Abstract
Wetlands, in particular salt marshes, are very interesting field laboratories to study metal biogeochemistry, namely, Cr. Due to the heavily industrialized history of most of estuarine systems, salt marshes became large deposits of heavy metals. Due to the large affinity of Cr to the medium organic matter, the removal of Cr throughout natural or enhanced processes occurs throughout plant-mediated processes. Naturally, plants acquire during their life cycle nutrients from their sediments but also some non-nutritional elements, like Cr, and store them in their tissues. In the last decades, this natural ability attracted the attention of several projects focusing on the enhancement of this process throughout the application of transporter molecules, like LMWOA, in order to increase the sediment-plant Cr transport. Due to its chemistry, Cr presents to oxidation states, Cr (III) and Cr (VI), being this last very toxic. Thus it became important to study not only the plant accumulation capacity but also the root-mediated processes of phyto-conversion of Cr (VI) toxic form to the less toxic Cr (III). Again, halophytes acquire an important role with high conversion efficiencies. All these passive and enhanced processes point out to a promising biotechnology using halophytes as potential cleaners of Cr-contaminated sediments, using environmental-friendly and low-cost technologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Salt Marshes, Halophytes and Contaminants
Several studies (Morris et al. 1986; Bewers and Yeats 1989; Vale 1990) describe estuaries as efficient filters of suspended particulate matter (SPM) and heavy metals, majorly through particle-solute interactions, flocculation processes and settling down of metal-charged particles. Since the industrial revolution, large amounts of contaminants have been released to the atmosphere, soils and watercourses. Most of these emissions will be transferred in ultimate analysis into a water matrix, either by atmospheric deposition or by soil erosion, where they will stay dissolved or associated to sediment particles (Nriagu 1988; Viers et al. 2009). This is a very dynamic mechanism greatly influenced by the river hydrological conditions.
Salt marshes are natural deposits of heavy metals in the estuarine system (Doyle and Otte 1997; Williams et al. 1994). When located near polluted areas, these ecosystems receive large amounts of pollutants from industrial and urban wastes that either drifts downstream within the river flow or of waste dumping from the near industrial and urban areas (Reboreda and Caçador 2007). When metals enter salt marshes, they spread along with the tides and periodic floods and interact with the sediment and the biotic community (Suntornvongsagul et al. 2007). Most salt marsh plants accumulate large amounts of metals in their aerial and belowground organs (Caçador et al. 1996). Their ability to phytostabilize those contaminants in the rhizosediment is an important aspect in the ecosystem self-remediative processes and biogeochemistry (Weis and Weis 2004).
In the last decades, phytoremediation has become a promising biotechnology for cleaning up contaminants, namely, metals (Cunningham et al. 1995; Cunningham and Ow 1996). Several works concerning a large variety of plant species have been published in the last decades identifying possible hyperaccumulator species (Salt et al. 1997; Brown et al. 1994). Besides accumulation, some other abilities have arisen as potential metal detoxification mechanisms to less harmful forms, either by chelation (Duarte et al. 2007) or redox reactions, changing the metal’s oxidation states. However two important aspects must be considered when choosing the best phytoremediator species for a specific location and level of contamination: the biomass production ability and the ecology of the species (Redondo-Gómez et al. 2011). When considering contaminated wetland phytoremediation, the number of potential species becomes reduced to a few due to the specificities of these environments, like tidal saltwater flooding and waterlogging. Even though a species is considered to fit in this description and with a phytoremediative potential, it is also important to consider its ecophysiological response (like oxidative feedback) to this metal accumulation. This oxidative feedback will be important to determine whether this species can tolerate high metal concentrations and with this maintain its ecophysiological health and carry out the phytoremediative process.
Chromium is used in many industrial processes, and its unregulated use and dumping have led to water, sediment and biota contamination (Vale et al. 2008; Duarte et al. 2008, 2009). Industrial activities such as plating, tanning, corrosion inhibition, glassware-cleaning solutions, wood preservation, metal finishing or chromite ore processing (COP), are the main sources of trivalent and hexavalent toxic chromium compounds (Barceloux 1999; Losi et al. 1994). Cr-elevated contents (up to 600 ppm) in some phosphate fertilizers may be also a significant source of this metal in soils, although the most hazardous addition of Cr to a soil is related to tannery sludge, which can contain up to 2.8 % of this metal (Kabata-Pendias and Pendias 2001). Estuarine areas are often affected by both these industrial and agricultural activities, unbalancing their critical environmental equilibrium (Pazos-Capeáns et al. 2010).
2 Chromium Sediment Storage
As stated before, one of the major sources of Cr supply to wetlands, and particularly in estuarine salt marshes, is the tidal flooding. When dissolved and particulate Cr reaches the salt marshes, it will be deposited in the sediments establishing chemical bounds with it. This can be easily observed by its speciation (how Cr is bound to each of the sediment components) as shown in Fig. 18.1.
The sediments become this way the major storage pool of Cr in the estuarine system. As seen in Fig. 18.1, Cr has high affinity for the organic matter, establishing relatively stable chemical bounds (Duarte et al. 2008, 2009). In fact, Cr is well described in the literature as being a strong oxidizer of the organic matter, being even used as reagent for the determination of some of the organic components of the sediments and water due to this property. This way, salt marsh sediments tend to store Cr in a rather stable form but which is accessible throughout oxidizable conditions or throughout organic matter hydrolysis, for example, by the microbial community (Duarte et al. 2008, 2009). This leads to shifts and fluctuations in the bioavailability of Cr, making it accessible to the biota. In fact, if the Cr concentrations in the estuarine environment are considered, the second largest pool of this metal appears in the roots of the halophytes.
3 Chromium Natural Phytoremediation
Considering the case of polluted salt marshes, the metal cycling throughout the sediment and the vegetation becomes of great interest. According to several works and as stated before (Duarte et al. 2010; Caçador et al. 2009), it is possible to observe that the two major pools of heavy metals in the salt marshes are the sediment and the root system of resident species (Fig. 18.2). The higher metal budgets in roots corroborate their increased ability for heavy metal accumulation. The calculated root decomposition rates suggest that these metal pools are quite mobile particularly in S. maritima. This mobility is very important since it creates a cycle of metals between the sediment and the root system. Although a higher fraction of biomass losses in the aboveground organs was found, their low metal concentration makes the detritus generated by the aboveground less contributing for the metal budgets. Conversely, the comparatively low losses of biomass in the root system generate less necromass, but with very high concentrations of metals. This necromass becomes important to the metal budget of the sediment, not only due to its input of heavy metals but also due to the increase of organic matter content of this matrix. The bioaccumulation of these elements in the belowground organs is rather mobile, being able to return to the sediment matrix due to necromass generation and mineralization processes subdue. The return of metals due to these decaying processes and consequent input of metals into the sediments, although in rather lower concentrations comparatively to the existent in this matrix, is very important to be considered not only due to the amount of metal released through this process but also by the metal forms it introduces into the sediment.
Chromium accumulation and exportations (due to senescence) per square metre by Spartina maritima in a low polluted salt marsh, during a year life cycle (adapted from Couto et al. 2013)
These organically bound metals were already reported as being one of the most important fractions of metals present in these sediments, being subjected to microbial degradation processes (Duarte et al. 2008), that can lead to more bioavailable metal forms and contamination of the pore waters. All this process is the equivalent to a phytoremediation process, occurring everyday in natural ecosystem such as salt marsh, indicating the suitability of these halophytes as potential applications in promoted phytoremediation processes. This natural process is a well-documented ecological role of these ecosystems, contributing naturally for the estuarine depuration and promoting the maintenance of the ecosystem ecological status (Caçador et al. 2013).
4 Chromium-Assisted Phytoremediation
Recently, a new approach has also been taken based on this natural phytoremediation processes. This approach is based on the enhancement of this mechanism throughout the addition of chemical compounds (organic or inorganic) that would intervene in the phytoremediative process (Duarte et al. 2007, 2010). Plants can modify metal speciation throughout several processes, like radial oxygen loss (ROL) and exudation of organic acids (Sundby et al. 1998; Jacob and Otte 2004; Duarte et al. 2007). This last process is well documented for plants with agricultural interest (Jones 1998 and paper herein referred), but there are much less papers investigating wetland plants. One of the groups of substances exudated by salt marsh plants are low-molecular-weight organic acids (LMWOA), such as malic, citric and acetic acids (Mucha et al. 2005). These LMWOA are able to bind to metals establishing complexes and changing their bioavailability (Parker et al. 2001). These exudates have been related to nutrient uptake (Marschner 1995), metal detoxification (Ma et al. 2001) and microbial communication (Jones 1998) in agricultural ecosystems. The use of these LMWOA can be a useful tool in the so-called assisted phytoremediation processes improving the metal uptake by plants. As observed in Fig. 18.3, the application of LMWOA improved not only the phyto-extractability of Cr but also in some cases its allocation within the plant (Duarte et al. 2010). Although some of the changes result directly from the application of the LMWOA in study, they are often products of reactions in which these molecules are involved at the root surface. Cr exhibited higher uptake upon application of organic acids. All of the applications showed important increases in the Cr uptake probably due to the interaction of Cr(III) with these organic ligands resulting in the formation of very mobile organic-bound Cr(III) complexes (Srivastava et al. 1999). Although the presence of manganese oxides could lead to the oxidation of Cr(III) to Cr(VI) and formation of Cr(VI) organic-bound complexes that are less taken up by plants, there are several side reactions that slow down this oxidation. These maintain the Cr(III) organic-bound complexes as the more abundant species of Cr(III) (Bartlett 1991). This is in agreement with the present data, which shows a high increase of Cr uptake upon organic acids application, due to the formation of this highly mobile organic-bound Cr(III) complexes. Previous work showed that most of the Cr found in the roots was present in the form of Cr acetate (Bluskov et al. 2005). This form is mostly stored in the cortex of the roots and lately translocated to the aboveground parts by conversion into Cr oxalate. In the plants treated with acetic acid, there was a very high increase in Cr content not only in the roots but also in the shoots. This should probably be attributed to this mechanism of acetate-oxalate conversion, in contrast to what was found in the other LMWOA treatments where only the root Cr content increased.
The tenfold increase in Cr uptake observed when acetic acid is applied points out to a very promising technique for bioremediation of Cr-contaminated sediments. The potential environmental risk must be recognized at the early stage of LMWOA application. With this application, the labile complexes-associated metals could be absorbed and taken up directly by plants. New techniques could facilitate the decomposition of these organo-metal complexes in the short term, increasing the proportion of free ions and enhancing the uptake by plants, thus minimizing the environmental risk. Metals can therefore be removed of the system by harvesting of the aboveground biomass. This points out to a research need to make the use of these environmental-friendly phytoextraction enhancers feasible for commercial phytoextraction. In addition, the use of natural compounds in contraposition to synthetic chelates sounds better for the public acceptance of phytoextraction as a technology to clean up metal-polluted soils.
5 The Phyto-transformation of Chromium (VI) to (III)
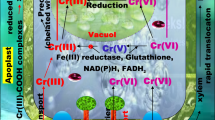
Chromium has two states of oxidation, Cr (III) and Cr (VI). The latter has been classified as a primary contaminant (Lytle et al. 1998) due to its mobility and reported harmful effects in animals and humans (Kortenkamp et al. 1996). Although this toxic effect is associated to Cr (VI) form, Cr is also an essential element in the nutrition of several organisms in its stable Cr (III) form (Katz and Salem 1994). This reduction of a toxic form to a stable nontoxic and beneficial form has gathered the attention of several investigative teams. Abiotically, the reduction of Cr (VI) can occur by the reactions with other ions, metallic or mineral surfaces and organic molecules (Wittbrodt and Palmer 1996). More important for phytoremediation proposes, the reduction of Cr (VI) to Cr (III) can be biologically mediated (Mikalsen et al. 1991; Stearns et al. 1995). Bacteria-mediated Cr (VI) to Cr (III) reduction pathway by a specific Cr reductase is well described (Shen and Wang 1993; Nies 1992; Romheld and Marschner 1983), although this mechanism has not been identified in non-engineered plants. Some recent studies (Duarte et al. 2012) point out some halophyte species as potential phyto-converters and phyto-accumulators of Cr. This has implications both at ecological and cellular levels. Ecologically it was found that, for example, H. portulacoides could accumulate very high Cr concentrations, in particular in the root system (Fig. 18.4).
Earlier reports pointed out in the same direction using non-halophyte-rooted aquatic plants (Gupta et al. 1999; Sinha et al. 2003; Suseela et al. 2002) and registered significantly higher values in the roots than their upper parts. High metal accumulation in the fine roots is also in agreement with earlier reports (Sinicrope et al. 1992). Qian et al. (1999) also reported the highest concentration of Cr in the plant roots and lower level in shoots similar to other ten elements studied in twelve aquatic plants. Also field-monitoring studies directed to this species showed that the main biological metal sink is the halophyte root system (Caçador et al. 2009; Duarte et al. 2010). This can be due to metal binding to organic ligands, thus reducing its mobility from roots to aerial parts. Another important aspect focused in this study (Duarte et al. 2012) was the H. portulacoides-mediated Cr (VI) to Cr (III) reduction (Fig. 18.5). It was found that this species can convert large Cr (VI) amounts to its less toxic form. This was already pointed out as a defence mechanism in sediments colonized by H. portulacoides, where a reduction of Cr (VI) to Cr (III) would have as consequence the retention, of this element, in Fe oxyhydroxide fraction, decreasing its bioavailability (Tanackovic et al. 2008). Lytle et al. (1998) found similar data concerning soluble Cr (VI) reduction by water hyacinth, suggesting that wetland plants uptake Cr in its less toxic form, throughout external reduction of Cr (VI) by their lateral fine roots, probably due to oxalate exudation.
This points out for two potential phytoremediative applications. Not only this species can accumulate large amounts of Cr withdrawn from its surrounding medium, but it can also convert high percentages of the remaining Cr (VI) into a less toxic form. This toxicity reduction is important not only for this plant species but also for the remaining surrounding biota, with a potentially essential role for environmental detoxification. This phytoremediation potential must be allied to a healthy and tolerant metabolism.
6 Final Remarks
Salt marshes are very interesting field laboratories to study metal biogeochemistry, namely, Cr. Salt marsh localization in estuarine systems, where large concentrations of industrial activities are gathered, makes them target systems to store metals. Being preferentially bound to the organic matter present in the sediment, the removal of Cr throughout natural or enhanced processes occurs throughout plant-mediated processes. Naturally, plants acquire during their life cycle nutrients from their sediments but also some non-nutritional elements, like Cr, and store them in their tissues. This natural ability can also be used and enhanced by the application of transporter molecules, like LMWOA, in order to increase the sediment-plant Cr transport. At this interface, it is also interesting to analyse the important root-mediated process of phyto-conversion of Cr (VI) toxic form to the less toxic Cr (III). Again, halophytes acquire an important role with high conversion efficiencies. All these passive and enhanced processes point out to a promising biotechnology using halophytes as potential cleaners of Cr-contaminated sediments, using environmental-friendly and low-cost technologies.
References
Barceloux D (1999) Chromium. J Tox Clin Tox 37:173–194
Bartlett RJ (1991) Cr cycling in soils and water: links, gaps and methods. Environ Health Persp 92:17–24
Bewers J, Yeats P (1989) Transport of river-derived trace metals through the coastal zone. Nether J Sea Res 23:359–368
Bluskov S, Arocena J, Omotoso O, Young J (2005) Uptake, distribution, and speciation of chromium in Brassica Juncea. Int J Phytoremediation 7:153–165
Brown S, Chaney R, Angle J, Baker A (1994) Phytoremediation potential of Thlaspi caerulescens and bladder campion for zinc- and cadmium-contaminated soil. J Environ Qual 23:1151–1157
Caçador I, Caetano M, Duarte B, Vale C (2009) Stock and losses of trace metals from salt marsh plants. Mar Environ Res 67:75–82
Caçador I, Neto JM, Duarte B, Barroso DV, Pinto M, Marques JC (2013) Development of an Angiosperm Quality Assessment Index (AQuA – Index) for ecological quality evaluation of Portuguese water bodies – a multi-metric approach. Ecol Ind 25:141–148
Caçador I, Vale C, Catarino F (1996) The influence of plants on concentration and fractionation of Zn, Pb, and Cu in salt marsh sediments (Tagus Estuary, Portugal). J Aquat Ecosyst Health 5:193–198
Couto T, Duarte B, Barroso D, Caçador I, Marques JC (2013) Halophytes as sources of metals in estuarine systems with low levels of contamination. Funct Plant Biol 40:931–939
Cunningham S, Ow D (1996) Promises and prospects of phytoremediation. Plant Physiol 110:715–719
Cunningham S, Berti W, Huang J (1995) Phytoremediation of contaminated soils. Trends Biotechnol 13:393–397
Doyle M, Otte M (1997) Organism-induced accumulation of Fe, Zn and AS in wetland soils. Environ Pollut 96:1–11
Duarte B, Caetano M, Almeida P, Vale C, Caçador I (2010) Accumulation and biological cycling of heavy metal in the root-sediment system of four salt marsh species, from Tagus estuary (Portugal). Environ Pollut 158:1661–1668
Duarte B, Delgado M, Caçador I (2007) The role of citric acid in cadmium and nickel uptake and translocation, in Halimione portulacoides. Chemosphere 69:836–840
Duarte B, Raposo P, Caçador I (2009) Spartina maritima (cordgrass) rhizosediment extracellular enzymatic activity and its role on organic matter decomposition and metal speciation processes. Mar Ecol 30:65–73
Duarte B, Reboreda R, Caçador I (2008) Seasonal variation of extracellular enzymatic activity (EEA) and its influence on metal speciation in a polluted salt marsh. Chemosphere 73:1056–1063
Duarte B, Silva V, Caçador I (2012) Hexavalent chromium reduction, uptake and oxidative biomarkers in Halimione portulacoides. Ecotoxicol Environ Saf 83:1–7
Gupta M, Cuypers A, Vangronsveld J, Clijsters H (1999) Copper affects the enzymes of the ascorbate-glutathione cycle and its related metabolites in the roots of Phaseolus vulgaris. Physiol Plant 106:262–267
Jacob D, Otte M (2004) Influence of Typha latifolia and fertilization on metal mobility in two different Pb Zn mine tailings types. Sci Total Environ 333:9–24
Jones DL (1998) Organic acids in the rhizosphere a critical review. Plant Soil 205:25–44
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants. CRC Press, Boca Ratón, FL
Katz S, Salem H (1994) The biological and environmental chemistry of chromium. VCH, New York
Kortenkamp A, Casadevall M, Faux SP, Jenner A, Shayer R, Woodbridge N, O’Brien P (1996) A role for molecular oxygen in the formation of DNA damage during the reduction of the carcinogen chromium(VI) by glutathione. Arch Biochem Biophys 329:199–207
Losi M, Amrhein C, Frankenberger W (1994) Environmental biochemistry of chromium. Rev Environ Contam Toxicol 136:91–121
Lytle C, Lytle F, Yang N, Qian J, Hanen D, Zayed A, Terry N (1998) Reduction of Cr (VI) to Cr (III) by wetland plants: potential for in situ heavy metal detoxification. Environ Sci Technol 32:3087–3093
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6:273–278
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press Limited, London
Mikalsen A, Alexander J, Wallin J, Ingelman-Sundberg M, Andersen R (1991) Reductive metabolism and protein binding of chromium(VI) by P450 protein enzymes. Carcinogenesis 12:825–831
Morris A, Bale A, Howland R, Millward G, Ackroyd D, Loring D, Rantala T (1986) Sediment mobility and its contribution to trace metal cycling and retention in a macrotidal estuary. Water Sci Technol 18:111–119
Mucha AP, Almeida CM, Bordalo AA, Vasconcelos MT (2005) Exudation of organic acids by a marsh plant and implications on trace metal availability in the rhizosphere of estuarine sediments. Estuarine Coastal Shelf Sci 65:191–198
Nies D (1992) Resistance to cadmium, cobalt, zinc and nickel in microbes. Plasmid 27:17–28
Nriagu J (1988) A silent epidemic of environmental metal poisoning. Environ Pollut 50:139–161
Parker D, Pedler J, Ahnstrom Z, Resketo M (2001) Re-evaluating the free-ion activity model of trace metal toxicity toward higher plants: experimental evidence with copper and zinc. Environ Toxicol Chem 20:899–906
Pazos-Capeáns P, Barciela-Alonso M, Herbello-Hermelo P, Bermejo-Barra P (2010) Estuarine increase of chromium surface sediments: distribution, transport and time evolution. Microchem J 96:362–370
Qian J-H, Zayed A, Zhu Y-L, Yu M, Terry N (1999) Phytoaccumulation of trace elements by wetland plants. III. Uptake and accumulation of trace elements by 12 plant species. J Environ Qual 28:1448–1455
Reboreda R, Caçador I (2007) Copper, zinc and lead speciation in salt marsh sediments colonised by Halimione portulacoides and Spartina maritima. Chemosphere 69:1655–1661
Redondo-Gómez S, Mateos-Naranjo E, Vecino-Bueno I, Feldman S (2011) Accumulation and tolerance characteristics of chromium in a cordgrass Cr-hyper-accumulator, Spartina argentinensis. J Hazard Mater 185:862–869
Romheld V, Marschner H (1983) Mechanism of iron uptake by peanut plants: I. FeIII reduction, chelate splitting, and release of phenolics. Plant Physiol 71:949–954
Salt D, Pickering I, Prince R, Gleba D, Dushenkov S, Smith R, Raskin I (1997) Metal accumulation by aquacultured seedlings of Indian mustard. Environ Sci Technol 31:1636–1644
Shen H, Wang Y (1993) Characterization of enzymatic reduction of hexavalent chromium by Escherichia coli ATCC 33456. Appl Environ Microbiol 59:3771–3777
Sinha S, Bhatt K, Pandey K, Singh S, Saxena R (2003) Interactive metal accumulation and its toxic effects under repeated exposure in submerged plant Najas indica Cham. Bull Environ Contam Toxicol 70:696–704
Sinicrope T, Langis R, Gergberg R, Busnardo M, Zedler J (1992) Removal of metals by wetland mesocosms subjected to different hydroperiods. Ecol Eng 1:309–322
Srivastava S, Prakash S, Srivastava M (1999) Chromium mobilization and plant availability – the impact of organic complexing ligands. Plant Soil 212:203–208
Stearns D, Kennedy L, Courtney K, Giangrande P, Phieffer L, Wetterhahn K (1995) Reduction of chromium(VI) by ascorbate leads to chromium-DNA binding and DNA strand breaks in vitro. Biochemistry 34:910–919
Sundby B, Vale C, Caçador I, Catarino F, Madureira MJ, Caetano M (1998) Metal-rich concretions on the roots of salt marsh plants: mechanism and rate of formation. Limnol Oceanogr 43:245–252
Suntornvongsagul K, Burke D, Hamerlynck E, Hahn D (2007) Fate and effects of heavy metals in salt marsh sediments. Environ Pollut 149:79–91
Suseela MR, Sinha S, Singh S, Saxena R (2002) Scanning electron microscopic studies of Scirpus lacustris L. treated with Cr and tannery effluent: accumulation of metal. Bull Environ Contam Toxicol 68:540–548
Tanackovic S, Caetano M, Vale C (2008) Effect of salt-marsh plants on the mobility of Cr in sediments. Ciencias Marinas 34:363–372
Vale C (1990) Temporal variations of particulate metals in the Tagus estuary. Sci Total Environ 97:137–154
Vale C, Canário J, Caetano M, Lavrado J, Brito P (2008) Estimation of the anthropogenic fraction of elements in surface sediments of the Tagus Estuary (Portugal). Mar Pollut Bull 56:1353–1376
Viers J, Dupré B, Gaillardet J (2009) Chemical composition of suspended sediments in World Rivers: new insights from a new database. Sci Total Environ 407:853–868
Weis J, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ Int 30:685–700
Williams TP, Bubb JM, Lester JN (1994) Metal accumulation within salt marsh environments: a review. Mar Pollut Bull 28:277–290
Wittbrodt P, Palmer C (1996) Effect of temperature, ionic strength, background electrolytes, and Fe(III) on the reduction of hexavalent chromium by soil humic substances. Environ Sci Technol 30:2470–2477
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Caçador, I., Duarte, B. (2015). Chromium Phyto-transformation in Salt Marshes: The Role of Halophytes. In: Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L. (eds) Phytoremediation. Springer, Cham. https://doi.org/10.1007/978-3-319-10969-5_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-10969-5_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10968-8
Online ISBN: 978-3-319-10969-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)