Abstract
Most plant studies of green roof taxa have only been conducted for a duration of 1 or 2 years. The problem with this scenario is that it can result in premature conclusions and misleading recommendations because green roofs are dynamic systems. Plants that initially survive may eventually experience reduced coverage or disappear completely due to competition, variability in climate, and other factors. Setting up long-term studies similar to the National Science Foundation (NSF) Long Term Ecological Research (LTER) model would provide the opportunity to follow changes to green roof habitats over time and also examine impacts and ecosystem service outputs on similarly designed roofs across geographic locations. Without consciously considering the effects and changes over time mistakes are not only made, but also repeated. We review several important longitudinal studies and discuss factors that impact long-term plant communities such as substrate composition and fertility, substrate depth, substrate moisture, microclimates, roof slope, orientation, and irradiance levels; as well as initial plant choices, functional diversity and complexity, and maintenance practices. In addition, we discuss the potential of applying the LTER model to green roofs and close with future research needs and questions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Long-term ecological research
- Plant performance
- Plant selection
- Plant succession
- Substrate composition
- Substrate depth
- Substrate moisture
13.1 Introduction
The long-term plant communities that exist on green roofs can have a major impact on the ecological services provided (Oberndorfer et al. 2007; Rowe and Getter 2010). If green roofs are to deliver these benefits over time, as well as to meet long-term client expectations, then plant selection and long-term plant performance are extremely important. To date, most green roof research has been conducted to measure performance of engineering traits such as stormwater retention and heat flux through roofing membranes or has focused on whether a particular species will survive. Unfortunately, there has been much less research involving green roof ecological principles (Cook-Patton and Bauerle 2012). These facts raise questions regarding how time will influence changes in plant communities, how these changes influence the ecosystem services that a roof provides, and what can be done to address these issues.
13.2 Review of Several Important Longitudinal Studies
One problem with the green roof literature dealing with plant evaluations is the lack of long-term studies published in peer-reviewed journals. Here we review several of the longest green roof studies of record that have been published in English. Studies where initial plantings were recorded which provide baseline information include the Paul-Lincke-Ufer project, Berlin (20 years) (Köhler 2006; Köhler and Poll 2010); Communication Arts Building, Michigan State University (9 years) (Getter et al. 2009a); Horticulture Teaching and Research Center, Michigan State University (7 years) (Durhman et al. 2007; Rowe et al. 2012); a commercial building in Sheffield, U.K. (6 years) (Dunnett and Nolan 2004; Dunnett et al. (2008); and Seaton Hall, Kansas State University (5 years) (Skabelund et al. 2014). The study on the Church of Jesus Christ of Latter-day Saints Conference Center in Salt Lake City (Dewey et al. 2004) was only conducted for 2 years, but the roof still exists and provides an excellent opportunity to go back and survey the roof after 14 years. The Ufa-Fabrik Cultural Center, Berlin (13 years) (Köhler 2006) and the Thuring and Dunnett (2014) paper that examined numerous old green roofs in Germany are included even though the original plantings are unknown.
13.2.1 Paul-Lincke-Ufer Project, Berlin (20-years)
In the longest plant evaluation study of record, Köhler (2006) evaluated long-term vegetation succession on the Paul-Lincke-Ufer (PLU) project located in Berlin. The study area consisted of ten sub-roofs each with a substrate depth of 10 cm (4 in) with varying orientation and slope. The green roofs were installed in 1985 as pre-vegetated mats seeded with ten species : wild chives (Allium schoenoprasum), cheatgrass (Bromus tectorum), orchardgrass (Dactylis glomerata), sheep fescue (Festuca ovina), red fescue (Festuca rubra), junegrass (Koeleria macrantha), perennial ryegrass (Lolium perenne), Canada bluegrass (Poa compressa), Kentucky bluegrass (Poa pratensis), and yellow stonecrop (Sedum acre). Data were recorded almost every year from 1985 until 2005 and measurements included the number of plants, coverage for each species, plant heights, and percentage of “standing dead” (living plants with dead leaves and stems). Over the 20-year period, 110 species were observed, but only about 10–15 were present in large numbers. The average number of plant species present at any given time was 15. Of the original ten species only five were present every year (A. schoenoprasum, B. tectorum, F. ovina, P. compressa, and S. acre). Dactylis glomerata no longer existed after the first year, and K. macrantha, P. pratensis, L. perenne, and F. rubra disappeared after 3, 5, 7, and 8 years, respectively. By 2005, A. schoenoprasum became by far the dominant species covering 56 % of the area followed by F. ovina, P. compressa, and B. tectorum. Initially, numerous weeds sprouted from the seed bank present in the growing substrate . However, these disappeared after a few years as the roofs were not irrigated. Wet summers tended to encourage spontaneous species and enrich plant diversity due primarily to colonization. Some colonizing species such as bulbous bluegrass (Poa bulbosa) persisted, likely because it forms a bulb, which allows it to survive during dry periods. Also, the lichen, Cladonia coniocrea, colonized and persisted because it can withstand dry periods .
Köhler attributed weather related factors such as temperature and rainfall to be more important than roof size, slope, or age in regards to species richness . After the initial decrease in plant diversity, roof age had limited impact on species richness (defined as the number of different species present in a given habitat). It varied from year to year due to weather conditions, but had more or less reached equilibrium. Some species such as loose silky-bent (Apera spica-venti) were more apparent during wet summers compared to dry ones .
13.2.2 Ufa-Fabrik Cultural Center, Berlin (13-years)
The Ufa-Fabrik Cultural Center located in suburban Berlin was installed in 1986, but the first data were not collected until 1992 and then data collection continued until 2005 (Köhler 2006) . The roof was planted with seed of wildflower meadow species collected from the Alps, but it is not known exactly what species were originally sown. This roof was irrigated the first 11 years and exhibited higher species richness during this time period. When irrigation was discontinued in 1997, herbaceous plant species started to decline and Sedum species began to dominate. In fact, in 1998, the common green roof plants, S. acre and Caucasian stonecrop (Phedimus spurius) appeared for the first time. It is difficult to make major conclusions regarding this roof since the exact original species are unknown and there wasn’t any data collected until 8 years after installation.
13.2.3 Communication Arts Building, Michigan State University, East Lansing (9-years)
A study is being conducted on the third-story rooftop of the Communications Arts and Sciences Building on the campus of Michigan State University to quantify the effect of solar radiation (full sun vs. full shade) on several U.S. native and non-native species (Getter et al. 2009a) (Fig. 13.1). Plugs of six native and three non-native species were planted in May 2005 on substrates of two different depths [8.0 cm (3.1 in) and 12.0 cm (4.7 in)] both in sun and shade. Species tested included wild nodding onion (Allium cernuum), heath sedge (Carex flacca), cascade stonecrop (Sedum divergens), narrow-petaled stonecrop (Sedum stenopetalum), largeflower fameflower (Talinum calycinum) (currently known as Phemeranthus calycinus by taxonomists), and sunbright (Talinum parviflorum)(currently known as Phemeranthus parviflorus), as well as three non-natives (Sedum acre (biting stonecrop), Sedum album (white stonecrop), and Sedum urvillei (stonecrop). Plots were irrigated during the first year of establishment, but relied on natural rainfall thereafter .
At the end of the first growing season, C. flacca was one of the most abundant species for both substrate depths in the shade. However, in subsequent years, it decreased in abundance during the driest portions of the summer that likely impacted overall regeneration. By the end of the 4 years, this species exhibited zero or near-zero absolute cover (AC) . Absolute cover is defined as the total number of contacts recorded for each species divided by the number of data collection points. In contrast, at the end of the second growing season, S. acre had established itself as the most abundant species for both substrate depths in the shade and exceeded an AC of 0.6 by the third growing season. For both substrate depths in the shade, A. cernuum was the next most abundant species by the fourth growing season, followed by S. album and T. calycinum. In the sun, by the second growing season both substrate depths were dominated by S. album, followed by T. calycinum and S. acre. At 12 cm (4.75 in), A. cernuum closely followed as the fourth most abundant, but this species was not nearly as abundant as it was in the shade at the same depth. By week 174 (23 Sept 2008), most species exhibited different AC within a depth between sun and shade . However, when all species were combined, overall AC did not differ between sun and shade within a depth. This indicated that while species make-up was changing among solar radiation levels, that overall coverage was not significantly different between sun and shade. For all substrate depths and solar levels, the most abundant species were S. acre, A. cernuum, S. album, and T. calycinum. With the exception of T. calycinum, native species were less abundant than non-native species. The native Talinum species (T. calycinum and T. parviflorum) were outside of their hardiness zone, but are prolific seeders. However, they need bare soil in order to germinate. By the end of 9 years, the only species that still existed were A. cernuum, S. acre, and S. album and represents a significant decrease in species richness over time. Plots were weeded the first 4 years, but have received no maintenance over the past 5 years .
13.2.4 Horticulture Teaching and Research Center, Michigan State University, East Lansing (7-years)
This study followed the succession of 25 succulents grown at three substrate depths over the course of 7 years (Durhman et al. 2007; Rowe et al. 2012) . Absolute cover was determined using a point-frame transect every two weeks during the first three growing seasons and monthly during years four through seven to measure community composition and change (Fig. 13.2). At the 7.5 cm (3 in) depth, 22 species were present at the end of the first growing season, but these numbers were reduced to 13, 8, and 7 after 2, 3, and 5 years, respectively. Similar results occurred at the shallower depths except that the number of species was reduced at a faster pace. For the most part, the species present did not change after 4 years, but the relative abundance for each species continued to change. At 5.0 cm (2 in) and 7.5 cm (3 in), both Caucasian stonecrop (Phedimus spurius) and Chinese mountain sedum (Sedum middendorffianum) continued to expand through year 7 at the expense of the other remaining species. At 2.5 cm (1 in), S. acre and S. album were the dominant species.
Results show that the length of the study can have a dramatic effect on conclusions and plant recommendations. The initial paper published from the first two seasons of data from this study (Durhman et al. 2007) recommended P. spurius, S. acre, S. album, S. middendorffianum, Jenny’s stonecrop (S. reflexum), pale stonecrop (S. sediforme), and P. spurius for extensive green roofs ranging from 2.5 cm (1 in) to 7.5 cm (3 in) in depth. Additional recommendations for subsidiary species that were present at specific substrate depths, but may not exhibit an ability to cover large areas included Burnatti sedum (S. dasyphyllum ‘Burnatii’), lilacmound sedum (S. dasyphyllum ‘Lilac Mound’), diffuse sedum (S. diffusum), Spanish sedum (S. hispanicum), and orange stonecrop (S. kamtschaticum syn. Phedimus kamtschaticus). As can be seen from the results following 7 years, recommendations were misleading as S. sediforme, S. dasyphyllum ‘Burnatii’, S. dasyphyllum ‘Lilac Mound’, S. diffusum, and S. hispanicum no longer existed at any depth .
13.2.5 A Commercial Building in Sheffield, U.K. (6 years)
Dunnett and Nolan (2004) and Dunnett et al. (2008) conducted a study on top of a three story commercial building in Sheffield, U.K. , over a period of 6 years from 2001–2006. The objectives of their study were to evaluate potential plant taxa for use on green roofs that experience a maritime U.K. climate and to test how substrate depth [10 cm (4 in) and 20 cm (8 in)] influenced plant establishment and survival, as well as visual and aesthetic criteria. Species included white thrift seapink (Armeria maritima ‘Alba’), lesser calamint (Calamintha nepeta), maiden pink (Dianthus deltoides) dwarf blue fescue (Festuca ovina glauca), bearskin fescue (Festuca scoparia), Lindheimer’s beeblossom (Gaura lindheimeri), white creeping babysbreath (Gypsophila repens ‘Alba’), Border Ballet red hot poker (Kniphofia X‘Border Ballet’), sea-lavender (Limonium platyphyllum), Fassen’s catnip (Nepeta Xfassenii), Herrenhuasen oregano (Origanum laevigatum ‘Herrenhausen’), roseroot (Rhodiola rosea), yellow stonecrop (Sedum acre), lambsear (Stachys byzantina), and dwarf spiked speedwell (Veronica spicata ‘Nana’).
Species tested were all native to dry and nutrient-stressed habitats, but differed widely in their heights, flowering times, life spans, growth forms, and locations where they are considered native. In addition to annually measuring plant height, spread, flowering performance and percent vegetation cover for the 15 planted species, they also recorded the numbers and percent cover for colonizing species .
The greatest survival, diversity, size, and flowering performance of planted species occurred at a substrate depth of 20 cm (8 in) relative to 4 in (10 cm) and the herbaceous species developed 85 and 58 % coverage at the end of two growing seasons at depths of 20 cm (8 in) and 10 cm (4 in), respectively. By the end of 5 years, all species survived at both depths, however, 14 of 15 species maintained at least 50 % of their original numbers at 20 cm (8 in) whereas, only eight did so at 10 cm (4 in). Bare ground and moss cover was greatest at 10 cm (4 in) as was diversity of colonizing species, presumably due to the presence of open space for invading seeds to germinate.
Species-richness (mean number of taxa per subplot) decreased over time at both substrate depths, but the rate of decline was greater at 10 cm (4 in). As mentioned, some species performed better at the 10 cm (4 in) or 20 cm (8 in) depth. The low-growing species such as sedum that are typical of shallow extensive roofs were not as competitive at 20 cm (8 in). Likewise, the perennial plants that normally possess greater biomass could not survive as well at 10 cm (4 in). Even when drought tolerant plant species are selected, the limiting factor for plant survival is often substrate moisture, which is often a function of substrate depth (Chaps. 4, 5). The authors emphasized the importance of long-term monitoring of green roofs because of the changes that occurred in plant communities from the first to the sixth year of their experiment.
13.2.6 Seaton Hall, Kansas State University, Manhattan, KS (5-years)
The first green roof project installed at Kansas State University was planted on Seaton Hall in May 2009 (Skabelund et al. 2014) . The main goal of the project was to see if a semi-intensive green roof consisting of native grasses and forbs growing in a substrate profile ranging from 10 cm (4 in) to 18 cm (7.1 in) was feasible in this relatively dry climate with minimal maintenance and irrigation. The roof is south facing and receives reflected light off windows and limestone especially during spring and fall. The 28.3 m2 (305 ft2) roof was planted with plugs of five species of grasses, ten forbs, and one forb-like shrub. Grasses included side-oats grama (Bouteloua curtipendula), blue grama (Bouteloua gracilis), little bluestem (Schizachyrium scoparium), prairie dropseed (Sporobolus heterolepis), and Indiangrass (Sorghastrum nutans). Forbs consisted of smooth aster (Aster laevis), purple poppy-mallow (Callirhoe involucrata), purple prairieclover (Dalea pupurea), tall gayfeather (Liatris aspera), dotted gayfeather (Liatris punctata), prairie coneflower (Ratibida columnifera), gray-headed prairie coneflower (Ratibida pinnata), wild blue sage (Salvia azurea), rigid goldenrod (Solidago rigida), and common spiderwort (Tradescantia ohiensis). The forb-like shrub was New Jersey tea (Ceanothus americanus). The study is still ongoing and has yet to be published other than in a proceedings from a meeting (Skabelund et al. 2014).
Along with plant survival and dynamics, a range of climatic variables was monitored. A subset of selected grasses was evaluated for height, basal diameter, and number of flowering stalks at the end of each growing season between 2009 and 2013. In 2009 and 2010, supplemental irrigation was provided on an as-needed basis and growing conditions were favorable, resulting in nearly 100 % plant survival. Most grasses exhibited flowering stalks and increased basal diameter. The west side of the green roof was not irrigated in 2011, the entire roof was irrigated in 2012, and then supplemental irrigation ceased during mid-August 2012. Between 2010 and 2011 the original plantings decreased from 130 to 98 for individual grasses and from 98 to 39 for forbs. At the end of 2012 grasses exhibiting visibly-green above ground biomass remained at 98 while forbs increased to 54. By November 2013 original grasses numbered 68 and forbs 21. After the first year many new native grasses and forbs established themselves from germinating seeds produced by the original plantings . This was particularly pronounced in 2010 and 2012. Notably, plants of B. gracilis were taller in deeper substrates, with 12–18 cm (4.75–7.1 in) depths producing plants approximately 10.5 cm (4.1 in) taller than 7.5–9 cm (3.0–3.5 in) depths. Between 2009 and 2012, 15–18 cm (5.9–7.1 in) substrate depths produced B. gracilis 10.8 cm (4.2 in) taller than those at 10 cm (4 in) depths.
13.2.7 Church of Latter-Day Saints Convention Center, Salt Lake City, Utah
An example of a short-term study is the Church of Jesus Christ of Latter-day Saints Conference Center in Salt Lake City , Utah (Dewey et al. 2004) (Fig. 13.3). However, because original substrate conditions and some information on plantings were recorded, the opportunity exists to monitor this roof into the future.
The objective of the original study was to observe the relative competitiveness of native grass and wildflower species growing in a range of different radiation/temperature environments. For research purposes, the roof was partitioned into seven radiation zones: (1) maximum sunlight, maximum reflection/radiation, (2) maximum sunlight, moderate refection/radiation, (3) maximum sunlight only, (4) minimal shading, (6) moderate shading, and (7) maximum shading. Zone 5 was eliminated from the study as it was considered similar to zone 4. The main component of the substrate was heat-expanded shale and it was placed at a depth of 1 m (3.3 ft). The roof was planted with plugs during the summer of 2000, overseeded in April 2001 with some of the same species in addition to others. Weeds were pulled as needed and the roof was irrigated twice a week.
During fall 2001, the roof was evaluated by counting the number of plants present for each species in a given sample area. Twenty one species were identified that should at least be considered for future grass/wildflower green roofs. However, Canada bluegrass (Poa compressa) and white sage (Artemisia ludoviciana) were too aggressive when planted in this grass and wildflower mixture. In contrast, the alpine bluegrass (Poa alpina), big bluegrass (Poa secunda), mutton bluegrass (Poa fendleriana), blue bellflower (Campanula rotundifolia), columbine (Aquilegia spp.), purple meadowrue (Thalictrum purpurea), and tickseed. (Coreopsis spp.) may not be competitive enough. Since there was no experimental design to the original planting, no replication, and only an estimate of the number of plugs planted in each zone, the study is only observational. Still, if monitored in to the future it would provide valuable information as to the long term succession of a grass and wildflower meadow on a green roof.
13.2.8 Old Green Roofs in Germany
It would be a travesty to discuss long-term plant communities on green roofs without acknowledging the long tradition of over 100-years of green roofs in Germany. Unfortunately, much of the original information on these roofs was never recorded, has been lost, or was anecdotal; studies were observational in nature without replication and thus not scientifically sound by today’s standards; were not published in peer-reviewed journals; or are not easily accessible to the scientific world as they were not written in English. However, in addition to the Paul-Lincke-Ufer project and the Ufa-Fabrik Cultural Center in Berlin (Köhler 2006) described above, two recent papers published in scientific journals have gone back and looked at some of these older German roofs (Köhler and Poll 2010; Thuring and Dunnett 2014).
The purpose of the Köhler and Poll (2010) study was to compare vegetation and substrate characteristics between the old Tar-Paper-Green roofs (TPG-roofs) that were installed between 1880 and 1930 to the first Modern Extensive Green roofs (MEG-roofs) that were established in the 1980’s. These roofs, subjects of previously published studies written in German from 1960, 1982, 1986, 1987, 1990, and 1995 were surveyed in 2008. While the Paul-Lincke-Ufer project (Köhler 2006) discussed earlier focused on ecological succession, this study concentrated on growing substrate, vegetative quality, and species richness .
According to the specified criteria set by Köhler and Poll (2010), they concluded that the performance of the MEG-roofs with engineered substrates composed of heat expanded clay, etc. was higher than the older TPG-roofs that originally utilized sandy soils. Even so, both roof types were still functional after many years and exhibited an increase in pedogenesis, a trend toward higher organic carbon , and a neutral pH. The old TPG-roofs were significantly richer in humus (mean organic C content of 4 %) than the MEG-roofs. Initial mean organic carbon content on the MEG-roofs was 2.5 % and then declined to 1.9 % due to microbial oxidation. After the roof stabilized after about 10 years, their organic carbon content increased steadily for the next 25 years up to the point that by 2008, the organic C content of both roof types were not significantly different. Total porosity of the MEG-roof substrates rose over a period of 10 years from 50 to 60 %. This change is likely due to processes such as the continuous formation and decay of plant roots, microbial activity, freezing and thawing.
Regarding plant species, 70 different species were recorded on the MEG-roofs, compared to 45 on TPG-roofs. Of course, this difference in species richness could be due to differences in substrate properties, as well as many other factors such as initial plantings. The most successful species were generally grasses such as cheatgrass (Bromus tectorum), poverty brome (Bromus sterilis), fescues Festuca spp., perennial ryegrass (Lolium perenne), annual bluegrass (Poa annua), and Canada bluegrass (Poa compressa) (most common).
The second study took place in southwestern Germany where Thuring and Dunnett (2014) surveyed vegetation and substrates on nine of the oldest extensive green roofs in the Stuttgart area during 2010 and 2011. Roof ages at the time of the survey ranged from 20 to 33-years-old. Unfortunately, there was little information on original substrate composition and depth or original species planted on these roofs so the results serve as a snapshot in time of present conditions. They could only speculate on how the substrates and plant communities changed over time. However, the roofs likely all adhered to early FLL standards and had a substrate depth less than 20 cm (8 in), a pH between 6.5 and 8.0, and organic content below 4.1 lbs/ft3 (65 g/L) when constructed (FLL 2008).
Results suggested a decrease in substrate depth, substrate pH, and plant biomass over time while substrate organic content increased. This increase in organic matter agrees with the Köhler and Poll (2010) study discussed above and with the findings of Getter et al. (2007) who reported that organic matter nearly doubled from 2.33 to 4.25 % in just 5 years where the primary component of the substrate was heat-expanded slate. Similarly, Getter et al. (2009b) reported that the amount of carbon sequestered on shallow sedum based roofs increased with age and that 100 g C/cm2 (57.8 oz/in3) were sequestered during the first 2 years after installation of 6 cm (2.4 in) deep plots. The increase in organic carbon makes sense when one considers that the engineered substrates often used on extensive green roofs have limited initial organic matter because they are designed to hold moisture by manipulating particle size distributions (FLL 2008). Also, low substrate pH could result in an accumulation of substrate organic matter because some microbes are adversely affected by low pH, thus reducing decomposition (Berendse 1998).
Regarding plant cover, Thuring and Dunnett (2014) reported that succulents dominated these roofs either by themselves or as a consistent groundcover underneath other herbaceous perennials or grasses. Over time species diversity decreased which agrees with the work of Liesecke (1998) who reported that one or two succulents, a single herb, and one or two moss species often dominated older, extensive green roofs or two moss species.
13.3 Factors Impacting Long-Term Plant Communities
Numerous factors impact long-term plant communities on green roofs . Factors include substrate composition and fertility, substrate depth, substrate moisture, microclimates, roof slope, orientation, and irradiance levels; as well as initial plant choices, functional diversity and complexity, and maintenance practices.
13.3.1 Substrate Composition and Fertility
Substrate composition influences plant communities primarily through moisture retention and nutrient availability. Ideally they should be lightweight, permanent, and able to sustain plant health without leaching nutrients that may pollute receiving water bodies. For these reasons substrates often incorporate aggregate materials such as heat expanded slate, shale, or clay as their main component (FLL 2008). Water holding capacity can be altered by manipulating the particle size distribution of the aggregates and by adding organic matter. Although organic matter will retain moisture and provide nutrients, high levels are not recommended because it decomposes resulting in substrate shrinkage and can leach nutrients such as nitrogen (N) and phosphorus (P) in the runoff (Rowe 2011). The same runoff problems can occur when fertilizer is applied. A detailed discussion of nutrient cycling in green roof ecosystems can be found in Chap. 5.
In a study that looked at the effects of substrate composition and fertility, Rowe et al. (2006) found that sedum achieved 100 % cover regardless of the percentage of heat-expanded slate in the substrate, but that the herbaceous perennials and grasses required greater percentages of organic matter or supplemental irrigation. They also reported that a greater number of smooth aster (Aster laevis), junegrass (Koeleria macrantha), and showy goldenrod (Solidago speciosa) survived when they were not fertilized. Presumably, these plants could survive drought conditions for a longer period of time since they had less biomass to maintain. In contrast, if the purpose of the green roof is urban agriculture then fertility levels must be relatively high to produce acceptable yields for fruits and vegetables (Whittinghill and Rowe 2012a; Whittinghill et al. 2013) .
Most commercial green roofs are built within German FLL guidelines (FLL 2008) and are composed of manufactured plastic layers topped with engineered growing substrates . These standards help to assure consistency of materials and success of green roof projects. However, many are being built without these expensive components, especially in Switzerland (Brenneisen 2006; Kiers 2013). Stephan Brenneisen, from the University of Applied Sciences Wädenswil, has been a proponent for the construction of green roofs with the primary purpose of promoting biodiversity. For example, some roofs utilize gravel or layers of straw or grasses such as maidengrass (Miscanthus sinensis) as the drainage layer , use native soils blended with other components such as lava rock or gravel, and are planted with native wildflowers. A commercial installer may be hesitant to go outside FLL substrate specifications, but other systems do work (Chaps. 3, 6).
An excellent example of a green roof constructed with non-standard green roofing materials is the Moos Lake water filtration plant in Wollishofen, Zürich, Switzerland. Installed in 1914, the roof was built long before German FLL guidelines were written and adopted (Fig. 13.4). The original drainage layer consisted of gravel topped with 12.5 cm (5 in) of sand and 15–20 cm (6–8 in) of local topsoil. After 100 years those layers are no longer distinguishable, but there are neither problems with drainage nor any negative effects on the vegetation, and the original roofing membrane is still in place. The 30,000 m2 (322,917 ft2) roof is home to 175 plant species, several of which are now endangered or rare. The roof consists of nine species of orchids and approximately 6000 specimens of green-winged orchid (Orchis morio) a species that is now extinct in the landscape surrounding Zürich. The roof reflects species richness of the surrounding area from 100 years ago as well as today. The original vegetation developed from the seed bank that was part of the original topsoil. Today, plant composition consists of these original species as well as any new species that colonized from the surrounding landscape .
13.3.2 Substrate Depth
Substrate depth has a major impact on plant survival and long-term plant communities. Depending on climate and the availability of supplemental irrigation, most shallow extensive green roofs are limited to drought tolerant species such as succulents. This is primarily due to a lack of moisture (Dunnett and Nolan 2004; Durhman et al. 2006), but some taxa such as Sedum spp. are naturally found in these conditions. However, even among succulents , substrate depth will influence total coverage and coverage of individual species. In Pennsylvania, Thuring et al. (2010) reported that white stonecrop (S. album) and six-sided stonecrop (S. sexangulare) survived in 3 cm (1.2 in), but produced greater biomass at depths of 6 cm (2.4 in) and 12 cm (4.7 in). Similarly, Getter and Rowe (2009) reported that the majority of the 12 species of Sedum tested in Michigan exhibited greater growth and coverage at a depth of 7.0 cm (2.7 in) and 10.0 cm (4 in) compared to 4.0 cm (1.6 in). At 5.0 cm (2 in) and 7.5 cm (3 in), Phedimus spurius and Sedum middendorfianum were the dominant species, but at 2.5 cm (1 in), S. acre and S. album covered the most area (Durhman et al. 2007).
In Sweden, S. acre and S. album were dominant at a depth of 4 cm (1.6 in) while the other succulents in the study, S. reflexum (syn. S. rupestre), S. sexangulare, pink Mongolian stonecrop (Hylotelephium ewersii), Chinese sedum (Phedimus floriferus), hybrid stonecrop (Phedimus hybridus), Phedimus kamtschaticus (syn. S. kamtschatium), and Caucasian stonecrop (Phedimus spurius) grown in various combinations had minimal coverage by the end of 3 years, generally 15 % or less for all other species combined (Emilsson and Rolf 2005; Emilsson 2008). Development over time varied depending on the original species mix planted, as well as substrate composition .
As depth increases, the number of potential species expands to grasses, many annual or herbaceous perennials, and even woody plants. Deeper substrates are beneficial for both increased water holding capacity (Durhman et al. 2006; VanWoert et al. 2005a; VanWoert et al. 2005b) and as a buffer for overwintering survival, as shallow substrates are more subject to fluctuations in temperature (Boivin et al. 2001). As discussed above, Dunnett et al. (2008) reported the greatest survival, diversity, size, and flowering performance of grasses and herbaceous perennials occurred at a substrate depth of 20 cm (8 in) compared to a depth of 10 cm (4 in). By the end of 5 years, all species survived at both depths, however, 14 of 15 species maintained at least 50 % of their original numbers at 20 cm (8 in) whereas, only eight did so at 10 cm (4 in). Likewise, in Southern Tuscany, most of the 20 Mediterranean xerophytic species tested exhibited greater growth and cover at 20 cm (8 in) relative to those grown at 15 cm (6 in) (Benvenuti and Bacci 2010). In addition to greater moisture stress, temperatures in the shallower substrate [15 cm (6 in)] reached a maximum of 90 °F (50 °C) and were on average 9 °F (5 °C) higher than the 20 cm (8 in) deep substrate. This could be partially explained by the fact that shallower substrate depths often have less coverage which exposes more substrate to direct sun resulting in higher substrate temperatures (Getter et al. 2009a) .
13.3.3 Substrate Moisture
Substrate moisture is a function of substrate composition and depth and is often the limiting factor for plant survival on green roofs (Dvorak and Volder 2010). In the Getter and Rowe (2009) study discussed above, mean volumetric moisture content at the three substrate depths were correlated with plant growth and coverage. Similarly, Thuring et al. (2010) reported that the herbaceous species tested were severely affected by drought when grown in shallower substrates. In addition, Monterusso et al. (2005) found that only four of 18 species of native herbaceous perennials and grasses still existed after 3 years when grown at a 10 cm (4 in) depth without irrigation. The majority of the plants tested were considered to be drought tolerant, but their survival in a native environment relies on deep tap roots to obtain moisture. Survival and persistence could have been improved by increasing substrate moisture through changes in substrate composition, depth, or by providing irrigation .
However, deeper substrate depths that hold more moisture are not beneficial to all plant species as long-term survival of stress tolerant species often depends on shallow soil depths with limited moisture. Otherwise, species with greater growth potential will outcompete them. This was even evident in the Rowe et al. (2012) study as S. acre and S. album were dominant at 2.5 cm (1 in) whereas P. spurius and S. middendorfianum were most prevalent at deeper depths. Similarly, Emilsson (2008) reported that S. acre decreased in area of coverage after 2 years. This may be because S. acre allocates a relatively small percentage of plant carbon to the root system (Getter et al. 2009b) and these roots also tend to be shallow and less able to compete for water. Increasing substrate depth is of no advantage to this species, as it must then compete against more aggressive plants with greater biomass (Getter and Rowe 2009). Likewise, in the Dunnett et al. (2008) study, Armeria maritima performed better at 10 cm (4 in) relative to 20 cm (8 in). Armeria maritima is a self-seeder and likely took advantage of the greater bare space at the shallower depth. Other species such as the succulents T. calycinum and T. parviflorum also depend on bare space for long-term survival in climates such as that found in Michigan. These species are perennials, but are killed by cold winter temperatures in Michigan and reappear each year by reseeding. However, as the roof obtains 100 % coverage, there is little open space for germination to continue from year to year and the species eventually disappears (Getter et al. 2009a).
Supplemental irrigation can alleviate substrate moisture problems, but the use of potable water on green roofs is often problematic. If irrigation is to be supplied, then it should be done so with the most efficient and sustainable method for the particular application (Rowe et al. 2014). Irrigation is critical when growing vegetables on roofs (Whittinghill and Rowe 2012a; Whittinghill et al. 2013) .
13.3.4 Microclimates, Roof Slope, Orientation, and Irradiance Levels
Microclimates present on a roof will dramatically influence short and long-term plant communities (see Chap. 3). They can be caused by variations in substrate composition and depth as described above, or from variations in irradiance levels due to shaded areas, roof slope, and roof orientation .
In the irradiance level (full sun versus full shade) study on the MSU Communication Arts Building described above it was found that regardless of depth, species differed depending on sun exposure (Getter et al. 2009a). After four growing seasons, heath sedge (Carex flacca), was still present at 12 cm (4.7 in), but only in the shade. After 9 years it has completely disappeared. Even though species mix was changing among solar radiation levels, overall coverage was not significantly different between sun and shade. Roof slope and orientation also influence substrate moisture and thus plant communities. Getter et al. (2007) reported that water retention was reduced by 10 % when slope increased from 2 to 25 %. Orientation is also important as evapotranspiration increases with solar exposure. Köhler and Poll (2010) reported that the greatest plant coverage was found on north-facing sections of the roof on the Paul-Lincke-Ufer Building in Berlin. The most dominant species was Allium schoenoprasum while on south facing slopes Sedum spp. dominated. Grasses were least competitive on west facing slopes .
One example of a roof designed to create various microclimates that in turn promote diversity is the California Academy of Sciences in San Francisco (Hauser 2013). The seven domes create different microclimates due to variations in slope and sun exposure and thus substrate moisture. This in turn influences the plant communities that find their niche among the various microclimates where they have a competitive advantage. The roof was originally planted in 15 cm (6 in) of substrate with four perennial and five annual species uniformly spaced over the entire roof. Today, there are approximately 70 native species thriving where the environmental conditions are best for each individual species. This increase in species richness is contrary to the decreases that occurred when only one substrate depth was employed on the other roofs described above .
Therefore, it seems logical that one way to increase plant diversity on green roofs is to create multiple microclimates. If roof slope and orientation are not options then variations in substrate depth and composition can be created. Because different species can compete best in a specific environment, each species will find its niche location where it has advantages over competing species. This will likely increase the biodiversity potential and improve the species richness of the long-term plant community as environmental conditions change over time and species increase and decrease in numbers .
13.3.5 Initial Plant Choices, Functional Diversity and Complexity, and Maintenance Practices
The plants present on a green roof at any given time also depend on what was initially planted, the functional diversity and complexity of these species, and the intensity or lack thereof of maintenance . Some plants may be originally chosen for factors such as aesthetics , but may be ill suited for the particular environmental conditions and destined to fail. Others may be too aggressive and will crowd out everything else (Getter and Rowe 2009; Rowe et al 2012). If the overly aggressive species was not planted to begin with, then the dynamic would be completely different.
Maintenance is also a major factor. If ‘weed’ species are removed on a regular basis then they clearly will not be able to colonize a roof. In this case, weeds are defined as any species that was not planted in the original design. Colonizing species will also be influenced by the proximity to local seed sources. The height of the roof and the surrounding landscape will influences seed sources (Chap. 15). Maintenance practices such as irrigation and fertility management are also major factors as discussed in Chaps. 4 and 5.
There are also complex interactions among plants (Chap. 8). Nagase and Dunnett (2010) studied how plant diversity on a green roof influenced survival by testing combinations of three major taxonomic and functional plant groups that are commonly used for extensive green roofs (forbs, sedums and grasses). They concluded that under drought conditions, combinations of species differing in functional diversity and complexity exhibited greater survival rates and visual qualities than monocultures. They attributed this result to the fact that plants of the same taxonomic group compete for the same resources when grown together.
In addition, Butler and Orians (2011) showed that the drought tolerant succulent, S. album, could have a positive or negative influence on neighboring plants depending on substrate moisture content . When ample substrate moisture was present, S. album had an adverse effect on growth of threadleaf giant hysop (Agastache rupestris) and whorled milkweed (Asclepias verticillata). In contrast, during drought conditions the presence of S. album as an understory cover facilitated growth of these more water dependent herbaceous plants. The favorable response during drought is likely due to S. album shading the surface, reducing evaporation from the substrate surface, and from a reduction in substrate temperatures (Butler and Orians 2011). One might expect the same result for other plant species although the use of S. album cover crop for green roof production of an assortment of vegetables had no effect on crop yields (Whittinghill and Rowe 2012b). Vegetables tested were tomatoes (Lycopersicon esculentum), bush beans (Phaseolus vulgaris), bush pickle hybrid cucumbers (Cucumis sativus), sweet peppers (Capsicum annuum), and large-leaf Italian basil (Ocimum basilicum). However, these plants were irrigated regularly so water deficit conditions were never an issue.
13.4 The Long Term Ecological Research (LTER) Model Applied to Green Roofs
Long-Term Ecological Research (LTER) is a National Science Foundation (NSF) funded program that was created in 1980 (Callahan 1984; Kratz et al. 2003) . The research network of scientists currently includes 26 research sites studying ecology over extended temporal and spatial scales . Long-term studies are important because the natural world is dynamic and with climate change, patterns of natural variation are occurring even faster. Plant communities take time to accumulate biomass, respond to disturbances such as invasions of native and non-native species, weather extremes, or disease and insect pressures, and there may be time lags between the cause and effect of ecological changes. They can provide a baseline from which to determine if an ecological system has changed over time and define the range of natural variability, they allow us to assess relationships and interactions among various components of the system, they allow us to detect cause and effect relationships among slowly changing variables, and data gathered across multiple sites can lead to stronger conclusions than those from single sites (Kratz et al. 2003) .
Although many would argue that placing plants on top of buildings in artificial substrates is not a natural system, the same LTER concepts apply to green roofs. One difference is that studies of natural landscapes could span decades, centuries, or even thousands of years. Buildings do not last that long. Green roofs are limited in time as most roofing membranes are replaced within 40–50 years. So what constitutes a long-term study on a green roof? Regardless, studies that span years are critical for making sound conclusions on long-term plant communities. The short 1 and 2 year studies that are common in the green roof literature do not really tell us anything about what species will be populating a green roof in the future. These short-term experiments are really just studies of plant establishment . However, the prevalence of 1 or 2 year studies at single sites is not surprising as research funding is rarely guaranteed for more than a few years. Also, many studies are graduate student projects, which cannot be dragged out for years and years. Even so, when studies have been conducted for three or more years, conclusions drawn are often dramatically different than what would have been concluded following just one or two seasons. Three to 5 years seems sufficient to predict long-term plant communities on shallow roofs consisting of sedum. However, on deeper roofs or roofs where species are allowed to colonize, then a much longer period of time is needed .
The few longer-term green roof studies where the original plantings were recorded in order to provide a baseline from which to work from all point to the importance of long-term studies. As outlined above from the 7 year study on the MSU Horticulture Teaching and Research Center, conclusions drawn at the end of 2 years were significantly different than what was present following 7 years (Durhman et al. 2007; Rowe et al. 2012). Similar results were drawn comparing 12 species of stonecrop in terms of absolute cover (Getter and Rowe 2008; Getter and Rowe 2009). Likewise, Dunnett et al. (2008) emphasized the importance of long-term monitoring of green roofs because of the changes that occurred in plant communities from the first to the fifth year of their experiment with 15 herbaceous perennials and grasses (Dunnett and Nolan 2004; Dunnett et al. 2008). In all of the above studies, changes in plant community development occurred faster at shallower substrate depths relative to deeper ones .
Setting up green roof research sites similar to the NSF LTER program would provide opportunities to follow changes to green roof habitats for longer periods of time and also look at similarly designed roofs across geographic distances. Because of the relatively short life spans of roofing membranes and modern buildings, it may be more feasible to conduct replicated studies over numerous geographic locations with varying climates, etc. As with most research, the primary roadblock to doing so is funding .
13.5 Future Research Needs and Questions
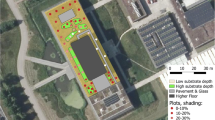
Since there have only been a handful of green roof plant studies that were carried out for more than 1 or 2 years, an obvious place to start is to initiate more of these studies. One example is a study that was initiated in 2011 to evaluate establishment, survival, and changes in plant community over time on the Molecular Plant Sciences Building at Michigan State University (Fig. 13.5). Plugs of four grasses and 13 herbaceous perennials native to Michigan were installed at substrate depths of 10 cm (4 in) and 20 cm (8 in). Up to 45 plugs of each species were planted on 20 cm (8 in) centers. Survival rates were recorded during June 2012 and as expected most species experienced greater survival when grown in 20 cm (8 in) relative to those at 10 cm (4 in). The roof will continue to be sampled every year into the distant future to record the presence of individual species.
In long-term studies it is important to record baseline plantings when the roof was first installed in order to know what changes occur over time. However, older existing roofs should to be sampled also even if it is not exactly known what existed there in the beginning. Estimates can often be made based on the type of roof, location, and who installed the roof. For example, even though it is not known exactly what was planted on day one and the roof was overseeded and additional species added the year after installation, the roof on the Church of Latter-day Saints Convention Center described above should be reevaluated. The roof is now 14-years-old and valuable information could be gleaned and compared to the original study. In addition, changes in substrate composition should be looked at on this roof as well as others. Since long-term studies may not always be possible, the LTER model could still be followed by replicating studies over multiple geographic locations to determine the role of site-specific environments on plant community development. Plants species should be tested by themselves and in combination with multiple species. Other factors that should be investigated include interactions among plant species, the effects of roof maintenance (pulling weeds or allowing other species to colonize), and how different plant combinations influence ecosystem services such as stormwater management, heat flux , aesthetics , and the ability of the roof to provide habitat for wildlife . Common sense would suggest that increasing plant diversity would increase the ability of a green roof to provide these services and reduce the impact of environmental change (Cook-Patton and Bauerle 2012). Although this statement is true for the most part, adding plant species without considering their interactions may actually decrease services (Lundholm et al. 2010, MacIvor et al. 2011). Research is needed to determine which combinations of species and functional groups will complement each other and maximize services over time (Chap. 8). It is a challenge to balance relative competition among species so that more aggressive species do not dominate the community and reduce biodiversity.
Lastly, more roofs need to be installed where multiple microclimates are created and then these interactions among the various microclimates need to be studied to see how they influence long-term plant communities. Different microclimates were achieved on the California Academy of Sciences Building in San Francisco due to roof slope and sun exposure. Other options include varying substrate compositions and depths on the same roof. All of these practices should increase plant diversity, green roof function, and long-term success.
References
Benvenuti S, Bacci D (2010) Initial agronomic performances of mediterranean xerophytes in simulated dry green roofs. Urban Ecosyst 13:349–363
Berendse F (1998) Effects of dominant plant species on soils during succession in nutrient poor ecosystems. Biogeochemistry 42:73–88
Boivin M, Lamy M, Gosselin A, Dansereau B (2001) Effect of artificial substrate depth on freezing injury of six herbaceous perennials grown in green roof system. Hort Technology 11(3):409–412.
Brenneisen S (2006) Space for urban wildlife: designing green roofs as habitats in Switzerland. Urban Habitats 4:27–36
Butler C, Orians C (2011) Sedum cools soil and can improve neighboring plant performance during water deficit on a green roof. Ecol Eng 37(11):1796–1803
Callahan J (1984) Long-term ecological research. BioScience 34(6):363–367
Cook-Patton S, Bauerle T (2012) Potential benefits of plant diversity on vegetated roofs: a literature review. J Environ Manage 106:85–92
Dewey D, Johnson P, Kjelgren R (2004) Species composition changes in a rooftop grass and wildflower meadow. Native Plants J 5(1):56–65
Dunnett N, Nolan A (2004) The effect of substrate depth and supplementary watering on the growth of nine herbaceous perennials in a semi-extensive green roof. Acta Hort. 643:305–309
Dunnett N, Nagase A, Hallam A (2008) The dynamics of planted and colonizing species on a green roof over six growing seasons 2001–2006: influence of substrate depth. Urban Ecosys 11:373–384
Durhman A, Rowe DB, Rugh C (2006) Effect of watering regimen on chlorophyll fluorescence and growth of selected green roof plant taxa. HortScience 41(7):1623–1628
Durhman A, Rowe DB, Rugh C (2007) Effect of substrate depth on initial growth, coverage, and survival of 25 succulent green roof plant taxa. HortScience 42(3):588–595
Dvorak BD, Volder A (2010) Green roof vegetation for North American ecoregions: a literature review. Landsc Urban Plan 96:197–213
Emilsson T (2008) Vegetation development on extensive vegetated green roof: Influence of substrate composition, establishment method and species mix. Ecol Eng 33:265–277
Emilsson T, Rolf K (2005) Comparison of establishment methods for extensive green roots in southern sweden. Urban for Urban Green 3:103–111
Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau (FLL) (Research Society for Landscape Development and Landscape Design) (2008) Introduction to the FLL: guidelines for the planning, construction and maintenance of green roofing. FLL, Bonn, Germany
Getter K, Rowe DB (2008) Media depth influences Sedum green roof establishment. Urban Ecosys 11:361–372
Getter K, Rowe DB (2009) Substrate depth influences sedum plant community on a green roof. HortScience 44(2):401–407
Getter K, Rowe DB, Andresen JA (2007) Quantifying the effect of slope on extensive green roof stormwater retention. Ecol Eng 31:225–231
Getter K, Rowe DB, Cregg B (2009a) Solar radiation intensity influences extensive green roof plant communities. Urban For Urban Green 8(4):269–281
Getter K, Rowe DB, Robertson G, Cregg B, Andresen J (2009b) Carbon sequestration potential of extensive green roofs. Environ Sci Technol 43(19):7564–7570
Hauser K (2013) An evolving landscape: the living roof at California Academy of Sciences. Pac Hortic 74(4):24–27
Kiers H (2013) Back to nature: an alternative approach to planting green roofs. Pac Hortic 74(4):28–30
Köhler M (2006) Long-term vegetation research on two extensive green roofs in Berlin. Urban Habitiats 4(1):3–26
Köhler M, Poll P (2010) Long-term performance of selected old Berlin greenroofs in comparison to younger extensive greenroofs in Berlin. Ecol Eng 36:722–729
Kratz T, Deegan L, Harmon M, Lauenroth W (2003) Ecological variability in space and time: insights gained from the US LTER program. BioScience 53(1):57–67
Liesecke H (1998) Langzeitentwicklung einer extensiven Dachbegrünung: Untersuchungenzum Substratverhalten und zur Vegetationsentwicklung eines 1985 ausgeführten Objektes (Long-term development of extensive roof vegetation: a study on substrate and vegetation development over 12 years). Stadt-und-Grün 47:428–436
Lundholm J, MacIvor J, MacDougall Z, Ranalli M (2010) Plant species and functional group combinations affect green roof ecosystem functions. PLoS ONE 5(3):11
MacIvor J, Ranalli M, Lundholm J (2011) Performance of dryland and wetland plant species on extensive green roofs. Ann Bot 107:671–679
Monterusso M, Rowe DB, Rugh C (2005) Establishment and persistence of Sedum spp. and native taxa for green roof applications. HortScience 40(2):391–396
Nagase A, Dunnett N (2010) Drought tolerance in different vegetation types for extensive green roofs: effects of watering and diversity. Landsc Urban Plan 97:318–327
Oberndorfer E, Lundholm J, Bass B, Connelly M, Coffman R, Doshi H, Dunnett N, Gaffin S, Köhler M, Lu K, Rowe DB (2007) Green roofs as urban ecosystems: ecological structures, functions, and services. BioScience 57(10):823–833
Rowe DB (2011) Green roofs as a means of pollution abatement. Environ Pollut 159(8–9):2100–2110
Rowe DB, Getter K (2010) Green roofs and roof gardens. In: Aitkenhead-Peterson J, Volder A (eds) Urban ecosystems ecology. Agron. Monogr. 55. American Society of Agronomy. Crop Science Society of America. Soil Science Society of America, Madison, WI, p 391–412
Rowe DB, Monterusso M, Rugh C (2006) Assessment of heat-expanded slate and fertility requirements in green roof substrates. HortTechnology 16(3):471–477
Rowe DB, Getter K, Durhman A (2012) Effect of green roof media depth on Crassulacean plant succession over seven years. Landsc Urban Plan 104(3–4):310–319
Rowe DB, Kolp M, Greer S, Getter K (2014) Comparison of irrigation efficiency and plant health of overhead, drip, and sub-irrigation for extensive green roofs. Ecol Eng 64:306–313
Skabelund L, Blocksome C, Hamehkasi M (2014) Semi-arid green roof research 2009–2014: resilience of native species. Proc. of 12th North American Green Roof Conference: Cities Alive, Nashville, TN
Thuring C, Dunnett N (2014) Vegetation composition of old extensive green roofs (from 1980s Germany). Ecol Process 3:4. doi:10.1186/2192-1709-3-4
Thuring C, Berghage R, Beattie D (2010) Green roof plant responses to different substrate types and depths under various drought conditions. HortTechnology 20(2):395–401
VanWoert N, Rowe DB, Andresen J, Rugh C, Fernandez R, Xiao L (2005a) Green roof stormwater retention: effects of roof surface, slope, and media depth. J Environ Qual 34(3):1036–1044
VanWoert N, Rowe DB, Andresen J, Rugh C, Xiao L (2005b) Watering regime and green roof substrate design affect Sedum plant growth. HortScience 40(3):659–664
Whittinghill L, Rowe DB (2012a) The role of green roof technology in urban agriculture. Renew Agric Food Sys 27(4):314–322
Whittinghill L, Rowe DB (2012b) Vegetable production on extensive green roofs. Proc. of 10th North American Green Roof Conference: Cities Alive, Chicago, IL. 17–20 October, 2012
Whittinghill L, Rowe DB, Cregg B (2013) Evaluation of vegetable production on extensive green roofs. Agroecol Sustain Food Syst 37(4):465–484
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rowe, B. (2015). Long-term Rooftop Plant Communities. In: Sutton, R. (eds) Green Roof Ecosystems. Ecological Studies, vol 223. Springer, Cham. https://doi.org/10.1007/978-3-319-14983-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-14983-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14982-0
Online ISBN: 978-3-319-14983-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)