Abstract
Renal cell cancer (RCC) accounts for 2–3 % of adult malignancies, and the incidence appears to be rising each year. Initially the increasing prevalence of computed tomography (CT) imaging was thought to be the reason for the increased incidence as it led to incidental findings of renal masses. However, even between the years 2000 and 2009, the incidence of localized and distant disease has continued to increase. Cigarette smoking, obesity, end-stage renal failure, and hypertension are some of the contributing risk factors, although strong associations have not been established. Clear cell RCC is the most common histologic subtype and accounts for approximately 70 % of RCC. A minority (<5 %) of RCC cases are considered hereditary. The pathophysiology of majority of clear cell cancers is driven by structural alterations in the von Hippel-Lindau gene. Clinical presentation of the disease is frequently an asymptomatic, incidental finding of a renal mass. Only 9 % of kidney cancers present with the classic triad of hematuria, flank pain, and fever. About a third of the patients have advanced disease at diagnosis. RCC is known as one of the “great masqueraders,” due to the wide variety of paraneoplastic manifestations and up to 20 % of patients present with these. The imaging gold standard for the diagnosis, staging, and surveillance of RCC is the CT scan. Tumor stage and grade remain the most important prognostic factors in localized disease. Nomograms incorporating these have been developed to predict the risk of relapse. In metastatic disease, the Memorial Sloan-Kettering and Heng criteria have been adopted to predict overall survival outcomes. Development of predictive biomarkers, for therapy selection, is the current critical challenge in RCC. This chapter highlights the clinical presentations, staging, and prognostic factors associated with RCC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Key Points-
Renal cancer is the tenth leading cause of cancer death in the United States.
-
For localized tumors, the 5-year survival rate exceeds 85 %; however, this falls to 20 % or less for advanced or metastatic tumors. Unfortunately, approximately 25–30 % of patients with RCC present with metastatic disease.
-
The critical gene involved in the pathogenesis of RCC is the von Hippel-Lindau tumor suppressor gene (VHL).
-
Clear cell histology accounts for 70 % of renal cancers and is the most aggressive form. Chromophobe and papillary are indolent and minimally symptomatic at presentation.
-
The most common paraneoplastic manifestations are hypertension and hypercalcemia.
-
Ultrasound is often the first imaging modality used to evaluate patients with suspected RCC, but the gold standard for diagnosis, staging, and surveillance is the computed tomography scan.
-
The staging system that is commonly employed is TNM system. Stage remains among the most important prognostic factors for the clinical behavior and outcome of RCC.
-
Several prognostic nomograms have been developed using clinicopathological features to predict patient outcome independent of treatment received.
1 Clinical Presentation
1.1 Symptoms and Signs (See Table 7.1)
The classic triad described in RCC is comprised of hematuria, flank pain, and fever but is seen in only 9 % of patients. Clinical presentation is actually extremely variable and is highly dependent on stage of presentation. The sequestered location of the kidney, in the retroperitoneum, results in asymptomatic and non-palpable masses that present only at an advanced or metastatic stage. Incidental detection has increased over time. Between 1935 and 1965, 7 % of tumors were discovered incidentally. In a National Cancer Institute (NCI) study conducted in metropolitan Detroit and Chicago from 2002 to 2007, the proportion of asymptomatic cases increased from 35 % in 2002 to 50 % in 2007. Cases before 1973 were found without the benefit of computed tomography (CT) or ultrasound scanning, whereas those after 1980 were discovered largely because of the widespread use of these technologies. What was once an internist’s tumor has transformed into a radiologist’s tumor. Incidental tumors diagnosed at an earlier stage obviously have a better prognosis. In a recent single-institution study, patients who underwent surgical resection from January 1, 1988, and December 31, 2007, were reviewed. Data were divided into four periods, with each time period encompassing 5 years. Over time the rate of incidental detection increased, from 10.6 to 27.6 % [1] largely because of imaging for evaluation of vague abdominal symptoms (see Fig. 7.1). The incidental tumors are more likely to be smaller (<4 cm) and have a lower grade (Fuhrman grade 1–2), which contributes to better cancer-related prognosis. The incidental finding of renal mass can also induce morbidity and the risks vs. benefits of therapy should be carefully considered, especially in cases with significant comorbidities (Table 7.2).
Comparison of incidental and symptomatic presentation of RCC from different time periods. Group 1:1988–1992; Group 2:1993–1997; Group 3:1998–2002; Group 4: 2003–2007 (Reprinted with permission. Medknow Publications & Media Pvt. Ltd. All rights reserved. Gupta et al. [1])
Approximately 25–30 % of patients with RCC present with locally advanced or metastatic disease. Expectedly, these patients can present with symptoms secondary to metastasis to distant sites. The most common sites of metastasis include:
-
Lung: 50–60 % (Fig. 7.2)
-
Bone: 30–40 % (Fig. 7.3)
Fig. 7.4 Postoperative nomogram to predict recurrence in localized clear cell RCC (Reprinted from Sorbellini et al. [54], January 2005 with permission from Elsevier)
-
Liver: 30–40 %
-
Soft tissue: 35 %
-
Central nervous system: 8 %
-
Cutaneous: 8 %
Depending on the organ involved, patients can present with hemoptysis, pleural effusion, cough, bone pain, back pain, pathological fracture, mental status changes, and headache.
Histology also appears to influence the initial clinical presentation. Clear cell RCC has a propensity for vascular invasion and is associated with distant metastasis at an early stage as compared to the papillary tumors that tend to have locoregional invasion with lymph node spread. Due to the low potential for early vascular invasion of papillary and chromophobe cancers distant metastases typically occur later in the disease course.
1.2 Paraneoplastic Manifestations
Paraneoplastic syndromes are defined as a collection of symptoms and clinical signs that occur in cancer patients, remotely from the tumor location. These are the result of humoral substances produced by the cancer cells (such as calcitriol production by RCC) or benign tissues generating humoral factors in response to malignancy (such as clubbing) or via modulation of the immune system.
Approximately 20 % of patients present initially with paraneoplastic symptoms, while up to 40 % can develop some form of paraneoplastic symptoms during their disease course. After nephrectomy, the recurrence of a previous paraneoplastic syndrome should alert for possible disease progression. Because of its propensity for causing paraneoplastic symptoms, RCC has historically been called one of the “great masqueraders” of medicine [2, 3].
1.2.1 Hypercalcemia
This is the most common of the paraneoplastic syndromes, affecting 13–20 % of patients with RCC. Approximately 75 % of patients presenting with hypercalcemia have advanced disease, while about half have bone metastasis. Non-metastatic hypercalcemia is secondary to the elaboration of humoral peptides by RCC. These include PTHrP, IL-1, TNF, TGF, and OAF. The clinical picture can be very polymorphic. Symptoms can range from nonspecific symptoms such as asthenia, headache, lack of appetite, nausea, vomiting, constipation, polyuria, and polydipsia (due to nephrogenic diabetes insipidus) to specific clinical syndromes such as hypercalcemia or erythrocytosis or anemia. Hypercalcemia is a life-threatening condition that typically manifests with confusion, constipation, or nausea. Profound lethargy or even comatose condition has been noted. Physical findings include decreased deep tendon reflexes and an impaired level of consciousness. Patients may be dehydrated secondary to loss of renal concentrating ability and subsequent polyuria. Laboratory studies in affected patients reveal hypercalcemia, decreased levels of PTH, and 1,25-vitamin D and renal phosphate wasting. ECG findings include increased PR and QT intervals with eventual bradyarrhythmias and asystole. Treatment is mainly with repletion of volume with IV fluids and loop diuretics as needed. Bisphosphonates such as pamidronate or zoledronate are effective for long-term management. It has been suggested that the most effective way to treat the hypercalcemia is to treat the cause, with nephrectomy for localized disease and systemic therapy for metastatic RCC [2, 4].
1.2.2 Hypertension
Up to 40 % of patients with RCC develop hypertension as a paraneoplastic manifestation. Hypertension is typically associated with low-grade, clear cell tumors. Potential mechanisms include renin secretion, ureteral or parenchymal compression, presence of an arteriovenous fistula, and polycythemia. The sequence of events is believed to be as follows: local renal parenchymal compression and ureteral obstruction causes renin secretion, which then contributes to hypertension. Elevated serum renin levels have been found in 37 % of patients with RCC. Treatment for hypertension caused by RCC is nephrectomy; 85 % will become normotensive after such a procedure [2, 3].
1.2.3 Polycythemia
This is seen in 1–8 % of RCC patients, mainly mediated by erythropoietin (EPO), a glycoprotein produced by tumor cells and peritubular renal interstitial cells that promotes red blood cell production in the bone marrow. Elevated EPO levels have no prognostic significance. Patients with high EPO levels develop anemia more often than polycythemia [2, 3]. Interestingly, although two-thirds of patients with RCC have elevated EPO levels, only 8 % experience erythrocytosis.
1.2.4 Non-metastatic Hepatic Dysfunction (Stauffer’s Syndrome)
In 1961, Stauffer noted hepatic laboratory abnormalities in a patient with RCC with no evidence of hepatic metastases. These resolved with nephrectomy but returned with disease recurrence. Incidence of this so-called Stauffer’s syndrome is 3–20 %. Patients with this syndrome present with hepatosplenomegaly, fevers, and weight loss. It is characterized by transaminitis and abnormal hepatic synthetic function. In two-thirds of patients, nephrectomy led to resolution of Stauffer’s syndrome. One-year survival was found to be 88 % in patients whose liver enzymes normalize after nephrectomy, compared to 26 % if they remain elevated [2, 3, 5, 6].
1.2.5 Constitutional Symptoms
One-third of RCC cases present with constitutional symptoms like fever, weight loss, and fatigue. Twenty to thirty percent can have fever, but only 2 % have it as a sole manifestation. In a study by Tsukamoto et al., 18 of 71 patients have elevated levels of IL-6, and 78 % of those with increased levels had fever [7]. In a study by Kim et al., cachexia, defined as hypoalbuminemia, weight loss, anorexia, or malaise, predicted worse survival after controlling for well-established indicators of prognosis including TNM stage, Fuhrman grade, and ECOG-PS [8].
1.2.6 Other Endocrine Abnormalities
Abnormal glucose metabolism has been described in RCC. There have been several case reports of either hyperglycemia or hypoglycemia. RCC tumors have been reported to have elevated intracellular levels of insulin, glucagon, and enteroglucagon when compared to controls.
RCC accounts for 2 % of all neoplasms that are responsible for Cushing’s syndrome. This is secondary to enzymatic conversion of pro-opiomelanocortin to ACTH by the tumor. This ectopic ACTH drives cortisol secretion by the adrenal glands. Post-nephrectomy these patient are at risk for postoperative Addisonian crisis [2, 3]; thus, clinicians should be cognizant of this potential complication.
Finally, elevated serum beta-HCG levels can be found in 6 % of patients with RCC.
1.2.7 Non-endocrine Paraneoplastic Syndromes
Amyloidosis is seen in 3–8 % of patients with RCC. The amyloid protein found is AA. The mechanism hypothesized for AA deposition is prolonged stimulation of the immune system by either the malignancy or tumoral necrosis, leading to a rise in the levels of the acute phase reactant SAA. Initial patient complaints are weakness, weight loss, and syncope. Eventually the symptoms depend on which organ is involved.
Neuromyopathies are also described in RCC. They can be sensory or motor. Severity varies from nonspecific myalgias to a symptom complex reminiscent of amyotrophic lateral sclerosis.
2 Imaging (Table 7.3)
With the implementation of modern cross-sectional imaging modalities in clinical practice, the diagnosis, treatment, and surveillance of RCC have changed dramatically in the past two decades. As the incidental detection of small renal tumors has increased, this allowed earlier detection and treatment, consequently improving long-term survival rates [9, 10].
The major goals of imaging techniques are to correctly differentiate benign from malignant lesions and for early diagnosis, precise staging, and response evaluation to systemic therapy [11].
2.1 Ultrasound
Ultrasound (US) is often the first imaging technique used to evaluate patients with suspected RCC. Vascular flow detected by color Doppler US was reported to be strongly suggestive of conventional clear cell histology. Color Doppler US had a diagnostic accuracy similar to dynamic CT in most patients with renal solid tumors, and the color flow pattern was different among RCC subtypes. These observations suggest the use of color Doppler US as an additional tool in patients whose tumor is poorly attenuated or in those with contraindications for contrast medium and radiation [12]. When compared to CT scans, the accuracy of US to detect small renal tumors is low. The sensitivity for tumors that are <3 cm in diameter is only 67 % [13]. The deficiencies with conventional US are definitive identification of the following: complex cystic lesions, venous tumor thrombus extension, and verification of metastatic lesions. These shortcomings are due to the well-known inherent limitations of US imaging such as reliance on operator experience and on patient’s constitution.
Contrast-enhanced US (CEUS) is a rapidly evolving technique using US-specific intravenous contrast agents in the form of microbubbles. A complete concordance between CEUS and CT in the differentiation of surgical and nonsurgical complex cysts was reported [14]. The sensitivity to detect tumor thrombus can reach 100 % if it involves the intrahepatic portion of the IVC, but it drops to 68 % if it lies below the level of the insertion of the hepatic veins. Depending on the patient’s constitution, in 43.5 % of cases the IVC is not completely visualized [15]. It is the only available intraoperative imaging modality to ensure nephron-sparing surgery and to identify additional tumors. Under US guidance, minimally invasive procedures like biopsies and radiofrequency ablations can be performed [16].
Dynamic contrast-enhanced US can potentially be used in the era of antiangiogenic therapies to evaluate tumor response. An ongoing French national study will be able to define its utility in monitoring antiangiogenic therapy [17].
2.2 Computed Tomography (CT) Scanning
The gold standard for the diagnosis, staging, and surveillance of RCC is the CT scan [18, 19]. With multidetector-row CT (MDCT) scanners, one is able to obtain a true volume scan and ultrathin sections (<0.5 mm) with minimal time for motion artifact [20]. With the advent of triphasic (unenhanced, corticomedullary or arterial phase, and nephrographic phase) MDCT and 3D reconstruction, there is provision of accurate preoperative planning, especially for nephron-sparing surgery [21]. The degree of enhancement is a unique finding to differentiate conventional clear cell RCC from other subtypes and from angiomyolipoma [22]. Jinzaki et al. reported that clear cell RCC showed a peak attenuation value in the cortical nephrographic phase of >100 HU, whereas for other subtypes it is <100 HU [23]. The presence of homogeneous and prolonged enhancement significantly differentiates angiomyolipoma with minimal fat from RCC [24].
The staging accuracy with CT scans is 90 %. The detection of a normal adrenal gland in MDCT is associated with 100 % negative predictive value for metastasis [25]. For lymph node metastasis, the false-negative rate is 10 %, and false-positive rate ranges from 3 to 43 % [26, 27]. For M staging, there is an excellent agreement between MDCT and surgical pathology [27]. With the MDCT, tumor thrombus is accurately identified and localized.
Tumor response to antiangiogenic therapy can also be assessed with CT scanning. The application of RECIST criteria is limited in tumors with irregularity and diffuse invasion. So volumetric mean tumor attenuation in contrast-enhanced MDCT has been proposed as an alternative potential response criterion.
2.3 Magnetic Resonance Imaging (MRI)
MRI is the imaging modality of choice in patients with contrast allergy and functional renal impairment or who are pregnant. It is mainly used as a complementary problem-solving tool in selected cases of undefined renal lesions and suspected perinephric tumor spread or recurrence. The advantages of MRI include: absence of radiation, lack of need for standard iodinated contrast medium, and its high inherent contrast among different soft tissues [16]. Disadvantages are longer examination times, higher cost, and inferior capacity to detect lung metastasis. In patients with renal insufficiency, the MRI contrast medium gadolinium has been associated with nephrogenic systemic fibrosis.
In a study by Pedrosa et al., the overall sensitivity and specificity of MRI to predict the histologic subtype were 92 and 83 % for clear cell and 80 and 94 % for papillary RCC, respectively [28]. MRI along with CT scans has difficulty in correctly identifying perinephric tumor invasion, distinguishing inflammation from tumor infiltration, and insensitivity in differentiating small collateral blood vessels from tumor extension in the lymphatics [29]. The sensitivity and specificity for detecting metastatic lymphadenopathy are low. It is highly sensitive and specific for detection of bone metastasis [30]. It is more sensitive than CT for detection of brain metastasis. MRI is a reliable method for evaluation of tumor thrombus. The accuracy is ranging from 65 to 100 % [16].
In regard to response evaluation to antiangiogenic therapy, it is still restricted to clinical trials because of poor standardization, methodologic challenges, limited sensitivity, and concerns related to potential harmful effects of MRI contrast agents.
2.4 FDG- PET
The increased background activity of healthy renal tissue and normal FDG excretion in urine can make visualization of primary renal cancers by PET difficult. 2-Deoxy-2-[18F]-fluoro-D-glucose (FDG) thus far has not offered any advantage over a standard imaging modality such as MDCT. In a retrospective review [31], the sensitivity and specificity of PET for detection of primary RCC were 60 % and 100 %, respectively, and with CT scan, these were 91.7 % and 100 %, respectively. It is also less sensitive than CT in the detection of metastasis to retroperitoneal lymph nodes and/or renal bed recurrence (75 vs. 92.6 %), lung metastases (75 vs. 91.1 %), and bony metastases (77.3 vs. 93.8 % of CT + bone scan). By using PET with an iodine-124-labelled antibody chimeric G250 (124I-cG250) against carbonic anhydrase-IX (“immuno-PET”) for clear cell RCC, sensitivity was 94 % and specificity was 100 % [32]. Other markers under investigation are 18F-fluoromisonidazole (FMISO), a noninvasive tumor marker of tissue hypoxia, and 18F-fluorothymidine, a tracer that mirrors cellular proliferation.
FDG-PET/CT has the advantage to detect the metabolic activity of local recurrence that is not influenced by factors that jeopardize diagnosis of local recurrence with CT, such as migration of the adjacent normal organs into the renal fossa, postoperative scarring, and artifacts from surgical clips [33]. FDG-PET/CT can examine the whole body in one procedure without contrast agents. Park et al. demonstrated that, for the surveillance of high-risk RCC, FDG-PET/CT had results as good as conventional methods and were not influenced by the Fuhrman grade or the histological subtype. FDG-PET/CT is 89.5 % sensitive, 83.3 % specific, and 85.7 % accurate in detection of recurrence or metastasis.
3 Staging
Tumor stage, which reflects the anatomic spread and involvement by disease, is recognized as the most important prognostic factor for the clinical behavior and outcome of RCC. The first formal staging system proposed by Flocks and Kadesky in 1958 was based on the physical characteristics of the tumor and the location of tumor spread.
Currently the staging system that is followed is the tumor-node-metastasis (TNM) system. It was most recently revised in 2010 and is supported by both the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control (UICC). This is a dynamic staging method that changes continually on the basis of new evidence from clinical studies. It is based on data from large multicenter studies with a fairly good level of evidence.
The first TNM staging system was developed in 1978. Tumors are characterized on the basis of the degree of local extension of the tumor at the primary site (T), the involvement of regional lymph nodes (N), and the presence or absence of distant metastases (M). The classification may be clinical (cTNM) or histopathological (pTNM). Regional lymph nodes for RCC are defined as the hilar, abdominal para-aortic, and paracaval nodes [34, 35]. Refer to Table 7.4 for a full description of the TNM staging system for RCC.
4 Clinical Prognostic/Predictive Markers
Prognostic factors in RCC include:
-
Anatomical (TNM classification, tumor size)
-
Histological (Fuhrman grade, histologic subtype)
-
Clinical (symptoms and performance status)
-
Molecular features (described in Chap. 4)
All these factors are not accurate by themselves, but when combined, they improve accuracy of predicting outcome independent of treatment received. Hence, various prognostic models or nomograms have been proposed and designed. These models can be valuable tools for patient counseling, follow-up, clinical trial design, analysis, and interpretation [36].
4.1 Prognostic Factors in Non-metastatic RCC
Classical prognostic factors for non-metastatic disease include anatomical, histological, clinical, and molecular features.
Anatomic features are integrated in the TNM staging system. RCCs with higher T stage and lymph node and distant metastasis are associated with a worse prognosis and shorter survival [37, 38]. Involvement of the renal sinus fat appears to have worse prognosis [39, 40]. The current TNM staging does not distinguish between perirenal fat and renal sinus fat invasion, or between invasion of a muscular branch of the renal vein, and involvement of the entire renal vein (both staged as pT3a). Involvement of ipsilateral adrenal gland confers dismal prognosis, and the outcomes are equivalent to stage IV disease [41]. Involvement of the IVC whether above or below the diaphragm is not prognostically different, but it has been shown that these patients have better prognosis when compared to patients with perinephric fat or nodal involvement [42].
Histological features include Fuhrman nuclear grade, histologic subtype, presence of sarcomatoid component, microvascular invasion, tumor necrosis, and collecting system invasion. The most widely accepted histologic prognostic factor is Fuhrman nuclear grade developed in 1982 by Fuhrman et al. [43]. Four nuclear grades (1–4) were defined in order of increasing nuclear size, irregularity, and nucleolar prominence. Nuclear grade was more effective than each of the other parameters in predicting development of distant metastasis following nephrectomy. The value of Fuhrman grade in histological subtypes other than clear cell RCC has been disputed. The simplified version was as accurate as the classical four grades scheme when the grade was integrated into a prognostic nomogram [44].
Many studies have observed a significant association between histologic subtype and disease-specific survival in univariate analysis, with clear cell being the most aggressive tumor followed by papillary and chromophobe RCC. This prognostic value disappears in multivariable analysis suggesting that stage and grade have a higher impact on prognosis than the histology [45, 46]. RCC with sarcomatoid features have a dismal prognosis. Papillary tumors are divided into two groups with very different prognosis. Type I papillary tumors are low grade and multifocal and display a very favorable outcome, and type II are usually high grade and have an increased metastatic potential.
The presence of tumor necrosis is also a well-established independent indicator of poor prognosis for localized disease. Invasion of the collecting system is relatively rare but is associated with a worse prognosis, especially in lower stage disease.
Clinical prognostic features include performance status, local symptoms, cachexia, and anemia. The University of Michigan found that the mode of presentation (symptomatic vs. incidental) was an independent prognostic factor in the multivariate analysis for both disease-free and disease-specific survival [47]. Thrombocytosis is an independent prognostic marker, and it reflects a cascade of biological events correlated with tumor aggressiveness.
Several molecular and genetic tissue markers are investigated for prognostic significance. The prognostic role of von Hippel-Lindau (VHL) gene alterations and of hypoxia-induced factor 1alpha is controversial [48, 49]. VEGF is associated with more aggressive tumor phenotype. High carbonic anhydrase 9 (CA IX) levels have been associated with improved prognosis in advanced clear cell RCC [50]. Ki-67 has been found to be an independent prognostic factor in a multivariate analysis [50, 51], with high levels associated with poorer outcomes. Molecular markers have the potential to be used for screening, diagnosis, and follow-up, but at present have not been validated in well-designed multicenter prospective studies, hence limiting their clinical utility. Chapter 4 provides a more detailed description of molecular biomarkers in RCC.
A single prognostic feature does not yield sufficient predictive accuracy. Thus, investigators have combined different established parameters into algorithms or nomograms in order to improve prognostic accuracy. These tools are simple to use and are superior over standard multivariate regression models since they provide an estimate of the individual probability of outcome in a specific patient.
4.2 Prognostic Nomograms in Localized Disease
The first prognostic model was developed by Elson et al. in 1988, in 610 patients with recurrent or metastatic renal cell carcinoma to predict cancer-specific mortality. In 2001, investigators from Memorial Sloan-Kettering Cancer Center (MSKCC) introduced a postoperative nomogram for patients with localized RCC, which assigned points based on a combination of variables that included histology, tumor size, 1997 T stage, and symptoms at presentation. The aim was to predict the probability of RCC recurrence after nephrectomy in 601 patients. The predictive accuracy was 74 %, which however is no different from the TNM staging [52]. External validation was carried out in a European series and showed variable results [53]. The Kattan nomogram was updated by Sorbellini in 2005 [54]. These achieved 82 % accuracy in external validation but only in clear cell subtype (Fig. 7.4).
UISS categorization Table with 2- and 5-year projected survivorships (Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved. Zisman et al. [68])
The Mayo Clinic introduced a prediction model to assess cancer-specific survival, in patients with clear cell RCC who underwent radical nephrectomy. In multivariable analysis TNM stage, tumor size, nuclear grade, and tumor necrosis are found to be significant. The predictive accuracy of the SSIGN was 81–88 % in external validation [55].
In 2003, Leibovich et al. developed an algorithm to predict progression to metastases after radical nephrectomy in clinically localized clear cell RCC. Tumor stage, size, grade, necrosis, and regional lymph node status were statistically significantly associated with progression to metastases. The metastases-free survival rates were 86.9 % at 1 year and 74.1 % at 5 years [56].
Another prognostic model has been the UCLA Integrated Staging System (UISS). The UISS was developed using the kidney cancer database from the University of California Los Angeles Kidney Cancer Program with the goal of providing a simple and accurate algorithm for predicting survival using variables that are available in any modern medical practice. In the initial study by Zisman et al. [57], patients were grouped based on TNM stage, Fuhrman grade, and ECOG performance status. This algorithm differed from the MSKCC nomogram, as it is limited to patients with clear cell histology and included other factors like nuclear grade and histologic tumor necrosis. The presence of symptoms at presentation, which was a prominent feature in the Kattan’s nomogram, was not significant in this analysis after adjusting tumor stage, size, regional lymph node status, nuclear grade, and necrosis. In this study it was found that tumors measuring >10 cm were 48 % more likely to metastasize when compared to tumors <10 cm, after adjusting for other statistically significant pathologic features (see Fig. 7.5). The purpose was mainly to define subgroups with different risks of death following nephrectomy (Fig. 7.6).
Kaplan-Meier survival analysis of the study population according to the UISS categories. Black triangles mark the ten patients at risk point (Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved. Zisman et al. [68])
Overall survival probability according to time after therapy initiation and risk group (Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved. Heng et al. [65])
In an international multicenter study by Patard et al., UISS was used to stratify both localized and metastatic RCC into three different risk groups. For localized disease, the 5-year survival rates were 92 %, 67 %, and 44 % for low-, intermediate-, and high-risk groups, respectively. A trend toward a higher risk of death was observed with increasing UISS risk category. This study confirmed the general applicability and accuracy of the UISS for predicting survival in localized RCC. The predictive accuracy was 86 % at 2 years, which is significantly superior to that of the TNM system alone. The high predictive accuracy combined with its validity and robustness across different populations made it a reliable and useful tool for clinical practice [58].
In 2007, Karakiewicz et al. [59] proposed a nomogram for prediction of RCC-specific survival. This is similar to the UISS, but tumor size is used as a continuous variable and the ECOG performance status is replaced by symptoms that distinguish asymptomatic and local and systemic symptoms. The predictive accuracy at 10 years was 89 % in the external cohort validation and had the highest predictive accuracy.
4.3 Prognostic Factors in Metastatic Disease
In metastatic setting, the prognostic impact of the primary tumor characteristics disappears. The classic anatomic factors (stage, size, perinephric fat, venous or adrenal invasion) have very limited prognostic role. The location, multiplicity, and resectability of the metastasis play a significant role in prognosis. Presence of multiple lung and brain metastasis and involvement of bone especially spinal location indicate worse prognosis. Presence of sarcomatoid differentiation is associated with very poor prognosis. However, the most important clinical prognosticator appears to be performance status.
Biological prognostic factors include low hemoglobin, elevated lactate dehydrogenase, and high corrected serum calcium and inflammatory markers. Several of these pretreatment clinical features have been associated with shorter survival, and thus identification of these prognostic factors has led to the development of risk stratification models.
In the metastatic setting, the combination of several variables has higher predictive accuracy than independent variables. The two most adopted are classification systems of the French group of immunotherapy and the MSKCC model(s).
The Groupe Franc¸ais d’immunotherapie enrolled 782 mRCC patients over a 6-year period. This group developed and validated a prognostic model based on performance status, number and location of metastases, interval between diagnosis and systemic treatment, hemoglobin level, neutrophil count, and other biological signs of inflammation. This was designed to predict progression and survival following cytokine-based immunotherapy and stratified patients according to the number of adverse prognostic factors into three prognostic groups—good, intermediate, and poor risk—with median survival rates of 42, 15, and 6 months, respectively. The four independent factors predictive of rapid progression under treatment were: presence of hepatic metastases, short interval from renal tumor to metastases (<1 year), more than one metastatic site, and elevated neutrophil counts. Patients with at least three of these factors have over 80 % probability of rapid progression despite treatment [60].
The MSKCC model was developed by Motzer et al. and used data from patients with RCC who received treatment with IFN-alpha. The database was a retrospective study of 670 advanced renal cancer patients treated in successive clinical trials at MSKCC to define pretreatment features predictive of survival. The five risk factors associated with shorter survival were low Karnofsky performance status (<80 %), high lactate dehydrogenase (>1.5 times upper limit of normal), low serum hemoglobin (< lower limit of normal), high corrected serum calcium (>10 mg/dL), and interval from diagnosis to treatment of less than 1 year. Three-year survival for the favorable-risk (0), intermediate-risk (1–2), and poor-risk (>/=3) groups were 31 %, 7 %, and 0 %, respectively. The median survival rates in the three risk groups were 20, 10 and 4 months [61], respectively.
The MSKCC criteria were validated and additionally elaborated by an independent group at the Cleveland Clinic in a cohort of 308 untreated mRCC patients. In addition to the MSKCC criteria, prior radiotherapy and the presence of more than one site of metastases also had negative prognostic value [62].
MSKCC investigators then developed another prognostic model for patients who have failed cytokine therapy. Factors associated with shorter survival were low Karnofsky performance status, low hemoglobin level, and high corrected serum calcium. The median survival times with 0, 1,>/=2 risk factors were 22, 11.9, and 5.4 months, respectively [63].
These prognostic risk profiles are derived from the era of immunotherapy, and it is unclear if these prognostic factors are relevant to contemporary patients predominantly treated with VEGF-targeted therapy. It was essential to validate these prognostic models in the era of targeted therapy. In the study by Motzer et al., treatment-naïve mRCC patients were randomized to either sunitinib or INF. The predefined MSKCC risk factors predicted longer PFS with sunitinib [64]. In a recent multicenter, retrospective study led by Heng et al. in metastatic RCC patients, treated with VEGF-targeted therapies, four out of the five MSKCC adverse prognostic factors (anemia, hypercalcemia, poor performance status, shorter time from diagnosis to initiation of therapy) were identified as independent determinants of OS outcome. Additionally, presence of bone metastases, neutrophilic leukocytosis, and thrombocytosis were noted to be independent adverse prognostic factors. Patients were segregated into three prognostic groups depending on these six factors. Two-year survival rates for the favorable-risk (0), intermediate-risk (1–2), and poor-risk (3–6) groups were 75 %, 53 %, and 7 %, respectively (Fig. 7.7). This study established a contemporary prognostic model that is clinically applicable to determine OS outcomes in the targeted therapy era [65].
Majority of the targeted therapies in mRCC were approved based on PFS benefit; however, it was not clear if PFS is an adequate surrogate of OS in advanced RCC. In a retrospective study evaluating 1158 RCC patients who received targeted therapy, median OS for patients who progressed at 3 months was 7.8 months, compared with 23.6 months for patients who did not progress at the 3-month time point (P < 0.0001). Similarly, using a 6-month cutoff instead of 3 months, progressing patients had a median OS of 8.6 months compared with 26 months for patients who did not progress (P < 0.0001). This study concluded that patients with advanced RCC who progressed on contemporary targeted therapy had an approximately three times increased risk of death compared to patients who are progression free at the same time point. This study suggested that PFS may be a meaningful intermediate endpoint for OS in patients with mRCC who receive treatment with novel agents [66].
SWOG 8949 prospectively evaluated the role of debulking nephrectomy in advanced RCC. Patients on the nephrectomy arm continued to have survival benefit at 9 years of follow-up, with risk reduction by 26 %. This benefit was seen across all predefined strata, including performance status and the presence or absence of lung metastasis and measurable disease. The role of cytoreductive nephrectomy (CN) in this new era of VEGF-targeted therapy was retrospectively evaluated by Choueiri et al. After adjusting for established prognostic risk factors, CN reduced the risk of death by 32 % (95 % CI: 0.46–0.99, P = 0.04). In the subgroup analysis, marginal survival benefit is seen in patients in the poor-risk group (p = 0.06) and Karnofsky performance status <80 % (p = 0.08) [67].
Case Vignette
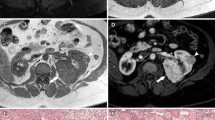
A 58-year-old man presented with flank pain and left hip discomfort. He has no other medical problems, never smoked, and only takes a statin for hyperlipidemia. His urinalysis showed microscopic hematuria. A complete blood count showed anemia with a hemoglobin concentration of 10 g/dL. His kidney and liver function tests were normal. Computed tomography (CT) scans revealed a large 12 cm solid right renal mass. Regional lymph nodes were not enlarged (Fig. 7.8a). A plain radiograph of the pelvis demonstrated a sclerotic lesion in the left femoral head (Fig. 7.8b). There was no other evidence for metastatic disease in the rest of the CT images. Bone scan showed only uptake in the left femoral head. This patient underwent a right radical nephrectomy and was found to have clear cell RCC; no nodes were involved. Biopsy of the femoral head mass was positive for metastatic clear cell cancer. An MRI of the left hip suggested that surgical resection was feasible. Because of the oligometastatic nature of this patient’s disease, he was considered for surgical metastasectomy, subsequently undergoing an R0 resection of the femoral head mass with placement of an artificial hip. He also received postoperative radiation therapy to the left hip. He was started on bisphosphonate therapy. Final pathologic stage was T2N0M1. He remains metastasis-free 2 years after his last operation. He is ambulating normally. Systemic therapy is planned only at the time of tumor recurrence.
References
Gupta NP, Ishwar R, Kumar A, Dogra PN, Seth A (2010) Renal tumors presentation: changing trends over two decades. Indian J Cancer 47(3):287–291
Palapattu GS, Kristo B, Rajfer J (2002) Paraneoplastic syndromes in urologic malignancy: the many faces of renal cell carcinoma. Rev Urol 4(4):163–170
Sacco E, Pinto F, Sasso F, Racioppi M, Gulino G, Volpe A, Bassi P (2009) Paraneoplastic syndromes in patients with urological malignancies. Urol Int 83(1):1–11
Pepper K, Jaowattana U, Starsiak MD, Halkar R, Hornaman K, Wang W, Dayamani P, Tangpricha V (2007) Renal cell carcinoma presenting with paraneoplastic hypercalcemic coma: a case report and review of the literature. J Gen Intern Med 22(7):1042–1046
Giannakos G, Papanicolaou X, Trafalis D, Michaelidis I, Margaritis G, Christofilakis C (2005) Stauffer’s syndrome variant associated with renal cell carcinoma. Int J Urol 12(8):757–759
Tomadoni A, Garcia C, Marquez M, Ayala JC, Prado F (2010) Stauffer’s syndrome with jaundice, a paraneoplastic manifestation of renal cell carcinoma: a case report. Arch Esp Urol 63(2):154–156
Tsukamoto T, Kumamoto Y, Miyao N, Masumori N, Takahashi A, Yanase M (1992) Interleukin-6 in renal cell carcinoma. J Urol 148(6):1778–1781; discussion 1781–1772
Kim HL, Belldegrun AS, Freitas DG, Bui MH, Han KR, Dorey FJ, Figlin RA (2003) Paraneoplastic signs and symptoms of renal cell carcinoma: implications for prognosis. J Urol 170(5):1742–1746
Smith SJ, Bosniak MA, Megibow AJ, Hulnick DH, Horii SC, Raghavendra BN (1989) Renal cell carcinoma: earlier discovery and increased detection. Radiology 170(3 Pt 1):699–703
Tsui KH, Shvarts O, Smith RB, Figlin R, de Kernion JB, Belldegrun A (2000) Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol 163(2):426–430
Mueller-Lisse UG, Mueller-Lisse UL (2010) Imaging of advanced renal cell carcinoma. World J Urol 28(3):253–261
Kitamura H, Fujimoto H, Tobisu K, Mizuguchi Y, Maeda T, Matsuoka N, Komiyama M, Nakagawa T, Kakizoe T (2004) Dynamic computed tomography and color Doppler ultrasound of renal parenchymal neoplasms: correlations with histopathological findings. Jpn J Clin Oncol 34(2):78–81
Jamis-Dow CA, Choyke PL, Jennings SB, Linehan WM, Thakore KN, Walther MM (1996) Small (< or = 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology 198(3):785–788
Lassau N, Chebil M, Chami L, Bidault S, Girard E, Roche A (2010) Dynamic contrast-enhanced ultrasonography (DCE-US): a new tool for the early evaluation of antiangiogenic treatment. Target Oncol 5(1):53–58
Trombetta C, Liguori G, Bucci S, Benvenuto S, Garaffa G, Belgrano E (2007) Evaluation of tumor thrombi in the inferior vena cava with intraoperative ultrasound. World J Urol 25(4):381–384
Sacco E, Pinto F, Totaro A, D’Addessi A, Racioppi M, Gulino G, Volpe A, Marangi F, D’Agostino D, Bassi P (2010) Imaging of renal cell carcinoma: state of the art and recent advances. Urol Int 39:17–20
Lassau N, Chami L, Benatsou B, Peronneau P, Roche A (2007) Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol 17(Suppl 6):F89–F98
Coll DM, Smith RC (2007) Update on radiological imaging of renal cell carcinoma. BJU Int 99(5 Pt B):1217–1222
Herts BR (2003) Imaging for renal tumors. Curr Opin Urol 13(3):181–186
Coppenrath EM, Mueller-Lisse UG (2006) Multidetector CT of the kidney. Eur Radiol 16(11):2603–2611
Sheth S, Scatarige JC, Horton KM, Corl FM, Fishman EK (2001) Current concepts in the diagnosis and management of renal cell carcinoma: role of multidetector ct and three-dimensional CT. Radiographics 21 Spec No:S237-254
Kim JK, Kim TK, Ahn HJ, Kim CS, Kim KR, Cho KS (2002) Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol 178(6):1499–1506
Jinzaki M, Tanimoto A, Mukai M, Ikeda E, Kobayashi S, Yuasa Y, Narimatsu Y, Murai M (2000) Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr 24(6):835–842
Kim JK, Park SY, Shon JH, Cho KS (2004) Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at biphasic helical CT. Radiology 230(3):677–684
Sawai Y, Kinouchi T, Mano M, Meguro N, Maeda O, Kuroda M, Usami M (2002) Ipsilateral adrenal involvement from renal cell carcinoma: retrospective study of the predictive value of computed tomography. Urology 59(1):28–31
Raj GV, Bach AM, Iasonos A, Korets R, Blitstein J, Hann L, Russo P (2007) Predicting the histology of renal masses using preoperative Doppler ultrasonography. J Urol 177(1):53–58
Turkvatan A, Akdur PO, Altinel M, Olcer T, Turhan N, Cumhur T, Akinci S, Ozkul F (2009) Preoperative staging of renal cell carcinoma with multidetector CT. Diagn Interv Radiol 15(1):22–30
Pedrosa I, Chou MT, Ngo L, HB R, Genega EM, Galaburda L, DeWolf WC, Rofsky NM (2008) MR classification of renal masses with pathologic correlation. Eur Radiol 18(2):365–375
Ergen FB, Hussain HK, Caoili EM, Korobkin M, Carlos RC, Weadock WJ, Johnson TD, Shah R, Hayasaka S, Francis IR (2004) MRI for preoperative staging of renal cell carcinoma using the 1997 TNM classification: comparison with surgical and pathologic staging. AJR Am J Roentgenol 182(1):217–225
Griffin N, Gore ME, Sohaib SA (2007) Imaging in metastatic renal cell carcinoma. AJR Am J Roentgenol 189(2):360–370
Kang DE, White RL Jr, Zuger JH, Sasser HC, Teigland CM (2004) Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. J Urol 171(5):1806–1809
Divgi CR, Pandit-Taskar N, Jungbluth AA, Reuter VE, Gonen M, Ruan S, Pierre C, Nagel A, Pryma DA, Humm J, Larson SM, Old LJ, Russo P (2007) Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol 8(4):304–310
Park JW, Jo MK, Lee HM (2009) Significance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography for the postoperative surveillance of advanced renal cell carcinoma. BJU Int. doi:10.1111/j.1464-410X.2008.08150.x
Delahunt B (2009) Advances and controversies in grading and staging of renal cell carcinoma. Mod Pathol 22(Suppl 2):S24–S36
Leibovich BC, Pantuck AJ, Bui MH, Ryu-Han K, Zisman A, Figlin R, Belldegrun A (2003) Current staging of renal cell carcinoma. Urol Clin North Am 30(3):481–497
Volpe A, Patard JJ (2010) Prognostic factors in renal cell carcinoma. World J Urol 28(3):319–327
Delahunt B, Kittelson JM, McCredie MR, Reeve AE, Stewart JH, Bilous AM (2002) Prognostic importance of tumor size for localized conventional (clear cell) renal cell carcinoma: assessment of TNM T1 and T2 tumor categories and comparison with other prognostic parameters. Cancer 94(3):658–664
Zisman A, Pantuck AJ, Dorey F, Chao DH, Gitlitz BJ, Moldawer N, Lazarovici D, deKernion JB, Figlin RA, Belldegrun AS (2002) Mathematical model to predict individual survival for patients with renal cell carcinoma. J Clin Oncol 20(5):1368–1374
Masuda H, Kurita Y, Fukuta K, Mugiya S, Suzuki K, Fujita K (1998) Significant prognostic factors for 5-year survival after curative resection of renal cell carcinoma. Int J Urol 5(5):418–422
Sene AP, Hunt L, McMahon RF, Carroll RN (1992) Renal carcinoma in patients undergoing nephrectomy: analysis of survival and prognostic factors. Br J Urol 70(2):125–134
Han KR, Bui MH, Pantuck AJ, Freitas DG, Leibovich BC, Dorey FJ, Zisman A, Janzen NK, Mukouyama H, Figlin RA, Belldegrun AS (2003) TNM T3a renal cell carcinoma: adrenal gland involvement is not the same as renal fat invasion. J Urol 169(3):899–903; discussion 903–894
Ficarra V, Righetti R, D’Amico A, Rubilotta E, Novella G, Malossini G, Mobilio G (2001) Renal vein and vena cava involvement does not affect prognosis in patients with renal cell carcinoma. Oncology 61(1):10–15
Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6(7):655–663
Hong SK, Jeong CW, Park JH, Kim HS, Kwak C, Choe G, Kim HH, Lee SE (2011) Application of simplified Fuhrman grading system in clear-cell renal cell carcinoma. BJU Int 107(3):409–415
Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27(5):612–624
Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC, Lobel B, Guille F, Chopin DK, Mulders PF, Wood CG, Swanson DA, Figlin RA, Belldegrun AS, Pantuck AJ (2005) Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol 23(12):2763–2771
Lee CT, Katz J, Fearn PA, Russo P (2002) Mode of presentation of renal cell carcinoma provides prognostic information. Urol Oncol 7(4):135–140
Yao M, Yoshida M, Kishida T, Nakaigawa N, Baba M, Kobayashi K, Miura T, Moriyama M, Nagashima Y, Nakatani Y, Kubota Y, Kondo K (2002) VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst 94(20):1569–1575
Schraml P, Struckmann K, Hatz F, Sonnet S, Kully C, Gasser T, Sauter G, Mihatsch MJ, Moch H (2002) VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J Pathol 196(2):186–193
Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao SY, Stanbridge E, Lerman MI, Palotie A, Figlin RA, Belldegrun AS (2003) Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 9(2):802–811
Shvarts O, Seligson D, Lam J, Shi T, Horvath S, Figlin R, Belldegrun A, Pantuck AJ (2005) p53 is an independent predictor of tumor recurrence and progression after nephrectomy in patients with localized renal cell carcinoma. J Urol 173(3):725–728
Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P (2001) A postoperative prognostic nomogram for renal cell carcinoma. J Urol 166(1):63–67
Cindolo L, Patard JJ, Chiodini P, Schips L, Ficarra V, Tostain J, de La Taille A, Altieri V, Lobel B, Zigeuner RE, Artibani W, Guille F, Abbou CC, Salzano L, Gallo C (2005) Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European study. Cancer 104(7):1362–1371
Sorbellini M, Kattan MW, Snyder ME, Reuter V, Motzer R, Goetzl M, McKiernan J, Russo P (2005) A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol 173(1):48–51
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H (2002) An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 168(6):2395–2400
Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, Weaver AL, Parker AS, Zincke H (2003) Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 97(7):1663–1671
Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, deKernion JB, Figlin RA, Belldegrun AS (2002) Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol 20(23):4559–4566
Patard JJ, Kim HL, Lam JS, Dorey FJ, Pantuck AJ, Zisman A, Ficarra V, Han KR, Cindolo L, De La Taille A, Tostain J, Artibani W, Dinney CP, Wood CG, Swanson DA, Abbou CC, Lobel B, Mulders PF, Chopin DK, Figlin RA, Belldegrun AS (2004) Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol 22(16):3316–3322
Karakiewicz PI, Briganti A, Chun FK, Trinh QD, Perrotte P, Ficarra V, Cindolo L, De la Taille A, Tostain J, Mulders PF, Salomon L, Zigeuner R, Prayer-Galetti T, Chautard D, Valeri A, Lechevallier E, Descotes JL, Lang H, Mejean A, Patard JJ (2007) Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol 25(11):1316–1322
Negrier S, Escudier B, Gomez F, Douillard JY, Ravaud A, Chevreau C, Buclon M, Perol D, Lasset C (2002) Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d’Immunotherapie. Ann Oncol 13(9):1460–1468
Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17(8):2530–2540
Mekhail TM, Abou-Jawde RM, Boumerhi G, Malhi S, Wood L, Elson P, Bukowski R (2005) Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 23(4):832–841
Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, Mazumdar M (2004) Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 22(3):454–463
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27(22):3584–3590
Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27(34):5794–5799
Heng DY, Xie W, Bjarnason GA, Vaishampayan U, Tan MH, Knox J, Donskov F, Wood L, Kollmannsberger C, Rini BI, Choueiri TK (2010) Progression-free survival as a predictor of overall survival in metastatic renal cell carcinoma treated with contemporary targeted therapy. Cancer 28:1061–1068
Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, McDermott DF, Rini BI, Heng DY (2011) The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol 185(1):60–66
Zisman A, Pantuck AJ, Dorey F et al (2001) Mproved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol 19(6):1649–1657
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing
About this chapter
Cite this chapter
Yadlapalli, S.B., Shi, D., Vaishampayan, U. (2015). Renal Cell Carcinoma: Clinical Presentation, Staging, and Prognostic Factors. In: Lara, P., Jonasch, E. (eds) Kidney Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-17903-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-17903-2_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17902-5
Online ISBN: 978-3-319-17903-2
eBook Packages: MedicineMedicine (R0)