Abstract
Our previous studies demonstrated that thrombin is an important factor in brain injury after intracerebral and intraventricular hemorrhage. This study examined the effect of acetazolamide, a carbonic anhydrase inhibitor, on thrombin-induced hydrocephalus. There were two parts in this study. First, rats had an injection of either 50 μl saline or 3 U thrombin into the right lateral ventricle. Second, rats had an injection of 3 U thrombin into the right lateral ventricle and were treated with either vehicle or acetazolamide (30 mg/kg, intraperitoneally (IP)) at 1 h after thrombin infusion. Lateral ventricle volumes were measured in magnetic resonance imaging T2 images and the brains were used for histology analysis at 24 h later. Intraventricular injection of thrombin induced significantly larger ventricle volume (27.8 ± 3.7 vs 8.5 ± 1.3 mm3, n = 6, p < 0.01) and more ventricular wall damage (the breakdown of the ependymal layer, 20.2 ± 3.1 vs 2.4 ± 0.8 %, n = 6, p < 0.01) compared with saline injection. Acetazolamide treatment (30 mg/kg, IP) markedly attenuated thrombin-induced hydrocephalus (16.1 ± 4.2 mm3 vs 29.5 ± 5.3 mm3, n = 6, p < 0.01). These results suggest decreasing CSF production by acetazolamide attenuated thrombin-induced hydrocephalus in rats.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Studies have found intraventricular hemorrhage (IVH) is a predictor of poor outcome after intracerebral hemorrhage (ICH) [2, 8]. For example, the presence of IVH in patients with ICH lowered the rate of favorable outcome from 31 to 15 % and was an independent predictor of worse outcome in the International Surgical Trial in ICH [2]. That trial also showed that hydrocephalus develops in more than 50 % of patients with IVH [2]. IVH is also an independent prognostic factor for poor outcome in SAH patients and acute hydrocephalus occurs in 20–30 % of such patients [15]. However, the mechanisms of brain hemorrhage-induced hydrocephalus are not well understood.
Thrombin is a serine protease and an essential component in the coagulation cascade. Thrombin forms immediately after brain hemorrhage. Our recent study found that thrombin has a role in hydrocephalus development after IVH [6]. Acetazolamide is a carbonic anhydrase inhibitor and can reduce CSF production [3]. This study examined the effect of acetazolamide on thrombin-induced hydrocephalus.
Materials and Methods
Animal Preparation and Intraventricular Injection
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. A total of 24 male Sprague-Dawley rats (Charles River Laboratories, Portage, MI, USA), at the weight of 270–300 g, were used in this study. Animals were anesthetized with pentobarbital (50 mg/kg intraperitoneally (IP)) and the right femoral artery was catheterized to monitor arterial blood pressure, blood pH, PaO2, PaCO2, hematocrit, and glucose levels. Core body temperature was maintained at 37.5 °C with a feedback-controlled heating pad. Rats were then positioned in a stereotaxic frame. A cranial burr hole (1 mm) was drilled and a 26-gauge needle was inserted stereotaxically into the right lateral ventricle (coordinates: 0.6 mm posterior, 4.5 mm ventral, and 1.6 mm lateral to the bregma). Saline or thrombin was infused using a microinfusion pump. The needle was removed after injection, the burr hole was filled with bone wax, and the skin incision was sutured closed.
Experimental Groups
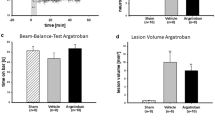
There were two parts in this study. First, rats had an injection of either 50 μl saline (n = 6) or 3 U of rat thrombin in 50 μl saline (n = 6) into the right lateral ventricle. Second, rats had an injection of 3 U thrombin into the right lateral ventricle and were treated with either vehicle (n = 6) or acetazolamide (30 mg/kg, IP, n = 6) at 1 h after thrombin infusion. All rats underwent magnetic resonance imaging (MRI) 24 h after the intraventricular injection and were then euthanized. Lateral ventricle volumes were measured in T2-weighted MRI images and the brains were used for histology.
MRI Scanning and Ventricle Volume Measurement
Rats were anesthetized with 2 % isoflurane throughout the MRI examination. MRI scanning was performed in a 7.0-T Varian MR scanner (Varian Inc., Palo Alto, CA) with a T2 fast spin-echo sequence, using a view field of 35 × 35 mm and 25 coronal slices [17]. Ventricular volumes were measured and calculated as described previously [4, 12, 13]. Bilateral ventricles were outlined and the areas were measured. Ventricular volume was calculated by summing the ventricle areas over all slices and multiplying by the section thickness. All image analysis was performed using the ImageJ program by a blinded observer.
Ventricular Wall Damage Analysis
Ventricular wall damage was analyzed by calculating the percentage of the ependyma that was damaged, as previously described [13]. Briefly, brain sections underwent hematoxylin and eosin staining. The length of the ependyma that was disrupted or detached from the periventricular parenchyma was determined and divided by the total ventricular surface perimeter. All the analysis was performed using ImageJ software by a blinded observer.
Statistical Analysis
Values are given as means ± standard deviation (SD). Student’s t-tests or Mann-Whitney U tests were used to analyze the data. Differences were considered significant at p < 0.05.
Results
MRI showed that intraventricular injection of thrombin (3 U) resulted in hydrocephalus. Thus, lateral ventricular volume in thrombin-injected rats was significantly larger than in saline-injected rats at 24 h (27.8 ± 3.7 vs 8.5 ± 1.3 mm3 in saline, p < 0.01, Fig. 1). In addition, intraventricular thrombin injection, but not saline injection, caused severe ventricular wall damage (20.2 ± 1.7 % vs 2.8 ± 0.4 % in saline, p < 0.01) at 24 h (Fig. 2).
Systemic treatment with acetazolamide, a carbonic anhydrase inhibitor, 1 h after intraventricular injection of 3 U of thrombin resulted in less ventricular enlargement compared with vehicle treatment (16.1 ± 4.2 vs 29.5 ± 5.3 mm3 in the vehicle-treated group, p < 0.01) (Fig. 3).
Discussion
In this study, we found intraventricular injection of thrombin caused hydrocephalus and ventricular ependymal wall damage. Acetazolamide, given at 30 mg/kg IP 1 h after thrombin injection, attenuated thrombin-induced hydrocephalus.
Thrombin is a serine protease and an essential component in the coagulation cascade. Experimental investigations have indicated that thrombin formation plays a major role in ICH-induced injury [9, 11, 16]. Thrombin is responsible for early brain edema formation after ICH, and that edema results partly from a direct opening of the blood-brain barrier (BBB). We have demonstrated that intraventricular injection of thrombin can cause significant ventricular dilatation and periventricular parenchyma injury [6]. At present, the mechanisms associated with thrombin-induced ventricular dilatation are unknown. Ependymal injury such as reduced cilia and abnormal of organelle structure were observed in thrombin-injected rats [6]. Ependymal damage may lead to increased periventricular brain injury and to hydrocephalus [4, 14]. Activities of normal ependymal cilia are thought to direct cerebrospinal fluid (CSF) current toward the ventricular outlets. Previous study showed absent or functionally defective ependymal cell motile cilia may be a cause of hydrocephalus in a mouse model [1]. Therefore, ependymal damage and abnormal ependymal cilia may play a role in hydrocephalus induced by thrombin.
Acetazolamide, a carbonic anhydrase inhibitor, decreases CSF production in animal models [5, 10, 17]. Acetazolamide has a rapid onset of action. Treatment with 10 mg/kg of acetazolamide resulted in a significant decrease in CSF production and absorption within 3 h of ingestion in dogs [17]. Acetazolamide is also used in both children and adults to treat hydrocephalus and pseudotumor cerebri [6, 7]. We have shown that co-injection of acetazolamide reduced ICH-induced brain injury [7]. In the present study, acetazolamide treatment significantly reduced thrombin-induced hydrocephalus, most likely by reducing CSF production.
Conclusions
Intraventricular injection of thrombin caused ventricular wall damage and hydrocephalus in rats. Inhibition of carbonic anhydrase by acetazolamide effectively reduced thrombin-induced hydrocephalus. The results suggest that thrombin-induced hydrocephalus may result from reduced absorption and/or increased production of CSF.
References
Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK (2005) Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132:5329–5339
Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD (2006) Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl 96:65–68
Carrion E, Hertzog JH, Medlock MD, Hauser GJ, Dalton HJ (2001) Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts. Arch Dis Child 84:68–71
Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G (2011) Role of iron in brain injury after intraventricular hemorrhage. Stroke J Cereb Circ 42:465–470
Cheng Y, Xi G, Jin H, Keep RF, Feng J, Hua Y (2014) Thrombin-induced cerebral hemorrhage: role of protease-activated receptor-1. Transl Stroke Res 5:472–475
Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G (2014) Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab 34:489–494
Guo F, Hua Y, Wang J, Keep RF, Xi G (2012) Inhibition of carbonic anhydrase reduces brain injury after intracerebral hemorrhage. Transl Stroke Res 3:130–137
Hanley DF (2009) Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke J Cereb Circ 40:1533–1538
Hua Y, Keep RF, Hoff JT, Xi G (2007) Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke J Cereb Circ 38:759–762
Jin H, Xi G, Keep RF, Wu J, Hua Y (2013) DARPP-32 to quantify intracerebral hemorrhage-induced neuronal death in basal ganglia. Transl Stroke Res 4:130–134
Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11:720–731
Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G (2009) Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke J Cereb Circ 40:1858–1863
Okubo S, Strahle J, Keep RF, Hua Y, Xi G (2013) Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke J Cereb Circ 44:547–550
Pang D, Sclabassi RJ, Horton JA (1986) Lysis of intraventricular blood clot with urokinase in a canine model: part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery 19:553–572
Rosen DS, Macdonald RL, Huo D, Goldenberg FD, Novakovic RL, Frank JI, Rosengart AJ (2007) Intraventricular hemorrhage from ruptured aneurysm: clinical characteristics, complications, and outcomes in a large, prospective, multicenter study population. J Neurosurg 107:261–265
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5:53–63
Zhao J, Chen Z, Xi G, Keep RF, Hua Y (2014) Deferoxamine attenuates acute hydrocephalus after traumatic brain injury in rats. Transl Stroke Res 5:586–594
Acknowledgment
This study was supported by grants NS-073959, NS079157, and NS084049 from the National Institutes of Health (NIH), 973 Program-2014CB541600, and grant NSFC 81471168 from National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gao, F., Zheng, M., Hua, Y., Keep, R.F., Xi, G. (2016). Acetazolamide Attenuates Thrombin-Induced Hydrocephalus. In: Applegate, R., Chen, G., Feng, H., Zhang, J. (eds) Brain Edema XVI. Acta Neurochirurgica Supplement, vol 121. Springer, Cham. https://doi.org/10.1007/978-3-319-18497-5_64
Download citation
DOI: https://doi.org/10.1007/978-3-319-18497-5_64
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18496-8
Online ISBN: 978-3-319-18497-5
eBook Packages: MedicineMedicine (R0)