Abstract
Timely removal of shed photoreceptor outer segments by retinal pigment epithelial cells (RPE) plays a key role in biological renewal of these highly peroxidizable structures and in maintenance of retina health. How environmental stress cause RPE cell dysfunction is undefined however. AMP-activated protein kinase (AMPK), a heterotrimer of a catalytic α subunit and regulatory β and γ subunits, maintains energy homeostasis by limiting energy utilization and/or promoting energy production when energy supply is compromised. Intriguingly, AMPK has been shown to be important in functions of RPE cells. In this mini-review, the role and mechanisms of AMPK in controlling RPE cell phagocytosis are discussed.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The retinal pigment epithelium (RPE) is a monolayer of cuboidal cells where its basal membrane is in contact with Bruch’s membrane and apical membrane is associated with the outer segments of retinal photoreceptor cells. The major function of RPE cells is to support the survival and normal functioning of photoreceptors by phagocytizing shed photoreceptor outer segment (POS) membrane discs for photoreceptor renewal (Nguyen-Legros and Hicks 2000) . Efficient disposal of shed POS by RPE is essential to prevent RPE and photoreceptor cells from the damaging effects of POS build-up. Phagocytosis of POS by RPE cells occurs by a complex process that includes binding, uptake, and degradation. POS first bind to the vitronectin receptor αvβ5 at the apical membrane of the RPE and initiates a downstream cytoplasmic signaling cascade that results in the reorganization of the RPE plasma membrane and engulfment of POS (Finnemann et al. 1997; Nandrot et al. 2004) . POS binding activates and recruits focal adhesion kinase (FAK) to the apical surface of RPE cells (Finnemann 2003) . In the meantime, POS binding relocates MER tyrosine kinase (MerTK) to the sites of internalized POS (Feng et al. 2002; Finnemann 2003) whereas MerTK is activated by FAK (Finnemann 2003). Activated MerTK mediates RPE engulfment of POS (Feng et al. 2002). Engulfed POS are degraded in RPE lysosomes (Deguchi et al. 1994) .
Age-related macular degeneration (AMD) is an idiopathic retinal degenerative disease that predominates in the elderly in the Western world as a cause of irreversible, profound vision loss (Evans 2001; Qin and Rodrigues 2008) . Growing evidence indicates that oxidative stress injury of RPE plays an important role in the etiology of AMD. The RPE is at high risk for oxidative injury due to its location in a highly oxygenated environment, its high levels of light exposure, and generation of reactive oxygen species during POS phagocytosis (Kindzelskii et al. 2004; Yu and Cringle 2005) . In the early stage of AMD development, oxidative insult induces a set of profound physiological responses in RPE, leading to dysfunction without initiation of cell death (Honda et al. 2001) . Although not much data are available regarding dysregulation of RPE cell phagocytosis by sub-lethal oxidative injury, AMP activated protein kinase (AMPK) , a metabolic-sensing Ser/Thr kinase consisting of a catalytic α subunit and regulatory β and γ subunits (Carling 2004) , has emerged as an important player in regulating RPE cell functions (Qin 2012) . AMPK has been demonstrated to play roles in regulating various RPE cell processes such as survival (Li et al. 2013; Qin and Rodrigues 2010; Yao et al. 2013) , immune responses (Qin et al. 2008), migration (Liu et al. 2012) , phagocytosis (Qin and De Vries 2008) and permeability (Qin and Rodrigues 2010; Villarroel et al. 2011) . In this review, possible mechanisms by which AMPK regulates RPE phagocytosis are discussed.
2 Inhibition of RPE Cell Phagocytosis by AMPK Activation
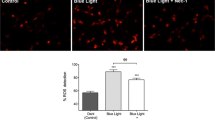
Activation of AMPK by oxidative stress is associated with inhibition of RPE cell phagocytosis (Qin and De Vries 2008). To demonstrate a causal-effect relationship between AMPK activation and phagocytosis inhibition, effects of 5-aminoimidazole-4-carboxamide riboside (AICAR) , an AMPK activator that mimics AMP to activate AMPK after its phosphorylation by adenosine kinase, on RPE cell phagocytosis were investigated. AICAR treatment activates AMPK signaling in ARPE19 cells as revealed by increased Thr172 phosphorylation of AMPKα and Ser79 phosphorylation of acetyl-CoA carboxylase (ACC), an AMPK substrate (Fig. 103.1a). Phosphorylation of AICAR by an adenosine kinase is essential for its activation of AMPKas adenosine kinase inhibitor 5-iodotubercidin completely abrogated activation of AMPK (Fig. 103.1b), revealing that AICAR activates AMPK in RPE cells via directly mimicking AMP effect. Incubation with AICAR inhibits RPE cell phagocytosis by 50 % and this inhibition is completely restored by inhibiting AMPK (Fig. 103.1c). Activation of AMPK is therefore directly linked to the inhibition of RPE cell phagocytosis.
Inhibition of RPE cell phagocytosis by AMPK. a AMPK activation by AICAR. Confluent ARPE19 cells were treated with 2 mM AICAR for 30 min. AMPK activation was assessed by immunoblotting with anti-pThr172 AMPKα and anti-pS79 ACC (Acetyl-CoA carboxylase, AMPK substrate). b Inhibition of AMPK activation by IODO. Confluent cells were treated with 0.5 μM iodotubercidin (IODO) for 30 min and then stimulated with 2 mM AICAR for 30 min. c Inhibitory effect of AICAR on phagocytosis. Confluent ARPE19 cells in 24-well plate were pre-incubated with 0.5 μM IODO for 30 min prior exposure to 2 mM AICAR for 1h followed by 4 h incubation with 5 × 106 POS particles in 300 μL growth medium in the presence of AICAR. Phagocytosis was determined by flow cytometer
3 Abrogation of MerTK Activation by AICAR
FAK and MerTK are two important tyrosine kinases in mediating RPE cell phagocytosis with Fak upstream of MerTK (Finnemann 2003) . Phagocytic challenge activates both FAK and MerTK in ARPE19 cells in a time-dependent manner (Fig. 103.2) (Qin and Rodrigues 2012) . To address how AMPK regulates RPE cell phagocytic machinery, cells were treated with AICAR before POS addition. AICAR treatment does not alter basal activity of FAK and MerTK. However, AICAR selectively abolishes POS-induced activation of MerTK with no effect on FAK (Fig. 103.2). This observation indicates that activated AMPK limits RPE cell phagocytic activity by abolishing POS-induced activation of MerTK.
Inhibition of POS-induced MerTK activation by AICAR. Confluent RPE cells were pre-incubated with 2 mM AICAR for 1 h. Cells were incubated with 5 × 106 POS in 300 μL complete medium for various lengths of times with presence of AICAR. Activation of FAK and MerTK was determined by activation-specific anti-pY397 FAK a and anti-pY729/753/754 MerTK antibody b respectively
4 Regulation of RPE Cell Phagocytosis by AMPK
RPE cells maintain survival and functions of photoreceptors via phagocytizing shed POS. Knockdown of AMPKα2 reduces the ability of RPE cells to phagocytize POS by 40 % whereas there is no effect with knockdown of AMPKα1 (Qin and De Vries 2008) . Under stress conditions, sub-lethal oxidative stress-activated AMPKα2 but not AMPKα1 inhibits RPE cell phagocytosis. It is unclear why oxidative stress-induced inhibition of RPE cell phagocytosis is selectively regulated by AMPKα2, however, AMPKα2 rather than AMPKα1 knock-out causes a dramatic decrease in oxidative stress-induced AMPK signaling (Qin and De Vries 2008) . Continued RPE phagocytosis of POS may add more insult to the already stressed RPE cells. Thus, reduction of RPE cell phagocytosis by AMPKα2 activation likely protects RPE cells from further photo-toxic damage caused by the oxidized POS. How does AMPK inhibit RPE cell phagocytosis? As proposed in Fig. 103.3, POS binding recruits FAK/MerTK to the membrane and initiates FAK-MerTK signaling cascade, triggering engulfment of POS and subsequent degradation in lysosome. Selective inhibition of POS-induced activation of MerTK by AMPKα2 suggests that AMPKα2 terminates FAK-MerTK signaling cascade by blocking signal relay at MerTK . This isoform-specific role of AMPKα in regulating RPE cell phagocytosis may provide novel therapeutic tools for retinal diseases by developing isoform-selective inhibitors of AMPK. Furthermore, sub-lethal oxidative stress can also inactivate basal and POS-induced activation of FAK and slow down RPE cell capability of phagocytizing POS (Qin and Rodrigues 2012) , showing that oxidative stress can regulate phagocytic activity of RPE cells in more than one mechanism.
Proposed regulation of RPE cell phagocytosis by AMPK. Phagocytosis starts with binding of POS to αvβ5 initiating a coordinate signal transduction, interaction of αvβ5 with MerTK through FAK that in turn results in engulfment of the bound POS and subsequent degradation in lysosome. Activated AMPK can selectively block activation of MerTK, thereby reducing RPE cell capacity of phagocytizing POS
References
Carling D (2004) The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci 29:18–24
Deguchi J, Yamamoto A, Yoshimori T et al (1994) Acidification of phagosomes and degradation of rod outer segments in rat retinal pigment epithelium. Invest Ophthalmol Vis Sci 35:568–579
Evans JR (2001) Risk factors for age-related macular degeneration. Prog Retin Eye Res 20:227–253
Feng W, Yasumura D, Matthes MT et al (2002) Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem 277:17016–17022
Finnemann SC (2003) Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J 22:4143–4154
Finnemann SC, Bonilha VL, Marmorstein AD et al (1997) Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A 94:12932–12937
Honda S, Hjelmeland LM, Handa JT (2001) The use of hyperoxia to induce chronic mild oxidative stress in RPE cells in vitro. Mol Vis 7:63–70
Kindzelskii AL, Elner VM, Elner SG et al (2004) Toll-like receptor 4 (TLR4) of retinal pigment epithelial cells participates in transmembrane signaling in response to photoreceptor outer segments. J Gen Physiol 124:139–149
Li KR, Zhang ZQ, Yao J et al (2013) Ginsenoside Rg-1 protects retinal pigment epithelium (RPE) cells from cobalt chloride (CoCl2) and hypoxia assaults. PLoS ONE 8:e84171
Liu Y, Cao GF, Xue J et al (2012) Tumor necrosis factor-alpha (TNF-alpha)-mediated in vitro human retinal pigment epithelial (RPE) cell migration mainly requires Akt/mTOR complex 1 (mTORC1), but not mTOR complex 2 (mTORC2) signaling. Eur J Cell Biol 91:728–737
Nandrot EF, Kim Y, Brodie SE et al (2004) Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med 200:1539–1545
Nguyen-Legros J, Hicks D (2000) Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int Rev Cytol 196:245–313
Qin S (2012) Roles for AMP-activated protein kinase in RPE cell function. Adv Exp Med Biol 723:745–751
Qin S, De Vries GW (2008) alpha2 But not alpha1 AMP-activated protein kinase mediates oxidative stress-induced inhibition of retinal pigment epithelium cell phagocytosis of photoreceptor outer segments. J Biol Chem 283:6744–6751
Qin S, Rodrigues GA (2008) Progress and perspectives on the role of RPE cell inflammatory responses in the development of age-related macular degeneration. J Inflamm Res 1:49–65
Qin S, Rodrigues GA (2010) Differential roles of AMPKalpha1 and AMPKalpha2 in regulating 4-HNE-induced RPE cell death and permeability. Exp Eye Res 91:818–824
Qin S, Rodrigues GA (2012) Roles of alphavbeta5, FAK and MerTK in oxidative stress inhibition of RPE cell phagocytosis. Exp Eye Res 94:63–70
Qin S, Ni M, De Vries GW (2008) Implication of S-adenosylhomocysteine hydrolase in inhibition of TNF-alpha- and IL-1beta-induced expression of inflammatory mediators by AICAR in RPE cells. Invest Ophthalmol Vis Sci 49:1274–1281
Villarroel M, Garcia-Ramirez M, Corraliza L et al (2011) Fenofibric acid prevents retinal pigment epithelium disruption induced by interleukin-1beta by suppressing AMP-activated protein kinase (AMPK) activation. Diabetologia 54:1543–1553
Yao J, Bi HE, Sheng Y et al (2013) Ultraviolet (UV) and hydrogen peroxide activate ceramide-ER stress-AMPK signaling axis to promote retinal pigment epithelium (RPE) cell apoptosis. Int J Mol Sci 14:10355–10368
Yu DY, Cringle SJ (2005) Retinal degeneration and local oxygen metabolism. Exp Eye Res 80:745–751
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Qin, S. (2016). Blockade of MerTK Activation by AMPK Inhibits RPE Cell Phagocytosis. In: Bowes Rickman, C., LaVail, M., Anderson, R., Grimm, C., Hollyfield, J., Ash, J. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 854. Springer, Cham. https://doi.org/10.1007/978-3-319-17121-0_103
Download citation
DOI: https://doi.org/10.1007/978-3-319-17121-0_103
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17120-3
Online ISBN: 978-3-319-17121-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)