Abstract
Brain arteriovenous malformation (BAVM) is a rare but serious cerebrovascular disease whose pathogenesis has not been fully elucidated. Studies have found that epigenetic regulation, genetic variation and their signaling pathways, immune inflammation, may be the cause of BAVM the main reason. This review comprehensively analyzes the key pathways and inflammatory factors related to BAVMs, and explores their interplay with epigenetic regulation and genetics. Studies have found that epigenetic regulation such as DNA methylation, non-coding RNAs and m6A RNA modification can regulate endothelial cell proliferation, apoptosis, migration and damage repair of vascular malformations through different target gene pathways. Gene defects such as KRAS, ACVRL1 and EPHB4 lead to a disordered vascular environment, which may promote abnormal proliferation of blood vessels through ERK, NOTCH, mTOR, Wnt and other pathways. PDGF-B and PDGFR-β were responsible for the recruitment of vascular adventitial cells and smooth muscle cells in the extracellular matrix environment of blood vessels, and played an important role in the pathological process of BAVM. Recent single-cell sequencing data revealed the diversity of various cell types within BAVM, as well as the heterogeneous expression of vascular-associated antigens, while neutrophils, macrophages and cytokines such as IL-6, IL-1, TNF-α, and IL-17A in BAVM tissue were significantly increased. Currently, there are no specific drugs targeting BAVMs, and biomarkers for BAVM formation, bleeding, and recurrence are lacking clinically. Therefore, further studies on molecular biological mechanisms will help to gain insight into the pathogenesis of BAVM and develop potential therapeutic strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain arteriovenous malformation (BAVM) is a rare developmental abnormality of the brain vasculature, in which proliferative blood vessels connect the feeding arteries and draining veins without the presence of capillaries, causing high-flow, low-resistance arteriovenous shunting (Lawton et al. 2015). The incidence of BAVM is 1.10–1.42 cases per 100,000 individuals, and it is most commonly diagnosed in adolescents and young adults aged 20–40. BAVM is typically characterized by intracranial hemorrhage and seizures but may also present as progressive neurological deficits, psychiatric disorders, dizziness, or migraines (Thomas et al. 2016). The rate of rupture and hemorrhage of BAVM is about 3% (Gross and Du 2013), and the mortality rate of patients with hemorrhage exceeds 20% (Karlsson et al. 2020). Patients who survived often carry a variety of sequelae, which seriously affect the quality of family life (Karlsson et al. 2020; Fukuda et al. 2017). The vast majority of BAVM are sporadic, and only 5% of patients are associated with genetic syndromes such as hereditary hemorrhagic telangiectasia (HHT) and capillary malformations-arteriovenous malformations (CM-AVM) (Pan et al. 2021). Traditionally, BAVM have been considered to be caused by congenital vascular anomalies. However, with the development of molecular biology and diagnostic imaging techniques, more and more research studies suggest that BAVMs may be dynamic diseases. Recent case reports have shown that patients with BAVM were de novo lesions many years later, and that BAVM lesions can grow and remodel rapidly even after surgical resection (Morales-Valero et al. 2014; Walcott et al. 2018). Moreover, some patients with a history of intracranial injury developed BAVMs, suggesting the possibility of an acquired form of the disease (Nagai et al. 2020; Park et al. 2021a). For example, patients with BAVMs acquired after cerebral aneurysm occlusion, stereotactic radiosurgery radiation therapy, hemorrhagic and ischemic stroke, seizures, traumatic brain injury, brain tumors, or encephalitic demyelinating lesions are thought to have experienced a “second hit” after brain injury (Florian et al. 2021a). Furthermore, various factors that contribute to abnormal vascular development, including epigenetic changes, cytokines, vascular injury repair-related proteins, environmental factors, and tumor-related gene mutations, are associated with the occurrence of BAVM. For example, single nucleotide polymorphisms (SNP) are involved in BAVM inflammatory response or angiogenesis. Interleukin-6 (IL-6), hypoxia-inducible factor (HIF‐1), vascular endothelial growth factor (VEGF), nuclear factor kappa-light-chain of activated B (NFκB), interleukin-1β (IL-1β) and interleukin-8 (IL-8) affect angiogenesis by participating in endothelial cell proliferation (Chen et al. 2006; Yao et al. 2007), migration, reducing apoptosis and increasing the expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) (Storer et al. 2008; Ardelt et al. 2005; Li et al. 2003), increase the risk of BAVM. In addition, the researchers also found that mutations in tumor-related genes such as KRAS, BRAF and the RAS-MAPK-ERK signaling pathway might also lead to endothelial cell dysfunction and play important roles in the formation of BAVM (Hong et al. 2019). Therefore, it is necessary to summarize the molecular and cellular signaling mechanisms involved in BAVM.
Epigenetics is the regulation of different physiological processes in organisms by causing changes in gene expression levels without changing the DNA sequence, such as non-coding RNA, DNA methylation, and histone modification. Epigenetic modifications may serve as a bridge between the external environment and the vascular microenvironment, and affect the occurrence, development and prognosis of BAVM by regulating related signaling pathways (Thomas et al. 2016). Therefore, this review will combine genetic variation, epigenetics, immune inflammation and related signaling pathways to elucidate their roles in the pathogenesis of BAVM.
Genetic variations
SNP refers to the variations in a single nucleotide in DNA that are typically present in non-coding segments (Florian et al. 2020). Multiple studies have revealed that SNPs present in sporadic BAVM are associated with susceptibility to BAVM and bleeding risk in BAVM and are involved in vascular formation, inflammation, or coding genes, such as those for fibrillin, laminin, and integrin, which may have a vital role in BAVM pathophysiology (Sturiale et al. 2013). Recent research has revealed genetic mutations associated with BAVM. About 5% of patients with BAVM associated with genetic syndromes may be associated with germline gene mutations (such as ENG, ACVRL1, SMAD4 in HHT, RASA1 or EPHB4 in CM-AVM), while in the remaining 95% of sporadic BAVM patients, it is more likely to be related to somatic mutations (Pan et al. 2021). Among these, KRAS gene has the highest mutation rate, which may be a direct genetic basis of BAVM. Studies have conducted genomic testing on surgical and blood samples and found 60% of the patients with BAVMs had somatic KRAS gene mutations. They also found that the upstream and downstream molecular signaling pathways were closely related to mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) MAPK/ERK pathway activation, causing endothelial dysfunction (Nikolaev et al. 2018). Mutations in endoglin (ENG) and activin a receptor like type 1 (ACVRL1) lead to hereditary hemorrhagic telangiectasia 1 (HHT1) and hereditary hemorrhagic telangiectasia (HHT2), respectively, whereas mutations in SMAD Family Member 4 (SMAD4) are linked with the combination of HHT and juvenile polyposis (Gallione et al. 2004). The genes related to these three loci are the components of the transforming growth factor-β (TGF-β) signal path, suggesting that the TGF-β signaling may play a crucial role in BAVM development. Capillary malformation is related with mutations in RAS P21 Protein Activator 1 (RASA1) and EPH Receptor B4 (EPHB4), and the EPHB4-RAS-ERK pathway plays an indispensable role in this condition (Amyere et al. 2017). Weinsheimer et al. conducted a large-scale multicenter whole-genome SNP detection and showed significant SNP differences in receptor tyrosine kinase and matrix metalloproteinase-3 (MMP-3) using univariate analysis (Sturiale et al. 2013). The AG genotype of RS522616 in the MMP-3 promoter region is related with a low risk of developing BAVM (Zhao et al. 2010). Previous studies have revealed that the IL-6-174G > C promoter polymorphism is linked with the symptoms of BAVM bleeding and that plasma IL-6 can be used as a predictive indicator for BAVM bleeding. In a mouse model, IL-6 induced the expression and activity of MMP-3 and MMP-9 in the brain, increased endothelial cell proliferation and migration, and promoted intracranial hemorrhage (Chen et al. 2006). Kim et al. found that IL-1β gene promoter polymorphism is related to the bleeding risk and disease susceptibility to BAVMs, suggesting that inflammatory reactions and cytokines also have a significant function in BAVM pathogenesis (Kim et al. 2009). VEGFA is a well-recognized vascular-associated protein that plays an important role in the migration, proliferation and survival of endothelial cells, and can increase vascular permeability and induce vasodilation during vascular disease (Claesson-Welsh and Welsh 2013). VEGFA gene polymorphisms was shown to be associated with susceptibility and bleeding risk of BAVM (Chen et al. 2011). Notch receptor 4 (NOTCH4) (6p21.32) mutations may be associated with clinical manifestations (rupture and seizures) and susceptibility to BAVM (Florian et al. 2020; Sturiale et al. 2013). Bendjilali et al. also showed that ACVRL1, and angiopoietin like 4 (ANGPTL4) mutations were significantly associated with sporadic BAVM (Bendjilali et al. 2013). In addition, SNPs in transforming growth factor beta receptor 2 (TGFR-β2), interleukin 1 alpha (IL-1α), interleukin 1 receptor antagonist (IL-1RN), MMP-3, MMP-9, RB binding protein 8 (RBBP8), adhesion G protein-coupled receptor A2 (GPR124), SRY-Box transcription factor 17 (SOX-17), CDKN2B Antisense RNA 1 (CDKN2BAS1), and VEGFA were related with susceptibility to BAVM. SNPs in IL-6, TNF-α, IL-1β, interleukin 17A (IL-17A), TGFR-β2, apolipoprotein E(APOE), EPHB4, MMP-9, and VEGFA were related with bleeding risk in BAVM (Sturiale et al. 2013; Kremer et al. 2015). Polymorphisms in brain-derived neurotrophic factors (BDNF) are linked with poor surgical outcomes in patients with unruptured BAVM (Westbroek et al. 2012). Through relative risk calculation, we found that EPHB4 (rs314308 and rs314313), IL-17A (rs2275913), MMP-3 (rs522616), MMP-9 (rs9509), and GPR124 (rs12676965, rs7823249 and rs7015566) are protective factors for BAVM, while other gene mutations such as ACVRL1 (rs2071219), ANGPTL4 (rs11672433), APOE (e-/e +), CDKN2BAS1 (rs1333040), EPHB4 (rs314346 and rs314353), IL-1α (rs1800587), IL-1β (rs16944 and rs1143627), IL-1RN (rs2234663), IL-6 (rs1800795), ITGB8 (rs10486391 and rs11982847), NOTCH (rs443198 and rs915895), VEGFA (rs1547651 and rs833069), RBBP8 (rs11082043), TGFR-β2 (rs3087465), TNF-α (rs361525), BDNF (rs6265), and SOX-17 (rs9298506) are risk factors for BAVM (Fig. 1). The abundance of KRAS/BRAF mutations in BAVM tissue was inversely proportional to the size of the lesion, suggesting that KRAS/BRAF mutations in endothelial cells were the initiating factors for the occurrence of BAVM (Hong et al. 2019). Recent study had also demonstrated through animal models that over-activating KRAS mutations might promote the occurrence and development of BAVM through the MEK/ERK pathway, and treatment with trametinib cloud effectively inhibit the growth of BAVM (Park et al. 2021b). Therefore, research on genetic mutations and SNPs can assist in BAVM diagnosis and monitoring, and drugs can be developed to target these genetic mechanisms (Li et al. 2021).
Copy number variation (CNV) and insertion/deletion (InDel) are another structural variation of genetic factors. CNV refers to genomic structural variations > 1 kb in length, manifested as an increase or decrease in the copy number of large genomic segments compared with the reference genome (Conrad et al. 2010). Similar to SNPs, CNVs affect the expression and variation of human genes and phenotypes. Current research has demonstrated a correlation between CNV and the occurrence of neurological and psychiatric disorders (Rees and Kirov 2021). Bendjilali et al. conducted a whole-genome CNV analysis of BAVM; however, owing to the limitations in the number of cases, common CNVs were not detected in patients with sporadic BAVM (Bendjilali et al. 2013). InDel are defined as genomic differences due to deletions or insertions of < 1 kb of nucleotides, but there is no clear evidence of a link between InDel and BAVM. Further research is needed to investigate CNVs and InDel associated with BAVM.
Epigenetics
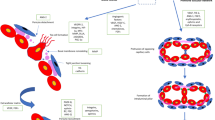
Epigenetics refers to mechanisms that alter gene expression without altering the DNA sequence. These alterations persist throughout development, can be passed on to future generations, and play a wide range of roles in various human diseases (Portela and Esteller 2010; Heard and Martienssen 2014). Epigenetic modifications can be divided into 3 major types: DNA methylation, non-coding RNA, and histone modifications, which regulate pathological processes including cell proliferation, development, and differentiation (Portela and Esteller 2010; Xia et al. 2021). Epigenetics responds to internal and external environmental stimuli under certain circumstances, leading to changes in disease susceptibility and progression. Previous studies have found that hemodynamics may be the most indispensable epigenetic factor in BAVM development (Fig. 2). Exposure of endothelial cells to hemodynamic factors, including blood pressure and shear stress, can induce chromatin modifications that alter gene expression, leading to abnormal blood vessel formation and remodeling, resulting in BAVM development (Thomas et al. 2016).
The possible relationship between epigenetic regulation and the occurrence of BAVM. DNA methylation of ZNF24, FAAH, AGGF1, CDKN2A, ANKRD65, PLA2G7, PDGFD, and NOS1AP is closely linked to BAVM occurrence. ZNF24 regulates the production of VEGF; FAAH regulates angiogenesis; AGGF1 regulates the proliferation, migration, and venous fate of endothelial cells; and CDKN2A may mediate the occurrence of BAVM by regulating the proliferation and repair of endothelial cells. MiR-18a can improve BAVM by inhibiting BMP4 and HIF-1α production. WTAP may be involved in the pathogenesis of BAVM via angiogenesis regulation through the Wnt pathway
DNA methylation
DNA methylation is a key epigenetic mechanism that primarily occurs in CpG dinucleotides in the gene promoter and is catalyzed by DNA methyltransferases with S-adenosyl-L-methionine as the methyl donor. It can prevent the interaction of transcription factors with specific binding sites, thereby mediating gene silencing (Stanzione et al. 2020; Jaenisch and Bird 2003). DNA methylation patterns vary among tissues, have indispensable functions in regulating the normal development of organisms, and are involved in many central nervous system (CNS) diseases (Chen et al. 2022; Deng et al. 2020; Dock et al. 2015; Dinicola et al. 2017; Rawlik et al. 2016; Wang et al. 2022; Numata et al. 2014).
Abnormal hemodynamics at the junction of the supplying artery and draining vein may lead to epigenetic changes in endothelial cells, affecting the regulation of blood vessel development. Thomas et al. measured the DNA methylation levels of blood vessel development-related genes in the lesion tissue of ten patients with BAVM and found high methylation levels in the promoters of Zinc Finger Protein 24 (ZNF24), fatty acid amide hydrolase (FAAH), angiogenic factor with G patch and FHA domains 1 (AGGF1), and ankyrin repeat domain 65 (ANKRD65). ZNF24 is a VEGF transcriptional repressor, and after being silenced by methylation, it activates VEGF transcription; FAAH can produce various vasoconstrictors, and its inactivation can inhibit vascular generation in vivo and in vitro; AGGF1 regulates various stages of vascular development, including endothelial cell migration, proliferation, and venous differentiation, and high methylation inhibits its expression, leading to abnormal venous development (Thomas et al. 2022). Chen et al. have confirmed that the methylation levels of the CPG1, CPG5, and CPG8 regions of the CDKN2A in the blood leukocytes of patients with BAVM were markedly higher than those in patients with intracranial aneurysms and healthy individuals, suggesting that CDKN2A is closely related with BAVM pathogenesis and has potential for use in early diagnosis (Chen et al. 2019). CDKN2A is a tumor suppressor protein, and previous studies on intracranial aneurysms have indicated that CDKN2A may have a crucial function in adjusting endothelial cell proliferation and repair to prevent vascular damage (Bilguvar et al. 2008). However, its role in venous development requires further study. Liu et al. found that CpG3 methylation of the phospholipase A2 group VIIG7 (PLA2G7) gene is related to the risk of BAVM, and that the average methylation level of the gene is related to the risk of BAVM in men and to APOE and apolipoprotein B (APOB) levels; however, the mechanism of action remains unclear (Liu et al. 2020). The methylation level of the platelet-derived growth factor-D (PDGFD) promoter is associated with the risk of BAVM (Zhou et al. 2017). The methylation of the nitric oxide synthase 1 adaptor protein (NOS1AP) promoter is also related to the risk of BAVM, and sex and smoking may have a synergistic effect on the methylation mechanism, which requires further verification in a large sample (Portela and Esteller 2010; Wang et al. 2016).
Abnormal DNA methylation is linked with BAVM, and the use of DNMT-specific inhibitors (DNMTIs) to reverse DNA methylation may help prevent BAVM recurrence. However, the downstream pathways remain unclear, and the role of methylation and related genes in blood vessel development requires further investigation. DNMTIs have been used in related diseases, such as malignant tumors; however, related drug research on BAVM is lacking.
Non-coding RNA
Non-coding RNAs, such as short non-coding microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), are transcribed from the genome and have indispensable function in adjusting gene expression and protein function (Portela and Esteller 2010; Heard and Martienssen 2014; Winkle et al. 2021).
miRNAs, which are approximately 20–22 nucleotides long, control gene expression via binding to complementary sequences and inhibiting translation or promoting mRNA degradation (Florian et al. 2020). miRNAs behave differently and regulate vascular development differently in various diseases and systems, which is influenced by vascular formation pathways and hemodynamics. miRNAs in different cell types act on different target cells through various signaling pathways, leading to abnormal vessel formation and decreased endothelial cell migration, resulting in BAVM development (Florian et al. 2021b). miR-18a can improve BAVM by inhibiting bowel management program 4 (BMP4) and hypoxia-inducible factor 1 subunit alpha (HIF-1α) and have important clinical value in preventing, reducing, and possibly reversing BAVM (Marín-Ramos et al. 2020). miR-137 and miR-195 regulate downstream signaling proteins involved in vascular development, including phosphoinositide 3-kinase (PI3K), protein kinase B (AKT), NFκB, MAPK, ERK, and VEGF, and their levels are significantly reduced in BAVM smooth muscle cells. Increasing the levels of these miRNAs can inhibit smooth muscle cell migration, vessel formation, and in vitro survival of BAVM. miR-137 and miR-195 act as angiogenesis inhibitors by altering the phenotype of smooth muscle cells in BAVM. The absence of miR-137 and miR-195 expression causes abnormal vascular formation (Huang et al. 2017).
N6-Methyladenosine (m6A) is one of the most abundant internal RNA modifications in eukaryotic cells and is involved in the functional regulation of various biological processes. The enzymes participated in m6A modification primarily include methyltransferases (writers), demethylases (erasers), and methylation readers (readers). The m6A modification is primarily facilitated by m6A methyltransferase and eliminated by m6A demethylase, thereby playing a crucial role in this process. The modified RNA undergoes specific recognition and binding by m6A recognition proteins. This protein complex facilitates various RNA processes including splicing, maturation, nucleation, degradation, and translation. Furthermore, m6A modification modulates gene expression and controls vital cellular processes such as self-renewal, differentiation, apoptosis, and invasion (Liu et al. 2023). Recent evidence suggests that the brain has the highest abundance of m6A RNA methylation among all organs, indicating its regulatory role in CNS development and cerebrovascular remodeling (Fan et al. 2023). Notably, the catalyzed methyltransferase 3 (METTL3) and methyltransferase 14 (METTL14) subunits and the regulated Wilms tumor 1-associated protein (WTAP) and Vir Like M6A Methyltransferase Associated (KIAA1429) subunits form the core methyltransferase complex and catalyze the m6A modification of adenosine in RNA (Bokar et al. 1997, 1994; Liu et al. 2014).
Recent research has found that METTL3 content is downregulated in large BAVM teratomas, suggesting that METTL3 can be used as a biomarker for BAVM lesion size to predict treatment outcomes. Further experiments have revealed that the direct downstream targets of METTL3, deltex E3 ubiquitin ligase 3L(DTX3L), and deltex E3 ubiquitin ligase 1(DTX1) synergistically regulate the NOTCH pathway to control angiogenesis. Knocking out METTL3 significantly affects tube formation and migration of endothelial cells, indicating that the characteristics of vascular endothelial cells can be regulated via m6A modification. Blocking the NOTCH pathway with DAPT restores the phenotype of METTL3-deficient endothelial cells (Wang et al. 2020a). NOTCH directly regulates the response of endothelial cells to BMP and the formation of new blood vessel branches by regulating SMAD family member 6 (SMAD6) expression in the TGF-β pathway (Wang et al. 2020a). In this study, SMAD6 activation was also observed, downregulating BMP responsiveness, which is consistent with previous studies (Wang et al. 2020a). Wilms’ tumor 1-associating protein (WTAP) is the most important modified subunit of m6A, and its absence may lead to various developmental disorders in embryos (Ping et al. 2014). WTAP is downregulated in BAVM lesions, and WTAP knockout significantly inhibits human endothelial cell tube formation. The absence of WTAP rapidly degrades the downstream bridging protein desmoplakin through m6A modification, enhances the activity of Wilms’ tumor 1, and negatively regulates the Wnt pathway, thereby regulating blood vessel formation (Wang et al. 2020b). These results provide important evidence for elucidating the pathogenesis of BAVM and identifying new potential targets for treatment.
lncRNA molecules are longer than 200 nucleotides and do not encode proteins. These RNA molecules regulate DNA, RNA, and proteins via base pairing or chemical interactions in their secondary structures. lncRNAs are involved in a lot of biological mechanisms; however, there is limited research on lncRNAs associated with BAVM (Winkle et al. 2021). Li et al. used an Arraystar-lncRNA array to analyze lncRNA and mRNA expression in lesion tissues from four surgical patients. They found that NADPH-downregulated lncRNA (ENST00000423394) was associated with symptoms of epilepsy, and the downregulation of lncRNA (TCOS_00013855) related to the mitochondrial fusion protein OPA1 may decrease the stability of vascular smooth muscle cells (VSMCs) and surrounding cells in BAVM. Both LncRNAs were not related to BAVM hemorrhage; however, the sample size was small, and further research is needed (Li et al. 2018).
Histone modification and environmental factors
The N-termini of histone tails in eukaryotic organisms undergo many posttranslational modifications. Lysine acetylation and methylation are the main modifications that regulate chromatin structure and local gene activity. Changes in histone acetyltransferases and histone deacetyl transferases (HDACs) may be associated with BAVMs (Portela and Esteller 2010). Previous researches have confirmed that HDAC1/2/3 could adjust endothelial cell proliferation and may be influenced by hemodynamic forces, whereas HDAC7 plays a crucial role in the differentiation and regeneration of vascular endothelial cells (Mottet et al. 2007; Margariti et al. 2010). Alterations in HDACs can affect endothelial cell function and impair vascular formation. However, their specific correlation with BAVMs remains unclear.
Lysine Demethylase 8 (JMJD5) is a pluripotent histone demethylase that regulates cancer cell proliferation, embryonic development, and embryonic stem cells. Sox2 regulates JMJD5 expression in endothelial cells and activates the expression of mesenchymal markers, which affects vascular development (Yao et al. 2019). In addition, other RNA modifications such as m5C, m7G, and ac4C, as well as histone modifications, require further research on their association with the occurrence and development of BAVMs to improve our understanding of the disease (Portela and Esteller 2010; Pandolfini et al. 2019; Shen et al. 2018; Arango et al. 2018).
Tumors, strokes, inflammation, and seizures can also act as environmental factors mediating the development of acquired BAVMs. This phenomenon has been referred to as “secondary hits” in previous studies. Trauma is necessary for BAVM development in adult mice with ACVRL1-deficient vasculature. Inflammatory reactions may contribute to BAVM development as a result of inflammatory diseases, demyelination, and Bell’s palsy, as evidenced by the detection of inflammatory factors around BAVM lesions. Inflammatory factors may lead to excessive expression of VEGF and cause vascular remodeling by inducing hemodynamic abnormalities. The effect of hypoxia on BAVMs may be related to HIF-1, and the development of BAVMs after aneurysm clipping surgery may be associated with partial ischemia following vessel occlusion (Nagai et al. 2020; Wang et al. 2015; Santos et al. 2018; Lee et al. 2022).
Signaling pathways
Many genetic changes also affect angiogenesis and vascular development by regulating VEGFs and signaling pathways (Fig. 3). Abnormalities in angiogenesis and vascular development that led to structural and functional abnormalities in blood vessels are key factors in the development of BAVMs. Multiple signaling pathways regulate angiogenesis and vascular homeostasis. During development, the process of hemangioblast aggregation and differentiation into primitive vascular plexuses is called HIF-1 vasculogenesis; however, the formation of new blood vessels through proliferation, differentiation, and migration of endothelial cells from pre-existing blood vessel networks is called angiogenesis (Folkman 2007; Jeltsch et al. 2013; Patan 2000). The expression of arterial, venous, and capillary markers determines the structure of blood vessels. BAVM lesions show abnormalities in blood vessel differentiation, maturation, and capillary-specific gene expression, and abnormal blood vessels do not undergo terminal differentiation or complete maturation (Thomas et al. 2018). The control of angiogenesis and vascular homeostasis is jointly controlled via multiple complex signaling pathways including VEGF/vascular endothelial growth factor receptor (VEGFR), Ang-Tie2, NOTCH, and platelet-derived growth factor subunit B (PDGF-B), BMP-9/ACVRL1/ENG, which interact to maintain a balance between promoting and inhibiting angiogenesis and play an indispensable role in the physiological and pathological processes (Patan 2000; Jain 2003; Goumans et al. 2009; Niessen and Karsan 2008; David et al. 2009). Epigenetics plays a crucial role in normal and pathological vascular development, and hemodynamics may be an important contributor (Matouk and Marsden 2008). Abnormal blood flow dynamics at the arteriovenous junction can change endothelial cell epigenetic factors, alter their control on vascular development, and lead to BAVM development (Thomas et al. 2016).
The possible relationship between gene mutations and BAVM. KRAS mutations may cause endothelial dysfunction by activating the downstream MAPK/ERK pathway or indirectly activating NOTCH signaling to regulate cell migration. METTL3 can reduce abnormal angiogenesis by inhibiting the NOTCH signaling pathway and promoting the phosphorylation of SMAD1/5/9 and SMAD2/3. NOTCH, TGF-β, Wnt, and mTOR-FABP4 pathways and PDGF-B/PDGFR-β signaling are involved in the development of BAVM
RAS-MAPK-ERK pathway
The BAVM phenotype is linked to the inhibition of TGF-β pathway and activation of the MAPK signaling pathway (Nikolaev et al. 2018). The RAS pathway includes cascading signals that regulate key cellular functions including proliferation, growth, and senescence (Prado et al. 2019). Mutations in genes participated in the RAS/MAPK pathway include those in KRAS, NRAS, B-raf proto-oncogene (BRAF), and mitogen-activated protein kinase kinase 1 (MAP2K1). KRAS mutations have the highest reported mutation rate in BAVMs. Nikolaev et al. proposed that KRAS may induce the activation of the MAPK-ERK pathway in brain endothelial cells (BECs), leading to BAVM development. In vitro KRAS mutations induce a rise of ERK activity, causing a rise in the expression of genes associated with angiogenesis and NOTCH pathway, as well as enhancing migration. In addition, an increase in the level of phosphorylation of ERK1/2 is observed in cells expressing mutant KRAS. ERK5 regulates several pathways participated in angiogenesis and suppresses VEGF expression during hypoxic responses (Sohn et al. 2002). A significant increase in the phosphorylation level of ERK1/2 indicates the activation of the RAS/RAF/MAPK/ERK pathway and is a marker of BAVM development (Nikolaev et al. 2018).
KRAS is a downstream effector molecule of receptor tyrosine kinases that activates multiple cell-signaling networks including the KRAS-MAPK-ERK and KRAS-PI3K-AKT-mTOR pathways. The activation of MAPK-ERK pathway by KRASG12V causes the disruption of endothelial cell calcium-binding protein connections and inhibits the formation of adhesion connections. Inhibition of the MAPK-ERK pathway signal transduction with MEK inhibitors, which suppress ERK phosphorylation, can reverse the inhibition of adhesion connection formation, thereby enabling normal actin function and restoring normal vascular cell morphology (Nikolaev et al. 2018).
Animal experiments have confirmed the function of the MAPK/ERK pathway in KRAS mutations in BAVMs. Fish et al. and Jason et al. used adult mouse and zebrafish models to verify that somatic KRAS (mainly G12D or G12V) gene mutations are sufficient to cause endothelial cell dysfunction in the absence of trauma, resulting in the changes in endothelial cell morphology, increased cell size, enlargement of the vascular lumen, and vascular ectopic budding, causing the direct connection of arteries and veins and the formation of BAVMs. They also demonstrated that gene mutations activate the MEK signaling pathway rather than the PI3K pathway (Fish et al. 2020). In zebrafish models, KRAS-dependent BAVM formation is reversible (Nikolaev et al. 2018; Fish et al. 2020). Currently, there are no pharmacological inhibitors that directly target KRAS. The mechanism underlying MEK inhibition provides a new direction for the prevention and treatment of BAVM.
VEGF/NOTCH pathway
Previous researchers have revealed that both the activation and inhibition of NOTCH can lead to BAVM development (Gale et al. 2004; Krebs et al. 2004; Murphy et al. 2012, 2009). VEGF is an important regulatory factor in vascular development, and its activity is mediated by VEGF receptors (VEGF1 and VEGF2). Endothelial cell expression of VEGF, together with its co-receptor, neuropilin 1, promotes differentiation toward arteries, while the ephrin and NOTCH pathways are crucial for maintaining arterial identity. Studies have revealed that the VEGF and NOTCH signaling pathways are associated with the development and maintenance of BAVMs. During angiogenesis, the NOTCH signaling pathway associated with the EFNB2/EphB4 phenotype determines the size of the blood vessels and the proportion of arterial and venous endothelial cells. Expression of the NOTCH family is a crucial marker of arteriovenous differentiation, and VEGF plays an important role in early vascular development (Kim et al. 2008). Various factors such as hypoxic environments and hemodynamics at the arteriovenous junction cause a series of chromatin modifications that lead to abnormal gene expression. Increased VEGF expression promotes the formation of disorganized abnormal blood vessels that are more prone to bleeding (Sohn et al. 2002; Tu et al. 2014; Illi et al. 2003).
In endothelial cells and zebrafish embryo METTL3 knockout models, the upregulation of the arterial marker HES related family BHLH transcription factor with YRPW motif 2 (HEY2) and activation of the NOTCH pathway are associated with the development of BAVM-like vessels and significantly reduce endothelial cell migration ability. Based on a zebrafish model, METTL3 directly acts on the downstream targets DTX3L and DTX1, inducing an overexpression of BHLH transcription factors, such as HES1 and HEY1, which leads to the overexpression of the arterial endothelial cell phenotype HEY2 through ligand binding and protein hydrolysis. HEY proteins have also been shown to directly inhibit transcription factors (Briot and Iruela-Arispe 2015; Kopan and Ilagan 2009). The NOTCH pathway inhibitor DAPT can rescue vascular defects caused by METTL3 knockdown and may be an important targeted drug for BAVM treatment. BMPs belong to the TGF-β superfamily of secreted growth factors and regulate vascular growth by inducing phosphorylation and nuclear translocation of SMAD transcription factors (SMAD1/5/9) through receptor binding (Mouillesseaux et al. 2016). Cross-talk between NOTCH pathways and BMP pathways regulates endothelial cell behavior during blood vessel development (Wang et al. 2020a; Luna-Zurita et al. 2010). BECs in BAVMs exhibit higher activity, proliferation, migration, and mobility than normal endothelial cells, which is related to the enhanced expression of the BMP and NOTCH signaling pathways and the regulation of VEGF expression levels (Nikolaev et al. 2018; Al-Olabi et al. 2018). Furthermore, the loss of NOTCH pathway in pericytes decreases PDGFR-B expressions and increases pericyte apoptosis, indicating that NOTCH plays a critical role in pericyte survival. Loss of vascular NOTCH signaling is manifested by a marked reduction in arterial pericytes and VSMCs, increased VSMCs in veins, and severe AVMs in retinal vessels in mice. Vascular malformations and pericyte loss have been confirmed in embryonic mouse forebrains lacking NOTCH signaling. This revealed a mechanism for AVM development and highlighted the important mediating role of NOTCH pathway in this process (Nadeem et al. 2020).
TGF-β pathway
The TGF-β pathway has played a crucial role in BAVM (Choi et al. 2023; Taniguchi et al. 2022; Scimone et al. 2020; Wang et al. 2018). The protein expressions of TGF-β1 and SMAD3 were increased in BAVM tissue, while the protein contents of BMP-9, ACVRL1, SMAD1, SMAD6, and SMAD8 were markedly reduced in AVM (Wei et al. 2022). The downregulation of SMAD6 and activation of TGF-β/BMP pathway in vascular endothelial cells have been linked with microhemorrhage in BAVMs. In vitro studies have revealed that the downregulation of SMAD6 in HUVECs promoted cell proliferation, migration, and invasion and increased the level of mesenchymal markers, and the endothelial-mesenchymal behavior was positively correlated with BAVM microhemorrhage (Fu et al. 2020). Targeting the components of the aberrantly expressed TGF-β/BMP pathway in AVM is a viable approach for the development of novel molecular therapies (Wei et al. 2022). Inhibition of TGF-β signaling markedly reduced the number of p-SMAD21-positive cells in the AVF wall and improved AVF patency. Endothelial cell-targeted TGF-β inhibition can be a translational strategy for improving AVF patency (Taniguchi et al. 2022). HHT, a genetic disorder caused by mutations in ENG, ACVRL1, or SMAD4, is strongly associated with TGF-β (Hwan Kim et al. 2020; Pardali and Dijke 2012). Germline mutations in several genes involved in TGF-β/BMP signaling are associated with sporadic genetic phenotypes (Scimone et al. 2020). Follow-up researches have revealed that BMP signaling attenuates the formation of cerebral AVMs in vertebrate models (Walcott 2014). In summary, TGF-β is expected to be a key target for overcoming BAVM.
Other pathways
The mTOR-FABP4 pathway is participated in endothelial cell proliferation, apoptosis, migration, and tube formation. Studies have revealed that mTOR-FABP4 signaling is activated in BAVMs. Rapamycin can inhibit the mTOR-FABP4 pathway, thereby inhibiting EC proliferation, apoptosis, migration, and angiogenesis (Yan et al. 2022). Rapamycin treatment reduced the number of BAVM in an HHT mouse model (Ruiz et al. 2020). RASA1 or EPHB4 deficiency causes vascular malformations in zebrafish models. Kawasaki et al. confirmed that in an EPHB4-deleted zebrafish model, the mTOR pathway was strongly overactivated, and inhibition of the mTOR pathway could rescue vascular structure and function (Kawasaki et al. 2014).
Pericyte loss leads to endothelial cell hyperplasia and endothelial luminal membrane over-folding. Significant reductions in smooth muscle cell number have been observed in mouse BAVM models (Walker et al. 2011; Chen et al. 2013). Imperfections and immaturity of the vessel wall structure are significantly associated with BAVM. Knockdown of PDGF-B and PDGFR-β in mice resulted in the loss of microvascular pericytes. PDGF-B and PDGFR-β play important roles in pericytes and VSMCs recruitment during angiogenesis (Winkler et al. 2018; Zhu et al. 2018). PDGFR-β expression is reduced in BAVM lesions in ACVRL1-deficient mice (Chen et al. 2013) and is related with reduced smooth muscle cell and pericyte coverage. Overexpression of PDGF-B in ACVRL1-deficient BAVMs increases BAVM pericyte coverage and reduces BAVM hemorrhage (Zhu et al. 2018). These results suggest that PDGF-B and PDGFR-β pathway have crucial function in maintaining the vascular integrity in BAVMs. FZD10 and MYOC exhibited elevated expression levels in the vascular endothelial cells and smooth muscle cells of BAVMs. The activation of the canonical Wnt signaling pathway involving FZD10 and MYOC is crucial for the development of BAVMs, specifically in the vascular endothelial and smooth muscle cells (Huo et al. 2019).
Inflammation and BAVM
Previous studies have indicated a close association between inflammation and BAVM (Fig. 4). Studies have found a significant increase in neutrophils and macrophages in BAVM tissues, whereas T- and B-lymphocytes have rarely been observed (Chen et al. 2008). Similarly, monocytic and macrophagic infiltration has been found around BAVM lesions (Singh et al. 2022). IL-6 and CD16 + monocyte levels also increase after BAVM embolization (Hakki et al. 2022). Microglia, the primary resident immune cells of the nervous system, are the primary triggers of neuroinflammation. Microglia secrete large amounts of cytokines, chemokines, and reactive oxygen species. In a BAVM mouse model, the depletion of microglia inhibitor reduced the risk of abnormal blood vessels and bleeding (Krithika and Sumi 2021). Low expression of integrin subunit beta 8(ITGB8) in perivascular astrocytes may regulate the interaction between astrocytes and glial cells and play a crucial role in the pathogenesis of BAVM (Su et al. 2010). IL-6, IL-1, TNF-α, and IL-17A play important roles in the occurrence and development of BAVMs (Kim et al. 2009; Krithika and Sumi 2021; Pawlikowska et al. 2004; Germans et al. 2022). Polymorphisms of inflammation-related genes such as IL-6, IL-1α, IL-1β, and TNF-α can affect gene expression and increase disease susceptibility or aggravate disease progression and increase the risk of BAVM rupture. Another study demonstrated that plasma levels of IL-1β, IL-6, IL-17A, and TNF-α were significantly higher in the BAVM rupture group than in the non-rupture group, and IL-6 can be used as a reliable predictor of bleeding risk in patients with BAVM (Li et al. 2013). IL-6 induces the expression and activity of MMP-3 and MMP-9 in the mouse brain and can increase the proliferation and migration of BECs, which is consistent with the hypothesis that the induction of angiogenic activity by inflammatory processes may lead to intracranial hemorrhage in BAVM (Chen et al. 2006). When blood vessels are stimulated by pathological factors, endothelial cells (ECs) inflammatory factors are up-regulated, cell adhesion molecules (CAM) are overexpressed, and leukocytes are recruited to diseased tissues (Jeong et al. 2021). Leukocytes release a variety of inflammatory cytokines, including IL-6, TNF-α, IL-1β, and MMPs (Al-Sadi et al. 2011). IL-6 is a pro-inflammatory cytokine secreted by T cells, mononuclear phagocytes and ECs, which can activate the early inflammatory responses by promoting the expressions of IL-1β, MMP-3, IL-8, MMP-9, TNF-α and MMP-12 (Tanaka et al. 2014). TNF-α is a potent pro-inflammatory cytokine involved in central nervous system homeostasis and disease states. TNF-α, which promotes inflammation by activating MMP-9 and IL-6, and triggers proteolytic processes leading to vessel rupture, is strongly associated with BAVM rupture and bleeding risk (Mouchtouris et al. 2015). MMP-9 expression promotes vascular instability and ECM degradation, which leads to vascular rupture (Mouchtouris et al. 2015). In addition, BAVM venous hypertension can cause high expression of HIF-1 and VEGF. Induction of VEGF by HIF‐1 causes EC proliferation, migration and overexpression of MMP‐9 (Krithika and Sumi 2021). The inflammatory response up-regulates the expression of VEGF, which in turn increases capillary permeability and up-regulates the expression of VCAM-1 and ICAM-1 on EC, thereby enhancing leukocyte recruitment, inflammatory response and vascular wall degradation through the MMPs secreted by leukocytes, and promoting vascular generation, vascular remodeling, increased permeability, and further inflammation of the BAVM wall, forming an inflammatory cascade.
Role of inflammation in BAVM. A large number of microglia and neutrophils were enriched near BAVM. Microglia release cytokines and reactive oxygen species. IL-6 promotes the proliferation and migration of endothelial cells, and IL-1β, IL-6, IL-17A, and TNF-α are associated with the rupture and hemorrhage of BAVM
Single cells and BAVM
Single-cell sequencing has identified the following major vascular cell types: endothelial cells (CLDN5), pericytes (KCNJ8), smooth muscle cells (MYH11), and vascular mural fibroblasts (DCN) (Winkler et al. 2022). Studies have used multiplex spatial transcriptomics to resolve vascular cell diversity in the adult human cerebral cortex, and the spatial distribution of brain vascular cells revealed a decrease in the vascular cell density in the white matter region. Based on CLDN5 and PECAM1 expression, endothelial cells were classified into six clusters corresponding to four arteriovenous segments: capillaries, arteries, venules, and veins. In addition, three clusters of endothelial cells were identified within the arterial bands, including a cluster rich in TXNIP, a regulator of glucose metabolism, and oxidative stress. This study confirmed the expression of MFSD2A, VEGFC, and ACKR1 in arteries, capillaries, and veins (Winkler et al. 2022). Notably, in human BAVMs, endothelial cells lose their normal banding, and a unique transcriptomic state of BAVM is observed, characterized by enhanced vascular generation potential and immune cell interference (Winkler et al. 2022). A heterogeneous spatial distribution of vascular-related antigens in BAVM lesions has been reported, with more IBA1 + P2RY12- macrophages or IBA1 + P2RY12 + microglia in discontinuous regions (Winkler et al. 2022). A significant perivascular myeloid response was observed, with IBA1 + P2RY12- macrophages found farther away from adjacent blood vessels, consistent with BAVM infiltration (P < 0.01) (Li et al. 2021; Winkler et al. 2022). This study found a clear population of mesenchymal cells in BAVMs co-expressing endothelial and mesenchymal cell markers, suggesting the presence of endothelial-mesenchymal transition (Li et al. 2021). The authors identified 17 immune cell populations. Nine immune cell populations comprised myeloid cells, including vascular-related microglia, three perivascular macrophage (pvMf) subpopulations, conventional dendritic cells, and three monocyte subpopulations. Eight lymphocyte populations were identified: CD4 + T cells, two CD8 + T cell subpopulations, regulatory T cells, NK cells, B cells, plasmacytoid dendritic cells, and a dividing lymphocyte population comprising Treg cells. Resident pvMfs were the most abundant immune cell population, accounting for 31.2% and 28.3% of the immune cells in the control and BAVM groups, respectively. More than 90% of the circulating immune cells (such as CD8 + T cells) in BAVMs are confined to the resting brain vessels (Winkler et al. 2022).
Other similar cerebrovascular diseases
In the cerebrovascular system, some diseases share pathological features similar to BAVM, such as cavernous malformations (CCMs) and venous malformations (Venugopal and Sumi 2022). BAVM is a vascular abnormality characterized by abnormal arteriovenous connections and the absence of normal capillary beds. CCM consists of multiple dilated blood vessel abnormalities. Venous malformations are vascular abnormalities characterized by abnormally dilated veins. All three disorders can cause severe nerve damage such as brain hemorrhages and seizures (Venugopal and Sumi 2022). Venous malformations are an acquired disease. BAVM and CCM are sporadic and hereditary, and familial CCM is often associated with mutations in the CCM1 (KRIT1), CCM2, and CCM3 genes (Spiegler et al. 2018). Previous studies reported that mutations at multiple sites were associated with higher risk factors for BAVM and CCM. BAVM-related genes such as IL-6, VEGFA, and ENG may also affect the pathophysiological mechanism of CCM through inflammatory response, angiogenesis, etc. (Kar et al. 2018). The animal models constructed by the two through gene knockout can also be mutually pathologically verified (Sati et al. 2021). Recently, it has been reported that there are somatic mutations in genes such as PIK3CA and MAP3K3 in CCM tissues (Hong et al. 2021). These genes are key genes in the PI3K, mTOR and ERK pathways, which can alter the activity of endothelial cells and lead to abnormal arteriovenous angiogenesis (Huo et al. 2023; Weng et al. 2021; Ren et al. 2023). Interestingly, KRAS and BRAF mutations in BAVM tissues have also been reported to be involved in vascular pathology through PI3K, mTOR and ERK pathways (Nikolaev et al. 2018; Fish et al. 2020). At the same time, somatic mutations in genes such as KRAS/BRAF, PIK3CA, and MAP3K3 have also been widely reported to be present in the pathogenesis of various tumors (Nakayama et al. 2017; Yu et al. 2020). This may be because the pathogenesis of some tumors has some common factors with the pathology of cerebrovascular system disorders (Dardiotis et al. 2019). Therefore, starting from the common factors of these basic biological mechanisms, developing clinical treatment strategies may have better value for the molecular mechanism research and clinical drug development of BAVM.
Conclusions
Most BAVMs are currently treated with surgical intervention or observation, and recurrence is possible after surgery. Currently, there are no specific drugs for BAVMs, and clinical biomarkers for their formation, hemorrhage, and recurrence are lacking. Investigating the genetic and epigenetic mechanisms underlying BAVMs can provide novel directions for preventing the development of BAVMs, hemorrhage, and postoperative recurrence. Although some possible biomarkers of elevated biological or epigenetic markers in the clinical tissues and blood samples of patients with BAVMs have been identified, our understanding of how they affect BAVMs remains limited. Importantly, these markers provide a direction for future research. Several studies and reviews on the impact of miRNAs in non-coding RNA on BAVMs have been conducted; however, limited research is available on m6A methylation, lncRNA, ceRNA, and other factors, warranting further exploration.
References
Al-Olabi L, Polubothu S, Dowsett K, Andrews KA, Stadnik P, Joseph AP, Knox R, Pittman A, Clark G, Baird W, Bulstrode N, Glover M, Gordon K, Hargrave D, Huson SM, Jacques TS, James G, Kondolf H, Kangesu L, Keppler-Noreuil KM, Khan A, Lindhurst MJ, Lipson M, Mansour S, O’Hara J, Mahon C, Mosica A, Moss C, Murthy A, Ong J, Parker VE, Rivière J-B, Sapp JC, Sebire NJ, Shah R, Sivakumar B, Thomas A, Virasami A, Waelchli R, Zeng Z, Biesecker LG, Barnacle A, Topf M, Semple RK, Patton EE, Kinsler VA (2018) Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest 128(4):1496–1508
Al-Sadi O, Schulze-Tanzil G, Kohl B, Lohan A, Lemke M, Ertel W, John T (2011) Tenocytes, pro-inflammatory cytokines and leukocytes: a relationship? Muscles Ligaments Tendons J 1(3):68–76
Amyere M, Revencu N, Helaers R, Pairet E, Baselga E, Cordisco M, Chung W, Dubois J, Lacour J-P, Martorell L, Mazereeuw-Hautier J, Pyeritz RE, Amor DJ, Bisdorff A, Blei F, Bombei H, Dompmartin A, Brooks D, Dupont J, González-Enseñat MA, Frieden I, Gérard M, Kvarnung M, Hanson-Kahn AK, Hudgins L, Léauté-Labrèze C, McCuaig C, Metry D, Parent P, Paul C, Petit F, Phan A, Quere I, Salhi A, Turner A, Vabres P, Vicente A, Wargon O, Watanabe S, Weibel L, Wilson A, Willing M, Mulliken JB, Boon LM, Vikkula M (2017) Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation 136(11):1037–1048
Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, Hosogane M, Sinclair WR, Nanan KK, Mandler MD, Fox SD, Zengeya TT, Andresson T, Meier JL, Coller J, Oberdoerffer S (2018) Acetylation of cytidine in mRNA promotes translation efficiency. Cell. https://doi.org/10.1016/j.cell.2018.10.030
Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD (2005) Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke 36(2):337–341
Barbosa Do Prado L, Han C, Oh SP, Su H (2019) Recent advances in basic research for brain arteriovenous malformation. Int J Mol Sci. https://doi.org/10.3390/ijms20215324
Bendjilali N, Kim H, Weinsheimer S, Guo DE, Kwok P-Y, Zaroff JG, Sidney S, Lawton MT, McCulloch CE, Koeleman BPC, Klijn CJM, Young WL, Pawlikowska L (2013) A genome-wide investigation of copy number variation in patients with sporadic brain arteriovenous malformation. PLoS ONE 8(10):e71434
Bilguvar K, Yasuno K, Niemelä M, Ruigrok YM, von Und Zu Fraunberg M, van Duijn CM, van den Berg LH, Mane S, Mason CE, Choi M, Gaál E, Bayri Y, Kolb L, Arlier Z, Ravuri S, Ronkainen A, Tajima A, Laakso A, Hata A, Kasuya H, Koivisto T, Rinne J, Ohman J, Breteler MMB, Wijmenga C, State MW, Rinkel GJE, Hernesniemi J, Jääskeläinen JE, Palotie A, Inoue I, Lifton RP, Günel M (2008) Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet 40(12):1472–1477
Bokar JA, Rath-Shambaugh ME, Ludwiczak R, Narayan P, Rottman F (1994) Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem 269(26):17697–17704
Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM (1997) Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3(11):1233–1247
Briot A, Iruela-Arispe ML (2015) Blockade of specific NOTCH ligands: a new promising approach in cancer therapy. Cancer Discov 5(2):112–114
Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, Lawton MT, Kwok PY, Yang GY, Young WL (2006) Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol 59(1):72–80
Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, Hashimoto T, Yang G-Y, Young WL (2008) Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. https://doi.org/10.1227/01.neu.0000333306.64683.b5
Chen H, Gu Y, Wu W, Chen D, Li P, Fan W, Lu D, Zhao F, Qiao N, Qiu H, Fu C, Mao Y, Zhao Y (2011) Polymorphisms of the vascular endothelial growth factor A gene and susceptibility to sporadic brain arteriovenous malformation in a Chinese population. J Clin Neurosci 18(4):549–553
Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, Degos V, Lawton MT, Tihan T, Davalos D, Akassoglou K, Nelson J, Pile-Spellman J, Su H, Young WL (2013) Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol 33(2):305–310
Chen X, Liu Y, Zhou S, Nie S, Lin Z, Zhou C, Sun J, Gao X, Huang Y (2019) Methylation of the CDKN2A gene increases the risk of brain arteriovenous malformations. J Mol Neurosci 69(2):316–323
Chen N, Peng C, Li D (2022) Epigenetic underpinnings of inflammation: a key to unlock the tumor microenvironment in glioblastoma. Front Immunol 13:869307
Choi H, Kim B-G, Kim YH, Lee S-J, Lee YJ, Oh SP (2023) BMP10 functions independently from BMP9 for the development of a proper arteriovenous network. Angiogenesis 26(1):167–186
Claesson-Welsh L, Welsh M (2013) VEGFA and tumour angiogenesis. J Intern Med 273(2):114–127
Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, Aerts J, Andrews TD, Barnes C, Campbell P, Fitzgerald T, Hu M, Ihm CH, Kristiansson K, Macarthur DG, Macdonald JR, Onyiah I, Pang AWC, Robson S, Stirrups K, Valsesia A, Walter K, Wei J, Tyler-Smith C, Carter NP, Lee C, Scherer SW, Hurles ME (2010) Origins and functional impact of copy number variation in the human genome. Nature 464(7289):704–712
Dardiotis E, Aloizou AM, Markoula S, Siokas V, Tsarouhas K, Tzanakakis G, Libra M, Kyritsis AP, Brotis AG, Aschner M, Gozes I, Bogdanos DP, Spandidos DA, Mitsias PD, Tsatsakis A (2019) Cancer-associated stroke: pathophysiology, detection and management (Review). Int J Oncol 54(3):779–796
David L, Feige J-J, Bailly S (2009) Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev 20(3):203–212
Deng Y, Chen D, Gao F, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Zhang J, Luo Y, Miao Z (2020) Silencing of long non-coding RNA GAS5 suppresses neuron cell apoptosis and nerve injury in ischemic stroke through inhibiting DNMT3B-dependent MAP4K4 methylation. Transl Stroke Res 11(5):950–966
Dinicola S, Proietti S, Cucina A, Bizzarri M, Fuso A (2017) Alpha-lipoic acid downregulates IL-1β and IL-6 by DNA hypermethylation in SK-N-BE neuroblastoma cells. Antioxidants (basel). https://doi.org/10.3390/antiox6040074
Dock H, Theodorsson A, Theodorsson E (2015) DNA methylation inhibitor zebularine confers stroke protection in ischemic rats. Transl Stroke Res 6(4):296–300
Fan Y, Lv X, Chen Z, Peng Y, Zhang M (2023) m6A methylation: critical roles in aging and neurological diseases. Front Mol Neurosci 16:1102147
Fish JE, Flores Suarez CP, Boudreau E, Herman AM, Gutierrez MC, Gustafson D, DiStefano PV, Cui M, Chen Z, De Ruiz KB, Schexnayder TS, Ward CS, Radovanovic I, Wythe JD (2020) Somatic gain of KRAS function in the endothelium is sufficient to cause vascular malformations that require MEK but not PI3K signaling. Circ Res 127(6):727–743
Florian IA, Timiș TL, Ungureanu G, Florian IS, Bălașa A, Berindan-Neagoe I (2020) Deciphering the vascular labyrinth: role of microRNAs and candidate gene SNPs in brain AVM development—literature review. Neurol Res 42(12):1043–1054
Florian IA, Beni L, Moisoiu V, Timis TL, Florian IS, Balașa A, Berindan-Neagoe I (2021a) “De Novo” brain AVMs-hypotheses for development and a systematic review of reported cases. Medicina (kaunas). https://doi.org/10.3390/medicina57030201
Florian IA, Buruiana A, Timis TL, Susman S, Florian IS, Balasa A, Berindan-Neagoe I (2021b) An insight into the microRNAs associated with arteriovenous and cavernous malformations of the brain. Cells. https://doi.org/10.3390/cells10061373
Folkman J (2007) Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6(4):273–286
Fu W, Huo R, Yan Z, Xu H, Li H, Jiao Y, Wang L, Weng J, Wang J, Wang S, Cao Y, Zhao J (2020) Mesenchymal behavior of the endothelium promoted by SMAD6 downregulation is associated with brain arteriovenous malformation microhemorrhage. Stroke 51(7):2197–2207
Fukuda K, Majumdar M, Masoud H, Nguyen T, Honarmand A, Shaibani A, Ansari S, Tan LA, Chen M (2017) Multicenter assessment of morbidity associated with cerebral arteriovenous malformation hemorrhages. J Neurointerv Surg 9(7):664–668
Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD (2004) Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A 101(45):15949–15954
Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJJ, Marchuk DA (2004) A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 363(9412):852–859
Germans MR, Sun W, Sebök M, Keller A, Regli L (2022) Molecular signature of brain arteriovenous malformation hemorrhage: a systematic review. World Neurosurg 157:143–151
Goumans M-J, Liu Z, ten Dijke P (2009) TGF-beta signaling in vascular biology and dysfunction. Cell Res 19(1):116–127
Gross BA, Du R (2013) Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg 118(2):437–443
Hakki S, Robinson EJ, Robson MG (2022) Circulating Interleukin-6 and CD16 positive monocytes increase following angioplasty of an arteriovenous fistula. Sci Rep 12(1):1427
Heard E, Martienssen RA (2014) Transgenerational epigenetic inheritance: myths and mechanisms. Cell. https://doi.org/10.1016/j.cell.2014.02.045
Hong T, Yan Y, Li J, Radovanovic I, Ma X, Shao YW, Yu J, Ma Y, Zhang P, Ling F, Huang S, Zhang H, Wang Y (2019) High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain 142(1):23–34
Hong T, Xiao X, Ren J, Cui B, Zong Y, Zou J, Kou Z, Jiang N, Meng G, Zeng G, Shan Y, Wu H, Chen Z, Liang J, Xiao X, Tang J, Wei Y, Ye M, Sun L, Li G, Hu P, Hui R, Zhang H, Wang Y (2021) Somatic MAP3K3 and PIK3CA mutations in sporadic cerebral and spinal cord cavernous malformations. Brain 144(9):2648–2658
Huang J, Song J, Qu M, Wang Y, An Q, Song Y, Yan W, Wang B, Wang X, Zhang S, Chen X, Zhao B, Liu P, Xu T, Zhang Z, Greenberg DA, Wang Y, Gao P, Zhu W, Yang G-Y (2017) MicroRNA-137 and microRNA-195* inhibit vasculogenesis in brain arteriovenous malformations. Ann Neurol 82(3):371–384
Huo R, Fu W, Li H, Jiao Y, Yan Z, Wang L, Wang J, Wang S, Cao Y, Zhao J (2019) RNA sequencing reveals the activation of Wnt signaling in low flow rate brain arteriovenous malformations. J Am Heart Assoc 8(12):e012746
Huo R, Yang Y, Sun Y, Zhou Q, Zhao S, Mo Z, Xu H, Wang J, Weng J, Jiao Y, Zhang J, He Q, Wang S, Zhao J, Wang J, Cao Y (2023) Endothelial hyperactivation of mutant MAP3K3 induces cerebral cavernous malformation enhanced by PIK3CA GOF mutation. Angiogenesis 26(2):295–312
Hwan Kim Y, Vu P-N, Choe S-W, Jeon C-J, Arthur HM, Vary CPH, Lee YJ, Oh SP (2020) Overexpression of activin receptor-like kinase 1 in endothelial cells suppresses development of arteriovenous malformations in mouse models of hereditary hemorrhagic telangiectasia. Circ Res 127(9):1122–1137
Illi B, Nanni S, Scopece A, Farsetti A, Biglioli P, Capogrossi MC, Gaetano C (2003) Shear stress-mediated chromatin remodeling provides molecular basis for flow-dependent regulation of gene expression. Circ Res 93(2):155–161
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33(Suppl):245–254
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9(6):685–693
Jeltsch M, Leppänen V-M, Saharinen P, Alitalo K (2013) Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a009183
Jeong JH, Ojha U, Lee YM (2021) Pathological angiogenesis and inflammation in tissues. Arch Pharm Res 44(1):1–15
Kar S, Bali KK, Baisantry A, Geffers R, Hartmann C, Samii A, Bertalanffy H (2018) Genome-wide sequencing reveals small nucleolar RNAs downregulated in cerebral cavernous malformations. Cell Mol Neurobiol 38(7):1369–1382
Karlsson B, Jokura H, Yang HC, Yamamoto M, Martinez R, Kawagishi J, Guo WY, Beute G, Chung WY, Soderman M, Yeo TT (2020) Clinical outcome following cerebral AVM hemorrhage. Acta Neurochir (wien) 162(7):1759–1766
Kawasaki J, Aegerter S, Fevurly RD, Mammoto A, Mammoto T, Sahin M, Mably JD, Fishman SJ, Chan J (2014) RASA1 functions in EPHB4 signaling pathway to suppress endothelial mTORC1 activity. J Clin Invest 124(6):2774–2784
Kim YH, Hu H, Guevara-Gallardo S, Lam MTY, Fong S-Y, Wang RA (2008) Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development 135(22):3755–3764
Kim H, Hysi PG, Pawlikowska L, Poon A, Burchard EG, Zaroff JG, Sidney S, Ko NU, Achrol AS, Lawton MT, McCulloch CE, Kwok P-Y, Young WL (2009) Common variants in interleukin-1-Beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc Dis 27(2):176–182
Kopan R, Ilagan MXG (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137(2):216–233
Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T (2004) Haplo insufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 18(20):2469–2473
Kremer PHC, Koeleman BPC, Pawlikowska L, Weinsheimer S, Bendjilali N, Sidney S, Zaroff JG, Rinkel GJE, van den Berg LH, Ruigrok YM, de Kort GAP, Veldink JH, Kim H, Klijn CJM (2015) Evaluation of genetic risk loci for intracranial aneurysms in sporadic arteriovenous malformations of the brain. J Neurol Neurosurg Psychiatry 86(5):524–529
Krithika S, Sumi S (2021) Neurovascular inflammation in the pathogenesis of brain arteriovenous malformations. J Cell Physiol 236(7):4841–4856
Lawton MT, Rutledge WC, Kim H, Stapf C, Whitehead KJ, Li DY, Krings T, terBrugge K, Kondziolka D, Morgan MK, Moon K, Spetzler RF (2015) Brain arteriovenous malformations. Nat Rev Dis Primers 1:15008
Lee JS, Cho HG, Ryu JY, Oh EJ, Kim HM, Kwak S, Lee S-J, Lee J, Lee SY, Huh S, Kim JY, Chung HY (2022) Hypoxia promotes angiogenic effect in extracranial arteriovenous malformation endothelial cells. Int J Mol Sci. https://doi.org/10.3390/ijms23169109
Li A, Dubey S, Varney ML, Dave BJ, Singh RK (2003) IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170(6):3369–3376
Li X, Wang R, Wang X, Xue X, Ran D, Wang S (2013) Relevance of IL-6 and MMP-9 to cerebral arteriovenous malformation and hemorrhage. Mol Med Rep 7(4):1261–1266
Li X, Lin F, Wu J, Wang S (2018) LncRNAs expression signatures of human brain arteriovenous malformation revealed by microarray. Medicine (baltimore) 97(30):e11308
Li H, Nam Y, Huo R, Fu W, Jiang B, Zhou Q, Song D, Yang Y, Jiao Y, Weng J, Yan Z, Di L, Li J, Wang J, Xu H, Wang S, Zhao J, Wen Z, Wang J, Cao Y (2021) De Novo germline and somatic variants convergently promote endothelial-to-mesenchymal transition in simplex brain arteriovenous malformation. Circ Res 129(9):825–839
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C (2014) A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10(2):93–95
Liu Y, Wu X, Nie S, Zhou S, Xiao S, Gao X, Lin Z, Sun J, Huang Y (2020) Methylation of phospholipase A2 group VII gene is associated with brain arteriovenous malformations in Han Chinese populations. J Mol Neurosci 70(7):1056–1063
Liu C, Wang X, Yang S, Cao S (2023) Research progress of m6A RNA methylation in skin diseases. Biomed Res Int 2023:3091204
Luna-Zurita L, Prados B, Grego-Bessa J, Luxán G, del Monte G, Benguría A, Adams RH, Pérez-Pomares JM, de la Pompa JL (2010) Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J Clin Invest 120(10):3493–3507
Margariti A, Zampetaki A, Xiao Q, Zhou B, Karamariti E, Martin D, Yin X, Mayr M, Li H, Zhang Z, De Falco E, Hu Y, Cockerill G, Xu Q, Zeng L (2010) Histone deacetylase 7 controls endothelial cell growth through modulation of beta-catenin. Circ Res 106(7):1202–1211
Marín-Ramos NI, Thein TZ, Ghaghada KB, Chen TC, Giannotta SL, Hofman FM (2020) miR-18a inhibits BMP4 and HIF-1α normalizing brain arteriovenous malformations. Circ Res 127(9):e210–e231
Matouk CC, Marsden PA (2008) Epigenetic regulation of vascular endothelial gene expression. Circ Res 102(8):873–887
Morales-Valero SF, Bortolotti C, Sturiale C, Lanzino G (2014) Are parenchymal AVMs congenital lesions? Neurosurg Focus 37(3):2
Mottet D, Bellahcène A, Pirotte S, Waltregny D, Deroanne C, Lamour V, Lidereau R, Castronovo V (2007) Histone deacetylase 7 silencing alters endothelial cell migration, a key step in angiogenesis. Circ Res 101(12):1237–1246
Mouchtouris N, Jabbour PM, Starke RM, Hasan DM, Zanaty M, Theofanis T, Ding D, Tjoumakaris SI, Dumont AS, Ghobrial GM, Kung D, Rosenwasser RH, Chalouhi N (2015) Biology of cerebral arteriovenous malformations with a focus on inflammation. J Cereb Blood Flow Metab 35(2):167–175
Mouillesseaux KP, Wiley DS, Saunders LM, Wylie LA, Kushner EJ, Chong DC, Citrin KM, Barber AT, Park Y, Kim J-D, Samsa LA, Kim J, Liu J, Jin S-W, Bautch VL (2016) Notch regulates BMP responsiveness and lateral branching in vessel networks via SMAD6. Nat Commun 7:13247
Murphy PA, Lu G, Shiah S, Bollen AW, Wang RA (2009) Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest 89(9):971–982
Murphy PA, Kim TN, Lu G, Bollen AW, Schaffer CB, Wang RA (2012) Notch4 normalization reduces blood vessel size in arteriovenous malformations. Sci Transl Med 4(117):117ra8
Nadeem T, Bogue W, Bigit B, Cuervo H (2020) Deficiency of Notch signaling in pericytes results in arteriovenous malformations. JCI Insight 5(21)
Nagai Y, Anan M, Fujiki M (2020) Cerebral arteriovenous malformations as acquired lesions: case reports and review of the literature. J Stroke Cerebrovasc Dis 29(10):105157
Nakayama I, Shinozaki E, Matsushima T, Wakatsuki T, Ogura M, Ichimura T, Ozaka M, Takahari D, Suenaga M, Chin K, Mizunuma N, Yamaguchi K (2017) Retrospective study of RAS/PIK3CA/BRAF tumor mutations as predictors of response to first-line chemotherapy with bevacizumab in metastatic colorectal cancer patients. BMC Cancer 17(1):38
Niessen K, Karsan A (2008) Notch signaling in cardiac development. Circ Res 102(10):1169–1181
Nikolaev SI, Vetiska S, Bonilla X, Boudreau E, Jauhiainen S, Rezai Jahromi B, Khyzha N, DiStefano PV, Suutarinen S, Kiehl T-R, Mendes Pereira V, Herman AM, Krings T, Andrade-Barazarte H, Tung T, Valiante T, Zadeh G, Tymianski M, Rauramaa T, Ylä-Herttuala S, Wythe JD, Antonarakis SE, Frösen J, Fish JE, Radovanovic I (2018) Somatic activating KRAS mutations in arteriovenous malformations of the brain. N Engl J Med 378(3):250–261
Numata S, Ye T, Herman M, Lipska BK (2014) DNA methylation changes in the postmortem dorsolateral prefrontal cortex of patients with schizophrenia. Front Genet 5:280
Pan P, Weinsheimer S, Cooke D, Winkler E, Abla A, Kim H, Su H (2021) Review of treatment and therapeutic targets in brain arteriovenous malformation. J Cereb Blood Flow Metab 41(12):3141–3156
Pandolfini L, Barbieri I, Bannister AJ, Hendrick A, Andrews B, Webster N, Murat P, Mach P, Brandi R, Robson SC, Migliori V, Alendar A, d’Onofrio M, Balasubramanian S, Kouzarides T (2019) METTL1 promotes let-7 microRNA processing via m7G methylation. Mol Cell. https://doi.org/10.1016/j.molcel.2019.03.040
Pardali E, Ten Dijke P (2012) TGFβ signaling and cardiovascular diseases. Int J Biol Sci 8(2):195–213
Park H, Koh EJ, Lee EJ, Cheon J-E, Kim S-K (2021a) An acquired cerebral arteriovenous malformation after brain abscess treatment: case report and a review of the literature. Childs Nerv Syst 37(9):2923–2926
Park ES, Kim S, Huang S, Yoo JY, Korbelin J, Lee TJ, Kaur B, Dash PK, Chen PR, Kim E (2021b) Selective endothelial hyperactivation of oncogenic KRAS induces brain arteriovenous malformations in mice. Ann Neurol 89(5):926–941
Patan S (2000) Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol 50(1–2):1–15.
Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, Hashimoto T, Zaroff J, Lawton MT, Marchuk DA, Kwok P-Y, Young WL (2004) Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke 35(10):2294–2300
Ping X-L, Sun B-F, Wang L, Xiao W, Yang X, Wang W-J, Adhikari S, Shi Y, Lv Y, Chen Y-S, Zhao X, Li A, Yang Y, Dahal U, Lou X-M, Liu X, Huang J, Yuan W-P, Zhu X-F, Cheng T, Zhao Y-L, Wang X, Rendtlew Danielsen JM, Liu F, Yang Y-G (2014) Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24(2):177–189
Portela A, Esteller M (2010) Epigenetic modifications and human disease. Nat Biotechnol 28(10):1057–1068
Rawlik K, Rowlatt A, Tenesa A (2016) Imputation of DNA methylation levels in the brain implicates a risk factor for Parkinson’s disease. Genetics 204(2):771–781
Rees E, Kirov G (2021) Copy number variation and neuropsychiatric illness. Curr Opin Genet Dev 68:57–63
Ren J, Huang Y, Ren Y, Tu T, Qiu B, Ai D, Bi Z, Bai X, Li F, Li JL, Chen XJ, Feng Z, Guo Z, Lei J, Tian A, Cui Z, Lindner V, Adams RH, Wang Y, Zhao F, Korbelin J, Sun W, Wang Y, Zhang H, Hong T, Ge WP (2023) Somatic variants of MAP3K3 are sufficient to cause cerebral and spinal cord cavernous malformations. Brain. https://doi.org/10.1093/brain/awad104
Ruiz S, Zhao H, Chandakkar P, Papoin J, Choi H, Nomura-Kitabayashi A, Patel R, Gillen M, Diao L, Chatterjee PK, He M, Al-Abed Y, Wang P, Metz CN, Oh SP, Blanc L, Campagne F, Marambaud P (2020) Correcting Smad1/5/8, mTOR, and VEGFR2 treats pathology in hereditary hemorrhagic telangiectasia models. J Clin Invest 130(2):942–957
Santos R, Aguilar-Salinas P, Entwistle JJ, Aldana PR, Beier AD, Hanel RA (2018) De Novo arteriovenous malformation in a pediatric patient: case report and review of the literature. World Neurosurg 111:341–345
Sati L, Soygur B, Goksu E, Bassorgun CI, McGrath J (2021) CTCFL expression is associated with cerebral vascular abnormalities. Tissue Cell 72:101528
Scimone C, Granata F, Longo M, Mormina E, Turiaco C, Caragliano AA, Donato L, Sidoti A, D’Angelo R (2020) Germline mutation enrichment in pathways controlling endothelial cell homeostasis in patients with brain arteriovenous malformation: implication for molecular diagnosis. Int J Mol Sci. https://doi.org/10.3390/ijms21124321
Shen Q, Zhang Q, Shi Y, Shi Q, Jiang Y, Gu Y, Li Z, Li X, Zhao K, Wang C, Li N, Cao X (2018) Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature 554(7690):123–127
Singh AK, Kilari S, Cai C, Misra S (2022) Bindarit encapsulated nanoparticles prevent venous neointimal hyperplasia and restenosis in a murine angioplasty model. Transl Res 248:68–86
Sohn SJ, Sarvis BK, Cado D, Winoto A (2002) ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxia-sensitive repressor of vascular endothelial growth factor expression. J Biol Chem 277(45):43344–43351
Spiegler S, Rath M, Paperlein C, Felbor U (2018) Cerebral cavernous malformations: an update on prevalence, molecular genetic analyses, and genetic counselling. Mol Syndromol 9(2):60–69
Stanzione R, Cotugno M, Bianchi F, Marchitti S, Forte M, Volpe M, Rubattu S (2020) Pathogenesis of ischemic stroke: role of epigenetic mechanisms. Genes (basel). https://doi.org/10.3390/genes11010089
Storer KP, Tu J, Karunanayaka A, Morgan MK, Stoodley MA (2008) Inflammatory molecule expression in cerebral arteriovenous malformations. J Clin Neurosci 15(2):179–184
Sturiale CL, Puca A, Sebastiani P, Gatto I, Albanese A, Di Rocco C, Maira G, Pola R (2013) Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: where do we stand? Brain 136(Pt 2):665–681
Su H, Kim H, Pawlikowska L, Kitamura H, Shen F, Cambier S, Markovics J, Lawton MT, Sidney S, Bollen AW, Kwok P-Y, Reichardt L, Young WL, Yang G-Y, Nishimura SL (2010) Reduced expression of integrin alphavbeta8 is associated with brain arteriovenous malformation pathogenesis. Am J Pathol 176(2):1018–1027
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10):a016295
Taniguchi R, Ohashi Y, Lee JS, Hu H, Gonzalez L, Zhang W, Langford J, Matsubara Y, Yatsula B, Tellides G, Fahmy TM, Hoshina K, Dardik A (2022) Endothelial cell TGF-β (transforming growth factor-beta) signaling regulates venous adaptive remodeling to improve arteriovenous fistula patency. Arterioscler Thromb Vasc Biol 42(7):868–883
Thomas JM, Surendran S, Abraham M, Rajavelu A, Kartha CC (2016) Genetic and epigenetic mechanisms in the development of arteriovenous malformations in the brain. Clin Epigenet 8:78
Thomas JM, Surendran S, Abraham M, Sasankan D, Bhaadri S, Rajavelu A, Kartha CC (2018) Gene expression analysis of nidus of cerebral arteriovenous malformations reveals vascular structures with deficient differentiation and maturation. PLoS ONE 13(6):e0198617
Thomas JM, Sasankan D, Abraham M, Surendran S, Kartha CC, Rajavelu A (2022) DNA methylation signatures on vascular differentiation genes are aberrant in vessels of human cerebral arteriovenous malformation nidus. Clin Epigenet 14(1):127
Tu J, Li Y, Hu Z (2014) Notch1 and 4 signaling responds to an increasing vascular wall shear stress in a rat model of arteriovenous malformations. Biomed Res Int 2014:368082
Venugopal V, Sumi S (2022) Molecular biomarkers and drug targets in brain arteriovenous and cavernous malformations: where are we? Stroke 53(1):279–289
Walcott BP (2014) BMP signaling modulation attenuates cerebral arteriovenous malformation formation in a vertebrate model. J Cereb Blood Flow Metab 34(10):1688–1694
Walcott BP, Winkler EA, Zhou S, Birk H, Guo D, Koch MJ, Stapleton CJ, Spiegelman D, Dionne-Laporte A, Dion PA, Kahle KT, Rouleau GA, Lawton MT (2018) Identification of a rare BMP pathway mutation in a non-syndromic human brain arteriovenous malformation via exome sequencing. Hum Genome Var 5:18001
Walker EJ, Su H, Shen F, Choi E-J, Oh SP, Chen G, Lawton MT, Kim H, Chen Y, Chen W, Young WL (2011) Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol 69(6):954–962
Wang L, Guo S, Zhang N, Tao Y, Zhang H, Qi T, Liang F, Huang Z (2015) The role of SDF-1/CXCR4 in the vasculogenesis and remodeling of cerebral arteriovenous malformation. Ther Clin Risk Manag 11:1337–1344
Wang Z, Zhao J, Sun J, Nie S, Li K, Gao F, Zhang T, Duan S, Di Y, Huang Y, Gao X (2016) Sex-dichotomous effects of NOS1AP promoter DNA methylation on intracranial aneurysm and brain arteriovenous malformation. Neurosci Lett 621:47–53
Wang K, Zhao S, Liu B, Zhang Q, Li Y, Liu J, Shen Y, Ding X, Lin J, Wu Y, Yan Z, Chen J, Li X, Song X, Niu Y, Liu J, Chen W, Ming Y, Du R, Chen C, Long B, Zhang Y, Tong X, Zhang S, Posey JE, Zhang B, Wu Z, Wythe JD, Liu P, Lupski JR, Yang X, Wu N (2018) Perturbations of BMP/TGF-β and VEGF/VEGFR signalling pathways in non-syndromic sporadic brain arteriovenous malformations (BAVM). J Med Genet 55(10):675–684
Wang L-J, Xue Y, Huo R, Yan Z, Xu H, Li H, Wang J, Zhang Q, Cao Y, Zhao J-Z (2020a) N6-methyladenosine methyltransferase METTL3 affects the phenotype of cerebral arteriovenous malformation via modulating Notch signaling pathway. J Biomed Sci 27(1):62
Wang L-J, Xue Y, Li H, Huo R, Yan Z, Wang J, Xu H, Wang J, Cao Y, Zhao J-Z (2020b) Wilms’ tumour 1-associating protein inhibits endothelial cell angiogenesis by m6A-dependent epigenetic silencing of desmoplakin in brain arteriovenous malformation. J Cell Mol Med 24(9):4981–4991
Wang S, Zeng Y, Pei P, He X, Liu F, Zhang T (2022) Abnormal transcriptome-wide DNA demethylation induced by folate deficiency causes neural tube defects. Front Genet 13:987210
Wei T, Richter GT, Zhang H, Sun RW, Smith CH, Strub GM (2022) Extracranial arteriovenous malformations demonstrate dysregulated TGF-β/BMP signaling and increased circulating TGF-β1. Sci Rep 12(1):16612
Weng J, Yang Y, Song D, Huo R, Li H, Chen Y, Nam Y, Zhou Q, Jiao Y, Fu W, Yan Z, Wang J, Xu H, Di L, Li J, Wang S, Zhao J, Wang J, Cao Y (2021) Somatic MAP3K3 mutation defines a subclass of cerebral cavernous malformation. Am J Hum Genet 108(5):942–950
Westbroek EM, Pawlikowska L, Lawton MT, McCulloch CE, Young WL, Kim H (2012) Brain-derived neurotrophic factor Val66Met polymorphism predicts worse functional outcome after surgery in patients with unruptured brain arteriovenous malformation. Stroke 43(8):2255–2257
Winkle M, El-Daly SM, Fabbri M, Calin GA (2021) Noncoding RNA therapeutics—challenges and potential solutions. Nat Rev Drug Discov 20(8):629–651
Winkler EA, Birk H, Burkhardt J-K, Chen X, Yue JK, Guo D, Rutledge WC, Lasker GF, Partow C, Tihan T, Chang EF, Su H, Kim H, Walcott BP, Lawton MT (2018) Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J Neurosurg 129(6):1464–1474
Winkler EA, Kim CN, Ross JM, Garcia JH, Gil E, Oh I, Chen LQ, Wu D, Catapano JS, Raygor K, Narsinh K, Kim H, Weinsheimer S, Cooke DL, Walcott BP, Lawton MT, Gupta N, Zlokovic BV, Chang EF, Abla AA, Lim DA, Nowakowski TJ (2022) A single-cell atlas of the normal and malformed human brain vasculature. Science 375(6584):eabi7377
Xia M, Wang B, Wang Z, Zhang X, Wang X (2021) Epigenetic regulation of NK cell-mediated antitumor immunity. Front Immunol 12:672328
Yan D, Hao Q, Chen Y, Li Z, Zhang H, Yuan K, Li R, Li R, Zhao Y, Wang K, Peng H, Zhang D, Chen X, Zhao Y (2022) mTOR-FABP4 signal is activated in brain arteriovenous malformations in humans. J Mol Med (berl) 100(9):1287–1297
Yao JS, Zhai W, Fan Y, Lawton MT, Barbaro NM, Young WL, Yang GY (2007) Interleukin-6 upregulates expression of KDR and stimulates proliferation of human cerebrovascular smooth muscle cells. J Cereb Blood Flow Metab 27(3):510–520
Yao J, Wu X, Zhang D, Wang L, Zhang L, Reynolds EX, Hernandez C, Boström KI, Yao Y (2019) Elevated endothelial Sox2 causes lumen disruption and cerebral arteriovenous malformations. J Clin Invest 129(8):3121–3133
Yu K, Lin CJ, Hatcher A, Lozzi B, Kong K, Huang-Hobbs E, Cheng YT, Beechar VB, Zhu W, Zhang Y, Chen F, Mills GB, Mohila CA, Creighton CJ, Noebels JL, Scott KL, Deneen B (2020) PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 578(7793):166–171
Zhao Y, Li P, Fan W, Chen D, Gu Y, Lu D, Zhao F, Hu J, Fu C, Chen X, Zhou L, Mao Y (2010) The rs522616 polymorphism in the matrix metalloproteinase-3 (MMP-3) gene is associated with sporadic brain arteriovenous malformation in a Chinese population. J Clin Neurosci 17(12):1568–1572
Zhou S, Gao X, Sun J, Lin Z, Huang Y (2017) DNA methylation of the PDGFD gene promoter increases the risk for intracranial aneurysms and brain arteriovenous malformations. DNA Cell Biol 36(6):436–442
Zhu W, Chen W, Zou D, Wang L, Bao C, Zhan L, Saw D, Wang S, Winkler E, Li Z, Zhang M, Shen F, Shaligram S, Lawton M, Su H (2018) Thalidomide reduces hemorrhage of brain arteriovenous malformations in a mouse model. Stroke 49(5):1232–1240
Funding
This study was supported by the grants from the Zhejiang Provincial Natural Science Foundation of China (LY22H090001), Ningbo Health Branding Subject Fund (PPXK2018-04), Ningbo Top Medical and Health Research Program (2022020304), Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province (2022E10026).
Author information
Authors and Affiliations
Contributions
YH and JY contributed to the conception and design of the study. SW, XD, YW, YW and SZ organized the database. SW and XD wrote the first draft of the manuscript. YH reviewed and edited.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Deng, X., Wu, Y. et al. Understanding the pathogenesis of brain arteriovenous malformation: genetic variations, epigenetics, signaling pathways, and immune inflammation. Hum. Genet. 142, 1633–1649 (2023). https://doi.org/10.1007/s00439-023-02605-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-023-02605-6