Abstract
In this chapter several aspects of ocular dosage forms are discussed with emphasis on eye drops, eye lotions and eye ointments prepared in pharmacies. Their formulation, method of preparation, packaging, storage and methods of administration are also discussed.
The availability of medicines in ocular dosage forms is low due to the efficient barrier function of the cornea, lachrymation, tear turn over and drainage. Formulations should take into account these constraints. The vehicle and excipients selected should improve the permeation of the active substances in the eye or the residence in the conjunctival sac and consequently the therapeutic effects, but also minimise irritation. Tolerance of the preparation is of utmost importance.
When formulating aqueous ophthalmic preparations attention should be given to osmolality, pH, solubility, chemical interactions, stability of the active substance, together with viscosity and the choice of a preservative. Sterility is of critical importance and therefore the most appropriate sterilisation method must be chosen.
Besides pharmaceutical factors, the correct administration of the eye drops is an important factor. Therefore, clear instructions to the patients about eye drop instillation and correct storage of the medicine is essential. It will add to the success of pharmaceutical care and patient compliance.
Based upon the chapter Oog by Adriaan van Sorge en Annick Ludwig in the 2009 edition of Recepteerkunde.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Eye
- Eye drops
- Eye ointment
- Eye lotion
- Eye cream
- Formulation
- Preparation
- Tear film
- Biopharmaceutics
- Osmotic value
- Lachrymal secretion
10.1 Orientation
Eye medication is intended for local use on or into the eye. Eye drops and semisolid preparations are usually applied topically, in the lower conjunctival sac. Absorption of active substances into the ocular blood vessels and also into the systemic circulation occurs at the conjunctiva and nasal mucosae. Due to absorption, systemic side effects could appear after instillation.
After permeation through the cornea, active substances reach the anterior chamber and afterwards the posterior chamber and vitreous. With cases of external infections absorption should not happen, because the active substance needs to be present in therapeutic concentrations at the cornea and conjunctiva. An example of a targeted local eye preparation are erodible inserts, the active substances diffuse slowly from the matrix at the ocular surface.
Some ocular diseases require specific treatment given via intravitreal or periocular injection into the eye.
The sensitivity of the eye requires that the formulation and sterility of ocular medication are of critical importance. An inappropriate formulation can cause irritation or disruption of the mechanisms responsible for the protection of the eye. Contaminated ophthalmic preparations could, especially in the case of an injured eye, cause infections or exacerbate the infection.
The preferred route of administration depends on the location of the disease (Table 10.1; [1, 2]).
Eye preparations are also employed for diagnostic purpose or in connexion with surgery. Combinations of fluorescein with oxybuprocaine, proxymethacaine or lidocaine are applied for the measurement of intra-ocular pressure with tonometry, diagnosis of corneal defects and choice of size and control of hard contact lenses. Strips with fluorescein are used to examine the integrity of the tear film. In some countries they are considered medical devices. Sodium hyaluronate eye drops, other eye drops which relieve the symptoms of dry eyes by increasing the viscosity of the tear film or eye preparations in the context of contact lenses are generally regarded as medical devices.
In order to improve bioavailability, active substance targeting and patient compliance new dosage forms with controlled release have been developed: colloidal carriers, implants, inserts, plugs, active substance eluting contact lenses and iontophoresis [3–6].
In community pharmacies contact lens solutions are delivered to customers as medical devices. It is noteworthy to mention that many contact lens wearers do not clean their lenses properly. The careless use of their lenses can result in eye infections. During application of medicated eye preparations contact lenses should not be worn.
In some countries eye preparations are prepared in pharmacies for the special needs of animals. E.g. some breeds of dogs frequently suffer from dry eyes or vascular keratitis. Formulas for veterinary use generally do not differ from those designated for human use.
10.2 Definitions
The description of eye preparations to be used as medicinal products is similar in the European, British, Japanese and US-American Pharmacopoeias. Several categories may be distinguished:
-
Eye drops
-
Eye lotions
-
Powders for eye drops and powders for eye lotions
-
Semisolid eye preparations (ointments, creams and gels)
-
Ophthalmic inserts
Eye drops are sterile aqueous or oily solutions, emulsions or suspensions of one or more active substances intended for administration upon the eyeball or instillation into the conjunctival sac.
Eye lotions are sterile aqueous solutions intended for use in rinsing or bathing the eye or for impregnating eye dressings in order to cover the eye.
Semisolid eye preparations are sterile ointments, creams or gels intended for application to the conjunctiva or to the eyelids. They contain one or more active substances dissolved or dispersed in a suitable base. They have a homogeneous appearance.
Ophthalmic inserts are sterile, solid or semisolid preparations of suitable size and shape, designed to be inserted in the conjunctival sac, to produce an ocular effect. They generally consist of a reservoir of active substances embedded in a matrix or bounded by a rate-controlling membrane. The active substance, which is more or less soluble in the lachrymal liquid, is released over a determined period of time. Ophthalmic inserts are individually distributed into sterile containers.
These pharmacopoeial general monographs on eye preparations do not comprise parenteral preparations to be administered in the eye.
10.3 Anatomy and Physiology
Anatomical characteristics and physiological mechanisms protect the eye against toxic external effects. These mechanisms include the specific structure of the cornea, blinking, baseline and reflex lachrymation, drainage, tear film composition and the corneal sensitivity. The combination of all mechanistic, anatomical and physiological characteristics maintains the integrity of the eye, together with immunological and antimicrobial properties of the lachrymal fluid [5, 7–10].
10.3.1 Structure of the Eye
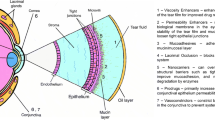
Figure 10.1 shows schematically the structure of the human eye. In detail the structure of the cornea is given. The cornea separates the aqueous humour from the lachrymal fluid and protects the delicate internal structures of the eye from external influences. The cornea is a clear, avascular tissue to which nutrients and oxygen are supplied by the lachrymal fluid and the aqueous humour. It is composed of five layers: a lipophilic multilayered epithelium, Bowman’s membrane, a hydrophilic stroma, Descemet’s membrane and a lipophilic endothelium.
Schematic representation the human eye and the structure of the cornea [77]
The epithelial cells are closely packed together like a pavement, forming not only an effective barrier to most micro-organisms, but also for active substance absorption. The low permeability of the cornea is due to the presence of tight junctions between the epithelial cells. The superficial corneal epithelial cells are exfoliated from the ocular surface, their average life is 4–8 days.
The cornea is highly innervated with sensory nerves, which serves important sensory and reflex functions.
The eyeball has a wall consisting of three layers: the outer coat or the sclera and cornea, a middle layer or uveal coat and the inner coat or retina.
The cornea has no blood vessels and the sclera only a few, consequently the supply of immunoglobulins to these tissues is limited. Therefore the treatment of infections is difficult.
The conjunctiva is a thin transparent membrane, which lines the inner surface of the eyelids and is reflected onto the globe. The conjunctiva consists of three parts: bulbar on the eye surface (sclera), fornix or conjunctival sac and palpebral on the inner side of the eyelids. The bulbar conjunctiva lies upon the sclera and only attaches to the sclera on the limbus. The structure resembles a palisade and is more permeable than the cornea.
The high corneal sensitivity is due to the specific innervation of the eye. The corneal surface possesses the highest nerve density of all organs in the human body: about circa 7,000 nociceptors per mm2. The nerve endings are located only one layer below the corneal surface. Consequently they are very sensitive and active substances elicit reflex blinking. Three types of stimuli are responsible for pain perception: mechanical, physico-chemical and temperature gradient dependent. The distribution of the various kinds of nerves and receptor is functional heterogeneous: 20 % mechanical, 70 % physico-chemical and 10 % temperature (cold) sensitive. The sensitivity of the cornea and conjunctiva seems to be dependent on the colour of the iris, age and gender [11, 12]. Pathophysiological processes, long term use of ocular medication could influence the corneal sensitivity [13, 14].
The high corneal sensitivity serves to protect the eye. Reducing pain perception is dangerous. Patients should be warned of the danger of anaesthesia dolorosa due to repeatedly instillation of local anaesthetic eye drops. Welding without protection or snow blindness due to excessive exposure to UV light can cause photoelectrical keratitis, which is a very painful condition [15]. Administration of local anaesthetics will be recommended. It is true that local anaesthetics reduce corneal sensitivity but they delay the renewal of corneal epithelium layers with nerve endings. Repeated instillations of anaesthetics result in the duration of action being shortened resulting in pain breakthrough and are responsible for the serious condition where the stroma melts away [16]. Therefore, local anaesthetics should only be delivered in single-dose containers and their use should be limited.
10.3.2 Tear Film and Lachrymal Secretion
The lachrymal glands secrete lachrymal fluid, which spreads on the exposed part of the eye forming the precorneal tear film. An intact film protects the ocular surface from desiccation. The tear film results from the lachrymal functional unit [8] which consists of the:
-
Lachrymal glands
-
Ocular surface
-
Sensory nerves involved
The tear film is a mixture of several excretion products:
-
Aqueous fluid (95 % of water, salts, glucose, urea, proteins) secreted by the lachrymal glands
-
Soluble mucins produced by the goblet cells present in the conjunctiva
-
Lipids from the Meibomian glands embedded in the tarsal plate of the eyelids
The lipid composition is kept within physiological limits by androgens [17]. The decrease of their secretion in elderly people is one of the reasons for development of dry eye syndrome. Patients with Meibomian gland dysfunction show a high tear film evaporation rate and a high tear osmolality [18, 19].
The structure of the tear film
According to the “three layers theory” the tear film consists of a superficial lipid layer, the central aqueous layer and an inner mucus layer.
No clear separation exists between the aqueous layer and the mucus layer because mucins are dissolved in the aqueous layer (see Fig. 10.2).
Schematic representation of the tear film structure [77]
To maintain the integrity of the tear film is of utmost importance during the administration of eye drops. The role of the glycocalyx is essential. The glycocalyx consists of anionic membrane-spanning or membrane-associated mucins secreted by corneal and conjunctival epithelial cells [20, 21]. Due to its moisture binding characteristics it stabilises the tear film (see Fig. 10.2). Moreover the superficial lipid layer prevents evaporation of the central viscous aqueous layer.
About 1.2 microlitres of lachrymal fluid is secreted per minute. The functions of the lachrymal fluid are:
-
Improvement or maintenance of the optical quality of vision (homeostasis)
-
Lubrication of the eyeball
-
Elimination of foreign bodies
-
Supply of nutrition to the ocular surface
-
Defence against infection (viral and bacterial)
-
Oxygen transport to the avascular corneal epithelium
The secreted lachrymal fluid is spread over the ocular surface by the eyelids (precorneal tear film) and distributed to the conjunctival sac during blinking. Meanwhile the tears are swept to the medial canthus and drained through puncta, canaliculi, lachrymal sac and nasolachrymal duct which opens into the inferior nasal passage. The volume of the precorneal tear film amounts to about 7 microlitres. The conjunctival sac can accommodate about 30 microlitres, but in some persons only 20 microlitres or even less [22]. The tear film evaporates at a rate of 6–12 microlitres per hour [23].
Immunological and Antibacterial Mechanisms of the Eye
The ocular surface is the domain of the mucosa-bonded immune system [24]. This system plays an important role in combating infections by killing micro-organisms. It consists of the lachrymal gland, conjunctiva and related structures. Besides immunoglobulins, enzymes and bactericidal components are present: IgA, lysozyme, lactoferrin, lipocalins, cathelicidine and probably beta-defensins [25]. Lipocalin is considered the most important component in eliminating toxic (phospho)lipids and fatty acids from the ocular surface [26]. Elimination is necessary, otherwise only partial hydration of the corneal epithelium will occur, which could result in ulceration [27].
10.3.2.1 Tear Film Stability
The tear film is only temporarily stable. The time period of stability of an intact tear film is named the tear film break up time (TFBUT) [28]. TFBUT is measured using fluorometry [29]. Tear film stability is reduced by tensioactive preservatives, which solubilise the superficial lipid layer. The example is the preservative benzalkonium chloride. Reduction of stability causes an increase in blinking frequency [30]. The tear film breaks up after 10–20 s and dry spots form on the corneal surface (dewetting of the cornea). These dry spots irritate the corneal nerve endings and activate the lachrymal functional unit, which triggers the blink reflex. During eyelid opening a new protective film spreads over the ocular surface. Patients suffering from dry eye syndrome exhibit formation of dry spots even when eye drops without benzalkonium chloride are instilled. The reduced film stability can be due to lower lipid-, tear- or mucin production [31].
Improvement of the Diagnosis of Dry Eye Syndrome
The Ocular Protection Index (OPI) was proposed to get a better insight into the uncomfortable condition, dry eye syndrome [32]. To obtain an optimal hydration of the corneal epithelium the ratio between the time period of an intact tear film and the time between two reflex blinks must be greater or equal to 1 (OPI ≥ 1). Investigation of tear film stability using sodium fluorescein improves the diagnosis of dry eye syndrome when 1–5 microlitres solution is used instead of larger volumes [33]. As a result the certainty of diagnosis is increased. However, the use of small drop volumes has not yet been introduced, as this technique is insufficiently developed.
Improvement of Tear Film Stability
Patients suffering from dry eye syndrome complain about tear film instability. Stability can be improved by increasing the viscosity of the tear film (see also Sect. 10.4.4) [34]. Viscoelastic polymers increase viscosity but also possess elastic properties. During blinking sodium hyaluronate (Na-HA) exhibits a kind of cushioning action and induces improved protection of the ocular surface compared to classical pseudoplastic viscosity enhancing polymers. Consequently the movement of the eyelids during blinking is smoother. Na-HA is effective in providing relief to dry eyes.
Other viscosity enhancing polymers are natural anionic polysaccharides such as gellan gum (E-418) and xanthan gum (E-415). These macromolecules are used as gelling agents. Artificial tears may contain dextran, hypromellose and carbomer sometimes with or without polyvinylalcohol and povidone. Unfortunately the ideal non crust forming and stability improving gelling agent has not yet been developed. It seems that hydroxypropyl-guar possess more specific adhesive properties to injured ocular surfaces [35–37].
10.4 Biopharmaceutics
After administration active substances should reach their target tissue. Therefore eye drops should fulfil certain requirements. The following properties are important:
-
The lipophilicity of the active pharmaceutical ingredient (active substance)
-
Active substance concentration
-
Dilution by the lachrymal fluid and drainage
-
Viscosity of the tear film
-
pH value and buffer capacity of the preparation
-
Osmotic value of the preparation
First the absorption of the active substances through the cornea is discussed and afterwards how the previously mentioned factors will influence the absorption.
10.4.1 Lipophilicity and Ionisation of Active Substance
As shown in Fig. 10.2 the cornea consists of various layers. The permeation of the active substances occurs transcellular or paracellular. Lipophilic, non-ionised molecules will diffuse via the transcellular pathway, while ionised, hydrophilic molecules pass through the paracellular space (tight junctions). The pore size at the corneal surface is about 6 nm (60 Å). The lipophilic epithelium prevents the passage of 90 % of the hydrophilic active substance dose, but only 10 % in the case of a lipophilic active substance [38]. Most ophthalmic active substances are salts of weak bases, which are completely dissociated at a low pH value. The contrary is true in the case of most NSAIDs. The permeation of pilocarpine [39] and some mydriatics [40] is higher when the molecule is not dissociated, resulting in a higher therapeutic effect compared to the protonated molecule (see Fig. 10.3).
The degree of lipophilicity and ionisation of the active substance determines the extent of corneal permeation. Many examples are reported in the literature [41, 42].
However, recent research proved the presence of a number of transporters in the cornea and conjunctiva such as amino acid/peptide, nucleoside, organic anionic and organic cationic. These systems will influence the absorption of active molecules. Moreover active substance efflux pumps at the cell surface could restrict active substance penetration into ocular tissues [5].
10.4.2 Active Substance Concentration, Drop Size, Surface Tension
The amount of active substance applied to the eye depends not only on the concentration but also on the drop size, which is influenced by the surface tension of the solution. The design, dimensions of the dropper tip, the cross-sectional surface area on which the drop is formed and the dispensing angle at which the patient manipulates the bottle influence the drop volume instilled [43].
Drop size Variation
Research performed in the eighties in the USA showed that most dropper containers delivered drop volumes between 25 and 75 microlitres [44]. A similar study performed about 10 years later demonstrated that drop volume of products on the market had decreased [45]. This trend will continue as production techniques of dropper tips became more precise.
Active substances such as antazoline and tetracaine reduce the surface tension. Eye drops with a surface tension below 35 mN/m (normal surface tension of lachrymal fluid is 40–46 mN/m) are painful and uncomfortable [30].
10.4.3 Dilution and Drainage
The maximum volume of solution the lower conjunctival sac can accommodate is about 30 microlitres. After instillation the normal volume of the precorneal tear film (7–10 microlitres) is established again due to drainage of the extra volume of fluid present. The drainage rate is directly proportional to the volume of ophthalmic solution instilled. A high percentage of hydrophilic active substances are eliminated and lost to the eye. The drained active substance reaches, via the nasolachrymal duct, the nasal mucosae and after absorption enters the systemic circulation. As lipophilic substances are absorbed much more rapidly, these systemic effects are less prominent.
If the decision is taken to administer very small volumes (1–10 microlitres) of concentrated active substance solutions in order to compensate for dilution in the tear film, irritation becomes a problem. Consequently excessive lachrymation, drainage and wash out will occur, resulting in low active substance availability. To avoid irritation the surface tension, osmolality, pH and buffer capacity should be within certain limits (see Sects. 10.4.5 and 10.4.6).
Tolerance and availability of ophthalmic preparations are closely related [46]. The condition being treated can also play a role: i.e. the drainage rate in Sjögren’s patients is slower than in healthy people [47].
Administration of more than one eye drop makes no sense. The second drop or a drop of double volume will be drained almost immediately. The availability could only be improved by lengthen the residence time of the preparation in the lower conjunctival sac. Closing the puncta by applying pressure with the thumb or fingers, but also closing the eyelids for approximately three minutes, increases the residence time and decreases the drainage of the ophthalmic solution to the nasal mucosae [48].
Animal experiments evaluating drop size and percentage of drained active substance demonstrated that after instillation of a 50 microlitres solution more than 50 % was lost [49]. When three drops of flurbiprofen (0.3 mg/mL) were instilled in rabbits, one drop every 30 min, a 2–3 times higher concentration was measured only in the corneal tissues [50].
10.4.4 Viscosity of the Tear Film
To improve the therapeutic effect of a medicine, one should try to increase the absorption of the active substance. Eye ointments and eye creams stay much longer in the conjunctival sac and on the ocular surface than eye drops. Consequently the active substance is delivered during a longer period of time to the eye. Ointments or creams could be considered as prolonged release dosage forms (depot preparations) for ophthalmic use. Viscous eye drops can increase residence time in the eye but are generally less successful. The use of viscous ophthalmic gels is under discussion. Xerogel forming polymers such as cellulose derivatives are in theory able to block for example puncta on the eyelids and canaliculi when used in high concentration and after desiccation. Carbomer, which does not form xerogels, used in the concentration 2 mg/mL, improves the availability and the prolonged activity of some active substances. No blurred vision was reported [51].
10.4.5 pH Value and Buffer Capacity of the Solution
The pH value of the lachrymal fluid is about 7.4. Due to evaporation of CO2 from the tear film when the eyes are open, the pH value increases to 8 and even higher values [52]. Three buffer systems are present in tear fluid: bicarbonate-carbonate, mono-dibasic phosphate and amphoteric proteins; the buffer capacity is low [53]. The acid-neutralising capacity of the tear fluid of one eye is equal to about 8–10 microlitres 0.01 M NaOH.
The pH value influences the active substance availability from an ophthalmic preparation in two ways:
-
A pH value outside the physiological range causes extra lachrymation and reduces the residence time of the active substance on the ocular surface.
-
The pH value influences the permeation of the active substance through the cornea (see Sect. 10.4.1).
Solutions with pH values below 5.0 and above 8.5 are uncomfortable and not well tolerated [54]. The intensity and the duration of pain sensation after instillation are related to the acidity (pH) and buffer capacity of the eye drop.
10.4.6 Osmotic Value of the Preparation
Eye drops should be in principal iso-osmotic with lachrymal fluid, which means the NaCl concentration 9 mg/mL or approximately 0,9 % (280 mosmol/L). This value corresponds to the tear fluid of patients suffering from conjunctivitis. In healthy persons the osmolarity of the lachrymal fluid equals 290–310 mosmol/L, but varies during the day [55]. Tears of keratoconjunctivitis sicca and Sjögren’s patients show higher values (343 mosmol/L) [56]. Therefore these patients welcome hypotonic eye drops [57].
Almost no pain sensation occurs within the range 0.5–2 % NaCl [58]. Strong hypo-osmotic solutions could damage the corneal epithelium. This should not happen during normal application of eye drops, because one minute after instillation of distilled water the baseline osmolarity of the tear film is restored.
10.5 Adverse Effects and Toxicity
The irritating properties of substances have been investigated using the Draize irritating test on rabbit eyes [59]. This method was used to test many different substances and resulted in serious consequences for the rabbit eye. Nowadays the test is performed according to contemporary acceptable procedures. The redness and its rate of development are an indication of the irritating potential of the substance examined. No satisfactory alternative method is available. Caution should be taken regarding interpretation of the observations collected. For example the frequency of (reflex) blinking influences the results obtained after application of ophthalmic preparations. The frequency differs between rabbit and human being. The rabbit blinks about every 20 min, while humans every 10 s. This difference in blinking frequency is relevant during investigation of viscous solutions.
Alternative in vitro or ex vivo methods have been developed and validated for assessing ocular irritation [60, 61].
Ophthalmic preparations should not contain substances, which could mechanically injure the eye during the blinking of the eyelids (see also Sect. 10.8). The cornea is extremely sensitive to solid particles especially when larger than 50 μm. Particles of 20–25 μm could, depending on their shape, irritate the eye. Due to the induced lachrymation the active substance will be washed away rapidly.
Even if eye drops are applied topically, undesirable systemic side effects could occur after absorption [43, 62]. The effects could be dangerous to life. Administration of scopolamine eye drops in children resulted in a toxic coma [63]. A substantial amount of active substance administered is drained through the nasolachrymal tube, reaches the nasal mucosae and will be absorbed in the systemic circulation. The correct instillation of eye drops reduces the risk of drainage to the nose but this cannot be completely eliminated. The correct methodology for instilling eye drops will be discussed under Sect. 10.9.
A special mention concerns the use of chloramphenicol in ointment and eye drops, see also Sect. 22.2.4. Chloramphenicol is degraded by light. During preparation and storage the degradation product 4-nitrobenzaldehyde is formed by photolysis. This degradation should be avoided because 4-nitrobenzaldehyde is responsible for a non-dose dependent aplastic anaemia, which condition is rare but lethal. This photochemical reaction can also occur on the ocular surface and skin. Therefore it may be better to apply chloramphenicol as eye ointment at night instead of eye drops at daytime. This might be anyway as effective as the general recommendation of 0,5 % eye drops 3 times a day.
After administration of eye drops, chloramphenicol appeared to disappear very rapidly from the tear film and aqueous humour contrary to the prolonged concentration after administration of the ointment [64, 65].
10.6 Product Formulation
A reliable source of information concerning this section can be found under “Codex der Augenarzneistoffe und Hilfsstoffe” published in Ophthalmika [66]. The pharmaceutical, physico-chemical and pharmacological properties of many active substances used for the preparation of eye drops are described.
Initially the formulation of eye drops will be discussed followed by eye lotions, eye ointments and eye creams.
10.6.1 Eye Drops
10.6.1.1 Choice of Active Substance
A soluble active substance is preferred when eye drops are being formulated. When the active substance prescribed is not (or not sufficiently) aqueous soluble, a suspension will be prepared.
Active substances employed in suspension eye drops are usually micronised. Polysorbate 80 or 20 may be used for wetting of the powdered active substances.
Hydrocortisone eye drops (see Table 18.12) is an example of a suspension formulation, where micronised raw material is used. Povidone is used mainly as wetting agent for an effective dispersion of the hydrocortisone acetate. This improves the settling behaviour (see also Sect. 18.4.2.2).
Precipitation or opalescence could occur when the concentration of one of the formulation components is near to its limit of solubility or due to an incompatibility between two formulation components. The appropriate choice of excipients can solve these problems. For example, the addition of citrate to an eye lotion containing zinc sulphate prevents precipitation of zinc hydroxide (see Table 10.2).
Borax in aqueous solution associates to form a 2:1 complex with chloramphenicol. Therefore, chloramphenicol 0.5 % eye drops could be prepared with the pH value of the solution adjusted to 7 (see Table 10.3).
Frequently the active substance is not or not readily available for pharmacy preparation. Then a sterile licensed pharmaceutical preparation must be used as starting material. Usually powder for solution for injection (i.v.) is used, sometimes solution for injection, powder for bladder irrigation or other products. The active substances comprise the range of antifungals (e.g. fluconazole, voriconazole, amphotericin B), antibiotics (e.g. vancomycin hydrochloride, cefuroxime sodium, tobramycin, bacitracin) or others, e.g. mitomycin. Detailed information is necessary about the overage with respect to the labelled value, the quantity of excipients, the resulting pH and osmolality. Suitability of the reconstituted solution for intravenous injection does not necessarily mean suitability for topical ophthalmic use.
10.6.1.2 Vehicle
Usually eye drops are formulated as an aqueous solution. If an oil is employed medium chain triglycerides [69] are suitable as a vehicle along with refined castor oil, refined peanut oil, refined sesame oil or mixtures of triglycerides (see Table 10.4 and Sect. 23.3.5).
10.6.1.3 pH and Buffer Capacity
The buffer capacity (see Sect. 18.1.1) of the tear film is low. Consequently the buffer capacity of eye drops should be as low as possible. The acid neutralising capacity of tear fluid of one eye is about 8–10 microlitres 0.01 M NaOH (see Sect. 10.4.5). In order to avoid eye irritation the following rule of thumb is used. The volume of 0.01 M NaOH necessary to adjust the pH of the tear film to 7.4 should be less than 25 microlitres 0.01 M NaOH per dose. Sometimes even the equivalent of 10–15 microlitres 0.01M NaOH may be uncomfortable.
The volume of 0.01 M NaOH needed depends on the acidic ingredients, including the buffer. In simple cases knowledge of the pKa value and the molar concentration of the active substance or the buffer substances enables the estimation of the pH of the solution (see Sect. 18.1.1).
Information on the maximum acceptable amount of H+ ions at a pH > 7.4 is not available and of less relevance.
A drop of 1 % pilocarpine HCl solution has a pH value of about 5.5, which after instillation must be adjusted to 7.4 by the lachrymal functional unit. 2 % and 4 % pilocarpine HCl solutions exhibit a pH value of 5.3 and 4.0 respectively, and more NaOH will be required to compensate for the pH difference. If the amount of NaOH needed is higher than the neutralising capacity of the tear fluid, instillation will be painful. The choice of a different salt of the active substance can potentially reduce the irritation caused by ophthalmic preparations. E.g. epinephrine HCl eye drops are less painful compared to epinephrine bitartrate.
For example, pilocarpine HCl, phenylephrine HCl and lidocaine HCl solutions in concentrations higher than 10 mg/mL possess such a high buffer capacity that during administration substantial pain is experienced resulting in lachrymation and wash out of the eye drop. This is due to their high therapeutic concentrations and their pKa values in the neutral or slightly acidic range. Therefore, the pH value of these eye drops should be adjusted as near as possible to 7.4.
The pH value of pilocarpine solutions on the market is 4 and is irritating due to the low buffer capacity of the tear fluid. Therefore the LNA formulated pilocarpine eye drops with a pH value of 6.5, this improves the tolerance of the preparation [71].
When the pH value of the eye solution deviates from 7.4, it will take time to get the normal pH restored in the tear fluid. The greater the buffering capacity is, the longer it will take [72]. Therefore, it is advisable not to use buffering solution outside the pH range 6.5–8.5.
In order to obtain well tolerated eye drops, the pH of the active substance solution is measured and if necessary a combination of excipients is added to adjust to the required value (see Table 10.5).
Addition of the excipients mentioned in Table 10.5 increases the osmotic value of the preparation. Due to incompatibility or a high osmotic value of the active substance solution, the substances cannot be always employed. In these cases a diluted HCl or NaOH solution is recommended. The disadvantage is of course that an amount of solution instead of solid powder must be weighed or measured. A pH increase can also be carried out with trometamol (pKa > 8).
Exact buffer compositions and osmotic values are reported in [66].
It is preferable to use a boric acid-borax buffer, because this buffer system has a very low buffer capacity at the pH value of the tear film and at any lower pH. Boric acid is a weak acid. Boric acid-borax buffer solutions reacts neutral to weakly basic.
Boric acid and borax are regarded as reproductive toxicants. The use of boric acid in eye drops for children younger than 3 years old is not recommended, but it is permitted since a clarification in 2003 [73]. Boric ions do not permeate through the intact corneal epithelium [74].
The EMA’s committee for medicinal products for human use (CHMP) considered that the benefits of phosphate-containing eye drops outweigh their risks, but that in very rare cases patients with significant damage to the cornea may develop corneal calcification during treatment with eye drops that contain phosphate [75].
10.6.1.4 Viscosity
The mean viscosity of tear fluid is between 1.3 and 5.9 mPa∙s [76]. As expected, increasing the viscosity of eye drops increases the residence time in the conjunctival sac [77]. Not only the viscosity, but also tensioactive properties, adhesion on the ocular surface and interactions with mucins play a role in increasing residence time.
Viscosity enhancing agents intended for use in eye drops must fulfil several requirements. Their chemical and physical characteristics must be stable during and after sterilisation. Sterilisation induces an important viscosity decrease for some polymers. Moreover viscous polymer solutions should be free of particles, colourless, be optically clear and have a refractive index comparable to tear fluid (\( {\eta}_D^{20} \) = 1.336–1.338). The concentration used should not cause discomfort and irritation.
10.6.1.5 Viscosity Enhancing Polymers
In Table 10.6 the characteristics of most frequently used viscosity enhancing polymers are reviewed. More information is available in literature [77] and Sect. 23.7.
Apart from the polymers mentioned in Table 10.6 some authorised medicines contain other viscosity enhancing agents such as dextran, hydroxyethylcellulose, hyaluronic acid and hydroxypropylguar gum (HP-guar; Systane®) [36, 37, 57]. Hyaluronic acid possesses good adhesive properties. In situ-gelling systems, such as gellan gum, are used in order to increase the precorneal residence time of the eye drop and to obtain a sustained active substance release [78, 79].
Nowadays interest in poloxamers has increased [77, 80]. Their solution viscosity increases at body temperature, however poloxamers are not added frequently to artificial tears. Excellent overviews of non-ionic poloxamers and surface active substances can be found in literature [81].
10.6.1.6 Preservatives
During the development of an eye preparation whose formulation contains an antimicrobial preservative, the necessity for and the efficacy of the chosen preservative must be demonstrated. The effectiveness of the preservative in the final preparation is tested according to the Ph. Eur. Efficacy of antimicrobial preservation (see Sect. 32.8).
Testing of Antimicrobial Activity
The methodology used to test the antimicrobial activity is still under debate.
A high storage temperature could reduce the antimicrobial activity as seen during the use of a new contact lens solution ReNu with moistureLoc® formulated with a new preservative alexidine [85]. The use of this commercial product caused a Fusarium keratitis epidemic worldwide. Research at room temperature and at high temperature (60 °C) has shown, contrary to other preservatives, that alexidine loses its antimicrobial activity at higher temperature. The cold supply chain of the product is of primary importance. The possible contribution to the development of the biofilm on the contact lens surface was also investigated, but was not considered to have contributed to the problem [86]. The researchers concluded that temperature control during production, storage and transport is of utmost importance. Examination of possible biofilm formation was relevant, because other studies investigated this phenomenon as possible origin of infections. In general, attention is drawn during antimicrobial efficacy tests to planktonic free moving bacteria contrary to microorganisms fixed in biofilm structures. Nowadays interest in biofilm formation (see also Sect. 19.3.5) has increased, because bacteria associated with such systems are more difficult to kill [87].
If eye drops do not contain antimicrobial preservatives (Tables 10.7 and 10.8) they are supplied in single-dose containers or in multidose bottles preventing microbial contamination of the content after opening.
Antimicrobial preservatives should be omitted in eye drops intended for use in surgical procedures. Tetracaine hydrochloride eye drops (Table 10.7) comply with the Ph. Eur. efficacy of antimicrobial preservation.
The use of preservatives is not possible when the patient is sensitive or allergic to the preservative or if eye drops will be administered just before, during or after surgery, because of its toxicity. Commercial ophthalmic products without preservatives are popular because of their better tolerance and lower irritancy potential [88–90].
The preference is given to the combination of benzalkonium chloride and sodium edetate (EDTA). Edetate is added in order to improve the activity of benzalkonium chloride against Pseudomonas aeruginosa.
Benzalkonium Chloride / Edetate and Active Substance Effect
Could the combination of benzalkonium chloride and edetate present in so many ophthalmic solutions influence the therapeutic activity of the active substance? In the case of an intact cornea a higher active substance availability is assumed because benzalkonium chloride acts as a penetration and solubility enhancer increasing passive diffusion of the active substances through the corneal epithelial cells (transcellular pathway). Additionally, edetate is a penetration enhancer, active at the tight junctions between cells, and has an effect on intercellular passive diffusion. Research performed using ketorolac eye drops on rabbits with intact and de-epithelialized corneas [91] demonstrated that the availability of ketorolac in the case of intact corneal epithelium was similar after application of drops with or without benzalkonium chloride and edetate, whilst in the case of the injured cornea a lower availability was measured in the presence of benzalkonium chloride. The researchers speculate that the non-irritating ketorolac formed an irritating combination with benzalkonium chloride resulting in lachrymation (active substance wash out) and lower availability. Combination of edetate with boric acid and an experimental active substance in an ophthalmic solution seems to exhibit permeation increasing properties ex vivo on intact rabbit cornea [92]. The results of both studies do not provide sufficient information to draw a meaningful conclusion as to whether benzalkonium chloride and edetate influence the availability of ophthalmic medicines.
When the combination of benzalkonium chloride and edetate cannot be used because of incompatibilities, thiomersal sodium can be used. Phenylmercuric borate is not available anymore because of toxicological problems to the environment.
A third preservative is chlorhexidine in the form of chlorhexidine acetate or chlorhexidine digluconate at a concentration of 0.1 mg/mL. However, chlorhexidine induces many chemical incompatibilities (see Sect. 23.8).
The preservative selected reduces the choice of other excipients required to adjust pH and osmotic values. Table 10.9 shows the possible combinations of preservatives, pH modifiers and excipients that can add up to the right osmotic value. Table 10.10 shows how these possibilities have led to standard basic solutions (vehicles) for eye drops and concentrates for further dilution.
An overview of preservatives is given in Sect. 23.8. More information concerning specific preservatives suitable for ophthalmic preparations can be found in the literature [66, 72, 88, 93, 94]. Benzalkonium chloride is the most frequently used preservative in ophthalmic products. However its use is under discussion, because of its toxicity in chronic treatment [95, 96]. Therefore new preservatives are used in the development of licensed products [94, 97–100].
The efficacy of a preservative is pH dependent. Therefore the pH value of the ophthalmic solution determines the choice of the preservative. Sorbic acid and benzoic acid present in other dosage forms are active at a pH value lower than 5, and therefore unsuitable in most ophthalmic solutions.
Phenylethanol alone is not routinely used as preservative in eye drops due to too low an antimicrobial activity especially against gram-positive bacteria. Moreover phenylethanol cannot be combined with other preservatives because of its potential to irritate the eye [101]. Hydroxybenzoic esters are also reported to cause a high incidence of eye irritation. Thiomersal is not routinely used due to low antimicrobial activity, allergic reactions and penetration of mercury into the eye. The same is also true but to a much lesser extent for phenylmercuric salts [102–104] (see also Sect. 23.8.4).
The preservative should remain effective throughout the period of use by the patient. The substance should be chemical stable, even after heat sterilisation. Moreover the preservative should be physically stable during preparation and storage. The preservative should be compatible with the other ingredients of the preparation, filters and packaging. Significant adsorption can reduce the antimicrobial efficacy partially or completely [105, 106].
Organic phenylmercuric derivates and thiomersal are known for their strong adsorption onto rubber and various plastics such as low density polyethylene (LDPE).
Benzalkonium chloride and chlorhexidine are also adsorbed onto plastics and rubber, but to a lesser extent.
Chlorobutanol is not recommended because of the relative strong adsorption on and permeation through the packaging material, degradation by heat, relative low dissolution rate and the chemical instability of the raw material.
Chlorhexidine degrades during heating but not to such an extent that autoclaving is impossible. The degradation product 4-chloraniline and related substances are formed. The degradation is strongly dependent on the pH of the solution. The lower the pH the lower the decomposition, with maximum stability at pH 5–6. During autoclaving the concentration of 4-chloraniline is less than 0.125 % (pH range 5–8). According to the Ph. Eur. monograph the maximum tolerable values are 0.25 % chloraniline and 3 % related substances for the chlorhexidine raw material (see also Sect. 23.8.7).
As already mentioned for contact lens solutions (see box Testing of Microbial Activity in Sect. 10.6.1.6) storage temperature also influences the preservation of eye drops. At room temperature a solution of borax, boric acid and edetate exhibit an effective antimicrobial activity but not at 4 °C. Non preserved pilocarpine-hypromellose eye drops can be used for a longer period of time when stored at room temperature compared to storage in the refrigerator.
10.6.1.7 Sterility
Sterility is the most important requirement concerning ophthalmic preparations. A diseased or injured eye is extremely sensitive to infections with catastrophic consequences. Pseudomonas aeruginosa is the most feared organism due to the organism causing serious and difficult to treat corneal ulceration, which can result in rapid loss of vision. Other bacteria such as Bacillus subtilis, Staphylococcus aureus and Haemophilis influenzae as well as yeasts and moulds such as Aspergillus fumigatus, Fusarium species and Candida albicans (or non-albicans) are responsible for serious eye infections.
Therefore ophthalmic preparations must be sterile when dispensed to the patient, and this sterility must be guaranteed throughout storage. When using single-dose packaging for eye drops, no issues with sterility should occur. When multidose packaging is chosen, the risk of contamination should be reduced by the following measures:
-
Preservation
-
Adequate design of primary packaging
-
Adequate instructions to the patient concerning correct application technique and hygiene
-
Limited storage time once the container is opened
-
Refrigeration once the container is opened
The probability of eventual growth of bacteria contaminating the preparation depends on:
-
Presence of a preservative
-
pH value of the preparation
-
Adequate antimicrobial properties of the active substance or the excipients
-
Presence of water and water activity (see Sect. 19.2.2)
-
Temperature
Literature reports are published on a regular basis concerning ophthalmic preparations which have been contaminated during use, even when the solution complies with the criteria of the antimicrobial efficacy test of the Ph. Eur. After 4 weeks of use caps, dropper tips or even solutions can be contaminated. The reasons are: careless administration, transfer of tear fluid into the dropper tip at instillation, cross-contamination in hospitals and nursing homes and resistance of (gram-negative) bacteria against preservatives [107–109]. This phenomenon is underestimated and is one of the reasons why after opening the contents of the container must not be used for longer than 4 weeks (unless otherwise justified). To reduce cross-contamination patients should be instructed how to correctly instil eye drops (see Sect. 10.9) and snap-cap containers (see Sect. 24.4.2.3) should be selected.
10.6.1.8 Osmotic Value
The osmotic value (see Sect. 18.5) of ophthalmic solutions should be in the range equivalent to 0.5–2 % sodium chloride solution in order to avoid pain sensation. However, in practice the upper limit should be set to 1.6 % NaCl to make sure the eye drops are well tolerated by all patients.
Isotonicity of eye drops is obtained by adding boric acid, borax or a combination thereof. If their use is not possible due to chemical incompatibilities, sodium chloride solution can be employed. Other tonicity substances are mentioned in Table 10.9.
10.6.1.9 Container and Labelling
A review of ophthalmic dropper packaging is given under Sect. 24.4.2. In community pharmacies glass containers with dropper tips are usually used to dispense multidose preparations. Polyethylene containers are becoming more popular. Chloro-or bromobutyl rubber teats should not be used with oily eye drops and with iodinated povidone only if previously tested, well defined and standardised cases. The dropper tip and the cap should be made of polypropylene. Packaging should protect the eye drops against exposure to light. If impossible the preparation must be placed into a protecting secondary packaging, e.g. a carton.
The label (see also Sect. 37.3) should mention the storage conditions, shelf life of unopened containers and for multidose bottles and the in-use shelf life after which the contents must be discarded. This period must not exceed 4 weeks. In order to guarantee sterility during use, the Ph. Eur. requires that multidose preparations are supplied in containers containing at most 10 mL solution.
The use of tamper-evident packaging makes it clear to the patient that he is the first person to open the container.
Non preserved ophthalmic solutions are preferably delivered in single-dose packaging such as Redipac® plastic tubes. In pharmacy preparation alternatives may be:
-
1 mL syringes (Luer) with stopper
-
10 mL polyethylene dropper bottles filled to only 250 microlitres or maximum 1 mL
Multidose containers could be used if sterility during storage and in use has been proven and guaranteed. Research on the storage of non-preserved eye drops delivered in Gemo-type containers with snap-cap (see Sect. 24.4.2.2) has been undertaken. In the case of acetylcysteine 5 % eye drops integrity during storage of the containers was guaranteed from a microbiological point of view, even after freezing and thawing [110]. Whether non-preserved eye drops supplied in this packaging could be administered for longer than 24 h, was not investigated.
10.6.1.10 Storage and Stability
A general discussion concerning stability and assignation of storage times is provided in Sect. 22.7.
Hydrolysis and oxidation play an important role in the stability of ophthalmic preparations. Degradation can be maintained within acceptable limits when an appropriate pH is selected and by addition of antioxidants if necessary. Degradation is also reduced by a lower sterilisation temperature, a lower storage temperature or a shorter shelf life.
Non-preserved aqueous eye drops sterilised by autoclaving may be stored in unopened containers for a maximum of 1 year in Redipac plastic tubes and 2 years in dropper bottles. After opening of the container storage should not exceed 28 days in the case of preserved solutions, eye drops with adequate antimicrobial properties imparted by the active substance, and oily eye drops. But for use on wards, 1 week is considered more appropriate.
The storage of aseptically prepared eye drops without preservative may be at maximum 6 months at –15 °C. If no freezer is available, the preparation should only be stored for 1 week in the refrigerator. After opening of the container there is no storage in the narrower sense, because non-preserved eye drops must be packaged in single-use containers. This must be strictly observed without exception when application to different patients cannot be excluded or with immunosuppressive eye drops. In practice storage and application on one and the same patient within some hours after opening of the container occurs frequently and is widely accepted. When justified the period of use after opening is always a maximum of 24 h and the volume of the preparation should be adjusted. When the patient‘s eye is injured or infected a shorter time limit should be considered. Research has demonstrated that some non-preserved preparations are not easily contaminated. If this is the case, a period after opening of longer than 24 h may be acceptable.
10.6.2 Eye Lotions
Eye lotions are defined as aqueous solutions. Thus active substances must be soluble at the concentration needed. Eye lotions must be sterile. According to Ph. Eur. eye lotions intended for use in surgical procedures or in first-aid treatment do not contain an antimicrobial preservative and are supplied in single-dose containers, see for example an eye lotion with iodinated povidone (Table 10.11).
Antiseptic eye lotions frequently used pre-, intra- and postoperatively at eye surgery may contain polihexanide (PHMB), iodinated povidone or chlorhexidine salts.
If a preservative is required for multidose containers, sterile and preserved vehicles can be used (see Table 10.10). The same considerations regarding the use of preservatives in eye drops apply to eye lotions.
Compared to eye drops a higher volume of eye lotion will be in contact with the eye. Therefore the pH value should be adjusted very close to 7.4. If this is not possible, the buffer capacity of the solution must be low in order not to cause discomfort and pain. Irritation is a great challenge in the case of eye lotions. The results of a German study evaluating eye lotions are surprising and showed that about 16 % of the commercial products showed a pH value outside the range 6.4–8.0 [112].
10.6.2.1 Osmotic Value
As high volumes of eye lotions are applied, the product must be isotonic to avoid irritation. However, eye lotions intended to treat ocular oedema should be hypertonic. As discussed under Sect. 10.6.1 the tonicity of eye drops is frequently adjusted with boric acid, borax or a combination thereof. The same is valid for eye lotions. If these excipients are chemically incompatible, or as an alternative, sodium chloride can be used (see Tables 10.11 and 10.12). Suitable excipients adjusting tonicity are summarised in Table 10.9.
Hypertonic Eye Lotions to Prevent Oedema
Research on the use of eye lotions to treat chemical burns noted the importance of hypertonicity. In vitro and ex vivo (rabbit and pigs eyes) a 2 M NaOH solution was applied resulting in tissue damage. The pH value of the aqueous humour increased by 5 pH units, the increase being quickest in eyes that were not rinsed. The rinsing solutions examined were: tap water (hypotonic), phosphate buffered saline solution (PBS, isotonic), physiological saline solution (0.9 % NaCl, isotonic), saline in hypertonic borate buffer solution and a hypertonic saline solution with an amphoteric chelator. Immediately after chemical burning, intensive rinsing for 15 min was carried out, according to American guidelines. PBS induces calcium phosphate precipitation at the ocular surface due to complexation of calcium ions released from the damaged cells with phosphate ions. Physiological saline solution exhibits the same effect as tap water. Without debate, the hypertonic eye lotions showed the best results. The use of hypertonic rinsing solutions prevents the development of corneal oedema. The amphoteric molecule diphoterine neutralises acids and bases and prevents chemical wounds. If only tap water is available, it should be used immediately but there is the risk of corneal swelling. The dilution of the chemical substance by tap water will reduce pain until a more suitable eye lotion is available, but rinsing as soon as possible is of utmost importance [113, 114].
10.6.2.2 Packaging and Labelling
High volume eye lotions prepared in pharmacies may be packed in sterile, clean polypropylene bottles with an appropriate closure. Also type I glass bottles can be used. The volume is a maximum of 200 mL, except if the solution is intended for first-aid treatment where a dispensed volume of 1,000 mL is more appropriate. Aseptically prepared eye lotions should be packed in sterilised containers.
An example of an eye lotion prepared in pharmacies is a low volume antiseptic solution for eye surgery. The lotion is filled in sterile injection vials, polyethylene dropper bottles or other suitable single-dose containers. If eye lotions do not contain antimicrobial preservatives they must be supplied in single-dose containers too.
The label states:
-
Where applicable, that the contents are to be used on one occasion only
-
For multidose containers - the period after opening after which the contents must be used or discarded: this period should not exceed 4 weeks
According to national legislation the label mentions the dosage form (eye lotion), the route of administration (ocular use), the patient information for the intended use. If necessary an eye cup should be supplied. The patient should be instructed as to the proper use of the eye lotion and eye cup, the contact time of bathing the eye and cleaning of the eye cup. The device should be thoroughly rinsed and cleaned before and after use.
10.6.3 Eye Ointments and Eye Creams
Apart from eye ointments and eye creams also eye gels could be seen as semisolid eye preparations. But many of the so called eye gels are not actually semisolid but they are high-viscous liquids (see Sect. 10.6.1).
10.6.3.1 Choice of the Dosage Form
The choice of the type of dosage form will depend on the salt form, particle size and solubility of the substance. In principal there are three categories of semisolid eye preparations:
-
The active substance is dissolved in a lipophilic ointment base.
-
The aqueous active substance solution is emulsified in the lipophilic ointment base (resulting in an eye cream).
-
The active substance is dispersed in the ointment base.
The first ointment type is applicable for only a few active substances dissolved in the non-aqueous ointment bases. To date, almost only paraffin-based lipophilic ointments are used for semisolid ophthalmic products. An example of this type of preparation is eye ointment with 0.5 % erythromycin (Table 10.13). Suitable triglyceride-based vehicles may lead to more solution-type eye ointments (see Sect. 10.7.3).
The second category of semisolid eye preparations is a lipophilic cream: the active substance is dissolved in water or a (preserved) aqueous vehicle and emulsified in the ointment base. An example to mention is a sodium chloride 5 % eye cream (Table 10.14).
The most common category of semisolid eye preparations is a suspension ointment as in chloramphenicol 1 % eye ointment (see Table 10.15). A microfine powdered chloramphenicol substance is used as starting material. The particle size of the powder to be dispersed must comply with Ph. Eur. requirements (see Sect. 10.8).
10.6.3.2 Vehicle
The base must be non-irritant to the conjunctiva. Non-aqueous lipophilic ointment bases consist of a mixture of white or yellow soft paraffin, liquid paraffin and lipophilic surfactants, such as cholesterol or wool fat.
Triglyceride-based vehicles may also be suitable and advantageous in respect to their dissolving power for active substances (see also Sect. 10.7.3).
Wool fat or cholesterol in an eye ointment emulsify with lachrymal fluid resulting in a water-in-oil emulsion-type cream. Cetostearyl alcohol is not a muco- or bioadhesive substance.
Common eye ointment bases are given in Tables 10.16 and 10.17.
10.6.3.3 Preservatives
Micro-organisms are not able to grow in ointments, as no water is present. Therefore, the addition of a lipophilic preservative to a non-aqueous ointment makes little sense. However for lipophilic eye creams the addition of a preservative to the aqueous phase is recommended.
The strong hypertonic aqueous phase of sodium chloride 5 % eye ointment FNA (Table 10.14) prevents bacterial growth.
10.6.3.4 Packaging and Labelling
Eye ointments are packed in small, clean, sterilised collapsible tubes fitted or provided with a sterilised cannula (see Sect. 24.4.9). According to Ph. Eur. the tube contains a maximum of 10 g of the preparation.
Eye ointments and eye creams are applied in the same manner as eye drops: in the lower conjunctival sac and after administration the eyelid is pulled forward. Due to body temperature the ointment melts and is spread by the eyelids over the ocular surface during blinking. When the eye is injured the ointment is applied on the eyelid rim, not in the conjunctival sac. Ointments and creams are not well tolerated, because they produce a film over the eye and thereby blur vision [121]. Therefore, application in the evening is preferred.
Patients should be instructed to the proper use and administration of eye ointments and eye creams. It is important to avoid contamination by contact with the skin or surface of the eye. Consequently, one preparation should be used only by one patient. The same tube can eventually be used by care providers for several persons, however, nursing home staff should be aware of the contamination risk.
According to national legislation the label mentions the dosage form (eye cream or eye ointment), the route of administration (ocular use), the intended use, the storage conditions, the expiry date and, for multidose containers, the beyond-use date after which the opened preparation must not be used. This period should not exceed 4 weeks. If necessary the label also bears warnings and mentions that the contents should be brought to room temperature before administration if the tube is stored in the refrigerator.
10.7 Method of Preparation
The preparation process for eye drops, eye lotions, eye creams and eye ointments will be described subsequently.
10.7.1 Eye Drops
Eye drops are prepared using materials and methods designed to ensure sterility and to avoid the introduction of contaminants and the growth of micro-organisms as also stated by the various Pharmacopoeias. The preparation method consists of several steps: dissolution of the ingredients, (sterile) filtration, filling and packaging and (when possible) heat sterilisation.
10.7.1.1 Dissolution of the Ingredients
For the dissolution process see Sect. 29.5. For small-scale preparation of preserved eye drops the use of autoclaved stock solutions may be convenient. They contain a preservative and often boric acid and borax (see Table 10.5). The other ingredients will be dissolved in these vehicles. When viscous eye drops are prepared, the viscous hypromellose stock solution containing the preservative (see Table 10.10) is always diluted 1:1 with a stock solution containing the same preservative. Vehicles for eye drops prepared on stock often show a weak acidic reaction. Benzalkonium chloride solutions with high pH values, containing alkaline substances such as borax, attack glass material, i.e. the borosilicate glass (type I) of Schott Duran bottles (see also Sect. 24.2.1).
In-process control of the dissolution of the active substances may include pH measurement of the bulk solution immediately before filtration to confirm that the correct ingredients and vehicles have been used.
10.7.1.2 Filtration
Foreign particles can be removed by (pre)filtration over a membrane filter (≤1.2 μm pore size). The use of this filter reduces the initial viable contamination as well. When autoclaving or steam sterilisation is not suitable for the product in order to remove viable contamination, i.e. bacteria, the solution is passed through 0.2 μm membrane which will retain all bacteria. In practice a one-step procedure is preferred using only one membrane filter with a nominal pore size of 0.2 μm. For use in pharmacies this type is readily available.
Polyethersulfone (PES) material for the membrane filter is preferred because of low active substance adsorption and superior filtration. It is unclear whether PES filters are suitable for oily eye drops. Usually fluoropolymer filters are used in these cases.
A viscous benzalkonium chloride solution, for instance with 0,5 % hypromellose 4,000 mPa∙s, is filtered through a membrane with pore size ≤ 1.2 μm to eliminate non dissolved hypromellose fibres. The solution is too viscous to be forced through a 0.2 μm membrane filter. When eye drops are prepared by dissolving a dry powder in a container with the supplied vehicle, the solution obtained should be withdrawn using a 5 μm filter needle to remove any undissolved powder particles [122].
The integrity of membrane filters with a pore size of 0.2 and 1.2 μm should be verified using a bubble-point test after use as an in-process control. During this test a 0.2 μm membrane filter should resist the air pressure produced by moving the plunger over 80–85 % of the total syringe volume and in the case of a 1.2 μm membrane filter over 50–60 % without continuous bubble formation on the opposite of the membrane (see also Sect. 30.6.5).
10.7.1.3 Sterilisation
Sterilisation is generally dealt with in Chap. 30. The preferred method is a 15 min steam sterilisation at 121 °C of the active substance solution filled into the final container. Sterilisation in the final container is however not always feasible because the container is not heat resistant or the active substance degrades at elevated temperatures. In order to keep the risk of non-sterile eye drops as low as possible a combination of measures must in that case be taken. The possible measures are:
-
Use of sterile vehicles (sterile stock solutions, sterile purified water or water for injections)
-
Addition of preservatives
-
Heating 30 min 100 °C over boiling water
-
Filtration through bacterial-retentive membrane with the nominal pore size of 0.2 μm
-
Use of sterile final container
-
Aseptic preparation in a Class A laminar flow workbench
-
Storage in a refrigerator
-
Deep-freeze storage
These measures reduce microbial contamination or prevent an increase in contamination during preparation and storage. For extemporaneous preparation of eye drops in pharmacies the responsible pharmacist must select the most adequate sterilisation technique after performing a risk assessment.
Tables 10.18 and 10.19 summarise for preserved and non-preserved eye drops respectively the range of obvious combinations of methods, procedures, utensils and containers for small scale preparation for obtaining a sterile product. The presence of a preservative in the formulation makes heating at 100 °C during 30 min (over boiling water) much more effective (see Sect. 30.7) and is therefore an important parameter in the risk analysis.
10.7.1.4 Aseptic Handling
Aseptic handling in clinical practice often occurs when licensed parenteral medicines are used off-label for eye disorders, i.e. amphotericin B, fluconazole, mitomycin, and voriconazole [123–126]. A sterile product with the active substance (i.e. a powder for solution for infusion, a concentrate for solution for infusion, a solution for infusion or these dosage forms for injection) has to be adapted into eye drops. The first preparation step involves dissolution of the powder in the vial, thus resembling the reconstitution for the designated use. The sterile vehicle used may contain a preservative [123] or may be water for injections [124, 125], saline or buffer solution [126]. Sometimes dilution to a larger volume is necessary before finally filling the eye drops into the container. It depends on the outcome of a risk assessment of each individual case, if filling should include filtration [123–125] or not (see Table 10.19). Aseptic handling, outside a Class A environment, may be achieved by preparing in a ‘nearly closed system’, by filling the sterile dropper bottle by piercing the package wrapped around it, after suitable disinfection of the packaging surface (see Fig. 10.4). This technique can include filtration or just mixing of sterile solutions. The conditions for the preparation are best described by the term ‘aseptic handling’, see Sect. 31.3.
(a and b). Aseptic handling of eye drops preparation (see text under Sect. 10.7.1.3)
10.7.1.5 Handling Containers
During heating at 100 °C (over boiling water), the caps of the dropper bottles should be closed or open depending on the kind of container (see Sect. 24.4.2). If the closure is open, the dropper bottles should be placed immediately after heat treatment in a Class A laminar flow workbench. After cooling down the cap has to be closed.
After filling, sealing and sterilisation of single-dose containers (for example Redipac plastic tubes, see Sect. 24.4.2.6), the integrity of the container should be confirmed by squeezing and inspecting for leakage. For prevention of water evaporation during storage, Redipac tubes should be wrapped individually in foil, already before sterilisation. This makes drying after sterilisation necessary: approximately 10 min at 80 °C in an oven has showed to be sufficient.
When heating at 100 °C (over boiling water) during 30 min in combination with membrane filtration sterile containers (dropper bottle or Redipac) and sterile solutions of excipients are required. The preparation will be performed in a Class A laminar flow workbench. After sterilisation the containers must be stored in the freezer.
When only aseptic preparation is possible Redipac plastic tubes, dropper bottles and syringes could be used as containers. The same requirements such as sterile container, sterile solution of excipients, aseptic preparation in a Class A laminar flow workbench and storage in a freezer are valid to ensure sterility.
10.7.2 Eye Lotions
The preparation of eye lotions is similar to eye drops (see Sect. 10.7.1). As in most cases non-preserved stock vehicles are used, eye lotions should be sterilised by autoclaving for 15 min at 121 °C in the final container. If not possible, several measures may be combined in order to keep the risk of contamination as low as possible, analogously to Tables 10.18 and 10.19.
Low-volume eye lotions with antiseptics (iodinated povidone, polihexanide or chlorhexidine salts) for use in eye surgery must not contain preservatives. They are usually prepared aseptically in pharmacies using water for injection and sterile excipients, analogously to Table 10.18. Iodinated povidone eye lotion is thermally unstable and membrane filtration (≤0.2 μm) has to be applied.
10.7.3 Eye Ointments and Eye Creams
An ointment base is prepared by melting the ingredients together. Sterilisation can be performed by dry heating (see Sect. 30.5.2) or membrane filtration (see Sect. 30.6.1). Heat sterilisation requires a validated heat steriliser, which may be expensive. In addition, a disadvantage of dry heat sterilisation is the partial decomposition of the fat components. The degradation products could negatively influence the stability of the active substance and probably cause irritation of the eye.
Some types of tubes can resist 3 h at 140 °C (see Sect. 24.4.9). Although this is not exactly the Ph. Eur. requirement for dry heat sterilisation, the use of this method has the advantage of a much easier and thereby safer aseptic preparation of the medicine. Heat sterilisation of an eye ointment can only be performed if the active substance is dissolved in the base and is stable to elevated temperatures.
Sterilisation in the final container obviously is not possible for eye creams. It is practical to distinguish solution-type preparations from suspension-type preparations.
10.7.3.1 Solution-Type Preparations
Preparation of a solution-type eye ointment starts with the melting and mixing all ingredients as described above.
Preparation of an eye cream (see Sect. 10.6.3) includes the preparation and sterilisation of the aqueous phase in a similar manner to eye drops. The aqueous phase is then incorporated into the sterile ointment base by aseptic processing. Using an oily solution of the active ingredient instead results in an eye ointment.
A semisolid triglyceride (Softisan 378®) that meets the monograph Hard fat Ph. Eur. may establish the option to prepare not only eye creams with water-soluble active substances but also solution-type eye ointments and eye creams with active substances soluble in fatty oils (i.e. clotrimazole, ciclosporin). Softisan 378® shows delayed solidification when molten and drawn into a syringe, thus making membrane filtration (≤0.2 μm pore size) possible at about 30 °C. However, specific formulas of triglyceride-based eye ointments and creams have not been fully developed yet. For example the ratio Softisan 378®)/refined peanut oil or the optimum cholesterol concentration as an emulsifier still has to be investigated.
For reading the temperature as an in-process control a non-contact laser infrared digital thermometer is used. The consistency could be measured using two glass plates as a simple extensometer.
Mixing Technique with Connected Syringes
For extemporaneous preparation an aseptic procedure is suitable for the preparation of eye ointments and eye creams in pharmacies. It requires 2 or more Luer-Lock-syringes consecutively conjoined by a sterile Luer-Lock-connector [127]. By pushing liquid and semisolid intermediate product from one syringe to the other and back through the connector, homogeneous ointments or creams can be prepared (see Fig. 10.5b, c). With the help of additional syringes, connectors and a membrane filter (≤0.2 μm pore size) aqueous or triglyceride-based solutions and certain types of molten ointment bases can be filtrated (see Fig. 10a) and kept into sterile syringes prior to mixing. Molten sterile ointment base can also be drawn into a syringe directly. Mixing in the ‘nearly closed system’ reduces the risk of microbial contamination. This method has of course to be validated for each formulation, especially with suspension-type ointments if agglomerates have to be broken up.
10.7.3.2 Suspension-Type Preparations
Sterilisation in the final container is not possible for suspension-type eye ointments. During heating the ointment base melts and the dispersed powder particles will settle. Active substances intended for use in suspension ointments must be purchased sterile or sterilised by dry heat prior to use if their thermal stability is sufficient. The container with the raw material should only be used for the preparation of eye ointments.
The substance must comply with Ph. Eur. requirements concerning particle size (see Sect. 10.8). During incorporation agglomerates should be broken down. This best may be performed using a stone or porcelain mortar and pestle. The use of plastic mortar and pestle or glass plate and flexible spatula is usually not sufficient to break down the agglomerates.
The laminar flow is disturbed more by operating with the open product as happens with the preparation of suspension-type eye ointments, than with eye drop preparation or by the mixing technique with connected syringes for semisolid eye preparations (see Fig. 10.5). Consequently, a higher risk of contamination exists (see Sect. 31.3.2), which has to be accounted for in the risk assessment.
As an in-process control the presence of agglomerates and the homogeneity shall be carried out visually after placing a sample of the preparation between two glass slides. No particles or agglomerates should be visible. The control of the particle size is performed using a microscope. For temperature and consistency measurement as in-process controls see Sect. 10.7.3.1.
The preparation of tetracycline eye ointment can be problematic and it is preferable to use micronised active substance to overcome particle size issues, knowing at the same time that the raw material must comply with chemical purity specification. The microcrystalline raw material as described in USP meets both requirements.Another way to solve the problem is the preparation of a semi-finished product using tetracycline base, dissolved in semisolid base which significantly reduces the decomposition rate [66, 128, 129].
10.8 Release Control and Quality Requirements
For ophthalmic preparations following quality requirements apply (see also Table 32.2):
-
Identity
-
Appearance (homogeneity, for eye drops: clarity and no precipitation)
-
Content of active substance(s) and preservative
-
pH (for eye drops)
-
Sterility
-
Foreign particles
-
Uniformity of dosage units
Solution-type eye drops must be practically free from particles. Eye drops that are suspensions may show a sediment that is readily resuspended on shaking to give a suspension which remains sufficiently stable to enable the correct dose to be delivered.
Suspension eye ointments should be prepared with powder as fine as possible, because large particles could mechanically injure the eye. Even small needle-shaped crystals (smaller than 50 μm) could damage the corneal surface.
Suspension-type eye drops and eye ointments must, according to the Ph. Eur. comply with following test: For each 10 microgram of solid active substance, not more than 20 particles have a maximum dimension greater than 25 μm and not more of two of these particles have a maximum dimension greater than 50 μm. None of the particles has a maximum dimension greater than 90 μm. The investigation is carried out using a microscope.
The Ph. Eur. has no test for metal particles originating from poor quality metal ointment tubes. The Japanese Pharmacopoeia has a specification for the presence of metal particles, number and dimensions. In 10 samples no more than 50 particles of 50 μm or greater should be present, the shape is not specified. In addition, in 1 sample not more than 8 particles should be found.
10.9 Administration of Ophthalmic Preparations
Each type of ophthalmic dosage form has advantages and drawbacks. The administration of aqueous eye drops appears to be the most practical and comfortable dosage form for an ocular medication. However, the residence time on the eye is very short. Viscous eye drops and eye ointments adhere better to and stay longer on the ocular surface.
The patient, carer or nurse should apply each type of ophthalmic preparation in the correct and reproducible way. Table 10.20 describes the best instillation technique [130, 131] for eye drops.
Many patients, especially the elderly, experience difficulties in administrating eye drops. It has been demonstrated that standardisation of administration instructions and the use of mechanical aids can improve patient compliance. Certain commercial products are supplied with devices to facilitate instillation such as Xal-Ease and Eyot, and also Autosqueeze developed by the British Royal National Institute for the Blind [133]. An overview of the various mechanical aids can be found in Sect. 24.4.19.
For patients on an intensive care ward following procedures were developed: in principle they should be applied conscientiously at each hospital ward and nursing home [132]:
-
Avoid ocular contact with dropper(tip) and tip of the ointment tube.
-
Do not use the same dropper tip or tube for both eyes of the patient. This prevents the possible spread of infection from one eye to the other. This implies that a separate preparation for each eye is preferable.
-
Do not forget to remove contact lenses prior to administering the ophthalmic preparation.
-
Do not use an eye covering wound dressing in cases of secreting ocular wounds.
-
Do not perform pulmonary suction over patient’s head without a procedure involving protection of his eyes against infection.
-
Do not use swabs imbibed with ethanol when patient is in coma.
Arthritic patients, elderly people and children all experience difficulties with administering eye drops [43, 134–137]. In the group of elderly persons studied less than 33 % were able to instil eye drops or even to squeeze the dropper bottle. Only 50 % of the persons, who were able to administer their eye drops could apply the eye drops into the conjunctival sac. This illustrates that clear and practical instructions are required. For children clear instructions were described for an alternative method of administration. The technique was initially tested on volunteers (20–33 year old). The patients should lay down and close their eyes. The caregiver instils a drop on the inner canthus (near the nose), the patient then slowly opens their eye lids and the drop runs into the tear film. The pharmacological effect of pilocarpine nitrate 0.25 % and 0.5 % applied using the above described technique, exhibits an effect between that of pilocarpine nitrate 0.25 % and 0.5 % with nasolachrymal occlusion. The ‘closed eye’ technique is thus an effective and easy to use alternative for reluctant children.
References
Gaudana R, Jwala J, Boddu SHS, Mitra AK (2009) Recent perspectives in ocular drug delivery. Pharm Res 26:1197–1216
del Amo EM, Urtti A (2008) Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today 13:135–43
Jain GK, Warsi MH, Nirmal J et al (2012) Therapeutic stratagems for vascular degenerative disorders of the posterior eye. Drug Discov Today 17:748–59
Diebold Y, Calonge M (2010) Applications of nanoparticles in ophthalmology. Prog Retin Eye Res 29:596–609
Molokhia SA, Thomas SC, Garff KJ et al (2013) Anterior eye segment drug delivery systems: current treatments and future challenges. J Ocul Pharmacol Ther 29:92–105
Guzman-Aranguez A, Colligris B, Pintor J (2013) Contact lenses: promising devices for ocular drug delivery. J Ocular Pharmacol Ther 29:189–99
Friedenwald JS, Hughes WF Jr, Herrmann H (1944) Acid-base tolerance of the cornea. Arch Ophthalmol 31:279–83
Stern ME, Gao J, Siemasko KF et al (2004) The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res 78:409–16
Dartt DA (2009) Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res 28:155–77
Barabino S, Chen Y, Chauhan S, Dana R (2012) Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res 31:271–85
Guthoff RF, Wienss H, Hahnel C et al (2005) Epithelial innervation of human cornea. A three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea 24:608–13
Acosta MC, Alfaro ML, Borras F et al (2006) Influence of age, gender and iris color on mechanical and chemical sensitivity of the cornea and conjunctiva. Exp Eye Res 83:932–8
Hirata H, Meng ID (2010) Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci 51:3969–76
Labbé A, Alalwani H, Van Went C et al (2012) The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci 53:4926–31
Behar-Cohen F, Martinsons C, Viénot F et al (2011) Light-emitting diodes (LED) for domestic lighting: any risks for the eye? Prog Retin Eye Res 30:239–57
Polak BCP, Henkes HE (1987) Bijwerkingen van geneesmiddelen in de oogheelkunde. Ned Tijdschr Geneeskd 131:2254–7
Khandelwal P, Liu S, Sullivan DA (2012) Androgen regulation of gene expression in human meibomian gland and conjunctival epithelial cells. Mol Vis 18:1055–67
McGinnigle S, Naroo SA, Eperjesi F (2012) Evaluation of dry eye. Surv Ophthalmol 57:293–316
Cuevas M, Gonzalez-Garcia MJ, Castellanos E et al (2012) Correlations among symptoms, signs, and clinical tests in evaporative-type dry eye disease caused by meibomian gland dysfunction (MGD). Curr Eye Res 37:855–63
Argüeso P, Gipson IK (2001) Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp Eye Res 73:281–9
Govindarajan B, Gipson IK (2010) Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res 90:655–63
Stein R, Hurwitz JJ (1995) Anatomy and physiology of tear secretion. In: Hurwitz JJ (ed) The lacrimal system. Lippincott-Raven, Philadelphia, pp 1–8
Guillon M, Maïssa C (2010) Tear film evaporation – effect of age and gender. Cont Lens Anterior Eye 33:171–5
Forrester JV (2009) Privilege revisited: an evaluation of the eye’s defence mechanisms. Eye 23:756–66
Zhou L, Beuerman RW (2012) Tear analysis in ocular surface diseases. Prog Retin Eye Res 31:527–50
Green-Church KB, Butovich I, Willcox M et al (2011) The international workshop on Meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci 52:1979–93
Caffery B, Joyce E, Boone A et al (2008) Tear lipocalin and lysozyme in Sjögren and non-Sjögren dry eye. Optom Vis Sci 85:661–7
Alcon. Tear film break up time. www.systane.com. Accessed 1 July 2014
Nichols JJ, King-Smith PE, Hinel EA et al (2012) The use of fluorescent quenching in studying the contribution of evaporation to tear thinning. Invest Ophthalmol Vis Sci 53:5426–32
Tiffany JM, Winter N, Bliss G (1989) Tear film stability and tear surface tension. Curr Eye Res 8:507–15
Stevenson W, Chauhan SK, Dana R (2012) Dry eye disease. An immune-mediated ocular surface disorder. Arch Ophthalmol 130:90–100
Ousler GW III, Hagberg KW, Schindelar M et al (2008) The ocular protection index. Cornea 27:509–13
Ousler GW III, Michaelson C, Christensen MT (2007) An evaluation of tear film breakup time extension and ocular protection index scores among three marketed lubricant eye drops. Cornea 26:949–52
McCann LC, Tomlinson A, Pearce EI, Papa V (2012) Effectiveness of artificial tears in the management of evaporative dry eye. Cornea 31:1–5
Report of the International Dry Eye Workshop (DEWS) April 2007; 5: 2 Special issue of The Ocular Surface. www.journals.elsevier.com/the-ocular-surface
Rolando M, Autori S, Baino F et al (2009) Protecting the ocular surface and improving the quality of life of dry eye patients: a study of the efficacy of an HP-Guar containing ocular lubricant in a population of dry eye patients. J Ocul Pharmacol Ther 25:1–7
Baudouin C, Cochener B, Pisella P-J et al (2012) Randomized, phase III comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye disease. Eur J Ophthalmol 22:751–61
Ghate D, Edelhauser HF (2008) Barriers to glaucoma drug delivery. J Glaucoma 17:147–56
Longwell A, Birss S, Keller N et al (1976) Effect of topically applied pilocarpine on tear film pH. J Pharm Sci 65:1654–7
Wang ESN, Hammarlund ER (1970) Corneal absorption reinforcement of certain mydriatics. J Pharm Sci 59:1559–63
Grass GM, Robinson JR (1988) Mechanisms of corneal drug penetration I: in vivo and in vitro kinetics. J Pharm Sci 77:3–14
Keister JC, Cooper ER, Missel PJ et al (1991) Limits on ocular drug delivery. J Pharm Sci 80:50–3
Van Santvliet L, Ludwig A (2004) Determinants of eye drop size. Surv Ophthalmol 49:197–213
Lederer CM, Harold ME (1986) Drop size of commercial glaucoma medications. Am J Ophthalmol 101:691–4
Sorensen SJ, Abel SR (1994) Drop size of ocular carteolol hydrochloride. Am J Hosp Pharm 51:1470–3
Ludwig A, Van Ooteghem M (1987) The influence of the osmolality on the precorneal retention of ophthalmic solutions. J Pharm Belg 42:259–66
Suzuki M, Massingale ML, Ye F et al (2010) Tear osmolarity as a biomarker for dry eye disease severity. Invest Ophthalmol Vis Sci 51:4557–61
Ellis PP, Wu P-Y, Pfoff DS et al (1992) Effect of nasolacrimal occlusion on timolol concentrations in the aqueous humor of the human eye. J Pharm Sci 81:219–20
Chrai SS, Makoid MC, Eriksen SP et al (1974) Drop size and initial dosing frequency problems of topically applied drugs. J Pharm Sci 63:333–8
Anderson JA, Chen CC (1988) Multiple dosing increases the ocular bioavailability of topically administered flurbiprofen. Arch Ophthalmol 106:1107–9
Rahman MQ, Chuah KS, Macdonald ECA et al (2012) The effect of pH, dilution, and temperature on the viscosity of ocular lubricants -shift in rheological parameters and potential clinical significance. Eye 26:1579–84
Coles WH, Jaros PA (1984) Dynamics of ocular surface pH. Br J Ophthalmol 68:549–52
Carney LG, Mauger TF, Hill RM (1989) Buffering in human tears: pH responses to acid and base challenge. Invest Ophthalmol Vis Sci 30:747–54
Trolle-Lassen C (1959) Investigations into the sensitivity of the human eye to hypo- and hypertonic solutions as well as solutions with unphysiological hydrogen ion concentrations. Pharm Weekbl 93:148–55
Li M, Du CX, Zhu DX et al (2012) Daytime variations of tear osmolarity and tear meniscus volume. Eye Contact Lens 38:282–7
Gaffney EA, Tiffany JM, Yokoi N, Bron AJ (2010) A mass and solute balance model for tear volume and osmolarity in the normal and the dry eye. Prog Retin Eye Res 29:59–78
Baeyens V, Bron A, Baudouin C (2012) Efficacy of 0.18% hypotonic hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye disease. J Fr Ophthalmol 35:412–9
Riegelman S, Vaughan DG Jr, Okumoto M (1955) Compounding ophthalmic solutions. J Am Pharm Assn 16:742–6
Draize JH, Woodward G, Calvery HO (1944) Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther 82:377–90
Schrage A, Kolle SN, Moreno MCR et al (2011) The bovine corneal opacity and permeability test in routine ocular irritation testing and its improvement within the limits of OECD test guideline 437. ATLA-Altern Lab Anim 39:37–53
Engelke M, Zorn-Kruppa M, Gabel D et al (2013) A human hemi-corneal model for eye irritation testing: quality control of production, reliability and predictive capacity. Toxicol in Vitro 27:458–68
Salminen L (1990) Review: systemic absorption of topically applied ocular drugs in humans. J Ocul Pharmcol 6:243–9
Nadal J, de la FuenteV, Aradias M et al (1987) Toxic coma induced by anticholinergic eye drops. Br Med J 295:1352
Hanna C, Massey JY, Hendrikson RO et al (1978) Ocular penetration of topical chloramphenicol in humans. Arch Ophthalmol 96:1258–61
Fraunfelder FW, Fraunfelder FT (2013) Restricting topical ocular chloramphenicol eye drop use in the United States. Did we overreact? Am J Ophthalmol 156:420–22.66
Anonymous (1990) Codex der Augenarzneistoffe und Hilfsstoffe. In: Dolder R, Skinner FS (eds) Ophthalmika: Pharmakologie, Biopharmazie und Galenik der Augenarzneimittel. 4. Auflage. Wissenschaftliche Verlagsgesellschaft, Stuttgart
Zinksulfaatoogwassing 0.25% FNA. Jaar 2009. Formularium der Nederlandse Apothekers. Den Haag : Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie KNMP
Chloramphenicol-Augentropfen 0,5%, NRF 15.10. Fassung 2009. In: Deutscher Arzneimittel-Codex/Neues Rezeptur-Formularium (DAC/NRF). ABDA. Eschborn (D): Govi-Verlag Pharmazeutischer Verlag Eschborn. Deutscher Apotheker Verlag Stuttgart
Product information Miglyol ®, neutral oils for pharmaceuticals and cosmetic. (www.sasoltechdata.com)
Ölige Ciclosporin-Augentropfen 1%, NRF 15.21. Fassung 2007. In: Deutscher Arzneimittel-Codex/Neues Rezeptur-Formularium (DAC/NRF). ABDA. Eschborn (D): Govi-Verlag Pharmazeutischer Verlag Eschborn. Deutscher Apotheker Verlag Stuttgart
Irritatie bij pilocarpineoogdruppels. Pharm Weekbl. 2000;135:31
Chowdan M, Lang JC, Missel O (2013) Ophthalmic preparations. Section 5: Pharmaceutical dosage forms: manufacturing and compounding, In: Allen LV (ed) Remington: the science and practice of pharmacy 22nd edn. Pharmaceutical Press. London – Philadelphia. p 920
Horikx A (2005) WINap. Boorzuur in oogdruppels ook beneden 3 jaar. Pharm Weekbl 140:182
Proosdij- van Hartzema EG (1966) Boriumverbindingen. Verleden heden en toekomst. Ned Tijdsch Geneesk 110:2260–2269
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Questions and answers on the use of phosphates in eye drops, 2012. www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2012/12/WC500136247.pdf. Accessed 1 July 2014
Schuller WO, Young WH, Hill RM (1972) Clinical measurements of the tears: viscosity. J Am Optom Assoc 43:1358–61
Ludwig A (2005) The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev 57:1595–639
Agrawal AK, Das M, Jain S (2012) In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin Drug Deliv 9:383–402
Rupenthal ID, Green CR, Alany RG (2011) Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 2: precorneal retention and in vivo pharmacodynamic study. Int J Pharm 411:78–85
Zigani M, Tabatabay C, Gurny R (1995) Topical semi-solid drug delivery: kinetics and tolerance of ophthalmic hydrogels. Adv Drug Deliv Rev 16:51–60
Jiao J (2008) Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv Drug Deliv Rev 60:1663–73
Tromp THFJ, Dankert J, De Rooy S et al (1976) De conservering van oogdruppels III. Een onderzoek naar de interactie van hydroxypropylmethylcellulose en benzalkoniumchloride. Pharm Weekbl 111:561–9
Tetracainhydrochlorid-Augentropfen 0,5% / 1% pH 6,5, NRF 15.12. Fassung 2013. In: Deutscher Arzneimittel-Codex/Neues Rezeptur-Formularium (DAC/NRF). ABDA. Eschborn (D): Govi-Verlag Pharmazeutischer Verlag Eschborn. Deutscher Apotheker Verlag Stuttgart
Neutrale Indometacin-Augentropfen 0,1% / Neutrale Indometacin-Augentropfen 0,1% ohne Konservierung, NRF 15.21. Fassung 2009. In: Deutscher Arzneimittel-Codex/Neues Rezeptur-Formularium (DAC/NRF). ABDA. Eschborn (D): Govi-Verlag Pharmazeutischer Verlag Eschborn. Deutscher Apotheker Verlag Stuttgart
Bullock JD (2008) Temperature instability of ReNu with MoistureLoc. A new theory to explain the worldwide Fusarium keratitis epidemic of 2004-2006. Arch Ophthalmol 126:1493–8
Behlau I, Gilmore MS (2008) Microbial biofilms in ophthalmology and infectious disease. Arch Ophthalmol 126:1572–81
Liu Y, Pinzon-Arango PA, Strauss J et al (2009) Fundamentals of bacterial adhesion applied toward infection prevention: focus on two case studies. Pharm Eng 29:56–66
Furrer P, Mayer JM, Gurny R (2002) Ocular tolerance of preservatives and alternatives. Eur J Pharm Biopharm 53:263–80
Martone G, Frezotti P, Tosi GM et al (2009) An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservatives on corneal innervation and morphology. Am J Ophthalmol 147:725–35
Rouland JF, Traverso CE, Stalmans I et al (2013) Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol 97:196–200
Madhu C, Rix PJ, Shackleton MJ et al (1996) Effect of benzalkonium chloride/EDTA on the ocular bioavailability of ketorolac tromethamine following ocular instillation to normal and de-epithelialized corneas of rabbits. J Pharm Sci 85:415–8
Kikuchi T, Suzuki M, Kusai A et al (2005) Synergistic effect of EDTA and boric acid on corneal penetration of CS-088. Int J Pharm 290:83–9
Baudouin C, Labbé A, Liang H et al (2010) Preservatives in eyedrops: the good, the bad and the ugly. Progr Retin Eye Res 29:312–34
Huber- van der Velden KK, Thieme H, Eichhorn M (2012) Morphologische Veränderungen durch Konservierungsmittel in Augentropfen. Ophthalmologe 109:1077–1081
Brignole-Baudouin F, Desbenoit N, Hamm G et al (2012) A new safety concern for glaucoma treatment demonstrated by mass spectrometry imaging of benzalkonium chloride distribution in the eye, an experimental study in rabbits. Plos One 7(11):e50180, doi:10.1371/journal.pone.0050180
Sarkar J, Chaudhary S, Namavari A et al (2012) Corneal neurotoxicity due to topical benzalkonium chloride. Invest Ophthalmol Vis Sci 53:1792–1802
Rolando M, Crider JY, Kahook MY (2011) Ophthalmic preservatives: focus on polyquaternium-1. Expert Opinion Drug Deliv 8:1425–38
Liang H, Brignole-Baudouin F, Riancho L, Baudouin C (2012) Reduced in vivo ocular toxicity with Polyquad-preserved travoprost versus benzalkonium chloride-preserved travoprost or latanoprost ophthalmic solutions. Ophthalmic Res 48:89–101
Aihara M, Oshima H, Araie M (2013) Effects of SofZia – preserved travoprost and benzalkonium chloride-preserved latanoprost on the ocular surface – a multicentre randomized single-masked study. Acta Ophthalmol 91:e7–e14
Anwar Z, Wellik SR, Galor A (2013) Glaucoma therapy and ocular surface disease: current literature and recommendations. Curr Opin Ophthalmol 24:136–43
Boer Y (1981) Irritation by eye drops containing 2-phenylethanol. Pharm Weekbl Sci Ed 3:122–3
Tan M, Parkin JE (2000) Route of decomposition of thiomersal (thimerosal). Int J Pharm 208:23–34
van t’Veen AJ, van Joost Th (1996) Bron en praktische betekenis van allergie voor thiomersal, een organische kwikverbinding. Ned Tijdschr Geneeskd 140:297–300
Winder AF, Sheraidah GAK, Astbury NJ et al (1980) Penetration of mercury from ophthalmic preservatives into the human eye. Lancet 2:237–9
Boer Y, Nijland CJ (1981) Conserveermiddelen en oogdruppelverpakking. Pharm Weekbl 116:371–4
Boer Y, Nijland CJ (1981) Sorptie van conserveermiddelen door membraanfilters bij de bereiding van oogdruppels. Pharm Weekbl 116:374–6
Stevens JD, Matheson MM (1992) Survey of the contamination of eyedrops of hospital inpatients and recommendations for the changing of current practice of in eyedrop dispensing. Br J Ophthalmol 76:36–8
Geyer O, Bottone EJ, Podos SM et al (1995) Microbial contamination of medications used to treat glaucoma. Br J Ophthalmol 79:376–9
Wilson LA (1996) To preserve or not to preserve, is that the question? Br J Ophthalmol 80:583–4
Panday PVN, van der Heiden J, Dillingh J et al (2007) Microbiologische validatie van de oogdruppelflacon met snap-cap: de casus acetylcysteineoogdruppels 5%. Pharm Weekbl Wetenschappelijk Platform 1:16–21
Povidon-Iod-Augenbad 1,25%, NRF 15.27. Fassung 20014/1. In: Deutscher Arzneimittel-Codex/Neues Rezeptur-Formularium (DAC/NRF). ABDA. Eschborn (D): Govi-Verlag Pharmazeutischer Verlag Eschborn. Deutscher Apotheker Verlag Stuttgart
Pitz S, Haber M, Pfeiffer N (1998) Beobachtungen über den pH-Wert verschiedener Tränenersatzmittel. Klin Monatsbl Augenheilkd 213:123–4
Schrage NF, Struck HG, Gerard M (2011) Empfehlungen zur Akutbehandlung von Verätzungen und Verbrennungen der Augen und Lider. Ophthalmologe 108:916–20
Goldich Y, Barkana Y, Zadok D et al (2013) Use of amphoteric rinsing solution for the treatment of ocular tissues exposed to nitrogen mustard. Acta Ophthalmol 91:e35–e40
Edetaatoogwassing 2% FNA. Jaar 2009. Formularium der Nederlandse Apothekers. Den Haag: Koninklijke Nederlandse Maatschappij ter bevordering van de Pharmacie KNMP
Erythromycine-oogzalf 0.5% FNA. Jaar 2009. Formularium der Nederlandse Apothekers. Den Haag: Koninklijke Nederlandse Maatschappij ter bevordering van de Pharmacie KNMP
Natriumchloride-oogzalf 5% FNA. Jaar 2009. Formularium der Nederlandse Apothekers. Den Haag: Koninklijke Nederlandse Maatschappij ter bevordering van de Pharmacie KNMP
Chlooramfenicol-oogzalf 1% FNA. Jaar 2014. Formularium der Nederlandse Apothekers. Den Haag: Koninklijke Nederlandse Maatschappij ter bevordering van de Pharmacie KNMP
Oogzalfbasis. Jaar 2012. Formularium der Nederlandse Apothekers. Den Haag: Koninklijke Nederlandse Maatschappij ter bevordering van de Pharmacie KNMP
Emulgierende Augensalbe DAC. Fassung 2014/1. In: Deutscher Arzneimittel-Codex/Neues Rezeptur-Formularium (DAC/NRF). ABDA. Eschborn (D): Govi-Verlag Pharmazeutischer Verlag Eschborn. Deutscher Apotheker Verlag Stuttgart
Hiraoka T, Yamamoto T, Okamoto F, Oshika T (2012) Time course of changes in ocular wave front aberration after administration of eye ointment. Eye 26:1310–7
American Society of Hospital Pharmacists (1993) ASHP technical assistance bulletin on pharmacy prepared ophthalmic products. Am J Hosp Pharm 50:1462–1463
Al-Badriyeh D, Li J, Stewart K et al (2009) Stability of extemporaneously prepared voriconazole ophthalmic solution. Am J Health-Syst Pharm 66:1478–83
Behrens-Baumann W (2009) Keratomycosis: diagnosis and therapy. Ophthalmologe 106:471–81
Dupuis A, Tournier N, Le Moal G, Venisse N (2009) Preparation and stability of voriconazole eye drop solution. Antimicrob Agents Chemother 53:798–9
Maas B, Krämer I, Simon K, Hoppe-Tichy T (1996) Mitomycin-C-Zubereitungen für die Anwendung am Auge. Krankenhauspharmazie 17:396–7
Daniels R (2010) Herstellung von Ophthalmika in der Apotheke. Pharm Unserer Zt 39:306–11
Wagenaar R (2006) Tetracycline oogzalf: conflicterende eisen. Pharm Weekbl 141:956
Romijn M (2006) Een betere oplossing. Tetracycline oogzalf: conflicterende eisen. Pharm Weekbl 141:1019
Fraunfelder FT (1976) Extraocular fluid dynamics: how best to apply topical medication. Trans Am Ophthalmol Soc 74:457–87
van Drooge MJ, de Smet PAGM (2000) Hoe glaucoompatiënten oogdruppels gebruiken. Instructieprotocol onderzocht in proefproject. Pharm Weekbl 135:1016–20
Dua HS (1998) Bacterial keratitis in the critically ill and comatose patient. Lancet 351:387–8
Nordmann JP, Baudouin C, Bron A (2009) Xal-Ease®: impact of an ocular hypotensive delivery device on ease of eyedrop administration, patient compliance, and satisfaction. Eur J Ophthalmol 19:949–56
Burns E, Mulley GP (1992) Practical problems with eye-drops among elderly ophthalmology outpatients. Age Ageing 21:168–70
Smith SE (1991) Eyedrop instillation for reluctant children. Br J Ophthalmol 75:480–1
Parkkari M, Latvala T, Ropo A (2010) Handling test of eye drop dispenser – comparison of unit-dose pipettes with conventional eye drop bottles. J Ocul Pharmacol Ther 26:273–6
Connor AJ, Severn PS (2011) Force requirements in topical medicine use- the squeezability factor. Eye 25:466–9
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 KNMP and Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ludwig, A., Reimann, H. (2015). Eye. In: Bouwman-Boer, Y., Fenton-May, V., Le Brun, P. (eds) Practical Pharmaceutics. Springer, Cham. https://doi.org/10.1007/978-3-319-15814-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-15814-3_10
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15813-6
Online ISBN: 978-3-319-15814-3
eBook Packages: MedicineMedicine (R0)