Abstract
Recent advances in high-throughput phenotyping allow breeders to collect phenotypic data with a level of accuracy that was impossible to achieve previously. However, many of these technologies depend on leveraging-controlled environments like green houses or growth chambers. While these controlled phenotypes can have strategic value for gene discovery, their relevance for breeding and understanding genotype x environment interactions to predict field performance is an active field of study and currently limited, at best. This chapter deals with various technologies that have empowered the collection of phenotypic data directly under field conditions and the relative advantages and disadvantages of using them to collect agronomic phenotypes. Important considerations to be aware of before planning a high-throughput phenotyping experiment that use technologies like field spectroscopy and remote sensing are also discussed including a review of various publically available and/or commercial aerial, ground-based and root phenotyping platforms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Normalize Difference Vegetation Index
- Unmanned Aerial Vehicle
- Canopy Temperature
- Unmanned Aircraft System
- Leaf Area Density
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
The human population is predicted to increase to 9.6 billion from the current world population of 7.2 billion by the year 2050 (United Nations 2013). Not the least of the global challenges associated with this population growth is the issue of supplying sufficient food, fuel, and fiber to feed a global economy driven by a population of this magnitude. The difficulty of meeting this demand is further compounded by a 5–10 million hectare reduction in agricultural land area annually due to land degradation (GEF/UNCCD 2011), as well as declines in agricultural productivity associated with predicted patterns of climate change (Melillo et al. 2014). Plant breeding and genetics sits at the center of the multidisciplinary solutions that will form to meet the challenge of feeding the future. Innovations in plant breeding will lead to the de novo generation of novel genetic material suitable to perform under such demanding environmental, economic, and political constraints.
Despite thousands of years of technological advancement since the origin of plant breeding, genetic recombination has remained the primary driver of genetic gain from selection . Selection in plant breeding can be defined as the science of discriminating among biological variants in a population to identify and pick desirable recombinants. However, identifying the recombinants that lead to superior phenotypes can be challenging as the phenotype is driven both by the genetic constituency of an organism as well as the influence of the environment within which it is grown. Complicating this further is the principle of genotype–environment interactions that favor genotypes differentially according to the environmental conditions. For the purposes of this discussion, the phenotype is defined as the observable effect of the genotype and its interaction with a given environment (Acquaah 2007).
The advent of genetic marker technologies has allowed breeders to visualize the effects of selection on the genome and in some measure predict recombinants that have a higher probability of producing superior phenotypes. Because this has been most easily accomplished via marker-assisted selection (MAS) in cases where the desirable phenotype is controlled by a single dominant gene, it has driven the development of diversity panels and mapping populations in several species, which attempt to explore existing genetic diversity in search of simple traits (McCouch et al. 2012).
In the more complex cases where the infinitesimal model of gene action applies (i.e., many genes all contributing small effects on a quantitative phenotype), this has proven more difficult. In these cases, genomic selection has emerged as one strategy to predict desirable recombinants based on a smaller training population that is carefully phenotyped and genotyped (Heffner et al. 2009; Asoro et al. 2011).
The reduction in the cost of genotyping that has come about with improvements in next-generation sequencing and single nucleotide polymorphism genotyping technology has resulted in the generation of an abundant amount of genotypic data in a very short time period. This deluge of genomic information has driven a strong focus on strategies for the integration of molecular information in the breeding pipeline.
However, despite these advances, there is still tremendous need for high-quality phenotype data—not only to empower genetic mapping efforts and genomic selection models, but also for direct phenotypic selection. No matter how much genetic or genomic information is used to predict phenotypic information, the phenotype is still driven by both the genome and the environment. To select desirable recombinants, the environment must either be sampled or controlled in order to separate genetic ‘signal’ from environmental ‘noise.’ This is a challenge because phenotyping is more expensive, labor intensive, and prone to error than genotyping. However, unlike with the evolution of genotyping technology, cost per phenotypic data point is unlikely to change dramatically. Tuberosa (2012) put it best by stating that “phenotyping is [now] king, and [has taken] heritability [a]s queen.”
Increasing heritability (the proportion of phenotypic variance explained by the genotype) of any phenotyping activity is partly a function of managing the influence of the environment. This can be done either by controlling the environmental variation directly (as with the use of a growth chamber or other managed environments) or by sampling the environmental variation by replicating observations of a genotype across a target population of environments. In this chapter, we primarily treat field-based phenotyping systems . For a more detailed comparison of field-based versus controlled phenotyping systems, see Cobb et al. (2013). Suffice it to mention that one of the major challenges of the field-based high-throughput phenotyping systems when compared to phenotyping in controlled environments is that field-based phenotyping systems require technology suitable for varied field conditions and data collection strategies that are simultaneously cheap and high throughput, but also avoid increasing error.
6.2 Major Considerations Before Planning a Phenotyping Experiment
Before conducting any study that requires high-throughput phenotyping (HTP) , it is vital to understand the objective of the experiment. HTP for selection of desirable recombinants at a 50 % selection intensity will require a different level of accuracy than phenotyping a mapping population with the intention of identifying a marker linked to an important gene. Cost should be the primary driver of any analysis; if all a researcher needs to do is throw away the bottom half of a population, the cost per phenotypic data point can be substantially reduced compared to the approach that would be needed for more sophisticated genetic analyses. The trait(s) that the researcher is looking to phenotype should be well articulated beforehand, and relevance to selection targets should be established conclusively. For example, in a naturally self-pollinating organism, phenotyping a population for stigma exertion in order to increase cross pollination is only useful if stigma exertion has been demonstrated to contribute to increased cross pollination a priori.
Even though the application of HTP data is broad and is used in various experiments, such as genomic selection, gene discovery, germplasm characterization, QTL mapping, and phenotypic selection of breeding material, it is important to have clearly defined objectives prior to the collection of phenotypic data (Araus and Cairns 2014). Information on the genetic architecture of a trait and the heritability of the trait under various phenotyping protocols is also necessary to help the researcher prioritize phenotyping activities and plan costs before using a HTP platform.
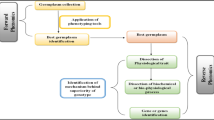
A detailed description of many of the factors that need to be considered collecting HTP data have been described by Cobb et al. (2013), as shown in Fig. 6.1. One often overlooked detail is the use of standardized ontologies and careful management of phenotypic databases. If done properly and in alignment across groups, such strategies integrate results across experiments and can drive powerful meta-analyses, which can leverage information across a diversity of environments in order to enhance genetic signal.
Factors to be considered before obtaining data from a high-throughput phenotyping experiment (Cobb et al. 2013; used with permission)
6.3 Major Phenotypes Measured in Field Conditions
Agronomically relevant phenotypes that are also most amenable to HTP tend to be observable at the canopy level, are measurable at different points throughout the crop lifecycle, and also discriminate plants based on their ability to capture natural resources and use them efficiently (Araus et al. 2008). Examples of such traits would be canopy temperature, leaf area, normalized difference vegetation index (NDVI), and chlorophyll density. Each of these traits serves as a proxy for more relevant agronomic metrics, such as water use efficiency, biomass, harvest index, and disease pressure. Researchers have developed a variety of inexpensive and novel field-based HTP approaches for a number of these traits, with the goal of automating the phenotyping process without sacrificing the power of prediction (Araus and Cairns 2014). In recent years, improvements in various technologies such as remote sensing, digital image analysis, infrared thermography, robotics, data management, and farm machinery have accelerated the rate at which HTP data is collected and catalogued in a database. The challenge moving forward, however, is to address the question of how this data can be translated into actionable information upon which breeding decisions can be made.
The literature contains a few good examples of this, including Munns et al. (2010), who recorded the temperature gradients in wheat and barley fields using an infrared camera. This indicated the levels of energy dissipation in plants and allowed the researchers to visualize stress response capacity and photosynthetic rates, which serve as reliable indicators for drought tolerance (Munns et al. 2010). Additionally, estimation of leaf area density (LAD) by using a three-dimensional portable LIDAR (light detection and ranging) enabled the more precise phenotyping of the vertical canopy structure in Japanese Zelkova trees (Hosoi and Omasa 2007). Furthermore, drought tolerance in cotton (Gossypium barbadense L.) was estimated by evaluating canopy height, NDVI, and temperature using four sets of sensors mounted on a tractor (Andrade-Sanchez et al. 2013). This technique allowed the researchers to collect time series data from the field in order to understand physiological and developmental traits that are typically harder to phenotype under relevant growing conditions.
Advances in field-based HTP technology have also opened areas of research to the below-ground parts of an agro-ecosystem, which have been historically hard to phenotype. One good example comes from Zenone et al. (2008), who used ground penetrating radar and electrical resistivity tomography as an indirect nondestructive technique for estimating the shape and behavior of pine tree roots in the subsoil. Perhaps the earliest foray into this space is the rapid root biomass detection in maize, done using a portable capacitance meter by Van Beem et al. (1998).
Each of these examples highlights three key questions that need to be asked before funding an effort to collect and manage HTP data:
-
(1)
What data are going to be collected?
-
(2)
What mechanism is going to be used to collect that data?
-
(3)
How is that data going to be analyzed and applied to meet the objectives of a breeding or genetics program?
6.4 Field Spectroscopy
In terms of what data should be collected, there are many options. Field spectroscopy is the most common technique used in field-based phenotyping systems due to the speed with which the data can be collected and the quantitative nature of the information itself. Spectroscopy is the study of the interaction between electromagnetic radiation and matter. Field Spectroscopy , specifically, is the measurement of the reflectance properties of soils, vegetation, rocks, and bodies of water under natural light. It is used for quantitative measurement of radiance, irradiance, reflectance, or transmission in the field; the resulting image-based measurements are converted to estimations of relevant phenotypes through an established calibration. Some the examples are RGB/CIR cameras, multispectral cameras, hyperspectral imagers, thermal imaging cameras, and even conventional handheld digital cameras (Araus and Cairns 2014). However, there are challenges associated with the collecting of spectroscopy data that, if not addressed, pose barriers to the precision and accuracy of the information a researcher collects.
Milton (1987) proposed several guidelines to help a researcher ensure quality data from a spectroscopy experiment. To increase the accuracy and consistency of field data, several methodologies have been suggested by various authors (Jackson and Robinson 1985; Kimes and Kirchner 1982). The following are the guidelines provided by Milton (1987) to standardize field spectroscopy experiments across different research groups:
-
(1)
To ensure a fixed geometry between the sensor , the standard panel, and the target, a mast or tripod should be used. The variable geometry and the proximity of the operator to the target and of the target to the radiometer leads to the reduced precision of the handheld measurements.
-
(2)
The sensor should be at least 1–2 m above the upper surface of the target.
-
(3)
The sensor support should be pointed directly towards the sun. This is to be consistent with orienting sensor horizontal support and positioning other field equipment in same positions relative to the sun.
-
(4)
The standard panels should be placed in such a way that it fills the field-of-view of all the bands of the sensor .
-
(5)
While taking the measurements, use of a continuously recording solarimeter within the field area provides three benefits:
-
All data from the primary sensor will be screened and any anomalies in them will be corrected.
-
It helps to quantify the atmospheric variability on a range of time scales.
-
The effects of variable cloud cover can be corrected using the data obtained from solarimeter .
-
-
(6)
Operators are advised to wear dark clothing while taking measurements. Errors in the measurement of radiance in red and near-infrared wavelengths can be as high as 10 and 12 %, respectively, if an operator with white clothing approaches the target at 0.5 m. However, if the operator wears black clothing, the errors in both bands were reduced to less than 2 % (Kimes and Kirchner 1982). The distance from the target and the vehicles should be more than 3 m for the same reason.
Of course, as a general guideline, Milton (1987)’s principles are useful, but it would behoove any researcher who is thinking of seriously investing in an HTP experiment to conduct pilot studies to optimize the collection of the phenotype data to the specific conditions of the activity. Such pilot studies form the basis of the establishment of a truly empowering HTP platform.
An essential part of the phenotypic optimization process will be to determine the optimum wavelength of light to use as the spectroscopic metric. The most widely used field spectroscopy-based techniques are remote sensing and imaging, infrared thermometry and thermal imaging, and near infrared spectroscopy (NIRS) analysis (Araus and Cairns 2014).
6.4.1 Remote Sensing and Imaging
remote sensing refers to collection of data about an object or phenomenon without any physical contact with the object. It usually refers to the data collected through satellites using different sensors of various platforms with a wide range of spatio-temporal, radiometric, and spectral resolutions (Khorram et al. 2012). Remote sensing instruments function as either passive or active sensors .
Passive sensors are used to measure the natural radiation emitted or reflected by objects. The most common source of radiation detected by Passive sensors is reflected sunlight. Most of the Passive sensors operate in the visible, infrared, thermal infrared, and microwave portions of the electromagnetic spectrum. Some examples of Passive sensors include accelerometers, radiometers, imaging radiometers, spectrometers, spectro-radiometers, and hyperspectral radiometers.
Unlike Passive sensors , an active sensor emits its own radiation towards the target of interest. The reflected or backscattered radiation from the target is detected by the active sensor to predict the properties of the target. Most of the active sensors operate in the microwave portion of the electromagnetic spectrum. Some of the examples of active sensors are RADAR (sound), LIDAR (light), a scatterometer, or a laser altimeter (Allen 1998).
The electromagnetic waves emitted by water sources, vegetation, and fields from earth can be captured by these sensors embedded in satellites and used to detect their characteristics. Plants are easily distinguishable in these systems because they exhibit stronger radiation in the electromagnetic spectra of visible-near infrared (VIS-NIR). Field-based HTP approaches using remote sensing use this VIS-NIR data to infer attributes of plants or field conditions. This technique can be particularly valuable because it is possible to phenotype the same individuals multiple times across the growing season, as the methods are both noninvasive and nondestructive. remote sensing can measure a wide array of traits including green biomass, photosynthetic transpirative gas exchange, and even grain yield (Araus et al. 2008). Phenotypic data generated from remote sensing can be used for a broad range of breeding experiments related to increasing yield potential or tolerance to abiotic and biotic stress factors.
6.4.2 Infrared Thermometry and Thermal Imaging
Infrared thermometers can be used to measure the temperature of an object by capturing the thermal radiation it emits. They are typically attached to the vehicles used in HTP systems as sensors (Furbank and Tester 2011). Long-wave infrared cameras and thermal imaging cameras are used to predict a variety of traits in crop plants, including water stress, disease and pathogen detection, and the detection of bruises on fruits and vegetables. These cameras can be attached either to aerial platforms or proximal sensing platforms, depending on the needs and funds of the researcher. High-resolution infrared cameras can enhance the quality of data and also reduce the time required to collect that data compared to lower resolution cameras (Araus and Cairns 2014).
6.4.3 Near-Infrared Spectroscopy
NIRS uses the waves emitted from the near-infrared region of the electromagnetic spectrum to analyze proteins, nitrogen, starch, and oil content, as well as grain texture and grain weight of individual seeds (Cabrera-Bosquet et al. 2011). This technique aids in evaluating large numbers of plot material and covers a broad distribution of measurements within plots. In addition, NIRS can be also used to measure more complex traits, such as stress adaptation, by using prediction models to correlate physiochemical properties to stresses of interest (Araus and Cairns 2014). Even though the precision of these estimates might be lower than the data points obtained from direct analysis, the lower cost of estimation often justifies its use. For example, in early-generation plant breeding material where thousands of genotypes need to be screened with relatively low selection intensity, this approach can help in identifying the bottom end of a phenotypic distribution in a reasonably cost-effective manner.
6.5 Data Collection Platforms
Even after the question of what data to collect is answered, the mechanism for collecting that data still remains a challenge for researchers. The efficiency and accuracy of collecting data from the field depends heavily on the mechanism for moving the sensors along the field. It is important to maintain consistency in data collection and—especially in the case of time series experiments—not to damage plants. There are two obvious and major methods used to collect data across fields: ground-based platforms and aerial platforms.
6.5.1 Ground-Based Phenotyping Platforms
Several ground-based high-throughput phenotyping systems have been developed over the past several years. These are usually modified vehicles equipped with global positioning systems (GPS) and sensors, often referred to as “phenomobiles ” (Araus and Cairns 2014). In an effort to balance cost, accuracy, and relevance, researchers have strived to develop both expensive as well as very cost-effective phenomobiles . Some examples of data collection using phenomobiles are as follows:
-
Ruixiu et al. (1989) used a three-wheeled cart with mounted multiple ultrasonic proximity sensors for measuring the morphological characters of bush-type plants (Fig. 6.2a).
Fig. 6.2 Phenomobiles used in field-based phenotyping systems. a Three wheeled cart (Ruixiu et al. 1989); b All-terrain motorized platform (Rundquist et al. 2004); c Hand cart made by welding two bicycle frames (White and Conley 2013); Tractor mounted with sensors for high-throughput phenotyping (Andrande-Sanchez et al. 2013); (used with permission.)
-
Rundquist et al. (2004) used an all-terrain motorized platform with a boom extendable to 12 m. The boom was mounted with a spectroradiometer that was used to estimate the biophysical characteristics of vegetation in a corn field (Fig. 6.2b).
-
White and Conley (2013) used a hand cart that was built by welding two bicycle frames to a steel scaffold. It was capable of positioning two monochrome cameras and three infrared thermometers over two rows (Fig. 6.2c).
-
Andrande-Sanchez et al. (2013) used a near infrared spectrometer sensor mounted on a tractor to measure canopy height, reflectance, and temperature (Fig. 6.2d).
One of the most commonly used phenomobiles is the combine/harvester. It is used to collect data on grain yield, moisture, and, in many cases, grain quality. Several other tractor- and harvester-mounted sensors are used for collecting data from field experiments. Multiple sensors are attached to these phenomobiles and are used in the detection of various traits, such as grain yield, crop canopy height, leaf area index (LAI), NDVI, multispectral imaging, and hyperspectral reflectance (Comar et al. 2012; Montes et al. 2011).
6.5.2 Unmanned Aerial Platforms
Aerial platforms for field-based phenotyping are particularly useful for noninvasive measurements of a large number of phenotypes quickly and over large distances. However, the throughput offered by these platforms often comes at the cost of accuracy. Various types of carriers are used for aerial phenotyping, including unmanned polycopters , blimps , and small unmanned helicopters (Fig. 6.3).
Chapman et al. (2014) used a “pheno-copter ” (Fig. 6.3a), a gas-powered robotic helicopter, to collect field data using three different cameras. The device was mounted with a visual camera, NIR camera, and a thermal camera. The three different types of images were captured using a GNC system and image capture computer, which allowed the pheno-copter to measure ground cover in sorghum, canopy temperature in sugarcane, and three-dimensional measures of crop lodging in wheat. One of the main advantages of using a pheno-copter is that it can fly low at a distance of up to 10–40 m above the field, which allows it to collect high-resolution image data for every plot and also lets the user collect data from many numbers of plots in a limited amount of time. Figure 6.3a depicts the pheno-copter platform, which can be flown with a radio transmitter or touchscreen control, and the ground station (Chapman et al. 2014).
The Centro Experimental de Norman E. Borlaug (CENEB) station, near Ciudad Obregon, associated with CIMMYT in Mexico, used an AB1100, 8-m-long helium-filled blimp (Fig. 6.3b) to evaluate wheat plants for abiotic stress tolerance during flowering time. The blimp was capable of floating up to 300 m above the fields while thermal infrared imagers and multispectral cameras were attached to the blimp for collecting data on canopy temperature, stomatal conductance, canopy water content, vegetation indices, and pigment indices (Goth 2014).
The Lancaster Hawkeye Mark II (Fig. 6.3c) is an unmanned aerial vehicle (UAV) built by the Precision Hawk Company. This vehicle is a completely autonomous fixed-wing system. It weighs only 3 pounds but can survey about 300 acres in 40 min. The company offers imagery, video, thermal, and multispectral imaging. Onboard sensors also help in adjusting the device for weather variability. The field information is programmed into the device before being tossed into the air by the user. The device then collects the phenotypic data and returns on its own. Additionally, it also supports an onboard Wi-Fi system for wireless data transmission (Hunting 2013).
The Octane multirotor system (Fig. 6.3d) is a bit heavier than Lancaster Hawkeye Mark II , but it is capable of taking high-resolution, GPS-referenced imagery and video. Manufactured by Volt Aerial Robotics, the company claims that the system is easier to operate under field conditions than the fixed-wing UAVs (Frey 2013). In addition to these examples, other commonly used UAVs include the Astec Falcon 8 (an 8-rotor UAV), the Airelectronics Skywalker, the Yamaha RMAX, and the Penguin series UAVs and unmanned aircraft systems (Hunting 2013).
6.6 Root Phenotyping Platforms
Any treatment of HTP platforms would be incomplete without at least making mention of one of the greatest phenotyping challenges of the modern age. One of the most difficult parts of the plant to be phenotyped in the field is the roots. However, roots form often up to 50–60 % or more of total plant biomass and are essential to understand such agronomically important traits as drought tolerance, nutrient use efficiency, and lodging resistance. Scientists have increasingly used ground-penetrating radar (GPR) to study root characteristics in plants over the past few years. Figure 6.4 depicts a researcher using GPR to measure wheat root biomass (Tanikawa et al. 2013).
This figure depicts a researcher using GPR to measure wheat root biomass (Thompson et al. 2013; used with permission)
GPR is essentially a broadband electromagnetic pulse radar system that is used to predict the depth, size and position of matter buried in the soil using the timing and characteristics of the reflected waves. A transmitting antenna from the GPR transmits pulses of electromagnetic energy, which penetrate the soil. This energy is reflected by the boundary layers of the objects with different physical characteristics. The receiving antenna in the GPR intercepts the reflected waves and the variation in the reflected wavelength is caused due to the contrasts in the dielectric permittivity between the bulk medium and buried objects (Barton and Montagu 2004).
Both soil depth and root water content affect the reflected radar waves, making it harder to get accurate data. However collection of data in sandy soils rather than clay have been shown to be more accurate (Borem and Fritsche-Neto 2014; Hirano et al. 2009).
6.7 Commercially Available Field-Based Phenotyping Systems
Apart from the various methodologies used by the public institutions to phenotype plants efficiently, several private companies have capitalized on the need to develop field-based phenotyping systems that are both powerful and accurate. The following sections discuss some of the commercially available field-based phenotyping platforms.
6.7.1 ScanalyzerField
ScanalyzerField is a phenotyping platform built by the Lemna-Tech Company. The company has been in the business of building high-throughput phenotyping platforms since 1998, and most of their platforms have been dedicated to greenhouse and growth chamber-based phenotyping systems. However, due to the need to measure agronomically important traits under field conditions, they have developed the ScanalyzerField platform, which is depicted in Fig. 6.5.
The Lemnatech ScanalyzerField platform for field-based phenotyping (Schwartz et al. 2013; used with permission.)
The ScanalyzerField platform is built around a stationary portal crane system that acts as a support for various sensors in the field. The system allows the user to phenotype plants from a height of 3–6 m over several hundred square meters of area, with a maximum size of 10 × 40 × 6 m for the stationary portal crane (ScanalyzerField 2014).
Even though the data collected from the system is more precise than the various aerial platforms, the portability and cost of the stationary portal crane makes the equipment less accessible for public research projects.
6.7.2 Smartfield TM
SmartField is a company that manufactures equipment designed to track relevant metrics of the field environment. This affords them the advantage of marketing both to researchers and to growers. Figure 6.6a shows a SmartProfile™ instrument loaded with four soil moisture sensors and four soil temperature sensors. Even though it is not used to measure field data on plants directly, it can be used to monitor stability and range of environmental conditions the plants are exposed to over time.
Smart Crop® sensor a SmartProfile™ b SmartCrop® c SmartWeather™ (Smartfield 2014; used with permission.)
Figure 6.6b depicts an image of a SmartCrop® sensor that has an infrared thermometer to collect the canopy temperature of plants in a single field. The company’s SmartCrop® sensor is capable of collecting canopy temperature readings every minute and averaging them at 15-min intervals and reports the data to a base station. Like SmartCrop®, the SmartWeather™ system collects field data on wind speed, wind direction, solar radiation, and barometric pressure in conjunction with the ambient temperature, relative humidity, and rainfall data collected by the base station (Smartfield™ 2014).
One of the most challenging components of phenotypic information for a researcher to understand is genotype–environment interaction. This is the phenomenon of adaptation that gives some genotypes favorable phenotypic expression in one environment, but allows it to be outcompeted by other genotypes when the environment changes. Critical to the understanding of genotype–environment interaction in any system is accurate and timely collection of environmental data that allows for the identification of the mechanisms that drive it. The Smartfield™ products are one of many market-based solutions for understanding a target population of environments.
6.8 Conclusion
In an age where genotype information is becoming cheaper, more reliable, and more readily available to smaller groups, and where the importance of phenotypic selection to plant breeding is often overlooked in favor of marker-based selection techniques, innovations in field-based HTP have the opportunity to accelerate the development of high-yielding, stress-tolerant, and disease-resistant plant varieties. Field-based HTP reduces the time and increases the accuracy of the parameters estimated from phenotypic data and allows for the selection of varieties and the identification of genetic loci with a precision not seen prior to the 21st century. However, just like in the case with high volumes of cheap genotype data, sorting through high volumes of cheap phenotype data will pose a significant logistical challenge to the scientific community. These challenges will be exacerbated by a dynamic and changing environment that is constantly shaping patterns of genotype–environment interaction and undermining the practices of selection and mapping. Meeting these challenges will take coordinated effort across groups and stable dedicated funding sources committed to the success of agriculture and plant breeding. Furthermore, these funds must be spent wisely to ensure that only the most relevant data are collected and employed for making genetic gain.
Looking to the future, once high volumes of genotype and phenotype data are available on genetically stable and agronomically important shared germplasm resources, high-throughput modeling of changes in the environment and their effects on the phenotype will be needed to complete the triangle and truly empower the mapping of genotype to phenotype.
References
Acquaah G (2007) Principles of plant genetics and breeding. Blackwell Publisher, Malden
Allen B (1998) Remote Sensing and Lasers. Accessed 17 June 2014 http://www.nasa.gov/centers/langley/news/factsheets/RemoteSensing.html
Andrade-Sanchez P, Gore M, Heun J, Thorp K, Carmo-Silva A, French A, Salvucci M, White J (2013) Development and evaluation of a field-based high-throughput phenotyping platform. Funct Plant Biol 41(1):68–79
Araus JL, Cairns JE (2014) Field high-throughput phenotyping: the new crop breeding frontier. Trends Plant Sci 19(1):52–61
Araus J, Slafer G, Royo C, Serret M (2008) Breeding for yield potential and stress adaptation in cereals. Crit Rev Plant Sci 27(6):377–412
Asoro F, Newell M, Beavis W, Scott M, Jannink J (2011) Accuracy and training population design for genomic selection on quantitative traits in elite north american oats. Plant Genome 4(2):132–144
Barton CVM, Montagu KD (2004) Detection of tree roots and determination of root diameters by ground penetrating radar under optimal conditions. Tree Physiol 24(12):1323–1331
Borém A, Fritsche-Neto R (2014) Omics in plant breeding. Ames: Wiley Blackwell
Cabrera-Bosquet L, Sanchez C, Rosales A, Palacios-Rojas N, Araus J (2011) Near-infrared reflectance spectroscopy (NIRS) assessment of delta O-18 and nitrogen and ash contents for improved yield potential and drought adaptation in maize. J Agric Food Chem 59(2):467–474
Chapman S, Merz T, Chan A, Jackway P, Hrabar S, Dreccer F, Holland E, Zheng B, Ling J, Jimenez-Berni J (2014) Pheno-copter: a low-altitude, autonomous remote-sensing robotic helicopter for high-throughput field-based phenotyping. Agronomy 4:279–301
Cobb JN, DeClerck G, Greenberg A, Clark R, McCouch S (2013) Next-generation phenotyping: requirements and strategies for enhancing our understanding of genotype–phenotype relationships and its relevance to crop improvement. Theor Appl Genet 126(4):867–887
Comar A, Burger P, de Solan B, Baret F, Daumard F, Hanocq J (2012) A semi-automatic system for high throughput phenotyping wheat cultivars in-field conditions: description and first results. Funct Plant Biol 39(10–11):914–924
Frey T (2013) Agriculture, the new games of drones. The futurist—a magazine of forecasts, trends and ideas about the future
Furbank RT, Tester M (2011) Phenomics–technologies to relieve the phenotyping bottleneck. Trends Plant Sci 16(12):635–644
GEF/UNCCD (2011) Land for life: securing our common future. Global Environment Facility, Washington, DC
Goth B (2014) Behind the science: researcher helps remote sensing soar. Accessed 17 July 2014 http://blog.cimmyt.org/behind-the-science-researcher-helps-remote-sensing-soar/
Heffner EL, Sorrells ME, Jannink J (2009) Genomic selection for crop improvement. Crop Sci 49(1):1–12
Hirano Y, Dannoura M, Aono K, Igarashi T, Ishii M, Yamase K, Makita N, Kanazawa Y (2009) Limiting factors in the detection of tree roots using ground-penetrating radar. Plant Soil 319(1):15–24
Hosoi F, Omasa K (2007) Factors contributing to accuracy in the estimation of the woody canopy leaf area density profile using 3D portable lidar imaging. J Exp Bot 58(12):3463–3473 doi: 10.1093/jxb/erm203
Hunting K (2013) Do you really know your fields? http://farmindusrynews.com/site/farmindusrynews.com/files/uploads/2013/07/FIN_22-25_UAVs.pdf
Jackson RD, Robinson BF (1985) Field evaluation of the temperature stability of a multispectral radiometer. Remote Sens Environ 17(1):103–108
Khorram S, Koch FH, van der Wiele CF, Nelson SAC (2012) Remote sensing. Springer, Boston
Kimes DS, Kirchner JA (1982) Irradiance measurement errors due to the assumption of a lambertian reference panel. Remote Sens Environ 12(2):141–149
McCouch SR, McNally KL, Wang W, Hamilton RS (2012) Genomics of gene banks: a case study in rice. Am J Bot 99(2):407–423
Melillo JM, Richmond TC, Yohe GW (eds) (2014) Climate change impacts in the united states: the third national climate assessment. U.S. Global Change Research Program, Washington, DC
Milton EJ (1987) Review article principles of field spectroscopy. Int J Remote Sens 8(12):1807–1827
Montes JM, Technow F, Dhillon BS, Mauch F, Melchinger AE (2011) High-throughput non-destructive biomass determination during early plant development in maize under field conditions. Field Crops Res 121(2):268–273
Munns R, James RA, Sirault XRR, Furbank RT, Jones HG (2010) New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J Exp Bot 61(13):3499–3507
Ruixiu S, Wilkerson JB, Wilhelm LR, Tompkins FD (1989) A microcomputer-based morphometer for bush-type plants. Comput Electron Agric 4(1):43–58
Rundquist D, Perk R, Leavitt B, Keydan G, Gitelson A (2004) Collecting spectral data over cropland vegetation using machine-positioning versus hand-positioning of the sensor. Comput Electron Agric 43(2):173–178
ScanalyzerField (2014) A wide range of choices for the quantitative, non-destructive analysis of different crops or model plants in field conditions [Internet]. Accessed 7 July 2014 http://www.lemnatec.com/products/hardware-solutions/scanalyzer-field/#.U7r4yvldV8E
Smartfield™ (2014) Growing a greener future: equipment [Internet]. Accessed 7 July 2014 http://www.smartfield.com/smartfield-products/equipment/
Stefan S, Jörge V, Dirk V, Matthias E (2013) Digital phenotyping of field crops under field conditions. LemnaTec—plant phenomics workshop, 1/11/2013. Plant and Animal Genome XXI, San Diego
Tanikawa T, Hirano Y, Dannoura M, Yamase K, Aono K, Ishii M, Igarashi T, Ikeno H, Kanazawa Y (2013) Root orientation can affect detection accuracy of ground-penetrating radar. Plant Soil 373(1):317–327
Thompson SM, Cossani CM, Ibrahim AMH, Reynolds MP, Goodman D, Hays DB (2013) Estimating wheat root biomass using ground penetrating radar. ASA, CSSA & SSSA International Annual Meetings, 9/3, Tampa, Florida, USA. ASA, CSSA & SSSA International Annual Meetings, Tampa
Tuberosa R (2012) Phenotyping for drought tolerance of crops in the genomics era. Front Physiol 3:347
United Nations (2013) World population prospects: the 2012 revision, key findings and advance tables. Department of Economic and Social Affairs, New York
Van Beem J, Smith ME, Zobel RW (1998) Estimating root mass in maize using a portable capacitance meter. Agron J 90(4):566–570
White J, Conley M (2013) A flexible, low-cost cart for proximal sensing. Crop Sci 53(4):1646–1649
Zenone T, Seufert G, Morelli G, Teobaldelli M, Fischanger F, Matteucci M, Sordini M, Armani A, Ferre C, Chiti T (2008) Preliminary use of ground-penetrating radar and electrical resistivity tomography to study tree roots in pine forests and poplar plantations. Funct Plant Biol 35(10):1047–1058
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Palanichamy, D., Cobb, J.N. (2015). Agronomic Field Trait Phenomics. In: Fritsche-Neto, R., Borém, A. (eds) Phenomics. Springer, Cham. https://doi.org/10.1007/978-3-319-13677-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-13677-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13676-9
Online ISBN: 978-3-319-13677-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)