Abstract
The world has long experienced the impact of surfaces fouling with biofilms, not only in economic terms, but also, importantly, the adverse effect that biofilms can have with regard to public health. In the USA alone, billions of dollars are spent every year cleaning equipment, decontaminating products and cleaning ship hulls, while over 100,000 mortalities are reported annually as a result of infections resulting from medical device implant surgeries that have been compromised by the presence of pathogenic bacteria. Of great concern is that the heavy use of chemicals for neutralising bacterial colonies has resulted in the production of tougher, more resistant strains of pathogenic bacteria, which challenges the scientific community to find new approaches for controlling the formation of biofilms. Recently, the hierarchical structures found on the surfaces of some organisms, such as plant leaves and insect cuticles, have been shown to be superhydrophobic, self-cleaning, and possess bactericidal activity. Since the self-cleaning properties of the lotus leaf were reported in 1997, there has been a great deal of effort put into exploring this approach as a potential method for controlling the formation of biofilms. These discoveries may provide alternative approaches for controlling bacterial behaviour, either before or after the bacteria have attached to a substrate surface. This chapter provides a summary of some of the strategies employed by nature for controlling the colonisation of bacteria on surfaces.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Antibiofouling
- Superhydrophobicity
- Self-cleaning
- Bactericidal activity
- Wettability
- Plant leaves
- Insect cuticule
- Mechanobactericidal activity
2.1 Introduction
Biofouling has remained a complex, problematic issue for a long period of time. Its consequences impact not only upon the economy, but also public health. For this reason, antibacterial materials have been developed in order to design advanced strategies for limiting the colonisation of bacteria on their surfaces (Zhang et al. 2013). Traditionally, antibacterial surfaces were designed so that their surfaces would leach biocides, which would kill bacterial cells in situ and in areas surrounding the surface. For example, peptides and chitosan have been used as chemical-based methods for controlling the colonisation of bacteria on surfaces (Gazit 2007; Qi et al. 2004). Antibacterial metal nanoparticle s such as silver (Rai et al. 2009), copper (Hsiao et al. 2006), and molybdenum (Yasuyuki et al. 2010) have also been used as an additive for controlling bacterial attachment. The effects of these metals on human health and the environment are of growing concern. In addition, ever-increasing doses are now being required for chemical-based methods to effectively sterilise surfaces. This increased use of chemical agents has led to another problem; bacterial resistance to antibiotics. Therefore, the scientific community must continue to find alternative methods for effectively controlling bacterial attachment.
More recently, new approaches for preventing bacterial attachment, which use photocatalytic metal oxides such as TiO2 (Gelover et al. 2006) and ZnO (Franklin et al. 2007; Jones et al. 2008), have been developed. These materials produce highly reactive species such as hydroxyl radicals, hydrogen peroxide and superoxide, which are lethal to Escherichia coli and some other types of bacterial cells (Maness et al. 1999; Ibáñez et al. 2003). These metal oxides are, however, mainly activated by UVA light sources, which limits their potential biomedical applications (In et al. 2007; Fu et al. 2005).
Superhydrophobic /self-cleaning surfaces based on natural materials such as plant leaves and insect cuticles are currently being developed for controlling bacterial colonisation. Traditionally, only those materials that could induce bacterial cell death were considered to be antibacterial materials (Zhang et al. 2013), however antibiofouling materials, many of which are also superhydrophobic, can also classified in this category due to their potential application in controlling bacterial attachment. Many natural surfaces have been subjected to harsh environmental conditions in that they are constantly in contact with pollutants and changing weather conditions. Over millions of years of evolution, organisms have developed strategies that enable them to survive. Lotus leaves have been studied in detail since 1997 and have given rise to the archetypal “lotus effect” due to their self-cleaning nature (Barthlott and Neinhuis 1997). The properties that afford the lotus leaf these self-cleaning properties are their high water contact angle (θ > 150°) and low tilting angle (θ < 10°), the angle to which the leaf needs to be tilted in order for the water droplet to roll off the surface. These properties allow water droplets to collect dirt as they move over the surface, hence the term ‘self-cleaning’ (Webb et al. 2011) being applied to such surfaces. If artificial surfaces can be synthetically produced to possess similar surface characteristics and therefore cause water to behave in a similar way, bacterial cells could potentially also be cleaned from such surfaces before they have a chance to develop a biofilm. A similar phenomenon was also observed on the surfaces of sections of some insects , such as cicada and dragonfly wings. Interestingly, some insect surfaces possess not only self-cleaning properties, but also act as bactericidal surfaces (Ivanova et al. 2012; Pogodin et al. 2013).
2.2 Basics of Biofilms
A biofilm is defined as the attachment and development of microorganism community embedded in extracellular matrix on a surface (O’Toole et al. 2000). The organisms undergo a transition state between having the ability to be free swimming in their native environment (planktonic cells) to being cells that form part of the surface-attached community. The essential factors necessary for the formation of a biofilm are microbes and a substratum (Garrett et al. 2008). There are numerous advantages for bacteria to be part of a biofilm; these include resistance to antibiotics (Schmidt et al. 2012) and disinfectants (Ryu and Beuchat 2005; Simões et al. 2009) and being part of a dynamic environment (Liu and Tay 2002; Di Iaconi et al. 2005). Intercellular communication within the biofilm community also enhances the regulation of gene expression, which enables the bacterial cells to temporally adapt to any phenotypic variations in the surrounding environment, in addition to any deficiency in the available nutrient supply (Dalton and March 1998; Kjelleberg and Molin 2002; Daniels et al. 2004).
Biofilm formation can involve a single microbial sp ecies or multiple microbial species adhering onto a range of surfaces. On most environmental surfaces, mixtures of various species will dominate the biofilm. It is usually a single bacterial species, however, that is responsible for the infection of medical devices and implants (Holmes et al. 2008; Behlau and Gilmore 2008; Seo et al. 2008; Bulgarelli et al. 2013; Wu et al. 2012). According to a public health report in 2002 (Klevens et al. 2007), approximately 64 % of hospital attending cases resulted from the viable bacterial infection of medical devices and implants. These biofilms have been associated with 100,000 mortalities annually in the US alone. Researchers began studying biofilms over three decades ago, with the discovery that under natural living conditions, microorganisms dominantly attach themselves to surfaces (Geesey et al. 1977). The first recorded observation was published in 1933 by Henrici (1933), however the impact of biofilm formation had been recognised even before this time in the form of the fouling of ships in marine environments (Angst 1923). It has been estimated that the fouling of US Navy ships costs approximately US$ 180M–$260M per year. This represents only 0.5 % of the total number of ships world-wide (Schultz et al. 2011).
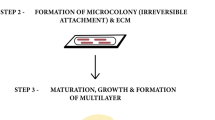
The initial development of a biofilm is described by a two stage kinetic binding model (Fig. 2.1). The first stage involves the initial reversible interaction that takes place between bacterial cells and the material surface, followed by the second stage where specific and non-specific interactions take place at the molecular level (Lichter et al. 2009; Bos et al. 1999). The interactions that occur in the second stage involve proteins that are expressed on the bacterial surface and on molecules on the material surfaces. The second stage occurs slowly and is irreversible once a mature biofilm has been formed. Apart from these two main steps of biofilm maturation, O’Toole et al. proposed that the starvation response pathway can also be considered as part of the biofilm development (O’Toole et al. 2000). This pathway is developed when the source of nutrients becomes depleted, and single microbial cells detach from the surface and return to their planktonic state, and commence infecting new areas of the surface.
Schematic repre sentation of the two stages of biofilm formation on substrate surfaces (Reproduced with permission from American Chemical Society (Lichter et al. 2009))
For these reasons, controlling bacterial attachment on material surfaces has been a long-standing battle for science. Several approaches have been developed to limit the colonisation of microbes onto the surfaces, however most of these have focussed on using chemical-based methods for bacterial control, which has led to the new and rising problem of bacterial resistance to these agents. Preventing bacterial adhesion from occurring by modifying the surface topography of substrates has been id entified as an approach that may provide attractive alternative strategies for controlling biofilm formation .
2.3 Antibiofouling Based on the Self-Cleaning Properties of a Surface
2.3.1 Wettability Theory
Wettability is a measure of the ability of a liquid to wet and spread over a solid surface. The contact angle, which is a function of surface energy of the solid, is formed when the liquid/vapour interface meets that of the liquid/solid interface. The wettability of solid surfaces plays an important role in daily life, industry and agriculture. Surfaces with special wettability properties, exhibiting for example high degrees of hydrophobici ty or hydrophilicity, have been the subject of much research due to the potential advantages associated with these types of surfaces (Nosonovsky and Bhushan 2005, 2007; Su et al. 2010). According to the most commonly agreed definitions, surfaces can be classified into one of four categories:
-
surfaces with a water contact angle greater than 150° and a tilting angle less than 10°. These surfaces are considered to be superhydrophobic and self-cleaning.
-
surfaces with a water contact angle between 90° and 150°. These surfaces are described as being hydrophobic.
-
surfaces with a water contact angle between 10° and 90°. These surfaces are described as being hydrophilic.
-
surfaces with a water contact angle less tha n 10°. These surfaces are considered to be superhydrophilic.
The measurement of water contact angle (WCA, θ) is the most common method for determining the wettability of surfaces. Originally, the contact angles were determined by Young’s Eq. 2.1 where the surfaces were assumed to be smooth, rigid, chemically homogeneous, insoluble and non-reactive (Zhang et al. 2013; Young 1805):
where θ is the contact angle; γ is the surface tension which is determined as the force per unit length; s, l, v represent solid, liquid and vapour, respectively. Surface tension is also known as surface energy, which is the energy required to br eak an intermolecular bond (Nosonovsky and Bhushan 2008). Numerically, surface tension and surface energy are equivalent, however they are thermodynamically different (Yan et al. 2011). Surface tension is used when dealing with liquids, whilst surface energy is a general term used for the description of solid surfaces.
In practice, most surfaces are both rough and chemically heterogeneous, and this complexity at the interface between the solid and liquid surfaces causes difficulties in determining the real contact angle. Wenzel first proposed a model to explain the relationship between surface roughness and the measured contact angle (Wenzel 1949), while Cassie and Baxter (1944) described the relationship between the surface fractions of different chemical composition and the contact angle. Wenzel’s equation is shown as:
where θ rough and θ smooth are water con tact angles on rough and ideal smooth surfaces, respectively, and r is the Wenzel roughness factor. The roughness factor is calculated as the ratio between the actual surface area and the projected surface area, which can be used to explain the change in surface hydrophobicity that arises through roughness, not surface chemistry. According to the theory, there are two separate cases where θ rough will behave differently as the roughness factor incre ases, depending on the value of θ smooth :
-
(i)
if θ smooth <90°, as r increases, θ rough will reduce to 0°
-
(ii)
if θ smooth >90°, as r increases, θ rough will approach 180°.
According to the Cassie and Baxter model, surface heterogeneity induces air entrapment between the topographical structures on a surface, which causes increased surface hydrophobicity , as given in the equation:
where, θ is the composite contact angle of t he heterogeneous surface, f 1 and f 2 are the area fractions of surface components 1 and 2, and θ 1 and θ 2 are their respective contact angles. This equation has been used widely to explain and/or predict the hydrophobicity of the surfaces with both a micro- and nano-hierarchical structure. When a water droplet sits on a rough surface, the two surface components that affect surface wettability are the surface itself and the air trapped between the surface features. Since the water contact angle on air can be taken as 180° (i.e. θ 2 = 180°), and f2 = 1 – f1, then Eq. 2.3 becomes:
$$ \cos \theta ={f}_1\left( \cos {\theta}_1+1\right)-1 $$(2.4)
According to Cassie-Baxter theory, superhydrophobicity arises from the combination of hierarchical surface structures that enable the entrapment of air on low surface energy materials. The sliding angle, another parameter that is important in determining the degree of hydrophobicity , is defined as the critical angle at which the water droplets start to slide along a tilted surface (Bhushan et al. 2009; Jung and Bhushan 2006; Yan et al. 2011). The scientific community has become aware of this principle only in the past century, whereas nature has adapted and evolved over millions of years to develop mechanisms that function according to this principle. Lotus leaves have long been regarded as a symbol of purity in many Asian cultures, and this originates from their clean nature despite being often found in unclean environmental conditions. It is now well established that the self-cleaning ability of the l otus leaf is a direct result of surface micro- and nanostructures that maximise the quantity of entrapped air in the surface, resulting in the condition of superhydrophobicity , in accordance to the Cassie-Baxter wetting regime. Several other organisms have been identified to utilise similar mechanisms, including other plant species and some insects. Some marine organisms are also known to remain clean through the different, but related concept of superoleophobicity. The following sections will focus on these organisms and the mechanisms by which their surfaces exhibit antibiofouling properties for c ontrolling bacterial colonisation onto the surfaces.
2.3.2 Plant Leaves
Since Barthlott and Neinhuis first reported the ‘lotus effect’, the lotus has become the archetype surface for exhibiting superhydrophobicity and self-cleaning abilities (Barthlott and Neinhuis 1997). Lotus leaves satisfy the two factors that are reflected in the Cassie-Baxter theory. The surface is covered by a layer of lipids, which are low in surface energy. The lipids appear as a layer of multiscale structures that enable a large quantity of air to be trapped in between the surface features. This results in a surface with very high WCA (θ ≈ 165°) and low tilting angle, hence the surface can remain clean as the water droplets collect dirt and contaminating particles as they roll off the surface. In Fig. 2.2, a mercury droplet that is spherical in shape can be seen to roll across the surface of a leaf. Cont aminants also adhere to the droplet rather than the surface. This demonstrates how superhydrophobic and self-cleaning surfaces can be very useful templates for designing antibiofouling materials.
Many other plants exhib it very similar properties to that of the lotus leaf, for example the Indian canna, taro and cabbage leaves. Plants first moved from water onto land approximately 480–360 million years ago; this was an important event in the history of life as it highlighted the consequences of the evolutionary changes of terrestrial organisms and global environments (Kenrick and Crane 1997). To cope with their new environments, plants developed a protective ‘skin’, known as the cuticle. The plant cuticle is a thin layer of lipophilic compounds that function as a protective barrier to perform various physiological, ecological and developmental roles. These roles include minimising water loss, reducing the leaching of cellular content, decreasing the adhesion of pathogenic spores and dust, protecting tissues from ultraviolet radiation, and mediation of their interaction with the surrounding environment (Van Maarseveen et al. 2009). The cuticle contains a continuous extracellular membrane that is made of biopolymers. These polymers cover the primary above-ground organs such as the flowers, leaves, stems and fruit of all land plants (Koch and Ensikat 2008). A mixture of hydrophobic compounds is integrated and superimposed on the cuticles, which is composed of various waxes (Jetter et al. 2000; Barthlott et al. 1998).
Plant waxes that are embed ded within the cutin network are called “intracuticular waxes”, whereas “epicuticular waxes” are located on the outer su rface of the cuticle (Barthlott and Neinhuis 1997; Barthlott et al. 1998; Buschhaus et al. 2007; Buschhaus and Jetter 2011; Ensikat et al. 2011; Koch et al. 2009). Cutin is a comprised of a polymer of predominantly ω- and mid-chain hydroxyl and epoxy C16 and C18 fatty acids in addition to glycerol (Samuels et al. 2008). The epicuticular waxes are organised within themselves to form three-dimensional crystals with highly variable morphologies, e.g., nano/micro projections, platelets, rods and tubules (Barthlott et al. 1998; Koch et al. 2006). Some examples of plants with superhydrophobic surfaces are pre sented in Fig. 2.3.
Images of some superhydrophobic plant surfaces, and their corresponding epicuticular wax structures: (a) Lotus leaves; (b) Indian canna leaves; (c) Rear face of purple Setcreasea leaves; (d) Rear face of ramee leaves (Guo and Liu 2007)
Both India canna leaves and purple Setcreasea are covered by many wax platelets, distributed randomly on a series of rod-like structures. This increases the proportion of air that can be trapped within the surface, producing water contact angles in excess of the 150° contact angle condition for superhydrophobicity (i.e. 165°). In the case of ramee leaves (Fig. 2.3d), the rear face is covered by a randomly distributed fiber-like structure which forms the layers of a web. This also allows for large amounts of entrapped air to be present on the surface, causing the surface to exhibit a large WCA (164°). The front of ramee leaves are significantly different in nature. They are composed of a web of micro-fibers, with many larger micrometer-size spheres without any further nanoscale-structure, and the sur face exhibits a WCA of 38° (Guo and Liu 2007).
There are many more le af surfaces that possess similar properties. Up to 200 water repellent plant species have been screened to measure their WCA and the majority were reported to possess superhydrophobic properties (Neinhuis and Barthlott 1997). The common feature shared by these surfaces is that each of them possessed a very dense layer of three-dimensional cuticular wax crystals arranged randomly or uniformly on their corresponding micro-scale surface features (e.g. papillae). This hierarchical structure enables the plant surfaces to remain clean, and therefore resistant against a wide range of contaminants. Many attempts have been made to understand how the lipids self-assemble into such useful and systematic structures, and while no clear understanding has yet been obtained regarding this process, it has been postulated that the cutin network may act as a template in controlling the orientation of the wax crystals (Jeffree 2006).
2.3.3 Insect Cuticle
Insects first ev olved the ability to fly at least 400 million years ago, and were the first organisms to develop powered flight; taking to the skies at least 90 million years prior to the earliest winged vertebrates (Grimaldi and Engel 2005). Nowadays they represent half of all eukaryotic species on earth. Insect wings are composed of lightweight building materials of thicknesses ranging from 0.5 μm to about 1 mm (Wan et al. 2008; Wootton 1992). In order to adapt to ever-changing environments, insects have evolved to possess geometric, non-smooth structures on their wing surface (Fig. 2.4) (Arsene et al. 2002; Boeve et al. 2011; Nelson and Charlet 2003). The presence of a thin superficial layer of waxes in the epicuticle was first reported by Ramsay in 1935 (Ramsay 1935).
As is the case with plant leaves, insec t surfaces are covered by a layer of cuticle, which is the barrier that directly interacts with the environment. Their terminology might be different, but in principle they are very similar in construction. The insect cuticle is secreted by a single layer of epidermal cells, forming a lipophilic structure that consists of two major sublayers, which are the epicuticle and the intracuticle (Lockey 1980, 1985; Nelson and Blomquist 1995; Buckner 2010; Jetter and Kunst 2008). The intracuticular layer, located beneath the epicuticle, is a mixture of chitin (poly-N-acetylglucosamine) and protein (Lockey 1980, 1985, 1988). The epicuticle is located in the outermost layer and is com posed of a mixture of aliphatic hydrocarbons and their derivatives; these compounds contain one or more oxygenated functional groups including esters, ketones, alcohols, aldehydes and fatty acids (Samuels et al. 2008; Koch and Ensikat 2008). This mixture of organic components is self-organized in the epicuticular layer of the cuticle, a highly-ordered, rough structure, composed of numerous micro- and nanometer-scale features. For some insects, e.g. dragonflies, the epicuticular waxes self-assemble into a three-dimensional layer of “nanopillars”, which enable air to be trapped in the spaces between and hence exhibit a high WCA (Ivanova et al. 2013b; Nguyen et al. 2013). Insect wing membranes are composed of lightweight building materials with a thickness ranging from 0.5 μm to approximately 1 mm (Wootton 1992). Their wings are framed by a system of veins that aid in stabilizing the wing as a whole (Kreuz et al. 2001; Gorb 1999; Moussian 2010). The highly-ordered, rough structure of the epicuticle enables insects to minimize their mass but still retain the ability to protect themselves from being wet by rain and coated with pollutants (Fig. 2.4).
A sy stematic terminology to describe the 2D and 3D micro- and nano-scale structures of the insect cuticle has not thus far been developed. Byun et al. used the terms ‘layered cuticle’, ‘setae’, ‘denticles’ and ‘fractal’ to describe the morpho logical features present on the surfaces of the insect wings, and this is the system that will be adopted here. The term ‘layered cuticle’ refers to a surface that contains scale-like structures that overlap, such as those typically found on butterfly wings. Surfaces with ‘setae’ contain high aspect ratio nanopillars or hairs. ‘Denticle’ structures refer to tooth-like projections, and these can vary greatly in their morphology, ranging from small hemispheres to taller nanopillars. ‘Fractal’ structures are composed of an irregular array of fine nanoscale protrusions (Byun et al. 2009). Among these structural types, the presence of layered cuticles, denticles and fractal structures result in the production of the most hydrophobic surfaces in a majority of cases, whilst the presence of setae alone on a surface usually produces a surface exhibiting hydrophilic properties (Table 2.1).
The superhydrophobicity of an insect wing surface, together with its ability to self-clean, are very important factors that contribute to an insect’s ability to survive. The nanoarray structures present on the surfaces of some insect wings such as those of the cicada and dragonfly afford the insect antireflective properties, which can assist in protecting them from predators (Watson et al. 2008). The superhydrophobic and self-cleaning properties can also assist in keeping their surfaces clean and free from contaminants that may also adversely impact their antireflective properties. The self-cleaning properties of these insect wings can be further enh anced due to the presence of turbulent conditions during their flight (Nishimoto and Bhushan 2013).
2.3.4 Superoleophobicity
Superhydrophobicity is the key for terrestrial organisms to deal with contaminants, however it is not a practical option for aquatic organisms, since their living conditions require constant contact with water. In order to cope with this differen ce in living conditions, nature has employed a different, but similar concept. The surfaces of these organisms are modified so that their surfaces remain wet but unable to be wet by oils, the main source of contaminants, particularly with modern types of marine pollution. Several aquatic species exhibit superoleophobicity rather than superhydrophobicity, exhibiting oil contact angles (OCA) greater than 150° when submerged in water. These organisms possess hierarchical surface structures that are self-cleaning, antifouling and promote low-drag conditions when moving through water (Bixler and Bhushan 2013).
For example, dolphin (Fish and Hui 1991), whale (Baum et al. 2002) and shark skin is known to reduce drag and improve fouling resistance. The skin of bottlenose dolphins Tursiops truncatus and the k iller whale Orcinus orca are covered by dermal ridges positioned such that they are transverse to the direction of flow (Ridgway and Carder 1993; Fish 2006). Another whale, Globicephala melas has enclosed nanopores on their patterned ridges, which exhibit great antifouling ability (Baum et al. 2002). Shark skin is covered by dermal denticles shaped like small ribs (or ‘riblets’). The denticles are oriented so that they align with the direction of fluid flow as the shark swims through the water. The low drag riblet microstructure, together with a mucous layer on the surface, allows the shark to remain flexible and clean (Bushnell and Moore 1991; Bechert et al. 1997; Dean and Bhushan 2010). This surface structure also provides protection from abrasion, which in turn minimises the opportunities for microorganisms to adhere (Bhushan 2012). Fish scales are another example of self-cleaning surfaces in aquatic environments (Hay 1996). They perform in a manner that is very similar to the shark skin. Their surfaces are covered by sector-like scales (diameter of 4–5 mm), which are covered by papillae (100–300 μm in length and 30–40 μm in width), and exhibit a particularly high oil contact angle in water (163°).
The surface structures of snail shells have been commercially exploited in the construction of snail shell-inspired self-cleaning surfaces for outdoor walls (Nishimoto and Bhushan 2013). These surfaces exhibit the ability to remai n clean, despite their dwelling environment and their appearance on rainy days. The surface of snail shells is comprised of a regularly rough structure consisting of line grooves (pitch of 0.5 mm), smaller grooves crossing the line groove (pitch of 0.1 mm) and micro-grooves between the line grooves (pitch of 10 μm). The surface of snail shells is covered by a regular hierarchical structure that ranges in size from micrometers to millimeters, which may facilitate water entrapment. Compared to superhydrophobic surfaces, which entrap air within their hierarchical structure, superoleophobic surfaces trap water molecules. This water-entrapment system helps the shells remain wet, yet remain clean under their semi-aquatic living conditions. This is a key factor that contributes to their ability to self-clean, in the way that their usually wetted surface is rarely able to be contamin ated (Nishimoto and Bhushan 2013).
2.4 Mechanobactericidal Activity
The inspiration that can be obtained from insects appears to be almost unlimited. Ivanova et al. recently found that the robust hexagonal arrays of ‘nanopillars’ on the surfaces of Psaltoda claripennis cicada wings are bactericidal (Ivanova et al. 2012). This nanopattern present on the wing surfaces penetrated attaching Pseudomonas aeruginosa cells, killing them with extreme efficiency (Fig. 2.5a, a1). The surface of the cicada wings retained its lethality against these G ram negative pathogenic bacteria even after the surface was coated with a 10 nm-thick layer of gold, which indicated that the bactericidal properties of the cicada wing surfaces arose from the physical properties of the wing surfaces, rather than from their chemical composition. It was also reported that the wings consistently killed other Gram-negative bacteria, i.e., Branhamella catarrhalis, E. coli, and Pseudomonas fluorescens, however Gram-positive cells (Bacillus subtilis, Planococcus maritimus, and Staphylococcus aureus) were found to be resistant to the action of the wing surface (Hasan et al. 2012). Cicada wings were the first example of a surface with bactericidal properties that arose as a result of purely physical action.
To explain this phenomenon, biophysical models were constructed to describe the interaction taking place between the bacterial cells and the nanopatterns present on the surface of the cicada wings (Pogodin et al. 2013). Mathematical calculations revealed that the nanopillars did not pierce the cells but rather the cells were stretched in the regions between the nanopillars as they adsorbed onto the wing surface, until the point of cell rupture. It was also found that the more rigid the cell membrane, the harder they were to break, which was consistent with the results obtained for the Gram-positive bacteria that attached to the wing surface, but were not killed by the action of the nanopillars; the thicker layer of peptidoglycan present in the cell wall afforded the cells a greater dgree of rigidity, making them resistant to the action of the wing nanopillars. This was supported experimentally by decreasing the rigidity of Gram-positive cells though microwave treatment. B. subtilis, S. aureus, and Planococcus maritimus were used as bacterial species. After microwave treatment, all three bacterial species showed a high level of susceptibility to the action of the cicada wing surfaces.
In contrast to cicada wings, which only showed effectiveness against Gram negative bacteria, the surfaces of dragonfly wings were shown to have the ability to kill a large range of bacterial species, including Gram-negative (Pseudomonas aeruginosa), and Gram-positive, (Staphylococcus aureus and Bacillus subtilis) bacteria and even endospores. Similar to cicada wings, dragonfly wings surfaces are covered by a layer of nanopillar-like structures, which punctured all types of bacterial cells that came into contact with the surface, as demonstrated in Fig. 2.5, b1–3. A synthetic material known as black silicon that mimics the surface structure of these dragonfly wings also demonstrated antibacterial properties against these different types of bacterial cells (Ivanova et al. 2013a). The discovery of the bactericidal properties possessed by th ese insect wings has brought them into focus as promising new prospects as templates for the production of synthetic biocidal surfaces.
References
Angst EC (1923) The fouling of ships bottoms by bacteria. Rep., Bur. Constr. Repair. US Navy Department, Washington, DC
Arsene C, Schulz S, Van Loon JJA (2002) Chemical polymorphism of the cuticular lipids of the cabbage white Pieris rapae. J Chem Ecol 28(12):2627–2631
Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202(1):1–8
Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H (1998) Classification and terminology of plant epicuticular waxes. Bot J Linn Soc 126(3):237–260
Baum C, Meyer W, Stelzer R, Fleischer LG, Siebers D (2002) Average nanorough skin surface of the pilot whale (Globicephala melas, Delphinidae): considerations on the self-cleaning abilities based on nanoroughness. Mar Biol 140(3):653–657
Bechert DW, Bruse M, Hage W, Van Der Hoeven JGT, Hoppe G (1997) Experiments on drag-reducing surfaces and their optimization with an adjustable geometry. J Fluid Mech 338:59–87
Behlau I, Gilmore MS (2008) Microbial biofilms in ophthalmology and infectious disease. Arch Ophthalmol 126(11):1572–1581
Bhushan B (2012) Bioinspired structured surfaces. Langmuir 28(3):1698–1714
Bhushan B, Jung YC, Koch K (2009) Self-cleaning efficiency of artificial superhydrophobic surfaces. Langmuir 25(5):3240–3248
Bixler GD, Bhushan B (2013) Fluid drag reduction and efficient self-cleaning with rice leaf and butterfly wing bioinspired surfaces. Nanoscale 5(17):7685–7710
Boeve JL, Voigt D, Gorb SN (2011) Crystalline wax coverage of the cuticle in easy bleeding sawfly larvae. Arthropod Struct Dev 40(2):186–189
Bos R, Van Der Mei HC, Busscher HJ (1999) Physico-chemistry of initial microbial adhesive interactions – its mechanisms and methods for study. FEMS Microbiol Rev 23(2):179–229
Buckner JS (2010) Oxygenated derivatives of hydrocarbons. In: Blomquist GJ, Bagnères A-G (eds) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press, Cambridge, pp 187–203
Bulgarelli D, Schlaeppi K, Spaepen S, Van Themaat EVL, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838
Buschhaus C, Jetter R (2011) Composition differences between epicuticular and intracuticular wax substructures: how do plants seal their epidermal surfaces? J Exp Bot 62(3):841–853
Buschhaus C, Herz H, Jetter R (2007) Chemical composition of the epicuticular and intracuticular wax layers on adaxial sides of Rosa canina leaves. Ann Bot 100(7):1557–1564
Bushnell DM, Moore KJ (1991) Drag reduction in nature. Annu Rev Fluid Mech 23(1):65–79
Byun D, Hong J, Saputra KJH, Lee YJ, Park HC, Byun BK, Lukes JR (2009) Wetting characteristics of insect wing surfaces. J Bionic Eng 6(1):63–70
Cassie ABD, Baxter S (1944) Wettability of porous surfaces. Trans Faraday Soc 40:546–551
Dalton HM, March PE (1998) Molecular genetics of bacterial attachment and biofouling. Curr Opin Biotechnol 9(3):252–255
Daniels R, Vanderleyden J, Michiels J (2004) Quorum sensing and swarming migration in bacteria. FEMS Microbiol Rev 28(3):261–289
Dean B, Bhushan B (2010) Shark-skin surfaces for fluid-drag reduction in turbulent flow: a review. Philos Trans A Math Phys Eng Sci 368(1929):4775–4806
Di Iaconi C, Ramadori R, Lopez A, Passino R (2005) Hydraulic shear stress calculation in a sequencing batch biofilm reactor with granular biomass. Environ Sci Technol 39(3):889–894
Ensikat HJ, Ditsche-Kuru P, Neinhuis C, Barthlott W (2011) Superhydrophobicity in perfection: the outstanding properties of the lotus leaf. Beilstein J Nanotechnol 2(1):152–161
Fish FE (2006) The myth and reality of Gray’s paradox: implication of dolphin drag reduction for technology. Bioinspir Biomim 1(2):R17–R25
Fish FE, Hui CA (1991) Dolphin swimming – a review. Mammal Rev 21(4):181–195
Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS (2007) Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol 41(24):8484–8490
Fu G, Vary PS, Lin CT (2005) Anatase TiO2 nanocomposites for antimicrobial coatings. J Phys Chem B 109(18):8889–8898
Garrett TR, Bhakoo M, Zhang Z (2008) Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 18(9):1049–1056
Gazit E (2007) Self-assembled peptide nanostructures: the design of molecular building blocks and their technological utilization. Chem Soc Rev 36(8):1263–1269
Geesey GG, Richardson WT, Yeomans HG, Irvin RT, Costerton JW (1977) Microscopic examination of natural sessile bacterial populations from an alpine stream. Can J Microbiol 23(12):1733–1736
Gelover S, Gómez LA, Reyes K, Leal MT (2006) A practical demonstration of water disinfection using TiO2 films and sunlight. Water Res 40(17):3274–3280
Gorb SN (1999) Serial elastic elements in the damselfly wing: mobile vein joints contain resilin. Naturwissenschaften 86(11):552–555
Grimaldi D, Engel MS (2005) Insects take to the skies. In: Evolution of the insects. Cambridge University Press, New York, pp 155–178
Guo Z, Liu W (2007) Biomimic from the superhydrophobic plant leaves in nature: binary structure and unitary structure. Plant Sci 172(6):1103–1112
Hasan J, Webb HK, Truong VK, Pogodin S, Baulin VA, Watson GS, Watson JA, Crawford RJ, Ivanova EP (2012) Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl Microbiol Biotechnol 97(20):1–6
Hay ME (1996) Marine chemical ecology: what’s known and what’s next? J Exp Mar Biol Ecol 200(1–2):103–134
Henrici AT (1933) Studies of freshwater bacteria. I. A direct microscopic technique. J Bacteriol 25(3):277–287
Holmes A, Doré CJ, Saraswatula A, Bamford KB, Richards MS, Coello R, Modi N (2008) Risk factors and recommendations for rate stratification for surveillance of neonatal healthcare-associated bloodstream infection. J Hosp Infect 68(1):66–72
Hsiao MT, Chen SF, Shieh DB, Yeh CS (2006) One-pot synthesis of hollow Au3Cu1 spherical-like and biomineral botallackite Cu2(OH)3Cl flowerlike architectures exhibiting antimicrobial activity. J Phys Chem B 110(1):205–210
Ibáñez JA, Litter MI, Pizarro RA (2003) Photocatalytic bactericidal effect of TiO2 on Enterobacter cloacae. Comparative study with other Gram (-) bacteria. J Photochem Photobiol A Chem 157(1):81–85
In S, Orlov A, Berg R, García F, Pedrosa-Jimenez S, Tikhov MS, Wright DS, Lambert RM (2007) Effective visible light-activated B-doped and B, N-codoped TiO2 photocatalysts. J Am Chem Soc 129(45):13790–13791
Ivanova EP, Hasan J, Webb HK, Truong VK, Watson GS, Watson JA, Baulin VA, Pogodin S, Wang JY, Tobin MJ, Löbbe C, Crawford RJ (2012) Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 8(16):2489–2494
Ivanova EP, Hasan J, Webb KW, Gervinskas G, Juodkazis S, Truong VK, Wu AHF, Lamb RN, Baulin VA, Watson GS, Watson JA, Mainwaring DE, Crawford RJ (2013a) Bactericidal activity of black silicon. Nat Commun 4:2838–2844
Ivanova EP, Nguyen SH, Webb HK, Hasan J, Truong VK, Lamb RN, Duan X, Tobin MJ, Mahon PJ, Crawford RJ (2013b) Molecular organization of the nanoscale surface structures of the dragonfly Hemianax papuensis wing epicuticle. PLoS ONE 8(7):e67893
Jeffree CE (2006) In: Riederer M, Müller C (eds) Biology of the plant cuticle. Blackwell Publishing, Oxford, pp 11–125
Jetter R, Kunst L (2008) Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. Plant J 54(4):670–683
Jetter R, Schäffer S, Riederer M (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23(6):619–628
Jones N, Ray B, Ranjit KT, Manna AC (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279(1):71–76
Jung YC, Bhushan B (2006) Contact angle, adhesion and friction properties of micro- and nanopatterned polymers for superhydrophobicity. Nanotechnology 17(19):4970–4980
Kenrick P, Crane PR (1997) The origin and early evolution of plants on land. Nature 389(6646):33–39
Kjelleberg S, Molin S (2002) Is there a role for quorum sensing signals in bacterial biofilms? Curr Opin Microbiol 5(3):254–258
Klevens RM, Edwards JR, Richards CL Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM (2007) Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep 122(2):160–166
Koch K, Ensikat HJ (2008) The hydrophobic coatings of plant surfaces: epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 39(7):759–772
Koch K, Dommisse A, Barthlott W (2006) Chemistry and crystal growth of plant wax tubules of lotus (Nelumbo nucifera) and nasturtium (Tropaeolum majus) leaves on technical substrates. Cryst Growth Des 6(11):2571–2578
Koch K, Bhushan B, Ensikat HJ, Barthlott W (2009) Self-healing of voids in the wax coating on plant surfaces. Philos Trans A Math Phys Eng Sci 367(1894):1673–1688
Kreuz P, Arnold W, Kesel AB (2001) Acoustic microscopic analysis of the biological structure of insect wing membranes with emphasis on their waxy surface. Ann Biomed Eng 29(12):1054–1058
Lichter JA, Van Vlietpa KJ, Rubner MF (2009) Design of antibacterial surfaces and interfaces: polyelectrolyte multilayers as a multifunctional platform. Macromolecules 42(22):8573–8586
Liu Y, Tay JH (2002) The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res 36(7):1653–1665
Lockey KH (1980) Insect cuticular hydrocarbons. Comp Biochem Physiol B: Biochem Mol Biol 65(3):457–462
Lockey KH (1985) Insect cuticular lipids. Comp Biochem Physiol B: Biochem Mol Biol 81(2):263–273
Lockey KH (1988) Lipids of the insect cuticle: origin, composition and function. Comp Biochem Physiol B: Biochem Mol Biol 89(4):595–645
Maness PC, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA (1999) Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol 65(9):4094–4098
Moussian B (2010) Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem Mol Biol 40(5):363–375
Neinhuis C, Barthlott W (1997) Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann Bot 79(6):667–677
Nelson DR, Blomquist LG (1995) Insect waxes. In: Hamilton RJ, Christie WW (eds) Waxes: chemistry, molecular biology and functions. The Oily Press Ltd Hamilton RJ, Dundee, pp 1–90
Nelson DR, Charlet LD (2003) Cuticular hydrocarbons of the sunflower beetle, Zygogramma exclamationis. Comp Biochem Physiol B: Biochem Mol Biol 135(2):273–284
Nguyen SH, Webb HK, Hasan J, Tobin MJ, Crawford RJ, Ivanova EP (2013) Dual role of outer epicuticular lipids in determining the wettability of dragonfly wings. Colloids Surf B: Biointerfaces 106:126–134
Nishimoto S, Bhushan B (2013) Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv 3(3):671–690
Nosonovsky M, Bhushan B (2005) Roughness optimization for biomimetic superhydrophobic surfaces. Microsyst Technol 11(7):535–549
Nosonovsky M, Bhushan B (2007) Multiscale friction mechanisms and hierarchical surfaces in nano- and bio-tribology. Mater Sci Eng R Rep 58(3–5):162–193
Nosonovsky M, Bhushan B (2008) Multiscale dissipative mechanisms and hierarchical surfaces: friction, superhydrophobicity and biomimetics. Springer, Berlin
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79
Pogodin S, Hasan J, Baulin VA, Webb HK, Truong VK, Nguyen THP, Boshkovikj V, Fluke CJ, Watson GS, Watson JA, Crawford RJ, Ivanova EP (2013) Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys J 104(4):835–840
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339(16):2693–2700
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83
Ramsay JA (1935) The evaporation of water from the cockroach. J Exp Biol 12:373–383
Ridgway SH, Carder DA (1993) Features of dolphin skin with potential hydrodynamic importance. IEEE Eng Med Biol Manag 12(3):83–88
Ryu JH, Beuchat LR (2005) Biofilm formation and sporulation by Bacillus cereus on a stainless steel surface and subsequent resistance of vegetative cells and spores to chlorine, chlorine dioxide, and a peroxyacetic acid-based sanitizer. J Food Protect 68(12):2614–2622
Samuels L, Kunst L, Jetter R (2008) Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu Rev Plant Biol 59:683–707
Schmidt S, Winter J, Gallert C (2012) Long-term effects of antibiotics on the elimination of chemical oxygen demand, nitrification, and viable bacteria in laboratory-scale wastewater treatment plants. Arch Environ Contam Toxicol 63(3):354–364
Schultz MP, Bendick JA, Holm ER, Hertel WM (2011) Economic impact of biofouling on a naval surface ship. Biofouling 27(1):87–98
Seo YS, Lee DY, Rayamahji N, Kang ML, Yoo HS (2008) Biofilm-forming associated genotypic and phenotypic characteristics of Staphylococcus spp. isolated from animals and air. Res Vet Sci 85(3):433–438
Simões M, Simões LC, Vieira MJ (2009) Species association increases biofilm resistance to chemical and mechanical treatments. Water Res 43(1):229–237
Su Y, Ji B, Zhang K, Gao H, Huang Y, Hwang K (2010) Nano to micro structural hierarchy is crucial for stable superhydrophobic and water-repellent surfaces. Langmuir 26(7):4984–4989
Van Maarseveen C, Han H, Jetter R (2009) Development of the cuticular wax during growth of Kalanchoe daigremontiana (Hamet et Perr. de la Bathie) leaves. Plant Cell Environ 32(1):73–81
Wan Y, Cong Q, Wang X, Yan Z (2008) The wettability and mechanism of geometric non-smooth structure of dragonfly wing surface. J Bionic Eng 5(SUPPL):40–45
Watson GS, Myhra S, Cribb BW, Watson JA (2008) Putative functions and functional efficiency of ordered cuticular nanoarrays on insect wings. Biophys J 94(8):3352–3360
Webb HK, Hasan J, Truong VK, Crawford RJ, Ivanova EP (2011) Nature inspired structured surfaces for biomedical applications. Curr Med Chem 18(22):3367–3375
Wenzel RN (1949) Surface roughness and contact angle. J Phys Colloid Chem 53(9):1466–1467
Wootton RJ (1992) Functional morphology of insect wings. Annu Rev Entomol 37(1):113–140
Wu MY, Sendamangalam V, Xue Z, Seo Y (2012) The influence of biofilm structure and total interaction energy on Escherichia coli retention by Pseudomonas aeruginosa biofilm. Biofouling 28(10):1119–1128
Yan YY, Gao N, Barthlott W (2011) Mimicking natural superhydrophobic surfaces and grasping the wetting process: a review on recent progress in preparing superhydrophobic surfaces. Adv Colloid Interface Sci 169(2):80–105
Yasuyuki M, Kunihiro K, Kurissery S, Kanavillil N, Sato Y, Kikuchi Y (2010) Antibacterial properties of nine pure metals: a laboratory study using Staphylococcus aureus and Escherichia coli. Biofouling 26(7):851–858
Young T (1805) An essay on the cohesion of fluids. Philos Trans R Soc Lond 95:65–87
Zhang X, Wang L, Levänen E (2013) Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv 3(30):12003–12020
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Nguyen, S.H., Webb, H.K., Crawford, R.J., Ivanova, E.P. (2015). Natural Antibacterial Surfaces. In: Ivanova, E., Crawford, R. (eds) Antibacterial Surfaces. Springer, Cham. https://doi.org/10.1007/978-3-319-18594-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-18594-1_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18593-4
Online ISBN: 978-3-319-18594-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)