Abstract
We have previously demonstrated that βA3/A1-crystallin, a member of the β/γ-crystallin superfamily, is expressed in the astrocytes and retinal pigment epithelial (RPE) cells of the eye. In order to understand the physiological functions of βA3/A1-crystallin in RPE cells, we generated conditional knockout (cKO) mice where Cryba1, the gene encoding βA3/A1-crystallin, is deleted specifically from the RPE using the Cre-loxP system. By utilizing the cKO model, we have shown that this protein is required by RPE cells for proper lysosomal degradation of photoreceptor outer segments (OS) that have been internalized in phagosomes and also for the proper functioning of the autophagy process. We also reported that βA3/A1-crystallin is trafficked to lysosomes, where it regulates endolysosomal acidification by modulating the activity of the lysosomal V-ATPase complex. Our results show that the V-ATPase activity in cKO RPE is significantly lower than WT RPE. Since, V-ATPase is important for regulating lysosomal pH, we noticed that endolysosomal pH was higher in the cKO cells compared to the WT cells. Increased lysosomal pH in cKO RPE is also associated with reduced Cathepsin D activity. Cathepsin D is a major lysosomal aspartic protease involved in the degradation of the OS and hence we believe that reduced proteolytic activity contributes to impaired degradation of OS in the cKO RPE. Reduced lysosomal activity in the cKO RPE also contributes to the incomplete degradation of the autophagosomes. Our results also suggest that βA3/A1-crystallin regulates V-ATPase activity by binding to the V0 subunit of the V-ATPase complex. Taken together, these results suggest a novel mechanism by which βA3/A1-crystallin regulates lysosomal function by modulating the activity of V-ATPase.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The Retinal Pigmented Epithelium (RPE) is a single layer of pigmented and polarized cells, with the apical surface facing the photoreceptors and the basal side facing Bruch’s membrane. It serves many physiological roles that are crucial for maintaining homeostasis of the retina (Strauss 2005) . The RPE cells are among the most active phagocytic cell types in the body, phagocytosing 10 % of total photoreceptor volume daily (Kevany and Palczewski 2010) . With advancing age, senescent RPE cells accumulate metabolic debris from remnants of incomplete degradation of ingested photoreceptors. This leads to accumulation of lipofuscin, an undegradable byproduct of OS metabolism (Sparrow et al. 2010) . Knowledge of the mechanisms that lead to the clearance of cellular material by RPE cells can help us develop strategies that lead to the restoration of the clearance functions in the RPE cells. Autophagy , a process by which cellular constituents are degraded and recycled as part of normal cellular remodeling, is likely to be of particular importance in post-mitotic cells with high metabolic demand, such as the RPE. This process begins with the formation of autophagosomes containing engulfed cytoplasmic organelles and protein complexes. The autophagosomes later fuse with the lysosomes to form autophagolysosomes and their contents are degraded by the acid hydrolases present in the lysosomes (Glick et al. 2010; Tong et al. 2010) . A disruption of autophagy in postmitototic cells like the RPE, would result in the accumulation of undigested or partially digested cellular aggregates, leading to degenerative cell death of the RPE (Kaarniranta et al. 2013) . Therefore, proper functioning of the RPE requires that both phagocytosis and autophagy processes be in balance.
2 Importance of Lysosomes in Clearance Functions in the RPE
Lysosomes , which are acidic subcellular organelles, are involved in the terminal events of both autophagy and phagocytosis (Luzio et al. 2007) . Although autophagy and phagocytosis are regarded as two separate biological processes, they share many morphological and topological similarities. The termination events in the processing of the phagosome and autophagosome are essentially similar (Deretic 2008) . Once formed, both phagosomes and autophagosomes fuse with lysosomes to from mature, acidified degradative organelles, called phagolysosomes and autophagolysosomes, respectively (Deretic 2008). Since lysosomes are a common element in both the processes, impaired lysosomal function is expected to result in dysregulated clearance of both phagosomes and autophagosomes. In a phagocytically active cell like the RPE, the degradative capacity of the lysosomes is indispensable for the proper clearance of ingested outer segments and cellular debri (Kaarniranta et al. 2010) . Previous studies have suggested that mutations affecting the activity of lysosomal proteases lead to accumulation of lipofuscin-like material in the RPE. These reports suggest the importance of proper functioning of lysosomal enzymes in the maintenance of physiological functions in the RPE (Siakotos et al. 1978 and Elner 2002) . Most lysosomal enzymes in the RPE are known to function in a narrow pH range in the acidic environment of the lysosomal lumen (Liu et al. 2008) . The lysosomal endopeptidases, Cathepsin B, D and E are known to be highly important in protein degradation and turnover in a majority of cell types (Luzio et al. 2007) . In the RPE cells, cathepsin D is the major protease involved in the lysosomal degradation of the outer segments. The activity of cathepsin D is tightly regulated by lysosomal pH, a rise in pH to 5.0 is known to reduce the activity of Cathepsin D by 80 % (Hayasaka et al. 1975) Studies have suggested that chronic use of drugs like chloroquine that alter lysosomal pH induce pathological changes in the RPE. Animals chronically exposed to chloroquine showed increased lysosomal pH and accumulation of phagosomes containing ingested outer segments. Undigested phagosomes and their contents are known to accumulate between Bruch’s membrane in RPE in chloroquine-treated animals (Mahon et al. 2004; Peters et al. 2006) . These studies suggest a stringent requirement of lysosomal pH for the proper functioning of lysosomal clearance functions in the RPE.
3 Mechanisms of Lysosomal Acidification
Lysosomes are acidic organelles involved in the degradation of macromolecules and play important roles in cellular maintenance7. The acidity of the lysosomes is generated and maintained by the lysosomal proton pump, vacuolar ATP-ase (V-ATPase). V-ATPase pumps protons into the lysosomal lumen against the electrochemical gradient by utilizing the free energy derived from ATP hydrolysis (Mindell 2012) . V-ATPases are multi-subunit complexes, composed of a cytosolic V1 domain that catalyzes ATP hydrolysis and an integral V0 domain that translocates protons from the cytoplasm to the lysosomal lumen. The V1 domain is composed of eight subunits (A-H) and the V0 domain is composed of five subunits (a, d, c, c’ and c”). In mammals, the ‘a’ subunit of the V0 domain is composed of multiple isoforms that have been shown to target V-ATPase to distinct cellular compartments (Mindell 2012).
4 Involvement of βA3/A1-Crystallin in the Maintenance of Lysosomal Function in the RPE
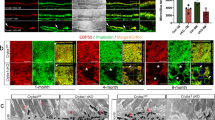
We recently reported that βA3/A1-crystallin , a lens structural protein, is expressed in RPE cells and trafficked to lysosomes, where it is involved in degradation of ingested OS and also in autophagy (Valapala et al. 2014) . We have recently generated a conditional knockout (cKO) mouse where βA3/A1-crystallin has been deleted specifically from the RPE. In our initial characterization of these animals, we found that while OS discs are ingested, the RPE cells are unable to degrade them and consequently accumulate ingested phagosomes. These mice also show impaired clearance of autophagosomes, hyper-vacuolation and loss of retinal function. These cellular abnormalities in the cKO RPE are also accompanied by an increase in lysosomal pH and a reduction in the activity of lysosomal proteases like cathepsin D. Our studies also suggested that loss of βA3/A1-crystallin inhibits the activity of the lysosomal V-ATPase in the cKO RPE. In order to investigate the mechanisms by which βA3/A1-crystallin modulates the activity of V-ATPase, we performed subcellular fractionation of lysosomes, extracted the lysosomal lumen and membrane fractions. Later, immunoprecipitation was performed using a polyclonal antibody to βA3/A1-crystallin and we immunoprecipitated the V-ATPase subunit ATP6V0A1 from the lysosomal membrane fractions in the Cryba1 floxed (Cryba1fl/fl) RPE cells (Fig. 104.1a). Since, the V0 subunit of the V-ATPase complex regulates its catalytic function; we believe that βA3/A1-crystallin modulates the catalytic efficiency of this complex (Valapala et al. 2014) . The exact mechanism by which βA3/A1-crystallin regulates this process is currently under investigation. Furthermore, molecular modeling studies have shown that the molecular surface of the βA3/A1-crystallin complex possesses a distinct binding site for the ATP6V0A1 subunit (Fig. 104.1b). Since, the major function of V-ATPase is to generate a pH gradient in the lysosomal compartments, loss of its function significantly alters and lysosomal pH and the activity of the lysosomal proteases in the cKO RPE. Our results show that dysregulated lysosomal degradation in the cKO RPE leads to incomplete degradation and accumulation of autophagosomes (Valapala et al. 2014). In summary, our studies suggest that βA3/A1-crystallin has critical function in the lysosome-mediated processing during both phagocytosis and autophagy in the RPE .
Regulation of lysosomal V-ATPase by βA3/A1-crystallin.a Lysosomal fractionation was performed to extract the lysosomal lumen and membrane fractions. Co-immunoprecipitation of these fractions with βA3/A1-crystallin antibody and immunoblotting with ATP6V0a1 antibody revealed the pull down of ATP6V0A1 predominantly in the membrane fraction. Immunoblotting with IgG heavy chain (IgGHc) served as a loading control. b Hypothetical complex of βA3/-crystallin and the N-terminal domain of V0a1 obtained by Hex protein docking is shown. Molecular surface of βA3/-crystallin is shown in green. The V0a1-Nterminus is shown as a ribbon model where β-sheet and α-helical structures are shown by red arrows and blue cylinders, respectively Reproduced with permission from the journal autophagy
References
Deretic V (2008) Autophagosome and phagosome. Meth Mol Biol 445:1–10
Elner VM (2002) Retinal pigment epithelial acid lipase activity and lipoprotein receptors: effects of dietary omega-3 fatty acids. Trans Am Ophthalmol Soc 100:301–338
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Path 221:3–12
Hayasaka S, Hara S, Mizuno K (1975) Degradation of rod outer segment proteins by cathepsin D. J Biochem 78:1365–1367
Kaarniranta K, Hyttinen J, Ryhanen T et al (2010) Mechanisms of protein aggregation in the retinal pigment epithelial cells. Front Biosci (Elite Ed) 2:1374–1384
Kaarniranta K, Sinha D, Blasiak J et al (2013) Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 9:973–84
Kevany BM, Palczewski K (2010) Phagocytosis of retinal rod and cone photoreceptors. Physiol (Bethesda) 25:8–15.
Liu J, Lu W, Reigada D et al (2008) Restoration of lysosomal pH in RPE cells from cultured human and ABCA4-/- mice: Pharmacologic approaches and functional recovery. Invest Ophthalmol Vis Sci 49:772–780
Luzio JP, Pryor PR, Bright NA (2007) Lysosomes: fusion and function. Nature Reviews Mol Cell Biol 8:622–632
Mahon GJ, Anderson HR, Gardiner TA et al (2004). Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res 28:277–284.
Mindell JA (2012) Lysosomal acidification mechanisms. Annu Rev Physiol 74:69–86
Peters S, Reinthal E, Blitgen-Heinecke P et al (2006). Inhibition of lysosomal degradation in retinal pigment epithelium cells induces exocytosis of phagocytic residual material at the basolateral plasma membrane. Ophthal Res 38:83–88
Siakotos AN, Armstrong D, Koppang N et al (1978) Studies on the retina and the pigment epithelium in hereditary canine ceroid lipofuscinosis, II: the subcellular distribution of lysosomal hydrolases and other enzymes. Invest Ophthalmol Vis Sci 17:618–633
Sparrow JR, Hicks D, Hamel CP (2010) The retinal pigment epithelium in health and disease. Curr. Mol. Med. 10:802–823
Strauss O (2005) The Retinal Pigment Epithelium in Visual Function. Phys Rev 85:841–881
Tong J, Yan X, Yu L (2010) The late stage of autophagy:cellular events and molecular regulation. Protein Cell 1:907–915
Valapala M, Wilson C, Hose S et al (2014) Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/bA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy 10:480–496
Acknowledgements
DS is supported by National Institutes of Health: RO1EY019037-S, Bright focus and Research to Prevent Blindness (an unrestricted grant to The Wilmer Eye Institute).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Valapala, M., Sergeev, Y., Wawrousek, E., Hose, S., Zigler, J., Sinha, D. (2016). Modulation of V-ATPase by βA3/A1-Crystallin in Retinal Pigment Epithelial Cells. In: Bowes Rickman, C., LaVail, M., Anderson, R., Grimm, C., Hollyfield, J., Ash, J. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 854. Springer, Cham. https://doi.org/10.1007/978-3-319-17121-0_104
Download citation
DOI: https://doi.org/10.1007/978-3-319-17121-0_104
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17120-3
Online ISBN: 978-3-319-17121-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)