Abstract
Caspases are the terminal proteases involved in apoptosis, as well as being involved in inflammation. The apoptotic caspases can be classified as either initiator or effector caspases based on both their position in the caspase cascade and their activation mechanism. Initiator caspases require dimerization to be activated, and cleavage of a loop called the intersubunit linker stabilizes the active enzyme. Effector caspases, on the other hand, are found as dimers in the cell and cleavage of the intersubunit linker is the key step in their activation.
The name caspase is short for cysteinyl aspartate-specific protease. As their name suggests, these enzymes hydrolyze peptide bonds after certain aspartate residues using a catalytic cysteine (with the aid of an active-site histidine residue). Caspases can be inhibited by endogenous inhibitors such as XIAP, by synthetic inhibitors which target either the active site or an allosteric site, or by post-translational modification. Further research is needed to find novel activators and inhibitors of caspases to treat diseases which involve misregulation of apoptosis.
Funding: This work was supported by a grant from the NIH (National Institutes of Health) [grant number GM065970 (to A.C.C.)].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara CaspasesCaspases (cysteinalaspartate-specific proteases) [1] are enzymes which utilize a catalytic cysteine to cleave their peptide substrates after specific aspartate residues. The first caspase was discovered in 1992 and because of its function was named interleukin-1-β converting enzyme (ICE) [2, 3] but was later renamed to caspase-1. In 1993, Ced-3 from C. elegans was found to be homologous to ICE [4] and the corresponding human protein CPP32 (later named caspase-3) was found in 1994 [5]. The official caspase nomenclature was decided on in 1996 to alleviate the confusion that went along with discovery of ten different caspases, some with multiple names [1].
1 Structure
Caspases are expressed as proenzymes (zymogens) called procaspases, which then become activated to the mature caspase form. Procaspase structure can be divided into three domains: an N-terminal prodomain, a large subunit, and a small subunit. The first step in maturation is dimerization. Then, proteolytic processing removes the prodomain and cleaves a loop called the intersubunit linker between the large and small subunits.
The secondary structure of mature caspases consists of six core β-strands in a slightly twisted sheet in each monomer, with two main helices on one face (the “front”) of the protein and three helices on the other face (the “back”) of the protein (Fig. 2.1). The first four core β-strands and helices 1–3 form the large subunit, whereas the last two core β-strands and helices 4–5 form the small subunit.
The dimer interface consists of the final β-strand from each monomer, side-by-side in an antiparallel manner. The two monomers are related through a C2 axis of symmetry such that one monomer is “upside-down” compared to the other monomer.
Five loops are important for the formation of the active site. Once the intersubunit linker is cleaved, the two halves of the cleaved linker are called L2 and L2′. Active site loops L1, L2, L3, and L4 come from one monomer, and loop L2′ comes from the other. The catalytic cysteine is part of loop L2, and the catalytic histidine is part of a loop extending from the C terminal end of β3.
2 Classification
Caspases are divided into two main categories based on their function: apoptotic caspases and inflammatory caspases. The apoptotic caspases are further divided into two categories based on time of entry into the apoptotic cascade: initiator caspases and effector caspases.
2.1 Apoptotic Caspases
2.1.1 Initiator Caspases
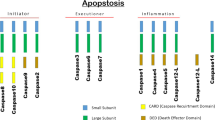
Initiator caspases are stable monomers in the cell until they are activated by dimerization. Once dimerized, initiator caspases have sufficient activity to autoprocess, cleaving their prodomain and intersubunit linker. An induced proximity model for dimerization was first invoked for caspases-8 and -10 but now seems to be generalizable to initiator caspases as a whole. This model says that activation complexes increase the local concentration of the initiator caspases, enabling them to dimerize [6]. The prodomains of initiator caspases contain either a CARD (caspase activation and recruitment domain) or DED (death effector domain), which allow initiator caspases to bind to activation complexes (Fig. 2.2).
The initiator caspases-2 and -9 are involved in the intrinsic pathway, which is activated by mitochondrial damage, cytotoxic stress, chemotherapeutic drugs or certain developmental cues [7]. Activation of caspase-2 leads to release of cytochrome c from the mitochondria, which then binds to Apaf-1 and forms the heptameric apoptosome. The apoptosome binds procaspase-9 to dimerize and therefore activate it. Once active, caspase-9 activates downstream effector caspases.
The initiator caspases-8 and -10 are activated by the extrinsic pathway: in order to eliminate excess cells created during development or remove cells with tumorigenic properties, a molecule binds to a death receptor at the membrane which is part of the tumor necrosis factor receptor (TNFR) superfamily [8, 9]. One such ligand/receptor pair is FasL (Fas ligand) and CD95(APO-1/Fas). The cytosolic death domains (DD) of the receptor recruit an adaptor molecule such as FADD (Fas-associated death domain), allowing the complex to bind initiator procaspases-8 or -10 to forma death-inducing signaling complex (DISC). Once the procaspases are part of the DISC, they are able to dimerize and therefore become active. The active caspase-8 or -10 then activates downstream effector caspases such as caspase-3.
2.1.2 Effector Caspases
The effector caspases-3, -6, and -7, are found as inactive dimers in the cell. They are activated once an initiator caspase cleaves their intersubunit linkers. Because they do not require death scaffolds for dimer formation [10, 11], their prodomains are short and lack the CARD and DED domains typical of initiator caspases. Their prodomains are, however, likely to be involved in targeting within the cell [12–15].
2.2 Inflammatory Caspases
Similarly to the initiator caspases, the inflammatory caspases-1, -4, -5, -11, -12, and -13 are activated by dimerization. Their prodomains contain a CARD which allows them to bind to activation complexes. Similarly to apoptosome formation, a multiprotein complex called the inflammasome consists of a NOD-like receptor such as NLRP1, an adaptor protein such as ASC (apoptosis-associated speck-like protein containing a CARD), and the inflammatory procaspase, particularly procaspase-1 [16]. In some cases, the procaspase can also be recruited to CARD domains in the receptor directly, without the aid of an adaptor molecule [17].
Once the inflammatory caspases become active, they are activators of cytokines through cleavage of their preforms. In monocytes and macrophages, caspase-1 activates interleukin-1β (IL-1β) [3] and interleukin-18 (IL-18). These cytokines mediate innate immunity and inflammation [18].
The mouse caspase-11 is a homolog of human caspase-4 [19]. In humans, caspase-12 is generally truncated due to a premature stop codon, but in some people of African descent, a read-through mutation causes expression of the full-length protein, causing increased risk of sepsis due to decreased inflammatory and immune response to endotoxins [20]. Caspase-13 is a bovine ortholog of human caspase-4 [21].
2.3 Other or Unclassified Caspases
Caspase-14 expression is restricted to epidermal keratinocytes and is involved in differentiation [22]. Like the effector caspases, it has a short prodomain with no adaptor regions. Several caspases are not yet classified: 15, 16, and 17 [23]. Caspase-15 is expressed in several mammalian species including pigs, dogs, and cattle [24]. It contains a pyrin-like region in its prodomain similar to that found in zebrafish caspases caspy and caspy2 [25]. Caspase-16 is found in marsupials and placental mammals and contains a short prodomain with no adaptor regions [23]. Caspase-17 is found in vertebrates except for marsupials and placental mammals and also does not contain adaptor regions in its prodomain. Caspase-18 is found in opossums and chickens and, like caspases-8 and -10, contains two DED regions in its prodomain, so it is likely also an initiator apoptotic caspase [23].
3 Mechanisms
3.1 Activation
Activation of caspases generally requires two events: they must be a dimer and the intersubunit linker must be cleaved. Removal of the prodomain is not necessary for activation; in fact, the prodomain may serve to stabilize the active enzyme [26].
After dimerization, cleavage of the intersubunit linker occurs first, followed by cleavage of the prodomain. Prior to cleavage, the intersubunit linker from one monomer occupies the dimer interface. Upon cleavage of the intersubunit linker, the C-terminal portion of the linker, L2′, vacates the central cavity and rotates about 180 degrees toward the active site, forming contacts with L2, L3, and L4 from the opposite monomer. These loop bundle contacts stabilize the active site. The movement of L2′ out of the dimer interface allows L3 to slide in towards the interface and form the substrate binding pocket. Rotation of a key arginine on L2 from a solvent-exposed position into the interface allows its neighboring residue, the catalytic cysteine, to assume its proper position for catalysis.
For effector caspases, equilibrium favors the inactive dimer. For initiator caspases, however, dimerization is the main challenge to be overcome for activation. Addition of kosmotropes such as sodium citrate causes caspase-8 to dimerize and become activated [27]. At least partly because the initiator caspases have longer intersubunit linkers than effector caspases, cleavage of the intersubunit linker is not necessary for activation, but rather, stabilizes the active conformation.
Effector caspase mutants, particularly procaspase-3 V266E, can also be activated without cleavage of the intersubunit linker [28]. This mutant is even more effective at inducing apoptosis than the wild-type (WT) enzyme [29]. The enhancement of activation caused by the mutation is predicted to occur because the mutation keeps the intersubunit linker from binding to the dimer interface. In general, when the intersubunit linker is in the dimer interface, the protein is inactive, whereas when it is out of the interface it can become active.
The conformational ensemble of effector procaspases includes both active and inactive conformers. Although the inactive ensemble is favored, binding of allosteric activators could shift the equilibrium to the active ensemble. On the other hand, binding of allosteric inhibitors to the active caspase could inactivate it. Manipulating the position of the intersubunit linker could lead to allosteric activation or inhibition. A drug which binds at the dimer interface and holds the intersubunit linker in place could inactivate the enzyme. Conversely, a drug which binds at the dimer interface and keeps the intersubunit linker from binding could activate the procaspase. In fact, a small molecule has been suggested to activate procaspase-3 by this mechanism [30].
Additionally, Wells and coworkers have found a small molecule termed 1541 which forms nanofibrils that act as a scaffold for (pro)caspase-3 binding and increase activation of the procaspase [31]. They suggest that the procaspase is activated through induced proximity, similar to the activation of initiator caspases. In vitro, amyloid-β (residues 1–40) fibrils were also shown to activate procaspase-3. The activation of caspases by fibrils may play a role in neurodegenerative diseases [32].
3.2 Catalysis
Proteases all have some mechanistic features in common. The trigonal planar peptide bond of the substrate is forced into a tetrahedral intermediate [33]. As this tetrahedral intermediate forms, a nucleophile attacks the carbonyl carbon of the peptide bond. Then, the amino nitrogen of the leaving group is protonated.
Caspases contain a catalytic dyad consisting of a cysteine and a histidine [33]. Based on the catalytic mechanism accepted for cysteine proteases, the mechanism for caspases has been thought to be as follows (See Fig. 2.3a): First, the catalytic histidine abstracts a proton from the catalytic cysteine. The catalytic cysteine acts as the nucleophile to form a covalent tetrahedral intermediate with the peptide substrate. Once the cysteine has bound, the histidine donates the proton to the amino moiety of the peptide leaving group. The peptide bond is cleaved, with the N-terminal part of the peptide remaining covalently attached to the cysteine while the C-terminal part of the peptide leaves. Finally, hydrolysis frees the N-terminal part of the peptide and re-protonates the catalytic histidine.
Two proposed mechanisms of caspase catalysis (Adapted from Miscione et al. [34])
An oxyanion hole, a pocket in the enzyme that hydrogen bonds to the carbonyl oxygen of the substrate, is also thought to be key for catalysis [33]. It is formed by the backbone nitrogens of a conserved glycine (238 in caspase-1) and the catalytic cysteine (285 in caspase-1). The oxyanion hole is thought to be important for polarizing and stabilizing the scissile carbonyl group [34].
However, there are some problems with the proposed mechanism. The 6–7 Å distance between the two catalytic residues is larger than found in most proteases, and makes direct hydrogen transfer unlikely [33]. Molecular dynamics simulations have shown that the catalytic residues cannot exist as a charged pair prior to catalysis [35]. Therefore, the deprotonation of the cysteine likely occurs during catalysis. Also, the histidine residue is not in an optimal location for protonating the amino leaving group [36].
A DFT study of the first part of the catalytic process (Fig. 2.3b, part 1) has been carried out for caspase-7 [34]. Miscione and coworkers found that first, a proton is transferred from the backbone nitrogen of the P1 aspartate to the carboxylate group of the P1 aspartate. In the second step, a proton is transferred from the aspartate to a water molecule, and from that water to the catalytic histidine. In the third step, a proton is transferred from the catalytic cysteine to the backbone nitrogen of the P1 aspartate. The overall result of these first three steps is the protonation of the catalytic histidine and the deprotonation of the catalytic cysteine. In a fourth step, the catalytic cysteine nucleophile attacks the carbonyl carbon of the substrate to form a tetrahedral intermediate, the peptide bond is cleaved, and a proton is transferred from the catalytic histidine to a second water, which transfers a proton to the amino nitrogen of the leaving group.
A QM/MM simulation focused on the hydrolysis of the covalent adduct (Fig. 2.3b, part 2) [37]. In the reaction scheme proposed by Sulpizi and coworkers, the catalytic histidine deprotonates a water molecule, which attacks the scissile carbonyl carbon (as in the original proposed mechanism). Then the proton from the catalytic histidine is abstracted by the now negatively-charged carbonyl oxygen, such that a diol is formed. A second water molecule interacts with the catalytic histidine and one of the diolhydroxy groups. Finally, a proton is transferred from that diol hydroxyl group to the P1 aspartate residue, causing cleavage of the covalent adduct. If this is true, it could more cogently explain the specificity for a P1 aspartate residue.
4 Functions
4.1 Apoptosis
The activation of caspases commits the cell to apoptosis. The main hallmarks of apoptosis include rounding of cells and retraction from neighbors, membrane blebbing to form vesicles called apoptotic bodies, nuclear fragmentation, chromatin condensation, hydrolysis of genomic DNA to approximately 200 bp fragments, and translocation of phosphatidylserine (PS) to the external surface of cells as an “eat me” signal to phagocytes. The apoptotic caspases are necessary for conferring all of these phenotypes.
In addition to the systematic dismantling of the cell, caspases are also involved in producing “find-me” signals to cause chemotaxis of phagocytes to apoptotic cells [38–40]. The recruitment of phagocytes keeps cells from releasing their contents into extracellular space and activating an immune response which could be harmful to the tissue.
When the number of apoptotic cells is too great for consumption by phagocytes, secondary necrosis can occur. When this happens, the cell releases its contents into extracellular space. However, immune cells are somehow able to recognize the cells undergoing apoptosis (and secondary necrosis) differently from necrotic cells. This is likely due to the actions of caspases. Caspases keep danger-associated molecular patterns (DAMPs) and alarmins from being activated [41]. This can be thought of as a “tolerate me” signal.
Caspases are also involved in turning off transcription and translation [42]. This keeps any infecting viral particles from replicating using the host’s machinery. They also fragment the Golgi, ER, and mitochondria [43, 44].
4.2 Inflammatory Response
In contrast to the actions of apoptotic caspases, which systematically dismantle the cell to avoid an immune response, the actions of inflammatory caspases lead to cell lysis and activation of the immune response in a process called pyroptosis [45]. In order to activate an immune response, caspases cleave cytokine IL-1β and IL-18 to produce the mature form [46].
In addition to activation of cytokines, procaspase-1 is also able to activate the pro-inflammatory transcription factor NF-κB [47]. Rather than using its catalytic activity, the CARD domain of procaspase-1 binds to a CARD domain in the kinase RIP2, which is involved in NF-κB activation.
4.3 Other Functions
Caspase expression is kept below a certain threshold required for apoptosis by IAPs (inhibitor of apoptosis proteins). At these subthresholdlevels they are able to play roles that are neither apoptotic nor inflammatory. Caspase-3 activity was found to be important for differentiation of erythroblasts, [48] skeletal muscle, [49] bone marrow stromal stem cells, [50] and neural stem cells [51].
Caspase-3 has several other non-apoptotic functions in nerve cells. In addition to neural cell differentiation, caspase-3 has also been implicated in neuronal migration and plasticity, [52] axon pruning, and synapse elimination [53].
Caspases have been shown to play a role in cell migration and invasion under certain circumstances [54]. They can also induce neighboring cells to proliferate to replace dying cells in a process called apoptosis-induced proliferation [55]. These roles for caspases have implications for cancer: moderate activation of caspases could, in fact, cause cancer to progress rather than regress [54, 55].
In addition to its apoptotic function, caspase-8 has an anti-apoptotic function when it forms a heterodimer with FLIPL (a protein similar to caspase-8 but lacking a catalytic site) [56]. This protein complex is able to activate the NF-κB signaling pathway leading to proliferation [57]. In another pro-survival capacity, the caspase-8/FLIPL complex is also able to inhibit RIPK3-dependent necrosis [56].
5 Substrates and Inhibitors
5.1 Synthetic Substrates and Substrate Specificity
Caspase substrate specificity is determined by a series of 4–5 substrate residues which bind to the active site of the caspase. These residues are named P1-P4 or P5, with P1 always being an aspartate residue (Fig. 2.4). The P4 residue is especially important in determining specificity for a given caspase [58].
Caspase substrate binding site (Adapted from Stennicke and Salvesen [33])
Because of this 4–5 residue contribution to specificity, substrates used for measuring caspase activity typically have a tetrapeptide preceded by an acetyl group (Ac) on the N terminus and followed by a fluorophore on the C terminus: for example, Ac-DEVD-AFC. When the peptide is cleaved by the caspase, the fluorophore is released and activity can be determined by fluorescence. Some typical fluorophores include AMC (7-amino-4-methylcoumarin) and AFC (7-amino-4-trifluoromethylcoumarin). Addition of p-nitroanilide (pNA) instead of a fluorophore to the C-terminus allows caspase activity to be determined colorimetrically.
A positional scanning combinatorial library approach has been used with these synthetic substrates to determine the substrate specificity for most of the mammalian caspases [58, 59]. Caspases-3 and -7 share the same substrate specificity: DEVD. The optimal sequence for caspase-1 is WEHD, and the optimal sequence for both caspase-4 and caspase-5 is (W/L) EHD. The optimal sequence for caspase-2 is DEHD, for caspase-6 is VEHD, for caspase-9 is LEHD, for caspase-8 is LETD, and for caspase-10 is LE(Nle)D (Nle = norleucine).
The P1-P4 residues fit into the S1-S4 pockets in the active site of the caspase. The S1 pocket, consisting of R179, R341, and Q283 (caspase-1 numbering), is nearly 100 % conserved; its basicity and its size make it ideally suited for binding an aspartate residue [60].
The S2 pocket of caspases-3 and -7 is formed by aromatic residues and accommodates small aliphatic amino acids [61]. A substitution of a valine or alanine in place of a tyrosine opens up the S2 subsite to larger residues in the initiator and inflammatory caspases.
The S3 pocket consists of main-chain interactions with R341 (caspase-1 numbering) [61]. In caspases-8, and -9, nearby basic residues enhance the binding of glutamic acid residues to the S3 subsite [27, 62, 63].
The S4 subsite of inflammatory caspases is long, shallow, and hydrophobic, accommodating bulky aromatic side chains such as a tryptophan [59]. On the other hand, in apoptotic caspases, a tryptophan (214 in caspase-3) reduces the size of the subsite, causing a preference for an aspartate or a small aliphatic residue in the S4 pocket [60]. An asparagine in caspases-2 and -3 or a glutamine in caspase-7 enhances interaction with a P4 aspartate [60].
Caspase-2 requires a P5 residue to occupy a S5 subsite [60]. The reason for this specificity may be that binding of a small hydrophobic residue to this subsite may enhance the burial of a P4 aspartate [64].
5.1.1 Endogenous Substrates
To date more than 700 substrates of caspases have been catalogued [65]. A searchable database can be found at http://bioinf.gen.tcd.ie/casbah/. Caspase substrates are involved in conferring an apoptotic phenotype to cells. They are also involved in producing “find-me” and “tolerate-me” signals during apoptosis.
5.1.1.1 Substrates Involved in the Apoptotic Phenotype
The following are some of the substrates of caspases which are involved in producing the apoptotic phenotype. The rounding of cells is likely in part due to caspase cleavage of components of actin microfilaments and microtubular proteins [42]. Retraction of cells from their neighbors likely facilitates phagocytosis and is caused in part by caspase cleavage of components of focal adhesion sites, components of cell-cell adherens junctions, cadherins, and desmosome-associated proteins [42]. Caspase cleavage of Rho effector ROCK1 which regulates movement of the actin cytoskeleton is a factor in blebbing and nuclear fragmentation [42]. Nuclear fragmentation also involves caspase cleavage of lamins A, B, and C [66]. Chromatin condensation is caused by caspase cleavage of Mst1 kinase [67]. Hydrolyisis of genomic DNA to small fragments is carried out by caspase-activated DNAse (CAD/DFF) [68]. Translocation of PS to the external surface of the cell is also caspase-dependent, but not fully understood [69].
5.1.1.2 Substrates Involved in Other Aspects of Apoptosis
Caspases are also involved in producing “find-me” signals to cause chemotaxis of phagocytes to apoptotic cells. Caspase-3 cleaves calcium-independent phospholipase A2, causing phosphatidylcholine in the membrane to become hydrolyzed to produce lysophatidylcholine (LPC) [38]. The C-terminal fragment of endothelial monocyte-activating polypeptide II (EMAPII) is produced by caspase-dependent proteolysis and acts as a “find-me” signal to attract monocytes [39]. Caspase-dependent cleavage of the membrane channel pannexin-1 causes release of modest amounts of ATP, which may also act as a “find-me” signal [40].
Caspases also function to keep danger-associated molecular patterns (DAMPs) and alarmins from being activated. This function can be thought of as a “tolerate-me” signal, and is important for avoiding autoimmunity [41]. As mentioned above, caspase activation leads to hydrolysis of genomic DNA (which acts as a DAMP) into short fragments [68]. Additionally, the alarmin IL-33 is inactivated by caspase-3/-7-dependent proteolysis [70].
5.1.2 Synthetic Inhibitors
5.1.2.1 Active Site Inhibitors
Active-site inhibitors bind in the place of substrate and are therefore competitive inhibitors. These inhibitors can be peptidic or nonpeptidic and can bind reversibly or irreversibly.
Peptidic inhibitors can have as few as one amino acid (for example, Boc-Asp-FMK), but typically have four (for example, Ac-DEVD-FMK) [71]. Peptides linked to leaving groups such as halomethylketones [for example, chloromethylketone (CMK) and fluoromethylketone (FMK)], acylomethylketones, and (phosphinyloxy) methyl ketones bind irreversibly, whereas peptides linked to non-leaving groups such as aldehyde (CHO) bind reversibly. The electrophilic carbonyl of the aldehyde or ketone binds to the catalytic cysteine, inhibiting it.
Several different peptidomimetics have been designed as inhibitors for caspases. These include urazolopyrazine-based β-strand peptidomimeticsdesigned as inhibitors for caspase-3 and caspase-8, [72] hydantoin-based peptidomimetics as inhibitors of caspase-3 [73], dipeptidylaspartylfluormethylketones with unnatural amino acids [74], 1-(2-acylhydrazinocarbonyl)-cycloalkyl carboxamides, [75] 8,5-fused bicyclic compounds, [76] and peptidomimetics containing a caprolactam ring [77].
Non-peptide inhibitors have also been discovered. These include isatins, [78, 79] indole aspartyl ketones, fuchsone derivatives, and pyrrolo[3,4-c]quinolone-1,3-diones [80].
5.1.2.2 Allosteric Inhibitors
Caspases-3 and -7 were found to contain an allosteric site at the dimer interface [81]. The drugs FICA and DICA form disulfide bonds with cysteines in the dimer interface of those caspases and inactivate the protein. The structural changes brought about by binding of these drugs involves massive loop rearrangements to a structure very similar to that of the proenzyme.
Mutation of valine 266 to a histidine at the dimer interface of caspase-3 also caused allosteric inactivation of the protein [28]; however, the structural changes brought about by the mutation were much more subtle than those that occurred upon binding of FICA or DICA [82]. Instead of conversion to a structure like that of the proenzyme, inactivation may be caused by a series of steric clashes, disordering of loop L1, and/or destabilization of helix 3.
A drug called compound 34 was found to bind to cysteines near the dimer interface of caspase-1 [83]. Similarly to the binding of FICA and DICA, the inactive structure was like that of the proenzyme.
Another set of allosteric inhibitors was found to inhibit caspases-3, -7, -8, and -9 [84]. A crystal structure with caspase-7 indicates that one and likely all of these compounds binds to the dimer interface. One of the compounds, Comp-A, inhibits dimerization of caspase-8; however, caspase-7 remained a dimer upon binding of the drug. As with FICA and DICA inhibition, the inhibited form was similar to that of the zymogen. However, these new compounds are reversible inhibitors, unlike FICA and DICA.
One of the urazolering peptomimetic inhibitors which bind at the active site was also found to bind near the dimer interface of caspase-8 [72]. Some of the interacting residues of caspase-8 are Tyr334, Thr337, Glu396, and Phe399.
Caspase-2 was allosterically inhibited through binding of a designed ankyrin repeat protein (DARPin) [85]. Binding causes the caspase to be fixed in an inactive conformation different from that of the proenzyme.
A novel allosteric site was found on caspase-6 [86]. Phage display produced a peptide pep419 which binds near helix 2 and causes tetramerization and therefore inactivation of caspase-6. Interestingly, it was found that at pH 8, the zymogen of caspase-6 is a tetramer in solution, whereas at pH 5.5, the zymogen is a dimer, but can be induced to form a tetramer through the binding of pep419 or a related peptide pep420. The pH changes in the cell brought about by apoptosis could potentially lead to dissociation of caspase-6 tetramers to the dimeric form, leading to activation of the protein.
5.1.3 Endogenous Inhibitors
Both viral and endogenous inhibitors can block caspase activity by competing for binding to activation complexes. Viral inhibitors target caspase activity of their host cells in order to counter an immune response. Several γ-herpesviruses and molluscipoxvirus use v-FLIPs to block caspase access to the DISC. Similarly, endogenous FLIPs blocks procaspase-8 recruitment to DISC, [87] and ICEBERG blocks caspase-1 recruitment to form the inflammasome [88].
Most protease inhibitors bind to the protease and block substrate access [60]. Suicide inhibitors are cleaved and cause a conformational change to occur in either the inhibitor or the protease. Although it is typically a serine protease inhibitor, the serpinCrmA is also able to inhibit caspases-1, [89] -8, [90] and -9, [91] likely by forming a covalent attachment with the caspase and undergoing a conformational change upon cleavage of the scissile P1–P1′ bond to place the caspase on the “bottom” of the inhibitor [92]. Similarly, the baculovirus protein p35 becomes covalently attached to the catalytic cysteine, the scissile bond is cleaved, but the protein is not liberated because it blocks the hydrolytic water from gaining access to the active site [93, 94].
Anothercategory of inhibitors are IAPs (inhibitor of apoptosis proteins). They were first discovered using baculovirus lacking a functional p35 gene [95]. They contain a 70–80 residue Zn2+ binding module named BIR. The most well-studied is X-linked inhibitor of apoptosis protein (XIAP) [96].
XIAP targets caspases in two different ways. A linker to the BIR1 domain and The BIR2 domain of XIAP target effector caspases-3 and -7 [97]. Residues in the active site, particularly in loop L1 make critical contacts with the inhibitor. Loop L2′ also makes contacts with XIAP. The necessity of ordered active site loops and cleaved intersubunit linker to form L2′ mean that XIAP only binds the active caspase rather than the inactive procaspase.
Unlike XIAP binding to the effector caspases, BIR3 and RING of XIAP target initiator caspase-9 [97, 98]. Also, instead of binding to the active site, it binds to the dimer interface of the monomer and blocks dimer formation. Loop L2′ of caspase-9 binds BIR3 in a similar manner to how loop L2′ of caspase-3 binds BIR2. The pocket where loop L2′ of caspase-9 binds BIR3 is called the Smac (second mitochondrial activator of caspases) pocket because Smac can also bind there to derepress caspase activation.
Caspase activity is also controlled endogenously through the use of post-translational modifications. The RING domain of XIAP acts as an E3 ubiquitin ligase toubiquitylate effector caspases-3 and -7, leading to proteasomal degradation [99, 100]. Sumoylation of procaspase-2 [101] and caspase-8 [102] likely leads to localization of the protein in the nucleus.
Phosphorylation is a third post-translational modification which affects caspase activity. p38-MAPK phosphorylates S150 of caspase-3, inhibiting it [103]. Phosphorylation by PKC-δ at an as yet unknown site, on the other hand, enhances caspase-3 activity [104]. PAK2 phosphorylates caspase-7 at three sites, decreasing its activity [105]. For caspase-9, ERK phosphorylates T125, [106] c-Abl phosphorylates Y153, [107] and Akt phosphorylates S196, [108] leading to decreased activity of the protein.
Abbreviations
- ASC:
-
Apoptosis-associated speck-like protein containing a CARD
- CARD:
-
Caspase activation and recruitment domain
- Caspase:
-
Cysteinal aspartate-specific protease
- DAMPs:
-
Danger-associated molecular patterns
- DD:
-
Death domain
- DISC:
-
Death inducing signaling complex
- FADD:
-
Fas-associated death domain
- FasL:
-
Fas ligand
- FLICE:
-
FADD-like interleukin 1β-converting enzyme
- FLIP:
-
FLICE-like inhibitory protein
- FLIPL :
-
Long splice variant of FLIP which forms a heterodimer with caspase-8
- FLIPS :
-
Short splice variant of FLIP which blocks caspase-8 from binding death receptor
- ICE:
-
Interleukin 1β-converting enzyme
- IL-1β:
-
Interleukin 1β
- IL-18:
-
Interleukin 18
- PS:
-
Phosphatidylserine
- Smac:
-
Second mitochondrial activator of caspases
- TNFR:
-
Tumor necrosis factor receptor
- XIAP:
-
X-linked inhibitor of apoptosis protein
References
Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J (1996) Human ICE/CED-3 protease nomenclature. Cell 87:171
Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA (1992) Molecular cloning of the interleukin-1 beta converting enzyme. Science 256:97–100
Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768–774
Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J (1993) Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75:653–660
Fernandes-Alnemri T, Litwack G, Alnemri ES (1994) CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem 269:30761–30764
Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM (1998) An induced proximity model for caspase-8 activation. J Biol Chem 273:2926–2930
Lowe SW, Lin AW (2000) Apoptosis in cancer. Carcinogenesis 21:485–495
Ashkenazi A, Dixit VM (1998) Death receptors: signaling and modulation. Science 281:1305–1308
Smith CA, Farrah T, Goodwin RG (1994) The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 76:959–962
Milam SL, Clark AC (2009) Folding and assembly kinetics of procaspase-3. Protein Sci 18:2500–2517
Pop C, Chen YR, Smith B, Bose K, Bobay B, Tripathy A, Franzen S, Clark AC (2001) Removal of the pro-domain does not affect the conformation of the procaspase-3 dimer. Biochemistry 40:14224–14235
Mao PL, Jiang Y, Wee BY, Porter AG (1998) Activation of caspase-1 in the nucleus requires nuclear translocation of pro-caspase-1 mediated by its prodomain. J Biol Chem 273:23621–23624
Baliga BC, Colussi PA, Read SH, Dias MM, Jans DA, Kumar S (2003) Role of prodomain in importin-mediated nuclear localization and activation of caspase-2. J Biol Chem 278:4899–4905
Colussi PA, Harvey NL, Kumar S (1998) Prodomain-dependent nuclear localization of the caspase-2 (Nedd2) precursor. A novel function for a caspase prodomain. J Biol Chem 273:24535–24542
Yaoita Y (2002) Inhibition of nuclear transport of caspase-7 by its prodomain. Biochem Biophys Res Commun 291:79–84
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426
Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC (2007) Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 25:713–724
Newton K, Dixit VM (2012) Signaling in innate immunity and inflammation. Cold Spring Harbor Perspect Biol 4:a006049, 006019 pp
Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J (1998) Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92:501–509
Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, Steinberg MH, Nolan V, Baldwin CT, Hotchkiss RS, Buchman TG, Zehnbauer BA, Hayden MR, Farrer LA, Roy S, Nicholson DW (2004) Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429:75–79
Koenig U, Eckhart L, Tschachler E (2001) Evidence that caspase-13 is not a human but a bovine gene. Biochem Biophys Res Commun 285:1150–1154
Rendl M, Ban J, Mrass P, Mayer C, Lengauer B, Eckhart L, Declerq W, Tschachler E (2002) Caspase-14 expression by epidermal keratinocytes is regulated by retinoids in a differentiation-associated manner. J Invest Dermatol 119:1150–1155
Eckhart L, Ballaun C, Hermann M, VandeBerg JL, Sipos W, Uthman A, Fischer H, Tschachler E (2008) Identification of novel mammalian caspases reveals an important role of gene loss in shaping the human caspase repertoire. Mol Biol Evol 25:831–841
Eckhart L, Ballaun C, Uthman A, Kittel C, Stichenwirth M, Buchberger M, Fischer H, Sipos W, Tschachler E (2005) Identification and characterization of a novel mammalian caspase with proapoptotic activity. J Biol Chem 280:35077–35080
Masumoto J, Zhou W, Chen FF, Su F, Kuwada JY, Hidaka E, Katsuyama T, Sagara J, Taniguchi S, Ngo-Hazelett P, Postlethwait JH, Núñez G, Inohara N (2003) Caspy, a zebrafish caspase, activated by ASC oligomerization is required for pharyngeal arch development. J Biol Chem 278:4268–4276
Feeney B, Clark AC (2005) Reassembly of active caspase-3 is facilitated by the propeptide. J Biol Chem 280:39772–39785
Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS (2003) A unified model for apical caspase activation. Mol Cell 11:529–541
Pop C, Feeney B, Tripathy A, Clark AC (2003) Mutations in the procaspase-3 dimer interface affect the activity of the zymogen. Biochemistry 42:12311–12320
Walters J, Pop C, Scott FL, Drag M, Swartz P, Mattos C, Salvesen GS, Clark AC (2009) A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem J 424:335–345
Schipper JL, MacKenzie SH, Sharma A, Clark AC (2011) A bifunctional allosteric site in the dimer interface of procaspase-3. Biophys Chem 159:100–109
Zorn JA, Wolan DW, Agard NJ, Wells JA (2012) Fibrils colocalize caspase-3 with procaspase-3 to foster maturation. J Biol Chem 287:33781–33795
Wellington CL, Hayden MR (2000) Caspases and neurodegeneration: on the cutting edge of new therapeutic approaches. Clin Genet 57:1–10
Stennicke HR, Salvesen GS (1999) Catalytic properties of the caspases. Cell Death Differ 6:1054–1059
Miscione GP, Calvaresi M, Bottoni A (2010) Computational evidence for the catalytic mechanism of caspase-7. A DFT investigation. J Phys Chem B 114:4637–4645
Sulpizi M, Rothlisberger U, Carloni P (2003) Molecular dynamics studies of caspase-3. Biophys J 84:2207–2215
Brady KD, Giegel DA, Grinnell C, Lunney E, Talanian RV, Wong W, Walker N (1999) A catalytic mechanism for caspase-1 and for bimodal inhibition of caspase-1 by activated aspartic ketones. Bioorg Med Chem 7:621–631
Sulpizi M, Laio A, VandeVondele J, Cattaneo A, Rothlisberger U, Carloni P (2003) Reaction mechanism of caspases: insights from QM/MM Car-Parrinello simulations. Proteins 52:212–224
Lauber K, Bohn E, Kröber SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S (2003) Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113:717–730
Knies UE, Behrensdorf HA, Mitchell CA, Deutsch U, Risau W, Drexler HC, Clauss M (1998) Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc Natl Acad Sci U S A 95:12322–12327
Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467:863–867
Martin SJ, Henry CM, Cullen SP (2012) A perspective on mammalian caspases as positive and negative regulators of inflammation. Mol Cell 46:387–397
Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9:231–241
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1:515–525
Lane JD, Lucocq J, Pryde J, Barr FA, Woodman PG, Allan VJ, Lowe M (2002) Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J Cell Biol 156:495–509
Lamkanfi M (2011) Emerging inflammasome effector mechanisms. Nat Rev Immunol 11:213–220
Miao EA, Rajan JV, Aderem A (2011) Caspase-1-induced pyroptotic cell death. Immunol Rev 243:206–214
Lamkanfi M, Kalai M, Saelens X, Declercq W, Vandenabeele P (2004) Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J Biol Chem 279:24785–24793
Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O (2001) Caspase activation is required for terminal erythroid differentiation. J Exp Med 193:247–254
Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA (2002) Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A 99:11025–11030
Miura M, Chen XD, Allen MR, Bi Y, Gronthos S, Seo BM, Lakhani S, Flavell RA, Feng XH, Robey PG, Young M, Shi S (2004) A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest 114:1704–1713
Fernando P, Brunette S, Megeney LA (2005) Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J 19:1671–1673
Gulyaeva NV (2003) Non-apoptotic functions of caspase-3 in nervous tissue. Biochemistry (Mosc) 68:1171–1180
Hyman BT, Yuan J (2012) Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci 13:395–406
Rudrapatna VA, Bangi E, Cagan RL (2013) Caspase signalling in the absence of apoptosis drives Jnk-dependent invasion. EMBO Rep 14:172–177
Ryoo HD, Bergmann A (2012) The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol 4:a008797
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471:363–367
Kataoka T, Tschopp J (2004) N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol 24:2627–2636
Thornberry NA, Chapman KT, Nicholson DW (2000) Determination of caspase specificities using a peptide combinatorial library. Methods Enzymol 322:100–110
Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW (1997) A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 272:17907–17911
Fuentes-Prior P, Salvesen GS (2004) The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J 384:201–232
Chéreau D, Kodandapani L, Tomaselli KJ, Spada AP, Wu JC (2003) Structural and functional analysis of caspase active sites. Biochemistry 42:4151–4160
Blanchard H, Kodandapani L, Mittl PR, Marco SD, Krebs JF, Wu JC, Tomaselli KJ, Grütter MG (1999) The three-dimensional structure of caspase-8: an initiator enzyme in apoptosis. Structure 7:1125–1133
Renatus M, Stennicke HR, Scott FL, Liddington RC, Salvesen GS (2001) Dimer formation drives the activation of the cell death protease caspase 9. Proc Natl Acad Sci U S A 98:14250–14255
Schweizer A, Briand C, Grutter MG (2003) Crystal structure of caspase-2, apical initiator of the intrinsic apoptotic pathway. J Biol Chem 278:42441–42447
Lüthi AU, Martin SJ (2007) The CASBAH: a searchable database of caspase substrates. Cell Death Differ 14:641–650
Rao L, Perez D, White E (1996) Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol 135:1441–1455
Ura S, Masuyama N, Graves JD, Gotoh Y (2001) Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc Natl Acad Sci U S A 98:10148–10153
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50
Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182:1545–1556
Lüthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ (2009) Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31:84–98
Callus BA, Vaux DL (2007) Caspase inhibitors: viral, cellular and chemical. Cell Death Differ 14:73–78
Wang Z, Watt W, Brooks NA, Harris MS, Urban J, Boatman D, McMillan M, Kahn M, Heinrikson RL, Finzel BC, Wittwer AJ, Blinn J, Kamtekar S, Tomasselli AG (2010) Kinetic and structural characterization of caspase-3 and caspase-8 inhibition by a novel class of irreversible inhibitors. Biochim Biophys Acta 1804:1817–1831
Vázquez J, García-Jareño A, Mondragón L, Rubio-Martinez J, Pérez-Payá E, Albericio F (2008) Conformationally restricted hydantoin-based peptidomimetics as inhibitors of caspase-3 with basic groups allowed at the S3 enzyme subsite. ChemMedChem 3:979–985
Wang Y, Jia S, Tseng B, Drewe J, Cai SX (2007) Dipeptidyl aspartyl fluoromethylketones as potent caspase inhibitors: peptidomimetic replacement of the P(2) amino acid by 2-aminoaryl acids and other non-natural amino acids. Bioorg Med Chem Lett 17:6178–6182
Soper DL, Sheville J, O’Neil SV, Wang Y, Laufersweiler MC, Oppong KA, Wos JA, Ellis CD, Fancher AN, Lu W, Suchanek MK, Wang RL, De B, Demuth TP (2006) Synthesis and evaluation of novel 1-(2-acylhydrazinocarbonyl)-cycloalkyl carboxamides as interleukin-1beta converting enzyme (ICE) inhibitors. Bioorg Med Chem Lett 16:4233–4236
Soper DL, Sheville JX, O’Neil SV, Wang Y, Laufersweiler MC, Oppong KA, Wos JA, Ellis CD, Baize MW, Chen JJ, Fancher AN, Lu W, Suchanek MK, Wang RL, Schwecke WP, Cruze CA, Buchalova M, Belkin M, Wireko F, Ritter A, De B, Wang D, Demuth TP (2006) Synthesis and evaluation of novel 8,5-fused bicyclic peptidomimetic compounds as interleukin-1beta converting enzyme (ICE) inhibitors. Bioorg Med Chem 14:7880–7892
Wang Y, O’Neil SV, Wos JA, Oppong KA, Laufersweiler MC, Soper DL, Ellis CD, Baize MW, Fancher AN, Lu W, Suchanek MK, Wang RL, Schwecke WP, Cruze CA, Buchalova M, Belkin M, De B, Demuth TP (2007) Synthesis and evaluation of unsaturated caprolactams as interleukin-1beta converting enzyme (ICE) inhibitors. Bioorg Med Chem 15:1311–1322
Lee D, Long SA, Adams JL, Chan G, Vaidya KS, Francis TA, Kikly K, Winkler JD, Sung CM, Debouck C, Richardson S, Levy MA, DeWolf WE, Keller PM, Tomaszek T, Head MS, Ryan MD, Haltiwanger RC, Liang PH, Janson CA, McDevitt PJ, Johanson K, Concha NO, Chan W, Abdel-Meguid SS, Badger AM, Lark MW, Nadeau DP, Suva LJ, Gowen M, Nuttall ME (2000) Potent and selective nonpeptide inhibitors of caspases 3 and 7 inhibit apoptosis and maintain cell functionality. J Biol Chem 275:16007–16014
Lee D, Long SA, Murray JH, Adams JL, Nuttall ME, Nadeau DP, Kikly K, Winkler JD, Sung CM, Ryan MD, Levy MA, Keller PM, DeWolf WE (2001) Potent and selective nonpeptide inhibitors of caspases 3 and 7. J Med Chem 44:2015–2026
Kravchenko DV, Kysil VM, Tkachenko SE, Maliarchouk S, Okun IM, Ivachtchenko AV (2005) Pyrrolo[3,4-c]quinoline-1,3-diones as potent caspase-3 inhibitors. Synthesis and SAR of 2-substituted 4-methyl-8-(morpholine-4-sulfonyl)-pyrrolo[3,4-c]quinoline-1,3-diones. Eur J Med Chem 40:1377–1383
Hardy JA, Lam J, Nguyen JT, O’Brien T, Wells JA (2004) Discovery of an allosteric site in the caspases. Proc Natl Acad Sci U S A 101:12461–12466
Walters J, Schipper JL, Swartz P, Mattos C, Clark AC (2012) Allosteric modulation of caspase 3 through mutagenesis. Biosci Rep 32:401–411
Datta D, Scheer JM, Romanowski MJ, Wells JA (2008) An allosteric circuit in caspase-1. J Mol Biol 381:1157–1167
Feldman T, Kabaleeswaran V, Jang SB, Antczak C, Djaballah H, Wu H, Jiang X (2012) A class of allosteric caspase inhibitors identified by high-throughput screening. Mol Cell 47:585–595
Schweizer A, Roschitzki-Voser H, Amstutz P, Briand C, Gulotti-Georgieva M, Prenosil E, Binz HK, Capitani G, Baici A, Plückthun A, Grütter MG (2007) Inhibition of caspase-2 by a designed ankyrin repeat protein: specificity, structure, and inhibition mechanism. Structure 15:625–636
Stanger K, Steffek M, Zhou L, Pozniak CD, Quan C, Franke Y, Tom J, Tam C, Elliott JM, Lewcock JW, Zhang Y, Murray J, Hannoush RN (2012) Allosteric peptides bind a caspase zymogen and mediate caspase tetramerization. Nat Chem Biol 8:655–660
Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J (1997) Inhibition of death receptor signals by cellular FLIP. Nature 388:190–195
Humke EW, Shriver SK, Starovasnik MA, Fairbrother WJ, Dixit VM (2000) ICEBERG: a novel inhibitor of interleukin-1beta generation. Cell 103:99–111
Komiyama T, Ray CA, Pickup DJ, Howard AD, Thornberry NA, Peterson EP, Salvesen G (1994) Inhibition of interleukin-1 beta converting enzyme by the cowpox virus serpin CrmA. An example of cross-class inhibition. J Biol Chem 269:19331–19337
Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS (1997) Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem 272:7797–7800
Ryan CA, Stennicke HR, Nava VE, Burch JB, Hardwick JM, Salvesen GS (2002) Inhibitor specificity of recombinant and endogenous caspase-9. Biochem J 366:595–601
Gettins PG (2002) Serpin structure, mechanism, and function. Chem Rev 102:4751–4804
Xu G, Cirilli M, Huang Y, Rich RL, Myszka DG, Wu H (2001) Covalent inhibition revealed by the crystal structure of the caspase-8/p35 complex. Nature 410:494–497
Xu G, Rich RL, Steegborn C, Min T, Huang Y, Myszka DG, Wu H (2003) Mutational analyses of the p35-caspase interaction. A bowstring kinetic model of caspase inhibition by p35. J Biol Chem 278:5455–5461
Crook NE, Clem RJ, Miller LK (1993) An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol 67:2168–2174
Rehm M, Huber HJ, Dussmann H, Prehn JH (2006) Systems analysis of effector caspase activation and its control by X-linked inhibitor of apoptosis protein. EMBO J 25:4338–4349
Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC (1998) A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem 273:7787–7790
Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC (1999) Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 18:5242–5251
Vucic D, Dixit VM, Wertz IE (2011) Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol 12:439–452
Suzuki Y, Nakabayashi Y, Takahashi R (2001) Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A 98:8662–8667
Shirakura H, Hayashi N, Ogino S, Tsuruma K, Uehara T, Nomura Y (2005) Caspase recruitment domain of procaspase-2 could be a target for SUMO-1 modification through Ubc9. Biochem Biophys Res Commun 331:1007–1015
Besnault-Mascard L, Leprince C, Auffredou MT, Meunier B, Bourgeade MF, Camonis J, Lorenzo HK, Vazquez A (2005) Caspase-8 sumoylation is associated with nuclear localization. Oncogene 24:3268–3273
Alvarado-Kristensson M, Melander F, Leandersson K, Rönnstrand L, Wernstedt C, Andersson T (2004) p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J Exp Med 199:449–458
Voss OH, Kim S, Wewers MD, Doseff AI (2005) Regulation of monocyte apoptosis by the protein kinase Cdelta-dependent phosphorylation of caspase-3. J Biol Chem 280:17371–17379
Li X, Wen W, Liu K, Zhu F, Malakhova M, Peng C, Li T, Kim HG, Ma W, Cho YY, Bode AM, Dong Z (2011) Phosphorylation of caspase-7 by p21-activated protein kinase (PAK) 2 inhibits chemotherapeutic drug-induced apoptosis of breast cancer cell lines. J Biol Chem 286:22291–22299
Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR (2003) Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol 5:647–654
Raina D, Pandey P, Ahmad R, Bharti A, Ren J, Kharbanda S, Weichselbaum R, Kufe D (2005) c-Abl tyrosine kinase regulates caspase-9 autocleavage in the apoptotic response to DNA damage. J Biol Chem 280:11147–11151
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cade, C.E., Clark, A.C. (2015). Caspases – Key Players in Apoptosis. In: Bose, K. (eds) Proteases in Apoptosis: Pathways, Protocols and Translational Advances. Springer, Cham. https://doi.org/10.1007/978-3-319-19497-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-19497-4_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19496-7
Online ISBN: 978-3-319-19497-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)