Abstract
Cancer is typically a consequence of imbalance between cell death and proliferation in a way favorable to cell proliferation and survival. Most conventional cancer therapies are based on targeting rapidly growing cancerous cells to block growth or enhance cell death, thereby, restoring the balance between these processes. In many instances, malignancies that develop resistance to current treatment modalities, for example photodynamic therapy (PDT), often present the greatest challenge in subsequent management of the patient. In this context, the role of survivin in resistance to anti-cancer therapies has become an area of intensive investigation. Survivin is a member of the inhibitor of apoptosis (IAP) family that correlates inversely with patient prognosis. The application of PDT resulted in an over-expression of survivin in tumor cells and, moreover, survivin has a specific role in modulating PDT-mediated apoptotic response. Tumor cells which present downregulated survivin and then are treated with PDT exhibit increased apoptotic indexes and cytotoxicity when compared to single agent-treated cells. There are several strategies under investigation to target survivin that include of molecular antagonists, small molecules and immunotherapy. The translation of these findings to the clinic is currently ongoing with a number of phase I/II clinical trials targeting survivin that are in progress. Therefore, combining PDT with a survivin inhibitor may attribute to a more favorable clinical outcome than the use of single-modality PDT.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- CPC:
-
Chromosomal passenger complex
- IAP:
-
Inhibitor of apoptosis protein
- IC:
-
Irradiated cells
- MAL:
-
Delta-aminoleavulinic acid methyl ester hydrochloride
- PDT:
-
Photodynamic therapy
- pSil_1:
-
Plasmid silencer against survivin
Introduction
The resistance of human tumors to cancer therapies is attributed to mutations, amplifications of genetic and epigenetic changes that influence in the take, transport and metabolism of the drug and a great network of survival and proliferation mechanisms . In this context, PDT-mediated oxidative stress induces a transient increase in the downstream early-response genes (c-fos, c-jun, c-myc and egr-l) and stress genes (coding for heat shock proteins [Hsp], glucose-regulated proteins and heme oxygenase) in mammalian cells [1–6]. The early-response genes function as transcription factors and act by regulating the expression of a variety of genes via specific regulatory domains. PDT appears to stimulate several different signaling pathways, some of which lead to cell death , by caspase-dependent [7] and –independent [8] apoptosis , whereas others mediate cell survival such that the ultimate survival of a given cell results from the combined action or interaction (or both) of these different pathways [9]. Therefore, survival cells may cooperate in tumor recurrences following PDT and underline the need to more fully understand the molecular responses initiated by PDT. In this context, there have been reports that showed that PDT induces the expression of heat-shock proteins (HSPs) such as HSP70 [10], HSP47 [11] and HSP60 [4], as well as other stress-inducible proteins [12]. HSPs are abundant. HSP70 and HSP90 correlate with a poor prognosis in acute myeloid leukemias and myelodysplastic syndromes [13, 14].
Conversely, it was observed that PDT induces overexpression and phosphorylation of a protein called “Survivin” in human cancer cells and tumors [15, 16]. Recently, there has been increasing clinical interest in this protein, because it possesses inherent properties that make it an ideal tumor marker and a potential therapeutic target [17–19].
Overview of Survivin
Survivin is the smallest member of the inhibitor of apoptosis protein (IAP) family [20], containing a single Baculovirus IAP Repeat (BIR), which is the hallmark of these molecules. The survivin gene encodes a 16.5 kDa protein consisting of an N-terminal Zn2+-binding BIR domain linked to a 65 Å amphipathic C-terminal-helix [21]. Survivin can function as a monomer for at least some protein–protein interactions, as well as mechanisms of subcellular localization [22]. The control of survivin gene expression at the tip of chromosome 17 in humans (17q25) is complex, and involves scores of positive and negative regulators. Several oncogenic transcription factors stimulate expression of the survivin gene, whereas multiple tumor suppressors actively repress the survivin gene [23]. A survivin protein is extensively post-translationally modified by degradative and non-degradative cycles of ubiquitylation and de-ubiquitylation, as well as phosphorylation [24], which control protein stability, binding to molecular partners and trafficking to various subcellular compartments.
Alternative splicing of survivin pre-mRNA from chromosome 17q25 produces five different mRNAs, which potentially encode distinct proteins, survivin, survivin DEx3 [25], survivin 3B [26] and survivin 2α [27]. Full-length survivin is derived from exons 1–4. Survivin 2B is also derived from exons 1–4 but retains an additional 69 bp from intron 2 as a cryptic exon. Survivin DEx3 is derived from exons 1, 2 and 4, as a frameshift read-through leads to exclusion of exon 3 [25]. Survivin 3B is derived from exons 1, 2, 3 and 4, and includes a new sequence of 165 bp from intron three [25]. Acquisition of an in-frame TGA stop codon within the novel exon 3B results in an open reading frame of 363 nucleotides, predicting a truncated 120 amino acid protein [25]. Survivin 2α comprises exons 1 and 2 of the survivin gene as well as a 30,197 bp region of intron 2 [26]. The acquisition of an in-frame stop codon within intron 2 results in an open reading frame of 225 nucleotides and predicts for a truncated 74 amino acid protein. The survivin 3B protein is predicted to have 120 aa, while survivin DEx3, full length survivin and survivin 2B are predicted to have 137, 142 and 165 aa, respectively.
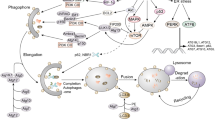
There are on-going interests in identifying the molecular functions of survivin since it is an interesting molecule that interferes with a variety of cellular process. It is known that survivin is a multifunctional protein and is essential, in that constitutive or conditional deletion of the survivin gene is incompatible with tissue or organism viability [24]. Orthologs of survivin have been found in lower organisms, such as yeast, worms, and flies, suggesting evolutionary conservation of this pathway. In mammalian cells, survivin participates in at least three homeostatic networks: the control of mitosis (1), the regulation of apoptosis and autophagy (2), and the cellular stress response (3) (Fig. 7.1). This classification is not restrictive, as novel functions of survivin are continuously proposed, as well as new roles for known properties. Even within the same network, survivin plays multiple roles .
The Role of Survivn in Cell Division
Some investigators have suggested that the primary function of survivin is in controlling cell division, rather than apoptosis inhibition [28–30]. During mitosis, survivin exists as a multi-protein complex, known as the chromosomal passenger complex (CPC) [31–33]. The CPC is a key regulator of mitosis, and this complex is composed of survivin, Borealin, and INCENP and Aurora B kinase. Structurally, the BIR domain of survivin can bind to the phosphorylated Thr3 site of histone H3 [34]. Upon CPC complex formation at the G2/M phase of cell cycle, survivin reads phosphorylated histone H3 and subsequently activates the mitotic kinase Aurora B [35–37] (Fig. 7.2). The formation of CPC and the interaction between CPC and Aurora B kinase through survivin’s BIR domain are both crucial for the completion of mitosis. In fact, it has been shown that survivin-depleted cells could exit mitosis prior to completion of sister chromatid segregation. An alternative possibility is that survivin promotes mitosis by acting as an interphase between the centromere/central spindle and the CPC [32, 33]. Coincident with its role in cell proliferation, survivin expression is predominantly regulated by a cell cycle-dependent and cell cycle homology region within the promoter, which leads to maximum expression during the G2/M phase of the cell cycle [38]. Interestingly, survivin may interfere with the dynamic formation of microtubules. Over-expression of survivin has been shown to reduce centrosomal microtubule nucleation and suppress both microtubule dynamics instability in mitotic spindles and bidirectional growth of microtubules in midbodies during cytokinesis [39]. It has been proposed that the splice variants function to modulate the function of full-length survivin [40]. While this may be true for apoptosis inhibition, where survivin and survivin DEx3 interact within the mitochondria to inhibit mitochondrial-dependent apoptosis [40], recent evidence suggests that the splice variants cannot modulate survivin’s function during cell division [41]. Structural comparison studies have supported this finding [22] .
The Role of Survivin in Apoptosis and Autophagy
Multiple in vitro and in vivo studies have shown that survivin inhibits cell death , especially apoptosis [42–45]. Survivin interferes with the process of apoptosis by inhibiting the activity of different caspases in cancer cells [43–46]. It is not surprising that survivin can interfere with the activity of caspases, given that survivin contains a single BIR domain and that the presence of the BIR domain was widely shown to be important in targeting caspases in various IAP family members [21]. Survivin, like all other IAPs except XIAP [20], does not directly inhibit caspases, but interacts with protein partners, most notably XIAP [47]. This complex promotes increased XIAP stability against degradation, activates multiple signaling pathways, including NF-κB, synergistically inhibits caspase-3 and -9, suppresses apoptosis, and accelerates tumor progression, in vivo (Fig. 7.3). Other mechanisms of survivin cytoprotection have been proposed, including the ability of mitochondrial localized pool of survivin to sequester pro-apoptotic Smac away from XIAP [48], or altogether prevent its release from the mitochondria [49] (Fig. 7.3) .
Survivin’s function in apoptosis. Survivin binds to XIAP, this complex promotes increased XIAP stability against degradation and synergistically inhibits caspase-3 and -9 and suppressing apoptosis. Also, a pool of survivin localized in the mitochondrial sequesters pro-apoptotic Smac away from XIAP. Moreover, survivin prevents AIF release from the mitochondrial intermembrane space and inhibiting the caspase-independent apoptosis
Furthermore, survivin play a role in inhibiting the caspase-independent apoptosis of cancer cells. Translocation of the apoptosis -inducing factor (AIF) from the cytoplasm to the nucleus is a molecular indicator of the caspase-independent apoptosis of cells . Down-regulation of survivin by siRNA induces the translocation of AIF from the cytoplasm to the nucleus in various cancer cells [50]. A study carried out by Pavlyukov et al. further showed that monomeric survivin (not dimeric survivin) prevents AIF release from the mitochondrial intermembrane space, protecting human fibrosarcoma HT1080 cells from caspase-independent apoptosis [51]. On the other hand, AIF knockout was shown effective in reversing the pro-apoptotic effect caused by the dominant-negative survivin in acute lymphoblastic leukemia (ALL) cells in vitro [52]. Taken together, these studies suggest that AIF plays critical roles in survivin-mediated caspase-independent apoptosis (Fig. 7.3).
Conversely, multiple evidences indicate that survivin interferes with the process of cell autophagy and down-regulation of survivin may induce apoptosis through autophagy-dependent mechanisms. First of all, an interaction between the autophagy regulator, Beclin 1, and survivin has been shown in human glioma cells in response to TRAIL [53]. Second, results from Roca et al.’s study showed that CCL2 (Chemokine (C–C motif) ligand 2) protected human PC3 prostate cancer cells from autophagic cell death via the phosphatidylinositol 3-kinase/Akt/survivin pathway [54]. Induction of autophagy-dependent apoptosis has further been shown in prostate cancer cells treated by the survivin suppressant YM155 [55]. Taken altogether, these studies showed that up-regulation of survivin inhibit autophagy, whereas downregulation of survivin promotes cell autophagy. However, the mechanisms by which survivin regulates autophagy remains to be determined .
The Role of Survivin in the Cellular Stress Response
A third function of survivin in the cellular stress response is beginning to emerge, and involves the association of survivin with various molecular chaperones, including the aryl hydrocarbon receptor-interacting protein (AIP) [56], Hsp60 [57], and Hsp90 [58] . These interactions may promote adaptation under conditions of cellular stress by maintaining survivin protein stability, folding, and subcellular trafficking .
For instance, HSP90 associated with survivin is overexpressed in cancers with roles in mitotic control and apoptosis inhibition . The cytoprotection mechanism of survivin–HSP90 association is centred on the mitochondrial pathway, where survivin has a role in the regulation of mitochondrial apoptosis specifically in tumors [58]. However, the possible disruption of the survivin–HSP90 complex destabilizes survivin leading to mitochondrial apoptosis and ultimately cell growth suppression [58]. HSP90 interaction with survivin enables stabilization of cofactors such as AKT, human epidermal growth factor receptor (Erb-2) and HIF-1a, which can lead to tumor progression [59].
As we saw so far, because of its nodal properties, and over-expression in virtually every human tumor, survivin has been intensely pursued as a drug target for novel cancer therapeutics [24, 60, 61]. Moreover, the ability of survivin to counteract apoptotic stimuli enhances cell survival, which in turn facilitates cell proliferation, including the proliferation of mutant cells. This proliferation may ultimately give rise to malignancy. The failure to execute apoptosis also renders malignant cells resistant to various forms of therapy including photodynamic therapy [15].
Photodynamic Therapy and Survivin Expression
PDT uses non-toxic dyes and harmless visible light in combination with oxygen to produce highly reactive oxygen species that kill cells. Our laboratory studies conducted on PDT-treatment using delta-aminoleavulinic acid methyl ester hydrochloride (MAL), such as a photosensitizer in T47D breast cancer cells, revealed increased both expression of survivin and its phosphorylated form [15]. Our results were in accordance with another study that observed increased survivin expression after PDT [62]. This suggests the possibility of interfering with the cellular response to photochemical therapy. Moreover, there are many signaling molecules up-regulated by PDT, including phosphatidylinositol 3-kinase/Akt, mitogen-activated protein kinase, hypoxia inducible factor-1a, activator protein-1, and nuclear factor-κB, all of which are inducers of survivin expression [63]. Similarly, inflammatory cytokines, vascular endothelial growth factor , vascular injury, and hypoxia are associated with increased expression and/or stability of survivin and these responses are also increased following PDT.
PDT stimulate several different signaling pathways, some of which lead to cell death, whereas others mediate cell survival such that the ultimate survival of a given cell results from the combined action or interaction (or both) of these different pathways. Our experience demonstrates that before treatment, the population of T47D cells has a level of survivin expression that is similar in all cells (Fig. 7.4). We argued that the increased expression observed on irradiated cells (IC) and cells treated with MAL alone (MAL) is attributed to some stressing condition given to the cells. But it is clear that only after treatment, a certain percentage of these cells could trigger signaling pathways which lead to cell survival involving survivin overexpression (Fig. 7.4).
Survivin and phosphorylated survivin expression after PDT treatment. Tumor cells were incubated with MAL and then exposed to ligth. After 24 h of light treatment, whole extracts from non-treated cells (NC), irradiated cells (IC), cells treated with MAL alone (MAL) and PDT-treated cells (PDT) were prepared and analyzed by Western blot to determine survivin, phosphorylated survivin (Phospho-Survivin), and GAPDH (internal control) levels
Enhanced survivin after PDT in T47D cells led us to hypothesize that this anti-apoptotic protein would be potentially relevant to the PDT outcome in sensitized cells. In an attempt to demonstrate the crucial role of survivin on moderate PDT response in metastatic breast cancer cells, we targeted specifically mRNA survivin by siRNA technology (pSil_1). Indeed, growth-inhibitory effects in T47D cells in the absence of any further cytotoxic stimulus was observed when survivin was downregulated, and this agrees with results previously reported [50]. Moreover, we performed a dose–response curve by combining treatment of T47D cells with MAL-PDT and survivin downregulation. We found the condition to sensitize T47D cells to PDT synergistically, suggesting a survivin specific role in modulating PDT. The synergistic combination increased apoptosis and cytotoxic effect when compared with single treatments (Fig. 7.5). We could observe that this procedure also led to enhanced PARP- and caspase-3 cleavage, a strong decrease in the Bcl-2/Bax ratio and activation of caspase-8. Furthermore, to confirm the specific role of survivin in the modulation of PDT, we overexpressed survivin. We observed the increase of cell viability and the reduction of cell death in breast cancer cells treated with PDT. Therefore, we suggest that survivin plays an important role in modulating cancer cell survival by PDT treatment during cancer therapy.
Evaluation of cytotoxicity and apoptosis rate on PDT-treated tumor cells with survivin down-regulated. Cells were pre-transfected with pSil_1 for 72 h and then incubated with MAL and exposed to light. a Tumor cells viability was measured using MTT assay 24 h after MAL-PDT. Values are expressed as means ± SDs of eight separate experiments. A statistically significant difference in the level of viability between cells treated with pSil_1/PDT combination therapy and pSil_1 or PDT monotherapy is denoted by ‘‘*’’ (p < 0.05). b Apoptotic indexes were measured 24 h after MAL-PDT using the Cell Death Apoptosis Detection ELISA Plus kit. Values are expressed as means ± SDs of two separate experiments. A statistically significant difference in the level of apoptosis between cells treated with pSil_1/PDT combination therapy and pSil_1 or PDT monotherapy is denoted by ‘‘*’’ (p < 0.05)
It has been proposed that survivin may inhibit apoptosis through suppression of caspase activity [43], but we have previously observed that silencing survivin in T47D cells by siRNA triggered apoptosis in a caspase-independent manner, involving nuclear translocation of mitochondrial AIF [50]. Interestingly, when the combined treatment was applied, apoptosis was triggered in a caspase-dependent manner. Therefore, our results demonstrated that in PDT protocols survivin directly or indirectly could interfere with caspase-3 activity.
Curiously, as a consequence of survivin downregulation, with or without PDT, a diminished Bcl-2/Bax ratio was observed (Fig. 7.6). It has been suggested that an alternatively spliced survivin variant, called survivin-DEx-3, which localizes in the mitochondria, interacts with Bcl-2 [64]. Since anti-apoptotic Bcl-2 proteins function as inhibitors of mitochondrial permeability transition, this recognition would position survivin, or at least one of its spliced variants, in the regulation of mitochondrial membrane integrity. Alternatives of this pathway have been suggested, involving hyperphosphorylation of Bcl-2, and a reduced activation of proapoptotic Bax by survivin, potentially upstream of caspase activation [65], thus, further dampening mitochondrial permeability. Furthermore, survivin-DEx-3 was recently shown to maintain mitochondrial membrane potential and to control the production of reactive oxygen species in response to cell-death stimuli [66]. Since the siRNA that we used for our experiments targets exon 1 of the human survivin mRNA, survivin-DEx-3 was blocked that would explain how survivin modulates the response of cancer cells to PDT (Fig. 7.7).
Analysis of Bcl-2/Bak relation in MAL-PDT apoptotic cells. Cell lysates from control (Control), photosensitizer alone (MAL), pSil_1 alone (pSil_1), and PDT-treated cells in the absence (PDT) or presence of pSil_1 (pSil_1 + PDT) were collected 24 h after light exposure. Expressions of survivin, Bcl-2, Bak, and GAPDH (internal control) were determined by Western blot analysis. Staurosporine (STS)-treated tumor cells were used as positive control of apoptosis
Role of survivin in modulating PDT-mediated apoptotic response. a An alternatively spliced survivin variant, called survivin-DEx-3 which localizes in mitochondria, interacts with Bcl-2 and Bax. Survivin-DEx-3 hyperphosphorylated Bcl-2, and reduced activation of proapoptotic Bax. Furthermore, survivin-DEx-3 maintains mitochondrial membrane potential and controls the production of reactive oxygen species in response to cell-death stimuli, like in PDT. b Absence of survivin-DEx-3 mitochondrial permeability change. The expression of Bak and ROS increases, proapoptotic proteins are released from the mitochondria and all these manifestations produce apoptosis
In summary, the intricate relationship between programmed cell death and cell survival may directly or indirectly be dependent on survivin . Since this protein is overexpressed in MAL-PDT treated cells, this could result in aggressive tumor behavior yielding a poor survival rate and resistance to cancer therapies. Moreover, down-regulation of survivin sensitizes breast cancer cells to PDT-induced cytotoxicity . Since a number of different strategies are now employed to treat metastatic breast cancer, it is promising to demonstrate that a combined modality and sequential therapy can prove beneficial to treatment. Therefore, our data suggest that emerging strategies in targeting protective proteins may increase the clinical effectiveness of cancer treatments.
Targeting Survivin: Which is the best option?
As survivin is not a cell surface protein and does not have an intrinsic enzymatic activity, targeting of survivin for therapeutic purposes might be expected to be difficult. In addition, crystallographic data have revealed few potential drugable sites on the survivin protein [21]. Despite these problems, several research groups have attempted to target survivin using different strategies (Table 7.1), which are discussed below.
Molecular Antagonists
The first described molecular antagonist of survivin is a phosphorothioate antisense oligonucleotide [67], which suppressed survivin mRNA and protein expressions, and produced a strong anticancer activity in preclinical models. Sponsored by Ely Lilly and Co., this agent designated LY2181308 showed a favorable toxicity profile with evidence of survivin downregulation in a phase I trial in patients with advanced cancers [68]. LY2181308 (also called ISIS23722 and Gataparsen) is a survivin-specific second generation antisense oligonucleotide with 2´-O-methoxyethyl modified 18-mer structure (5´-TGTGCTATTCTGTGAATT-3´) [69, 70]. The major mechanism of action of LY2181308 is relatively straightforward: it selectively binds to the 3´-untranslated region of the survivin transcript by Watson–Crick base pairing, resulting in the destruction of survivin RNA by RNase H [69–71]. In the first-in-human dose (FHD) study performed by Talbot et al. [72], patients with advanced solid tumors (gastrointestinal, melanoma , lung and breast cancers) were treated with LY2181308 using the following schedule: daily i.v. infusion on day 1–3, then weekly infusion. The most common symptoms observed in patients treated with LY2181308 were shown to be fatigue, fever, vomiting and thrombocytopenia, and the recommended dose of LY2181308 was suggested as 750 mg [72]. In another study, Japanese patients with advanced solid tumors (lung, pancreatic, and breast cancers) were administered with the drug, and the maximum tolerated dose (MTD) of LY2181308 determined was shown to be 750 mg (daily i.v. infusion on day 1–3, then weekly infusion), which is similar to the MTD reported in Talbot et al.’s study [72]. Pharmacokinetic analysis of LY2181308 in the same clinical study revealed that the terminal half-life, distribution clearance and Vss were 21 days, 2.0 L/h and 2.05–105 L, respectively. Thrombocytopenia and fatigue were reported as common reversible grade ½ toxicities related to the therapy, similar to those reported in Talbot et al.’s study [72]. It is worth noting that a study carried out by Gurbuxani et al. [73] has found that murine survivin plays a role in hematopoietic cell development [73]. Therefore, human survivin may also play a role in erythropoiesis. However, further investigations are needed to determine whether thrombocytopenia is a mechanism-based toxicity induced by LY2181308.
Santaris Pharma, and, more recently, ENZON Pharmaceuticals [74] have also developed an agent that showed excellent safety in a phase I clinical, called SPC3042 (or ENZ3042 [75]. SPC3042 is an antisense 16-mer locked nucleic acid (LNA) oligonucleotide (5´-CTCAATCCATGGCAGC-3´) that targets exon 4 of the survivin transcript [74]. A study published in 2010 revealed that treatment with SPC3042 alone induced 60 % down-regulation of survivin mRNA in tumors and 37–45 % tumor growth inhibition (TGI) in the A549 and Calu-6 lung xenograft models [76].
Additional strategies to acutely lower survivin levels in tumor cells involved small interfering RNA (siRNA) or hammerhead ribozymes [19]. In preclinical studies, these agents consistently showed anticancer activity, alone or in combination with chemotherapy, with no detectable systemic toxicity [19]. The delivery of siRNA in vivo is challenging, but the apparent success of recent studies [77] suggests that survivin-directed gene silencing may at some point be evaluable in cancer patients.
Small Molecules
Small molecules that directly target survivin have been developed [24], and several clinical trials with these agents have been completed, with more underway. YM155 monobromide(1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1Hnaphtho [2,3-d] imidazolium bromide) is a small molecule survivin gene suppressant, and it is the most functionally evaluated survivin inhibitor in both pre-clinical and clinical studies so far [29]. Originally developed by Yamanouchi Pharmaceuticals, and, more recently, by Astellas Pharma [78]. At the molecular level, YM155 binds to the Sp1 rich region of the promoter of survivin, and inhibits the transcription of survivin in cells [79, 80].
Two phase I studies of YM155 in heavily-pretreated cancer patients have been published. The trial conducted in the US reported impressive responses, with tumor shrinkage and durable remissions in patients with advanced prostate cancer, large cell non-Hodgkin’s lymphoma and non-small cell lung cancer [81]. The Japan phase I trial of YM155 also provided evidence of disease stabilization in nine patients [78]. Importantly, both studies showed a favorable toxicity profile with minimal and rapidly reversible side effects.
Despite various pre-clinical and phase I clinical studies indicating that YM155 could be an effective anti-cancer reagent [78], phase II clinical trials showed disappointing results. In a phase II clinical trial, 34 patients with un-resectable stage III or IV melanoma were infused with YM155 according to the following schedule: 168 h (7 days) continuous infusion at a dosage of 4.8 mg/m2/day, followed by a 14 rest period, for 6 cycles [19]. The most common adverse events of YM155 monotherapy reported in this study were fatigue, nausea, pyrexia, headache, arthralgia and back pain. The same study also reported that one patient developed Grade 3 acute renal failure during Cycle 1 of therapy and four patients (11.8 %) developed cardiac adverse events (AE). However, the development of cardiac adverse events was not likely related to YM155. The objective tumor response rate (ORR) of patients with the YM155 treatment was approximately 3 % in such study [19]. On the other hand, Giaccone et al.’s study[82] reported that YM155 only exhibited modest single-agent activity in patients, and the ORR to YM155 treatment (similar dosage schedule as the abovementioned study) was approximately 5.4 % in patients with advanced refractory nonsmall-cell lung carcinoma [82]. Notably, a recent study showed that YM155 is a substrate of the multi-drug resistant protein (MDR1/ABCB1/P-gp), suggesting that YM155 treatment may not be useful in treating cancer patients with MDR1-related drug resistance after prolonged chemotherapy [83].
Another direct small molecule inhibitor of survivin is tetra-O-methyl nordihydroguaiaretic acid (M(4)N), which also acts as a transcriptional repressor of the survivin promoter, potentially by antagonizing Sp1-dependent gene expression [84]. This compound, designated Terameprecol (EM-1421) [85], has shown good preclinical activity with an impressive 88 % bioavailability, in vivo [86]. Terameprecol has been formulated for systemic delivery to cancer patients, and a phase I study in patients with advanced solid malignancies has shown favorable safety and disease stabilization in 8 out of 25 evaluable patients [85]. Another phase I study of Terapremecol in 16 heavily pretreated patients with adult myelogenous leukemia (AML) has also shown a favorable safety, one partial response and disease stabilization in five patients [87]. In addition, Terameprecol has been formulated as a 1 % or 2 % vaginal ointment for local application in women with papillomavirus- or herpes simplex virus-associated carcinogenesis. Two phase I studies with Terameprecol ointment have shown excellent safety, no adverse events and no systemic absorption of the agent [88, 89].
Cancer Vaccine/Immunotherapy
Immunotherapy is the use of the immune system to either cure a disease or to avoid the development of a disease. Several studies have reported successful application of cell-based immunotherapy as cancer treatment [90–92]. Cell-based cancer immunotherapy involves the use of immune cells such as the natural killer cells, dendritic cells, and cytotoxic T lymphocytes, which are isolated from the patient, activated in vitro and transfused back to the patient to target cancer cells. Because of its differential expression in cancer, as opposed to normal tissues, it has been hypothesized that cancer patients may recognize survivin as a non-self protein, and mount an immune response to it [93]. This concept has been validated in the clinic, and sera from cancer patients contained antibodies [94], and cytolytic T cells against survivin [95]. This immune recognition has been mapped in detail [96, 97], and dendritic cells pulsed with survivin peptides, survivin-containing tumor lysates or transduced/transfected with survivin, elicit cytolytic T cell responses and MHC-restricted anticancer activity in vitro [98, 99] and in preclinical models [100]. Several phase I/II trials of survivin-directed immunotherapy have been reported [101]. In these studies, survivin-based vaccination was invariably safe, devoid of significant side-effects, and associated with antigen-specific immunologic responses. Although no objective responses were noted, two phase I/II trials with infusion of dendritic cells pulsed with survivin showed durable (> 6 months) disease stabilization in 24 % of melanoma patients [102], and 50 % of renal cell carcinoma patients [103]. A phase I/II trial of systemic delivery of protamine-protected survivin mRNA in melanoma was also safe, produced detectable T cell responses, and achieved one complete response out of seven evaluable patients [104]. Anecdotal reports also suggest that survivin-based vaccination may be effective against metastatic disease [105]. Based on these encouraging findings, several additional phase I and II clinical trials using survivin -based immunotherapy are ongoing (Table 7.1). Currently, studies are focusing on the specific epitopes that elicit the most potent immunodominant, immunoprevalent T cell responses against survivin, with the likelihood that those inducing both a CD8+ and CD4+ response will be most effective [106].
Conclusion
PDT induces considerable stress within the tumor microenvironment . This includes both oxidative stress produced by the photochemical generation of reactive oxygen species and hypoxia resulting from the rapid vascular damage produced by PDT and/or by the photochemical consumption of oxygen [107]. A consequence of PDT-mediated stress is the induction of a survival phenotype associated with increased expression of angiogenic growth factors, cytokines, proteinases, and antiapoptotic molecules. Our increasing knowledge of PDT responses at a molecular level provides significant opportunities to further improve the therapeutic effectiveness of PDT. In particular, over the last period, it has become increasingly clear that survivin may have an important role in the survival phenotype observed in PDT treatments [15–108]. Survivin has many functions involved in cell survival including complex intracellular signaling, stabilizing mitosis and facilitating cellular adaptation. So, it is clinically relevant that inhibitors of survivin expression may enhance PDT responsiveness.
Survivin antagonists may function not as single protein inhibitors, but rather as global pathway inhibitors that may disable multiple signaling circuits in tumors. Clinical trials have highlighted the problems with attempts to correlate survivin expression with clinical outcome. Small sample numbers, nonuniform treatments, the presence of multiple alternatively spliced survivin mRNAs with differing effects on apoptosis and the different methods of detection of survivin, all lead to difficulties in trial interpretations. Further efforts are required to achieve a greater understanding of the biology of survivin and the other IAPs and more effectively exploit strategies that target this protein in cancer .
No Conflict of Interest
No potential conflicts of interest were disclosed.
References
Gomer CJ, Ferrario A, Rucker N, Wong S, Lee AS. Glucose regulated protein induction and cellular resistance to oxidative stress mediated by porphyrin photosensitization. Cancer Res. 1991;51:6574–9.
Gomer CJ, Luna M, Ferrario A, Rucker N. Increased transcription and translation of heme oxygenase in Chinese hamster fibroblasts following photodynamic stress or Photofrin II incubation. Photochem Photobiol. 1991;53:275–9.
Luna MC, Gomer CJ. Isolation and initial characterization of mouse tumor cells resistant to porphyrin-mediated photodynamic therapy. Cancer Res. 1991;51:4243–9.
Hanlon JG, Adams K, Rainbow AJ, Gupta RS, Singh G. Induction of Hsp60 by Photofrin-mediated photodynamic therapy. J Photochem Photobiol B. 2001;64:55–61.
Tong Z, Singh G, Rainbow AJ. Sustained activation of the extracellular signal-regulated kinase pathway protects cells from photofrin-mediated photodynamic therapy. Cancer Res. 2002;62:5528–35.
Tong Z, Singh G, Valerie K, Rainbow AJ. Activation of the stress-activated JNK and p38 MAP kinases in human cells by Photofrin-mediated photodynamic therapy. J Photochem Photobiol B. 2003;71:77–85.
Alvarez MG, Prucca C, Milanesio ME, Durantini EN, Rivarola V. Photodynamic activity of a new sensitizer derived from porphyrin-C60 dyad and its biological consequences in a human carcinoma cell line. Int J Biochem Cell Biol. 2006;38:2092–101.
Vittar NB, Awruch J, Azizuddin K, Rivarola V. Caspase-independent apoptosis, in human MCF-7c3 breast cancer cells, following photodynamic therapy, with a novel water-soluble phthalocyanine. Int J Biochem Cell Biol. 2010;42:1123–31.
Moor AC. Signaling pathways in cell death and survival after photodynamic therapy. J Photochem Photobiol B. 2000;57:1–13.
Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumor immunity. Nat Rev Cancer. 2006;6:535–45.
Verrico AK, Haylett AK, Moore J V. In vivo expression of the collagen-related heat shock protein HSP47, following hyperthermia or photodynamic therapy. Lasers Med Sci. 2001;16:192–98.
Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–26.
Duval A, Olaru D, Campos L, Flandrin P, Nadal N, Guyotat D. Expression and prognostic significance of heat-shock proteins in myelodysplastic syndromes. Haematologica. 2006;91:713–4.
Thomas X, Campos L, Mounier C, Cornillon J, Flandrin P, Le Q-H et al. Expression of heat-shock proteins is associated with major adverse prognostic factors in acute myeloid leukemia. Leukemia Res. 2005;29:1049–58.
Cogno IS, Vittar NB, Lamberti MJ, Rivarola VA. Optimization of photodynamic therapy response by survivin gene knockdown in human metastatic breast cancer T47D cells. J Photochem Photobiol B. 2011;104:434–43.
Ferrario A, Rucker N, Wong S, Luna M, Gomer CJ. Survivin, a member of the inhibitor of apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response. Cancer Res. 2007;67:4989–95.
Zaffaroni N, Pennati M, Daidone MG. Survivin as a target for new anticancer interventions. J Cell Mol Med. 2009;9:360–72.
Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis. 2007;28:1133–39.
Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy. Expert Opin Ther Targets. 2008;12:463–76.
Srinivasula SM, Ashwell JD. IAPs: what’s in a name?. Mol Cell. 2008;30:123–35.
Chantalat L, Skoufias DA, Kleman JP, Jung B, Dideberg O, Margolis RL. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol Cell. 2000;6:183–89.
Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–85.
Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle. 2009; 8:2708–10.
Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70.
Mahotka C, Wenzel M, Springer E, Gabbert HE, Gerharz CD. Survivin-deltaEx3 and survivin-2B: two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties. Cancer Res. 1999;59:6097–102.
Badran A, Yoshida A, Ishikawa K, Goi T, Yamaguchi A, Ueda T, Inuzuka, M. Identification of a novel splice variant of the human anti-apoptopsis gene survivin. Biochem Biophys Res Commun. 2004;314:902–7.
Caldas H, Honsey LE, Altura RA. Survivin 2alpha: a novel Survivin splice variant expressed in human malignancies. Mol Cancer. 2005;4:11–20.
Yang D, Welm A, Bishop JM. Cell division and cell survival in the absence of survivin. Proc Natl Acad Sci USA. 2004;101:15100–105.
Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumor cells. Nat Rev Cancer. 2004;4:592–603.
Wolanin K, Piwocka K. Role of survivin in mitosis. Postepy Biochemii. 2007;53:10–8.
Vader G, Kauw JJW, Medema RH, Lens SMA. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. 2006;7:85–92.
Ruchaud S, Carmena M, Earnshaw WC. The chromosomal passenger complex: one for all and all for one. Cell. 2007;131:230–31.
Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 2004;166:179–91.
Jeyaprakash AA, Basquin C, Jayachandran U, Conti E. Structural basis for the recognition of phosphorylated histone h3 by the survivin subunit of the chromosomal passenger complex. Structure. 2011;19:1625–34.
Bolton MA, Lan W, Powers SE, McCleland ML, Kuang J, Stukenberg PT. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol Biol Cell 2002;13:3064–77.
Lens SM1, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, Kops G, Medema RH. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J. 2003;22:2934–47.
Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330:235–9.
Li F, Altieri DC. Transcriptional analysis of human survivin gene expression. Biochem J. 1999;344Pt 2:305–11.
Rosa J, Canovas P, Islam A, Altieri DC. Doxsey SJ. Survivin modulates microtubule dynamics and nucleation throughout the cell cycle. Mol Biol Cell. 2006;17:1483–93.
Caldas H1, Jiang Y, Holloway MP, Fangusaro J, Mahotka C, Conway EM, Altura RA. Survivin splice variants regulate the balance between proliferation and cell death. Oncogene. 2005;24:1994–2007.
Noton EA, Colnaghi R, Tate S, Starck C, Carvalho A, Ko Ferrigno P, Wheatley SP. Molecular analysis of survivin isoforms: evidence that alternatively spliced variants do not play a role in mitosis. J Biol Chem. 2006;281:1286–95.
Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–21.
Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–20.
Zaffaroni N1, Pennati M, Colella G, Perego P, Supino R, Gatti L, Pilotti S, Zunino F, Daidone MG. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Life Sci. 2002;59:1406–12.
Grossman D, Kim PJ, Schechner JS, Altieri DC. Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc Natl Acad Sci USA. 2001;98:635–40.
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–23.
Dohi T, Xia F, Altieri DC. Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol Cell. 2007;27:17–28.
Song Z, Yao X, Wu M. Direct interaction between survivin and Smac/DIABLO is essential for the anti-apoptotic activity of survivin during taxol-induced apoptosis. J Biol Chem. 2003;278:23130–40.
Ceballos-Cancino G, Espinosa M, Maldonado V, Melendez-Zajgla J. Regulation of mitochondrial Smac/DIABLO-selective release by survivin. Oncogene. 2007;26:7569–75.
Croci DO, Cogno IS, Vittar NB, Salvatierra E, Trajtenberg F, Podhajcer OL, Osinaga E, Rabinovich GA, Rivarola VA. Silencing survivin gene expression promotes apoptosis of human breast cancer cells through a caspase-independent pathway. J Cell Biochem. 2008;105:381–90.
Pavlyukov MS, Antipova N V, Balashova M V, Vinogradova T V, Kopantzev EP, Shakhparonov MI. Survivin monomer plays an essential role in apoptosis regulation. J Cell Biochem. 2011;286:23296–307.
Okuya M, Kurosawa H, Kikuchi J, Furukawa Y, Matsui H, Aki D, Matsunaga T, Inukai T, Goto H, Altura RA, Sugita K, Arisaka O, Look AT, Inaba T. Up-regulation of survivin by the E2A-HLF chimera is indispensable for the survival of t(17;19)-positive leukemia cells. J Biol Chem. 2010;285:1850–60.
Niu T-K, Cheng Y, Ren X, Yang J-M. Interaction of Beclin 1 with survivin regulates sensitivity of human glioma cells to TRAIL-induced apoptosis. FEBS Lett. 2010;584:3519–24.
Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem. 2008;283:25057–73.
Wang Q, Chen Z, Diao X, Huang S. Induction of autophagy-dependent apoptosis by the survivin suppressant YM155 in prostate cancer cells. Cancer Lett. 2011;302:29–36.
Kang BH, Altieri DC. Regulation of survivin stability by the aryl hydrocarbon receptor-interacting protein. J Biol Chem. 2006;281:24721–27.
Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J Biol Chem. 2008;283:5188–94.
Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, Sessa WC, Altieri DC. Regulation of survivin function by Hsp90. Proc Natl Acad Sci USA. 2003;100:13791–96.
Cheung CHA, Chen H-H, Cheng L-T, Lyu KW, Kanwar JR, Chang J-Y. Targeting Hsp90 with small molecule inhibitors induces the over-expression of the anti-apoptotic molecule, survivin, in human A549, HONE-1 and HT-29 cancer cells. Mol Cancer. 2010;9:77–89.
Ryan BM, O’Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–62.
Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5.
Ferrario A, Rucker N, Wong S, Luna M, Gomer CJ. Survivin, a member of the inhibitor of apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response. Cancer Res. 2007;67:4989–95.
Gomer CJ, Ferrario A, Luna M, Rucker N, Wong S. Photodynamic therapy: combined modality approaches targeting the tumor microenvironment. Lasers Surg Med. 2006;38:516–21.
Wang H-W, Sharp T V, Koumi A, Koentges G, Boshoff C. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi’s sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 2002;21:2602–15.
Vogel C, Hager C, Bastians H. Mechanisms of mitotic cell death induced by chemotherapy-mediated G2 checkpoint abrogation. Cancer Res. 2007;67:339–45.
You R-I, Chen M-C, Wang H-W, Chou Y-C, Lin C-H, Hsieh S-L. Inhibition of lymphotoxin-beta receptor-mediated cell death by survivin-DeltaEx3. Cancer Res. 2006;66:3051–61.
Li F1, Ackermann EJ, Bennett CF, Rothermel AL, Plescia J, Tognin S, Villa A, Marchisio PC, Altieri DC. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol. 1999;1:461–66.
Molckovsky A, Siu LL. First-in-class, first-in-human phase I results of targeted agents: highlights of the 2008 American society of clinical oncology meeting. J Hematol Oncol. 2008;1:20.
Hayashi N, Asano K, Suzuki H, Yamamoto T, Tanigawa N, Egawa S, Manome Y. Adenoviral infection of survivin antisense sensitizes prostate cancer cells to etoposide in vivo. Prostate. 2005;65:10–9.
Olie RA, Simões-Wüst AP, Baumann B, Leech SH, Fabbro D, Stahel RA, Zangemeister-Wittke U. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60:2805–09.
Kim KW, Mutter RW, Willey CD, Subhawong TK, Shinohara ET, Albert JM, Ling G, Cao C, Gi YJ, Lu B. Inhibition of survivin and aurora B kinase sensitizes mesothelioma cells by enhancing mitotic arrests. Int J Radiat Oncol Biol Phys. 2007;67:1519–25.
Talbot DC, Ranson M, Davies J, Lahn M, Callies S, André V, Kadam S, Burgess M, Slapak C, Olsen AL, McHugh PJ, de Bono JS, Matthews J, Saleem A, Price P. Tumor survivin is downregulated by the antisense oligonucleotide LY2181308: a proof-of-concept, first-in-human dose study. Clin Cancer Res. 2010;16:6150–58.
Gurbuxani S, Xu Y, Keerthivasan G, Wickrema A, Crispino JD. Differential requirements for survivin in hematopoietic cell development. Proc Natl Acad Sci USA. 2005;102:11480–85.
Hansen JB, Fisker N, Westergaard M, Kjaerulff LS, Hansen HF, Thrue CA, Rosenbohm C, Wissenbach M, Orum H, Koch T. SPC3042: a proapoptotic survivin inhibitor. Mol Cancer Ther. 2008;7:2736–45.
Walker K, Padhiar M. AACR-NCI-EORTC-21st International Symposium. Molecular targets and cancer therapeutics-Part 1. IDrugs. 2010;13:7–9.
Sapra P, Wang M, Bandaru R, Zhao H, Greenberger LM, Horak ID. Down-modulation of survivin expression and inhibition of tumor growth in vivo by EZN-3042, a locked nucleic acid antisense oligonucleotide. Nucleosides Nucleotides Nucleic Acids. 2010;29:97–112.
Davis ME1, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70.
Satoh T1, Okamoto I, Miyazaki M, Morinaga R, Tsuya A, Hasegawa Y, Terashima M, Ueda S, Fukuoka M, Ariyoshi Y, Saito T, Masuda N, Watanabe H, Taguchi T, Kakihara T, Aoyama Y, Hashimoto Y, Nakagawa K. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:3872–80.
Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10.
O’Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol. 2000;156:393–8.
Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, Patnaik A, Papadopoulos K, Takimoto C, Bartels P, Keating A, Antonia S. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–203.
Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, Kuta M, van Klaveren RJ, Chaudhary S, Gunther A, Shamsili S. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27:4481–86.
Rödel C, Haas J, Groth A, Grabenbauer GG, Sauer R, Rödel F. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: survivin as a radioresistance factor. Int J Radiat Oncol Biol Phys. 2003;55:1341–47.
Chang C-C, Heller JD, Kuo J, Huang RCC. Tetra-O-methyl nordihydroguaiaretic acid induces growth arrest and cellular apoptosis by inhibiting Cdc2 and survivin expression. Proc Natl Acad Sci USA. 2004;101:13239–44.
Smolewski P. Terameprocol, a novel site-specific transcription inhibitor with anticancer activity. IDrugs. 2008;11:204–14.
Park R, Chang CC, Liang YC, Chung Y, Henry RA, Lin E, Mold DE, Huang RC. Systemic treatment with tetra-O-methyl nordihydroguaiaretic acid suppresses the growth of human xenograft tumors. Clin Cancer Res. 2005;11:4601–09.
Zhu X, Ma Y, Liu D. Novel agents and regimens for acute myeloid leukemia: 2009 ASH annual meeting highlights. J Hematol Oncol. 2010;3:17.
Khanna N, Dalby R, Connor A, Church A, Stern J, Frazer N. Phase I clinical trial of repeat dose terameprocol vaginal ointment in healthy female volunteers. SexTransm Dis. 2008;35:577–82.
Khanna N, Dalby R, Tan M, Arnold S, Stern J, Frazer N. Phase I/II clinical safety studies of terameprocol vaginal ointment. Gynecol Oncol. 2007;107:554–62.
Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26:161–72.
Saji H, Song W, Furumoto K, Kato H, Engleman EG. Systemic antitumor effect of intratumoral injection of dendritic cells in combination with local photodynamic therapy cancer therapy. Clin Cancer Res. 2006;12:2568–74.
Sanlorenzo M, Vujic I, Posch C, Dajee A, Yen A, Kim S, Ashworth M, Rosenblum MD, Algazi A, Osella-Abate S, Quaglino P, Daud A, Ortiz-Urda S. Melanoma immunotherapy. Cancer Biol Ther. 2014;15:665–74.
Andersen MH, Sørensen RB, Schrama D, Svane IM, Becker JC, Thor Straten P. Cancer treatment: the combination of vaccination with other therapies. Cancer Immunol Immunother. 2008;57:1735–43.
Rohayem J, Diestelkoetter P, Weigle B, Oehmichen A, Schmitz M, Mehlhorn J, Conrad K, Rieber EP. Antibody response to the tumor-associated inhibitor of apoptosis protein survivin in cancer patients. Cancer Res. 2000;60:1815–17.
Schmidt SM, Schag K, Müller MR, Weck MM, Appel S, Kanz L, Grünebach F, Brossart P. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571–76.
Casati C, Dalerba P, Rivoltini L, Gallino G, Deho P, Rini F, Belli F, Mezzanzanica D, Costa A, Andreola S, Leo E, Parmiani G, Castelli C. The apoptosis inhibitor protein survivin induces tumor-specific CD8+ and CD4+ T cells in colorectal cancer patients. Cancer Res. 2003;63:4507–15.
Idenoue S1, Hirohashi Y, Torigoe T, Sato Y, Tamura Y, Hariu H, Yamamoto M, Kurotaki T, Tsuruma T, Asanuma H, Kanaseki T, Ikeda H, Kashiwagi K, Okazaki M, Sasaki K, Sato T, Ohmura T, Hata F, Yamaguchi K, Hirata K, Sato N. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res. 2005;11:1474–82.
Pisarev V, Yu B, Salup R, Sherman S, Altieri DC, Gabrilovich DI. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9:6523–33.
Schmitz M, Diestelkoetter P, Weigle B, Schmachtenberg F, Stevanovic S, Ockert D, Rammensee H, Rieber EP. Generation of Survivin-specific CD8+ T Effector Cells by Dendritic Cells Pulsed with Protein or Selected Peptides. Cancer Res. 2000;60:4845–49.
Siegel S, Wagner A, Schmitz N, Zeis M. Induction of antitumor immunity using survivin peptide-pulsed dendritic cells in a murine lymphoma model. Br J Haematol. 2003;122:911–14.
Friedrichs B, Siegel S, Andersen MH, Schmitz N, Zeis M. Survivin-derived peptide epitopes and their role for induction of antitumor immunity in hematological malignancies. Leuk Lymphoma. 2006;47:978–85.
Trepiakas R, Berntsen A, Hadrup SR, Bjørn J, Geertsen PF, Straten PT, Andersen MH, Pedersen AE, Soleimani A, Lorentzen T, Johansen JS, Svane IM. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. 2010;12:721–34.
Berntsen A, Trepiakas R, Wenandy L, Geertsen PF, thor Straten P, Andersen MH, Pedersen AE, Claesson MH, Lorentzen T, Johansen JS, Svane IM. Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: a clinical phase 1/2 trial. J Immunother. 2008;31:771–80.
Weide B1, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, Pawelec G, Hoerr I, Rammensee HG, Garbe C. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498–507.
Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294–98.
Wang R, Wang X, Li B, Lin F, Dong K, Gao P, Zhang HZ. Tumor-specific adenovirus-mediated PUMA gene transfer using the survivin promoter enhances radiosensitivity of breast cancer cells in vitro and in vivo. Breast Cancer Res Treat. 2009;117:45–54.
Macdonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyrins Phthalocyanines. 2001;5:105–29.
Ferrario A, Rucker N, Wong S, Luna M, Gomer CJ. Survivin, a member of the inhibitor of apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response. Cancer Res. 2007;67:4989–95.
Acknowledgements
We want to thanks to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (PICT), Secretaría de Ciencia y Técnica (SECyT), Universidad Nacional de Rio Cuarto, Argentina.
VR is member of the Scientific Researcher Career at CONICET. ISC hold fellowship from CONICET.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rivarola, V., Cogno, I. (2015). Optimization of Photodynamic Therapy Response by Survivin Gene. In: Rapozzi, V., Jori, G. (eds) Resistance to Photodynamic Therapy in Cancer. Resistance to Targeted Anti-Cancer Therapeutics, vol 5. Springer, Cham. https://doi.org/10.1007/978-3-319-12730-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-12730-9_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12729-3
Online ISBN: 978-3-319-12730-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)