Abstract
Brain arteriovenous malformation (bAVM) is an important cause of intracranial hemorrhage (ICH), particularly in the young population. ICH is the first clinical symptom in about 50 % of bAVM patients. The vessels in bAVM are fragile and prone to rupture, causing bleeding into the brain. About 30 % of unruptured and non-hemorrhagic bAVMs demonstrate microscopic evidence of hemosiderin in the vascular wall. In bAVM mouse models, vascular mural cell coverage is reduced in the AVM lesion, accompanied by vascular leakage and microhemorrhage. In this review, we discuss possible signaling pathways involved in abnormal vascular development in bAVM.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brain arteriovenous malformations
- Delta-like ligand-4
- Microhemorrhage
- Mural cell coverage
- Notch
- Platelet-derived growth factor receptor beta
- Vascular integrity
- Vascular leakage
Introduction
Patients harboring brain arteriovenous malformation (bAVM) are at life-threatening risk of vessel rupture and intracranial hemorrhage (ICH) [1, 2], and the malformed vessels are fragile and prone to rupture, causing bleeding into the brain. ICH is the first clinical symptom in about 50 % of bAVM patients. In a past study, we showed that 30 % of unruptured and non-hemorrhagic bAVMs demonstrated microscopic evidence of hemosiderin deposition in the vascular wall [3]. The presence of silent intralesional microhemorrhage may be a biomarker for the risk of ICH. However, the underlying mechanisms for bAVM rupture and microhemorrhage are not fully understood.
Current treatment options for bAVM are invasive. Approximately 20 % of patients are not offered interventional therapy because of excessive treatment-related risks [4, 5]. Furthermore, treatment of unruptured bAVMs—half of all cases—has become increasingly controversial because the natural history for these patients may be less morbid than invasive therapy [6–10]. So far, there is no specific medical therapy to treat bAVMs.
Previous studies have focused on the association of bAVM angioarchitecture and the risk of hemorrhage. These studies have found that a small number of draining veins, excessive deep-draining veins, vein stenosis, deep locations in the brain, and diffused bAVM morphology are risk factors for bAVM rupture [11–16]. Analysis of mean pressure of feeding arteries, in conjunction with other morphological or clinical risk factors, indicates that high arterial input pressure and venous outflow restriction (exclusively deep venous drainage) are the most powerful risk predictors for hemorrhagic bAVM presentation [17]. Previously, because of the lack of an animal model, the biology behind the abnormal vascular remodeling could not be tested.
We have established several bAVM mouse models through conditional knockout of endoglin (Eng) or Activin-like kinase 1 (Alk1; Acvlr1) genes, causative genes for an autosomal-dominated genetic disorder, Hereditary hemorrhagic telangiectasia (HHT) [18–21]. HHT is characterized by solid organ AVMs (i.e., in the lung, liver, and brain) and mucocutaneous telangiectasias [22]. As much as 5 % of bAVMs may be due to HHT [23]. As a familial form, bAVM in HHT possesses a similar phenotype to sporadic bAVM so that knowledge of these inherited gene pathways can shed light on sporadic disease pathogenesis [24]. Our mouse models resemble some phenotypes of human bAVM that are related to rupture risk, for example, iron deposition from red blood cell (RBC) extravasation [3] and macrophage infiltration [25–27].
In this review, we provide evidence that vascular structure is abnormal in bAVM, which might be formatted through the following pathways (Fig. 1): (1) ALK1, ENG, or matrix Gla protein (MGP) deletion leads to reduced expression of the Notch ligand, delta-like ligand-4 (DLL4), in microvascular endothelial cells; (2) decreased DLL4 results in a reduction of platelet-derived growth factor-B (PDGFB) signaling. As a result, mural cell recruitment is impaired [19, 28].
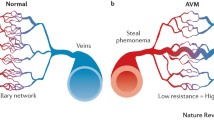
Speculated pathways involved in AVM pathogenesis. Angiogenic factors, such as VEGF, induce endothelial mitogenesis. When mutation of AVM causative genes occurs, the angiogenic response leads to the formation of leaky vessels with abnormal vessel wall structure through altered Notch signaling and reduced Pdgf-b signaling
Impaired Vessel Wall Structure Correlates with Vascular Leakage and Microhemorrhage
Structural imperfection and immaturity of the vascular wall in bAVM suggest that vessels are histoembryogenically maldeveloped. Prominent caliber dilation, hypertrophy of muscular layer, hyalinization, and an abnormal increase of elastic fibers might be the result of vascular remodeling in response to changed cerebral hemodynamics caused by an arteriovenous shunt [29].
Abnormal expression pattern of collagen (Col) I and Col III has been found in bAVMs [30]. Compared with control brain samples, bAVMs have a higher level of Col I and a lower level of Col III. The collagen fibers in bAVM vessels are disorganized and interrupted in the internal elastic lamina. Col I is a stiff fibrillar protein that provides resistance to tension, whereas Col III forms an elastic network [31] that prevents rupture of the vessel wall. Col I/Col III ratio is markedly increased in bAVM, which can increase the stiffness of the bAVM vessels. Interestingly, ruptured AVMs have higher type-I and -III collagen content than unruptured AVMs [32].
In a study analyzing surgical specimens of four adult cases of cerebral pial AVM [33], severe mural fibrosis was found in arteriovenous shunts larger than 700 μm located in the subarachnoid space. The authors found that vessels adjacent to shunting segments were arterialized veins and have segmental loss of the internal elastic membrane (IEM) and/or smooth muscle cells (SMCs). The smaller shunts in the cerebral parenchyma were dilated small arteries, which showed abrupt loss of IEM and gradual loss of SMCs and transformed into dilated and tortuous veins [33]. These findings suggest that AVM rupture is caused not only by dilated veins but also by a segmental loss of IEM and SMCs.
SMCs in AVMs are in various stages of differentiation [34]. The expression of smoothelin is less prevalent in large AVM vessels than in the normal brain, which may reflect the loss of contractile property associated with hemodynamic stress [34]. Hoya et al. [35] analyzed the expression of SMC marker proteins, including smooth muscle alpha-actin and four myosin heavy chain isoforms (SM1, SM2, SMemb, and NMHC-A) in bAVM specimens. Although the arterial components of AVM showed the same staining pattern as mature normal arteries, two different types of abnormal veins were noticed in the AVM specimens: large veins with a thick and fibrous wall (so-called “arterialized” veins) and intraparenchymal thin-walled sinusoidal veins. The former express alpha-actin, SM1, SM2, and SMemb, and the latter, alpha-actin, SM1, and SM2. These markers are normally expressed in cerebral arteries. The results were compatible with arterialization of the cerebral veins caused by arteriovenous shunting [35, 36].
Abnormal vessel wall structure has also been noticed in the bAVM vessels in our mouse models [18, 37]. Compared with normal brain angiogenic foci, the lesion in bAVM mouse models have more vessels with diameters larger than 15 μm that lack α-SMA positive cells and have fewer pericytes. Reduced SMC and pericyte coverage is associated with increased vascular permeability and microhemorrhage.
All of the data cited thus far suggest that vessels in bAVMs have impaired wall structure, which may be the cause of AVM microhemorrhage and rupture. Currently, it is not clear which molecular signaling pathway is involved in the formation of these abnormal vascular structures. Through analysis of the surgically sectioned human bAVM specimens and bAVM in the HHT mouse models, we found Notch and Pdgf-b signaling pathways are involved in abnormal vascular formation and remodeling.
Altered Notch Signaling Causes Abnormal Angiogenesis in bAVM
There is empirical evidence that proteins involved in Notch signaling—including receptors, ligands, and downstream signals—are expressed in excised surgical specimens [38, 39]. Animal experiments support a potential link between Notch signaling and human diseases. In mice, both gain and loss of Notch function cause arteriovenous shunts to form during prenatal development [40]. Endothelial overexpression of a constitutive active Notch-4 intracellular domain (Notch 4*) results in bAVMs in young mice [41, 42], and normalizing Notch 4* expression results in lesion regression [43]. In addition, the expression of Notch ligands, Jagged 1 and 2, are increased in Mgp deficient mice [44]. All these data indicate that Notch signaling is involved in bAVM pathogenesis.
In mammals, there are 4 Notch receptors (Notch-1, -2, -3 and -4) and 5 ligands (Jagged1 and 2, Dll1, 3, and 4). Notch interacts with VEGF signaling during tip-cell and stalk-cell specification [45]. Dll4 is predominantly expressed in tip cells, and Jagged-1 in stalk cells. Dll4-Notch1 signaling suppresses tip cell formation leading to nonproductive sprouting, whereas Jagged-1 antagonizes the Dll4 ligand, thereby promoting sprouting angiogenesis [46]. We and others have found through analysis of human bAVM specimens and animal model development that a pro-angiogenic signal is needed for bAVM development [18, 47–51]. Without Dll4 signaling, a pro-angiogenic state is favored, for example, proliferation of tip at the expense of stalk cells [46, 52]. Blocking Dll4 in tumor models leads to an excessively branched, chaotic vascular network and impaired mural cell recruitment [53, 54].
In addition to its interaction with angiogenesis, Notch signaling is also essential in regulating arterial fate specification [55]. Notch and its downstream signaling are important in directing arterial-venous segregation and stabilizing brain endothelial-pericyte interaction during vasculogenesis in the embryos [55–57]. Absence of Notch results in expression of venous markers in the arteries [58]. We found that endothelial cells in some vessels in the bAVM lesion in a mouse model express both arterial and venous markers [18], suggesting that Alk1 deletion impairs the endothelial cell specification during angiogenesis. This altered specification may be the cause of irregular SMC coverage of AVM vessels.
The interaction of Alk1 or Eng with Notch signaling is just beginning to be examined [52, 59]. Notch signaling is important in vascular homeostasis and response to injury (angiogenesis) [59–62].
Gain and loss of Notch function may affect venous and arterial cells differently [63]. ALK1 knockdown in human umbilical artery endothelial cells (HUAEC) causes a reduction in EPHRIN B2, a marker for artery endothelial cells [63]. Deficiency of Mgp, a bone morphogenetic protein (Bmp) inhibitor, causes alternation of Notch ligand-expression, dysregulation of endothelial differentiation, and development of bAVM [44]. Increased Bmp activity due to the lack of Mgp induces the expression of Alk1 in the cerebrovascular endothelium, which enhances the expression of Notch ligands (Jagged 1 and 2) and alters the expression of arterial and venous endothelial markers (Ephrin B2 and Eph B4). Expression of Alk1 does not change when Jagged expression is reduced [44], suggesting that Jagged 1 and 2 act downstream of Alk1.
Together, the data above suggest that Notch signaling is located downstream of bAVM causative genes, such as Alk1 or Mgp. Notch and its downstream signaling participate in bAVM pathogenesis in several ways: (1) enhancement of angiogenesis; (2) impairment of vessel wall structure; and (3) alteration of arterial and venous specification in endothelial cells.
Reduced PDGF-B Signaling Results in Abnormal Mural Cell Coverage in Brain AVM
PDGFs are important mitogens for various types of mesenchymal cells, such as fibroblasts, SMC, and pericytes [64]. They exert critical function during organogenesis in mammalian embryonic and early postnatal development. Increase or loss of function of PDGF is also noticed in diseases such as cancer, tissue fibrosis, and cardiovascular diseases in adults [65]. The PDGF family includes PDGF-A, -B, -C and -D, which are assembled as disulfide-linked homo- or heterodimers. PDGFs have two types of receptors: PDGFR-α and -β [64, 66]. Among PDGFs, PDGF-B has intrinsic pro-angiogenic effects. Microvascular integrity can be compromised when PDGF-B expression is too high [67] or too low [68, 69]. PDGF-B signaling through PDGFR-β regulates pericyte recruitment and differentiation to nascent capillaries. The differentiation of mesenchymal cells into the pericyte/SMC- lineage is dependent on PDGFR-β expression in mice [70].
Knockout Pdgf-b or Pdgfr-β in mice results in loss of pericytes from the microvessels [68]. The absence of pericytes also leads to endothelial hyperplasia (associated with abnormal endothelial junctions), and excessive endothelial luminal membrane folds [69]. Pdgfr-β or Pdgf-b null mice have cerebral hemorrhage with an absence of microvascular pericytes in the brain vessels and endothelial hyperplasia [69]. Reduction of vascular pericytes correlates with impairment of vascular integrity [71, 72]. Higher PDGF-B expression has been detected in some, but not all, resected sporadic human bAVM specimens compared with control tissue [73, 74]. Other cells in the brain can also express PDGF-B, which could obscure the analysis of PDGF-B expression [75].
We have demonstrated that expression of Pdgfr-β is reduced in the bAVM lesions of Alk1-deficient mice [19], suggesting a possible link between Alk1 and Pdfgr-β/Pdgf-b signaling pathways. However, it is not clear whether the reduced expression of Pdfgr-β is caused by the reduced number of pericytes in the tissue. Many AVM vessels in Alk1-deficient mice do not have the SMC-layer and have less pericyte coverage.
PDGF-B/PDGFR-β has also been implicated in skin and retina AVMs, as well as Eng-associated signaling pathway. Oral administration of thalidomide reduces the frequency and the duration of nosebleeds and blood transfusion requirements in a small group of HHT patients [28]. Thalidomide treatment does not inhibit endothelial cell proliferation and migration, but increases mural cell coverage of the vasculature through increasing Pdgf-b expression in endothelial cells [28].
The data above indicate that: (1) AVM-causative genes, such as Alk1 and Eng, play an important role in maintaining cerebrovascular integrity; (2) mutations of these genes result in abnormal angiogenic response, which leads to abnormal vessel formation; (3) PDGF-B signaling is one of the downstream signaling pathways involved in brain AVM pathogenesis; (4) upregulation of PDGF-B signaling may reduce the severity of bAVM phenotype, and thus could be developed into a therapeutic strategy to treat bAVM.
Other Signalings
Angiopoietin/TIE2 signaling also plays a role in the recruitment of peri-endothelial support structures. Alternations of angiopoietin/TIE2 expression in human bAVM specimens have been noticed [76], which could be a cause of defective vessel wall in bAVM. For example, angiopoietin-2 (ANG-2), which allows loosening of cell-to-cell contacts, is overexpressed in the perivascular region in AVM vascular channels [76].
A key downstream consequence of VEGF and ANG-2 signaling contributing to the angiogenic phenotype is matrix metalloproteinase (MMP) expression. MMP-9 expression, in particular, appears to be higher in bAVM than in control tissue [27, 77]. Similarly, TIMP-1 and TIMP-3, which are naturally occurring MMP inhibitors, are also increased in bAVM but to a lesser degree.
Exactly how the dysplastic response propagates and leads to bAVM formation is not known. Recruitment of progenitor cell populations may be one source influencing AVM growth and development, an area that needs further exploration. For example, endothelial progenitor cells are present in the nidus of the brain and spinal cord AVMs, and may mediate pathological vascular remodeling and impact the clinical course of AVMs.
More generally, circulating bone marrow-derived cells have a major role in both microcirculatory angiogenesis [78, 79] and conductance vessel remodeling [80, 81]. If bAVM pathogenesis involves these two processes, it is reasonable to infer that bone marrow-derived cells may have an underappreciated role in lesion formation and growth. An unresolved issue with stem cell interaction is the extent to which progenitor cells actually integrate into existing tissue compartments, or whether they provide a nursing function by supplying critical components of the repair response, such as cytokines, growth factors, and enzymes, to the tissue.
In summary, we have reviewed the possible roles of Notch and Pdgf-b signaling in bAVM pathogenesis. In both brain and non-brain endothelial cells, Alk1 is upstream of Notch signaling [59], and Notch signaling is upstream of Pdgf-b, which appears to be an important regulator of cerebrovascular Pdgf-b expression [67]. The relationship between Eng function and Pdgf-β [28] and Notch signaling is not as well understood [52]. However, animal studies show that either correcting Notch signaling [43] or increasing Pdgf-b [28] expression resumes abnormal vascular structure in bAVM. Thus, by modulating these two pathways, new therapies could be developed.
References
Fleetwood IG, Steinberg GK (2002) Arteriovenous malformations. Lancet 359(9309):863–873
Arteriovenous Malformation Study Group (1999) Arteriovenous malformations of the brain in adults. N Engl J Med 340(23):1812–1818
Guo Y, Saunders T, Su H, Kim H, Akkoc D, Saloner DA, Hetts SW, Hess C, Lawton MT, Bollen AW, Pourmohamad T, McCulloch CE, Tihan T, Young WL (2012) Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke 43(5):1240–1246
Spetzler RF, Martin NA (1986) A proposed grading system for arteriovenous malformations. J Neurosurg 65(4):476–483
Han PP, Ponce FA, Spetzler RF (2003) Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg 98(1):3–7
Stapf C, Mohr JP, Choi JH, Hartmann A, Mast H (2006) Invasive treatment of unruptured brain arteriovenous malformations is experimental therapy. Curr Opin Neurol 19(1):63–68
Cockroft KM, Jayaraman MV, Amin-Hanjani S, Derdeyn CP, McDougall CG, Wilson JA (2012) A perfect storm: how a randomized trial of unruptured brain arteriovenous malformations’ (ARUBA’s) trial design challenges notions of external validity. Stroke 43(7):1979–1981
Mohr JP, Moskowitz AJ, Stapf C, Hartmann A, Lord K, Marshall SM, Mast H, Moquete E, Moy CS, Parides M, Pile-Spellman J, Al-Shahi Salman R, Weinberg A, Young WL, Estevez A, Kureshi I, Brisman JL (2010) The ARUBA trial: current status, future hopes. Stroke 41(8):e537–e540
Mohr JP, Moskowitz AJ, Parides M, Stapf C, Young WL (2012) Hull down on the horizon: a randomized trial of unruptured brain arteriovenous malformations (ARUBA) trial. Stroke 43(7):1744–1745
Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, Salman RA, Vicaut E, Young WL, Houdart E, Cordonnier C, Stefani MA, Hartmann A, von Kummer R, Biondi A, Berkefeld J, Klijn CJ, Harkness K, Libman R, Barreau X, Moskowitz AJ (2014) Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 383(9917):614–621
Mansmann U, Meisel J, Brock M, Rodesch G, Alvarez H, Lasjaunias P (2000) Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery 46(2):272–279
Stefani MA, Porter PJ, terBrugge KG, Montanera W, Willinsky RA, Wallace MC (2002) Angioarchitectural factors present in brain arteriovenous malformations associated with hemorrhagic presentation. Stroke 33(4):920–924
Stefani MA, Porter PJ, terBrugge KG, Montanera W, Willinsky RA, Wallace MC (2002) Large and deep brain arteriovenous malformations are associated with risk of future hemorrhage. Stroke 33(5):1220–1224
Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ, Kondziolka D (1996) Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke 27(1):1–6
Hademenos GJ, Massoud TF (1996) Risk of intracranial arteriovenous malformation rupture due to venous drainage impairment. A theoretical analysis. Stroke 27(6):1072–1083
Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES, Pile-Spellman J, Mohr JP (2006) Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 66(9):1350–1355
Duong DH, Young WL, Vang MC, Sciacca RR, Mast H, Koennecke HC, Hartmann A, Joshi S, Mohr JP, Pile-Spellman J (1998) Feeding artery pressure and venous drainage pattern are primary determinants of hemorrhage from cerebral arteriovenous malformations. Stroke 29(6):1167–1176
Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, Lawton MT, Kim H, Chen Y, Chen W, Young WL (2011) Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol 69(6):954–962
Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, Degos V, Lawton MT, Tihan T, Davalos D, Akassoglou K, Nelson J, Pile-Spellman J, Su H, Young WL (2013) Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol 33(2):305–310
Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, Mao L, Arnold T, Young WL, Su H (2014) De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 45(3):900–902
Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H (2014) Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One 9(2), e88511
Braverman IM, Keh A, Jacobson BS (1990) Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol 95(4):422–427
Bharatha A, Faughnan ME, Kim H, Pourmohamad T, Krings T, Bayrak-Toydemir P, Pawlikowska L, McCulloch CE, Lawton MT, Dowd CF, Young WL, Terbrugge KG (2012) Brain arteriovenous malformation multiplicity predicts the diagnosis of hereditary hemorrhagic telangiectasia: quantitative assessment. Stroke 43(1):72–78
Shovlin CL (2010) Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev 24(6):203–219
Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, Hashimoto T, Yang GY, Young WL (2008) Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery 62(6):1340–1349
Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, Lawton MT, Kwok PY, Yang GY, Young WL (2006) Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol 59(1):72–80
Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, McCulloch CE, Hashimoto T, Lee C, Barbaro NM, Bollen AW, Yang GY, Young WL (2006) MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci 11:3121–3128
Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, Breant C, Mathivet T, Larrivee B, Thomas JL, Arthur HM, Westermann CJ, Disch F, Mager JJ, Snijder RJ, Eichmann A, Mummery CL (2010) Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med 16(4):420–428
Isoda K, Fukuda H, Takamura N, Hamamoto Y (1981) Arteriovenous malformation of the brain – histological study and micrometric measurement of abnormal vessels. Acta Pathol Jpn 31(5):883–893
Guo Y, Qumu SW, Nacar OA, Yang JY, Du J, Belen D, Pan L, Zhao YL (2013) Human brain arteriovenous malformations are associated with interruptions in elastic fibers and changes in collagen content. Turk Neurosurg 23(1):10–15
Lee RM (1995) Morphology of cerebral arteries. Pharmacol Ther 66(1):149–173
Niu H, Cao Y, Wang X, Xue X, Yu L, Yang M, Wang R (2012) Relationships between hemorrhage, angioarchitectural factors and collagen of arteriovenous malformations. Neurosci Bull 28(5):595–605
Meng JS, Okeda R (2001) Histopathological structure of the pial arteriovenous malformation in adults: observation by reconstruction of serial sections of four surgical specimens. Acta Neuropathol 102(1):63–68
Uranishi R, Baev NI, Kim JH, Awad IA (2001) Vascular smooth muscle cell differentiation in human cerebral vascular malformations. Neurosurgery 49(3):671–679
Hoya K, Asai A, Sasaki T, Nagata K, Kimura K, Kirino T (2003) Expression of myosin heavy chain isoforms by smooth muscle cells in cerebral arteriovenous malformations. Acta Neuropathol 105(5):455–461
Hoya K, Asai A, Sasaki T, Kimura K, Kirino T (2001) Expression of smooth muscle proteins in cavernous and arteriovenous malformations. Acta Neuropathol 102(3):257–263
Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, Degos V, Lawton MT, Tihan T, Davalos D, Akassoglou K, Nelson J, Pile-Spellman J, Su H, Young WL (2013). Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol 33(2):305–310
ZhuGe Q, Zhong M, Zheng W, Yang GY, Mao X, Xie L, Chen G, Chen Y, Lawton MT, Young WL, Greenberg DA, Jin K (2009) Notch1 signaling is activated in brain arteriovenous malformation in humans. Brain 132(Pt 12):3231–3241
Murphy PA, Lu G, Shiah S, Bollen AW, Wang RA (2009) Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest 89(9):971–982
Gridley T (2010) Notch signaling in the vasculature. Curr Top Dev Biol 92:277–309
Murphy PA, Lam MT, Wu X, Kim TN, Vartanian SM, Bollen AW, Carlson TR, Wang RA (2008) Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci U S A 105(31):10901–10906
Murphy PA, Kim TN, Huang L, Nielsen CM, Lawton MT, Adams RH, Schaffer CB, Wang RA (2014) Constitutively active Notch4 receptor elicits brain arteriovenous malformations through enlargement of capillary-like vessels. Proc Natl Acad Sci U S A 111(50):18007–18012
Murphy PA, Kim TN, Lu G, Bollen AW, Schaffer CB, Wang RA (2012) Notch4 normalization reduced blood vessel size in arteriovenous malformations. Sci Transl Med 4(117):117ra118
Yao Y, Yao J, Radparvar M, Blazquez-Medela AM, Guihard PJ, Jumabay M, Bostrom KI (2013) Reducing Jagged 1 and 2 levels prevents cerebral arteriovenous malformations in matrix Gla protein deficiency. Proc Natl Acad Sci U S A 110(47):19071–19076
Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, Gerhardt H (2009) Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell 16(1):70–82
Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH (2009) The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137(6):1124–1135
Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL (2011) Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl 111:83–92
Xu B, Wu YQ, Huey M, Arthur HM, Marchuk DA, Hashimoto T, Young WL, Yang GY (2004) Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab 24(2):237–244
Hao Q, Su H, Marchuk DA, Rola R, Wang Y, Liu W, Young WL, Yang GY (2008) Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol 295(6):H2250–H2256
Hao Q, Zhu Y, Su H, Shen F, Yang GY, Kim H, Young WL (2010) VEGF induces more severe cerebrovascular dysplasia in Endoglin+/− than in Alk1+/− mice. Transl Stroke Res 1(3):197–201
Walker EJ, Su H, Shen F, Degos V, Amend G, Jun K, Young WL (2012) Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke 43(7):1925–1930
Benedito R, Trindade A, Hirashima M, Henrique D, da Costa LL, Rossant J, Gill PS, Duarte A (2008) Loss of Notch signalling induced by Dll4 causes arterial calibre reduction by increasing endothelial cell response to angiogenic stimuli. BMC Dev Biol 8:117
Djokovic D, Trindade A, Gigante J, Badenes M, Silva L, Liu R, Li X, Gong M, Krasnoperov V, Gill PS, Duarte A (2010) Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is highly effective in disrupting tumor angiogenesis. BMC Cancer 10(1):641
Trindade A, Djokovic D, Gigante J, Badenes M, Pedrosa AR, Fernandes AC, Lopes-da-Costa L, Krasnoperov V, Liu R, Gill PS, Duarte A (2012) Low-dosage inhibition of Dll4 signaling promotes wound healing by inducing functional neo-angiogenesis. PLoS One 7(1), e29863
Gridley T (2007) Notch signaling in vascular development and physiology. Development 134(15):2709–2718
Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY (2009) Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 326(5950):294–298
Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X (2011) Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell 20(3):291–302
Hofmann JJ, Iruela-Arispe ML (2007) Notch signaling in blood vessels: who is talking to whom about what? Circ Res 100(11):1556–1568
Larrivee B, Prahst C, Gordon E, Del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A (2012) ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell 22(3):489–500
Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J (2011) Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood 118(12):3436–3439
Outtz HH, Wu JK, Wang X, Kitajewski J (2010) Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. J Immunol 185(7):4363–4373
Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, Zwijsen A (2012) Stalk cell phenotype depends on integration of notch and smad1/5 signaling cascades. Dev Cell 22(3):501–514
Kim JH, Peacock MR, George SC, Hughes CC (2012) BMP9 induces EphrinB2 expression in endothelial cells through an Alk1-BMPRII/ActRII-ID1/ID3-dependent pathway: implications for hereditary hemorrhagic telangiectasia type II. Angiogenesis 15(3):497–509
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79(4):1283–1316
Betsholtz C, Keller A (2014) PDGF, pericytes and the pathogenesis of idiopathic basal ganglia calcification (IBGC). Brain Pathol 24(4):387–395
Shim AH, Liu H, Focia PJ, Chen X, Lin PC, He X (2010) Structures of a platelet-derived growth factor/propeptide complex and a platelet-derived growth factor/receptor complex. Proc Natl Acad Sci U S A 107(25):11307–11312
Yao H, Duan M, Hu G, Buch S (2011) Platelet-derived growth factor B chain is a novel target gene of cocaine-mediated Notch1 signaling: implications for HIV-associated neurological disorders. J Neurosci Meth 31(35):12449–12454
Lindahl P, Johansson BR, Leveen P, Betsholtz C (1997) Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277(5323):242–245
Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C (2001) Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 153(3):543–553
Crosby JR, Seifert RA, Soriano P, Bowen-Pope DF (1998) Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet 18(4):385–388
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68(3):409–427
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C (2010) Pericytes regulate the blood–brain barrier. Nature 468(7323):557–561
Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM, Young WL (2005) Co-expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery 56(5):1058–1065
Yildirim O, Bicer A, Ozkan A, Kurtkaya O, Cirakoglu B, Kilic T (2010) Expression of platelet-derived growth factor ligand and receptor in cerebral arteriovenous and cavernous malformations. J Clin Neurosci 17(12):1557–1562
Sasahara M, Sato H, Iihara K, Wang J, Chue CH, Takayama S, Hayase Y, Hazama F (1995) Expression of platelet-derived growth factor B-chain in the mature rat brain and pituitary gland. Brain Res Mol Brain Res 32(1):63–74
Hashimoto T, Lam T, Boudreau NJ, Bollen AW, Lawton MT, Young WL (2001) Abnormal balance in the angiopoietin-tie2 system in human brain arteriovenous malformations. Circ Res 89(2):111–113
Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, Barbaro NM, Higashida RT, Dowd CF, Halbach VV, Young WL (2003) Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke 34(4):925–931
Hao Q, Liu J, Pappu R, Su H, Rola R, Gabriel RA, Lee CZ, Young WL, Yang GY (2008) Contribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leucocytes and macrophages. Arterioscler Thromb Vasc Biol 28(12):2151–2157
Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, Young WL, Yang GY (2007) Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab 27(11):1853–1860
Ota R, Kurihara C, Tsou TL, Young WL, Yeghiazarians Y, Chang M, Mobashery S, Sakamoto A, Hashimoto T (2009) Roles of matrix metalloproteinases in flow-induced outward vascular remodeling. J Cereb Blood Flow Metab 29(9):1547–1558
Nuki Y, Matsumoto MM, Tsang E, Young WL, van Rooijen N, Kurihara C, Hashimoto T (2009) Roles of macrophages in flow-induced outward vascular remodeling. J Cereb Blood Flow Metab 29(3):495–503
Acknowledgments
We thank members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their support, and Voltaire Gungab for assistance with manuscript preparation. This study was supported by grants to Hua Su from the National Institutes of Health (R01 NS027713, R01 HL122774, and 1R21NS083788) and from the Michael Ryan Zodda Foundation and UCSF Research Evaluation and Allocation Committee (REAC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Zhang, R., Zhu, W., Su, H. (2016). Vascular Integrity in the Pathogenesis of Brain Arteriovenous Malformation. In: Applegate, R., Chen, G., Feng, H., Zhang, J. (eds) Brain Edema XVI. Acta Neurochirurgica Supplement, vol 121. Springer, Cham. https://doi.org/10.1007/978-3-319-18497-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-18497-5_6
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18496-8
Online ISBN: 978-3-319-18497-5
eBook Packages: MedicineMedicine (R0)