Abstract
Previous studies report that retinitis pigmentosa (RP) patients treated with the histone deacetylase inhibitor (HDACi) valproic acid (VPA) present with improved visual fields and delayed vision loss. However, other studies report poor efficacy and safety of HDACi in other cohorts of retinal degeneration patients. Furthermore, the molecular mechanisms by which HDACi can improve visual function is unknown, albeit HDACi can attenuate pro-apoptotic stimuli and induce expression of neuroprotective factors. Thus, further analysis of HDACi is warranted in pre-clinical models of retinal degeneration including zebrafish. Analysis of HDAC expression in developing zebrafish reveals diverse temporal expression patterns during development and maturation of visual function.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The 18 HDAC proteins are divided into two families, “classical” HDACs and SIR2 HDACs which are further subdivided into four classes based on homology to yeast HDAC orthologues and functional activity. In general, Class I members (HDAC1, 2, 3, 8) are localized to the nucleus while Class II members (HDAC4, 5, 6, 7, 9, 10) can be either localised in the nucleus or cytoplasm. Class III are a family of 7 NAD+ dependent proteins, known as sirtuins (SIRT1–7) , similar to yeast Sir2 proteins. Class IV HDACs show structural similarity to both Class I and II HDACs (Yang and Seto 2008) . These proteins can control gene transcription via epigenetic alteration of chromatin or modulate the activity of non-histone proteins by altering their acetylation (Choudhary et al. 2009) . Consequently, HDACs regulate cell cycle progression, differentiation and survival.
2 HDACi as Potential Therapeutics for Treatment of Retinal Degeneration
A retrospective study of 7 RP patients reported improved visual field (VF) and visual acuity (VA) scores and delayed vision loss in five patients following treatment for 4 months with a mean dose of 643(+/− 133) mg/day valproic acid (VPA) (Clemson et al. 2011) . Only mild side-effects, such as fatigue and stomach irritation were reported and liver function and blood chemistry remained normal. However, in a similar study of pigmentary dystrophy patients treated with 500–1000 mg/day VPA for 10 months; the five patients for which VF field tracings were available before and after treatment presented with a decline in VF, 22 patients had a decline in VA and 12 patients reported severe negative side effects inclding high alanine aminotransferase, aspartate aminotransferase and ammonia levels (Bhalla et al. 2013) . The Clemson study has been criticised regarding study design, patient numbers (van Schooneveld 2011), and statistical analyses (Sandberg et al. 2011) . Indeed, VPA may compromise photoreceptor function due to antagonistic effects on sodium and calcium channels in the retina (Sisk 2012) . Despite these concerns, a randomized, placebo-controlled trial of oral VPA for treatment of autosomal dominant RP (NCT01233609), and a non-randomized trial (NCT01399515) are in progress.
3 HDAC Inhibition in a Pre-Clinical Rodent Model of Retinal Degeneration
In the rd1 (retinal degeneration 1) mouse model of RP , histone acetylation is dramatically reduced in retinal cells. Retinal degeneration in rd1 mice is mediated by phosphodiesterase-6 (PDE6) dysfunction resulting in high cyclic guanosine-mono-phosphate (cGMP) levels and increased oxidative stress (Sahaboglu et al. 2013) . Increased expression of cell proliferation and oxidative stress genes is observed during rd1 photoreceptor degeneration (Hackam et al. 2004) as is increased HDAC activity, with class I/II HDACs contributing the majority of total HDAC activity (Sancho-Pelluz et al. 2010) . TUNEL positive cells in the degenerating rd1 mouse eye also have reduced histone acetylation. Overall, reduced histone acetylation due to aberrant HDAC activity appears to be a major contributing factor to retinal degeneration in the rd1 model. Notably, treatment of rd1 retinal explants with Class I/II HDAC inhibitors , 1 µM Trichostatin A (TSA) or 6 µM Scriptaid, reduced photoreceptor cell death and restored photoreceptor outer segments (Sancho-Pelluz et al. 2010) . These results suggest a major contribution of class I/II HDACs, to mutation-induced rd1 photoreceptor cell death.
4 Mechanism of Action
A number of mechanisms by which HDACi produce their therapeutic effects have been suggested. HDACi diminish the activity of the Hsp90 chaperone, by increased acetylation (Scroggins et al. 2007; Kekatpure et al. 2009) . Hsp90 inhibition increases expression of the neuroprotective chaperone Hsp70, which promotes neuronal survival (Wen et al. 2008). TNF-α is lowly expressed in wildtype retina but increased in models of ischemic injury (Genini et al. 2013) . Pharmacological inhibition of Class I/II HDACs with 2.5 mg/kg TSA blocks increases in TNF-α levels in the rat eye post ischemic injury (Crosson et al. 2010) . HDACi also modulate expression of brain derived neurotrophic factor (BDNF) via repression of its promoter. Selective pharmacological inhibition of class II HDACs with 5 µM MC1568 leads to rapid induction of BDNF expression while inhibition of class I HDACs with 5 µM MS-275 leads to a comparatively slower induction (Koppel and Timmusk 2013) . In agreement, treatment of rd1 retinal explants with BDNF and ciliary neurotrophic factor (CNTF) provides a neuroprotective effect (Azadi et al. 2007) .
5 HDAC Expression in The Zebrafish Model
Zebrafish eye development is rapid. At 11 hpf the optic vesicle is visible (Kimmel et al. 1995) . At 3 days post fertilisation (dpf) all cell types of the retina have differentiated and measurable cone mediated visual responses develop (Easter and Nicola 1996) . The zebrafish eye has a similar structure to other vertebrates, sharing the cell types and laminate structure present in humans. In early stages of development (2–16 hpf) hdac1 is ubiquitously expressed. At later stages (36–48 hpf), expression is partially restricted to the branchial arches, fin bud mesenchyme and hindbrain. Pharmacological inhibition of HDACs by TSA results in a failure of craniofacial cartilage to develop from these tissues (Pillai et al. 2004) . Similarly, in the hindbrain of hdac1 mutants there is reduced cell proliferation marked by defects in axial extension of hindbrain branchiomotor neurons caused by reduced activation of non-canonical Wnt/PCP pathway regulators (Cunliffe 2004) . In addition, inhibition of class I/II HDACs affects migration of the posterior lateral line primordium. Treatment disrupted neuromast deposition in a dose dependent manner (He et al. 2014) . Treatment with VPA also reduces proliferation of neural stem cells in the adult zebrafish optic tectum via inhibition of Notch signaling (Dozawa et al. 2014) .These reports underline the importance of HDAC activity for cell proliferation and migration.
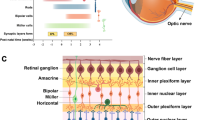
An analysis of publically available RNA-seq data (Fig. 61.1) depicts the expression of zebrafish HDACs during development in whole larvae (Aanes et al. 2011; Collins et al. 2012) . Hdac genes show diverse expression patterns during development. Hdac1 and hdac3 (Class I) are highly expressed from 2–4 cells until 7 dpf, when visual function is matured. Sirt7, hdac7 and hdac11 (Class III, II and IV respectively) show higher expression at earlier stages, while sirt2 and hdac9b (Class II) show increased gene expression after 6 hpf or at later developmental stages.
To begin to explore the importance of HDACs in the zebrafish eye , we profiled HDAC gene expression in eyes from 3, 4 and 5 dpf larvae (Yin et al. 2012) . As shown in Fig. 61.2, hdac1 and hdac3 show similar decreasing expression from 3–5 dpf. In contrast expression of hdac9b significantly increased from 3–5 dpf. The differential expression of hdacs during the development of visual function indicates a temporal importance of HDAC expression during eye development. Other hdac genes did not exhibit any significant difference in gene expression from 3 to 5 dpf.
Gene expression profiles of HDACs on microarray. Log2 transformed signal intensities of embryonic eyes on 3, 4 and 5 days post fertilization (dpf). The solid red line indicates high gene expression (log2 signal intensity of 9). The dashed red line indicates medium gene expression (log2 signal intensity of 6). *p-value < 0.05
With the notable exception of hdac1, the role of most HDAC genes in the zebrafish eye is poorly understood. The absence of hdac1 in the zebrafish retina results in increased cell proliferation, the optic stalk fails to terminally differentiate resulting in a reduced plexiform layer and number of retinal ganglion cells, photoreceptors are also absent (Stadler et al. 2005) . hdac1 is necessary for controlling transcription of the key cell cycle regulators cyclin D1 and E2. hdac1 appears to be required for the switch from proliferation to differentiation in the zebrafish retina mediated by the Wnt and Notch pathways (Yamaguchi et al. 2005) .
6 Conclusion
Clinical and pre-clinical studies suggest that HDACi may be effective therapeutics in certain models of retinal degeneration . Zebrafish are an excellent model to gain further insight into the requirement of HDACs for eye development and function. Aditionally, zebrafish models of inherited blindness can be utilised to determine the efficacy and safety of HDACi in genetically diverse models of retinal degeneration and to understand the neuroprotective mechanisms of HDACi .
Abbreviations
- BDNF:
-
Brain derived neurotrophic factor
- CNTF:
-
Ciliary neurotrophic factor
- DPF:
-
Days post fertilisation
- HAT:
-
Histone acetyltransferase
- HDAC:
-
Histone deacetylase
- HDACi:
-
Histone deacetylase inhibitor
- HPF:
-
Hours post fertilization
- rd1 :
-
Retinal degeneration 1
- RP:
-
Retinitis pigmentosa
- TSA:
-
Trichostatin A
- VA:
-
Visual acuity
- VF:
-
Visual field
References
Aanes H, Winata CL, Lin CH et al (2011) Zebrafish mRNA sequencing deciphers novelties in transcriptome dynamics during maternal to zygotic transition. Genome Res 21:1328–1338
Azadi S, Johnson LE, Paquet-Durand F et al (2007) CNTF+ BDNF treatment and neuroprotective pathways in the rd1 mouse retina. Brain Res 1129:116–129
Bhalla S, Joshi D, Bhullar S et al (2013) Long-term follow-up for efficacy and safety of treatment of retinitis pigmentosa with valproic acid. Br J Ophthalmol 97:895–899
Choudhary C, Kuman C, Gnad F et al (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325:834–840
Clemson CM, Tzekov R, Krebs M et al (2011) Therapeutic potential of valproic acid for retinitis pigmentosa. Br J Ophthalmol 95:89–93
Collins JE, White S, Searle SM et al (2012) Incorporating RNA-seq data into the zebrafish Ensembl genebuild. Genome Res 22:2067–2078
Crosson CE, Mani SK, Husain S et al (2010) Inhibition of histone deacetylase protects the retina from ischemic injury. Invest Ophthalmol Vis Sci 51:3639–3645
Cunliffe VT (2004) Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development 131:2983–2995
Dozawa M, Kono H, Sato Y et al (2014) Valproic acid, a histone deacetylase inhibitor, regulates cell proliferation in the adult zebrafish optic tectum. Dev Dyn 243(11):1401–1415
Easter SS, Nicola GN (1996) The development of vision in the zebrafish (Danio rerio). Dev Biol 180:646–663
Genini S, Beltran WA, Aguirre GD (2013) Up-regulation of tumor necrosis factor superfamily genes in early phases of photoreceptor degeneration. PLoS One 8:e85408
Hackam A, Strom R, Liu D et al (2004) Identification of gene expression changes associated with the progression of retinal degeneration in the rd1 mouse. Invest Opthalmol Vis Sci 45:2929–2942
He Y, Wu J, Mei H et al (2014) Histone deacetylase activity is required for embryonic posterior lateral line development. Cell Prolif 47:91–104
Kekatpure VD, Dannenberg AJ, Subbaramaiah K (2009) HDAC6 modulates Hsp90 chaperone activity and regulates activation of aryl hydrocarbon receptor signaling. J Biol Chem 284:7436–7445
Kimmel CB, Ballard WW, Kimmel SR et al (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310
Koppel I, Timmusk T (2013) Differential regulation of Bdnf expression in cortical neurons by class-selective histone deacetylase inhibitors. Neuropharm 75:106–115
Pillai R, Coverdale LE, Dubey G et al (2004) Histone deacetylase 1 (HDAC-1) required for the normal formation of craniofacial cartilage and pectoral fins of the zebrafish. Dev Dyn 231:647–654
Sahaboglu A, Paquet-Durand O, Dietter J et al (2013) Retinitis pigmentosa: rapid neurodegeneration is governed by slow cell death mechanisms. Cell Death Dis 4:e488
Sancho-Pelluz J, Alavi MV, Sahaboglu A et al (2010) Excessive HDAC activation is critical for neurodegeneration in the rd1 mouse. Cell Death Dis 1:e24
Sandberg MA, Rosner B, Weigel-DiFranco C et al (2011) Lack of scientific rationale for use of valproic acid for retinitis pigmentosa. Br J Ophthalmol 95:744
Scroggins BT, Robzyk K, Wang D et al (2007) An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell 25:151–159
Sisk RA (2012) Valproic acid treatment may be harmful in non-dominant forms of retinitis pigmentosa. Br J Ophthalmol 96:1154–1155
Stadler JA, Shkumatava A, Norton WH et al (2005) Histone deacetylase 1 is required for cell cycle exit and differentiation in the zebrafish retina. Dev Dyn 233:883–889
van Schooneveld, MJ et al (2011) The conclusions of Clemson et al concerning valproic acid are premature. Br J Ophthalmol 95(1):153-154
Wen XR, Li C, Zong Y et al (2008) Dual inhibitory roles of geldanamycin on the c-Jun NH2-terminal kinase 3 signal pathway through suppressing the expression of mixed-lineage kinase 3 and attenuating the activation of apoptosis signal-regulating kinase 1 via facilitating the activation of Akt in ischemic brain injury. Neuroscience 156:483–497
Yamaguchi M, Tonou-Fujimori N, Komori A et al (2005). Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development 132:3027–3043
Yang XJ, Seto E (2008) The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol 9:206–218
Yin J, Shine L, Raycroft F et al (2012) Inhibition of the pim1 oncogene results in diminished visual function. PLoS One 7:e52177
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Daly, C., Yin, J., Kennedy, B. (2016). Histone Deacetylase: Therapeutic Targets in Retinal Degeneration. In: Bowes Rickman, C., LaVail, M., Anderson, R., Grimm, C., Hollyfield, J., Ash, J. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 854. Springer, Cham. https://doi.org/10.1007/978-3-319-17121-0_61
Download citation
DOI: https://doi.org/10.1007/978-3-319-17121-0_61
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17120-3
Online ISBN: 978-3-319-17121-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)