Abstract
Kesner’s attribute model of memory endows the hippocampus with the ability to code both time and space. These two parameters are intertwined in their very essence and lend structure to the ongoing autobiographical record of an organism. Kesner’s addition of time and temporal processing to the notion that the hippocampus supports a spatial cognitive map, fused hippocampal theory into a coherent framework for human and non-human animals. The mechanism by which the hippocampus and its associated circuitry supports memory for time is a fertile area of research that was seeded by Kesner and his contemporaries. The inherent physiological properties of the hippocampus support Kesner’s original hypothesis, emphasizing that temporal and spatial inputs converge in the hippocampus. The temporal scale of this convergence is evident from patterns of neuronal firing to enduring memories.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
The “Tapestry of Memory” (Fig. 3.1)
What is the basic constituent of a memory? What is lost when we say that we have (alas) forgotten? (Underwood 1977)
This image shows the dentate gyrus of the hippocampus, a region of frequent discussion in this chapter. This photomicrograph was taken from a “Brainbow” transgenic mouse that allows distinction between neighboring neurons through color. (Photograph: J Livet (now Institut de la Vision, Paris), J W Lichtman, and J R Sanes (Harvard University))
Benton Underwood’s (1969) notion of memory asserted that memory was composed of many attributes, or different types of information. Building upon this idea, Kesner first proposed (1980) that all memories are composed of a set of six salient features or attributes: space, time, affect, sensory perception, response, and language (in humans). Each experience would incorporate a specific and unique combination of attributes, and would be supported by neural processes. This attribute-based model of memory greatly advanced memory research in two important ways. First, the model defined memory as a distributed neural process. This definition asserted that memory, by necessity, could not be accomplished by a single brain region, but would instead require the integration of multiple memory systems thought, traditionally, to act independently. Investigations of this multidimensional model of memory from a neurobiological approach would, therefore, rely heavily on both the anatomy of individual brain regions for examining their individual contributions to memory, and upon the connections between regions for their cooperative function at a systems level. Second, in theory, the inclusion of multiple attributes would increase the dimensionality of a given experience, thus capturing the brain’s computational ability to increase similarity or reduce interference between multiple experiences. The incorporation of multidimensional information processing into the function of a given brain region would dramatically affect computational models and experimental tests of memory processes, particularly in the hippocampus.
A primary assertion of the attribute-based model of memory is that functional circuits of the brain support attributes. In order for an organism to represent memory for an attribute, incoming sensory information must be encoded and temporarily stored within a neural system. In representing a spatial attribute, for example, the encoding and temporary storage of specific stimuli representing spatial locations, directions, and distances, which may or may not be independent of the subject’s own body schema, must occur. A temporal attribute represents the occurrence of an episode in time, separating the episode from past or future episodes, as well as coding the duration of the episode.
The inclusion of both sensory perception and motoric (i.e., response) functions as essential to memory processes places this active account of memory within the realm of modern embodied cognition, requiring the intrinsic and positional state of an organism to be part of the initial processing of memory. Within this realm, the attribute of affect can be experiential or retrospective in that it involves the encoding and temporary storage of reinforcement contingencies that result in positive or negative emotional experiences in the visceral sense, which could subsequently be categorized. The interaction between memory for individual attributes, as a function of the activity of various neurobiological regions and their processes, combine to represent a unique memory.
Additionally, the attribute model accounts for differential processing of information by incorporating interconnected memory systems. For example, a data or an event-based memory system that emphasizes encoding of incoming information, combined with an expectancy or knowledge-based system that emphasizes top-down processing, allow for fluid use of previous knowledge in interpreting incoming information. Kesner emphasizes (Kesner 1990) that most situations require multiple such memory systems with disproportionate involvement of a system or two at any particular time. The theory is deeply rooted in the anatomy of the system, with an early understanding that the connectivity maps of the brain (an early embrace of the basis of modern “connectomics”) are absolutely essential to the patterns of neural activity and the content of the ultimate recollection. Taking this perspective, memory is labile from the outset, and memories rely on the timing and availability of activation at the moment of recollection. Remember a time, for example, when you recalled an event (perhaps, a conversation with someone), without remembering when it occurred and you proceeded to reconstruct the context in order to remember the time of occurrence. This exemplifies the way in which the availability of a particular attribute can lead to an aggregation of the memory. From the perspective of memory processes, this can also be the point at which interference is reduced and the memory is effectively separated from other similar memories. Thus, memory is a multidimensional, distributed process.
The Functional Anatomy of Spatial and Temporal Memory Attributes
Whereas the theory concludes that behavioral or psychological processes are supported by brain function, the mapping of structure to function has taken an important turn towards a processing account of memory. Such an account acknowledges the important fact that the way in which behavioral or psychological functions are supported is reliant on a principled account of a brain that bears no obligation to function according to the psychological labels that are imposed on it. As such, a careful parsing of the computational processes subserved by the neural architecture is explored with respect to their ultimate role in mediating behavioral function (Kesner and Rolls 2001; Rolls and Kesner 2006).
Reducing Interference by Separating Attributes
The architecture of the hippocampus both constrains and allows for the separating or linking of specific types of information in the service of memory. For example, original computational models describing the hippocampal circuit endowed the dentate gyrus (DG) with the ability to pattern separate (McClelland et al. 1995; O’Reilly and McClelland 1994; Rolls 2010; Treves and Rolls 1992). The idea of pattern separation addresses the requirement that there must be a mechanism to reduce interference of input patterns in order to form separate representations that will be transmitted to downstream targets. Pattern separation in such networks is based on the notion of orthogonalization. The DG in the rat has approximately 1 million neurons. It has more principal cells than the upstream entorhinal cortex (EC) and downstream CA3 combined (van Strien et al. 2009). A network with the anatomical properties contained in the EC–DG circuit is ideally situated to achieve highly disparate (non-overlapping) outputs, as the number of nodes in the DG network is orders of magnitude higher than the number of input nodes, thus allowing for a sparse and independent representation of overlapping inputs. If not true orthogonalization, the anatomy of the EC–DG circuit suggests that the dentate would at least act as a sparsifying network that encodes inputs in a non-distributed manner. The encoding of an experience containing multiple attributes would, thus, create a highly unique pattern of activity in DG, with each attribute acting as an additional means to separate or reduce interference of the memory from other experiences. In support of this hypothesis, experimental evidence has shown that DG neurons create more distinct representations of experiences at the single cell and population level than other subregions of the hippocampus (Deng et al. 2013; Leutgeb et al. 2007; Neunuebel and Knierim 2014; Rangel and Eichenbaum 2013).
Reinstating Memories from Linked Attributes
Early models also proposed that through Hebbian learning, or repeated experience, disparate attributes could be linked together in the CA3 region of the hippocampus such that incomplete features of a memory could reinstate the full original experience, a process called pattern completion (McClelland et al. 1995; O’Reilly and McClelland 1994). Specifically, repeated experience would strengthen the synaptic connections among activated neurons within CA3 through a long-term potentiation (LTP)-like mechanism, and partial or noisy activation would utilize CA3 recurrent collaterals to recruit linked neurons. The encoding of multiple attributes in this system therefore provides more avenues from which to reconstruct existing links or associations. Thus, attributes make memories more distinct in one hippocampal subregion, and more similar through acquired associations in another (Fig. 3.2).
Space and Time in Context
Space and Place Cells
The Kesner attribute model proposes that the rich architecture of the hippocampus supports the ongoing processing of space and time (Kesner et al. 1989). The contemporary accounts of the function of the hippocampus were entrenched in the powerful discovery of “place-cells” by O’Keefe and Dostrovsky (O’Keefe and Dostrovsky 1971) and the proposition that the hippocampus was the locus of the spatial “Cognitive Map,” or our innate knowledge of space (O’Keefe and Nadel 1978). Spatial cognition in this case refers to a perception of the external world that is readily available and usable in every organism. The process of cognitive mapping, theoretically achieved by the hippocampus, can be described as:
… a construct that encompasses those processes that enable people to acquire, code, store, recall, and manipulate information about the nature of their spatial environment. It refers to the attributes and relative locations of people and objects in the environment, and is an essential component in the adaptive process of spatial decision-making such as finding a safe and quick route to and from work, locating potential sites for a new house or business, and deciding where to travel on a vacation trip. Cognitive processes are not constant, but undergo change with age or development and use or learning. (O’Keefe and Nadel 1978)
If spatial cognition relies upon a cognitive map that is an innate ability in all organisms, then spatial information should be available in the hippocampus during novel exposures to spatial environments and prior to any learning. Indeed, single cells in the hippocampus demonstrate spatially specific activity in the form of place fields during even the first few minutes of novel exposures to an environment (Kentros et al. 1998). These cells additionally demonstrate large coverage of spatial environments at predictable spatial resolution along the septo-temporal axis (Kjelstrup et al. 2008). The hippocampus, thus, has the means to provide a spatial construct at the single cell level.
Place cell activity over the course of familiarity with a new environment is additionally reflective of increasing perception of space. A large body of experimental evidence suggests that stable place fields are highly contingent upon experience. Although these cells demonstrate place specific activity immediately, they remain unstable and flexible during initial encounters with an environment before demonstrating stable fields (Bostock et al. 1991; Frank et al. 2006; Kentros et al. 2004; Rowland et al. 2011). As further indication that the activity of these cells is linked to spatial perception, spatially specific activity of these cells is closely associated with animal movement and perceived location, rather than absolute allocentric location. Specifically, the firing rate of place cells as an animal travels through its place field can be heavily modulated by speed, direction, and trajectory (Frank et al. 2000; McNaughton et al. 1983). Moreover, rotations of spatial cues surrounding an environment cause predictable shifts in place field location relative to the degree of cue rotation (Lenck-Santini et al. 2005; Poucet et al. 2000). Taken together, these findings suggest that place cells not only provide an internal representation of a spatial environment, but also encode these features in a behaviorally meaningful manner.
If place cells enable an internal spatial representation of the world, then testing the extent to which spatial firing properties of place cells account for learned features of an environment and changing behavioral conditions can help determine their ultimate contribution to learning and behavior. Experimental evidence has demonstrated that even cells with stable place fields can demonstrate changes in firing rate or location when fields are in close proximity to changing components of an environment (Lenck-Santini et al. 2005; Rivard et al. 2004). Moreover, their long-term stability has been correlated with spatial learning performance, and their instability in aging is correlated with a decline in spatial learning ability (Kentros et al. 2004; Shen et al. 1997). Thus, in addition to providing a flexible spatial representation of an environment, in a way that is behaviorally meaningful to an organism, these place cells may be utilized and perhaps required for specific types of spatial learning.
Lesions of the hippocampus result in a long-term inability to encode episodic memories, or the conscious knowledge of specific personal experience (Milner et al. 1998; Rosenbaum et al. 2000, 2005; Tulving 2002). Rats and humans with hippocampal lesions or inactivations maintain an ability to navigate novel environments and perform spatial learning tasks over time. In rats, lesions of the hippocampus impair performance in spatial learning tasks (Morris et al. 1990; Olton and Papas 1979; Olton et al. 1978). Over a significantly longer period of time, however, successful performance in these tasks can be achieved, suggesting secondary mechanisms for forming spatial associations with additional knowledge (DiMattia and Kesner 1988). This is consistent with other studies demonstrating that the behavior of rats with hippocampal lesions reflects maintained perceptual learning of their spatial environment (Jackson-Smith et al. 1993). Moreover, rats with fimbria/fornix lesions that demonstrate spatial learning impairment, still exhibit the presence of place cell activity, suggesting that place cells alone are insufficient for spatial knowledge (Whishaw et al. 1995). In clinical research, humans with lesions of the hippocampus maintain sufficient spatial orientation to demonstrate an ability to navigate their current living environments (Milner et al. 1968). These results suggest that in the absence of a hippocampus, an internal spatial map is available for use, and according to the attribute model this is likely supported by the parietal cortex (Chiba et al. 2002). It is thus possible that the hippocampus is not necessary for all spatial processing per se, but rather the knowledge of the map’s appropriate utility.
Yet, it is clear that humans and rodents without a hippocampus lack an explicit perception of changing environmental conditions. Whereas in the rat it is difficult to claim the presence of conscious spatial knowledge, previous research has used the ability to generate decisions (i.e., “declare”) based on appropriate knowledge of spatial learning contingencies as evidence of declarative memory (DeCoteau and Kesner 2000). Even though rats with hippocampal lesions can perform spatial learning tasks over long periods of time, they demonstrate inflexibility in their ability to adapt to changing spatial conditions (Jacobson et al. 2011). This is in high contrast to control rats, which instead demonstrate faster learning and adaptation to changing environmental conditions with increased experience (Tse et al. 2007). Humans with hippocampal lesions demonstrate a similar inflexibility in being able to update their perception of changing environmental conditions. This is coupled by an inability to consciously recall the utility of their current spatial surroundings (Milner et al. 1968).
Thus, in both rats and humans, the ability to assess the appropriate utility of a map is impoverished without a hippocampus, despite the availability of spatial knowledge in other areas of the brain. Indeed, cells in other areas of the brain, such as parietal cortex, demonstrate place specific firing in a manner analogous to place cells in the hippocampus but with additional properties allowing knowledge of spatial routes and position (Nitz 2009), that may account for the maintenance of spatial perception following hippocampal lesions (Chiba et al. 2002). Here, both systems are privy to the spatial code of the EC (Leutgeb et al. 2005), providing a map of the local spatial topography of the environment.
It has been hypothesized that the function of the hippocampal spatial map is to serve as a lattice for memory, on which episodes can be superimposed (Burgess et al. 2002; de Pontes et al. 2005). Kesner’s attribute model set forth the convergence of the spatiotemporal code as the defining feature of an episode. This view was influenced by Milner’s (Milner and Penfield 1956) early work with HM and exemplified his foresight in developing a model that could account both for the human amnestic syndrome (the inability to code new memories in both space and time) that arises from hippocampal damage, and the obligatory spatial code of the hippocampal architecture. Other current theories of the hippocampus suggest that the hippocampus creates relationships between important features across experiences and is thus essential for both spatial and nonspatial, or relational memory (Cohen et al. 1997). Kesner’s model specifically endows the CA3 “autoassociative network” with the capacity to form arbitrary or relational associations with the space (Kesner 2013). Both viewpoints assert that spatial encoding does not exist in isolation from the encoding of other attributes and that memories include the relationships between space and other dimensions that together compose rich contextual knowledge.
Time and Time Cells
The concept that time serves as an organizing principle for memory is age-old but not antiquated. Aristotle established, the “principle of contiguity” as one of his “Laws of Association” based on the general finding that recall of an item is facilitated by the presentation or recall of another item that occurred close in time to the target item (Aristotle and Barnes 1984). The role of temporal context in memory has since been extensively studied. After writing “Attributes of Memory,” Underwood elaborated on the role of temporal context and order in his book, “Temporal Codes for Memory.” There, he too emphasized that those items occurring in close temporal proximity were more likely to be conjoined whereas those occurring with greater temporal distance were more likely to be distinguishable. Shortly thereafter, Kesner designed a variety of tasks that paralleled contemporary human experiments, such as list-learning experiments, for use with rats. He demonstrated that serial position effects were constant across species and that both retroactive and proactive interference were present in rat models of list learning in which different places or maze arms represented the elements in the list (Kesner and Novak 1982; Kametani and Kesner 1989; Kesner et al. 1989). Since then, many studies have utilized the rat model organism to further investigate the neural substrates and underlying mechanisms of temporal order deficits and the encoding of temporal sequences (Allen et al. 2014; Howard et al. 2005; Howard and Kahana 2002). The creation of associations between temporally proximal events contributes greatly to episodic memory formation in humans. Specifically, when subjects bias their retrieval strategy to rely on temporal associations, they perform better on episodic recall tasks (Sederberg et al. 2010).
The role of the hippocampus in time and temporal order is likely to be fundamental to the role of the hippocampus in coding episodes that are essential to an individual’s ongoing autobiography. The role of the hippocampus with respect to time is complex and occurs in a series of nested timescales from the short duration of spike timing within oscillatory neural circuits to the construal of time with respect to place and context. With respect to memory, time has been studied regarding the basic substrates of neuronal coding (firing rates, spike-time dependent plasticity, and rhythmic oscillatory activity), the sequential order of events (including succession, temporal order, and relative recency), and memory for the duration of events including memory for intervals or time periods between events (Jackson-Smith et al. 1993; Pastalkova et al. 2008). Just as spatial memories require organized associations between spatial features of an environment, the encoding of events in time also requires a temporal organization that can account for similar and distinct temporal features. Experimental evidence suggests that single cells in the hippocampus can demonstrate reliable, and temporally selective, sequential activity during a given length of time in a manner similar to place cell activity for distinct spatial locations over a given spatial environment (MacDonald et al. 2011; Munn and Bilkey 2011). These cells, labeled time cells, are some of the first evidence to suggest that the hippocampus may have a temporal organization for episodes that is very similar to its characterized mechanism for spatial organization of environments. More importantly, these findings are evidence for single cell encoding of a temporal dimension or attribute.
Conjoining Space and Time
To better understand why brain regions such as the hippocampus would need to encode temporal as well as spatial features into memory, it is important to know that space and time are linked and inseparable in nature. In fact, the physical dimensions of space and time are often considered together and referred to as spacetime, whereby they do not have separate existences. In extreme cases such as the realm of relativistic physics, this can mean that events occurring billions of years ago at the farthest reaches of the universe can occur simultaneously with your thoughts as you look up at the light from that event shining in the earth’s sky. In our everyday lives, it can mean that the changes we observe in places we have known since childhood are measured by, and are indicators of the passage of time. In the latter case, which refers to our own egocentric view of the universe, we realize that memories of places (“where”) from our childhood are dependent upon an inseparable temporal (“when”) component. Since space and time are inseparable in nature, it should be tested whether the classical separation of space and time in the brain and in the encoding of memories is an artificial division. Although place and time cells provide a mechanism through which spatial and temporal associations can be made along two separate dimensions, it remains to be tested how associations are encoded across these dimensions. How are spatial features of memory linked to events in time? The unity of these dimensions could be accomplished by place cells dependent upon temporal features or time cells with spatial contingencies. To this end, it has been demonstrated that the temporal organization of place cell activity with respect to the phase of theta (4–12 Hz) frequency oscillations in the hippocampus is related to a rat’s movement through space, a phenomenon that has been termed phase precession (Dragoi and Buzsáki 2006; Skaggs et al. 1996; Tsodyks et al. 1996). This phenomenon is observed in each of the subregions of the hippocampus. Additionally, sharp wave ripple events in CA3 and CA1 in the hippocampus elicit activity resembling the sequential firing of place cells during task behavior, providing evidence for the encoding of temporal order for spatial experiences by the network (Davidson et al. 2009; Gupta et al. 2010; Jadhav et al. 2012). Both phenomena are promising evidence that the hippocampus represents both spatial and temporal information together at short-time scales.
Conjoining Space and Time in the DG
A subregion within the hippocampal formation, the DG, has been hypothesized to combine both spatial and temporal dimensions at the single cell level (Aimone et al. 2009). The DG is the only subregion of the hippocampus that demonstrates neurogenesis, or the continuous birth of new neurons, throughout adulthood. Adult-born neurons are born in the subgranular zone of the DG granule cell layer and demonstrate a characteristic development before becoming mature functional granule cells. Importantly, immature adult-born neurons exhibit a transient period of both intrinsic and synaptic hyperexcitability that is due to low membrane capacitance and less synaptic inhibition, respectively (Esposito et al. 2005; Laplagne et al. 2006, 2007; Piatti et al. 2006). This transient physiological difference between mature and immature granule cells may yield a unique role for adult-born cells in temporal encoding. Computational models demonstrate that temporally proximal events occurring within the transient period of hyperexcitability for a set of adult-born neurons elicit activity from common immature cells in an otherwise sparse firing mature network (Aimone et al. 2006). There then exists a similarity in DG output for temporally proximal events that does not exist for events separated in time, a temporal pattern integration, that is the direct result of adult-born neuron physiology during development (Aimone et al. 2006, 2009). This temporal pattern integration can then ultimately link disparate features in the spatial dimension through close proximity along the temporal dimension.
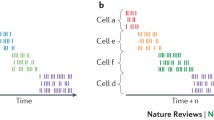
The transient period of hyperexcitability in adult-born neuron development is also a critical period for regulation of their survival and activity. Although a majority of adult-born cells die before becoming mature granule cells, exposure to learning paradigms or enriching environments during this critical period can greatly enhance their survival and bias their activity toward input received during their development (Aimone et al. 2006; Tashiro et al. 2007). This has led to the prediction that surviving adult-born neurons provide dedicated and selective activity to temporally proximal events during their development and thus can create new outputs from the dentate that are temporally distinct. Adult neurogenesis in the DG can therefore provide a mechanism for an additional type of pattern separation, a temporal pattern separation, through the continuous contribution of new temporal dimensions to distinguish between similar events separated in time (Aimone et al. 2011). Recent behavioral studies have demonstrated that the ability to make accurate spatiotemporal order judgments relies on the integrity of the DG. In fact, after lesions of the DG or the selective elimination of postnatal neurogenesis, rats cannot disambiguate the order of presentation of two spatial locations that were visited contiguously (Kesner et al. 2014; Morris et al. 2013). At the single cell level, one would predict that place cells in DG separate similar or identical spatial locations that are far apart along the temporal dimension by exhibiting spatial activity that is dependent upon time. To this end, it was shown that single cells, and even place cells in the DG of the hippocampus, demonstrate activity that is temporally selective (Rangel et al. 2014). Specifically, temporal separation between different experiences created a more distinct population code for each experience than experiences with no temporal separation, and manipulations to reduce levels of adult neurogenesis increased the similarity of responses to the different experiences. Cells in the DG thus support the integration of both spatial and temporal information through activity that is selective to both space and time, revealing the relationship between these dimensions by encoding experiences as distinct events in spacetime (Fig. 3.3).
Separating exposure to three different behavioral contexts during training (a circular track, an open-field cheeseboard, and a square foraging pot), resulted in place cells with activity selective for only one of three contexts during test exposures to all three contexts in the same day. This temporal selectivity was reduced in groups with shorter temporal separations between contexts and in groups with decreased levels of adult neurogenesis. (Taken from Rangel et al. 2014)
A Multidimensional Hippocampus
In addition to the DG, the integration of space and time has recently been shown to exist in the CA1 region of the hippocampus. Populations of CA1 place cells, but not CA3 place cells, demonstrate different patterns of activity across days with increasing temporal intervals between cell recordings (Mankin et al. 2012). This implies that subregions of the hippocampus may integrate time and space according to different timescales. It remains an open question, however, how the spatiotemporal coding observed in DG contributes to or interacts with the spatiotemporal coding observed in downstream CA1. The encoding of both spatial and temporal dimensions at the single cell level in the DG and downstream hippocampal subregions can provide a mechanism for a more complete theory of how the hippocampus accomplishes associations between complex features of memories. In relativistic physics, spacetime describes everything in the universe as events that occur in space and time. The utility of combining these dimensions is that highly disparate locations in the universe can be linked in time, and highly overlapping locations can become more distinct in time. In other words, spacetime has the ability to reveal the relationship of events along both dimensions. In the DG, the combination of these two dimensions means that associations can be made between spatial and temporal features of events. Multidimensional activity in the hippocampus may thus be a mechanism through which the hippocampus accounts for relationships across spatial and nonspatial features of memories.
The hippocampus is thus more than a cognitive map, and more equivalent to a multidimensional terrain well suited for the demands of the attribute-based approach to memory. The large advantage of this multidimensional view is that it removes the hippocampus from the constraint of encoding complex features of memories along a single dimension. Instead, single cells are given the ability to reveal the relationships across spatial, temporal, and perhaps other dimensions. By acknowledging that these dimensions exist in the hippocampus, we can begin to examine the exact dynamics of the relationships between these dimensions and determine the rules, if any, regarding how these relationships manifest themselves in the activity of single cells. As the attribute-based model of memory suggests, these studies support the idea that space is so integrally linked with other dimensions that it would be difficult and potentially unmeaningful to examine it as encoded separately in the brain.
Conclusion
Kesner’s attribute model theorizes that time and space are conjoined in the hippocampus and implicates the DG as essential to separating events that occur close in time. We further describe how the hippocampus, and more specifically the DG, may have the ability to create distinct spatial representations that also incorporate time, revealing an integrated spatiotemporal code. This code may be useful for segregating events that occur on long timescales. Here, spatiotemporal coding of contextual inputs may be accomplished through the continual generation of new neurons, which, due to their transient window of hyperexcitability and plasticity, allow for preferential encoding of information present during that temporal window. Thus, on a protracted timescale, the DG may act in large part as a sparsifying network, and temporally orthogonalize inputs, as computationally predicted (Aimone et al. 2009; Rangel et al. 2014).
By defining memory as composed of multiple complex features, the attribute model of memory first and foremost described memory as a systems level computation. The structures responsible for memory formation would need to have mechanisms for forming associations across features and for using different features to make memories distinct. This approach will continue to provide an inspirational framework for incorporating computational, behavioral, systems, cellular, and molecular level approaches towards investigating how rich contextual information aggregates to form our recollections.
Had there been an ageless observer at the sparkling moment of the creation of the egg—or of the hen—we would be no better off than we are today, for I am sure the observer would have soon forgotten which came first. Underwood 1977
References
Aimone, J. B., Wiles, J., & Gage, F. H. (2006). Potential role for adult neurogenesis in the encoding of time in new memories. Nature Neuroscience, 9(6), 723–727. doi:nn1707 [pii] 10.1038/nn1707.
Aimone, J. B., Wiles, J., & Gage, F. H. (2009). Computational influence of adult neurogenesis on memory encoding. Neuron, 61(2), 187–202. doi:S0896-6273(08)01019-2 [pii] 10.1016/j.neuron.2008.11.026.
Aimone, J. B., Deng, W., & Gage, F. H. (2011). Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70(4), 589–596. doi: 10.1016/j.neuron.2011.05.010.
Allen, T. A., Morris, A. M., Mattfeld, A. T., Stark, C. E. L., & Fortin, N. J. (2014). A Sequence of events model of episodic memory shows parallels in rats and humans. Hippocampus, 24, 1178–1188 doi:10.1002/hipo.22301.
Aristotle, & Barnes, J. (1984) The complete works of Aristotle: The revised Oxford translation (Bollingen Series LXXI-2) (Vol. 2). New Jersey: Princeton University Press.
Bostock, E., Muller, R. U., & Kubie, J. L. (1991). Experience-dependent modifications of hippocampal place cell firing. Hippocampus, 1(2), 193–205. doi:10.1002/hipo.450010207.
Burgess, N., Maguire, E. A., & O’Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron 35(4), 625–641.
Chiba, A. A., Kesner, R. P., & Jackson, P. A. (2002). Two forms of spatial memory: A double dissociation between the parietal cortex and the hippocampus in the rat. Behavioral Neuroscience, 116(5), 874–883.
Cohen, N. J., Poldrack, R. A., & Eichenbaum, H. (1997). Memory for items and memory for relations in the procedural/declarative memory framework. Memory (Hove, England), 5(1–2), 131–178. doi:10.1080/741941149.
Davidson, T. J., Kloosterman, F., & Wilson, M. A. (2009). Hippocampal replay of extended experience. Neuron, 63(4), 497–507. doi:10.1016/j.neuron.2009.07.027.
DeCoteau, W. E., & Kesner, R. P. (2000). A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behavioral Neuroscience, 114(6), 1096–1108.
Deng, W., Mayford, M., & Gage, F. H. (2013). Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife, 2, e00312. doi:10.7554/eLife.00312
de Pontes, J. C. A., Batista, A. M., Viana, R. L., & Lopes, S. R. (2005). Short-term memories with a stochastic perturbation. Chaos, Solitons & Fractals 23(5), 1689–1694.
DiMattia, B. D., & Kesner, R. P. (1988). Spatial cognitive maps: Differential role of parietal cortex and hippocampal formation. Behavioral Neuroscience, 102(4), 471–480.
Dragoi, G., & Buzsáki, G. (2006). Temporal encoding of place sequences by hippocampal cell assemblies. Neuron, 50(1), 145–157. doi:10.1016/j.neuron.2006.02.023.
Esposito, M. S., Piatti, V. C., Laplagne, D. A., Morgenstern, N. A., Ferrari, C. C., Pitossi, F. J., & Schinder, A. F. (2005). Neuronal differentiation in the adult hippocampus recapitulates embryonic development. The Journal of Neuroscience, 25(44), 10074–10086. doi:25/44/10074 [pii] 10.1523/JNEUROSCI.3114-05.2005.
Frank, L. M., Brown, E. N., & Wilson, M. (2000). Trajectory encoding in the hippocampus and entorhinal cortex. Neuron, 27(1), 169–178.
Frank, L. M., Brown, E. N., & Stanley, G. B. (2006). Hippocampal and cortical place cell plasticity: Implications for episodic memory. Hippocampus, 16(9), 775–784. doi:10.1002/hipo.20200.
Gupta, A. S., van der Meer, M. A. A., Touretzky, D. S., & Redish, A. D. (2010). Hippocampal replay is not a simple function of experience. Neuron, 65(5), 695–705. doi:10.1016/j.neuron.2010.01.034.
Howard, M. W., & Kahana, M. J. (2002). A distributed representation of temporal context. Journal of Mathematical Psychology, 46(3), 269–299. doi:10.1006/jmps.2001.1388.
Howard, M. W., Fotedar, M. S., Datey, A. V, & Hasselmo, M. E. (2005). The temporal context model in spatial navigation and relational learning: Toward a common explanation of medial temporal lobe function across domains. Psychological Review, 112(1), 75–116. doi:10.1037/0033-295X.112.1.75.
Jackson-Smith, P., Kesner, R. P., & Chiba, A. A. (1993). Continuous recognition of spatial and nonspatial stimuli in hippocampal-lesioned rats. Behavioral and Neural Biology, 59(2), 107–119.
Jacobson, T. K., Gruenbaum, B. F., & Markus, E. J. (2011). Extensive training and hippocampus or striatum lesions: Effect on place and response strategies. Physiology & Behavior, 105(3), 645–652. doi:10.1016/j.physbeh.2011.09.027.
Jadhav, S. P., Kemere, C., German, P. W., & Frank, L. M. (2012). Awake hippocampal sharp-wave ripples support spatial memory. Science (New York, N.Y.), 336(6087), 1454–1458. doi:10.1126/science.1217230.
Kametani, H., & Kesner, R. P. (1989). Retrospective and prospective coding of information: Dissociation of parietal cortex and hippocampal formation. Behavioral Neuroscience, 103, 84–89.
Kentros, C., Hargreaves, E., Hawkins, R. D., Kandel, E. R., Shapiro, M., & Muller, R. V. (1998). Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science (New York, N.Y.), 280(5372), 2121–2126.
Kentros, C. G., Agnihotri, N. T., Streater, S., Hawkins, R. D., & Kandel, E. R. (2004). Increased attention to spatial context increases both place field stability and spatial memory. Neuron, 42(2), 283–295.
Kesner, R. P. (1980). An attribute analysis of memory: The role of the hippocampus. Physiology Psychology, 8, 189–197.
Kesner, R. P. (1990). New approaches to the study of comparative cognition. NIDA Research Monograph, 97, 22–36 (http://www.ncbi.nlm.nih.gov/pubmed/2247138).
Kesner, R. P. (2013). A process analysis of the CA3 subregion of the hippocampus. Frontiers in Cellular Neuroscience, 7, 78. doi:10.3389/fncel.2013.00078.
Kesner, R. P., & Novak, J. (1982). Serial position curve in rats: Role of the dorsal hippocampus. Science, 218, 173–174.
Kesner, R. P., & Rolls, E. T. (2001). Role of long-term synaptic modification in short-term memory. Hippocampus, 11(3), 240–250. doi:10.1002/hipo.1040.
Kesner, R. P., Adelstein, T. B., & Crutcher, K. A. (1989). Equivalent spatial location memory deficits in rats with medial septum or hippocampal formation lesions and patients with dementia of the Alzheimer’s type. Brain and Cognition, 9(2), 289–300.
Kesner, R. P., Hui, X., Sommer, T., Wright, C., Barrera, V. R., Fanselow, M. S. (2014). The role of postnatal neurogenesis in supporting remote memory and spatial metric processing. Hippocampus, 24, 1663–1671. doi:10.1002/hipo.22346.
Kjelstrup, K. B., Solstad, T., Brun, V. H., Hafting, T., Leutgeb, S., Witter, M. P., et al. (2008). Finite scale of spatial representation in the hippocampus. Science, 321(5885), 140–143. doi:321/5885/140 [pii] 10.1126/science.1157086.
Laplagne, D. A., Esposito, M. S., Piatti, V. C., Morgenstern, N. A., Zhao, C., van Praag, H., et al. (2006). Functional convergence of neurons generated in the developing and adult hippocampus. Plos Biology, 4(12), e409. doi:06-PLBI-RA-0577R3 [pii] 10.1371/journal.pbio.0040409.
Laplagne, D. A., Kamienkowski, J. E., Esposito, M. S., Piatti, V. C., Zhao, C., Gage, F. H., & Schinder, A. F. (2007). Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. European Journal Neuroscience, 25(10), 2973–2981. doi:EJN5549 [pii] 10.1111/j.1460-9568.2007.05549.x.
Lenck-Santini, P.-P., Rivard, B., Muller, R. U., & Poucet, B. (2005). Study of CA1 place cell activity and exploratory behavior following spatial and nonspatial changes in the environment. Hippocampus, 15(3), 356–69. doi:10.1002/hipo.20060.
Leutgeb, S., Leutgeb, J. K., Moser, M. B., & Moser, E. I. (2005). Place cells, spatial maps and the population code for memory. Current Opinion Neurobiology, 15(6), 738–746. doi:S0959-4388(05)00152-2 [pii] 10.1016/j.conb.2005.10.002.
Leutgeb, J. K., Leutgeb, S., Moser, M. B., & Moser, E. I. (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315(5814), 961–966. doi:315/5814/961 [pii] 10.1126/science.1135801.
MacDonald, C. J., Lepage, K. Q., Eden, U. T., & Eichenbaum, H. (2011). Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron, 71(4), 737–749. doi:10.1016/j.neuron.2011.07.012.
Mankin, E. A., Sparks, F. T., Slayyeh, B., Sutherland, R. J., Leutgeb, S., & Leutgeb, J. K. (2012). Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci U S A. 109(47), 19462–19467. doi: 10.1073/PNAS.1214107109. Epub 2012 Nov 6. Erratum in: Proc Natl Acad Sci U S A. 2015, 112(10), E1169.
McClelland, J. L., McNaughton, B. L., & O’Reilly, R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419–457.
McNaughton, B. L., Barnes, C. A., & O’Keefe, J. (1983). The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Experimental Brain Research, 52(1), 41–49.
Milner, B., & Penfield, W. (1956). The effect of hippocampal lesions on recent memory. Transactions of the American Neurological Association, 42-8(80th Meeting).
Milner, B., Corkin, S., & Teuber, H. L. (1968). Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H.M. Neuropsychologia, 6(3), 215–234. doi:10.1016/0028-3932(68)90021-3.
Milner, B., Squire, L. R., & Kandel, E. R. (1998). Cognitive neuroscience and the study of memory. Neuron, 20(3), 445–468.
Morris, R. G. M., Schenk, F., Tweedie, F., & Jarrard, L. E. (1990). Ibotenate lesions of hippocampus and/or subiculum: Dissociating components of allocentric spatial learning. The European Journal of Neuroscience, 2(12), 1016–1028 (http://www.ncbi.nlm.nih.gov/pubmed/12106063).
Morris, A. M., Curtis, B. J., Churchwell, J. C., Maasberg, D. W., & Kesner, R. P. (2013). Temporal associations for spatial events: The role of the dentate gyrus. Behavioural Brain Research, 256, 250–6. doi:10.1016/j.bbr.2013.08.021.
Munn, R. G. K., & Bilkey, D. K. (2011). The firing rate of hippocampal CA1 place cells is modulated with a circadian period. Hippocampus, 22, 1325–1337. doi:10.1002/hipo.20969.
Neunuebel, J. P., & Knierim, J. J. (2014). CA3 retrieves coherent representations from degraded input: Direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron, 81(2), 416–427. doi:10.1016/j.neuron.2013.11.017.
Nitz, D. (2009). Parietal cortex, navigation, and the construction of arbitrary reference frames for spatial information. Neurobiology of Learning and Memory, 91(2), 179–185. doi:10.1016/j.nlm.2008.08.007.
O’Keefe, J., & Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34(1), 171–175.
O’Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Hippocampus (Vol. 3, p. 570). Oxford: Oxford University Press.
O’Reilly, R. C., & McClelland, J. L. (1994). Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus, 4(6), 661–682. doi:10.1002/hipo.450040605.
Olton, D. S., & Papas, B. C. (1979). Spatial memory and hippocampal function. Neuropsychologia, 17(6), 669–682.
Olton, D. S., Walker, J. A., & Gage, F. H. (1978). Hippocampal connections and spatial discrimination. Brain Research, 139(2), 295–308.
Pastalkova, E., Itskov, V., Amarasingham, A., & Buzsáki, G. (2008). Internally generated cell assembly sequences in the rat hippocampus. Science (New York, N.Y.), 321(5894), 1322–1327. doi:10.1126/science.1159775.
Piatti, V. C., Esposito, M. S., & Schinder, A. F. (2006). The timing of neuronal development in adult hippocampal neurogenesis. The Neuroscientist, 12(6), 463–468. doi:12/6/463 [pii] 10.1177/1073858406293538.
Poucet, B., Save, E., & Lenck-Santini, P. P. (2000). Sensory and memory properties of hippocampal place cells. Reviews in the Neurosciences, 11(2–3), 95–111.
Rangel, L. M., & Eichenbaum, H. (2013). What’s new is older. eLife, 2, e00605. doi:10.7554/eLife.00605.
Rangel, L. M., Alexander, A. S., Aimone, J. B., Wiles, J., Gage, F. H., Chiba, A. A., & Quinn, L. K. (2014). Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nature Communications, 5, 3181. doi:10.1038/ncomms4181.
Rivard, B., Li, Y., Lenck-Santini, P.-P., Poucet, B., & Muller, R. U. (2004). Representation of objects in space by two classes of hippocampal pyramidal cells. The Journal of General Physiology, 124(1), 9–25. doi:10.1085/jgp.200409015.
Rolls, E. T. (2010). A computational theory of episodic memory formation in the hippocampus. Behavioural Brain Research, 215(2), 180–196. doi:10.1016/j.bbr.2010.03.027.
Rolls, E. T., & Kesner, R. P. (2006). A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology, 79(1), 1–48. doi:S0301-0082(06)00036-0.
Rosenbaum, R. S., Priselac, S., Köhler, S., Black, S. E., Gao, F., Nadel, L., & Moscovitch, M. (2000). Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nature Neuroscience, 3(10), 1044–1048. doi:10.1038/79867.
Rosenbaum, R. S., Köhler, S., Schacter, D. L., Moscovitch, M., Westmacott, R., Black, S. E., et al. (2005). The case of K.C.: Contributions of a memory-impaired person to memory theory. Neuropsychologia, 43(7), 989–1021. doi:10.1016/j.neuropsychologia.2004.10.007.
Rowland, D. C., Yanovich, Y., & Kentros, C. G. (2011). A stable hippocampal representation of a space requires its direct experience. Proceedings of the National Academy of Sciences, 108(35), 14654–14658. doi:10.1073/pnas.1105445108.
Sederberg, P. B., Miller, J. F., Howard, M. W., & Kahana, M. J. (2010). The temporal contiguity effect predicts episodic memory performance. Memory & Cognition, 38(6), 689–699. doi:10.3758/MC.38.6.689.
Shen, J., Barnes, C. A., McNaughton, B. L., Skaggs, W. E., & Weaver, K. L. (1997). The effect of aging on experience-dependent plasticity of hippocampal place cells. The Journal of Neuroscience, 17(17), 6769–6782.
Skaggs, W. E., McNaughton, B. L., Wilson, M. A., & Barnes, C. A. (1996). Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus, 6(2), 149–172. doi:10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K.
Tashiro, A., Makino, H., & Gage, F. H. (2007). Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. The Journal of Neuroscience, 27(12), 3252–3259. doi:27/12/3252 [pii] 10.1523/JNEUROSCI.4941-06.2007.
Treves, A., & Rolls, E. T. (1992). Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus, 2(2), 189–199. doi:10.1002/hipo.450020209.
Tse, D., Langston, R. F., Kakeyama, M., Bethus, I., Spooner, P. A., Wood, E. R., et al. (2007). Schemas and memory consolidation. Science (New York, N.Y.), 316(5821), 76–82. doi:10.1126/science.1135935.
Tsodyks, M. V., Skaggs, W. E., Sejnowski, T. J., & McNaughton, B. L. (1996). Population dynamics and theta rhythm phase precession of hippocampal place cell firing: A spiking neuron model. Hippocampus, 6(3), 271–280. doi:10.1002/(SICI)1098-1063(1996)6:3<271::AID-HIPO5>3.0.CO;2-Q.
Tulving, E. (2002). Episodic memory: From mind to brain. Annual Review of Psychology, 53(1), 1–25. doi:10.1146/annurev.psych.53.100901.135114.
Underwood, B. J. (1969). Attributes of memory. Psychological Review, 76(6), 559–573.
Underwood, B. J. (1977). Temporal codes for memories: Issues and problems. Hillsdale: Erlbaum.
Van Strien, N. M., Cappaert, N. L. M., & Witter, M. P. (2009). The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nature Reviews. Neuroscience, 10(4), 272–282. doi:10.1038/nrn2614.
Whishaw, I. Q., Cassel, J. C., & Jarrad, L. E. (1995). Rats with fimbria-fornix lesions display a place response in a swimming pool: A dissociation between getting there and knowing where. The Journal of Neuroscience, 15(8), 5779–5788.
Acknowledgments
Recollections of the Kesner Lab
My (Chiba’s) memories of Ray Kesner’s lab in the context of graduate school surround the time of exciting theoretical advances, pushing the attribute model from a static to an active processing model. Daily candid exchanges were inspired by Ray’s openness to creatively and rigorously testing, rather than simply supporting his theories. Ray’s approach provided a platform for learning across several different labs working on similar questions. His genius for behavioral design and effervescence was contagious and as such all of us from that era inherited a portion of his passion and made his science part of our own. To our post-docs and students, there was nothing more inspiring than their first meal with Ray who is particularly facile at using restaurant condiments to represent all physical aspects of an experiment. The prize of the meal was the napkin covered with newly designed experiments to test the question of the evening. Each of us aspired to take at least some small aspect of Ray back to the lab. To Ray, we owe our intrinsic satisfaction from beautiful science; this is what makes a scientist for life and across many venues. What rich and perplexing lives he has given us. Thank you, Ray!
I (Chiba) also wish to acknowledge the late Dr. William H. Saufley II, a student of Underwood’s, who instilled my early desire to pursue science and directed me towards Ray’s Chapter in Learning and Memory: A Biological View (Eds. J L Martinez and R P Kesner 1986). This eye-catching book illuminated the path to Ray’s lab.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rangel, L., Quinn, L., Chiba, A. (2016). Space, Time, and the Hippocampus. In: Jackson, P., Chiba, A., Berman, R., Ragozzino, M. (eds) The Neurobiological Basis of Memory. Springer, Cham. https://doi.org/10.1007/978-3-319-15759-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-15759-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15758-0
Online ISBN: 978-3-319-15759-7
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)