Abstract
A large amount of hazardous materials including heavy metals were released into the environment from natural and extensive anthropogenic activities, which cause soil, air, and water pollution and deterioration. At higher concentration, these metals exert toxic effects on plant and animal health including human. Among various traditional soil remediation technologies, use of phytoremediation to clean up metal(loid)-contaminated sites has gained increasing attention as an inexpensive, eco-friendly, and publicly acceptable remediation technology but has experienced varied successes in practice. Recent scientific discoveries that resulted from the application of molecular biology, bioinformatics, omics, and next-generation DNA sequencing technologies have assisted the remarkable impact of these immensely parallel platforms on genetics. In this context, genetic engineering has contributed rapid and significant changes in the crop improvement by offering a wide array of novel genes and traits which can be effectively inserted into candidate plants to raise its phytoremediation potential for metal removal. This review summarizes recent advances in the field of transgenic plant research with particular emphasis on genetic engineering of plants for heavy metal removal from contaminated soils, potential target genes, plant transformation methods, model systems for transgenic studies, optimization of transgene expressions in transgenic plants, along with risk assessment and mitigation strategies. Besides, the role of transgenic hairy roots and genetic engineering of plant symbionts in heavy metal phytoremediation is also discussed. However, translation of this knowledge into usable technologies is the need of the hour to accelerate phytoremediation as an eco-friendly and cost-effective technology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Heavy metals are elements with metallic properties and have atomic mass >20 and specific gravity above 5 g cm−3 (Rascio and Navari-Izzo 2011). The most common heavy metal contaminants are arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), mercury (Hg), lead (Pb), and zinc (Zn) (Ha et al. 2014). Metals are natural components present in the soil (Tangahu et al. 2011). A large amount of hazardous materials including heavy metals were released into the environment from natural (e.g., geological erosion and saline seeps) and extensive anthropogenic (e.g., mining, agriculture, industry, wastewater treatment, construction) activities, which cause soil, air, and water pollution (Arthur et al. 2005). Some metals are micronutrients (requires at low concentrations) necessary for plant growth, such as Zn, Cu, manganese (Mn), nickel (Ni), and cobalt (Co), while others have unknown biological functions, such as Cd, Pb, and Hg (Appenroth 2010). At higher concentration these metals exert toxic effects on plant and animal health including human (Table 22.1). Unlike organic contaminants heavy metal does not undergo biodegradation and persist in the soil for a long time; therefore, its removal from the soil is receiving great attention.
The conventional (e.g., soil excavation, land filling, soil washing) and physicochemical (e.g., thermal treatment, chemical extraction, encapsulation) methods were employed to remediate contaminated soil. These methods are expensive, inefficient especially for large-scale cleanup, and non-eco-friendly as they destroy natural habitat and leave unsightly scars on the landscape. Consequently, alternative biological methods such as bioremediation (use of living organisms), phytoremediation (use of plants), and zoo remediation (use of animals) (Gifford et al. 2006) are developed for environmental remediation. Of these, phytoremediation has emerged as inexpensive, eco-friendly, and publicly acceptable remediation technology that utilizes plants and associated microorganisms not only to remove, transform, or stabilize contaminants in the water and soil but also help in CO2 sequestration, soil stabilization, watershed management, biodiversity improvement, providing diverse sources of energy, and aesthetics (Dickinson et al. 2009).
However, the slow rate of metal removal and incomplete metabolism, as an autotroph plant, lack catabolic enzyme machinery necessary to achieve degradation/full mineralization of xenobiotic substances which stymied the progress of phytoremediation. This results into the accumulation of toxic metabolites as it is into the plant tissues that could be released into the environment (Aken 2008). The possibility of the release of toxic substances into the food chain limits the widespread utilization of phytoremediation. Genetic engineering of plants has the potentiality to overcome these challenges. This chapter summarizes our current knowledge of transgenic plants for heavy metal removal from contaminated area.
2 Basic Strategies of Phytoremediation for Metals
The use of plants and their associated microbes to bioremediate contaminated soil, sediments, and water is known as phytoremediation (Arthur et al. 2005; Cherian and Oliveira 2005). The plants used several different phytoremediation strategies such as rhizofiltration, phytostabilization, phytodegradation, phytoextraction, and phytovolatilization to decontaminate soil and water contaminated with several organic pollutants (Arthur et al. 2005). Out of these, rhizofiltration-, stabilization-, extraction-/accumulation-, and volatilization-based phytoremediation strategies were utilized by the plants to remove metals from contaminant soils (EPA 1998).
In rhizofiltration, plant root system is employed for the elimination of metals from contaminated wastewater, where they absorb and accumulate metals in the roots (Dushenkov et al. 1995). Similarly, the hairy roots induced in some plants by the Agrobacterium infection were also utilized for rhizofiltration of radionuclides and heavy metals (Eapen et al. 2003; Straczek et al. 2009).
Phytostabilization simply prevents/reduces the mobility and bioavailability of metals in the environment through immobilization of soil by plant roots (Salt et al. 1995). Therefore, in phytostabilization, even if metal concentration is not reduced, the migration of metals in the surrounding environment is prevented (Li et al. 2000).
In phytoextraction, plants absorb metals from contaminated soils and concentrate/accumulate them into harvestable plant parts (shoot, leaves, etc.). After harvesting, the plant parts may be ashed or utilized for metal recovery followed by disposal of the ashes in a landfill (Kumar et al. 1995). It is the most effective among several phytoremediation methods, although technical difficulties exist in their applications (Krämer 2005).
Phytovolatilization involves the use of plants to take up the metals from the soil, transforming them into volatile form, and release them through transpiration into the atmosphere (Bizily et al. 2003; Rugh et al. 1998). The details of all of these technologies are summarized by Arthur et al. (2005).
3 Improving Metal Phytoremediation with Genetic Engineering of Plants
Widespread utilization of phytoremediation can be limited by the small habitat range or size of plants expressing remediation potential and insufficient abilities of native plants to tolerate and accumulate contaminants (Arthur et al. 2005). Several approaches such as agronomic practices (planting density, fertilization) (Chaney et al. 2000), use of soil amendments (organic acids, synthetic chelators) (Joner 2013), and conventional breeding are utilized to increase biomass and metal uptake capacity of the suitable plants used for metal phytoremediation (Pilon-Smits and Pilon 2002).
However, in order to achieve a better phytoremediation efficiency, plants should have the ability to grow outside their area of collection, extensive root system, and fast growth rate; accumulate high amounts of heavy metals in their easily harvestable parts; tolerate soil pollution; and also produce a great quantity of biomass in contamination condition (Pilon-Smits 2005). The development of plants having all these traits is not possible through conventional breeding methods as these are time-consuming and laborious and have several other ecological, physiological, and biological constraints. On the other hand, the precision of biotechnological approaches, mainly genetic engineering, contributed rapid and significant changes in the crop improvement by offering a wide array of novel genes and traits which can be effectively inserted into candidate plants to improve their phytoremediation potential for metal removal.
4 Transgenic Plants for Heavy Metal Removal
Transgenic plants expressing desirable genes from different organisms are developed to increase the heavy metal remediation efficiency of plants. Two principle strategies have been pursued in the phytoremediation techniques, i.e., improved in planta and ex planta metabolism that may lead to enhanced removal of xenobiotics/heavy metal (Fig. 22.1). In planta process includes uptake and diffusion through the roots, trunk, or leaves, sorption and transformation, and/or sequestration via tree metabolic activity manipulation. Alternatively, ex planta process includes genetic engineering of plants for extracellular synthesis of reactive enzymes, metal-selective ligands (phytosiderophores or chelating agents), or plant-associated microorganisms (bacteria and entophytes) in the rhizosphere (James and Strand 2009; Ma and Nomoto 1996; Raskin 1996).
4.1 Host Systems
The plant system which is transformed for enhanced phytoremediation ability should possess certain characteristics such as extensive deep rooting system, fast growth rate with high biomass, tolerance to contaminants, ease of genetic transformation, stability of gene expression, and amenable to breeding procedures, and it should not be a food or fodder crop, and it has an advantage if it has an economic value (Kotrba et al. 2009). No single plant species can have all these characteristics. However, depending on phytoremediation strategies, types of heavy metal, and its intended final use, the plant species have to be selected.
4.2 Model Systems for Genetic Engineering Study
There is a great diversity in biological species present on the earth, and it is practically impossible to analyze each of them in detail. Since, phylogenetically related species display strong similarities in their genetic makeup, physiology, and behavior, at the molecular level, principle cellular signaling pathways are highly conserved. Biologists recognized the merits of employing model organisms as representatives of their species/subspecies to investigate the phenomena and mechanisms of development in great depth. Moreover, for most model organisms, protocols for transgenesis, full genome sequence information, and numerous bioinformatic resources are available, which offer significant informative data and tools that helps not only to improve plant phytoremediation properties in a highly targeted manner but also to disentangle biological complexity, to unravel networks of molecular interactions, and finally to predict mechanisms in distantly related species (Pitzschke 2013).
4.2.1 Whole Plant Systems
Arabidopsis and tobacco are considered as two of the best model species for phytoremediation-related transformation studies on metal tolerance and accumulation and for testing in planta expression of potential target gene constructs prior to transformation of other candidate species. The development of optimized tissue culture protocols and well-established genetic transformation methods makes Arabidopsis and tobacco ideal candidates for transformation studies. Additionally, this small nonfood plant with short generation time and prolific seed production provides an advantage to test and clarify the roles/functions of transgenes in a very short time.
4.2.2 In Vitro Cultures
In vitro culture involves growing plant cells and tissues aseptically on defined medium in environmentally controlled conditions (temperature, photoperiod, and darkness) (George et al. 2008). The in vitro dedifferentiated cells (callus or cell suspension), differentiated organs (roots and shoots), and genetically transformed (hairy roots and shooty teratomas) culture are convenient laboratory tools for phytoremediation studies related to metal removal (Doran 2009).
The in vitro culture systems offer a number of advantages over a whole plant system as follows. (1) Once established, these in vitro culture systems can be propagated indefinitely and are available on demand. (2) The in vitro (callus, cell suspensions) culture systems used as a suitable material to carry out nuclear transformation studies and also play an important role in the selection and confirmation of successful transformants having desired trait prior to time-consuming process of plant regeneration are carried out. (3) Use of in vitro cultures allows experiments to be carried out using material derived from the same parent plant avoiding the effects of variability between individual specimens. (4) An in vitro screening reduces not only the growth period and the treatment time length of the plants but also the space required for the experiments. (5) The in vitro systems allow the independent study of the complex interaction among plant/soil/microbiota to evaluate the participation of specific enzymes, organic compounds, transporters, or peptides involved in the plant response to the pollutants (Boominathan and Doran 2002; Doran 2009; Flocco and Giulietti 2007). (6) Cell cultures are also a useful system for metabolic engineering and for obtaining rapid evidence of the ecotoxicological behavior of chemicals and heavy metals in plants with less analytical expense (Golan-Goldhirsh et al. 2004). (7) Moreover, the environmental factor variability is also reduced, physiological activities can be increased by modifying the culture conditions (e.g., employing biotic and abiotic stress), and it is easier to isolate and analyze metabolites (Hu and Du 2006; Shanks and Morgan 1999). (8) When non-differentiated tissues are employed, genetic and epigenetic changes can be observed due to somaclonal variations (Lee and Phillips 1988). However, this variation and in vitro selection seem to be an appropriate technology for the development of new plant variants with enhanced metal accumulation and extraction properties (Herzig et al. 2003; Jan et al. 1997; Nehnevajova et al. 2007). (9) This approach also allows the analysis of metal accumulation properties of each organ (Kartosentono et al. 2001; Nedelkoska and Doran 2000a) and the possibility to develop industrial bioreactor models (Giri and Narasu 2000; Kim et al. 2002).
In spite of the number of advantages offered by in vitro culture systems in phytoremediation research, it is neither practical nor a feasible technology for direct large-scale phytoremediation applications. However, in vitro cell suspensions and hairy root culture are frequently used as model systems for understanding the metabolic and tolerance mechanisms that function in whole plants (Doran 2009).
4.3 Methods of Gene Transfer to the Plants
Significant achievements in vector-mediated/indirect and vectorless/direct gene transfer strategies have tremendously been assisting scientists to incorporate genes from any organisms to plants in a specific and targeted approach. Among the various vector-mediated transformation strategies, Agrobacterium tumefaciens plant-pathogenic soil bacterium has been widely used as a vector to create transgenic plants. This bacterium is known as “natural genetic engineer” of plants because these bacteria have natural ability to transfer a portion of its Ti (tumor inducing) plasmid and T-DNA (transfer DNA) to the genome of the host plant upon infection of cells at the wound site and cause an unorganized growth of a cell mass known as crown gall. Ti plasmids are used as gene vectors for delivering useful foreign genes into target plant cells and tissues. The foreign gene is cloned in the T-DNA region of Ti plasmid in place of unwanted sequences (Block 1993; Gelvin 2003). The T-DNA transfer and its integration into the plant genome are governed by various Agrobacterium and plant tissue-specific factors which include genotype of the plant, type of explant, plasmid vector, bacterial strain, composition and pH of culture medium, temperature and time of cocultivation, tissue damage, and suppression or elimination of Agrobacterium infection after cocultivation (Ziemienowicz 2014). A. tumefaciens-mediated transformation method has been extensively utilized for engineering dicotyledonous plants including tobacco (Gisbert et al. 2003; Pomponi et al. 2006), A. thaliana (Kim et al. 2005; Shukla et al. 2013), and Indian mustard (Gasic and Korban 2007; LeDuc et al. 2004) for heavy metal phytoremediation. However, monocotyledon plants and forest trees are considered as recalcitrant to Agrobacterium-mediated transformation. Therefore, considering the limitations of vector-mediated (Agrobacterium) gene transfer methods, the vectorless/direct gene transfer methods have been developed. The microprojectiles or biolistics or particle gun for gene transfer is widely employed in plant genetic engineering studies (Chen et al. 1998; Rugh et al. 1998). In this method, tungsten or gold microparticles coated with DNA of interest are accelerated to high velocity by a particle gun apparatus into living plant tissue. These particles with high kinetic energy penetrate the cells and membranes and carry foreign DNA inside of the bombarded cells (Block 1993).
Over the years, gene transfer techniques have been refined, and many underlying mechanisms have been decoded. However, there are several experimental prerequisites to be followed for effective gene delivery. Each plant has its own requirements, and it is necessary to establish a separate protocol of transformation individually. The choice of gene to be incorporated, type of vector, genotype, promoter, and reproducible regeneration system are some of the parameters that determine success of plant genetic transformation.
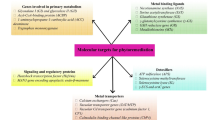
4.4 Target Genes
The plants were able to grow and colonize in heavy metal-contaminated sites by the development of several metal exclusion and tolerance mechanisms (Viehweger 2014). An integrated physiological, anatomical, biochemical, and molecular studies reveal the metal tolerance mechanism of plants as well as lead to the identification of target genes involved in various processes including metal uptake, translocation, and sequestration in plant. Therefore, the classical approach to engineer plants for enhanced heavy metal remediation consists in the strengthening of endogenous systems by upregulation of the expression or activity of target genes. Some of the target genes useful for genetic engineering of plants for enhanced heavy metal phytoremediation are enlisted below.
4.4.1 Membrane Transporters
Membrane transporters are likely to play a central role in translocation of heavy metals from source to root and to shoot and its further sequestration into specialized compartments (viz., vacuoles, chloroplast, mitochondria) to ensure sufficient levels to the necessary compartments while safely storing metals under times of excess (Cherian and Oliveira 2005; Palmer and Guerinot 2009). Recent developments in molecular biology, bioinformatics, omics, and sequencing technologies lead not only to the identification of novel vacuolar/plasma membrane-localized transporters but also understanding their role in heavy metal uptake, translocation, and sequestration. Many membrane transporters belonging to heavy metal ATPases, natural resistant-associated macrophage proteins (NRAMPs), cation diffusion facilitators (CDF), and ZIP (zinc-regulated transporter, iron-regulated transporter-related protein) gene families were identified in plant genome (Hall and Williams 2003; Krämer 2010). The manipulation of membrane transporter genes and its transfer to the non-accumulator plant potentially offer an enhanced phytoremediation process with increased heavy metal uptake and tolerance as shown in Table 22.2. Our knowledge of the molecular nature and regulation of transporters has expanded vastly over the past years. Fundamental research into transport mechanisms in plants is leading to the development of genetic-engineered plants provided with specialized membrane transporters can be geared up for cleanup of heavy metal-contaminated soils (phytoremediation), mining of rare metals (phytomining), and enhanced human nutrition through accumulation of nutritionally important metals in plant tissues (biofortification) which in turn could expand available arable land (Schroeder et al. 2013).
4.4.2 Phytochelatins (PCs)
Phytochelatins (PCs) are low molecular weight cysteine-rich metal-binding peptides whose synthesis is induced by heavy metals. PCs are synthesized nontranslationally from glutathione by the enzyme phytochelatin synthase to form molecules of (γ-EC) nG (where n ∼2–11) (Cobbett 2000; Krämer 2010). They chelate heavy metals with their thiol (-SH) group of cysteine, and the resulting metal–phytochelatin complexes are sequestered into vacuoles. This results into development of heavy metal tolerance in plants by decreasing free heavy metal ion concentration in plant fluids. A number of structural variants are identified in a wide variety of plant species, and different metals, including Cd, Hg, Ag, Cu, Ni, Au, Pb, and Zn, are found to induce PC production; however, Cd is by far the strongest inducer (Mejáre and Bülow 2001; Pal and Rai 2010). Transgenic plants with increased phytochelatin level through overexpression of phytochelatin synthase resulted in enhanced heavy metal tolerance (Table 22.2).
4.4.3 Metallothioneins (MTs)
Metallothioneins (MTs) are ubiquitous, low molecular weight (5–10 kDa), cysteine-rich proteins present in plants, animals, fungi, and cyanobacteria. In plants, MTs are suggested to be involved in metal tolerance or homeostasis, detoxification, and distribution as they are able to bind metal ions by the formation of mercaptide bonds with the numerous cysteine residues. Recent reports show that MTs are also involved in the scavenging of reactive oxygen species (Hassinen et al. 2011; Freisinger 2011; Leszczyszyn et al. 2013). Each MT exhibits preferences for a special metal ion due to coordination residues other than cysteine and differences in folding and stability in dependence on the bound metal (Leszczyszyn et al. 2007). However, various MT genes are cloned and transferred to several plants resulting in constitutive enhancement of heavy metal tolerance in transgenic plants (Eapen and D’Souza 2005) (Table 22.2).
4.4.4 Metal Chelators (MCs)
Metal chelators are mostly low molecular weight organic compounds, viz., organic acids (malate, citrate) and amino acids (nicotianamine, histidine), which can act as metal-binding ligands. MCs are involved in the uptake, transport, and sequestration of possibly toxic-free metal ions present in cytosol or plant fluids to the vacuolar compartment, providing metal tolerance to the plants (Haydon and Cobbett 2007). Overexpression of metal chelator genes in plants showed enhanced heavy metal tolerance (Table 22.2).
4.4.5 Manipulation of Enzymes Involved in Heavy Metal Phytoremediation
Genetic-engineered plants to express the enzymes responsible for detoxification, tolerance, and chelation of heavy metals, oxidative stress response/mechanisms, and plant architecture are developed and showed enhanced phytoremediation capacity (Table 22.2). Transgenic plants overexpressing the genes encoding the enzymes for histidine biosynthesis, ACC deaminase, Hg2 reductase, glutathione synthetase, arsenate reductase, and aldolase/aldehyde reductase were shown to become more tolerant to the toxic levels of metals and carried out phytoextraction with increasing potential (Chatterjee et al. 2013).
4.5 Optimization of Transgene Expressions in Transgenic Plants
The expression levels of transgene in transgenic plants are generally low in the case of heterologous gene expression system. Since the additional transcriptional and translational enhancers and constitutive and tissue-specific promoters are needed to optimize expression of transgenes that results into increased phytoremediation capacity of transgenic plants.
4.5.1 Use of Constitutive Promoters
The promoter is used to manage gene expressions. The selection of a promoter in a particular expression system depends on the entry and integration of align gene into the host genome. Phytoremediation-related plant transformation studies have largely utilized the cauliflower mosaic virus 35S (CaMV 35S) (Thomine et al. 2000), and actin promoters (Dhankher et al. 2002) have been employed to drive constitutive high level of expression of integrated genes in most of the plant tissues.
4.5.2 Inducible Promoters
The performance of inducible promoters is not conditioned to endogenous factors but to environmental conditions and external stimuli that can artificially be controlled. The expression pattern of the gene may also be programmed to be only under certain environmental conditions (e.g., stress, light) by using different promoters. The double transgenic plant with strong tolerance to the As and enhanced As accumulation was developed by co-expressing two bacterial genes. The E. coli arsenate reductase, arsC, gene was expressed in leaves as driven by a light-induced soybean RuBisCO small subunit 1 (SRS1) promoter and results into arsenate reduction in leaves. In addition, the E. coli γ-glutamylcysteine synthetase, γ-ECS, was expressed in both roots and shoots, driven by strong constitutive Actin2 promoter, and leads to enhanced biosynthesis of thiol-rich peptides for AsIII complexation. Thus, they provide increased AsV tolerance (Dhankher et al. 2002).
4.5.3 Tissue-Specific Expression of Transgenes
Tissue-specific promoters direct the expression of a gene in specific tissues (e.g., roots, leaves, tubers, fruits and seeds, etc.). The expression of metal hyper accumulator genes in easily harvestable plant tissues like leaves has an advantage in phytoextraction and phytomining. However, fruit-/seed-specific expression of transgene for heavy metal phytoremediation face objections due to fruits/seeds is eaten by birds and other organisms. This leads to the biomagnification of heavy metal in the food chain.
Addressing the problem of high Cd accumulation in rice plants including seeds, Li et al. (2007) focused their efforts on reducing Cd accumulation in rice seeds by silencing the expression of phytochelatin synthase (PCS) gene, OsPCS1. If OsPCS1 gene was constitutively silenced, the transgenic rice plant may become sensitive to Cd exposure that would affect the growth of plants on Cd-contaminated soil. Hence, the knockdown of OsPCS1 gene by RNAi under the control of the seed-specific promoter ZMM1 (from maize) in rice showed reduced Cd accumulation in rice seeds as compared to the wild plants. Furthermore, this transgene-induced RNA interference approach can be used to control heavy metal accumulation in the edible part of the food crops that would be grown on metal-contaminated soils or irrigated with metal-contaminated water. Thus, it is possible to enhance tolerance to heavy metal and restrict its entry into the food chain.
4.5.4 Organelle Targeting
Transgene integration in the various targeted organelle compartments like chloroplast, mitochondria, and vacuoles facilitates sequestration/detoxification of toxic heavy metals in the organelle. This prevents adverse interaction of heavy metals with cytoplasmic environment. Plant chloroplast genetic engineering offers several advantages over nuclear transformation, i.e., very high levels of transgene expression to 46 % of total leaf protein, transgene containment by the maternal inheritance/cytoplasmic inheritance of the plastid genome, the absence of gene silencing and positioning effect, the ability to express multiple genes in a single transformation event, and the ability to express bacterial genes without codon optimization. However, chloroplast transformation technology success was limited by several challenges, viz., it requires a species-specific vectors and achieving homoplasmy (integration of transgene into each chloroplast genome and elimination of untransformed genome).
Phytoremediation of organomercurial compounds via chloroplast genetic engineering was achieved by integrating the native mer operon containing the merA (mercuric ion reductase) and merB (organomercurial lyase) genes into the tobacco chloroplast genome. The transgenic plants showed better growth and resistance to very high concentrations of PMA (phenylmercuric acetate) up to 400 μM as compared to control untreated plants (Ruiz et al. 2003). Therefore, chloroplast engineering could be a beneficial approach for Hg phytoremediation as well.
4.6 Genetic Engineering of Plant Symbionts
The plant-associated bacteria including endophytic, phyllospheric, and rhizospheric bacteria can affect plant growth and development by fixing atmospheric nitrogen, increasing bioavailability of essential mineral nutrients, and synthesizing phytohormones and enzymes involved in plant growth hormone metabolism (Weyens et al. 2009a, b). The potential of plants and their associated microorganisms in degradation and removal of environmental pollutants/heavy metals has been recently recognized. However, not every plant-associated bacterium possesses the ability to degrade every toxic compound, and not every bacterium that has a degrading capacity toward a contaminant limits their widespread application in phytoremediation (Newman and Reynolds 2005). However, the success rate of phytoextraction of heavy metals using endophytic bacteria remains slow because of the lack of proper strains with heavy metal resistance and detoxification capacities (Luo et al. 2011). To overcome these constraints, plant-associated bacteria are engineered with the appropriate characteristics to achieve enhanced phytoremediation (Menn et al. 2000).
Several plant-associated bacteria are reported to accelerate the phytoremediation in metal-contaminated soils by promoting plant growth and health (Miransari 2011; Rajkumar et al. 2012). On the other hand, the rate of phytoremediation is limited by metal availability, uptake, translocation, and phytotoxicity (Weyens et al. 2009a, b). Therefore, to improve the efficiency of phytoremediation of toxic metal-contaminated soils, plant-associated bacteria can be engineered with pathways for the synthesis of natural metal chelators such as citric acid to increase metal availability for plant uptake or alternatively with metal sequestration systems to reduce phytotoxicity and increase metal translocation to aerial plant parts (Sessitsch and Puschenreiter 2008). Lupinus luteus grown on a nickel-enriched substrate and inoculated with an engineered nickel-resistant bacterium Burkholderia cepacia L.S.2.4 ncc-nre showed significantly increased nickel concentration in roots (Lodewyckx et al. 2001).
A. thaliana PCS1 gene along with a genetic fusion of four mammalian MT-coding sequence was expressed in Mesorhizobium huakuii subsp. rengei (strain B3) and resulted in approximately 25-fold increase in Cd accumulation as compared to its natural capability (Rajkumar et al. 2012). The colonization of Chinese milk vetch (Astragalus sinicus) with the B3 strain in rice paddy soil containing 1 mg kg−1 Cd promoted uptake of the metal in roots but not in nodules, by three times. This strategy would be useful in the rhizofiltration or transient phytostabilization of heavy metals in soil.
The possible advantages of using genetic-engineered endophytic microorganisms to improve xenobiotic remediation were summarized by Newman and Reynolds (2005), a major advantage being where genetic engineering of a xenobiotic degradation pathway is required, bacteria are easier to manipulate than plants. In addition, quantitative gene expression of pollutant catabolic genes within the endophytic populations could be a useful monitoring tool for assessing the efficiency of the remediation process. The unique niche of the interior plant environment provides the xenobiotic degrader strain with an ability to reach larger population sizes due to the reduced competition. Another important advantage of using endophytic pollutant degraders is that any toxic xenobiotics taken up by the plant may be degraded in planta, thereby reducing phytotoxic effects and eliminating any toxic effects on herbivorous fauna residing on or near contaminated sites (Ryan et al. 2008).
5 Hairy Root Cultures for Heavy Metal Removal
Agrobacterium rhizogenes is a plant-pathogenic gram-negative soil bacterium, initiating hairy root disease in most of dicotyledonous plants upon infection. These hairy roots no longer require the continuous presence of the inciting bacterium for proliferation, demonstrating that the plant cells have been transformed. The molecular basis of this transformation revolves around the activities of a large (∼200 kb) root-inducing (Ri) plasmid resident in A. rhizogenes virulent strains. Specifically, a portion of the Ri plasmid, the transferred DNA (T-DNA), delineated by 25 base pair border repeats, is transferred into the plant cells, integrated into the nuclear DNA, and expressed. This transformation process results into induction of hairy roots from infected plant tissue. A number of excellent review articles have been published describing Agrobacterium and plant genes involved in T-DNA transfer and integration (Gelvin 2000, 2003) and Agrobacterium and plant cell interaction (McCullen and Binns 2006); hence, these aspects are not described in this chapter.
The hairy root disease is characterized by plagiotropic root growth, a high degree of lateral branching, and the profusion of root hairs, although the tissue maintains a highly differentiated and functional root organ (Santos-Díaz 2013). “Hairy root” cultures have several properties—fast growth; greater genetic, phenotypic, and biochemical stability; growth in hormone-free media; and easily established—and maintenance and propagation in the laboratory offers a more reliable and reproducible in vitro model experimental system (cf. Sect. 22. 4.2.2), which have promoted their use in phytoremediation (Doran 2009; Georgiev et al. 2012; Shanks and Morgan 1999; Zhou et al. 2013). Table 22.3 highlights examples of hairy root cultures used for metal removal.
6 Transgenic Plants: Lab to Land Transfer/Testing
The myriad of reports on transgenic plants with enhanced heavy metal accumulation, tolerance, and volatilization published in recent years could be used for phytoremediation of heavy metal-contaminated sites. However, the majority of phytoremediation studies are restricted to the model plants and have been carried out under controlled laboratory conditions using metal-spiked hydroponic system or agar medium. Up to now, these transgenic plants never have been tested/used in real metal-contaminated sites for remediation. On the other hand, phytoextraction capacity of transgenic phytochelatin-overproducing mustard plant was tested in a greenhouse study using metal-contaminated soil from Leadville, Colorado (Bennett et al. 2003). Both types of transgenics accumulated significantly higher levels of Cd and Zn in their shoots than untransformed plants.
In another greenhouse pot experiment using naturally seleniferous soil, the ATP sulfurylase (APS) transgenic Brassica accumulated threefold higher Se levels than wild-type (WT) Brassica juncea, while cystathionine-γ-synthase (CgS) transgenics contained 40 % lower Se levels than WT plant (Huysen et al. 2004). In a field experiment on Se-contaminated sediment in the San Joaquin Valley (CA, USA), the APS transgenics accumulated fourfold higher Se levels than wild-type B. juncea (Bañuelos et al. 2005), and on the same site, the selenocysteine lyase (cpSL) and selenocysteine methyltransferase (SMT) transgenic showed twofold higher Se accumulation compared to WT B. juncea, in agreement with earlier laboratory experiments (Bañuelos et al. 2007). The results obtained from the different transgenics under controlled laboratory conditions were essentially the same as those obtained with greenhouse and field experiment (Pilon-Smits and LeDuc 2009).
Additionally, the number of plants with enhanced heavy metal phytoremediation capability was developed through the genetic engineering tools and tested under the laboratory conditions; however, this is often where the process of evaluating phytoremediation ends. Ideally, these transgenic plants need to be tested in in situ field trials to assess its phytoremediation capability. Such established field trials are, therefore, immediately required to make it a commercially viable and acceptable technology (Cherian and Oliveira 2005).
7 Transgenic Plants for Phytoremediation: Key Considerations for Risk Assessment and Mitigation Strategies
Genetic-engineered plants are rapidly being developed which deal with heavy metal accumulation, tolerance, and resistance and have been used not only for phytoextraction of various metals (Pilon-Smits and Pilon 2002) but also to enhance crop productivity in areas with suboptimal soil metal levels or heavy metal-contaminated soil which could expand available arable land (Guerinot and Salt 2001). Furthermore, transgenic plants are also used for biofortification of crop plants (Guerinot and Salt 2001; Schroeder et al. 2013). However, each GE plants/product undergoes an environmental risk assessment (ERA) prior to commercialization to assess potential harmful effects on the environment. The ERA directs the assessment based on the product concept, crop, trait(s), intended use (e.g., import versus cultivation), receiving environment, and potential interaction among these factors (Prado et al. 2014). The safety assessment strategy ensures that the safety of transgenic crops is reviewed by multiple regulatory agencies in accordance with different risk assessment strategies and with national and international safety assessment guidelines (Paoletti et al. 2008). This is essential to educate the community about the risks and benefits of transgenic plants for phytoremediation so that this technology can gain full regulatory and public acceptance and realize its full commercial potential (Linacre et al. 2003). The risk assessment scenario for transgenic plants is given in detail by Häggman et al. (2013) and Prado et al. (2014).
Some of the possible risks associated with transgenic plants for metal phytoremediation are accumulation of toxic metals in edible parts leading to metal entry into the food chain, which in turn affects animal and human health, and uncontrolled spread of transgene to the wild relatives leading to super weeds due to higher fitness offered by the transgene. The risk of biomagnification can be minimized by using nonedible plants and growing transgenic plants in restricted area. The various physical (placement of barriers to pollen spread, restriction of location or timing of crop planting, containment of engineered crops) and physiological (harvest engineered plants before flowering, engineer traits into plants that self-pollinate, engineer traits into plants that are sterile, perform genetic modifications on plastids) barriers are used to limit gene flow (through pollen) between related plant species (Daniell 2002; Häggman et al. 2013; Pilon-Smits and Pilon 2002; Ruiz and Daniell 2009). Additionally, transgenic for phytoremediation is likely to involve genetic use restriction technologies designed to impede transgene movement in the environment (Hills et al. 2007).
Further, considerations for the use of transgenics for the phytoremediation are the same as those involved with growing transgenics for other purposes and should also be evaluated and weighed against the risk of alternative remediation methods (Pilon-Smits and LeDuc 2009).
8 Future Perspectives and Conclusion
Phytoremediation is considered as an effective, low cost, environmental friendly, preferred remediation technology, and potentially applicable cleanup option to remove contaminants from soil- or water-contaminated areas (Dickinson et al. 2009). Transgenic plants expressing various genes from different sources have been developed as a means to increase heavy metal tolerance, accumulation, and volatilization that facilitates more effective heavy metal phytoremediation (Table 22.2). Most of the transgenic research was carried out on the model plant species, viz., Arabidopsis, tobacco, and Brassica. However, B. juncea (L.), an edible oil-producing crop, is being consumed by humans or animals in one form or another. Ecologically, use of edible crops for phytoremediation is not viable because the heavy metals enter into the food chain by consumption of either humans or animals. Therefore, further research is needed to explore more efficient nonedible candidate plant which is suitable for metal phytoremediation and genetic transformation. In this scenario, Gupta et al. (2013) suggested that aromatic plants (Vetiveria zizanioides, Cymbopogon martinii, C. flexuosus, C. winterianus, Mentha sp., Ocimum basilicum) producing essential oils could be a better choice for the heavy metal phytoremediation as these plants are nonedible and are not being consumed directly by humans or animals. In addition, the essential oil obtained from aromatic plants is free from the risk of heavy metal accumulation from plant biomass which offers economic benefits. Furthermore, the use of halophytes is suggested as the optimal candidate for phytostabilization or phytoextraction of heavy metal-contaminated saline soils as many halophytes can accumulate or excrete heavy metals (Wang et al. 2014).
The advent of the genomic era, next-generation sequencing technologies, and rapid progress in molecular biology research have led to the fast screening and identification of novel genes and proteins responsible for metal tolerance, accumulation, and volatilization. This will open up further avenues for the creation of new transgenic plants having one or several genes (gene stacking) with desirable properties for heavy metal phytoremediation. Additionally, it might be possible to control transgene expressions in specific tissues/compartments under specific conditions (Pilon-Smits and Pilon 2002; Ruiz and Daniell 2009).
The recent novel omics approaches (genomics, transcriptomics, proteomics, metabolomics, secretomics, high-throughput and next-generation technologies) combined with new bioinformatics techniques will allow us to understand how integrated biological communities (plants and microbes) interact to adapt to contaminant stress and enhance soil remediation. Furthermore, the plant–microbe metaorganism approach can be modified to maximize growth, appropriate assembly of microbial communities, and, ultimately, phytoremediation activity (Bell et al. 2014; Schenk et al. 2012). To achieve further improvements in phytoremediation, it will need coordinated efforts from plant physiologists, agronomists, soil scientists, molecular biologists, microbiologists, chemists, environmental engineers, and government regulators (Lee 2013; Pilon-Smits 2005).
The enormous amount of knowledge regarding transgenic plants for phytoremediation is generated through emerging scientific tools and methodologies combined with established practices and techniques. However, translation of this knowledge into usable technologies is the need of the hour to accelerate phytoremediation as an eco-friendly and cost-effective technology.
References
Aken BV (2008) Transgenic plants for phytoremediation helping nature to clean up environmental pollution. Trends Biotechnol 26:225–227
Appenroth KJ (2010) Definition of heavy metals and their role in biological systems. In: Sherameti I, Varma A (eds) Soil heavy metals, vol 19, Soil biology. Springer, Berlin, pp 19–29
Arazi T, Sunkar R, Kaplan B, Fromm H (1999) A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J 20:171–182
Arthur EL, Rice PJ, Rice PJ, Anderson TA, Baladi SM, Henderson KLD, Coats JR (2005) Phytoremediation - an overview. Crit Rev Plant Sci 24:109–122
Bañuelos G, LeDuc DL, Pilon-Smits EAH, Tagmount A, Terry N (2007) Transgenic Indian mustard overexpressing selenocysteine lyase or selenocysteine methyltransferase exhibit enhanced potential for selenium phytoremediation under field conditions. Environ Sci Technol 41:599–605
Bañuelos G, Terry N, LeDuc DL, Pilon-Smits EAH, Mackey B (2005) Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of selenium- contaminated sediment. Environ Sci Technol 39:1771–1777
Bell TH, Joly S, Pitre FE, Yergeau E (2014) Increasing phytoremediation efficiency and reliability using novel omics approaches. Trends Biotechnol 32:271–280
Bennett LE, Burkhead JL, Hale KL, Terry N, Pilon M, Pilon-Smits EAH (2003) Analysis of transgenic Indian mustard plants for phytoremediation of metal-contaminated mine tailings. J Environ Qual 32:432–440
Bizily SP, Kim T, Kandasamy MK, Meagher RB (2003) Subcellular targeting of methylmercury lyase enhances its specific activity for organic mercury detoxification in plants. Plant Physiol 131:463–471
Bizily SP, Rugh CL, Meagher RB (2000) Phytodetoxification of hazardous organomercurials by genetically engineered plants. Nat Biotechnol 18:213–217
Bizily SP, Rugh CL, Summers AO, Meagher RB (1999) Phytoremediation of methylmercury pollution: merB expression in Arabidopsis thaliana confers resistance to organomercurials. Proc Natl Acad Sci U S A 96:6808–6813
Block M (1993) The cell biology of plant transformation: current state, problems, prospects and the implications for the plant breeding. Euphytica 71:1–14
Boominathan R, Doran PM (2002) Ni-induced oxidative stress in roots of the Ni hyperaccumulator Alyssum bertolonii. New Phytol 156:205–215
Boominathan R, Doran PM (2003a) Organic acid complexation, heavy metal distribution and the effect of ATPase inhibition in hairy roots of hyperaccumulator plant species. J Biotechnol 101:131–146
Boominathan R, Doran PM (2003b) Cadmium tolerance and antioxidative defenses in hairy roots of the cadmium hyperaccumulator Thlaspi caerulescens. Biotechnol Bioeng 83:158–167
Chaney RL, Li YM, Brown SL, Homer FA, Malik M, Angle JS, Baker AJM, Reeves RD, Chin M (2000) Improving metal hyperaccumulator wild plants to develop commercial phytoextraction systems: approaches and progress. In: Terry N, Bañuelos G (eds) Phytoremediation of contaminated soil and water. Lewis, Boca Raton, FL, pp 129–158
Chatterjee S, Mitra A, Datta S, Veer V (2013) Phytoremediation protocols: an overview. In: Gupta DK (ed) Plant-based remediation processes. Springer, Berlin, pp 1–18
Cherian S, Oliveira M (2005) Transgenic plants in phytoremediation: recent advances and new possibilities. Environ Sci Technol 39:9377–9390
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF (2000) Involvement of Nramp1 from Arabidopsis thaliana in iron transport. Biochem J 347:749–755
Czakó M, Feng X, He Y, Liang D, Márton L (2006) Transgenic Spartina alterniflora for phytoremediation. Environ Geochem Health 28:103–110
Daghan H, Arslan M, Uygur V, Koleli N (2013) Transformation of tobacco with ScMTII gene-enhanced cadmium and zinc accumulation. Clean Soil Air Water 41:503–509
Daniell H (2002) Molecular strategies for gene containment in transgenic crops. Nat Biotechnol 20:581–586
de la Fuente JM, Ramírez-Rodríguez V, Cabrera-Ponce JL, Herrera-Estrella L (1997) Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science 276:1566–1568
Denkhaus E, Salnikow K (2002) Nickel essentiality, toxicity and carcinogenicity. Crit Rev Oncol Hemat 42:35–56
Dhankher OP, Li Y, Rosen BP, Shi J, Salt D, Senecoff JF, Sashti NA, Meagher RB (2002) Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γ- glutamylcysteine synthetase expression. Nat Biotechnol 20:1140–1145
Dickinson NM, Baker AJM, Doronila A, Laidlaw S, Reeves RD (2009) Phytoremediation of inorganics: realism and synergies. Int J Phytorem 11:97–114
Domínguez-Solís JR, López-Martín MC, Ager FJ, Ynsa MD, Romero LC, Gotor C (2004) Increased cysteine availability is essential for cadmium tolerance and accumulation in Arabidopsis thaliana. Plant Biotechnol J 2:469–476
Doran PM (2009) Application of plant tissue cultures in phytoremediation research: incentives and limitations. Biotechnol Bioeng 103:60–76
Dushenkov V, Nanda Kumar PBA, Motto H, Raskin I (1995) Rhizofiltration: the use of plants to remove heavy metals from aqueous streams. Environ Sci Technol 29:1239–1245
Eapen S, D’souza SF (2005) Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol Adv 23:97–114
Eapen S, Suseelan K, Tivarekar S, Kotwal S, Mitra R (2003) Potential for rhizofiltration of uranium using hairy root cultures of Brassica juncea and Chenopodium amaranticolor. Environ Res 91:127–133
EPA (1998) A citizen’s guide to phytoremediation. EPA, Washington, DC, EPA publication 542-F-98-011
Evans K, Gatehouse J, Lindsay W, Shi J, Tommey A, Robinson N (1992) Expression of the pea metallothionein-like gene PsMTa in Escherichia coli and Arabidopsis thaliana and analysis of trace metal ion accumulation: implications for PsMTa function. Plant Mol Biol 20:1019–1028
Flocco CG, Giulietti AM (2007) In vitro hairy root cultures as a tool for phytoremediation research. In: Willey N (ed) Phytoremediation methods in biotechnology. Humana, Totowa, NJ
Freisinger E (2011) Structural features specific to plant metallothioneins. J Biol Inorg Chem 16:1035–1045
Gasic K, Korban SS (2007) Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol Biol 64:361–369
Gelvin SB (2000) Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu Rev Plant Physiol Plant Mol Biol 51:223–256
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “Gene-Jockeying” tool. Microbiol Mol Biol Rev 67:16–37
George EF, Hall MA, De Klerk GJ (2008) Plant propagation by tissue culture, 3rd edn. Springer, Berlin
Georgiev MI, Agostini E, Ludwig-Müller J, Xu J (2012) Genetically transformed roots: from plant disease to biotechnological resource. Trends Biotechnol 30:528–537
Gifford S, Dunstan RH, O’Connor W, Koller CE, MacFarlane GR (2006) Aquatic zooremediation: deploying animals to remediate contaminated aquatic environments. Trends Biotechnol 25:60–65
Giri A, Narasu L (2000) Transgenic hairy roots: recent trends and applications. Biotechnol Adv 18:1–22
Gisbert C, Ros R, De Haro A, Walker DJ, Pilar Bernal M, Serrano R, Navarro-Aviñó J (2003) A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochem Biophys Res Commun 303:440–445
Golan-Goldhirsh A, Barazani O, Nepovim A, Soudek P, Smrcek S, Dufkova L, Krenkova S, Yrjala K, Schröders P, Vanek T (2004) Plant response to heavy metals and organic pollutants in cell culture and at whole plant level. J Soils Sedim 4:133–140
Guerinot ML, Salt DE (2001) Fortified foods and phytoremediation. Two sides of the same coin. Plant Physiol 125:164–167
Guo J, Dai X, Xu W, Ma M (2008) Overexpressing gsh1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere 72:1020–1026
Gupta AK, Verma SK, Khan K, Verma RK (2013) Phytoremediation using aromatic plants: a sustainable approach for remediation of heavy metals polluted sites. Environ Sci Technol 47:10115–10116
Ha H, Olson J, Bian L, Rogerson PA (2014) Analysis of heavy metal sources in soil using Kriging interpretation on principle components. Environ Sci Technol 48:4999. doi:10.1021/es405083f
Häggman H, Raybould A, Borem A, Fox T, Handley L, Hertzberg M, Lu M-Z, Macdonald P, Oguchi T, Pasquali G, Pearson L, Peter G, Quemada H, Séguin A, Tattersall K, Ulian E, Walter C, McLean M (2013) Genetically engineered trees for plantation forests: key considerations for environmental risk assessment. Plant Biotechnol J 11:785–798
Hall JL, Williams LE (2003) Transition metal transporters in plants. J Exp Bot 54:2601–2613
Hasanuzzaman M, Fujita M (2013) Heavy metals in the environment current status, toxic effects on plants and phytoremediation. In: Anjum NA, Pereira ME, Ahmad I, Duarte AC, Umar S, Khan NA (eds) Phytotechnologies: remediation of environmental contaminants. CRC Press Taylor & Francis Group, Boca Raton, FL, pp 7–74
Hasegawa I, Terada E, Sunairi M, Wakita H, Shinmachi F, Noguchi A, Nakajima M, Yazaki J (1997) Genetic improvement of heavy metal tolerance in plants by transfer of the yeast metallothionein gene (CUP1). Plant Soil 196:277–281
Hassinen VH, Tervahauta AI, Schat H, Kärenlampi SO (2011) Plant metallothioneins – metal chelators with ROS scavenging activity. Plant Biol 13:225–232
Haydon MJ, Cobbett CS (2007) Transporters of ligands for essential metal ions in plants. New Phytol 174:499–506
He YK, Sun JG, Feng XZ, Czakó M, Márton L (2001) Differential mercury volatilization by tobacco organs expressing a modified bacterial merA gene. Cell Res 11:231–236
Herzig R, Guadagnini M, Rehnert A, Erismann KH (2003) Phytoextraction efficiency of in vitro bred tobacco variants using a non-GMO approach. In: Vanek T, Schwitzguebel JP (eds) Phytoremediation inventory – COST Action 837 view. UOCHB AVČR, Prague
Hills MJ, Hall L, Arnison PJ, Good AG (2007) Genetic use restriction technologies (GURTs): strategies to impede transgene movement. Trends Plant Sci 12:177–183
Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ (2000) Expression of Arabidopsis CAX2 in tobacco. altered metal accumulation and increased manganese tolerance. Plant Physiol 124:125–133
Hsieh J, Chen C, Chiu M, Chein M, Chang J, Endo G, Huang CC (2009) Expressing a bacterial mercuric ion binding protein in plant for phytoremediation of heavy metals. J Hazard Mater 161:920–925
Hu ZR, Du M (2006) Hairy root and its application in plant genetic engineering. J Integr Plant Biol 48:121–127
Huysen T, Terry N, Pilon-Smits EAH (2004) Exploring the selenium phytoremediation potential of transgenic Brassica juncea overexpressing ATP sulfurylase or cystathionine-γ-synthase. Int J Phytorem 6:111–118
Ike A, Sriprang R, Ono H, Murooka Y, Yamashita M (2007) Bioremediation of cadmium contaminated soil using symbiosis between leguminous plant and recombinant rhizobia with the MTL4 and the PCS genes. Chemosphere 66:1670–1676
James CA, Strand SE (2009) Phytoremediation of small organic contaminants using transgenic plants. Curr Opin Biotech 20:237–241
Jan VV, Demacedo CC, Kinet JM, Bouharmont J (1997) Selection of Al-resistant plants from a sensitive rice cultivar using somaclonal variation, in vitro and hydroponic cultures. Euphytica 97:303–310
Jarup L (2003) Hazards of heavy metal contamination. Brit Med Bull 68:167–182
Joner EJ (2013) Effects of biotic and abiotic amendments on phytoremediation efficiency applied to metal-polluted soils. In: Anjum NA, Pereira ME, Ahmad I, Duarte AC, Umar S, Khan NA (eds) Phytotechnologies: remediation of environmental contaminants. CRC Press Taylor & Francis Group, Boca Raton, FL, pp 283–288
Kartosentono S, Nuraida A, Indrayanto G, Noor CZ (2001) Phytoremediation of Sr2+and its influence on the growth, Ca2+ and solasodine content of shoot culture of Solanum laciniatum. Biotechnol Lett 23:153–155
Kawashima CG, Noji M, Nakamura M, Ogra Y, Suzuki KT, Saito K (2004) Heavy metal tolerance of transgenic tobacco plants over-expressing cysteine synthase. Biotechnol Lett 26:153–157
Kim J, Wyskiyzuk BE, Weathers PJ (2002) Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell Dev Biol Plant 38:1–10
Kim S, Takahashi M, Higuchi K, Tsunoda K, Nakanishi H, Yoshimura E, Mori S, Nishizawa N (2005) Increased nicotianamine biosynthesis confers enhanced tolerance of high levels of metals, in particular nickel, to plants. Plant Cell Physiol 46:1809–1818
Koprivova A, Kopriva S, Jäger D, Will B, Jouanin L, Rennenberg H (2002) Evaluation of transgenic poplars over-expressing enzymes of glutathione synthesis for phytoremediation of cadmium. Plant Biol 4:664–670
Korenkov V, Hirschi K, Crutchfield JD, Wagner GJ (2007a) Enhancing tonoplast Cd/H antiport activity increases Cd, Zn, and Mn tolerance, and impacts root/shoot Cd partitioning in Nicotiana tabacum L. Planta 226:1379–1387
Korenkov V, Park S, Cheng N, Sreevidya C, Lachmansingh J, Morris J, Hirschi K, Wagner GJ (2007b) Enhanced Cd2+ -selective root-tonoplast-transport in tobaccos expressing Arabidopsis cation exchangers. Planta 225:403–411
Kotrba P, Najmanova J, Macek T, Ruml T, Mackova M (2009) Genetically modified plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotech Adv 27:799–810
Krämer U (2005) Phytoremediation: novel approaches to cleaning up polluted soils. Curr Opin Biotech 16:133–141
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61:517–534
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol 29:1239–1245
Chen L, Marmey P, Taylor NJ, Brizard J, Espinoza C, D’Cruz P, Huet H, Zhang S, Kochko A, Beachy RN, Fauquet CM (1998) Expression and inheritance of multiple transgenes in rice plants. Nat Biotechnol 16:1060–1064
LeDuc DL, AbdelSamie M, Móntes-Bayon M, Wu CP, Reisinger SJ, Terry N (2006) Overexpressing both ATP sulfurylase and selenocysteine methyltransferase enhances selenium phytoremediation traits in Indian mustard. Environ Pollut 144:70–76
LeDuc DL, Tarun AS, Montes-Bayon M, Meija J, Malit MF, Wu CP, AbdelSamie M, Chiang C, Tagmount A, deSouza M, Neuhierl B, Böck A, Caruso J, Terry N (2004) Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiol 135:377–383
Lee JH (2013) An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnol Bioprocess Eng 18:431–439
Lee M, Phillips RL (1988) The chromosomal basis of somaclonal variation. Annu Rev Plant Physiol Plant Mol Biol 39:413–437
Lee S, Moon JS, Ko T, Petros D, Goldsbrough PB, Korban SS (2003) Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol 131:656–663
Leszczyszyn OI, Imam HT, Blindauer CA (2013) Diversity and distribution of plant metallothioneins: a review of structure, properties and functions. Metallomics 5:1146–1169
Leszczyszyn OI, Schmid R, Blindauer CA (2007) Toward a property/function relationship for metallothioneins: histidine coordination and unusual cluster composition in a zinc-metallothionein from plants. Prot Struct Funct Bioinform 68:922–935
Li JC, Guo JB, Xu WZ, Ma M (2007) RNA Interference-mediated silencing of phytochelatin synthase gene reduce cadmium accumulation in rice seeds. J Integr Plant Biol 49:1032–1037
Li Y, Dhankher OP, Carreira L, Lee D, Chen A, Schroeder JI, Balish RS, Meagher RB (2004) Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol 45:1787–1797
Li YM, Chaney RL, Angle JS, Baker AJM (2000) Phytoremediation of heavy metal contaminated soils. Environ Sci Poll Con Ser 22:837–857
Linacre NA, Whiting SN, Baker AJM, Scott Angle J, Ades PK (2003) Transgenics and phytoremediation: the need for an integrated risk assessment, management, and communication strategy. Int J Phytorem 5:181–185
Lodewyckx C, Taghavi S, Mergeay M, Vangronsveld J, Clijsters H, van der Lelie D (2001) The effect of recombinant heavy metal resistant endophytic bacteria in heavy metal uptake by their host plant. Int J Phytorem 3:181–185
Luo S, Wan Y, Xiao X, Guo H, Chen L, Xi Q, Chen J (2011) Isolation and characterization of endophytic bacterium LRE07 from cadmium hyperaccumulator Solanum nigrum L. and its potential for remediation. Appl Microbiol Biotechnol 89:1637–1644
Ma JF, Nomoto K (1996) Effective regulation of iron acquisition in graminaceous plants. The role of mugineic acids as phytosiderophores. Physiol Planta 97:609–617
Macek T, Kotrba P, Suchova M, Skacel F, Demmerova K, Rumi T (1994) Accumulation of cadmium by hairy root cultures of Solanum niger. Biotechnol Lett 16:621–624
Macek T, Macková M, Pavlíková D, Száková J, Truksa M, Cundy A, Kotrba P, Yancey N, Scouten WH (2002) Accumulation of cadmium by transgenic tobacco. Acta Biotechnol 22:101–106
Martin S, Griswold W (2009) Human health effects of heavy metals. Environ Sci Technol Brief Cit 15:1–6
Martínez M, Bernal P, Almela C, Vélez D, García-Agustín P, Serrano R et al (2006) An engineered plant that accumulates higher levels of heavy metals than Thlaspi caerulescens, with yields of 100 times more biomass in mine soils. Chemosphere 64:478–485
McCullen CA, Binns AN (2006) Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu Rev Cell Dev Biol 22:101–127
Mejáre M, Bülow L (2001) Metal binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol 19:67–73
Menn F-M, Easter JP, Sayler GS (2000) Genetically engineered microorganisms and bioremediation. In: Klein J (ed) Biotechnology, vol 11b. Wiley, New York, NY, pp 441–463
Merlot S, Hannibal L, Martins S, Martinelli L, Amir H, Lebrun M, Thomine S (2014) The metal transporter PgIREG1 from the hyperaccumulator Psychotria gabriellae is a candidate gene for nickel tolerance and accumulation. J Exp Bot 65:1551–1564
Metzger L, Fouchault I, Glad C, Prost R, Tepfer D (1992) Estimation of cadmium availability using transformed roots. Plant Soil 143:249–257
Miransari M (2011) Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol Adv 29:645–653
Misra S, Gedamu L (1989) Heavy metal tolerant transgenic Brassica napus L. and Nicotiana tabaccum L. plants. Theor Appl Genet 78:161–168
Nedelkoska T, Doran PM (2000a) Hyperaccumulation of cadmium by hairy roots of Thlaspi caerulescens. Biotechnol Bioeng 67:607–615
Nedelkoska T, Doran PM (2000b) Characteristics of heavy metal uptake by plant species with potential for phytoremediation and phytomining. Miner Eng 13:549–561
Nedelkoska TV, Doran PM (2001) Hyperaccumulation of nickel by hairy roots of Alyssum species: comparison with whole regenerated plants. Biotechnol Prog 17:752–759
Nehnevajova E, Herzig R, Erismann KH, Schwitzguébel JP (2007) In vitro breeding of Brassica juncea L. to enhance metal accumulation and extraction properties. Plant Cell Rep 26:429–437
Newman LA, Reynolds CM (2005) Bacteria and phytoremediation: new uses for endophytic bacteria in plants. Trends Biotechnol 23:6–8
Ontañon OM, González PS, Ambrosio LF, Paisio CE, Agostini E (2014) Rhizoremediation of phenol and chromium by the synergistic combination of a native bacterial strain and Brassica napus hairy roots. Int Biodeter Biodegrad 88:192–198
Pal R, Rai JPN (2010) Phytochelatins: peptides involved in heavy metal detoxification. Appl Biochem Biotechnol 160:945–963
Palmer CM, Guerinot ML (2009) Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat Chem Biol 5:333–340
Pan A, Yang M, Tie F, Li L, Chen Z, Ru B (1994) Expression of mouse metallothionein-I gene confers cadmium resistance in transgenic tobacco plants. Plant Mol Biol 24:341–351
Paoletti C, Flamm E, Yan W, Meek S, Renckens S, Fellouse M, Kuiperf H (2008) GMO risk assessment around the world: some examples. Trends Food Sci Technol 19:S70–S78
Pavlíková D, Macek T, MacKová M, Száková J, Balík J (2004) Cadmium tolerance and accumulation in transgenic tobacco plants with a yeast metallothionein combined with a polyhistidine tail. Int Biodeter Biodegrad 54:233–237
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39
Pilon-Smits EAH, Hwang S, Lytle CM, Zhu Y, Tai JC, Bravo RC, Chen Y, Leustek T, Terry N (1999) Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol 119:123–132
Pilon-Smits EA, Pilon M (2002) Phytoremediation of metals using transgenic plants. Crit Rev Plant Sci 21:439–456
Pilon-Smits EAH, LeDuc DL (2009) Phytoremediation of selenium using transgenic plants. Curr Opin Biotechnol 20:207–212
Pitzschke A (2013) From bench to barn: plant model research and its applications in agriculture. Adv Genet Eng 2:110. doi:10.4172/2169-0111.1000110
Pomponi M, Censi V, DiGirolamo V, DePaolis A, di Toppi LS, Aromolo R, Costantino P, Cardarelli M (2006) Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta 223:180–190
Prado JR, Segers G, Voelker T, Carson D, Dobert R, Phillips J, Cook K, Cornejo C, Monken J, Grapes L, Reynolds T, Martino-Catt S (2014) Genetically engineered crops: from idea to product. Annu Rev Plant Biol 65:769–790
Rajkumar M, Sandhya S, Prasad MN, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30:1562–1574
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? and what makes them so interesting? Plant Sci 180:169–181
Raskin I (1996) Plant genetic engineering may help with environmental cleanup [commentary]. Proc Natl Acad Sci U S A 93:3164–3166
Rugh CL, Senecoff JF, Meagher RB, Merkle SA (1998) Development of Transgenic yellow poplar for mercury phytoremediation. Nat Biotechnol 16:925–928
Rugh CL, Wilde HD, Stack NM, Thompson DM, Summers AO, Meagher RB (1996) Mercuric ion reduction and resistance in transgenic Arabidopsis thaliana plants expressing a modified bacterial merA gene. Proc Natl Acad Sci U S A 93:3182–3187
Ruiz ON, Daniell H (2009) Genetic engineering to enhance mercury phytoremediation. Curr Opin Biotech 20:213–219
Ruiz ON, Hussein HS, Terry N, Daniell H (2003) Phytoremediation of organomercurial compounds via chloroplast genetic engineering. Plant Physiol 132:1344–1352
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278:1–9
Salt DE, Prince RE, Pickering IJ, Raskin I (1995) Mechanism of cadmium mobility and accumulation ion Indian mustard. Plant Physiol 109:1427–1433
Santos-Díaz MS (2013) Metal remediation via in vitro root cultures. In: Gupta DK (ed) Plant-based remediation processes, vol 35, Soil biology. Springer, Berlin, pp 101–116
Sasaki Y, Hayakawa T, Inoue C, Miyazaki A, Silver S, Kusano T (2006) Generation of mercury hyperaccumulating plants through transgenic expression of the bacterial mercury membrane transport protein MerC. Transgenic Res 15:615–625
Schenk PM, Carvalhais LC, Kazan K (2012) Unraveling plant–microbe interactions: can multi-species transcriptomics help. Trends Biotechnol 30:177–184
Schroeder JI, Delhaize E, Frommer WB, Guerinot ML, Harrison MJ, Herrera-Estrella L, Horie T, Kochian LV, Munns R, Nishizawa NK, Tsay YF, Sanders D (2013) Using membrane transporters to improve crops for sustainable food production. Nature 497:60–66
Sessitsch A, Puschenreiter M (2008) Endophytes and rhizosphere bacteria of plants growing in heavy metal-containing soils. In: Dion P, Nautiyal CS (eds) Microbiology of extreme soils. Springer, New York, NY, pp 317–332
Shanks JV, Morgan J (1999) Plant ‘hairy root’ culture. Curr Opin Biotech 10:151–155
Shaul O, Hilgemann DW, de-Almeida-Engler J, Van Montagu N, Inze D, Galili G (1999) Cloning and characterization of a novel Mg2+ ⁄H+ exchanger. EMBO J 18:3973–3980
Shukla D, Kesari R, Tiwari M, Dwivedi S, Tripathi RD, Nath P, Trivedi PK (2013) Expression of Ceratophyllum demersum phytochelatin synthase CdPCS1 in Escherichia coli and Arabidopsis enhances heavy metal(loid)s accumulation. Protoplasma 250:1263–1272
Sone Y, Nakamura R, Pan-Hou H, Sato MH, Itoh T, Kiyono M (2013) Increase methylmercury accumulation in Arabidopsis thaliana expressing bacterial broad-spectrum mercury transporter MerE. AMB Exp 3:52
Song W, Sohn EJ, Martinoia E, Lee YJ, Yang Y, Jasinski M, Forestier C, Hwang I, Lee Y (2003) Engineering tolerance and accumulation of lead and cadmium in transgenic plants. Nat Biotechnol 21:914–919
Soudek P, Petrová S, Benešová D, Vanĕk T (2011) Uranium uptake and stress responses of in vitro cultivated hairy root culture of Armoracia rusticana. Agrochimica 60:15–28
Straczek A, Wannijn J, Van Hees M, Thijs H, Tiry Y (2009) Tolerance of hairy roots of carrots to U chronic exposure in standardized in vitro device. Environ Exp Bot 65:82–89
Subroto MA, Priambodo S, Indrasti NS (2007) Accumulation of zinc by hairy root cultures of Solanum nigrum. Biotechnology 6:344–348
Sunitha MSL, Prashant S, Anilkumar S, Rao S, Narasu ML, Kavi Kishore PB (2013) Cellular and molecular mechanisms of heavy metal tolerance in plants: a brief overview of transgenic plants overexpressing phytochelatin synthase and metallothionein genes. Plant Cell Biotech Mol Biol 14:33–48
Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S (2001) Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol 19:466–469
Talano MA, Oller ALW, González P, González SO, Agostini E (2014) Effects of arsenate on tobacco hairy root and seedling growth, and its removal. In Vitro Cell Dev Biol Plant 50:217–225
Tangahu BV, Abdullah SRS, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A Review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 2011, Article ID: 939161, pp 1–31
Thomas JC, Davies EC, Malick FK, Endreszl C, Williams CR, Abbas M, Petrella S, Swisher K, Perron M, Edwards R, Osenkowski P, Urbanczyk N, Wiesend WN, Murray KS (2003) Yeast metallothionein in transgenic tobacco promotes copper uptake from contaminated soils. Biotechnol Prog 19:273–280
Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI (2000) Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci U S A 97:4991–4996
Uriu-Adams JY, Keen CL (2005) Copper oxidative stress and human health. Mol Aspects Med 26:268–298
van der Zaal BJ, Neuteboom LW, Pinas JE, Chardonnens AN, Schat H, Verkleij JA, Hooykaas PJ (1999) Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol 119:1047–1055
Van Huysen T, Abdel-Ghany S, Hale KL, LeDuc D, Terry N, Pilon-Smits EAH (2003) Overexpression of cystathionine-γ-synthase enhances selenium volatilization in Brassica juncea. Planta 218:71–78
Viehweger K (2014) How plants cope with heavy metals. Bot Stud 55:35–46
Wang H-L, Tian C-Y, Jiang L, Wang L (2014) Remediation of heavy metals contaminated saline soils: a halophyte choice? Environ Sci Technol 48:21–22
Weyens N, Lelie D, Taghavi S, Newman L, Vangronsveld J (2009a) Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27:591–598
Weyens N, Lelie D, Taghavi S, Vangronsveld J (2009b) Phytoremediation: plant–endophyte partnerships take the challenge. Curr Opin Biotech 20:248–254
Zhang Y, Liu J (2011) Transgenic alfalfa plants co-expressing glutathione S-transferase (GST) and human CYP2E1 show enhanced resistance to mixed contaminates of heavy metals and organic pollutants. J Haza Mat 189:357–362
Zhou ML, Tang YX, Wu YM (2013) Plant hairy roots for remediation of aqueous pollutants. Plant Mol Biol Rep 31:1–8
Zhu YL, Pilon-Smits EA, Jouanin L, Terry N (1999a) Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol 119:73–80
Zhu YL, Pilon-Smits EA, Tarun AS, Weber SU, Jouanin L, Terry N (1999b) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol 121:1169–1178
Ziemienowicz A (2014) Agrobacterium-mediated plant transformation: factors, applications and recent advances. Biocatal Agric Biotechnol 3:95. doi:10.1016/j.bcab.2013.10.004
Acknowledgment
UBJ thanks University Grants Commission, New Delhi, India, for providing Dr D. S. Kothari postdoctoral fellowship, and VAB thanks Indian National Science Academy, New Delhi, India, for providing INSA Senior Scientist Fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jagtap, U.B., Bapat, V.A. (2015). Genetic Engineering of Plants for Heavy Metal Removal from Soil. In: Sherameti, I., Varma, A. (eds) Heavy Metal Contamination of Soils. Soil Biology, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-319-14526-6_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-14526-6_22
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14525-9
Online ISBN: 978-3-319-14526-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)