Abstract
Taurine (2-aminoethanesulphonic acid) is an endogenous amino acid that has a number of protective roles in most mammalian cells, including modulation of cytoplasmic calcium levels, antioxidant effects and protection against mitochondrial dysfunction and endoplasmic reticulum stress associated with neurological disorders. Thiotaurine (2-aminoethane thiosulfonate), a molecule structurally related to hypotaurine and taurine, counteracts the damaging effect of oxidants and prevents apoptosis of human neutrophils.
In this study we have compared the effect of taurine and thiotaurine in protecting cerebellar granule neurons (CGNs) from apoptotic death. Two experimental paradigms were exploited to induce apoptosis: i) CGNs were continuously cultured in 5 mM K+-containing medium up to 6 days in the presence or absence of 1 mM either taurine or thiotaurine (chronic paradigm); ii) CGNs were cultured in 25 mM K+-containing medium for 6 days and then shifted to a 5 mM K+-containing medium in the presence or absence of 1 mM either taurine or thiotaurine (acute paradigm). In the first condition, CGNs survive up to 5 days, and then start to die; in the second condition, CGNs nicely differentiate elongating neurites but enter apoptosis within 30 min when shifted to the 5 mM K+-containing medium.
These assays showed that taurine and thiotaurine counteracted the apoptotic death induced by low potassium in both the acute and chronic paradigms. However, while they displayed a similar efficacy in the chronic paradigm, the thiotaurine showed a significantly higher (20 %) efficacy compared to taurine in the acute paradigm. This finding pinpoints the thiotaurine as a powerful anti-apoptotic molecule in neurons that are fully differentiated and have established synaptic connections.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Taurine
- Thiotaurine
- Neuroprotection
- Cerebellar granule neurons
- Apoptosis induced by low potassium
- Caspase-3
1 Introduction
Death by apoptosis is a normal phenomenon in animal development, essential to eliminate supernumerary, misplaced or damaged cells with high specificity (Meier et al. 2000).
It is known that taurine (TAU), an endogenous amino acid found at high concentrations in the brain, is a neuroprotective molecule acting as osmoregulator, modulating ionic movements, regulating intracellular level of free calcium and increasing mitochondrial buffering of these ions (Kumari et al. 2013). In particular, TAU protects neurons against glutamate-induced neurotoxicity by modulating glutamate-induced membrane depolarization, elevation of [Ca2+]i, activation of calpain, reduction of Bcl-2 and apoptosis (Wu et al. 2009). Moreover, TAU prevents the amyloid-β peptide neurotoxicity in chick cultured retinal neurons by binding to GABAA receptors and increasing Cl− conductance (Louzada et al. 2004). Several studies have recently reported novel protective effects of TAU against ischemic stroke (Menzie et al. 2013), age-related impairment of the GABAergic system (El Idrissi et al. 2013) and ethanol-induced apoptosis in the mouse cerebellum during postnatal life (Taranukhin et al. 2010).
Hypotaurine, an intermediate in the biosynthesis of TAU, reacts with sulfide to produce thiotaurine (TTAU), a thiosulfonate. Compared to TAU, the anti-apoptotic effect of TTAU has been poorly investigated so far. For instance, it is only know that addition of 100 μM TTAU to incubation medium determines a 55 % inhibition of Caspase-3 activity during the spontaneous apoptosis of human leukocytes (Capuozzo et al. 2013).
We have expanded present knowledge in the field by comparing the neuroprotective efficacy of TTAU and TAU in a well-established experimental system for studying cell survival and apoptosis, such as isolated cerebellar granule neurons (CGNs).
Since CGNs represent the largest homogeneous neuronal population of mammalian brain and are mostly generated postnatally, in vitro cultures of these cells can be easily obtained. Most CGNs die after 6 days in culture unless they are maintained under a chronic depolarizing condition that is obtained in a medium containing a high potassium concentration (25 mM K+). The 25 mM K+ condition mimics synaptic excitatory inputs these cells receive from mossy fibers in vivo. When CGNs are cultured in 25 mM K+ for 6 days and shifted to a medium containing a lower potassium concentration (5 mM K+) they undergo apoptosis within 30 min (Canterini et al. 2009) and die during the following 24–48 h, due to the hyperpolarization of plasma membrane. On the other hand, as originally performed by de Luca et al. (1996), CGNs continuously cultured in 5 mM K+-containing medium undergo spontaneous apoptosis and show progressive accumulation of DNA breaks, chromatin condensation and nuclear fragmentation.
Besides confirming the ability of TAU to prevent neuronal death of CGNs cultured in 5 mM K+-containing medium, our results enlighten a novel and more remarkable anti-apoptotic effect of TTAU in CGNs committed to apoptosis in the acute paradigm. In conclusion, TTAU may represent a novel class of TAU derivatives playing a key role in counteracting neuronal apoptosis.
2 Methods
2.1 Chemicals
TTAU was prepared from hypotaurine and elemental sulfur and their purity confirmed by HPLC as previously described (Capuozzo et al. 2013).
TAU was purchased from Fluka BioChemica (Sigma Aldrich, St. Louis, MO). In vitro culture media and related reagents were purchased from InVitrogen GIBCO (Invitrogen/GIBCO, Cralsbad, California). Chemicals were from Sigma Aldrich, unless otherwise specified.
2.2 Animals
CD1 mice of the Swiss-Webster strain were purchased from Charles River Italia (Calco, Italy) and raised in our colony. Pups were killed by decapitation without anaesthesia. Experimental protocols and procedures were approved by the Italian Ministry of Public Health and animals were raised in accordance with Sapienza University guidelines for the care and use of laboratory animals. All efforts were made to reduce the number of animals used.
2.3 In Vitro Cultures of CGNs
In vitro cultures of isolated CGNs were prepared from cerebella of PN5–6-day-old mice as previously described (Canterini et al. 2012). Briefly, cerebella were rapidly dissected from the brain, minced into small pieces, incubated at RT for 15 min in digestion buffer (containing 0.1 % trypsin and 100 μg/mL DNase in PBS) and repeatedly passed through a flame-polished glass pipette until a single-cell suspension was obtained. Cells were then recovered by centrifugation and suspended in DMEM culture medium containing 25 mM K+, 2 mM glutamine, 2 % B27 (Gibco), 100 units/mL penicillin and 100 μg/mL streptomycin, and 5 % fetal bovine serum (FBS), then plated at a density of 1 × 105 cells/well culture multiwell (previously coated with 0.1 μg/mL poly-l-lysine). CGNs were either continuously cultured for 6 days in a medium containing a standard potassium concentration (5 mM K+) in the presence/absence of 1 mM TAU or TTAU (chronic paradigm) or in a medium containing an high concentration of potassium (25 mM K+). Under the 25 mM K+ condition, CGNs differentiate and increase neurite sprouting and elongation. Cell shift from 25 to 5 mM K+ and treatment with 1 mM of TAU or TTAU for 16 h were performed after 6 days of in vitro culture (DIV6) and were preceded by a 12 h incubation in serum-free medium (acute paradigm).
Routinely, glial cell proliferation was inhibited by supplementing the culture medium with 10 μM cytosine-α-D arabinofuranoside (Ara-C; Sigma Aldrich) 18–22 h after plating.
2.4 Detection of Cellular Viability
In vitro cell viability was estimated by staining nuclei with Hoechst 33258 (Sigma Aldrich) and propidium iodide (PI) (Sigma Aldrich). Because plasma membranes of live cells are not permeant to PI, it was used to detect dead cells, whereas Hoechst staining allowed to count the total number of nuclei (Iyer et al. 1998).
Following treatments, cells were incubated with Hoechst 33258 (10 μM) and PI (10 μM) for 15 min at 37 °C and observed under an epifluorescence microscope (Leica Microsystem, Milan, Italy) to determine the number of Hoechst 33258- and PI-positive cells, respectively. The number of cells counted was approximately 1,000 cells for each independent observation (3 randomly acquired microscopic fields). Images were acquired and analyzed using the Metamorph 5.5 software.
2.5 Detection of Caspase-3 Protein Expression by Western Blotting Assays
To analyze the level of Caspase-3, CGNs were quickly rinsed with chilled PBS, detached/collected using a plastic scraper and homogenized in RIPA buffer (Sigma Aldrich) supplemented with protease-phosphatase inhibitors (Protease and Phosphatase Inhibitor Cocktails, Roche Diagnostics, Milan, Italy). Homogenates were centrifuged at 10,000 × g for 15 min at 4 °C and supernatants were analysed by Western Blotting (WB). Protein concentration was routinely determined by DC Protein Assay (Bio-Rad, Milan, Italy) loading equal amounts of total protein/lane on 4–20 % gradient Mini-Protean TGX precast gel for electrophoresis (Bio-Rad, Milan, Italy). Fractionated proteins were transferred to PVDF membranes (Roche, Milan, Italy) and then processed for WB using an anti-Caspase-3 rabbit polyclonal (Sigma Aldrich; 1:400 dilution), anti-cleaved Caspase-3 rabbit polyclonal (Cell Signaling Technology, Danvas, MA, USA; 1:600 dilution), and anti-β III Tubulin mouse polyclonal (AbCam, Cambridge, UK; 1:400 dilution) antibodies. Secondary antibodies used were anti-rabbit HRP-conjugated goat polyclonal (Pierce, Rockford, IL, USA; 1:650 dilution) and anti-mouse HRP-conjugated horse polyclonal (Pierce; 1:650 dilution) antibodies. Chemiluminescent protein bands were revealed using SuperSignal West Dura reagents (Pierce). Caspase-3 protein was normalized to β III Tubulin levels.
2.6 Statistics
In all figures, histograms represent the mean ± SEM of data obtained in three independent experiments and having at least three replicates for each data point. Graphics and statistical significance was determined using SPSS software.
3 Results
3.1 TAU and TTAU Likewise Rescue the Death of CGNs Cultured in 5 mM K+-Containing Medium (Chronic Paradigm)
We have first investigated the neuroprotective effect of TAU and TTAU on CGNs continuously cultured in 5 mM K+ up to 6 days (chronic paradigm).
To this end, CGNs were cultured for 6 days either in the absence (5 mM K+) or in the presence of 1 mM of TAU (5 mM K+ + TAU) or 1 mM of TTAU (5 mM K+ + TTAU). In parallel, CGNs were also continuously cultured in high potassium-containing medium (25 mM K+).
Cell viability was then assessed by determining the number of apoptotic nuclei after staining unfixed cells with Hoechst (to stain all nuclei) and PI dye (to stain dead cells). The fraction of dead cells was then determined by visualization under an epifluorescence microscope.
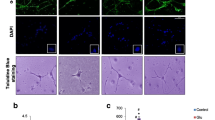
Figure 1 shows that CGNs cultured in 25 mM K+ were mostly alive with only a small fraction (8 %) of PI-positive cells, whereas CGN cultures maintained in 5 mM K+ displayed a 70 % of dead cells. The presence of 1 mM either TAU (5 mM K+ + TAU) or TTAU (5 mM K+ + TTAU) reduced the fraction of dead cells to 20 %. Together, these data indicate that TTAU is a potent protective molecule in this apoptotic system having an efficacy similar to that of TAU, a well-established neuroprotector (Louzada et al. 2004).
(a) Hoechst (nuclei) and propidium iodide (PI, apoptotic nuclei) double staining of CGNs that were cultured in K5+-containing medium for 6 days (chronic paradigm) either in the absence (5 mM K+) or in the presence of 1 mM of TAU (5 mM K+ + TAU) or 1 mM of TTAU (5 mM K+ + TTAU). Scale bar indicates 30 μm. (b) Histograms represent the fraction of dead cells, determined by PI staining (red)
3.2 Compared to TAU, TTAU Rescues More Efficiently CGNs from Potassium Deprivation-Induced Apoptosis (Acute Paradigm)
To gain more insights on the mechanisms underlying TAU and TTAU efficacy in counteracting apoptotic death, we next exploited the acute paradigm to induce apoptosis of CGNs. Since, as mentioned in the introduction, in this paradigm CGNs are triggered to apoptosis after they are fully differentiated, these experiments were aimed at investigating the rescuing ability of TAU and TTAU in a condition mimicking the physiological context in which these cells usually are. To this end, DIV6 CGNs continuously maintained in 25 mM K+ were triggered to apoptosis by lowering potassium in the culture medium in the absence of TAU or TTAU (5 mM K+) or in the presence of 1 mM either TAU (5 mM K+ + TAU) or TTAU (5 mM K+ + TTAU). After 24 h cell viability was assessed by Hoechst and PI staining, as described in the previous paragraph. In parallel, CGNs were also continuously cultured in high potassium-containing medium (25 mM K+).
Exposure to 5 mM K+ resulted in approximately 70 % cell death (Fig. 2), whereas 5 mM K+ medium supplementation with TAU partially protected CGNs from death, reducing the fraction of death cells to 35 % of total cells. Interestingly, the supplementation of 5 mM K+ medium with TTAU more efficiently improved cell survival further reducing the fraction of dead cells to 10 % of total cells.
(a) Hoechst (nuclei) and propidium iodide (PI, apoptotic nuclei) double staining of CGNs shifted to 5 mM K+ medium for 24 h (acute paradigm) after 6 days of culture in 25 mM K+ medium (depolarizing, pro-survival condition) either alone (5 mM K+, apoptotic condition) or in the presence of 1 mM TAU (5 mM K+ + TAU) or 1 mM TTAU (5 mM K+ + TTAU). Scale bar indicates 30 μm. (b) Histogram represents the fraction of dead cells, detected using propidium iodide (red staining)
Given that molecular events downstream from potassium dyshomeostasis in CGNs include the activation of Caspase-3 by proteolysis of the pro Caspase-3 (Mora et al. 2001), we decided to evaluate whether TTAU prevented the proteolytic cleavage of this pro-enzyme.
To this end, we determined the amount of activated Caspase-3 by performing WB assays of total proteins extracted from CGNs 16 h after the shifting to 5 mM K+ medium. Both the uncleaved and the cleaved form of Caspase-3 were detected using specific antibodies and their relative abundance was expressed as ratio between cleaved Caspase-3 and β III Tubulin standard.
While the expression levels of pro Caspase-3 were not changed under the various conditions, K+ deprivation was marked by a sevenfold increase of activated Caspase-3 compared to the 25 mM K+ condition. The supplementation of 5 mM K+ medium with TAU or TTAU significantly reduced and precluded Caspase-3 activation, respectively (Fig. 3).
(a) Total protein extracts of CGNs in acute paradigm were processed for WB with anti-pro Caspase-3 and anti-cleaved Caspase-3. (b) The relative abundance of cleaved Caspase-3 protein bands was expressed as ratio between pixels of cleaved Caspase-3 and β III Tubulin bands. Histograms represent the mean ± SEM of the ratios obtained in three independent experiments
Taken together these results strongly suggest that when apoptosis is induced by potassium-deprivation in the acute paradigm the TTAU directly prevents the activation of Caspase-3.
4 Discussion
This study provides novel inside on the anti-apoptotic activities of TAU and TTAU in a well-established model system of neuronal apoptosis (Gallo et al. 1987).
The chronic and acute paradigms exploited in this study allowed us to investigate the neuroprotective effects of TAU and TTAU in neuronal cells that markedly differ in terms of differentiative traits and spontaneously die or can be triggered to die, respectively. In fact, CGNs that are cultured in 5 mM K+-containing medium (chronic paradigm) are functionally immature and spontaneously die after DIV6 entering a death program that is not mediated by cytochrome complex (cyt-c) release from mitochondria and Caspase-9 activation (Alavez et al. 2003). By contrast, CGNs that are cultured in 25 mM K+-containing medium for more than 5–6 days fully differentiate and can be committed to apoptosis by the exposure to a 5 mM K+-containing medium (acute paradigm). Thereafter, they die within 24–48 h, showing typical feature of apoptotic death, including DNA condensation and Caspase-3 activation (Moran et al. 1999).
The responsiveness of CGNs to K+ deprivation is acquired during in vitro differentiation; DIV3-4 CGNs do not respond to the shift in 5 mM K+ in terms of commitment to apoptosis because they have not yet developed the dependence on membrane electrical activity for survival (de Luca et al. 1996).
Our findings indicate that under the chronic paradigm TAU and TTAU share a similar efficacy in rescuing the survival of CGNs, indicating that TAU and TTAU promote CGN survival through a similar pathway(s). However, in spite of the wealth of information available on the neuroprotective effect of TAU, the role of TTAU has been poorly investigated so far. Among the pathways responsible for the pro-survival effect of TAU in our chronic paradigm, we favor the hypothesis that a regulation of the homeostasis of intracellular K+ concentration plays a major role. In fact, it has been reported that TAU inhibits several classes of K+ channels, including Ca2+-activated K+ channels (Tricarico et al. 2001) and influences K+ conductance through the inhibition of inward voltage dependent K+ channels (Kv) (Bulley et al. 2013).
Under the acute paradigm, TTAU more efficiently improves cell survival compared to the very weak effect of TAU. This difference likely relies on the specific feature of the apoptosis induced by K+-deprivation in fully differentiated CGNs, as outlined above. Apoptosis triggered by the acute paradigm is marked by an early-phase (0–3 h after the apoptotic stimulus) in which ROS production increases and cyt-c release from mitochondria occurs to begin Caspase-3 activation. Then, during a later phase (3–15 h after the apoptotic stimulus) proteosomes activity decreases, Caspase-3 activity increases, cyt-c is degraded and ROS levels remain still high (Atlante et al. 2003).
Because TTAU is a potent anti-oxidant (Acharya and Lau-Cam 2013) and a biochemical intermediate in the transport, storage, and release of sulfide (Pruski and Fiala-Médioni 2003; Capuozzo et al. 2013), we believe that its strong efficacy in counteracting apoptosis in the acute paradigm mostly relies on the ability to reduce the level of ROS. However, Capuozzo et al. (2013) suggested that TTAU prevents apoptosis of human neutrophils by generating H2S from sulfane sulfur atom, a gaseous molecule that has a regulatory activity on inflammatory responses (Zanardo et al. 2006). Moreover, it is widely recognized that H2S promotes the short-term survival of neutrophils by inhibiting of Caspase-3 cleavage (Rinaldi et al. 2006) and exerts its anti-aging effects by directly increasing the inhibitory effects of GSH and SOD on ROS production and the redox enzyme levels improving the resistance of cell to stress (Zhang et al. 2013). In spite of the different efficacy displayed by TAU and TTAU in the acute paradigm, results of Fig. 3 indicate that their pro-survival effect is dependent on the ability to inhibit Caspase-3 activation, indicating a specific role in the early phase of cell commitment to apoptosis.
5 Conclusion
Our study adds novel inside on the anti-apoptotic activity of TTAU, the biological relevance of which is still a challenge to biochemical research, by showing that it strongly inhibits Caspase-3 activation. This pinpoints an important role of this molecule in the biochemical changes associated with early phases of apoptosis in differentiated and functionally mature neurons.
Abbreviations
- Ara-C:
-
Cytosine-α-D arabinofuranoside
- CGNs:
-
Cerebellar granule neurons
- cyt-c:
-
Cytochrome complex
- DIV:
-
Days in vitro
- PI:
-
Propidium iodide
- TAU:
-
Taurine
- TTAU:
-
Thiotaurine
References
Acharya M, Lau-Cam CA (2013) Comparative evaluation of the effects of taurine and thiotaurine on alterations of the cellular redox status and activities of antioxidant and glutathione-related enzymes by acetaminophen in the rat. Adv Exp Med Biol 776:199–215
Alavez S, Pedroza D, Morán J (2003) Mechanisms of cell death by deprivation of depolarizing conditions during cerebellar granule neurons maturation. Neurochem Int 43:581–590
Atlante A, Bobba A, Calissano P, Passarella S, Marra E (2003) The apoptosis/necrosis transition in cerebellar granule cells depends on the mutual relationship of the antioxidant and the proteolytic systems which regulate ROS production and cytochrome c release en route to death. J Neurochem 84:960–971
Bulley S, Liu Y, Ripps H, Shen W (2013) Taurine activates delayed rectifier Kv channels via a metabotropic pathway in retinal neurons. J Physiol 591:123–132
Canterini S, Bosco A, De Matteis V, Mangia F, Fiorenza MT (2009) THG-1pit moves to nucleus at the onset of cerebellar granule neurons apoptosis. Mol Cell Neurosci 40:249–257
Canterini S, Bosco A, Carletti V, Fuso A, Curci A, Mangia F, Fiorenza MT (2012) Subcellular TSC22D4 localization in cerebellum granule neurons of the mouse depends on development and differentiation. Cerebellum 11:28–40
Capuozzo E, Pecci L, Baseggio Conrado A, Fontana M (2013) Thiotaurine prevents apoptosis of human neutrophils: a putative role in inflammation. Adv Exp Med Biol 775:227–236
de Luca A, Weller M, Fontana A (1996) TGF-beta-induced apoptosis of cerebellar granule neurons is prevented by depolarization. J Neurosci 16:4174–4185
El Idrissi A, Shen CH, L’amoreaux WJ (2013) Neuroprotective role of taurine during aging. Amino Acids 45:735–750
Gallo V, Kingsbury A, Balázs R, Jørgensen OS (1987) The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J Neurosci 17:2203–2213
Iyer S, Chaplin DJ, Rosenthal DS, Boulares AH, Li LY, Smulson ME (1998) Induction of apoptosis in proliferating human endothelial cells by the tumor-specific antiangiogenesis agent combretastatin A-41. Cancer Res 58:4510–4514
Kumari N, Prentice H, Wu JY (2013) Taurine and its neuroprotective role. Adv Exp Med Biol 775:19–27
Louzada PR, Paula Lima AC, Mendonca-Silva DL, Noël F, De Mello FG, Ferreira ST (2004) Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J 18:511–518
Meier P, Finch A, Evan G (2000) Apoptosis in development. Nature 407:796–801
Menzie J, Prentice H, Wu JY (2013) Neuroprotective mechanisms of taurine against ischemic stroke. Brain Sci 3:877–907
Mora A, Sabio G, González-Polo RA, Cuenda A, Alessi DR, Alonso JC, Fuentes JM, Soler G, Centeno F (2001) Lithium inhibits caspase 3 activation and dephosphorylation of PKB and GSK3 induced by K+ deprivation in cerebellar granule cells. J Neurochem 78:199–206
Moran J, Itoh T, Reddy UR, Chen M, Alnemri ES, Pleasure D (1999) Caspase-3 expression by cerebellar granule neurons is regulated by calcium and cyclic AMP. J Neurochem 73:568–577
Pruski AM, Fiala-Médioni A (2003) Stimulatory effect of sulphide on thiotaurine synthesis in three hydrothermal-vent species from the East Pacific Rise. J Exp Biol 206:2923–2930
Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, Vitale M (2006) Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest 86:391–397
Taranukhin AG, Taranukhina EY, Saransaari P, Podkletnova IM, Pelto-Huikko M, Oja SS (2010) Neuroprotection by taurine in ethanol-induced apoptosis in the developing cerebellum. J Biomed Sci 24:17
Tricarico D, Barbieri M, Conte Camerino D (2001) Voltage-dependent antagonist/agonist actions of taurine on Ca(2+)-activated potassium channels of rat skeletal muscle fibers. J Pharmacol Exp Ther 298:1167–11671
Wu JY, Wu H, Jin Y, Wei J, Sha D, Prentice H, Lee HH, Lin CH, Lee YH, Yang LL (2009) Mechanism of neuroprotective function of taurine. Adv Exp Med Biol 643:169–179
Zanardo RCO, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL (2006) Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20:2118–2120
Zhang Y, Tang ZH, Ren Z, Qu SL, Liu MH, Liu LS, Jiang ZS (2013) Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol 33:1104–1113
Acknowledgements
This work was supported by grant “Progetti di ricerca di Ateneo” from “Sapienza” University of Rome, year 2012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Dragotto, J., Capuozzo, E., Fontana, M., Curci, A., Fiorenza, M.T., Canterini, S. (2015). Thiotaurine Protects Mouse Cerebellar Granule Neurons from Potassium Deprivation-Induced Apoptosis by Inhibiting the Activation of Caspase-3. In: Marcinkiewicz, J., Schaffer, S. (eds) Taurine 9. Advances in Experimental Medicine and Biology, vol 803. Springer, Cham. https://doi.org/10.1007/978-3-319-15126-7_41
Download citation
DOI: https://doi.org/10.1007/978-3-319-15126-7_41
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15125-0
Online ISBN: 978-3-319-15126-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)