity and stability of balsam fir forests. For Chron 60:273–279
MacLean DA (1996) The role of a stand dynamics model in the spruce budworm decision support system. Can J For Res 26:1731–1741
MacLean DA, Lidstone RG (1982) Defoliation by spruce budworm: estimation by ocular and shoot count methods and variability among branches, trees, and stands. Can J For Res 12:582–594
MacLean DA, MacKinnon WE (1996) The accuracy of aerial sketch mapping of spruce budworm defoliation in New Brunswick. Can J For Res 26:2099–2108
MacLean DA, MacKinnon WE (1997) Effects of stand and site characteristics on susceptibility and vulnerability of balsam fir and spruce to spruce budworm in New Brunswick. Can J For Res 27:1859–1871
MacLean DA, MacKinnon WE, Porter KB, Beaton KP, Cormier G, Morehouse S (2000) Use of forest inventory and monitoring data in the spruce budworm decision support system. Comput Electron Agric 28:101–118
MacLean DA, Beaton KP, Porter KB, MacKinnon WE, Budd M (2002) Potential wood supply losses to spruce budworm in New Brunswick estimated using the Spruce Budworm Decision Support System. For Chron 78:739–750
MacLean DA, Erdle TA, Porter KB et al (2001) The Spruce Budworm Decision Support System: forest protection planning to sustain long-term wood supply. Can J For Res 31:1742–1757
Maltais J, Régnière J, Cloutier C, Hébert C, Perry DF (1989) Seasonal biology of Meteorus trachynotus Vier. (Hymenoptera: Braconidae) and of its overwintering host Choristoneura rosaceana (Harr.) (Lepidoptera: Tortricidae). Can Entomol 121:745–756
Mason RR (1996) Dynamic behavior of Douglas-fir tussock moth populations in the Pacific Northwest. For Sci 42:182–191
McLeod IM, Lucarotti CJ, Hennigar CR et al (2012) Advances in aerial application technologies and decision support for integrated pest management. In: Soloneski S Larramendy ML (eds) Integrated Pest management and pest control. InTech Open Access Publisher, Rijeka
Miller A, Rusnock P (1993) The rise and fall of the silvicultural hypothesis in spruce budworm (Choristoneura fumiferana) management in eastern Canada. For Ecol Manage 61:171–189
Moran PAP (1953) The statistical analysis of the Canadian lynx cycle II. Synchronization and meteorology. Aust J Zool 1:291–298
Morin H, Laprise D, Simard A-A, Amouch S (2009) Spruce budworm outbreak regimes in eastern North America. In: Gauthier S, Vaillancourt M-A, Leduc A, et al (eds) Ecosystem management in the boreal forest. Presses de l’Université du Québec, Québec
Morris RF, Cheshire WF, Miller CA, Mott DG (1958) The numerical response of avian and mammalian predators during a gradation of the spruce budworm. Ecology 34:487–494
Morris RF (ed) (1963a) The dynamics of epidemic spruce budworm populations. Mem Entomol Soc Can 31
Morris RF (1963b) The development of predictive equations for the spruce budworm based on key-factor analysis. In: Morris RF (ed) The dynamics of epidemic spruce budworm populations. Mem Entomol Soc Can 31
Myers JH (1998) Synchrony in outbreaks of forest Lepidoptera: a possible example of the Moran effect. Ecology 79:1111–1117
Myers JH, Cory C (2013) Population cycles in forest Lepidoptera revisited. Ann Rev Ecol Evol Syst 44:565–592
Nealis VG, Régnière J (2004a) Insect-host relationships influencing disturbance by the spruce budworm in a boreal mixedwood forest. Can J For Res 34:1870–1882
Nealis VG, Régnière J (2004b) Fecundity and recruitment of eggs during outbreaks of the spruce budworm. Can Entomol 136:591–604
Nicholson AJ, Bailey VA (1935) The balance of animal populations—Part I. Proc Zool Soc London 105:551–598
Nisbet RM, Gurney WSC (1982) Modelling fluctuating populations. Wiley, New York
Osawa A (1989) Causality in mortality patterns of spruce trees during a spruce budworm outbreak. Can J For Res 19:632–638
Ostaff DP, MacLean DA (1995) Patterns of balsam fir foliar production and growth in relation to defoliation by spruce budworm. Can J For Res 25:1128–1136
Peltonen M, Liebhold AM, Bjørnstad ON, Williams DW (2002) Spatial synchrony in forest insect outbreaks: Roles of regional stochasticity and dispersal. Ecology 83:3120–3129
Powell J, Tams J, Bentz B, Logan J (1998) Theoretical analysis of “switching” in a localized model for mountain pine beetle mass attack. J Theor Biol 194:49–63
Quayle D, Régnière J, Cappuccino N, Dupont A (2003) Forest composition, host-population density, and parasitism of spruce budworm Choristoneura fumiferana eggs by Trichogramma minutum. Entomol Exp Appl 107:215–227
QMRNF (2013) Aires infestées par la tordeuse des bourgeons de l’épinette au Québec en 2013 Quebec Min Res natur et de la faune Québec, QC
Raffa KF, Aukema BH, Bentz BJ et al (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58:501–517
Ranta E, Kaitala V, Lindström J, Helle E (1997) The Moran effect and synchrony in population dynamics. Oikos 78:136–142
Ravenscroft C, Scheller RM, Mladenoff DJ, White MA (2010) Forest restoration in a mixed-ownership landscape under climate change. Ecol Appl 20:327–346
Régnière J, You M (1991) A simulation model of spruce budworm (Lepidoptera: Totricidae) feeding on balsam fir and white spruce. Ecol Model 54:277–297
Régnière J, Delisle J, Pureswaran DS, Trudel R (2013) Mate-finding allee effect in spruce budworm population dynamics. Entomol Exp Appl 146:112–122
Régnière J, Lysyk TJ (1995) Population dynamics of the spruce budworm, Choristoneura fumiferana. In: Armstrong JA, Ives WGH (eds) Forest insects pests in Canada. Natural Resources Canada, Canadian Forest Service, Ottawa, ON
Régnière J, Nealis VG (2007) Ecological mechanisms of population change during outbreaks of the spruce budworm. Ecol Entomol 32:461–477
Régnière J, St-Amant R, Duval P (2012) Predicting insect distributions under climate change from physiological responses: spruce budworm as an example. Biol Inv 14:1571–1586
Remsoft Spatial Planning System 2010 Woodstock 2010.5 User Guide. Remsoft Inc., Fredericton
Robert LE, Kneeshaw DD, Sturtevant BR (2012) Effects of forest management legacies on spruce budworm outbreaks. Can J For Res 42:463–475
Robert LE (2014) Landscapes level influence of forest management legacies on outbreaks of defoliating insects. Dissertation, Université du Québec à Montréal
Roland J, Taylor PD (1997) Insect parasitoid species respond to forest structure at different spatial scales. Nature 386:710–713
Roland J, Mackey BG, Cooke B (1998) Effects of climate and forest structure on duration of forest tent caterpillar outbreaks across central Ontario, Canada. Can Entomol 130:703–714
Roland J (2005) Are the “seeds” of spatial variation in cyclic dynamics apparent in spatially-replicated short time-series? An example from the forest tent caterpillar. Ann Zool Fenn 42:397–407
Roth D, Roland J, Roslin T (2006) Parasitoids on the loose—experimental lack of support of the parasitoid movement hypothesis. Oikos 115:277–285
Rose MR, Harmsen R (1981) Ecological outbreak dynamics and the cusp catastrophe. Acta Biotheor 30:229–253
Royama T (1971) A comparative study of models for predation and parasitism. Res Pop Ecol 13:1–91
Royama T (1980) Effect of adult dispersal on the dynamics of local populations of an insect species: a theoretical investigation. In: Berryman AA (ed) Dispersal of forest insects: evaluation, theory, and management implications. Cooperative Extension Service, Washington State University, Pullman, WA
Royama T (1984) Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol Monogr 54:429–462
Royama T (1992) Analytical population dynamics. Chapman and Hall, London
Royama T (1997) Population dynamics of forest insects: are they governed by single or multiple factors? In: Watt AD, Stork NE, Hunter MD (eds) Forests and insects. Papers from the 18th symposium of the Royal Entomological Society in London, Sept 1995. Chapman and Hall, London
Royama T (2001) Measurement, analysis, and interpretation of mortality factors in insect survivorship studies, with reference to the spruce budworm, Choristoneura fumiferana (Clem) (Lepidoptera: Tortricidae). Pop Ecol 43:157–178
Royama T (2005) Moran effect on nonlinear population processes. Ecol Monogr 75:277–293
Royama T, MacKinnon WE, Kettela EG, Carter NE, Hartling LK (2005) Analysis of spruce budworm outbreak cycles in New Brunswick, Canada, since 1952. Ecology 86:1212–1224
Ruel J-C, Huot M (1993) Impact de la tordeuse des bourgeons de l’épinette [Choristoneura fumiferana (Clem.)] sur la régénération des sapinières après la coupe à blanc. For Chron 69:163–172
Sanders CJ (1980) A summary of current techniques used for sampling spruce budworm populations and estimating defoliation in eastern Canada. Canadian Forest Service, Great Lakes Forest Research Centre, Inf Rep O-X-306
Sanders CJ, Stark RW, Mullins EJ, Murphy J (eds) (1985) Recent advances in spruce budworms research. In: Proceedings of the CANUSA spruce budworms research symposium, Bangor, Maine, 16–20 Sept 1984. Canadian Forest Service, Ottawa, ON
Scheller RM, Mladenoff DJ (2005) A spatially interactive simulation of climate change, harvesting, wind, and tree species migration and projected changes to forest composition and biomass in northern Wisconsin, USA. Global Change Biol 11:307–321
Scheller RM, Mladenoff DJ (2007) An ecological classification of forest landscape simulation models: tools and strategies for understanding broad-scale forested ecosystems. Landscape Ecol 22:491–505
Scheller RM, Mladenoff DJ (2008) Simulated effects of climate change, fragmentation, and inter-specific competition on tree species migration in northern Wisconsin, USA. Clim Res 36:191–202
Simard I, Morin H, Lavoie C (2006) A millennial-scale reconstruction of spruce budworm abundance in Saguenay, Québec, Canada. Holocene 16:31–37
Spence CE, MacLean DA (2012) Regeneration and stand development following a spruce budworm outbreak, spruce budworm-inspired harvest, and salvage harvest. Can J For Res 42:1759–1770
Stedinger JR (1984) A spruce budworm-forest model and its implications for suppression programs. For Sci 30:597–615
Sturtevant BR, Gustafson EJ, Li W, He HS (2004) Modeling biological disturbances in LANDIS: a module description and demonstration using spruce budworm. Ecol Model 180:153–174
Sturtevant BR, Miranda BR, Shinneman DJ, Gustafson EJ, Wolter PT (2012) Comparing modern and presettlement forest dynamics of a subboreal wilderness—does spruce budworm enhance fire risk? Ecol Appl 22:1278–1296
Sturtevant BR, Achtemeier GL, Charney JJ, Cooke BJ, Townsend PA (2013) Long-distance dispersal of spruce budworm (Choristoneura fumiferana Clemens) in Minnesota (USA) and Ontario (Canada) via the atmospheric pathway. Agr Forest Meteor 168:186–200
Swaine JM, Craighead FC (1924) Studies on the spruce budworm (Cacoecia fumiferana Clem.). Part 1: a general account of the outbreaks, injury and associated insects. Canada Dept Agric Tech Bull 37:3–27
Su Q, MacLean DA, Needham TD (1996) The influence of hardwood content on balsam fir defoliation by spruce budworm. Can J For Res 26:1620–1628
Turchin P (1990) Rarity of density dependence or population regulation with lags? Nature 344:660–663
Urban DL (2005) Modeling ecological processes across scales. Ecology 86:1996–2006
Volney WJA (1985) Comparative population biologies of North American spruce budworms. In: Sanders CJ, Stark RW, Mullins EJ, Murphy J (eds) Recent advances in spruce budworms research. Proceedings of the CANUSA spruce budworms research symposium, Bangor, Maine, 16–20 Sept 1984. Agriculture Canada, Ministry of State for Forestry, Ottawa
Volney WJA, Fleming RA (2000) Climate change and impacts of boreal forest insects. Agric Ecosyst Environ 82:283–294
Watt KEF (1963) How closely does the model mimic reality? Mem Entomol Soc Can 95:109–111
Watt KEF (1964) The use of mathematics and computers to determine optimal strategy and tactics for a given outbreak pest control problem. Can Entomol 96:202–220
Webb FE, Cameron DC, and MacDonald DR (1956) Studies of aerial spraying against the spruce budworm in New Brunswick. V. Techniques for large-scale egg and defoliation ground surveys 1953–55, Canada Department of Agriculture, Forest Biology Division, Interim Report 1955–58. Forest Biology Laboratory, Fredericton, New Brunswick. p. 24
Wermelinger B (2004) Ecology and management of the spruce bark beetle Ips typographus—a review of recent research. For Ecol Manage 202:67–82
Williams DW, Liebhold AM (2000) Spatial synchrony of spruce budworm outbreaks in eastern North America. Ecology 81:2753–2766
Wood DM, Parry D, Yanai RD, Pitel NE (2010) Forest fragmentation and duration of forest tent caterpillar (Malacosoma disstria Hübner) outbreaks in northern hardwood forests. For Ecol Manage 260:1193–1197
Zhao K, MacLean DA, Hennigar CR (2014) Spatial variability of spruce budworm defoliation at different scales. For Ecol Manage 328:10–19
Zografou K, Kati V, Grill A et al (2014) Signals of climate change in butterfly communities in a mediterranean protected area. PLoS ONE 9:e87245
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sturtevant, B.R., Cooke, B.J., Kneeshaw, D.D., MacLean, D.A. (2015). Modeling Insect Disturbance Across Forested Landscapes: Insights from the Spruce Budworm. In: Perera, A., Sturtevant, B., Buse, L. (eds) Simulation Modeling of Forest Landscape Disturbances. Springer, Cham. https://doi.org/10.1007/978-3-319-19809-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-19809-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19808-8
Online ISBN: 978-3-319-19809-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)