Abstract
Niacin is biosynthesized from l-tryptophan (Trp). There is a substantial amount of Trp in the body which can be efficiently converted to niacin especially during critical situations. Therefore, it is very important to know the factors affecting the conversion ratio of Trp to niacin. In animal experiments, the conversion ratios decreased with increasing dietary protein levels. Feeding of diets containing unsaturated fatty acids increased the conversion ratio, while feeding of diets with saturated fatty acids did not. In the effects of carbohydrate, the conversion ratio was higher in diets containing starch than in diets containing sucrose. Vitamin B1, vitamin B2, or vitamin B6 deficiency affects the metabolism of Trp to niacin. Mineral deficiency also affects the metabolism of Trp to niacin. With regard to hormones, thyroxin increased the Trp to niacin conversion ratio, while estrone, progesterone, and adrenaline decreased. Certain chemicals can also impact niacin synthesis; alloxane and streptozotocin decreased the conversion ratio, while pyrazinamide, phthalates, valproic acid, and clofibrate increased the ratio. Moderate food restriction greatly decreased the conversion. In human experiments, the urinary excretion ratio of anthranilic acid formation from Trp was calculated to be approximately 0.06 % against Trp intake, kynurenic acid 0.13 %, xanthurenic acid 0.11 %, 3-hydroxyanthranilic acid 0.13 %, and quinolinic acid 0.34 % in basal metabolic level. Supplementing healthy women with up to 5.0 g/day of Trp had no adverse effects. Urinary excretion of 3-hydroxykynurenine is a good surrogate biomarker for excess Trp ingestion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tryptophan
- Niacin
- Urine
- Nicotinamide
- N 1-methylnicotinamide
- N 1-methyl-2-pyridone-5-carboxamide
- N 1-methyl-4-pyridone-3-carboxamide
- Quinolinic acid
Introduction
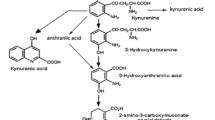
The vitamin niacin is biosynthesized from the essential amino acid the l-tryptophan (Trp) (Fig. 3.1), and one defines it as the conversion ratio of Trp to niacin. Over 20 % of enzymes need pyridine nucleotide coenzymes. When the metabolic rate is accelerated, the need of pyridine nucleotide coenzymes concomitantly increase. Such a case, the conversion ratio of Trp to niacin would be increased. Therefore, it is very important to know what affects the conversion ratio of TRP to niacin.
It has been generally accepted in the nutrition field that in the normal state, 60 mg of Trp has equal activity to 1 mg of nicotinamide (Nam) in humans based on several human studies [1–4]. During pregnancy, there is an observed increase in the TRP to niacin conversion ratio [5].
Analyses
Metabolites
TRP [6] and its metabolites, including kynurenine (KYN) [7], kynurenic acid (KA) [8], anthranilic acid (AnA) [9], 3-hydroxykynurenine (3-HK) [9], xanthurenic acid (XA) [10], 3-hydroxyanthranilic acid (3-HA) [10], 2-oxoadipic acid (2-OAA) [11], quinolinic aid (QA) [12], N 1-methylnicotinamide (MNA) [13], Nam [14], N1-methyl-2-pyridone-5-carboxamide (2-Py) [14], and N 1-methyl-4-pyridone-3-carboxamide (4-Py) [14], were measured as described.
Enzymes
Trp 2,3-dioxygenase (TDO) [EC 1.13.11.11] [15], kynureninase [EC 3.7.1.3] [15], kynurenine aminotransferase [EC 2.6.1.7] [15], 3-hydroxyanthranilic acid dioxygenase [EC 1.13.11.6] [15], 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase (ACMSD) [EC 4.1.1.45] [16], and quinolinic acid phosphoribosyltransferase (QPRT) [EC 2.4.2.19] [17] were measured as described.
Conversion Ratio
The conversion ratio was calculated from the following equation:
Rat Experiment
Effects of Dietary Protein Levels
The conversion ratio decreased with increasing dietary protein levels (Table 3.1). The activity of ACMSD increased with increasing dietary protein levels [18], while those of the other enzymes did not. The increased ACMSD activity means that ACMS, which is synthesized from Trp, is metabolized into acetyl-CoA but not into niacin. Therefore, the low conversion ratio in the group of high-protein diet might be attributed to the increase in the activity of liver ACMSD.
It is known that niacin itself is not necessary in rats when Trp is given in adequate amounts, because rats can biosynthesize niacin from Trp [19]. The rats fed the 20 % and 40 % casein diets did not require niacin for optimum growth, but the rats fed the 70 % casein diet needed it [20]. This phenomenon was attributed to the supposition that liver ACMSD activity increased according with the dietary casein levels. The conversion ratio in rats fed on the 70 % casein diet became extremely low, and then the rats needed niacin.
Effects of Dietary Constant Protein Levels but Differing Trp Level
As it had been known that the amounts of Nam increase with increasing intake of Trp, our data [18, 20] conflict common sense for the conversion. The sum total urinary excretion of Nam and its metabolites did not increase as the level of dietary casein increased [21]. While the sum total urinary excretion of Nam and its catabolites in the groups fed the 10 % casein-30 % gelatin, 20 % casein-20 % gelatin, and 40 % casein diets were about 5, 7, and 11 μmol/day, respectively, values were significantly different from each other [22]. Therefore, it was found that only when the total protein intake was constant the sum total urinary excretion increases with increasing intake of Trp.
Effects of Composition of Amino Acids
The conversion ratio in the group fed with a preformed niacin-free, 9 % casein-sucrose diet was 3.5 %. The ratio was decreased to 0.2 % by the addition of 2 % glycine, 0.078 % l-threonine, and 0.2 % l-cystine and restored to 3.2 % by the further addition of 0.1 % Trp [23]. The same phenomena were observed in other types of Trp-limiting diets [24]. These results clearly prove that the conversion ratio was lowest when the rats were fed with the Trp-limiting diets. Therefore, we think that the pellagragenic factor of corn is simply due to a low content of Trp, but the adverse effect is due to a low conversion ratio of Trp to niacin.
The conversion ratio was significantly lower in the groups fed with the 3 % l-leucine-, l-valine-, or l-isoleucine-added diet than in the group fed with the control diet [25]. Namely, the inhibition of the conversion was observed not only by the addition of l-leucine but also by the addition of l-valine or l-isoleucine. The ratio in the group fed with an amino acid diet-simulating rice protein was around 2.5 %. The ratio was decreased by the addition of the limiting amino acids, except for Trp, and increased by the addition of all the limiting amino acids, including Trp [26]. The composition of amino acids proved to greatly affect the conversion ratio.
Effects of Dietary Fat
Feeding of diets containing unsaturated fatty acids increased the conversion ratio, while diets containing saturated fatty acids did not affect it (Table 3.2). The increased conversion ratio in the group fed with unsaturated fatty acids might be attributed to the decreased activity of ACMSD because it is known that feeding of diets containing unsaturated fatty acids inhibits the ACMSD activity but that feeding diets containing saturated fatty acids does not [27].
Effects of Dietary Carbohydrates
The conversion ratio was higher in diets containing starch than in diets containing sucrose (Table 3.3) [18]. This phenomenon also might be due to the change of the activity of ACMSD.
Effects of Dietary Vitamins
Vitamin B1-Free Diet
We clarified the following two phenomena: (1) severe food restriction gave an increase of the conversion ratio compared to free access control group [28, 29] and (2) the conversion ratio also increased by vitamin B1 deficiency compared to free access control group [30]. So, another study was set up to clarify whether or not a true reason about an increase in the conversion ratio is vitamin B1 deficiency or severe food restriction [31].
Vitamin B2-Free Diet
In riboflavin-deficient rats, the activities of 2-Py-forming MNA oxidase and 4-Py-forming MNA oxidase were not detected in vitro [32]. These results show that the two MNA oxidases are FAD-dependent enzymes. On the contrary, the Nam methyltransferase activity was higher in riboflavin-deficient rats than in control rats. Therefore, the resulting excretion ratio of (2-Py + 4-Py/MNA) was greatly lower in the riboflavin-deficient rats than in the control rats.
Vitamin B6-Free Diet
The urinary excretion of KA decreased while that of XA increased drastically in the vitamin B6-free diet. The conversion ratio was lower in the vitamin B6-free diet than in the vitamin B6-containing diet [33].
Effects of Hormones
Thyroxine
It has been reported that thyroxine increased the activity of kynurenine aminotransferase and reduced the activity of kynurenine 3-monooxygenase, with a resulting increase in the reaction of kynurenine to KA [34]. Traditionally, these changes were taken to mean that thyroxine suppressed the conversion ratio; however, the effect of this hormone on the conversion ratio has not been actually demonstrated. We, therefore, investigated the effect on the conversion of feeding to rats, a preformed niacin-free, 20 % casein diet containing 0.002 % thyroxine [26]. The ratio was statistically higher in hyperthyroid rats than in the control rats. Furthermore, the present data suggests that ACMSD played a critical role in this conversion.
Sex Hormones
It is known that deaths attributable to pellagra, which is considered to be a disease caused by the disturbance of Trp metabolism, have been approximately twofold higher in women than in men [35]. Administration of ovarian hormones significantly decreased the conversion ratio [30, 36]. Feeding a diet containing testosterone had no effect on any parameter [30].
Adrenaline
The administration of glycemia-affecting chemicals such as alloxane [15], streptozotocin [37], and 6-aminonicotinamide [38] decreases the conversion ratio. Adrenaline is also known to increase the glucose level. The conversion ratio was reduced to half by the intraperitoneal injection of adrenalin [39].
Prednisolone
The urinary excretion of Nam, MNA, 2-Py, and 4-Py was each higher in the prednisolone group than in the physiological saline group after 1 day; however, each excretion was lower in the prednisolone group than in the saline group after 3, 4, 5, and 6 days [40]. On around the 7th day, the urinary excretion in the prednisolone group was restored to the initial values. The initial rapid increase of prednisolone decrease in the urinary excretion of Nam and its metabolites by an injection of prednisolone is considered to be attributable to a respective elevation of the liver TDO level and the liver ACMSD level.
Chemicals Substances
Table 3.4 summarizes the effects of some chemical substances on the metabolism of Trp to niacin.
Food Restriction
The conversion ratio was lower in both restricted groups than in the ad libitum-fed control group during the experimental period (control group, 1.37 ± 0.24 %; 80 %-restricted group, 0.20 ± 0.04 %; 65 %-restricted group, 0.15 ± 0.02 %. control vs. restricted groups, p < 0.01) [29]. Food restriction, even at mild levels, suppressed the conversion ratio compared to the ad libitum-fed control group.
Loading
Tryptophan
Young rats were fed on a 20 % casein diet with 0, 0.5, 1.0, 2.0, or 5.0 % added Trp for 30 days [46]. The apparent toxicity and growth retardation was observed in the 5.0 % Trp-added group. Metabolites of the Trp-Nam pathway such as AnA, KA, XA, 3-HA, and QA in urine increased in a dose-dependent manner. Of these metabolites, urine KA progressively increased, and urine AnA dramatically increased in the 2.0 and 5.0 % Trp-added groups. The urine ratio of AnA/KA is a useful index to monitor an excessive Trp intake.
Quinolinic Acid
Dietary QA had no adverse effects up to 35 mg/100 g of diet. Dietary QA is approximately 1/9 as active as niacin in growing rats [47].
Picolinic Acid
Picolinic acid (PiA) is an endogenous metabolite of Trp that has been reported to possess a wide range of physiological actions. Toxicity of PiA was higher compared with analogs such as nicotinic acid and QA. Feeding an ordinary diet containing 0.05 % and 0.1 % PiA did not elicit decreased intake of food or loss in body weight. PiA did not affect the in vitro liver activities of QPRT and ACMSD, a Zn-dependent enzyme. PiA administration did not affect Trp metabolites such as AnA, KA, and XA. However, QA and subsequent metabolites such as Nam and its catabolites were increased by administration of a diet containing 0.05 % PiA but not by a 0.1 % PiA diet. These results suggest that the in vivo activity of ACMSD is controlled by Zn level [48].
Human Experiments
Basal Metabolic Fate
Fate of dietary Trp in humans was investigated [49]. The urinary excretion amounts of Trp, kynurenine, AnA, KA, 3-HK, XA, 3-HA, and QA were about 40, 20, 4, 1, 10, 4, 3, 5, and 20 μmol/day, respectively. In this experiment, Trp was ingested at levels of 674 mg/day or 3300 μmol/day. The percentage of AnA formation from Trp was calculated to be approximately 0.06 %, KA 0.13 %, XA 0.11 %, 3-HA 0.13 %, and QA 0.34 %.
Tryptophan Loading
Healthy Japanese women were randomly assigned to receive placebo (0 g/day) or 1.0, 2.0, 3.0, 4.0, or 5.0 g/day of Trp for 21 days each with a 5-week washout period between trials [50]. Food intake, body weight, general biomarkers in blood and urine, and amino acid composition in blood and urine were not affected by any dose of Trp. Administration of up to 5.0 g/day Trp had no effect on a profile of mood states category measurement. The urinary excretion of NAm and its catabolites increased in proportion to the ingested amounts of Trp, indicating that participants could normally metabolize this amino acid. The urinary excretion of Trp metabolites, including kynurenine, AnA, KA, 3-HK, 3-HK, and QA, all of which are intermediates of the Trp–kynurenine–QA pathway, was in proportion to Trp loading. The response of 3-HK was the most characteristic of these Trp metabolites. This finding suggests that the urinary excretion of 3-HK is a good surrogate biomarker for excess Trp ingestion.
Nicotinamide Loading
To determine if de novo Nam synthesis from Trp is influenced by Nam intake itself, young women consumed controlled diets containing 0, 10.9, 37.8, or 68.6 mg/day respectively, and urinary excretion of Trp metabolites were measured [50]. None of the intermediates, including AnA, KA, XA, 3-HA, and QA, changed at all. That is, exogenous Nam did not affect de novo Nam synthesis.
Abbreviations
- ACMS:
-
2-Amino-3-carboxymuconate-6-semialdehyde
- ACMSD:
-
2-Amino-3-carboxymuconate-6-semialdehyde decarboxylase
- AnA:
-
Anthranilic acid
- 3-HA:
-
3-Hydroxyanthranilic acid
- 3-HK:
-
3-Hydroxykynurenine
- IDO:
-
Indoleamine 2,3-dioxygenase
- KA:
-
Kynurenic acid
- KYN:
-
Kynurenine
- MNA:
-
N 1-methylnicotinamide
- Nam:
-
Nicotinamide
- NiA:
-
Nicotinic acid
- 2-Py:
-
N 1-methyl-2-pyridone-5-carboxamide
- 4-Py:
-
N 1-methyl-4-pyridone-3-carboxamide
- PiA:
-
Picolinic acid
- QA:
-
Quinolinic acid
- TDO:
-
Tryptophan 2,3-dioxygenase
- Trp:
-
l-Tryptophan
- XA:
-
Xanthurenic acid
References
Horwitt MK, Harvey C, Rothwell W, Cutler J, Haffron D. Tryptophan-niacin relationships in man studies with diets deficient in riboflavin and niacin, together with observations on the excretion of nitrogen and niacin metabolites. J Nutr. 1956;60(1 Suppl):1–43.
Goldsmith GA, Miller ON, Unglaub WG. Efficiency of tryptophan as a niacin precursor in man. J Nutr. 1961;73(2):172–6.
Nakagawa I, Takahashi T, Sasaki A, Kajimoto M, Suzuki T. Efficiency of conversion of tryptophan to niacin in humans. J Nutr. 1973;103(8):1195–9.
Fukuwatari T, Ohta M, Kimtjra N, Sasaki R, Shibata K. Conversion ratio of tryptophan to niacin in Japanese women fed a purified diet conforming to the Japanese dietary reference intakes. J Nutr Sci Vitaminol. 2004;50(6):385–91.
Wertz AW, Lojkin ME, Bouchard BS, Derby MB. Tryptophan-niacin relationships in pregnancy. J Nutr. 1958;64(3):339–53.
Shibata K, Onodera M, Aihara S. High-performance liquid chromatographic measurement of tryptophan in blood, tissues, urine, and foodstuffs with electrochemical and fluorometric detections. Agric Biol Chem. 1991;55(6):1475–81.
Fukuwatari T, Ohsaki S, Fukuoka S, Sasaki R, Shibata K. Phthalate esters enhance quinolinate production by inhibiting alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase (ACMSD), a key enzyme of the tryptophan pathway. Toxicol Sci. 2004;81(2):302–8. doi:10.1093/toxsci/kfh204.
Shibata K. Fluorimetric micro-determination of kynurenic acid, an endogenous blocker of neurotoxicity, by high-performance liquid chromatography. J Chromatogr. 1988;430(2):376–80.
Shibata K, Onodera M. Measurement of 3-hydroxyanthranilic acid and anthranilic acid in urine by high-performance liquid chromatography (analytical chemistry). Agric Biol Chem. 1991;55(1):143–8.
Shibata K, Onodera M. Simultaneous high-performance liquid chromatographic measurement of xanthurenic acid and 3-hydroxyanthranilic acid in urine. Biosci Biotechnol Biochem. 1992;56(6):974.
Shibata K, Yasui M, Sano M, Fukuwatari T. Fluorometric determination of 2-oxoadipic acid, a common metabolite of tryptophan and lysine, by high-performance liquid chromatography with pre-chemical derivatization. Biosci Biotechnol Biochem. 2011;75(1):185–7. doi:10.1271/bbb.100723.
Mawatari K, Oshida K, Iinuma F, Watanabe M. Determination of quinolinic acid by liquid chromatography with fluorimetric detection. Adv Exp Med Biol. 1996;398:697–701.
Shibata K. Ultramicro-determination of N 1-methylnicotinamide in urine by high-performance liquid chromatography. Vitamins. 1987;61:599–604.
Shibata K, Kawada T, Iwai K. Simultaneous micro-determination of nicotinamide and its major metabolites, N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-3-carboxamide, by high-performance liquid chromatography. J Chromatogr. 1988;424(1):23–8.
Shibata K. Tryptophan-niacin metabolism in alloxan diabetic rats and partial prevention of alloxan diabetes by nicotinamide (biological chemistry). Agric Biol Chem. 1987;51(3):811–16.
Ichiyama A, Nakamura S, Kawai H, Honjo T, Nishizuka Y, Hayaishi O, et al. Studies on the metabolism of the benzene ring of tryptophan in mammalian tissues. II. Enzymic formation of alpha-aminomuconic acid from 3-hydroxyanthranilic acid. J Biol Chem. 1965;240:740–9.
Shibata K, Fukuwatari T, Sugimoto E. Reversed-phase high-performance liquid chromatography of nicotinic acid mononucleotide for measurement of quinolinate phosphoribosyltransferase. J Chromatogr B Biomed Sci Appl. 2000;749(2):281–5.
Shibata K. Effects of protein-amino acids, lipid, and carbohydrate on the conversion ratio of tryptophan to niacin. Vitamins (in Japanese). 1996;70:369–82.
Krehl WA, Teply LJ, Elvehjem CA. Corn as an etiological factor in the production of a nicotinic acid deficiency in the rat. Science. 1945;101(2620):283. doi:10.1126/science.101.2620.283.
Kimura N, Fukuwatari T, Sasaki R, Shibata K. The necessity of niacin in rats fed on a high protein diet. Biosci Biotechnol Biochem. 2005;69(2):273–9. doi:10.1271/bbb.69.273.
Shibata K, Nomamoto R, Iwai K. Effect of dietary protein levels on the urinary excretion of nicotinamide and its metabolites in rats (Food & Nutrition). Agric Biol Chem. 1988;52(7):1765–9.
Shibata K, Matsuo H. Effect of dietary tryptophan levels on the urinary excretion of nicotinamide and its metabolites in rats fed a niacin-free diet or a constant total protein level. J Nutr. 1990;120(10):1191–7.
Shibata K. Conversion ratio of tryptophan to niacin in rats fed with a nicotinic acid-free, tryptophan-limiting diet. Biosci Biotechnol Biochem. 1995;59(4):715–16.
Shibata K, Shimada H, Kondo T. Effects of feeding tryptophan-limiting diets on the conversion ratio of tryptophan to niacin in rats. Biosci Biotechnol Biochem. 1996;60(10):1660–6. doi:10.1271/bbb.60.1660.
Shibata K, Taniguchi I, Onodera M. Effect of adding branched-chain amino acids to a nicotinic acid-free, low-protein diet on the conversion ratio of tryptophan to nicotinamide in rats. Biosci Biotechnol Biochem. 1994;58(5):970–1.
Shibata K, Toda S. Effects of thyroxine on the conversion ratio of tryptophan to nicotinamide in rats. Biosci Biotechnol Biochem. 1994;58(10):1757–62.
Shibata K, Onodera M. Changes in the conversion rate of tryptophan-nicotinamide according to dietary fat and protein levels. Biosci Biotechnol Biochem. 1992;56(7):1104–8.
Shibata K, Kondo T, Miki A. Increased conversion ratio of tryptophan to niacin in severe food restriction. Biosci Biotechnol Biochem. 1998;62(3):580–3. doi:10.1271/bbb.62.580.
Shibata K, Nakata C, Fukuwatari T. Moderate food restriction suppresses the conversion of L-tryptophan to nicotinamide in weaning rats. Biosci Biotechnol Biochem. 2014;78(3):478–81. doi:10.1080/09168451.2014.890025.
Shibata K, Toda S. Effects of sex hormones on the metabolism of tryptophan to niacin and to serotonin in male rats. Biosci Biotechnol Biochem. 1997;61(7):1200–2. doi:10.1271/bbb.61.1200.
Shibata K, Kobayashi R, Fukuwatari T. Vitamin B1 deficiency inhibits the increased conversion of tryptophan to nicotinamide in severe food-restricted rats. Biosci Biotechnol Biochem. 2014;1–6. doi:10.1080/09168451.2014.962473.
Shibata K. The catabolism of nicotinamide in riboflavin-deficient rats. Vitamins (in Japanese). 1990;64:589–95.
Shibata K, Mushiage M, Kondo T, Hayakawa T, Tsuge H. Effects of vitamin B6 deficiency on the conversion ratio of tryptophan to niacin. Biosci Biotechnol Biochem. 1995;59(11):2060–3. doi:10.1271/bbb.59.2060.
Okamoto H, Okada F, Hayaishi O. Kynurenine metabolism in hyperthyroidism. A biochemical basis for the low NAD(P) level in hyperthyroid rat liver. J Biol Chem. 1971;246(24):7759–63.
Miller DF. Pellagra deaths in the United States. Am J Clin Nutr. 1978;31(4):558–9.
Shibata K, Kondo T. Effect of progesterone and estrone on the conversion of tryptophan to nicotinamide in rats. Biosci Biotechnol Biochem. 1993;57:1890–3.
Shibata K. Tryptophan-NAD metabolism in streptozotocin diabetic rats. Agric Biol Chem. 1988;52:1993–8.
Shibata K, Ogawa A, Taniguchi I. Effects of dietary 6-aminonicotinamide, an antagonist of nicotinamide, on the metabolism of tryptophan to nicotinamide in rats. Biosci Biotechnol Biochem. 1994;58(4):727–33.
Shibata K. Effects of adrenalin on the conversion ratio of tryptophan to niacin in rats. Biosci Biotechnol Biochem. 1995;59(11):2127–9.
Shibata K. Effect of prednisolone on the urinary excretion of nicotinamide and its metabolites in rats fed with a niacin-free diet. Agric Biol Chem. 1990;54(5):1195–200.
Shibata K. Effects of pyrazinamide on tryptophan-niacin conversion in rats (Biological Chemistry). Agric Biol Chem. 1990;54(9):2463–4.
Fukuwatari T, Sugimoto E, Shibata K. Growth-promoting activity of pyrazinoic acid, a putative active compound of antituberculosis drug pyrazinamide, in niacin-deficient rats through the inhibition of ACMSD activity. Biosci Biotechnol Biochem. 2002;66(7):1435–41. doi:10.1271/bbb.66.1435.
Shibata K, Fukuwatari T, Enomoto A, Sugimoto E. Increased conversion ratio of tryptophan to niacin by dietary di-n-butylphthalate. J Nutr Sci Vitaminol. 2001;47(3):263–6.
Shibata K, Kondo R, Sano M, and Fukuwatari T. Dietary valproate increases the conversion of tryptophan to nicotinamide in rats. Biosci Biotechnol Biochem. 2013;77(2):295–300.
Shibata K, Kondo T, Marugami M, Umezawa C. Increased conversion ratio of tryptophan to niacin by the administration of clofibrate, a hypolipidemic drug, to rats. Biosci Biotechnol Biochem. 1996;60(9):1455–9. doi:10.1271/bbb.60.1455.
Okuno A, Fukuwatari T, Shibata K. Urinary excretory ratio of anthranilic acid/kynurenic acid as an index of the tolerable amount of tryptophan. Biosci Biotechnol Biochem. 2008;72(7):1667–72. doi:10.1271/bbb.70630.
Shibata K, Tanaka K, Murata K. Efficiency of exogenous quinolinic acid as niacin in rats (Food & Nutrition). Agric Biol Chem. 1986;50(8):2025–32.
Shibata K, Fukuwartari T. Large amounts of picolinic acid are lethal but small amounts increase the conversion of tryptophan-nicotinamine in rats. J Nutr Sci Vitaminol. 2014;60:335–40.
Hiratsuka C, Fukuwatari T, Shibata K. Fate of dietary tryptophan in young Japanese women. Int J Tryptophan Res. 2012;5:33–47. doi:10.4137/IJTR.S10497.
Hiratsuka C, Fukuwatari T, Sano M, Saito K, Sasaki S, Shibata K. Supplementing healthy women with up to 5.0 g/d of L-tryptophan has no adverse effects. J Nutr. 2013;143(6):859–66. doi: 10.3945/jn.112.173823.
Fukuwatari T, Shibata K. Effect of nicotinamide administration on the tryptophan-nicotinamide pathway in humans. Int J Vitam Nutr Res. 2007;77(4):255–62.
Acknowledgements
This investigation was part of the project “Studies on the nutritional evaluation of amino acids and B-group vitamins” (principal investigator, Katsumi Shibata), which was supported by a Research Grant for Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science. This study was also part of the project entitled “Development of an index of metabolic upper intake level instead of tolerable upper intake level of tryptophan for humans” (principal investigator, Katsumi Shibata), which was supported by The International Council on Amino Acid Science (ICAAS) Research Funding.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Shibata, K. (2015). Nutritional Aspects of Tryptophan Metabolism. In: Mittal, S. (eds) Targeting the Broadly Pathogenic Kynurenine Pathway. Springer, Cham. https://doi.org/10.1007/978-3-319-11870-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-11870-3_3
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11869-7
Online ISBN: 978-3-319-11870-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)