Abstract
Obese versus lean humans show greater activation of reward valuation regions (caudate, amygdala, orbitofrontal cortex [OFC], insula) in response to palatable food images and to cues that signal impending palatable food receipt. Although this elevated responsivity theoretically results from conditioned associations between reward from palatable food and cues that signal impending palatable food receipt (the incentive sensitization theory), elevated amygdala response to palatable food images, nucleus accumbens response to palatable food images, OFC response to cues that predict palatable food image presentation, and striatal response to palatable food commercials predict future weight gain. These data suggest that elevated reward region responsivity to food cues trigger cravings that drive overeating. One prevention implication is that reducing intake of energy dense food should attenuate the conditioning process that gives rise to the hyper-responsivity of reward regions to food cues. Emerging evidence also suggests that thinking of the long-term health costs of eating unhealthy foods reduces reward region response to food cues, increases activation of inhibitory regions to food cues, and attenuates cravings, implying that prevention and treatment interventions should include cognitive reappraisal training. A pilot trial revealed that an intervention that trained young adults to apply cognitive reappraisals when confronted with unhealthy foods resulted in increased responsivity of a region implicated in inhibitory control and decreased responsivity of a region implicated in attention and expectation in response to palatable food images, but produced only pre-post reductions in body fat relative to participants in an obesity education control condition that did not persist over follow-up. It may be possible to use real time fMRI biofeedback to increase the effects of the cognitive reappraisal obesity prevention program. Yet obese versus lean adults also show less striatal D2 receptor availability, lower capacity of nigrostriatal neurons to synthesize dopamine, and weaker striatal activation (caudate, putamen) in response to high-fat/high-sugar food intake. Although animal experiments indicate that overfeeding results in reduced sensitivity of reward regions to food intake and pharmacologic stimulation, echoing effects from prospective weight gain studies with humans, individuals may overeat to compensate for an inborn or acquired reward deficit. Our pilot obesity prevention trial also encouraged participant-driven reductions dietary fat and sugar intake, but we did not find evidence of pre-post increases in reward region response to palatable beverages. Continued effort to translate neuroscience findings into obesity prevention and treatment interventions may yield more effective interventions for this pernicious public health problem.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Nearly 70 % of US adults are overweight or obese [1], which increases risk for coronary heart disease, atherosclerotic cerebrovascular disease, colorectal cancer, and all-cause mortality, is credited with 300,000 US deaths and $ 150 billion in health-related expenses yearly [2, 3]. Unfortunately, treatments almost never result in lasting weight loss and virtually all obesity prevention programs have not reduced future obesity onset [4, 5]. An improved understanding of the risk processes that give rise to weight gain should guide the design of more effective preventive programs and treatments. At present, most risk factors that predict future weight gain show only weak effects [6–9]. For example, the predictive effects for parental obesity, a well replicated risk factor for future weight gain, have only ranged from an r = 0.18 to 0.21 in large epidemiologic studies [e.g., 7, 9].
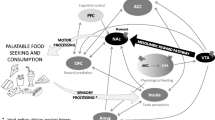
Theorists have focused on the role of reward circuitry in obesity because eating palatable food increases activation in regions implicated in reward, including the striatum, midbrain, amygdala, and orbitofrontal cortex (OFC) [10–12] and causes dopamine (DA) release in the dorsal striatum, with the amount released correlating with meal pleasantness ratings [13]. Indeed, even delivery of high-fat food directly to the gut, bypassing the oral cavity, has been shown to induce robust striatal dopamine release in rodents [14]. It has been posited that aberrant reward-related responses to food intake and/or cues override homeostatic processes of hunger and fullness, resulting in excess adipose tissue and weight gain [e.g., 15]. Further, data indicate that appetitive hormones thought to influence homeostatic determinants of food intake (e.g., leptin, peptide YY, glucagon-like peptide 1) act by altering reward value of food [16]. In support of hedonically driven food intake, direct pharmacological activation of the striatum prompts hyperphagia in animals, increasing preferential intake of high-fat/sugar foods, even in sated animals [17]. Agonist and antagonist experiments suggest that DA signaling plays a larger role in reward learning and that opioid peptide signaling plays a larger role in hedonic pleasure from food intake [18], though reward regions contain both DA and opioid receptors and opioid agonists cause DA signaling [19], implying the two neurotransmitter systems are tightly intertwined.
Reward Surfeit and Incentive Sensitization Theories of Obesity
The reward surfeit model holds that individuals who showed greater responsivity of reward regions to food intake, which is presumably inborn, are at elevated risk for overeating and consequent weight gain [20]. The incentive sensitization model posits that repeated intake of palatable foods results in an elevated responsivity of reward valuation regions to cues that are repeatedly associated with palatable food intake via conditioning, which prompts elevated food intake when these cues are encountered [18].
Consistent with these theories, obese versus lean humans show greater responsivity of brain regions associated with reward and motivation (striatum, amygdala, OFC) to pictures of high-fat/sugar foods versus low-fat/sugar foods and control images [21–28] and to pictorial cues that signal impending palatable food receipt [20, 29]. These data are supported by studies examining acute and longer-term food intake. Specifically, midbrain and medial OFC activity in response to milkshake receipt positively predicted subsequent ad libitum milkshake consumption and BOLD response in the ventral striatum during exposure to food images positively predicts later snack consumption [30, 31]. Using objectively measured energy intake over a two-week period in lean adolescents, a positive relation was observed between energy intake beyond basal metabolic needs and BOLD response during cues predicting food receipt in regions thought to encode visual processing and attention (visual and anterior cingulate cortices), salience (precuneus), as well as the primary gustatory cortex (frontal operculum) and (reward/motivation) striatum [32]. Animal experiments indicate that firing of striatal and ventral pallidum DA neurons initially occurs in response to receipt of a novel palatable food, but that after repeated pairings of palatable food intake and cues that signal impending receipt of that food, DA neurons begin to fire in response to reward-predictive cues and no longer fire in response to food receipt [33–35]. Theorists posit this shift during cue-reward learning acts to either update knowledge regarding the predictive cues or attribute reward value to the cues themselves thereby guiding behavior [36–39].
Critically, fMRI studies indicate that hyper-responsivity of reward regions (striatum, amygdala, OFC) to palatable food images [40, 41], palatable food odors [42] or cues that signal impending presentation of palatable food images [43] predicts future weight gain. Additionally, teens that exhibit greater striatal response to high-fat/sugar food commercials show elevated weight gain over 1-year follow-up (r = 0.47–0.51; Fig. 6.1; [44]). Elevated amygdala, midbrain, thalamus, hypothalamus, ventral pallidum, and nucleus accumbens responsivity to tastes of milkshake have also predicted weight gain over 1-year follow-up [42, 45].
Partial regression plots showing the relations of a activation in the caudate (MNI coordinates: 12, 14, 1) in response to food commercials > non-food commercials and b activation in the caudate (MNI coordinates: −9, 14, −2) in response to food commercials > television show to change in BMI over 1-year follow-up
Interestingly, there is evidence that the effects of hyper-responsivity of reward regions to food and food cues shows significantly stronger relations to future weight gain for individuals with a genetic propensity for greater DA signaling capacity in reward regions. Adolescents with elevated caudate and putamen responsivity to milkshake tastes who have a genetic propensity for greater DA signaling due to possessing an A2/A2 TaqIA allele showed significantly greater weight gain over 1-year follow-up [46]. Likewise, teens who show elevated striatal/OFC response to palatable food images and who had a genetic propensity for greater DA signaling due to possessing an A2/A2 TaqIA allele, also showed elevated future weight gain [27]. Similar effects have emerged for another genotype (no seven-repeat or longer alleles of the DRD4 gene [DRD4-S]) that has been associated with elevated DA signaling [27]. Data from a large (N = 155) ongoing study revealed that lean teens who showed greater OFC response to a cue that signals impending milkshake receipt were more likely to gain weight over 2-year follow-up (r = 0.29) and that this relation was significantly stronger for youth with a genetic propensity for greater DA signaling capacity in reward circuitry as indexed by a multilocus composite that reflects the number of alleles associated with elevated DA signaling (Fig. 6.2). We examined this multilocus score because it relates more strongly to reward region responsivity than the individual alleles used to calculate this composite genetic risk score [47, 48]. Theoretically, this is because a greater number of these genotypes, regardless of the particular combination, are associated with greater DA signaling capacity. The multilocus composite was scored as follows: TaqIA A1/A1, DRD2-141C Ins/Ins, DRD4-L, DAT1 10R/10R, and COMT Met/Met genotypes were assigned a score of 0 (‘low’); TaqIA A2/A2, DRD2-141C Ins/Del and Del/Del, DRD4-S, DAT1 9R, and COMT Val/Val genotypes were assigned a score of 1 (‘high’), and TaqIA A1/A2 and COMT Met/Val genotypes received a score of 0.5. Scores were summed to create the composite. Humans with the A2/A2 allele versus an A1 allele of the Taq1A polymorphism and the Del allele versus Ins/Ins genotype of the DRD2-141C Ins/Del polymorphism show more D2 receptors [49]. Humans with the shorter than seven alleles (DRD4-S) versus seven-repeat or longer allele (DRD4-L) of the DRD4 genotype show greater in vitro DA functioning and stronger response to DA agonists [50, 51]. Humans with the nine-repeat allele (DAT1-S) versus homozygous for the ten-repeat allele (DAT1-L) of the DAT1 show lower DAT1 expression [52], theoretically increasing synaptic DA clearance, producing lower basal DA levels and increased phasic DA release [53]. Val homozygotes versus Met homozygotes of the Catechol-O-methyltransferase (COMT val158met) gene putatively have lower basal striatal DA levels and greater phasic DA release [54]. Individuals with higher multilocus scores showed greater future weight gain in three separate studies (Fig. 6.3; [55]), confirming that this effect is replicable.
Partial regression plots showing the effects of the number of high DA signaling alleles (multilocus composite score) on change in BMI over 1-year follow-up in a 38 young females (M age = 20.9 ± 1.2; M BMI = 28.1; BMI range = 24.0–39.0), b 30 adolescents (56.7 % female; M age = 15.2 ± 1.0; M BMI =26.9; BMI range = 19.5–37.9), and c 161 adolescents (50.6 % female; M age = 15.2 ± 1.0; M BMI = 20.8; BMI range = 17.0–26.1), while controlling for baseline BMI
Thus, studies from multiple independent labs have found that individuals who show elevated reward region responsivity to palatable food intake are more likely to enter a prolonged period of positive energy balance and gain weight, providing key behavioral data in support of the reward surfeit theory of obesity. Studies from multiple independent labs have also found that individuals who show elevated reward region responsivity to cues that have been associated with palatable food intake show elevated future weight gain, providing behavioral support for the incentive sensitization theory of obesity. There was also evidence that the predictive relations of elevated reward region responsivity to palatable food intake and food cues to future weight gain are stronger for individuals with a genetic propensity for greater DA signaling capacity in reward regions, which might be construed as further support for the reward surfeit model. These results imply that reducing habitual intake of high-fat and high-sugar foods should theoretically reduce the conditioning process that leads to elevated reward region responsivity to food cues, which may be an effective method of reducing risk for weight gain. However, the fact that behavioral weight loss programs typically result in reduced intake of such foods, typically leading to a 10 % weight loss on average, but do not produced sustained weight loss implies that it is very difficult to reduce reward region hyper-responsivity to food cues once it has emerged.
Reward Deficit Theory of Obesity
The reward deficit model of obesity posits that individuals with lower sensitivity of DA-based reward regions overeat to compensate for this deficiency [56]. Apparently consistent with this theory, obese versus lean adults show lower striatal DA D2 receptor availability [57–59], though two other studies found no significant group differences [60, 61], which might have been due to the smaller samples sizes in the latter studies. Obese versus lean adults show lower capacity of nigrostriatal neurons to synthesize DA [62], and less striatal responsivity to tastes of high-fat/sugar beverages [20, 46, 63–65]. Obese versus lean rats likewise have lower basal DA levels and D2 receptor availability and less ex vivo DA release in response to electrical stimulation in nucleus accumbens and dorsal striatum tissue [66–69]. Interestingly, a recent study in humans [58] found a positive correlation between BMI and DA release in the dorsal striatum and substantia nigra in response to amphetamine, suggesting that D2 receptor availability may not be closely coupled with degree of DA response from rewarding experiences.
Although the above cross-sectional findings appear to provide some support for the reward deficit theory of obesity, prospective and experimental findings indicate that overeating contributes to reward region hypo-responsivity. Lean youth at risk for future obesity by virtue of parental obesity show hyper-responsivity of reward regions to palatable food receipt and monetary reward, rather than hypo-responsivity [70]. Young women who gained weight over a 6-month period showed a reduction in striatal responsivity to palatable food receipt relative to women who remained weight stable [71]. This finding converges with experimental overfeeding studies involving animals; rats randomized to overeating conditions that results in weight gain versus control conditions show down-regulation of post-synaptic D2 receptors, and reduced D2 sensitivity, extracellular DA levels in the nucleus accumbens and DA turnover, and lower sensitivity of DA reward circuitry to food intake, electrical stimulation, amphetamine administration, and potassium administration [69, 72–76]. Of note, rats that had consumed a high-fat/sugar diet continued to eat that food when subsequently paired with foot shocks, whereas chow-diet rats would not eat high-fat/sugar food when paired with foot shocks [76], suggesting that energy dense diets may induce compulsive eating. Further, pigs randomized to a weight gain intervention versus a stable weight condition showed reduced resting activity in the midbrain and nucleus accumbens [77]. The reduced DA signaling capacity appears to occur because habitual intake of high-fat diets causes decreased synthesis of oleoylethanolamine, a gastrointestinal lipid messenger [14]. Further, people who report elevated intake of particular foods show reduced striatal response during intake of that food, independent of BMI [78–80].
Given that animals that habitually use drugs of abuse that produce similar down-regulation of reward regions will work to keep DA levels in the nucleus accumbens above a certain level [81–83], Geiger and associated [74] speculate that rats which have experienced diet-induced down-regulation of DA circuitry may similarly overeat to increase DA signaling. However, a more recent study found that mice in which reduced striatal DA signaling from food intake was experimentally induced through intragastric feeding of high-fat food worked less for intragastric administration of high-fat food and consumed less food ad lib than control mice [14]. These experimental results seem incompatible with the notion that an induced down-regulation of DA reward circuitry leads to compensatory overeating. Results from the Tellez et al. [14] study also provided further evidence that intake of high-fat food can result in reduced DA response to food intake, independent of weight gain per se.
Interestingly, the relations between lower striatal response to milkshake receipt and weight gain over 1-year follow-up [20] and between lower putamen and OFC response to palatable food images and weight gain over 1-year follow-up [27] were significantly stronger for youth with the A1 allele, which is associated with less DA signaling, implying that any reduction in DA signaling caused by overeating may have a more pronounced reward deficit effect for those at genetic risk for lower DA signaling. Similar effects have emerged for individuals with the seven-repeat allele of the DRD4 gene, which is also associated with reduced DA signaling capacity [27].
Thus, studies have provided little prospective or experimental support for the reward deficit theory of obesity. Specifically, no prospective study has reported a main effect between reduced reward region responsivity to food intake or cues and future weight gain. Indeed, as noted, prospective studies have found that greater responsivity of reward circuitry, including the amygdala, midbrain, ventral pallidum, nucleus accumbens, and striatal, rather than reduced responsivity, to palatable milkshake intake predicts future weight gain [e.g., 42, 45]. And recent data found that inducing down-regulation of DA response to food intake resulted in less caloric intake and motivation for food than observed in control mice [14]. Thus, findings collectively suggest that the reduced DA signaling capacity of reward circuitry can be acquired from overeating, but provide little support for the notion that this contributes to overeating and subsequent weight gain.
Translating Findings from Brain Imaging Research into an Obesity Prevention Program
Thus, emerging research suggests that obese versus lean individuals show elevated reward region responsivity to images of high-fat/high-sugar foods and that this increases risk for future weight gain. Fortunately, there is evidence that prefrontal regions can reduce reward region responsivity to appetitive cues [84]. Cognitive reappraisals, such as thinking of the long-term health consequences of eating unhealthy food when viewing images of such foods, increases inhibitory region (dlPFC, vlPFC, vmPFC, lateral OFC, superior and inferior frontal gyrus) activation and decreases reward region (ventral striatum, amygdala, ACC, VTA, posterior insula) and attention region (precuneus, posterior cingulate cortex) activation relative to contrast conditions, such as imagined intake [85–88]. Stoeckel and associates [89] used real-time fMRI biofeedback to augment the effects of cognitive reappraisals in reducing reward region responsivity and increasing inhibitory region responsivity to images of palatable foods; the training resulted in significantly greater reduction in medial OFC, right ventral striatum, and right amygdala, as well as greater activation in an inhibitory control region (inferior frontal cortex [IFG]) in response to palatable food images. Thus, findings suggest that cognitive reappraisals may reduce hyper-responsivity of reward regions to food cues and increase inhibitory control region activation, which is crucial because our environment is replete with food images and cues (e.g., ads on TV) that contribute to overeating. For instance, US teens are exposed to over 5000 unhealthy food commercials yearly [90]. Indeed, exposure to unhealthy food commercials results in greater caloric intake of the advertised foods and other unhealthy foods [90–92]. Accordingly, we developed an obesity prevention program that trained participants to use cognitive reappraisals when confronted with unhealthy tempting foods. We hypothesize that if participants learn to automatically apply these cognitive reappraisals, they will show reduced reward and attention region responsivity and increased inhibitory region responsivity to food images and cues signaling impending delivery of a high-fat/high-sugar food, which should result in reduced caloric intake and weight gain.
Emerging data also suggests that obese versus lean individuals show reduced recruitment of reward regions during intake of high-fat/high-sugar foods, which is either inborn of acquired, with some evidence that this may increase risk for future weight gain. If overeating energy-dense food reduces reward region response to such food, which may prompt compensatory overeating to achieve the same satisfaction experienced previously, reducing fat and sugar intake may help people avoid this induced-reward deficit that may contribute to obesity. Such a “palate-retraining” intervention may also reduce preferences for high-fat/sugar foods, which may contribute to weight gain. Reducing intake of dietary fat decreases preferences and frequency of future consumption of previously preferred high-fat foods and increases acceptance of low-fat foods [93, 94], implying a relation between habitual fat intake and preferences for fat foods. Chronic intake of a high-fat diet theoretically leads to reduced oral sensitivity, prompting compensatory escalations in fat intake to experience same degree of reward [95]. We therefore included a palate-retraining component to our neuroimaging-informed obesity prevention program wherein participants reduce fat and sugar intake to decrease taste preferences for high-fat/sugar foods and avoid the reduced reward region responsivity to high-fat/sugar food intake observed in obese humans. We hypothesize that if intervention participants reduce overall consumption of fat and sugar, they will show an increase in striatal response to receipt of a high-fat/high-sugar milkshake, which may reduce risk for compensatory overeating.
We therefore evaluated an obesity prevention program that trained young adults to (a) use cognitive reappraisals when confronted with tempting palatable foods and (b) gradually reduce intake of fat and sugar in their diets to decrease risk for a blunted striatal response to palatable food intake [96]. Young adults at risk for future weight gain by virtue of weight concerns (N = 148) were randomized to this new Minding Health prevention program, an alternative prevention program promoting participant-driven gradual reductions in caloric intake and increases in physical activity (the Healthy Weight intervention), or an obesity education video control condition, completing assessments at pre, post, and 6-month follow-up. A subset of Minding Health and control participants completed an fMRI scan at pre and post assessing neural response to images of high-fat/sugar foods and to receipt and anticipated receipt of a high-fat/sugar food. Minding Health participants showed significantly greater reductions in body fat than controls and percentage of caloric intake from fat and sugar than Healthy Weight participants, though these effects attenuated somewhat by 6-month follow-up. However, Healthy Weight participants showed greater reductions in BMI and eating disorder symptoms than Minding Health participants. Minding Health participants showed greater activation of an inhibitory control region (IFG) and reduced activation of an attention/expectation region (mid cingulate gyrus) in response to palatable food images relative to pretest and controls. Although the Minding Health intervention produced some of the hypothesized effects, it only affected some outcomes and the effects often showed limited persistence.
Future Research Directions
This review highlights several important directions for future research. First, although a small number of prospective brain imaging studies have investigated neural vulnerability factors that predict future weight gain, the vast majority of the literature reviewed herein is cross-sectional. It will therefore be important for additional large sample prospective brain imaging studies to identify neural vulnerability factors that predict future weight gain. Second, it will be important to investigate environmental, social, and biological factors that amplify and mitigate the effects of these vulnerability factors on future weight gain. Third, it would be useful for additional prospective repeated-measures studies to attempt to capture the plasticity of reward region responsivity to food images/cues and food receipt, that appears to emerge secondary to overeating, which may play a role in maintaining overeating. If randomized experiments could be used to address these three directions for future research, much stronger inferences regarding these etiologic processes would be possible. Finally, we hope that future research will continue to try to translate findings from brain imaging studies into prevention and treatment interventions for obesity. For instance, we suspect it would be useful to test whether real-time fMRI biofeedback could be used to enhance the efficacy of the cognitive reappraisal-based obesity prevention program described above. It might likewise be possible to use non-invasive brain stimulation procedures to augment the efficacy of these types of obesity prevention programs.
References
Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307(5):491–497
Finkelstein EA, Trogdon JG, Cohen JW, Dietz W (2009) Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 28(5):w822–831
Flegal KM, Graubard BI, Williamson DF, Gail MH (2005) Excess deaths associated with underweight, overweight, and obesity. JAMA 293(15):1861–1867
Stice E, Shaw H, Marti CN (2006) A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychol Bull 132(5):667–691
Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE (2009) Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs 24(1):58–80
Haines J, Neumark-Sztainer D, Wall M, Story M (2007) Personal, behavioral, and environmental risk and protective factors for adolescent overweight. Obesity (Silver Spring) 15(11):2748–2760
Salbe AD, DelParigi A, Pratley RE, Drewnowski A, Tataranni PA (2004) Taste preferences and body weight changes in an obesity-prone population. Am J Clin Nutr 79(3):372–378
Stice E, Presnell K, Shaw H, Rohde P (2005) Psychological and behavioral risk factors for obesity onset in adolescent girls: a prospective study. J Consult Clin Psychol 73(2):195–202
Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH (1997) Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 337(13):869–873
Kringelbach ML, O’Doherty J, Rolls ET, Andrews C (2003) Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 13(10):1064–1071
Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M (2001) Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 124(Pt 9):1720–1733
Stice E, Burger KS, Yokum S (2013) Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am J Clin Nutr 98(6):1377–1384
Small DM, Jones-Gotman M, Dagher A (2003) Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 19(4):1709–1715
Tellez L, Medina S, Han W, Ferreira J, Licona-Limon P, Ren X et al (2013) A gut lipid messenger links excess dietary fat to dopamine deficiency. Science 341:800–802
Lutter M, Nestler EJ (2009) Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 139(3):629–632
Burger KS, Berner LA (in press) A functional neuroimaging review of obesity, appetitive hormones and ingestive behavior. Phys Behav. (available online)
Kelley AE, Baldo BA, Pratt WE, Will MJ (2005) Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86(5):773–795
Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG (2010) The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 1350:43–64
Mahmoud S, Thorsell A, Sommer W, Heilig M, Holgate J, Bartlett S, Ruiz-Velasco V (2011) Pharmacological consequences of the A118G opioid receptor polymorphims on morphine- and fentanyl-mediated modulation of CA2+ channels in humanized mouse sensory neurons. Anesthesiology 115(5):1054–1062
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM (2008b) Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 117(4):924–935
Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Savage CR (2010) Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obesity 34(10):1494–1500
Dimitropoulos A, Tkach J, Ho A, Kennedy J (2012) Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 58(1):303–312
Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, Jansen A (2011) Reward activity in satiated overweight women is decreased during unbiased viewing but increased when imaging taste: an event-related fMRI study. Int J Obes 36(5):1–11
Holsen L, Savage C, Martin L, Bruce A, Lepping R, Ko E, Brooks W, Goldstein JM (2012) Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. Int J Obes 36(5):638–647
Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Savage CR (2010) Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity 18(2):254–260
Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF (2007) Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37(2):410–421
Stice E, Yokum S, Bohon C, Marti N, Smolen A (2010) Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage 50(4):1618–1625
Stoeckel LE, Weller RE, Cook EW 3rd Twieg DB, Knowlton RC, Cox JE (2008) Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 41(2):636–647
Ng J, Stice E, Yokum S, Bohon C (2011) An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite 57(1):65–72
Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD (2012) Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage 63(1):415–422
Nolan-Poupart S, Veldhuizen MG, Geha P, Small DM (2013) Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger. Appetite 60(1):168–174
Burger KS, Stice E (2013) Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am J Clin Nutr 97(6):1188–1194
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275(5306):1593–1599
Tindell AJ, Berridge KC, Aldridge JW (2004) Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci 24(5):1058–1069
Tobler PN, Fiorillo CD, Schultz W (2005) Adaptive coding of reward value by dopamine neurons. Science 307(5715):1642–1645
Balleine BW, Daw ND, O’Doherty JP (2008) Multiple forms of value learning and the function of dopamine. In: Glimcher PW, Fehr E, Camerer C, Poldrack RA (eds) Neuroeconomics: decision making and the brain. Academic, New York, pp 367–385
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18(3):247–291
Smith K, Tindell A, Aldridge J, Berridge K (2009) Ventral pallidum roles in reward and motivation. Behav Brain Res 196(2):155–167
Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akil H (2011) A selective role for dopamine in stimulus-reward learning. Nature 469(7328):53–57
Chouinard-Decorte F, Felsted J, Small DM (2010) Increased amygdala response and decreased influence of internal state on amygdala response to food in overweight compared to healthy weight individuals. Appetite 54:639
Demos KE, Heatherton TF, Kelley WM (2012) Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci 32(16):5549–5552
Sun X, Veldhuizen M, Wray A, De Araujo I, Small D (2013) Amygdala response to food cues in the absence of hunger predicts weight change. Appetite 60(1):168–174
Yokum S, Ng J, Stice E (2011) Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 19(9):1775–1783
Yokum S, Gearhardt A, Harris J, Brownell K, Stice, E (in press). Individual differences in striatum activity to food commercials predicts weight gain in adolescence. Obesity
Geha PY, Aschenbrenner K, Felsted J, O’Malley SS, Small DM (2013) Altered hypothalamic response to food in smokers. Am J Clin Nutr 97(1):15–22
Stice E, Spoor S, Bohon C, Small DM (2008) Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322(5900):449–452
Nikolova YS, Ferrell RE, Manuck SB, Hariri AR (2011) Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology 36(9):1940–1947
Stice E, Yokum S, Burger KS, Epstein L, Smolen A (2012) Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci 32(29):10093–10100
Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC (1999) Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry 4(3):290–296
Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH (1995) Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem 65(3):1157–1165
Seeger G, Schloss P, Schmidt MH (2001) Marker gene polymorphisms in hyperkinetic disorder-predictors of clinical response to treatment with methylphenidate? Neurosci Lett 313(1–2):45–48
Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Weinberger DR (2000) Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 22(2):133–139
van Dyck CH Malison RT Jacobsen LK Seibyl JP Staley JK Laruelle M Gelernter J (2005) Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med 46(5):745–751
Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM (1996) Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6(3):243–250
Yokum S, Marti N, Smolen A, Stice E (submitted) Multilocus genetic composite reflecting elevated dopamine signaling predicts future weight gain.
Wang GJ, Volkow ND, Fowler JS (2002) The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets 6(5):601–609
de Weijer BA van de Giessen E van Amelsvoort TA Boot E Braak B Janssen IM Booij J (2011) Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res 1(1):37
Kessler RM, Zald DH, Ansari MS, Li R, Cowan RL (in press) Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity. Synapse
Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Pradhan K (2008) Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42(4):1537–1543
Eisenstein SA, Antenor-Dorsey JAV, Gredysa DM, Koller JM, Bihum EC, Ranck SA, Hershey T (2013) A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[11C]methyl)benperidol. Synapse 67(11):748–756
Haltia LT, Rinne JO, Merisaari H, Maguire RP, Savontaus E, Helin S, Kaasinen V (2007) Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse 61(9):748–756
Wilcox C, Braskie M, Kluth J, Jagust W (2010) Overeating behavior and striatal dopamine with 6-[F]-Fluoro-L-m-Tyrosine PET. J Obesity 2010:909348
Babbs RK, Sun X, Felsted J, Chouinard-Decorte F, Veldhuizen MG, Small DM (2013) Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol Behav 121:103–111
Frank GK, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, O’Reilly RC (2012) Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology 37(9):2031–2046
Green E, Jacobson A, Haase L, Murphy C (2011) Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Res 1386:109–117
Fetissov SO, Meguid MM, Sato T, Zhang LH (2002) Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am J Physiol Regul Integr Comp Physiol 283(4):R905–R910
Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN (2008) Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J 22(8):2740–2746
Huang XF, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, Deng C (2006) Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res 175(2):415–419
Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND (2008) Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse 62(1):50–61
Stice E, Yokum S, Burger KS, Epstein LH, Small DM (2011) Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci 31(12):4360–4366
Stice E, Yokum S, Blum K, Bohon C (2010) Weight gain is associated with reduced striatal response to palatable food. J Neurosci 30(39):13105–13109
Bello NT, Lucas LR, Hajnal A (2002) Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport 13(12):1575–1578
Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, Benoit SC (2008) Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci 122(6):1257–1263
Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN (2009) Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 159(4):1193–1199
Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN (2003) Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci 18(9):2592–2598
Johnson PM, Kenny PJ (2010) Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13(5): 635–641.
Val-Laillet D, Layec S, Guerin S, Meurice P, Malbert CH (2011) Changes in brain activity after a diet-induced obesity. Obesity (Silver Spring) 19(4):749–756
Burger KS, Stice E (2012) Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream-based milkshake. Am J Clin Nutr 95(4):810–817
Green E, Murphy C (2012) Altered processing of sweet taste in the brain of diet soda drinkers. Physiol Behav 107(4):560–567
Rudenga K, Small D (2012) Amygdala response to sucrose consumption is inversely related to artificial sweetener use. Appetite 58(2):504–507
Wise RA, Leone P, Rivest R, Leeb, K (1995 Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse 21(2):140–148
Wise RA, Newton P, Leeb K, Burnette B, Justice JB (1995) Fluctuations in nucleus accumbens dopamine concentrations during intravenous cocaine self-administration in rats. Psychopharmacology 120(1):10–20
Ranaldi R, Pocock D, Zereik R, Wise A (1999) Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous d-amphetamine self-administration. J Neurosci 19(10):4102–4109
Hare TA, Camerer CF, Rangel A (2009) Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324(5927):646–648
Hollmann M, Hellrung L, Pleger B, Schlogl H, Kabisch S, Stumvoll M, Horstmann A (2012) Neural correlates of the volitional regulation of the desire for food. Int J Obes 36(5):648–655
Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN (2010) Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A 107(33):14811–14816
Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A (2012) Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. NeuroImage 60(1):213–220
Yokum S, Stice E (2013) Cognitive regulation of food craing: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes 37(12):1565–1570
Stoeckel L, Ghosh S, Stern J, Keshavan A, Gabrieli J, Whitfield-Gabrieli, Evins A (2013) Real time fMRI neurofeedback: Effect on food and cigarette cue reactivity. Paper presented at the American College of Neuropsychopharmacology, Florida
Harris JL, Bargh JA, Brownell KD (2009) Priming effects of television food advertising on eating behavior. Health Psychol 28(4):404–413
Halford JC, Gillespie J, Brown V, Pontin EE, Dovey TM (2004) Effect of television advertisements for foods on food consumption in children. Appetite 42(2):221–225
Story M, French S (2004) Food advertising and marketing directed at children and adolescents in the US. Int J Behav Natr Phys Act 1(1):3
Ledikwe JH, Ello-Martin J, Pelkman CL, Birch LL, Mannino ML, Rolls BJ (2007) A reliable, valid questionnaire indicates that preference for dietary fat declines when following a reduced-fat diet. Appetite 49(1):74–83
Mattes RD (1993) Fat preference and adherence to a reduced-fat diet. Am J Clin Nutr 57(3):373–381
Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RS (2010) Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr 104(1):145–152
Stice E, Yokum S, Burger K, Rohde P, Shaw H, Gau J (in press). A pilot randomized trial of a cognitive reappraisal obesity prevention program. Physiology and Behavior
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Stice, E., Yokum, S., Burger, K. (2014). Neural Vulnerability Factors that Increase Risk for Weight Gain: Prevention and Treatment Implications. In: Nóbrega, C., Rodriguez-López, R. (eds) Molecular Mechanisms Underpinning the Development of Obesity. Springer, Cham. https://doi.org/10.1007/978-3-319-12766-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-12766-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12765-1
Online ISBN: 978-3-319-12766-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)