Abstract
The primary goals of breast reconstruction in patients with cancer are to improve body image and satisfy patient expectations. This chapter focuses on the effects of post-mastectomy radiotherapy (PMRT), which aims at eliminating residual tumor foci, reducing the risk of loco-regional relapse and improving overall survival, on implants or prostheses that are inserted during the mastectomy session. It also explores treatment- and patient- related risk factors for complications, current attempts to reduce the risk of complications after PMRT, improve cosmetic outcomes and increase patient satisfaction with the procedure. The chapter aims at providing updated information for the multi-disciplinary team that is involved in the care of women with breast cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Breast Reconstruction
- National Comprehensive Cancer Network
- Tissue Expander
- Capsular Contracture
- Internal Mammary Node
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Today reconstruction after mastectomy is available for many patients with breast cancer. When performed in the same surgical session as mastectomy, breast reconstruction avails of the following options: placing a temporary tissue expander which will be later replaced with a permanent implant; inserting the permanent implant although only in selected cases; using autologous tissues alone or with a prosthesis [1]. Choice is conditional on the patient’s physical characteristics and preference, feasibility and the surgeon’s expertise, bearing in mind that reconstruction with expander/prosthesis was associated with a higher complication rate [2–8].
Immediate breast reconstruction undoubtedly offers advantages that are not found if it is delayed for months or even years, despite a higher complication rate [9–14]. Since the supple and elastic breast skin and subcutaneous soft tissue have not yet been exposed to radiotherapy (RT), expansion to the appropriate reconstructed breast volume and shape is easier and more rapid, with better aesthetic results. Patients have the chance to maintain their body image, with no triggering of psychological trauma due to breast mutilation and thus their quality of life remains relatively unperturbed. Finally, a costly and risky second major operation is avoided, the success of which may be jeopardized because the breast tissue has been irradiated.
Post-mastectomy RT (PMRT) aims at eliminating residual tumor foci, reducing the risk of loco-regional relapse and improving overall survival [15]. Since the role of biological subtypes as risk factors for loco-regional relapse has not yet been investigated in depth [16, 17], PMRT is administered mainly according to disease stage. It is recommended for high risk patients, i.e. those with locally advanced disease or with four or more metastatic axillary lymph nodes in early stage disease (T1-2) [18–22]. When 1–3 axillary lymph nodes are positive, evidence is generally insufficient to recommend PMRT but the National Comprehensive Cancer Network (NCCN) Guidelines suggest strongly considering it even though the evidence level is only 2B [20]. Other guidelines [18, 22] propose taking into account risk factors like youth, tumor size >3.5–4 cm, negative hormone receptors, lympho-vascular invasion, high grade, nodal ratio >20–25 % [18, 23–28]. The results of the latest Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) metanalysis [29], which assessed the effect of RT on outcomes after mastectomy and axillary surgery in patients with 1–3 positive nodes, may impact upon current recommendations as it suggested RT reduced disease recurrence and mortality.
Target volumes for PMRT are the chest wall and the supra- infraclavicular nodes [19–22]. There is no indication for RT to the resected part of the axilla if there is no residual disease after surgery [18, 22]. RT to the internal mammary nodes is still controversial [18–22], as the evidence level is low [20] and conflicting results have emerged from recent studies [30–33]. Despite all the advantages it offers PMRT was, however, associated with more complications than were observed in non-irradiated patients [7–9, 34–46] (Table 36.1). These have the potential to impact negatively upon cosmetic outcome and upon the patient’s quality of life.
2 Concerns About Immediate Breast Reconstruction
Immediate breast reconstruction was, in the past, penalized by the view that medical adjuvant treatments could be delayed because of RT-related toxicity. However, it was shown that adjuvant systemic treatments were administered at the same times whether women underwent it or not [47–51]. Even though immediate breast reconstruction was feared to mask local relapse and delay its diagnosis, it was not associated with a higher incidence of recurrences and worse survival [51–56]. Indeed, a retrospective matched cohort study with a median follow-up of 11.5 years, analyzed the clinical outcome in 600 patients after mastectomy, 300 of whom received immediate breast reconstruction while 300 controls did not. About one-third of patients in each group received PMRT. The incidence of distant metastases, breast cancer and all-cause mortality were significantly higher in controls [57]. The higher percentage of receptor positive tumors in the reconstructed group may account for these results but when the statistical analysis was corrected for hormonal receptor status, the inter-group difference in breast cancer mortality was no longer significant. Since the authors did not describe their correction model one might hypothesize that a stratification was performed so the sample size in each sub-group was too low to reach significance.
Another obstacle to immediate breast reconstruction was that it created technical difficulties for PMRT delivery. In patients with expanders and/or prostheses the large rigid reconstructed breast volume creates steep slopes in medial and apical contours. Standard three-dimensional conformal RT techniques become arduous if the chest wall, the supra-infraclavicular, and particularly the internal mammary nodes have to be irradiated [58–60]. Chest wall and regional coverage may be impaired, raising the risk of relapse. The dose to the heart and lungs may be increased and consequently, more treatment-related toxicity might be expected to occur. For example, when an anterior electron field was used to treat the internal mammary nodes, the field junction between them and the non-uniform chest wall was imprecise and coverage was impaired because of under-dosage across the electron field [58–60]. Arthur et al. [61] compared RT techniques after breast conserving surgery with the aim of selecting the most suitable. The partially wide tangent fields avoided junction problems and, as it irradiated only the internal mammary nodes in the first three intercostal spaces, it spared the organs at risk of toxicity. This technique can be translated to the immediately reconstructed breast.
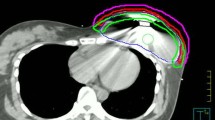
More advanced RT techniques like intensity modulated RT (IMRT) and tomotherapy were evaluated for delivering PMRT after reconstruction [62–64]. When available, they are generally reserved for patients in whom conformal three dimensional RT does not adequately cover target volumes or does not spare organs at risk. Both improved target volume irradiation, eliminated the field junction problem and delivered lower doses to organs at risk. Furthermore, tomotherapy treated only the tissue anterior to a sub-muscular implant, thus sparing it [64]. This is worth noting because irradiation may harden or alter the colour of some expander/prosthesis models [65]. However with IMRT and tomotherapy more healthy tissues received low-dose irradiation, which needs to be weighed up against their benefits. Figures 36.1 and 36.2 illustrate dosimetric results with these techniques. Proton therapy, which is at present offered by very few RT Centres because of costs, was proposed for some highly-selected patients with unfavorable anatomy [66, 67]. Protons provided full, homogeneous dose delivery to target volumes, completely sparing organs at risk. However, the following issues have emerged: uncertainties in dose deposition, skin dose and the impact of chancing set-up and respiratory motion on treatment delivery. Furthermore, since expanders with metal ports introduced dose uncertainty, patients with them had to be excluded [66]. With photon PMRT, on the other hand, metal ports did not interfere significantly with treatment planning as they caused only a slight variation in dose distribution to the surrounding area [68–70]. In any case, specific algorithms for the metal material and high energy photons (~15 MV) were proposed [70, 71].
Dose distribution to a right reconstructed breast and omolateral supra-infraclavicular nodes and dose volume histograms. Dose distribution: In panels (a–c) images are axial, sagittal and coronal. Panel (a) 3DRT, panel (b) IMRT, panel (c) tomotherapy. Dose volume histograms: panel (d) 3D and IMRT, panel (e) tomotherapy
Dose distribution to a left reconstructed breast, omolateral supra-infraclavicular and internal mammary nodes and dose volume histograms. Dose distribution: In panels (a) and (b) images are axial, sagittal and coronal. Panel (a) IMRT, panel (b) tomotherapy. Dose volume histograms: panel (c) IMRT, panel (d) tomotherapy
3 Complications After Expander/Implant Breast Reconstruction and PMRT
Short- and long-term RT-related complications may mitigate the benefits of reconstruction. Even though up to 68 % of patients were reported to develop them [3, 4, 7, 37, 38, 40, 44–46, 72–77] inter-study comparisons are difficult and a definitive picture is hard to obtain. Retrospective studies reported complications in diverse ways such as incidence or probability at different time–points e.g. 2 or 5 years and provided low-level evidence because they were limited by biases, confounding variables and lack of selection criteria and long-term follow-ups. They usually had relatively small cohorts of negatively selected patients who were ineligible for autologous procedures because of contraindications. There were often no separate analyses of the different types of reconstruction (expander alone, prosthesis, autologous reconstructive procedure with expander). The few available prospective studies also provided little definitive evidence because patient cohorts were relatively small and they were not randomized. It is worth noting that randomized studies will never be performed in this field because no group of patients should be deprived of the chance of breast reconstruction.
Early complications include infection, inflammation, hematoma, seroma, poor wound healing, skin necrosis and implant extrusion. Fibrosis, partial loss of skin and soft tissue elasticity and suppleness have a later onset as do capsular contracture, prosthesis rupture, displacement or loss of shape. Finally, pain may occur at any time after RT. Late complications were found to be more common in patients undergoing PMRT after breast reconstruction, while patients receiving PMRT beforehand tended to have more early complications [73].
Complications are defined as major if patients require an additional operation, premature expander or permanent implant removal or reconstruction with another implant and/or an autologous tissue flap. The incidence of implant/prosthesis removal ranged from 4 to 43 % [4, 37, 38, 41, 46, 74–81]. Presenting the latest Memorial Sloan Kettering Cancer Center (MSKCC) results Ho et al. distinguished between implant removal (explantation) and implant replacement [82] but whatever the final outcome, the original implant was always removed, which indicated treatment failure. One interesting point which emerged from this study was the time link between implant failure and the underlying cause i.e. infection in the first year after PMRT and capsular contracture alone or combined with other factors, such as dissatisfaction or suboptimal cosmesis in the second year or later, with the incidence increasing as follow-up lengthened [79, 83]. Attention has focussed mainly on implant removal and capsular contracture, the most serious side effects which also occur in non-irradiated patients. Like other reports, this chapter will analyze them together.
4 Capsular Contracture and Implant Removal
PMRT is the most significant risk factor for capsular contracture (Fig. 36.3) even though its aetiology is also linked to choice of implant filler material, implant position, inflammation, infection and patient age. Classified according to the Spear and Baker system (Table 36.2) [84], the most serious cases (Classes III and IV) of capsular contraction, were reported to range in incidence from 11 to 53 % [4, 38, 39, 41, 43, 46, 76, 77, 79, 80, 85]. Table 36.3 reports Grade III-IV capsular contracture and removal rates in a series of studies. Patients with capsular contracture are referred for surgery to correct it and substantially reduce high-grade fibrosis [43, 85, 86]. Indeed, in the Claßen et al. series, the grade ≥III fibrosis rate declined markedly from the predicted 43 % incidence at 3 years to the real 18 % rate [85].
Several strategies were proposed to reduce the incidence of capsular contracture. The MSKCC designed an algorithm to address PMRT timing [87] because no histological evidence of capsular or dermal alterations was found in an animal model of tissue expansion if irradiation was delivered after expansion was completed [88]. In the clinical setting RT delivery to the permanent prosthesis was expected to minimize unwanted side effects. Briefly, expander was positioned at the time of mastectomy and expanded 1–2 weeks after surgery, with expansion continuing throughout adjuvant chemotherapy. Approximately 4 weeks after systemic adjuvant treatment ended a permanent implant replaced the tissue expander. RT started 1 month later. Under this protocol 11 % of permanent implants were removed from 81 patients. A follow-up of at least 1 year (median 34 months) was available for 68 cases, 68 % of whom developed capsular contracture, (33.8 % grade III; 5.9 % grade IV) [87]. Results were confirmed 2 years later [41]. In a more recent series of 151 patients (median follow-up 86 months; range 11–161), the 7-year rates of implant replacement and removal were 17.1 % and 13.3 % respectively [82].
A low incidence of implant removal was observed with PMRT to expanders. Anderson et al. attributed their 4.8 % rate in 62 patients (median follow-up 48 months) to IMRT in approximately one-third of patients [74]. This retrospective study did, however, contain biases in cohort size and lack of separate analyses for standard RT and IMRT results. After following-up 101 patients (90 of whom were irradiated to the expander) for a median of 50 months, Aristei et al. observed Grades III and IV capsular contracture in 17.4 % and prosthesis removal in 11.9 % [77]. Like Piroth et al. [76] who reported a 22.7 % implant removal rate, the authors were of the view that complete expander filling, which was achieved in all patients before starting RT, might have determined the low removal rate. Results from the Milan Cancer Institute seemed to confirm these findings: 50/159 patients received neo-adjuvant chemotherapy and PMRT during tissue expansion as they could not wait to fill the expander completely without risking the oncological outcome. Reconstructions failed in 40 % vs 6.4 % in patients who received PMRT to the permanent implant, suggesting that patients who need neo-adjuvant chemotherapy may not be suitable candidates for immediate breast reconstruction with expander. Interestingly the incidence of Grade III and IV capsular contracture was very similar in the two groups (40 % expander vs 47.7 % permanent; 13.3 % vs 10.1 %) [80]. Further evidence in support of this view comes from Spear et al. who reported severe capsular contracture in 60.7 % of patients who received PMRT during expansion [86].
Besides complete tissue expansion before PMRT, time from the end of RT to expander/implant exchange was another factor in outcomes. Implant failure rate correlated with time to exchange, being highest (28.6 %) when under 3 months elapsed from the end of PMRT to expander/implant exchange. It dropped to 22.4 % in patients with under 6 months’ time to exchange and to 7.7 %, in those with over 6 months. Even though the series included only 88 patients who were finely analyzed in several sub-groups, the optimal time for expander exchange appeared to be more than 6 months after the end of PMRT [81].
For patients who demand immediate reconstruction but who might, or probably would, require PMRT, the “delayed/immediate breast reconstruction”, was developed at the M.D. Anderson Cancer Center [12, 13, 89, 90] to prevent problems that were reported to be associated with PMRT delivery to an immediately reconstructed breast. In this two-step approach, a filled textured saline tissue expander was inserted under the pectoral muscle immediately after skin-sparing mastectomy so as to prevent skin retraction and loss of breast shape. If histology findings indicated PMRT was not needed, the tissue expander was removed and the breast was reconstructed using autologous tissue or a permanent implant, achieving aesthetic outcomes that were similar to immediate reconstruction. If, on the other hand, PMRT was required, the tissue expander was deflated just before starting it, re-expanded after it ended and finally removed and replaced with autologous tissue or a permanent implant, 6–12 months after the end of RT. Infections in 53 % of patients who had a medium follow-up of 40 months, were associated with 32 % tissue expander loss which was attributed to a too long stay in situ of the drain. Interestingly, the expander loss rate dropped as the learning curve rose. Finally, no clinical evidence was found of irradiated skin or reconstructed breast contracture [55].
5 Risk Factors for Complications
Data on risk factors for complications after breast reconstructions are somewhat discordant. Attempts to identify them were hindered by several biases: risk factors could have been under-reported because of unreliable data collection, data were often retrospective, sample sizes and events were small in number, reconstruction techniques were heterogeneous, confounding variables were not always taken into consideration, factors that were significant in univariate analyses became insignificant in the multivariate, different statistical models (or even no model) were used. Some studies focused on one single risk factor, failing to take into account potential combinations; others concentrated on the impact of several factors on one single outcome; diverse risk factors were correlated with overall complications or with only specific complications.
Factors that are related to the patient and her lifestyle may increase the incidence of complications after breast reconstruction. As for any other form of surgery, older age [7, 46, 52, 75, 77, 91], obesity (body mass index –BMI- of 30 or greater) [7, 11, 46, 52, 75, 91], hypertension [52], and the smoking habit [52, 79, 91, 92] were recognized as risk factors. More specifically breast volume also plays a role [91, 93]. In patients receiving PMRT, large breasts were more susceptible to RT-related toxicity due to the difficulty in achieving homogenous dose distribution in the target volume. Tumor size vis-à-vis breast volume is another risk factor. Cowen et al. speculated that large tumors in small breasts involved sacrifice of a large skin area which made expansion more difficult. Indeed, 45.5 % of patients in this series had tumor ≥30 mm in size and, even though information on cup-size was missing in 32/141 patients, reconstruction failure occurred more frequently in patients with A or B cup-sizes (35.9 % vs 16.7 % in the others p = 0.009) [79].
One treatment-related risk factor for complications was the skill of operating surgeon, suggesting that reconstruction should be performed by, or in collaboration with, a plastic surgeon. A team of expert surgeons would reduce the risk of hemorrhage and underlying aponeurosis injury, which might impact upon vascularization [79]. Hormonal therapy was associated with tamoxifen-related toxicity [37, 79] which current use of aromatase inhibitors should reduce in post-menopausal patients. Chemotherapy [38], and RT [7–9, 34–46, 79, 86] are treatment-related risk factors for toxicity but in the Tallet et al. series their effects could not be distinguished because all but three patients received both [38].
RT-related factors were the irradiated volumes, irradiation techniques and, consequently, dose distribution in target volumes. Outcomes were adversely affected by the dose to the chest wall, a boost dose to the mastectomy scar, and a bolus i.e. tissue-equivalent material placed on the skin to avoid under-dosage [9, 94, 95]. For example, bolus was associated with a 51 % complication rate vs 23 % without it (p = 0.0009) [94]. Good-to-excellent cosmetic results were observed in 87 % of patients who were irradiated without bolus compared with 37 % with it (p = 0.016) [95]. A significantly lower rate of complications was reported with the use of a bolus customized to the reconstruction shape compared to the use of a standard bolus, as the 3-year complication rate was 9 % vs 24 % (p = 0.05). The custom-fashioned bolus eliminated the air gap and appeared to protect skin from overdoses due to contaminant electrons in the gap region [96].
To predict the patient complication rate and identify which patients were best suited for tissue expander/implants Berry et al. [7] created a nomogram with variables including administration or not of RT and chemotherapy, chemotherapy timing (neo-adjuvant or adjuvant), reconstruction type (autologous or expander). Like all nomograms this one needs validation before it can be applied to clinical practice. Cowen et al. [79] derived a mathematical model to predict the probability of reconstruction failure from the results of a prospective French multi-center study with 141 patients (median follow-up 37 months). Risk factors for the 22.7 % implant removal rate were smoking habit, tumor size and nodal positivity in uni- and multi-variate analyses. The probability of reconstruction failure increased progressively 7–100 % as the number of factors increased. The authors believed the model could be useful in routine clinical practice prior to proposing breast reconstruction but admitted a preoperative assessment of tumor size and lymph node status was problematic. Since no other data have linked tumor size and nodal stage with implant removal, these factors require validation and furthermore, the model itself requires validation.
6 Cosmesis and Quality of Life
Cosmetic outcome and patient satisfaction are major endpoints of breast reconstruction after mastectomy because the primary goals are to improve body image and satisfy patient expectations. Despite the complication rate, most patients affirmed cosmetic outcomes were excellent or good with rates rising up to 90 % [4, 38, 74, 77, 78, 95]. Results were not always comparable due to small series, varying lengths of follow-up, lack of control groups (women who underwent the same reconstructive procedure with or without RT), lack of, or different, scales and questionnaires for assessing toxicity, cosmesis and patient satisfaction. Furthermore, patient satisfaction is particularly hard to assess because responses to questionnaires are subjective and refer to the time of administration. They may not reflect the patient’s real perception of cosmetic outcome which can fluctuate with mood, time, expectancies, understanding of realistic alternatives and potential consequences, as well as the patient’s personal views of the entire reconstructive process. It is worth noting the patient’s satisfaction with the reconstruction decision is likely to be highest when she has been adequately informed and her involvement in decision-making was consistent with her own wishes and expectations.
Although aesthetic results and complications clearly impacted upon patient satisfaction, they might not have been the only factor contributing to satisfaction. Cowen et al. [79] reported young pre-menopausal women were more dissatisfied than the post-menopausal, because they attributed more importance to body image while Alderman et al. [97] reported that although age had no significant effect on satisfaction, older women tended to be less satisfied, perhaps because of late asymmetry produced by gradual contralateral breast ptosis. Furthermore, older women are at greater risk of complications which were reported to be a particularly important indicator of dissatisfaction with reconstruction [98].
Overall patient satisfaction was high after tissue expander/implant reconstruction and PMRT, with similar satisfaction rates in patients and controls (63.16 % vs 66.88 %) [44]. The Michigan questionnaire for patients with tissue expander/implant-based reconstruction found no significant differences in general and aesthetic satisfaction whether PMRT was delivered or not, despite a higher rate of expander/implant reconstruction failure and complications in the group receiving RT [37]. Cordeiro et al. [41] observed a 95 % satisfaction rate while 91.4 % of patients stated they would choose the same reconstruction again. In a very small series of 33 patients Piroth et al. [76] found that although 19 % of patients would not undergo breast reconstruction again, refusal did not correlate with outcome dissatisfaction (p = 0.79) because 5/11 patients (45.5 %) who were only somewhat (n = 4) or not (n = 1) satisfied would nonetheless repeat the procedure.
To investigate long-term satisfaction with breast reconstruction BREAST-Q, a new patient-reported outcome measure focusing on breast surgery outcomes, evaluated satisfaction in 110 patients with expander/implants in terms of the reconstructed breast appearance, shape, softness, size and projection [99]. Only 19 % of patients received PMRT. Dissatisfaction grew with the passage of time. For example at under five years after surgery 18 % were not happy with breast appearance compared with 55 % at 8 years and 16 % were dissatisfied with breast size vs 55 % after 8 years follow-up. In any case, with or without PMRT, results may change over time as implants do not become naturally ptotic with age, do not change in size as a patient gains or loses weight and may become distorted in shape due to capsular contracture (Fig. 36.3).
7 Conclusions
The benefits of breast reconstruction include improved body image, self-esteem and well-being. To ensure patients have realistic expectations they have to be clearly informed of the risks that can impact upon cosmesis and quality of life, or lead to prosthesis removal. As long as appropriate patients are selected and the oncological and reconstructive surgical teams are well-coordinated in a multidisciplinary approach, PMRT after breast reconstruction with tissue expander/implant is safe and provides excellent/good cosmetic outcomes and a high grade of satisfaction in most patients. It seems to be the preferred option in the USA while Europeans tend to opt more for autologous tissue flaps [100]. More widespread application of advanced RT and surgical techniques [86, 101] are expected to reduce the risk of complications in coming years.
In evaluating the indications for a reconstructive procedure, the risk of complications such as the effects of PMRT and the difficulties in delivering PMRT to reconstructed breasts have to be carefully considered. Expander/implant breast reconstruction has not yet been optimized but strategies include delayed-immediate reconstruction [12, 13, 89, 90], complete expander filling [76, 77] and a minimum delay of 6 months before prosthesis insertion [81] or expander replacement with the prosthesis before starting PMRT [1, 41, 82, 87]. As results do not vary greatly, choice of strategy is linked to institutional preferences. To have a comprehensive evaluation of breast reconstruction surgery specific modules to assess patient reported outcomes will need to be developed and validated [102, 103]. It is to be hoped they will also be forthcoming in the near future.
References
Cordeiro PG (2008) Breast reconstruction after surgery for breast cancer. N Engl J Med 359(15):1590–1601
Kroll SS, Baldwin B (1992) A comparison of outcomes using three different methods of breast reconstruction. Plast Reconstr Surg 90(3):455–462
Spear SL, Onyewu C (2000) Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg 105(3):930–942
Chawla AK, Kachnic LA, Taghian AG, Niemierko A, Zapton DT, Powell SN (2002) Radiotherapy and breast reconstruction: complications and cosmesis with TRAM versus tissue expander/implant. Int J Radiat Oncol Biol Phys 54(2):520–526
Jhaveri JD, Rush SC, Kostroff K, Derisi D, Farber LA, Maurer VE, Bosworth JL (2008) Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. Int J Radiat Oncol Biol Phys 72(3):859–865
Wong JS, Ho AY, Kaelin CM, Bishop KL, Silver B, Gelman R, Harris JR, Hergrueter CA (2008) Incidence of major corrective surgery after post-mastectomy breast reconstruction and radiation therapy. Breast J 14(1):49–54
Strålman K, Mollerup CL, Kristoffersen US, Elberg JJ (2008) Long-term outcome after mastectomy with immediate breast reconstruction. Acta Oncol 47(4):704–708
Berry T, Brooks S, Sydow N, Djohan R, Nutter B, Lyons J, Dietz J (2010) Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol 17(Suppl 3):S202–S210
Barry M, Kell MR (2011) Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat 127(1):15–22
Miller AP, Falcone RE (1991) Breast reconstruction: systemic factors influencing local complications. Ann Plast Surg 27(2):115–120
Alderman AK, Wilkins EG, Kim HM, Lowery JC (2002) Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg 109(7):2265–2274
Kronowitz SJ, Hunt KK, Kuerer HM, Babiera G, McNeese MD, Buchholz TA, Strom EA, Robb GL (2004) Delayed-immediate breast reconstruction. Plast Reconstr Surg 113(6):1617–1628
Chevray PM (2008) Timing of breast reconstruction: immediate versus delayed. Cancer J 14(4):223–229
Sullivan SR, Fletcher DR, Isom CD, Isik FF (2008) True incidence of all complications following immediate and delayed breast reconstruction. Plast Reconstr Surg 122(1):19–28
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106
Abdulkarim BS, Cuartero J, Hanson J, Deschênes J, Lesniak D, Sabri S (2011) Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol 29(21):2852–2858
Pignol J-P, Rakovitch E, Olivotto I (2011) Is breast conservation therapy superior to mastectomy for women with triple-negative breast cancer? J Clin Oncol 29(21):2841–2843
Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, Cardoso F, on behalf of the ESMO Guidelines Working Group (2013) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi7–vi23
Goldrisch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223
NCCN (National Comprehensive Cancer Network) Clinical practice guidelines in oncologyTM: Breast cancer. Version 3.2014. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 27 Apr 2014
Sautter-Bihl M-L, Sedlmayer F, Budach W, Dunst J, Feyer P, Fietkau R, Fussl C, Haase W, Harms W, Piroth MD, Souchon R, Wenz F, Sauer R (2014) DEGRO Practical Guidelines: radiotherapy of breast cancer III – radiotherapy of the lymphatic patways. Strahlenther Onkol 190(4):342–351
Associazione Italiana di Radioterapia Oncologica – Gruppo di Lavoro AIRO per la Patologia Mammaria. La radioterapia dei tumori della mammella. Indicazioni e criteri guida. Version 2013. http://radioterapiaitalia.it. Accessed 5/2/14
Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, Falkson G, Falkson HC, Taylor SG IV, Tormey DC (1999) Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol 17(6):1689–1700
Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, Hortobagyi G, Buzdar AU, Theriault R, Singletary SE, McNeese MD (2000) Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol 18(15):2817–2827
Taghian A, Jeong J-H, Mamounas E, Anderson S, Bryant J, Deutsch M, Wolmark N (2004) Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol 22(21):4247–4254
Wallgren A, Bonetti M, Gelber RD, Goldhrisch A, Castiglione-Gertsch M, Holmberg SB, Lindtner J, Thürlimann B, Fey M, Werner ID, Forbes JF, Price K, Coates AS, Collins J (2003) Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol 21(7):1205–1213
Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E (2005) Selecting breast cancer patients with T1-T2 tumors and one to three axillary positive nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys 61(5):1337–1347
Karlsson P, Cole BF, Price KN, Coates AS, Castiglione-Gertsch M, Gusterson BA, Murray E, Lindtner J, Collins JP, Holmberg SB, Fey MF, Thürlimann B, Crivellari D, Forbes JF, Gelber RD, Goldhirsch A, Wallgren A (2007) The role of the number of uninvolved lymph nodes in predicting locoregional recurrence in breast cancer. J Clin Oncol 25(15):2019–2026
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383(9935):2127–2135
Hennequin C, Bossard N, Servagi-Vernat S, Maingon P, Dubois JB, Datchary J, Carrie C, Roullet B, Suchaud JP, Teissier E, Lucardi A, Gerard JP, Belot A, Iwaz J, Ecochard R, Romestaing P (2013) Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys 86(5):860–866
Chang JS, Park W, Kim YB, Lee IJ, Keum KC, Lee CG, Choi DH, Suh CO, Huh SJ (2013) Long-term survival outcomes following internal mammary node irradiation in stage II-III breast cancer: results of a large retrospective study with 12 year follow-up. Int J Radiat Oncol Biol Phys 86(5):867–872
Jagsi R, Pierce L (2013) Radiation therapy to the internal mammary nodal region in breast cancer: the debate continues. Int J Radiat Oncol Biol Phys 86(5):813–815
Poortmans PSH, Kirkove C, Budach V, Maingon P, Valli MC, Collette S, Fourquet A, Bartelink H, Van den Bogaert W (2013) Irradiation of the internal mammary and medial supraclavicular lymph nodes in stage I to III breast cancer: 10 years results of the EORTC radiation oncology and breast cancer groups phase III trial 22922/10925. Eur J Cancer 47 (Suppl 2)
Barreau-Pouhaer L, Lê MG, Rietjens M, Arriagada R, Contesso G, Martins R, Petit JY (1992) Risk factors for failure of immediate breast reconstruction with prosthesis after total mastectomy for breast cancer. Cancer 70(5):1145–1151
Evans GRD, Schusterman MA, Kroll SS, Miller MJ, Reece GP, Robb GL, Ainslie N (1995) Reconstruction and the radiated breast: is there a role for implants? Plast Reconstr Surg 96(5):1111–1118
Vandeweyer E, Deraemaecker R (2000) Radiation therapy after immediate breast reconstruction with implants. Plast Reconstr Surg 106(1):56–58
Krueger EA, Wilkins EG, Strawderman M, Cederna P, Goldfarb S, Vicini FA, Pierce LJ (2001) Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys 49(3):713–721
Tallet AV, Salem N, Moutardier V, Ananian P, Braud A-C, Zalta R, Cowen D, Houvenaeghel G (2003) Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: complications and esthetic results. Int J Radiat Oncol Biol Phys 57(1):136–142
McCarthy CM, Pusic AL, Disa JJ, McCormick BL, Montgomery LL, Cordeiro PG (2005) Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg 116(6):1642–1647
Ascherman JA, Hanasono MM, Newman MI, Hughes DB (2006) Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg 117(2):359–365
Cordeiro PG, McCarthy CM (2006) A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: Part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg 118(4):832–839
Behranwala KA, Dua RS, Ross GM, Ward A, A’Hern R, Gui GPH (2006) The influence of radiotherapy on capsule formation and aesthetic outcome after immediate breast reconstruction using biodimensional anatomical expander implants. J Plast Reconstr Aesthet Surg 59(10):1043–1051
Benediktsson K, Perbeck L (2006) Capsular contracture around saline-filled and textured subcutaneously-placed implants in irradiated and non-irradiated breast cancer patients: five years of monitoring of a prospective trial. J Plast Reconstr Aesthet Surg 59(1):27–34
Lee BT, Adesiyun TA, Colakoglu S, Curtis MS, Yueh JH, Anderson KE, Tobias AM, Recht A (2010) Postmastectomy radiation therapy and breast reconstruction: an analysis of complications and patient satisfaction. Ann Plast Surg 64(5):679–683
Christante D, Pommier SJ, Diggs BS, Samuelson BT, Truong AL, Marquez C, Hansen J, Naik AM, Vetto JT, Pommier RF (2010) Using complications associated with postmastectomy radiation and immediate breast reconstruction to improve surgical decision making. Arch Surg 145(9):873–878
Brooks S, Djohan R, Tendulkar R, Nutter B, Lyons J, Dietz J (2012) Risk factors for complications of radiation therapy on tissue expander breast reconstructions. Breast J 18(1):28–34
Caffo O, Cazzolli D, Scalet A, Zani B, Ambrosini G, Amichetti M, Bernardi D, Brugnara S, Ciaghi G, Lucenti A, Natale N, Agugiaro S, Eccher C, Galligioni E (2000) Concurrent adjuvant chemotherapy and immediate breast reconstruction with skin expanders after mastectomy for breast cancer. Breast Cancer Res Treat 60(3):267–275
Alweiss TM, Boisvert ME, Otero SE, Perry DJ, Dubin NH, Priebat DA (2002) Immediate reconstruction after mastectomy for breast cancer does not prolong the time to starting adjuvant chemotherapy. Am J Surg 183(3):218–221
Wilson CR, Brown IM, Weiller-Mithoff E, George WD, Doughty JC (2004) Immediate breast reconstruction does not lead to a delay in the delivery of adjuvant chemotherapy. Eur J Surg Oncol 30(6):624–627
Taylor CW, Horgan K, Dodwell D (2005) Oncological aspects of breast reconstruction. Breast 14(2):118–130
Howard-McNatt MM (2013) Patients opting for breast reconstruction following mastectomy: an analysis of uptake rates and benefit. Breast Cancer 5:9–15
McCarthy CM, Pusic AL, Sclafani L, Buchanan C, Fey JV, Disa JJ, Mehrara BJ, Cordeiro PG (2008) Breast cancer recurrence following prosthetic, postmastectomy reconstruction: incidence, detection, and treatment. Plast Reconstr Surg 121(2):381–388
Wright JL, Cordeiro PG, Ben-Porat L, Van Zee KJ, Hudis C, Beal K, McCormick B (2008) Mastectomy with immediate expander-implant reconstruction, adjuvant chemotherapy, and radiation for stage II–III breast cancer: treatment intervals and clinical outcomes. Int J Radiat Oncol Biol Phys 70(1):43–50
Petit JY, Gentilini O, Rotmensz N, Rey P, Rietjens M, Garusi C, Botteri E, De Lorenzi F, Martella S, Bosco R, Khuthaila DK, Luini A (2008) Oncological results of immediate breast reconstruction: long term follow-up of a large series at a single institution. Breast Cancer Res Treat 112(3):545–549
Kronowitz SJ, Lam C, Terefe W, Hunt KK, Kuerer HM, Valero V, Lance S, Robb GL, Feng L, Buchholz TA (2011) A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg 127(6):2154–2166
Nedumpara T, Jonker L, Williams MR (2011) Impact of immediate breast reconstruction on breast cancer recurrence and survival. Breast 20(5):437–443
Eriksen C, Frisell J, Wickman M, Lidbrink E, Krawiec K, Sandelin K (2011) Immediate reconstruction with implants in women with invasive breast cancer does not affect oncological safety in a matched cohort study. Breast Cancer Res Treat 127(2):439–446
Buchholz TA, Strom EA, Perkins GH, McNeese MD (2002) Controversies regarding the use of radiation after mastectomy in breast cancer. Oncologist 7(6):539–546
Schechter NR, Strom EA, Perkins GH, Arzu I, McNeese MD, Langstein HN, Kronowitz SJ, Meric-Bernstam F, Babiera G, Hunt KK, Hortobagyi GN, Buchholz TA (2005) Immediate breast reconstruction can impact postmastectomy irradiation. Am J Clin Oncol 28(5):485–494
Motwani SB, Strom EA, Schechter NR, Butler CE, Lee GK, Langstein HN, Kronowitz SJ, Meric-Bernstam F, Ibrahim NK, Buchholz TA (2006) The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 66(1):76–82
Arthur DW, Arnfield MR, Warwicke LA, Morris MM, Zwicker RD (2000) Internal mammary node coverage: an investigation of presently accepted techniques. Int J Radiat Oncol Biol Phys 48(1):139–146
Koutcher L, Ballangrud A, Cordeiro PG, McCormick B, Hunt M, Van Zee KJ, Hudis C, Beal K (2010) Postmastectomy intensity modulated radiation therapy following immediate expander-implant reconstruction. Radiother Oncol 94(3):319–323
Ohri N, Cordeiro PG, Keam J, Ballangrud A, Shi W, Zhang Z, Nerbun CT, Woch KM, Stein NF, Zhou Y, McCormick B, Powell SN, Ho AY (2012) Quantifying the impact of immediate reconstruction in postmastectomy radiation: a large, dose-volume histogram-based analysis. Int J Radiat Oncol Biol Phys 84(2):e153–e159
Massabeau C, Fournier-Bidoz N, Wakil G, Castro Pena P, Viard R, Zefkili S, Reyal F, Campana F, Fourquet A, Kirova YM (2012) Implant breast reconstruction followed by radiotherapy: can helical tomotherapy become a standard irradiation treatment? Med Dosim 37(4):425–431
Klein EE, Kuske RR (1993) Changes in photon dose distributions due to breast prostheses. Int J Radiat Oncol Biol Phys 25(3):541–549
MacDonald SM, Patel SA, Hickey S, Specht M, Isakoff SJ, Gadd M, Smith BL, Yeap BY, Adams J, DeLaney TF, Kooy H, Lu H-M, Taghian AG (2013) Proton therapy for breast cancer after mastectomy: early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys 86(3):484–490
Jimenez RB, Goma C, Nyamwanda J, Kooy M, Halabi T, Napolitano BN, McBride SM, Taghian AG, Lu HM, MacDonald S (2013) Intensity modulated proton therapy for postmastectomy radiation of bilateral implant reconstructed breasts: a treatment planning study. Radiother Oncol 107(2):213–217
Moni J, Graves-Ditman M, Cederna P, Griffith K, Krueger EA, Fraass BA, Pierce LJ (2004) Dosimetry around metallic ports in tissue expanders in patients receiving postmastectomy radiation therapy: an ex vivo evaluation. Med Dosim 29(1):49–54
Thompson RCA, Morgan AM (2005) Investigation into dosimetric effect of a MAGNA-SITE tissue expander on post-mastectomy radiotherapy. Med Phys 32(6):1640–1646
Damast S, Beal K, Ballangrud A, Losasso TJ, Cordeiro PG, Disa JJ, Hong L, McCormick BL (2006) Do metallic ports in tissue expanders affect postmastectomy radiation delivery? Int J Radiat Oncol Biol Phys 66(1):305–310
Chen SA, Ogunleye T, Dhabbaan A, Huang EH, Losken A, Gabram S, Davis L, Torres MA (2013) Impact of internal metallic ports in temporary tissue expanders on postmastectomy radiation dose distribution. Int J Radiat Oncol Biol Phys 85(3):630–635
McCarthy CM, Mehrara BJ, Riedel E, Davidge K, Hinson A, Disa JJ, Cordeiro PG, Pusic AL (2008) Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg 121(6):1886–1892
Adesiyun TA, Lee BT, Yueh JH, Chen C, Colakoglu S, Anderson KEM, Nguyen M-DT, Recht AR (2011) Impact of sequencing of postmastectomy radiotherapy and breast reconstruction on timing and rate of complications and patient satisfaction. Int J Radiat Oncol Biol Phys 80(2):392–397
Anderson PR, Freedman G, Nicolaou N, Sharma N, Li T, Topham N, Morrow M (2009) Postmastectomy chest wall radiation to a temporary tissue expander or permanent implant- Is there a difference in complication rates? Int J Radiat Oncol Biol Phys 74(1):81–85
Hirsch EM, Seth AK, Dumanian GA, Kim JYS, Mustoe TA, Galiano RD, Fine NA (2014) Outcome of immediate tissue expander breast reconstruction followed by reconstruction of choice in the setting of postmastectomy radiation therapy. Ann Plast Surg 72(3):274–278
Piroth MD, Piroth DM, Pinkawa M, Woodruff SG, Holy R, Eble MJ (2009) Immediate reconstruction with an expander/implant following ablatio mammae because of breast cancer. Strahlenther Onkol 185(10):669–674
Aristei C, Falcinelli L, Bini V, Palumbo I, Farneti A, Petitto RP, Gori S, Perrucci E (2012) Expander/implant breast reconstruction before radiotherapy: outcomes in a single-institute cohort. Strahlenther Onkol 188(12):1074–1079
Baschnagel AM, Shah C, Wilkinson JB, Dekhne N, Arthur DW, Vicini FA (2012) Failure rate and cosmesis of immediate tissue expander/implant breast reconstruction after postmastectomy irradiation. Clin Breast Cancer 12(6):428–432
Cowen D, Gross E, Rouannet P, Teissier E, Ellis S, Resbeut M, Tallet A, Vaini Cowen V, Azria D, Hannoun-Levi JM (2010) Immediate post-mastectomy breast reconstruction followed by radiotherapy: risk factors for complications. Breast Cancer Res Treat 121(3):627–634
Nava MB, Pennati AE, Lozza L, Spano A, Zambetti M, Catanuto G (2011) Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg 128(2):353–359
Peled AW, Foster RD, Esserman LJ, Park CC, Hwang ES, Fowble B (2012) Increasing the time to expander-implant exchange after postmastectomy radiation therapy reduces expander-implant failure. Plast Reconstr Surg 130(3):503–509
Ho A, Cordeiro P, Disa J, Mehrara B, Wright J, Van Zee KJ, Hudis C, McLane A, Chou J, Zhang Z, Powell S, McCormick B (2012) Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer 118(9):2552–2559
Whitfield GA, Horan G, Irwin MS, Malata CM, Wishart GC, Wilson CB (2009) Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother Oncol 90(1):141–147
Spear SL, Baker JL Jr (1995) Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg 96(5):1119–1123
Claßen J, Nitzsche S, Wallwiener D, Kristen P, Souchon R, Bamberg M, Brucker S (2010) Fibrotic changes after postmastectomy radiotherapy and reconstructive surgery in breast cancer. Strahlenther Onkol 186(11):630–636
Spear SL, Seruya M, Rao SS, Rottman S, Stolle E, Cohen M, Rose KM, Parikh PM, Nahabedian MY (2012) Two-stage prosthetic reconstruction using alloderm including outcomes of different timings of radiotherapy. Plast Reconstr Surg 130(1):1–9
Cordeiro PG, Pusic AL, Disa JJ, McCormick B, VanZee K (2004) Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg 113(3):877–881
Goodman CM, Miller R, Patrick CW Jr, Zheng B, Duman H, Johnston C, Mani M, Cromeens D, Hanson WF, Evans GR (2002) Radiotherapy: effects on expanded skin. Plast Reconstr Surg 110(4):1080–1083
Kronowitz SJ, Robb GL (2004) Breast reconstruction with postmastectomy radiation therapy: current issues. Plast Reconstr Surg 114(4):950–960
Kronowitz SJ (2007) Immediate versus delayed reconstruction. Clin Plast Surg 34(1):39–50
Woerdeman LA, Hage JJ, Hofland MMI, Rutgers EJT (2007) A prospective assessment of surgical risk factors in 400 cases of skin-sparing mastectomy and immediate breast reconstruction with implants to establish selection criteria. Plast Reconstr Surg 119(2):455–463
Goodwin SJ, McCarthy CM, Pusic AL, Bui D, Howard M, Disa JJ, Cordeiro PG, Mehrara BJ (2005) Complications in smokers after postmastectomy tissue expander/implant breast reconstruction. Ann Plast Surg 55(1):16–19
Roostaeian J, Pavone L, Da Lio A, Lipa J, Festekjian J, Crisera C (2011) Immediate placement of implants in breast reconstruction: patient selection and outcomes. Plast Reconstr Surg 127(4):1407–1416
Kuske RR, Schuster R, Klein E, Young L, Perez CA, Fineberg B (1991) Radiotherapy and breast reconstruction: clinical results and dosimetry. Int J Radiat Oncol Biol Phys 21(2):339–346
Victor SJ, Brown DM, Horwitz EM, Martinez AA, Kini VR, Pettinga JE, Shaheen KW, Benitez P, Chen PY, Vicini FA (1998) Treatment outcome with radiation therapy after breast augmentation or reconstruction in patients with primary breast carcinoma. Cancer 82(7):1303–1309
Anderson PR, Hanlon AL, McNeeley SW, Freedman GM (2004) Low complications rates are achievable after postmastectomy breast reconstruction and radiation therapy. Int J Radiat Oncol Biol Phys 59(4):1080–1087
Alderman AK, Wilkins EG, Lowery JC, Kim M, Davis JA (2000) Determinants of patients satisfaction in postmastectomy breast reconstruction. Plast Reconstr Surg 106(4):769–776
Andrade WN, Baxter N, Semple J (2001) Clinical determinants of patients satisfaction in breast reconstruction. Plast Reconstr Surg 107(1):46–54
Hu ES, Pusic AL, Waljee JF, Kuhn L, Hawley ST, Wilkins E, Alderman AK (2009) Patient-reported aesthetic satisfaction with breast reconstruction during the long-term survivorship period. Plast Reconstr Surg 124(1):1–8
Chen SA, Hiley C, Nickleach D, Petsuksiri J, Andic F, Riesterer O, Switchenko JM, Torres MA (2013) Breast reconstruction and post-mastectomy radiation practice. Radiat Oncol 8:45
Korwar V, Skillman J, Matey P (2014) Skin reducing mastectomy and immediate reconstruction: the effect of radiotherapy on complications and patient reported outcomes. Eur J Surg Oncol 40(4):442–448
Winters ZE, Benson JR, Pusic AL (2010) A systematic review of the clinical evidence to guide treatment recommendations in breast reconstruction based on patient-reported outcome measures and health-related quality of life. Ann Surg 252(6):929–942
Thomson HJ, Winters ZE, Brandberg Y, Didier F, Blazeby JM, Mills J (2013) The early development phases of a European Organisation for Research and Treatment of Cancer (EORTC) module to assess patient reported outcome (PROs) in women undergoing breast reconstruction. Eur J Cancer 49(5):1018–1026
Acknowledgements
The authors would like to thank Drs Martina Iacco, Claudio Zucchetti and Marta Marcantonini for treatment planning and Dr Geraldine A Boyd for English editing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Aristei, C., Falcinelli, L., Perrucci, E. (2016). Expander/Implant Breast Reconstruction Before Radiotherapy. In: Shiffman, M. (eds) Breast Reconstruction. Springer, Cham. https://doi.org/10.1007/978-3-319-18726-6_36
Download citation
DOI: https://doi.org/10.1007/978-3-319-18726-6_36
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18725-9
Online ISBN: 978-3-319-18726-6
eBook Packages: MedicineMedicine (R0)