Abstract
Anthropogenic activities have been fundamental for the development of modern societies. However, they have resulted in the increase of environmental pollution which is a serious worldwide problem. Although phytoremediation has emerged as an attractive methodology to deal with contaminants, mainly present in water and soil, there are still some drawbacks to become a widely used practice, such as the problem of soils with diffuse mixed pollution (inorganic and organic). A useful strategy to overcome current limitations of phytoremediation is the use of genetically engineered plants. In this chapter we will discuss the most recent advances which use genetic engineering tools for enhancing phytoremediation of inorganic and organic pollutants. Advantages as well as disadvantages of using transgenic plants will also be discussed. In spite of the controversial aspects of field applications, efforts to minimize risks and reach public acceptance are very significant since the potential and usefulness of phytotechnologies are of great importance to clean up the environment.
All authors contributed equally to this book chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Environmental pollution
- Genetically engineered plants
- Inorganic pollutants
- Organic pollutants
- Phytotechnologies
- Phytotransformation
- Phytoextraction
1 Introduction
Phytoremediation is a green, eco-friendly, and emerging technology that uses plants and enzymes derived from them for the treatment of soil, water, and groundwater contaminated by toxic pollutants (Ali et al. 2013). The concept of using plants for these purposes emerged few decades ago with the understanding that plants were capable of accumulating high quantities of toxic metals and/or metabolizing organic compounds in their tissues or organs. However, the term “phytoremediation” has been used since the beginning of the 1990s, when scientists focused their research interests on plant-based technologies, as suitable alternatives to traditional cleanup procedures because of their low capital costs, low maintenance requirements, end-use value, and aesthetic nature. Since then, a number of related technologies were developed, and the definition later evolved in “phytotechnologies” (ITRC 2001) that includes a wide range of processes such as phytoextraction, phytostabilization, phytotransformation, phytovolatilization, rhizofiltration, and phytostimulation, which have been extensively described (Pilon-Smits 2005; Abhilash et al. 2009). In addition to the well-known advantages of phytoremediation over other remediation strategies, it offers potential benefic side effects, such as erosion control, site restoration, carbon sequestration, and feedstock for biofuel production (Van Aken et al. 2010).
Even though these technologies have shown to efficiently reduce the chemical hazard associated with various classes of inorganic and organic pollutants, they also have several limitations related to the metabolism of recalcitrant xenobiotic compounds. Thus, to improve the phytoremediation abilities of selected plants, research was oriented to the obtainment of genetically modified plants (Macek et al. 2008; Novakova et al. 2010). Genes involved in multistep degradation pathways of pollutants, transport, and sequestration can be isolated from bacteria, fungi, animals, or plants and introduced into candidate plants. Therefore, three main strategies have been employed: (a) transformation with genes from other organisms, (b) transformation with genes from other plant species, and (c) overexpression of genes from the same plant species. The target is to obtain a plant with high ability to tolerate, accumulate, detoxify, or degrade pollutants and with suitable agronomical characteristics. In fact, the possibility of using transgenics in phytoremediation depends on the availability of gene sequences, which can improve, modulate, or radically change plant metabolism, conferring to the plant a phenotype which does not naturally possess. The main objective of inorganic remediation is to enhance metal accumulation and tolerance by overexpressing in transgenic plant genes involved in homeostasis, metabolism, uptake, and/or translocation of the toxic elements (Kotrba 2013). On the contrary, for organic compounds, this approach could lead, in an optimal case, to the complete mineralization of the pollutants or the formation of less toxic metabolites than those produced by the existing pathway. Another potential advantage of the transgenic approach is the possibility of producing enzymes in root exudates through the expression (or overexpression) of secretory enzymes involved in pollutant removal or through the expression of heterologous enzymes with signal sequences to drive them to the secretary pathway.
In the case of a heterologous gene introduced with genetic engineering, a critical issue is its expression level in the new genetic and cellular context. For this reason, the introduced genes are frequently embedded in an “expression cassette” which can ensure an efficient transcription and translation (Maestri and Marmiroli 2011).
Since the first work describing transgenic plants modified for pollutant metabolism (Feng et al. 1997), a lot of research has been performed in this way. However, works dealing with inorganic contaminants have always been more abundant, as compared to works on organic contaminants, and there is only a small amount of papers addressing simultaneously with both compounds.
Regarding plant species used for transformation experiments, Arabidopsis thaliana and Nicotiana tabacum are among the most common model organisms due to well-developed and efficient protocols for DNA delivery and recovery of transformants (Meyers et al. 2010). However, it is important to note that for the past few years, the use of transgenic plants has evolved from transformation of model plants and laboratory tests to clarify the roles and functions of genes to transformation of plants effectively useful for phytoremediation in the field (Maestri and Marmiroli 2011). Such plants would posses some suitable properties like high biomass and deep roots, and they would also be amenable to easy growth in different climatic and soil conditions.
Despite its promising potential, plant genetic engineering still faces considerable technical challenges. Thus, further research into mechanisms underlying nuclear and/or plastid transformation, more efficient DNA delivery systems as well as plant tissue culture and regeneration protocols are essential for progressing in the field of plant genetic engineering. In addition, certain drawbacks related to the applicability of transgenic plants for phytoremediation in the field should be saved in order to successfully apply this technology, as it will be described in the present chapter.
2 Phytoremediation of Inorganic Contaminants
Inorganic pollutant phytoremediation involves mechanisms, enzymes, and processes different from those concerning organic pollutants. This is mainly due to the fact that metal(loid)s are not degradable. Thus, phytoextraction, phytostabilization, phytovolatilization, and rhizofiltration are suitable phytotechnologies for inorganic pollutant removal.
There are several factors that affect metal phytotoxicity such as metal type, concentration, redox state, and solubility. Metal type is the first aspect that needs to be considered. Some metals are essential for plants as micronutrients like Fe, Mn, Zn, Cu, Mg, Mo, B, Cl, and Ni, while others have no biological role such as As, Pb, Cd, Hg, Ag, and U and are toxic even at very low concentrations, being classified as nonessential metals. However, when some essential metals occurred at high concentrations, they can become toxic to plant cellular processes. Plants evolved several transport mechanisms to take up micronutrients from soil matrix. Contrarily, toxic metal(loid)s are not taken up by specific transpoter mechanisms and they are taken up by plants along with other micronutrients (Kabata-Pendias 2011).
Plants have developed defense mechanisms to minimize metal toxicity. For instance, plants can make metals insoluble mostly by the aid of chelating agents and after being accumulated. Besides, there are plant enzymes that can change the redox state to a less toxic form, whereas others can transform metal(loid)s to make them more volatile.
In this work, we are going to focus on plant genetic engineering directed to increase accumulation of nonessential metals since hyper-accumulation of essential metals is linked with food bio-fortification and it needs a different consideration and discussion. In this sense, food bio-fortification and phytoremediation are two sides of the same coin, although, as we already mentioned, essential metals in excess also become an environmental problem (Guerinot and Salt 2001). It is important to note that many transgenic plants have also been developed with the aim of studying both the function of metal(loid) transporter proteins and its relation with accumulation in edible parts to asses food security issues.
Transgenic approaches for improving inorganic phytoremediation include:
-
Increasing mobilization and uptake of metal(loid)s from the surrounding environment
-
Increasing sequestration of metals within cells
-
Increasing metal translocation to the aboveground tissues
-
Increasing or adding ligands to allow phytovolatilization
In this section and considering that each plant-metal system is so particular, i.e., it has specific molecular mechanism for uptaking, transporting, and sequestrating, we will review recent advances separately by metal(loid). However, most current findings provide evidence that the relationship between genetic engineering and the effect of the genetic modification is quite complex. In some cases, an increase in phytochelatins (PCs)/thiol peptide concentration has not always been correlated with an increase in metal(loid) accumulation and thus with plant resistance or tolerance. In other studies, it has been true for one metal and not for others.
2.1 Genetically Engineered Plants for Improving Phytoremediation of the Nonessential Metal(loid)s: As, Cd, Pb, and Hg
2.1.1 Arsenic
Arsenic (As) is a very toxic and ubiquitous metalloid. Although it has a geological origin, its concentration can be increased by anthropogenic activities such as pesticide application and wood preservatives, mining and smelting operations, and coal combustion (Wang and Mulligan 2006). Consequently, elevated levels of As have been reported in soils and groundwater worldwide. In soils and groundwater, inorganic arsenic is present mainly as As+5 and As+3. Arsenic is a nonessential element for plants. Thus, they do not have specific transporter systems for arsenic species. The lines below describe the targets for genetic engineering directed toward the improvement of arsenic phytoextraction and phytostabilization from soils and sediments.
2.1.1.1 Increasing Sequestration of As Within Cells
Phytochelatins play a central role in metal(loid) detoxification by chelating those toxic ions. Most research has focused in some of the three enzymes that constitute the PCs biosynthetic pathway: gamma-glutamylcysteine synthase (ECS), glutathione synthase (GS), and phytochelatin synthase (PCS). The expression of these genes from different organisms has contributed to the knowledge about tolerance and removal of As and other metals (Dhankher et al. 2002; Li et al. 2004, 2005, 2006). Moreover, the simultaneous overexpression of yeasts PCS and GS (AsPCS1 and GSH1) in A. thaliana successfully led to elevated total PCs production and increased tolerance and accumulation of As and also Cd (Guo et al. 2008). Furthermore, independent overexpression of different PCS enzymes (AtPCS1 gene from Arabidopsis or CePCS from Caenorhabditis elegans) in the same plant species (N. tabacum) resulted in distinctive metabolic changes accompanied by differences in Cd and As tolerance between the transgenic plants (Wojas et al. 2010). Recently, enhanced As tolerance of transgenic poplar plants was achieved by expressing the bacterial ECS (from E. coli) (LeBlanc et al. 2011). In addition, transgenic tobacco plants expressing PCS gene from Ceratophyllum demersum (CdPCS1), an aquatic macrophyte, showed severalfold increased PCs content and precursors of thiol peptides, with enhanced accumulation of Cd and As (Shukla et al. 2012).
Metallothioneins (MTs), another type of metal-binding ligands, are also targets of genetic engineering. MTs are low molecular mass proteins (from 2 to 16 kDa) with unique abundance of cysteine residues (more than 30 % from all amino acids). Even though the role of MTs in plant protective mechanisms against metals is not fully understood, they are known as effective free radical regulators by binding metals. Although As is not in the list of metals reacting with MTs, the expression of the MT 2b (AtMT2b) from A. thaliana in tobacco led to enhanced As+3 sensitivity and translocation (Grispen et al. 2009).
All current publications seem to indicate that multigene approach directed to increase sequestration of As within cells led to better results than simple gene transformation. This is a very important consideration since most contaminated sites, such as mining or industrial areas, contain a mixture of metals, and thus transgenic plant with phytoremediation capabilities must be able to cope with this situation.
2.1.1.2 Increasing As Translocation to the Aboveground Tissues
The mechanisms involved in As translocation are not fully understood; consequently the development of transgenic plants with genes involved in this process has been delayed. Recently, transgenic A. thaliana plants expressing an As+3 antiporter gene from P. vittata (PvACR3) accumulated approximately 7.5-fold more As in the aboveground tissues than WT plants (Chen et al. 2013). These results suggest the involvement of PvACR3 in As translocation.
2.1.1.3 Adding Ligands to Allow As Volatilization
Regarding As volatilization, no genes for methyltransferase activity have been identified in plants. However, an arsM gene from the soil bacterium Rhodopseudomonas palustris was expressed in rice (Oryza sativa), and the transgenic rice produced volatile methylated arsenic species (MMA+5 and DMA+5) after exposure to As+3, theoretically providing a potential strategy for phytoremediation (Meng et al. 2011).
2.1.2 Lead
Lead (Pb) contamination of soils is widespread at many industrial and mining sites throughout the world. In addition, Pb contamination also derives from the past use of lead pesticides, leaded paints, leaded gasoline, and some types of pressure-treated wood. Because of the immobility of Pb in soils, historical Pb contamination in urban, industrial, and high-traffic areas persists today despite the phase out of leaded gasoline and paints beginning in the 1970s (McBride et al. 2012). In a similar way as other nonessential and highly toxic elements, plant cells are not likely to possess specific Pb transporters (Arazi et al. 1999), but some cation transporters in the plasma membrane offer potential entry pathways into plant cells. There has been a lack of specific genes identified as conferring capacity for Pb resistance and accumulation (Song et al. 2003), which has delayed genetic engineering for the obtainment of Pb-extracting plants. However, many advances have taken place in the last years.
2.1.2.1 Increasing Pb Uptake from Soil
NtCBP4 gene isolated from N. tabacum codifies a membrane protein channel which can carry Pb ions through the plasma membrane into the plant cell. Transgenic plants overexpressing NtCBP4 exhibited increased accumulation of Pb (Arazi et al. 1999). Gupta et al. (2013) mentioned this strategy as the first example of a plant gene that can modulate Pb tolerance and accumulation, after which there have not been other advances in this aspect.
2.1.2.2 Increasing Sequestration of Pb Within Cells
This approach has been the most applied concerning lead phytoextraction. Several studies have evaluated Pb accumulation and translocation in plants transformed with genes involved in general processes of metal sequestration. For instance, three different plant species (A. thaliana, Brassica juncea, and Populus alba × P. tremula var glandulosa) overexpressing a yeast Cd factor 1 (ScYCF1, from Saccharomyces cerevisiae), a transporter that pumps GSH-conjugated Cd into the vacuole, tolerated and accumulated increased amounts of Pb conjugated with glutathione (GSH) from cytoplasm to vacuoles (Song et al. 2003; Bhuiyan et al. 2011b; Shim et al. 2013). In a similar approach, directed to enhance the expression of PCs, aspen transgenic lines expressing a PCS gene (TaPCS1) from wheat reached total biomass and Pb accumulation significantly greater than in the control plants (Couselo et al. 2010). These studies evidence the utility of overexpressing ligands able to bind Pb ions and transport them conjugated into vacuoles.
A different and successful approach was overexpression of AtATM3, a member of the ATP-binding cassette (ABC) transporter family localized at the mitochondrial membrane, under the control of the CaMV35S in B. juncea. This genetic modification conferred enhanced tolerance not only to Pb+2 but also to Cd+2 (Bhuiyan et al. 2011a).
2.1.3 Cadmium
Cadmium (Cd) is widespread in soils, water, and atmosphere. The main sources of Cd contamination into the environment are metallurgic industries, waste incinerators, urban traffic, cement factories, and phosphate fertilizers (Gratao et al. 2005).
The effect of Cd toxicity on plants has been largely explored (Gallego et al. 2012). The metal often produces plant growth inhibition and decrease of photosynthetic activities; thus, strategies of obtaining transgenic plants with different candidate genes have been used in order to improve plant tolerance and/or Cd phytoextraction and/or phytostabilization.
2.1.3.1 Increasing Sequestration of Cd in Vacuoles
As it was pointed out to other metal(loid)s, an efficient Cd detoxification is related to binding free ions in the cytoplasm, and their sequestration into vacuoles (Clemens 2006), one possible approach to generate plants suitable for Cd phytoremediation, might consist of introducing gene coding proteins able to transport heavy metals or their complexes to appropriate storage compartments. Vacuolar sequestration of Cd can be achieved through either PC-dependent or PC-independent pathways (Hirschi et al. 2000; Song et al. 2003; Korenkov et al. 2007; Martinoia et al. 2007).
Multidrug resistance-associated proteins (MRPs), a subfamily of ABC transporters, catalyze the export of substrates out of the cytosol in an ATP-dependent manner (Verrier et al. 2008), and they have been related with Cd sequestration (Klein et al. 2006). Wojas et al. (2009) showed that AtMRP7 overexpression in tobacco increased Cd tolerance by an efficient storage of the metal in vacuoles and higher Cd retention in roots, suggesting a contribution to the control of Cd root-to-shoot translocation.
Another strategy for Cd accumulation into vacuoles includes cations/H+ exchangers, such as CAXs (from calcium exchangers). Recently, Wu et al. (2011) demonstrated that the expression of a CAX1 protein in petunia enhances Cd accumulation and tolerance. The transport of Cd into vacuoles by cation/H+ antiporters is energized by the pH gradient established by proton pumps. Thus, Khoudi et al. (2012) studied the potential for enhancing proton pump expression as a strategy to improve Cd accumulation in plants. They found that transgenic tobacco plants which expressed TaVP1 cDNA, encoding wheat vacuolar H+-pyrophosphatase (V-H-PPase), were both more tolerant to Cd compared to wild-type (WT) plants and accumulated higher Cd concentration.
Recently, ScYCF1 has been overexpressed in poplar trees. Transgenic plants exhibited enhanced growth, reduced toxicity symptoms, and increased Cd content in the aerial tissue compared to WT plants (Shim et al. 2013). Furthermore, these plants established an extensive root system in mine tailing soil and accumulated high amounts of Cd, Zn, and Pb. Thus, YCF1-expressing poplar may be useful for phytostabilization, especially in highly contaminated regions, where WT plants cannot survive.
In addition, other authors have used transgenic plants with simultaneous expression of two genes to increase Cd tolerance and accumulation. Guo et al. (2012) assembled in transgenic A. thaliana plants AsPCS1and ScYCF1 genes for an effective metal chelation by thiols and the following inclusion in vacuoles.
Even though the overexpression of genes involved in Cd-tolerance mechanisms gives positive results, there are some cases which are not successful. For example, overexpression of AtPCS in the same plant species paradoxically produced hypersensitivity to Cd stress (Lee et al. 2003).
Regarding strategies that involve Cd and MTs, Krystofova et al. (2012) found that the expression of a yeast MT was responsible for higher Cd accumulation in roots of transgenic N. tabacum plants and its limited transport to aerial parts.
2.1.4 Mercury
Mercury (Hg) exists in different forms (HgS, Hg2+, Hg0, and methyl-Hg); however, in agricultural soil ionic form (Hg2+) is predominant (Han et al. 2006). Inorganic Hg forms are usually less harmful than organic forms, because the last ones are hydrophobic and move across cell membranes. In plants, ionic Hg tends to affect the plasmatic membrane producing damage to transporters such as aquaporins, leading to nutrient and water disruption (Zhang and Tyerman 1999), while organomercurials rapidly localize into plastids where they accumulate and disrupt important metabolic functions (Bernier and Carpentier 1995).
Since plants cannot successfully detoxify or interconvert Hg to less harmful forms, genetic engineering is directed to integrate foreign genes from other organisms to enhance their phytoremediation capabilities (Ruiz and Daniell 2009).
2.1.4.1 Increasing Hg Volatilization
A well-characterized Hg-phytoremediation system is the use of the bacterial merA (mercuric ion reductase) and merB (organomercurial lyase) genes to genetically engineer plants for the remediation of this metal (Bizily et al. 2003; Che et al. 2003; Lyyra et al. 2007). This mechanism is based in protonolysis of organic Hg to Hg2+ by the lyase enzyme and the following reduction of Hg2+ to Hg0 by the mercuric ion reductase, which is volatilized from plants. Despite the fact that the first attempts failed to express bacterial genes in eukaryotic organisms, the use of preferred codons for plants allowed obtaining transgenic plants highly Hg resistant (Rugh et al. 1996; Yang et al. 2003). For merA the best results were obtained with its root-specific expression, indicating that root is the main organ affected and that its protection is important for phytoremediation. These gene expressions have been directed to nuclei but also to chloroplast. Ruiz et al. (2003) hypothesized that expressing merA and merB genes within plant chloroplasts would confer protection for essential metabolic reactions occurring within plastids, since chloroplast has shown to be the main target for Hg poisoning. More recently, considering these explored aspects, Hussein et al. (2007) obtained transgenic tobacco plants with the combined expression of merAB via the chloroplast genome and showed enhanced conversion of Hg+2 into Hg0, rapid volatilization, and increased shoot accumulation of different forms of Hg, even surpassing the concentrations found in soil.
2.1.4.2 Increasing Hg Scavenging by Chelation
One limitation regarding the use of the merAB system is the release of Hg0 into the atmosphere. Therefore, an alternative approach would be the chelation of ionic Hg inside the cell by molecules negatively charged, as polyphosphates. Tobacco plants expressing the bacterial ppk gene, which codifies for the enzyme involved in polyphosphate synthesis (polyphosphate kinase), showed enhanced tolerance and accumulation of Hg2+ (Nagata et al. 2006a, b). With the same purpose of Hg chelation, Hsieh et al. (2009) expressed the bacterial merP gene in plants that codified for a cell membrane protein, providing enhanced resistance to HgCl2. Then, Nagata et al. (2009) reported that the expression of bacterial merT (Hg transporter gene) for a in ppk-transgenic tobacco resulted in accelerated and enhanced Hg uptake and accumulation. More recently, Nagata et al. (2010) combined several of the genes mentioned below and obtained tobacco plants that coexpressed three bacterial genes: ppk from Klebsiella aerogenes and merT and merB, both from Pseudomonas K-62. The ppk/merT/merB-transgenic tobacco callus showed more resistance to methylmercury (CH3Hg+) and accumulated more Hg from CH3Hg+-containing medium than the ppk/merT-transgenic and WT progenitors. These results indicate that the MerB enzyme degraded the incorporated CH3Hg+ to Hg2+, which then was accumulated as a less toxic Hg-polyP complex in tobacco cells. Hence, it is believed that these engineered ppk/merT/merB-transgenic plants would have more public acceptance since they prevent the release of volatile Hg into the atmosphere.
Another possible Hg-chelation strategy could be mediated by disulfide compounds (copper-zinc superoxide dismutase, Cu/Zn SOD) and sulfhydryl compounds (GSH, PCs, and MTs), since Hg cations have a high affinity for sulfhydryl groups. Thus, Chen et al. (2012) studied the relationship between Hg detoxification and a disulfide isomerase-like protein (PDIL) with a chaperone function and disulfide isomerase activity. For that reason, the authors expressed MTH1745, a gene that codifies a PDIL from thermophilic archaea Methanothermobacter thermoautotrophicum in Oryza sativa L. cv. Nipponbare. The transgenic rice seedlings showed more effective photosynthesis, lower levels of ROS and malondialdehyde, as well as higher levels of antioxidant enzymes than WT plants indicating an enhanced Hg tolerance.
In order to avoid releasing volatile Hg into the environment, Hg accumulation inside plants would be a useful strategy. Ruiz et al. (2011) reported the development of a transplastomic approach, which consists in the insertion of foreign DNA in chloroplasts, to express the mouse MT gene (mt1). The transplastomic lines accumulated high Hg concentrations and maintained high chlorophyll content. This study reported the usefulness of chloroplast genetic engineering approach to express Hg-scavenging proteins.
As it could be noted, transgenic plants with increased metal tolerance and accumulation rely on overexpressing genes involved in the biosynthesis pathways of metal-binding proteins and peptides, genes that can convert a toxic ion into a less toxic or easy to handle form, as well as genes coding transport proteins involved in vacuolar accumulation or a combination of some of them. There are no reports about improving a more efficient translocation of metal(loid)s from root to shoot, constituting a challenge since this is the more interesting character of hyperaccumulator plants. In this sense, studies based on genomic and functionality of target elements related to metal tolerance of hyperaccumulator plants would allow interesting advances in this aspect.
3 Phytoremediation of Organic Compounds
The efficiency of transgenic plants in the phytoremediation of organic contaminants has been investigated. For that, two main strategies have been pursued: (1) the manipulation of metabolic activities to enhance in planta degradation rates or to impart a novel metabolic activity and (2) the enhanced secretion of enzymes from roots leading to accelerated ex planta degradation of organic contaminants. One of the main processes involved in organic pollutant phytoremediation is phytotransformation, also known as phytodegradation. In this process, plants uptake pollutants and subsequently metabolize or transform them into less toxic metabolites. Once taken up, the organic chemicals generally undergo three transformation phases: (I) chemical modification (oxidations, reductions, hydrolysis), (II) conjugation, and (III) sequestration or compartmentalization (Ohkawa et al. 1999; Cherian and Oliveira 2005). Plant enzymes that typically catalyze the reactions of phase I are cytochrome P450 monooxygenases, carboxylesterases, peroxidases, and laccases (Coleman et al. 1997; Burken 2003). The second phase involves conjugation with GSH, sugars, or amino acids, catalyzed by glutathione, glucosyl, and malonyl transferases, resulting in more soluble, polar compounds (Marrs 1996). The third phase of plant metabolism is compartmentalization and storage of soluble conjugates either in vacuoles or in the cell wall. The conjugates are actively transported to the vacuole or apoplast by ATP-dependent membrane pumps (Martinoia et al. 1993).
A great diversity of organic pollutants has been introduced into the environment by human activities. However, phytoremediation using transgenic plants of only some of these compounds has been extensively studied. The first attempts for these purposes were targeted to herbicides, explosives, and halogenated organic compounds (Feng and Kennedy 1997; French et al. 1999; Doty et al. 2000). In the following sections, the more recent examples related to the use of transgenic plants for phytoremediation of these contaminants and others will be presented.
3.1 Pesticides
Pesticides include a wide range of chemicals used to kill, repel, or control pests and weeds. Among them, DDT [2,2-bis(chlorophenyl)-1,1,1-trichloroethane] is one of the 21 POPs that require immediate elimination, according to the 2010 Stockholm Convention (Sudharshan et al. 2012). Since World War II DDT has accumulated in the environment because of its use against forest and agricultural pests and against insect vectors of typhus and malaria (Lunney et al. 2004). Cytochrome P450 enzymes from pig, human, and other living organisms have been expressed in different genetically engineered plant species for pesticide removal (Hussain et al. 2009). In this sense, Mouhamad et al. (2012) evaluated the phytoremediation of TCE and DDT polluted water using transgenic Sesbania grandiflora and A. thaliana plants harboring rabbit cytochrome P450 2E1. Arabidopsis transgenic plants exposed to both contaminants accumulated more DDT and TCE compared with WT plants.
Lindane (g-1,2,3,4,5,6-hexachlorocyclohexane), also known as gammaxene or benzene hexachloride (BHC), is another organochlorine insecticide used worldwide in agriculture, as well as to control insect-borne diseases relevant to human and animal health (Singh et al. 2011). Although lindane has been banned from the European Community and United States markets due to its toxicity and recalcitrance, there are still several areas polluted with this insecticide (Rigas et al. 2009). Therefore many research studies focused on its remediation. In this context, Singh et al. (2011) showed that stable integration and expression of human cytochrome P450 2E1 (CYP2E1) in tobacco plants produced great tolerance as well as enhanced removal of this compound from liquid solution and soil.
Herbicides play an important role in agriculture worldwide but have negative effects on the environment (Dowling and Doty 2009). Even though phytoremediation of herbicides has been well studied using conventional plants, field trials suggested that the rate of contaminant removal was inadequate. Transgenic technology has been used with some success considering that two enzymes play main roles in the increased degradation of pesticides: cytochrome P450 monooxygenases (P450s) and glutathione S-transferases (GSTs) (Inui and Ohkawa 2005; Kawahigashi et al. 2005, 2006; Karavangeli et al. 2005). For example, Kawahigashi et al. (2008a) engineered rice plants expressing human cytochrome P450 genes (CYP1A1, CYP2B6, and CYP2C19) that were more tolerant to several herbicides than WT plants. Besides, transgenic plants were able to remove atrazine and metolachlor from soil. In addition, the accumulation of the OsGSTL1 protein (with GST activity) in the vegetative tissues of transgenic rice plants enhanced their tolerance to chlorsulfuron and glyphosate (Hu et al. 2009).
3.2 Explosives
The most widespread explosives are 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-triazine, hexogen (RDX), and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetraazocine (HMX) (Octogen). These toxic and mutagenic explosives are stable in the environment and recalcitrant to remediation (Panz and Miksch 2012).
As it was mentioned before, the use of genetic engineering is also a powerful tool for enhancing the efficiency of explosive phytoremediation (French et al. 1999; Hannink et al. 2001, 2007). In particular for TNT remediation, the overexpression of two glycosyltransferases in Arabidopsis resulted in an increase of conjugate production and detoxification (Gandia-Herrero et al. 2008). Bacterial nitroreductases efficiently reduce the nitro side groups of TNT to different isomers of aminonitrotoluene. Accordingly, Van Dillewijn et al. (2008) showed that the expression of the bacterial nitroreductase gene (pnrA) improved the natural capacity of transgenic hybrid aspen (Populus tremula × tremuloides var. Etropole) to tolerate, grow, and more importantly eliminate TNT not only from contaminated hydroponic medium but also from contaminated soil where its bioavailability is reduced.
RDX is a nitramine, often found along with TNT (Rylott and Bruce 2009). The first study demonstrating the use of transgenic plants to remove simultaneously TNT and RDX was carried out by Rylott et al. (2011) using A. thaliana. These plants were transformed with the bacterial genes xplA and the associated reductase xplB, an unusual explosive-degrading P450 system, (RDX degrading) from Rhodococcus rhodochrous strain 11Y, in combination with the gene nfsI (TNT-detoxifying nitroreductase) from Enterobacter cloacae. The transgenic plants obtained, removed RDX from soil leachate, and grew on soil contaminated with both explosives at inhibitory concentrations for plants that only expressed XplA.
On the other hand, HMX is less susceptible to phytoremediation than RDX and TNT. Since this octogen has a chemical structure that is similar to the hexogen, there have been attempts to remove this compound using genetically modified plants expressing the xplA gene. However, transgenic lines did not assimilate more HMX than WT plants (Rylott and Bruce 2009; Panz and Miksch 2012). Thus, it constitutes a challenge that should continue under investigation.
3.3 Polychlorinated Biphenyls
Polychlorinated biphenyls (PCBs) are persistent organic pollutants (POPs), a group of chemical with long half-life in the environment and potential bioaccumulation through the food chain. PCBs are characterized by two linked aromatic rings substituted by 1–10 chlorine atoms. There are about 209 of their congeners and are identified by chlorine numbers and position (Anyasi and Atagana 2011). PCBs have been used for a variety of industrial applications, including lubricants, dielectric fluids, and plasticizers. Due to their hydrophobicity and chemical stability, PCBs are slowly taken up and degraded by plants, resulting in an incomplete metabolism and potential release of toxic metabolites into the environment. In order to overcome these limitations, bacterial genes involved in PCB metabolism, such as biphenyl dioxygenases, enzymes that catalyze the first steps in their degradation, have been introduced into plants (Mohammadi et al. 2007; Sylvestre et al. 2009; Van Aken et al. 2010). Among these enzymes, the 2,3-dihydroxybiphenyl-1,2-dioxygenase (BPHC) is the third enzyme in the biphenyl degradation pathway, and its function is the cleavage of biphenyl ring. Chrastilová et al. (2008) and Novakova et al. (2009) obtained 12 lines of transgenic N. tabacum plants expressing bphC gene from Comamonas testosteroni B356. The presence and expression of the bphC gene as well as the enzyme were detected in transgenic plants. One transgenic line, namely, H2, showed high biomass, high viability on toxic substrates, and increased phytoremediation of high 2,3-dihydroxybiphenyl (2,3-DHB) concentrations (Novakova et al. 2010).
3.4 Volatile Organic Compounds
Another group of organic contaminants called volatile organic compounds (VOCs) includes trichloroethylene (TCE), carbon tetrachloride (CT), vinyl chloride (VC), benzene, chloroform, toluene, and bromodichloromethane (BDCM). Many VOCs are used and produced in the manufacture of paints, adhesives, petroleum products, pharmaceuticals, and refrigerants. James et al. (2008) developed transgenic tobacco plants capable to remove VOCs expressing CYP2E1, a key enzyme in the mammalian metabolism of several low molecular weight VOCs. These transgenic plants showed increased removal of TCE, VC, CT, benzene, toluene, chloroform, and BDCM, compared to WT plants but not of perchloroethylene or 1,1,1-trichloroethane. In a similar way, transgenic petunia plants expressing the same enzyme showed a significant increase in absorption capacity of benzene and toluene and improved resistance to formaldehyde (Zhang et al. 2011). This study revealed that the CYP2E1 gene enhances plant resistance to formaldehyde and also provides a method for reducing VOCs, by using transgenic flowering horticultural plants.
In view of the large size and extensive root systems of trees, transgenic poplars would constitute a useful tool to effectively remediate sites contaminated with a variety of pollutants at faster rates and lower costs. In this sense, Doty et al. (2007) obtained transgenic poplars expressing CYP2E1 that showed enhanced metabolism and removal of TCE, chloroform, and benzene. More recently, these transgenic poplars were evaluated in field conditions. They showed enhanced degradation of TCE in the field but in a lesser extent than that observed in laboratory studies (Legault 2013). All the studies carried out until now have shown that CYP2E1 would be the most common gene used for engineering plants with enhanced VOC phytoremediation ability.
3.5 Phenol Compounds
Phenol and its derivatives are widely distributed in the environment due to their multiple applications in petrochemical and pharmaceutical industries, in the synthesis of resins, perfumes, solvents, and lubricating oils, as well as in the preparation of other chemicals (e.g., plastics, drugs, explosives, pesticides, and detergents) (Iurascu et al. 2009). Different strategies were used to enhance phenol remediation capabilities, such as the overexpression of enzymes involved in phase I or in phase II of plant xenobiotic metabolism.
For many years our research group has developed expertise in phenol phytoremediation using mainly hairy roots (HRs) as model system from different plant species. Removal efficiencies and optimization of the process were determined, as well as the involvement of different peroxidase isoenzymes (Agostini et al. 2003; González et al. 2006; Coniglio et al. 2008; Talano et al. 2010). In order to increase the efficiency and to evaluate physiological and biochemical mechanisms involved in phytoremediation of phenols, transgenic tomato and tobacco plants and HRs were developed (Wevar Oller et al. 2005; Sosa Alderete et al. 2009, 2012; Talano et al. 2012). For instance, the involvement of basic peroxidase isoenzymes, TPX1 and/or TPX2, in phenol removal was evaluated by overexpression in tomato and tobacco (Wevar Oller et al. 2005; Sosa Alderete et al. 2009). The increased removal efficiency obtained with transgenic HRs contributed to give more evidence that reinforces the hypothesis that basic peroxidases would be the main isoenzymes involved in phenol removal process. In addition, an increase in removal efficiency of 2,4-DCP and a decrease in toxicity of treated solutions were obtained using double transgenic (tpx1 and tpx2) tobacco plants (Talano et al. 2012). Recently, the effects on the phospholipid turnover and phospholipase D activity after phenol treatment were also studied, using WT and double transgenic tobacco HRs. The results obtained suggest that the pollutant may induce changes of lipid kinase activities, involved in the synthesis of signaling phospholipids (Sosa Alderete et al. 2012).
Sonoki et al. (2012) carried out studies to enhance the remediation of bis-phenol A (BPA; 2,2-bis(4-hydroxyphenyl)propane), a widely distributed alkyl phenol. This compound is one of the major chemicals used in plastics and resins, and it is well known that it disrupts endocrine systems in humans and animals. Tobacco plants were genetically modified with fungal enzymes such as lignin peroxidase (LiP), laccase (Lac), and manganese peroxidase (MnP) that can degrade and polymerize BPA (phase I). An increase of BPA removal efficiency by fungal peroxidase expression in these plants was observed.
Another strategy has been to obtain plants with an enhanced ability to secrete detoxifying enzymes. In this context, Wang et al. (2004) overexpressed a secretory laccase to enhance ex planta phytoremediation of phenolics leading to more competitive plants. Recently, Chiaiesea et al. (2011) cloned the fungal laccase gene poxA1b that codifies for an enzyme involved in phenol metabolism and transformed tobacco plants and microalgae cells of Chlamydomonas, Ankistrodesmus, and Chlorella genera. Transgenic plants and microalgae were able to secrete the laccase and to remove high phenol concentrations from an olive oil mill wastewater. These authors suggest further studies to evaluate the application of a consortium of algae or a combination of plants and microalgae expressing fungal laccase for phenol removal.
On the other hand, the xenobiotic glycosylation ability of uridine diphosphate-glucose-dependent glucosyltransferase (UGTs) is known to function in phase II of plant detoxification pathway. In this sense, previous works have reported that PtUGT72B1 enzyme from Populus trichocarpa has high activity in detoxifying trichlorophenol by conjugation with glucose. Xu et al. (2013) analyzed the substrate specificity of PtUGT72B1 toward phenols and determined that this enzyme was able to catalyze the o-glucosylation of phenol, hydroquinone, and catechol when it was expressed in A. thaliana. Transgenic plants removed these compounds more efficiently than WT plants.
3.6 Hydrocarbons
Other important targets for plant-based decontamination are hydrocarbons that are mainly produced during fuel combustion. Many plants have been transformed with foreign genes aimed to remove different kinds of hydrocarbons. For instance, tobacco plants overexpressing fungal GSTs from Trichoderma virens showed enhanced tolerance to anthracene (Dixit et al. 2008, 2011). Two bacterial enzymes, haloalkane dehalogenase (DhlA) and haloacid dehalogenase (DhlB) from the bacterium Xanthobacter autotrophicus GJ10, with ability to dehalogenate a range of halogenated aliphatic hydrocarbons, including 1,2-dichloroethane (1,2-DCA), were also studied. Focused on these enzymes, Mena-Benitez et al. (2008) expressed dhlA and dhlB genes into N. tabacum plants and used 1,2-DCA as a model substrate to demonstrate the ability of transgenic tobacco to remediate a range of halogenated aliphatic hydrocarbons.
3.7 Textile Dyes
Dyes are recalcitrant, and thus they remain in the environment for a long period without being degraded. Although dye wastewaters are usually treated by physicochemical processes, these technologies are generally ineffective in color removal, expensive, and less adaptable to a wide range of dye wastewater (Vinayak et al. 2012). Few studies are reported of textile dye phytoremediation with conventional plants (Ghodake et al. 2009; Kagalkar et al. 2009; Khandare et al. 2011; Telke et al. 2011; Vinayak et al. 2012; Kabra et al. 2012) and even fewer using transgenic plants. Several microorganisms have been reported to be capable of decolorizing triphenylmethane dyes. Recent studies using A. thaliana transgenic plants showed that overexpression of a triphenylmethane reductase from Citrobacter sp. enhanced plant tolerance to crystal violet (CV) and malachite green by converting CV to nontoxic leucocrystal violet (Fu et al. 2013). This finding is an important contribution to this area of research and will surely lead to further studies in this topic.
As it was already described, the development of transgenic plants for organic compound remediation is mainly based on overexpression of genes codifying enzymes involved in xenobiotic transformation (phase I) or conjugation (phase II). To our knowledge, there are no genetic engineering studies that involve sequestration or compartmentalization (phase III). Moreover, genetic engineering of plants is important and necessary since they rarely mineralize hazardous organic compounds; thus, transgenic plants will be necessary to achieve this goal.
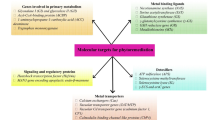
Figure 8.1 summarizes different strategies used to develop transgenic plants with enhanced abilities for inorganic (As, Cr, Cd, Hg) and organic phytoremediation.
Scheme with different strategies of transgenic plants with improved abilities for inorganic (As, Pb, Cd, Hg) and organic phytoremediation. Increased uptake of metal(loid)s in transgenic plants has been possible for Pb and Hg incorporation through overexpression of different cell membrane proteins. Regarding increased sequestration, it has been realized by increasing the expression of enzymes from PC synthesis pathway like ECS (gamma-glutamylcysteine synthase), GS (glutathione synthase), and PCS (phytochelatin synthase) or through the expression of genes coding for metallothioneins. Also, metal(loid) transport into vacuoles has been improved by overexpressing ABC transporter genes, cation/H+ exchanger, and proton pumps. In addition, an efficient chelation of metal(loid)s has been achieved by the expression of genes involved in polyphosphate synthesis or disulfide compounds. Increased volatilization in transgenic plants has been attained for As and Hg. Increasing translocation from roots to shoots has been less explored. For organic compound phytoremediation, the more deepened strategies have been those related with phase I (chemical modification), and there have been some examples involving phase II, such as the overexpression of glucosyltransferases. Phase III has not been explored for developing transgenic plants with improved organic phytoremediation
4 Limitations of Using Genetically Modified Plants to Clean Up the Environment
It is clear that biotechnology has opened new gateways in phytoremediation allowing plants to be genetically modified to enhance their pollutant remediation capabilities. Thus, plants with high biomass, rapid growth rate, and climatic adaptability can be genetically engineered to produce elite plants with enhanced remediation abilities (Czakó et al. 2006). It is noteworthy that although transgenic plants used in phytoremediation will not be intended as human and animal food, so food safety, allergenicity, and labeling are not relevant issues, they still have a number of drawbacks to be widely used (Davison 2005).
One of these limitations is related with the increased invasiveness of transgenic plants and decreased genetic variability of native plants due to interbreeding or cross-pollination (Davison 2005). To minimize the risk of interbreeding to WT relatives, it is better to choose transgenic plant species that have no compatible WT relatives. Other gene flow containment measures are using male sterility, planting away from WT relatives, and/or harvesting the plants before flowering (Pilon-Smits and Pilon 2002; Ruiz and Daniell 2009; Kotrba 2013). In addition, an alternative to contain transgenes is integrating them into the chloroplast genome instead of the nucleus, since plastid inheritance is almost entirely maternal and its transmission via pollen rarely occurs (Hails 2000; Davison 2005; Kotrba 2013). Another suitable technique is the use of several constructions that confer conditional lethality on transgenic plants. One of the constructions that have been proposed is based on poison/antidote idea and employs lethal ribonuclease barnase of Bacillus amyloliquefaciens as poison and protein barstar as antidote. The barnase gene is expressed from a sulfhydryl endopeptidase promoter, active at the time of seed pod development and preventing, consequently, seed germination. The “antidote” is the expression of barstar gene, which is placed under the control of a heat shock promoter. Seed development and germination is only possible when the barstar is produced due to the controlled heating of developing seeds to 40 °C. Such conditions are unlikely in the field, making the germination of progeny likely to fail there (Davison 2005; Kotrba 2013).
Another drawback regarding the use of transgenic plants is that most data on the performance of phytoremediating transgenic plants are based on observations made in controlled conditions, rather than in the field (Abhilash et al. 2009; Ruiz and Daniell 2009; Bhargava et al. 2012). One of the reasons for the discrepancy between the number of scientific papers based on laboratory test over those dealing with field conditions is the high cost for maintenance, monitoring of installations, and waste disposal (Maestri and Marmiroli 2011). In this sense, several methods of contaminated plant disposal after phytoremediation process have been researched, including ashing, incineration, and liquid extraction (Sas-Nowosielska et al. 2004). Currently, incineration is proposed as the most feasible, economically acceptable, and environmentally thorough disposal method (Rayu et al. 2012).
Public acceptance is another barrier to the use of genetically engineered plants for phytoremediation. In this sense, although the creation of the first transgenic organisms took place during the early 1970s, the debate about their risks continues today. The public receive scientific information through the massive media and depending on their financial and political influence; the media can manipulate the public, causing scientific controversies that are rarely about science (Farre et al. 2011). Media involvement can also affect government decisions and policy. The impact of phytoremediation with transgenics should be carefully evaluated and weighed against the risks of doing nothing and with the known disadvantages of traditional remediation techniques (Pilon-Smits and Pilon 2002; Bhargava et al. 2012; Kotrba 2013; Pathak et al. 2013).
It is important to note that after more than twenty years of research and after different transgenic plants with enhanced phytoremediation capabilities have been developed, none of them reached commercial existence (Maestri and Marmiroli 2011). This fact is related with time and money and with the strict regulations necessary to bring a genetically modified organism to market compared to a nongenetically modified one. The regulatory process is bureaucratic and unwarranted by science: despite rigorous investigation over more than a decade of the commercial use of genetically engineered plants, environmental or health risks have not been noticed (Potrykus 2010a). Meanwhile, a new plant created by traditional breeding methods, which also modify the genome, requires less or no safety data, only the demonstration that it performs at least as well as others (Potrykus 2010b). In some countries, like the United States, Canada, the United Kingdom, Germany, and Italy, among others, there are companies specialized in conducting air, soil, sediment, and groundwater phytoremediation protocols. Some of them are supported by more than 10 years of experience offering effective solutions to environmental pollution. Thus, although nowadays the use of transgenic plants is still associated to perceived risks for ecosystems, their application could be perceived more favorably in the future, allowing their large-scale application and leading to cleaning up the environment more efficiently.
5 Future Trends Using Genetically Engineered Plants
Most of the research till now has been focused with single or double traits/genes to introduce or enhance a phytoremediation capacity of a vegetal species. However, the expression of complete pathways for metabolism, including the uptake, translocation, and sequestration, needs to be developed (Dowling and Doty 2009; Seth 2012). Although the major problem encountered with plants transformed with multiple traits is gene silencing, plants for agricultural applications have been successfully developed (James and Strand 2009).
Since the majority of polluted sites contain complex mixtures of chemicals, including both inorganic and organic compounds, thus it is important to develop plants that can cope simultaneously with multiple contaminants. Even though only a small proportion of papers have dealt with both types of pollutants, they have obtained promising results (Maestri and Marmiroli 2011; Zhang and Liu 2011; Zhang et al. 2013).
Although phytotechnologies have greatly contributed to reduce and control environmental pollution, there are still many challenges to overcome. Thus, scientific community should make an effort to address the most important questions that limit the application of transgenic plants for phytoremediation.
References
Abhilash P, Jamil S, Singh N (2009) Transgenic plants for enhanced biodegradation and phytoremediation of organic xenobiotics. Biotechnol Adv 27:474–488. doi:10.1016/j.biotechadv.2009.04.002
Agostini E, Coniglio MS, Milrad SR, Tigier HA, Giulietti AM (2003) Phytoremediation of 2,4-dichlorophenol by Brassica napus hairy root cultures. Biotech Appl Biochem 37(2):139–144
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals – concepts and applications. Chemosphere 91:869–881, http://dx.doi.org/10.1016/j.chemosphere.2013.01.075
Anyasi RO, Atagana HI (2011) Biological remediation of polychlorinated biphenyls (PCB) in the environment by microorganisms and plants. African J Biotechnol 10(82):18916–18938. doi:10.5897/AJB10.557
Arazi T, Sunkar R, Kaplan B, Fromm H (1999) A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J 20:171–182. doi:10.1046/j.1365-313x.1999.00588.x
Bernier M, Carpentier R (1995) The action of mercury on the binding of extrinsic polypeptides associated with water oxidizing complex of photosystem II. FEBS Lett 360:251–254. doi:10.1016/0014-5793(95)00101-E
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manag 105:103–120. doi:10.1016/j.jenvman.2012.04.002
Bhuiyan MSU, Min SR, Jeong WJ, Sultana S, Choi KS, Lee Y, Liu JR (2011a) Overexpression of AtATM3 in Brassica juncea confers enhanced heavy metal tolerance and accumulation. Plant Cell Tiss Org 107:69–77. doi:10.1007/s11240-011-9958-y
Bhuiyan MSU, Min SR, Jeong WJ, Sultana S, Choi KS, Song WY, Lee Y, Lim YP, Liu JR (2011b) Overexpression of a yeast cadmium factor 1 (YCF1) enhances heavy metal tolerance and accumulation in Brassica juncea. Plant Cell Tiss Org 105:85–91. doi:10.1007/s11240-010-9845-y
Bizily SP, Kim T, Kandasamy MK, Meagher RB (2003) Subcellular targeting of methylmercury lyase enhances its specific activity for organic mercury detoxification in plants. Plant Physiol 131:463–471. doi:10.1104/pp. 010124
Burken JG (2003) Uptake and metabolism of organic compounds: green-liver models. In: McCutcheon SC, Schnoor JL (eds) Phytoremediation: transformation and control of contaminants. Wiley, New York
Che D, Meagher RB, Heaton AC, Lima A, Rugh CL, Merkle SA (2003) Expression of mercuric ion reductase in Eastern cottonwood (Populus deltoides) confers mercuric ion reduction and resistance. Plant Biotechnol J 1:311–319. doi:10.1046/j.1467-7652.2003.00031
Chen Z, Pan Y, Wang S, Ding Y, Yang W, Zhu C (2012) Overexpression of a protein disulfide isomerase-like protein from Methanothermobacter thermoautotrophicum enhances mercury tolerance in transgenic rice. Plant Sci 197:10–20. doi:10.1016/j.plantsci.2012.08.005
Chen Y, Xu W, Shen H, Yan H, Xu W, He Z, Ma M (2013) Engineering arsenic tolerance and hyperaccumulation in plants for phytoremediation by a PvACR3 transgenic approach. Environ Sci Technol 47:9355–9362. doi:10.1021/es4012096
Cherian S, Oliveira MM (2005) Transgenic plants in phytoremediation: recent advances and new possibilities. Environ Sci Technol 39(24):9377–9390. doi:10.1021/es051134l
Chiaiesea P, Palombaa F, Tatinoa F, Lanzilloa C (2011) Engineered tobacco and microalgae secreting the fungal laccase POXA1b reduce phenol content in olive oil mill wastewater. Enzyme Microb Technol 49:540–546. doi:10.6088/ijes.2012030131038
Chrastilová Z, Macková M, Nováková M, Szekeres M, Macek T (2008) Phytoremediation of polychlorinated biphenyls by transgenic tobacco. 4th bioremediation conference ID240. http://www.srcosmos.gr/srcosmos/showpub.aspx?aa=11512
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719. doi:10.1016/j.biochi.2006.07.003
Coleman JOD, Blake-Kalff MMA, Davies TGE (1997) Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci 2:144–151. doi:10.1016/S1360-1385(97)01019-4
Coniglio MS, Busto VD, González PS, Medina MI, Milrad S, Agostini E (2008) Application of Brassica napus hairy root cultures for phenol removal from aqueous solutions. Chemosphere 72:1035–1042. doi:10.1016/j.chemosphere.2008.04.00
Couselo JL, Navarro-Aviñó J, Ballester A (2010) Expression of the phytochelatin synthase TaPCS1 in transgenic aspen, insight into the problems and qualities in phytoremediation of Pb. Int J Phytoremediation 12(4):358–370. doi:10.1080/15226510902968134
Czakó M, Liang D, Márton L, Pollock R, Feng X, He Y (2006) Phytoremediation with transgenic plants. In: Fári MG, Holb I, Bisztray GD (ed) Proceedings of 5th IS on in vitro culture and Hort. Breeding Acta Hort
Davison J (2005) Risk mitigation of genetically modified bacteria and plants designed for bioremediation. J Ind Microbiol Biotechnol 32:639–650. doi:10.1007/s10295-005-0242-1
Dhankher OP, Li Y, Rosen BP, Shi J, SaltD SJF, Sashti NA, Meagher RB (2002) Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and gamma-glutamylcysteine synthetase expression. Nat Biotechnol 20(11):1140–1145. doi:10.1038/nbt747
Dixit P, Singh S, Mukherjee PK, Eapen S (2008) Development of transgenic plants with cytochrome P4502E1 gene and glutathione-S-transferase gene for degradation of organic pollutants. J Biotechnol 136:S692–S693. doi:10.1016/j.jbiotec.2008.07.1607
Dixit P, Mukherjee PK, Sherkhane PD, Kale SP, Eapen S (2011) Enhanced tolerance and remediation of anthracene by transgenic tobacco plants expressing a fungal glutathione transferase gene. J Hazard Mater 192:270–276. doi:10.1016/j.jhazmat.2011.05.018
Doty SL, Shang TQ, Wilson AM, Tangen J, Westergreen AD, Newman LA, Strand SE, Gordon MP (2000) Enhanced metabolism of halogenated hydrocarbons in transgenic plants containing mammalian cytochrome P450 2E1. Proc Natl Acad Sci U S A 97:6287–6291
Doty SL, James CA, Moore AL, Vajzovic A, Singleton GL, Ma C et al (2007) Enhanced phytoremediation of volatile environmental pollutants with transgenic trees strand. Proc Natl Acad Sci U S A 104:16816–16821, doi: 10.1073pnas.0703276104
Dowling DN, Doty SL (2009) Improving phytoremediation through biotechnology. Curr Opin Biotechnol 20:204–206. doi:10.1016/j.copbio.2009.03.007
Farre G, Twyman RM, Zhu C, Capell T, Christou P (2011) Nutritionally enhanced crops and food security: scientific achievements versus political expediency. Curr Opin Biotechnol 22:245–251. doi:10.1016/j.copbio.2010.11.002
Feng L, Kennedy IR (1997) Biodegradation and plant protection from the herbicide 2,4-D by plant-microbial associations in cotton production systems. Biotechnol Bioeng 54:513–519
Feng PCC, Ruff TG, Rangwala SH, Rao SR (1997) Engineering plant resistance to thiazopyr herbicide via expression of a novel esterase deactivation enzyme. Pestic Biochem Physiol 59:89–103
French CE, Rosser SJ, Davis GJ, Nicklin S, Bruce NC (1999) Biodegradation of explosives by transgenic plants expressing pentaerythritol tetranitrate reductase. Nat Biotechnol 17:49–494
Fu XY, Zhao W, Xiong AS, Tian YS, Zhu B, Peng RH, Yao QH (2013) Phytoremediation of triphenylmethane dyes by overexpressing a Citrobacter sp. triphenylmethane reductase in transgenic Arabidopsis. Appl Microbiol Biotechnol 97(4):1799–1806. doi:10.1007/s00253-012-4106-0
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46. doi:10.1016/j.envexpbot.2012.04.006
Gandia-Herrero F, Lorenz A, Larson T, Graham IA, Bowles J, Rylott EL, Bruce NC (2008) Detoxification of the explosive 2,4,6-trinitrotoluene in Arabidopsis: discovery of bifunctional O-and C-glucosyltransferases. Plant J 56(6):963–974. doi:10.1111/j.1365-313X.2008.03653.x
Ghodake G, Telke A, Jadhav J, Govindwar S (2009) Potential of Brassica juncea in order to treat textile effluent contaminated sites. Int J Phytoremediation 11:297–312. doi:10.1080/15226510802429518
González PS, Capozucca CE, Tigier HA, Milrad SR, Agostini E (2006) Phytoremediation of phenol from wastewater, by peroxidases of tomato hairy root cultures. Enzyme Microb Technol 39:647–653. doi:10.1016/j.enzmictec.2005.11.014
Gratao PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal stressed plants a little easier. Funct Plant Biol 32:481–494. doi:10.1071/FP05016
Grispen VMJ, Irtelli B, Hakvoort HWJ, Vooijs R, Bliek T, ten Bookum WM, Verkleij JAC, Schat H (2009) Expression of the Arabidopsis metallothionein 2b enhances arsenite sensitivity and root to shoot translocation in tobacco. Environ Exp Bot 66(1):69–73. doi:10.1016/j.envexpbot.2008.12.021
Guerinot ML, DE Salt. Fortified Foods and Phytoremediation. Two sides of the same coin (2001) Plant Physiol 125:164–167. doi: 10.1104/pp.125.1.164
Guo J, Dai X, Xu W, Ma M (2008) Overexpressing gsh1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere 72:1020–1026. doi:10.1016/j.chemosphere.2008.04.018
Guo J, Xua W, Ma M (2012) The assembly of metals chelation by thiols and vacuolar compartmentalization conferred increased tolerance to and accumulation of cadmium and arsenic in transgenic Arabidopsis thaliana. J Hazard Mater 199–200:309–313. doi:10.1016/j.jhazmat.2011.11.008
Gupta DK, Huang HG, Corpas FJ (2013) Lead tolerance in plants: strategies for phytoremediation. Environ Sci Pollut Res 20:2150–2161. doi:10.1007/s11356-013-1485-4
Hails RS (2000) Genetically modified plants – the debate continues. Tree 5:14–18
Han FX, Su Y, Monts DL, Waggoner AC, Plodinec JM (2006) Binding, distribution, and plant uptake of mercury in a soil from Oak Ridge, Tennessee, USA. Sci Total Environ 368:753–768. doi:10.1016/j.scitotenv.2006.02.026
Hannink NK, Rossser SJ, French CE, Basran A, Murray JAH, Nicklin S, Bruce NC (2001) Phytodetoxification of TNT by transgenic plants expressing a bacterial nitroreductase. Nat Biotechnol 19(12):1168–1172
Hannink NK, Subramanian M, Rosser SJ, Basran A, Murray JAH, Shanks JV, Bruce NC (2007) Enhanced transformation of TNT by tobacco plants expressing a bacterial nitroreductase. Int J Phytoremediation 9(5):385–401. doi:10.1080/15226510701603916
Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ (2000) Expression of Arabidopsis CAX2 in tobacco altered metal accumulation and increased manganese tolerance. Plant Physiol 124:125133. doi:10.1104/pp. 124.1.125
Hsieh JL, Chen CY, Chiu MH, Chein MF, Chang JS, Endo G, Huang CC (2009) Expressing a bacterial mercuric ion binding protein in plant for phytoremediation of heavy metals. J Hazard Mater 161:920–925. doi:10.1016/j.jhazmat.2008.04.079
Hu T, Qv X, Xiao G, Huang X (2009) Enhanced tolerance to herbicide of rice plants by over-expression of a glutathione S-transferase. Mol Breeding 24:409–418. doi:10.1007/s11032-009-9302-y
Hussain S, Siddique T, Arshad M, Saleem M (2009) Bioremediation and phytoremediation of pesticides: recent advances. Critic Rev Environ Sci Technol 39:843–907. doi:10.1080/10643380801910090
Hussein H, Ruiz ON, Terry N, Daniell H (2007) Phytoremediation of mercury and organomercurials in chloroplast transgenic plants: enhanced root uptake, translocation to shoots and volatilization. Environ Sci Technol 41:8439–8446. doi:10.1021/es070908q
Interstate Technology and Regulatory Cooperation (ITRC) (2001) Phytotechnology technical and regulatory guidance document. www.itrcweb.org/Documents/PHYTO-2.pdf. Accessed Jan 2001
Inui H, Ohkawa H (2005) Herbicide resistance in transgenic plants with mammalian P450 monooxygenase genes. Pest Manag Sci 61:286–291
Iurascu B, Siminiceanu I, Vione D, Vicente MA, Gil A (2009) Phenol degradation in water through a heterogeneous photo-Fenton process catalyzed by Fe-treated laponite. Water Res 43:1313–1322. doi: 10.1016/j.watres.2008.12.032
James CA, Strand S (2009) Phytoremediation of small organic contaminants using transgenic plants. Curr Opin Biotechnol 20:1–5. doi:10.1016/j.copbio.2009.02.014
James CA, Xin G, Doty SL, Strand SE (2008) Degradation of low molecular weight volatile organic compounds by plants genetically modified with mammalian cytochrome P450 2E1. Environ Sci Technol 42:289–293. doi:10.1021/es071197z CCC
Kabra AN, Khandare RV, Waghmode TR, Govindwar SP (2012) Phytoremediation of textile effluent and mixture of structurally different dyes by Glandularia pulchella (Sweet) Tronc. Chemosphere 87(3):265–272. doi:10.1016/j.chemosphere.2011
Kabata-Pendias A (2011) Trace elements in soils and plants. CRC, Boca Raton, FL. ISBN-10: 1420093681. ISBN-13: 978-1420093681
Kagalkar AN, Jagtap UB, Jadhav JP, Bapat VA, Govindwar SP (2009) Biotechnological strategies for phytoremediation of the sulfonated azo dye Direct Red 5B using Blumea malcolmii Hook. Bioresour Technol 100:4104–4110. doi:10.1016/j.biortech.2009.03.049
Karavangeli M, Labrou NE, Clonis YD, Tsaftaris A (2005) Development of transgenic tobacco plants overexpressing maize glutathione S-transferase I for chloroacetanilide herbicides phytoremediation. Biomol Eng 22:121–128
Kawahigashi H, Hirose S, Ohkawa H, Ohkawa Y (2005) Phytoremediation of metolachlor by transgenic rice plants expressing human CYP2B6. J Agric Food Chem 53:9155–9160
Kawahigashi H, Hirose S, Ohkawa H, Ohkawa Y (2006) Phytoremediation of the herbicides atrazine and metolachlor by transgenic rice plants expressing human CYP1A1, CYP2B6, and CYP2C19. J Agric Food Chem 54:2985–2991
Kawahigashi H, Hirose S, Ohkawa H, Ohkawa Y (2008) Transgenic rice plants expressing human P450 genes involved in xenobiotic metabolism for phytoremediation. J Mol Microbiol Biotechnol 15:212–219. doi:10.1159/000121332
Khandare RV, Kabra AN, Tamboli DP, Govindwar SP (2011) The role of Aster amellus Linn. in the degradation of a sulfonated azo dye remazol red: a phytoremediation strategy. Chemosphere 82:1147–1154
Khoudi H, Maatar Y, Gouiaa S, Masmoudi K (2012) Transgenic tobacco plants expressing ectopically wheat H+-pyrophosphatase (H+-PPase) gene TaVP1 show enhanced accumulation and tolerance to cadmium. J Plant Physiol 169:98–103. doi:10.1016/j.jplph.2011.07.016
Klein M, Burla B, Martinoia E (2006) The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. FEBS Lett 580:1112–1122. doi:10.1016/j.febslet.2005.11.056
Korenkov V, Hirschi K, CrutchWeld JD, Wagner GJ (2007) Enhancing tonoplast Cd/H antiport activity increases Cd, Zn, and Mn tolerance, and impacts root/shoot Cd partitioning in Nicotiana tabacum L. Planta 226:1379–1387. doi:10.1007/s00425-007-0577-0
Kotrba P (2013) Transgenic approaches to enhance phytoremediation of heavy metal-polluted soils. In: Gupta DK (ed) Plant-based remediation processes, soil biology. Springer, Berlin
Krystofova O, Zitka O, Krizkova S, Hynek D, Shestivska V, Adam V, Hubalek J, Mackova M, Macek T, Zehnalek J, Babula P, Havel L, Kizek R (2012) Accumulation of cadmium by transgenic tobacco plants (Nicotiana tabacum L.) carrying yeast metallothionein gene revealed by electrochemistry. Int J Electrochem Sci 7:886–907
LeBlanc MS, Lima A, Montello P, Kim T, Meagher RB, Merkle S (2011) Enhanced arsenic tolerance of transgenic eastern cottonwood plants expressing gamma-glutamylcysteine synthetase. Int J Phytoremediation 13(7):657–673. doi:10.1080/15226514.2010.499917
Lee S, Moon JS, Ko TS, Petros D, Goldsbrough PB, Korban SS (2003) Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol 131:656–663. doi:10.1104/pp. 014118
Legault EK (2013) A mass balance field study of the phytoremediation of trichloroethylene with transgenic poplars genetically modified with cytochrome P450 2E1. Thesis. University of Washington
Li Y, Dhankher OP, Carreira L, Lee D, Chen A, Schroeder JI, Balish RS, Meagher RB (2004) Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol 45(12):1787–1797. doi:10.1093/pcp/pci034
Li Y, Dhankher OP, Carreira L, Balish RS, Meagher RB (2005) Arsenic and mercury tolerance and cadmium sensitivity in Arabidopsis plants expressing bacterial gamma-glutamylcysteine synthetase. Environ Toxicol Chem 24(6):1376–1386. doi:10.1897/04-340R.1
Li Y, Heaton ACP, Carreira L, Meagher RB (2006) Enhanced tolerance to and accumulation of mercury, but not arsenic, in plants overexpressing two enzymes required for thiol peptide synthesis. Physiol Plantarum 128:48–57. doi:10.1111/j.1399-3054.2006.00732.x
Lunney AI, Zeeb BA, Reimer KJ (2004) Uptake of weathered DDT in vascular plants: potential for phytoremediation. Environ Sci Technol 38:6147–6154
Lyyra S, Meagher RB, Kim T, Heaton A, Montello P, Balish RS, Merkle SA (2007) Coupling two mercury resistance genes in Eastern cottonwood enhances the processing of organomercury. Plant Biotechnol J 5:254–262. doi:10.1111/j.1467-7652.2006.00236.x
Macek T, Kotrba P, Svatos A, Novakova M, Demnerova K, Mackova M (2008) Novel roles for genetically modified plants in environmental protection. Trends Biotechnol 26:146–152. doi:10.1016/j.tibtech.2007.11.009
Maestri E, Marmiroli N (2011) Transgenic plants for phytoremediation. Int J Phytoremediation 13:264–279. doi:10.1080/15226514.2011.568549
Marrs KA (1996) The functions and regulation of glutathione S-transferase in plants. Annu Rev Plant Physiol Plant Mol Biol 47:127–158. doi:10.1146/annurev.arplant.47.1.127
Martinoia E, Grill E, Tomasini R, Kreuz K, Amrehin N (1993) ATP-dependent glutathione S-conjugate ‘export’ pump in the vacuolar membrane of plants. Nature 364:247–249. doi:10.1038/364247a0
Martinoia E, Maeshima M, Neuhaus HE (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58:83–102. doi:10.1093/jxb/erl183
McBride MB, Simon T, Tam G, Wharton S (2012) Lead and arsenic uptake by leafy vegetables grown on contaminated soils: effects of mineral and organic amendments. Water Air Soil Pollut 224:1378. doi:10.1007/s11270-012-1378-z
Mena-Benitez GL, Gandia-Herrero F, Graham F, Larson TR, McQueen-Mason SJ, French CE, Rylott EL, Bruce NC (2008) Engineering a catabolic pathway in plants for the degradation of 1,2-dichloroethane. Plant Physiol 147:1192–1198. doi:10.1104/pp. 108.119008
Meng XY, Qin J, Wang LH, Duan GL, Sun GX, Wu HL, Chu CC, Ling HQ, Rosen BP, Zhu YG (2011) Arsenic biotransformation and volatilization in transgenic rice. New Phytol 191:49–56. doi:10.1111/j.1469-8137.2011.03743.x
Meyers B, Zaltsman A, Lacroix B, Kozlovsky SV, Krichevsky A (2010) Nuclear and plastid genetic engineering of plants: comparison of opportunities and challenges. Biotechnol Adv 28:747–756. doi:10.1016/j.biotechadv.2010.05.022
Mohammadi M, Chalavi V, Novakova-Sura M, Laliberté J, Sylvestre M (2007) Expression of bacterial biphenyl-chlorobiphenyl dioxygenase genes in tobacco plants. Biotechnol Bioeng 97(3):496–505. doi:10.1002/bit.21188
Mouhamad R, Ghanem I, AlOrfi M, Ibrahim K, Ali N, Al-Daoude A (2012) Phytoremediation of trichloroethylene and dichlorodiphenyltrichloroethane-polluted water using transgenic Sesbania grandiflora and Arabidopsis thaliana plants harboring rabbit cytochrome p450 2E1. Int J Phytoremediation 14(7):656–668. doi:10.1080/15226514.2011.619232
Nagata T, Ishikawa C, Kiyono M, Pan-Hou H (2006a) Accumulation of mercury in transgenic tobacco expressing bacterial polyphosphate. Biol Pharm Bull 29(12):2350–2353. doi:10.1248/bpb.29.2350
Nagata T, Kiyono M, Pan-Hou H (2006b) Engineering expression of bacterial polyphosphate kinase in tobacco for mercury remediation. Appl Microbiol Biotechnol 72:777–782. doi:10.1007/s00253-006-0336-3
Nagata T, Nakamura A, Akizawa T, Pan-Hou H (2009) Genetic engineering of transgenic tobacco for enhanced uptake and bioaccumulation of mercury. Biol Pharm Bull 32:1491–1495. doi:10.1248/bpb.32.1491
Nagata T, Morita H, Akizawa T, Pan-Hou H (2010) Development of a transgenic tobacco plant for phytoremediation of methylmercury pollution. Appl Microbiol Biotechnol 87:781–786. doi:10.1007/s00253-010-2572-9
Novakova M, Mackova M, Chrastilova Z, Viktorova J, Szekeres M, Demnerova K, Macek T (2009) Cloning the bacterial bphC gene into Nicotiana tabacum to improve the efficiency of PCB phytoremediation. Biotechnol Bioeng 102(1):29–37. doi:10.1002/bit.22038
Novakova M, Mackova M, Antosova Z, Viktorova J, Szekeres M, Demnerova K, Macek T (2010) Cloning the bacterial bphC gene into Nicotiana tabacum to improve the efficiency of phytoremediation of polychlorinated biphenyls. Bioeng Bugs 1(6):419–423. doi:10.4161/bbug.1.6.12723
Ohkawa H, Imaishi H, Shiota N (1999) In: Brooks JT, Roberts TR (eds) Pesticide chemistry and biosciences: the food-environment challenge. Cytochromes P450 and other xenobiotic metabolizing enzymes in plants. Royal Society of Chemistry, Cambridge, UK
Panz K, Miksch K (2012) Phytoremediation of explosives (TNT, RDX, HMX) by wild-type and transgenic plants. J Environ Manage 113:85–92. doi:10.1016/j.jenvman.2012.08.016
Pathak B, Khan R, Fulekar J, Fulekar MH (2013) Biotechnological strategies for enhancing phytoremediation. In: Gupta DK (ed) Biotechnology of crucifers. Springer: New York
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39. doi:10.1146/annurev.arplant.56.032604.144214
Pilon-Smits E, Pilon M (2002) Phytoremediation of metals using transgenic plants. Critical Rev Plant Sci 21:439–456. doi:10.1080/0735-260291044313
Potrykus I (2010a) Constraints to biotechnology introduction for poverty alleviation. N Biotechnol 27:447–448. doi:10.1016/j.nbt.2010.07.004
Potrykus I (2010b) Regulation must be revolutionized. Nature 466(7306):561
Rayu S, Karpouzas DG, Singh BK (2012) Emerging technologies in bioremediation: constraints and opportunities. Biodegradation 23:917–926. doi:10.1007/s10532-012-9576-3
Rigas F, Papadopoulou K, Philippoussis A, Papadopoulou M, Chatzipavlidis J (2009) Bioremediation of lindane contaminated soil by Pleurotus ostreatus in non sterile conditions using multilevel factorial design. Water Air Soil Pollut 197:121–129. doi:10.1007/s11270-008-9795-8
Rugh CC, Summers AO, Meagher RB (1996) Mercuric ion reductase and resistance in transgenic Arabidopsis thaliana expressing modified bacterial merA gene. Proc Natl Acad Sci U S A 93:3182–3187
Ruiz ON, Daniell H (2009) Genetic engineering to enhance mercury phytoremediation. Curr Opin Biotechnol 20:213–219. doi:10.1016/j.copbio.2009.02.010
Ruiz ON, Hussein HS, Terry N, Daniell H (2003) Phytoremediation of organomercurials via the chloroplast genetic engineering. Plant Physiol 132:1344–1352. doi:10.1104/pp. 103.020958
Ruiz ON, Alvarez D, Torres C, Roman L, Daniell H (2011) Metallothionein expression in chloroplasts enhances mercury accumulation and phytoremediation capability. Plant Biotechnol J 9(5):609–617. doi:10.1111/j.1467-7652.2011.00616.x
Rylott EL, Bruce NC (2009) Plants disarm soil: engineering plants for the phytoremediation of explosives. Trends Biotechnol 27:73–81. doi:10.1016/j.tibtech.2008.11.001
Rylott EL, Budarina MV, Barker A, Lorenz A, Strand SE, Bruce NC (2011) Engineering plants for the phytoremediation of RDX in the presence of the co-contaminating explosive TNT. New Phytol 192(2):405–413. doi:10.1111/j.1469-8137.2011.03807.x
Sas-Nowosielska A, Kucharski R, Malkowski E, Pogrzeba M, Kuperberg JM, Krynski K (2004) Phytoextraction crop disposal – an unsolved problem. Environ Pollut 128:373–379. doi:10.1016/j.envpol.2003.09.012
Seth CS (2012) A review on mechanisms of plant tolerance and role of transgenic plants in environmental clean-up. Bot Rev 78:32–62. doi:10.1007/s12229-011-9092-x
Shim D, Kim S, Choi YI, Song WY, Park J, Youk ES, Jeong SC, Martinoia E, Noh EW, Lee Y (2013) Transgenic poplar trees expressing yeast cadmium factor 1 exhibit the characteristics necessary for the phytoremediation of mine tailing soil. Chemosphere 90:1478–1486. doi:10.1016/j.chemosphere.2012.09.044
Shukla D, Kesari R, Mishra S, Dwivedi S, Tripathi RD, Nath P, Kumar Trivedi P (2012) Expression of phytochelatin synthase from aquatic macrophyte Ceratophyllum demersum L. enhances cadmium and arsenic accumulation in tobacco. Plant Cell Rep 31:1687–1699. doi:10.1007/s00299-012-1283-3
Singh S, Sherkhane PD, Kale SP, Eapen S (2011) Expression of a human cytochrome P4502E1 in Nicotiana tabacum enhances tolerance and remediation of γ-hexachlorocyclohexane. N Biotechnol 28(4):423–429. doi:10.1016/j.nbt.2011.03.01
Song WY, Sohn EJ, Martinoia E, Yong YY, Jasinski M, Forestier C, Hwang I, Lee Y (2003) Engineering tolerance and accumulation of lead and cadmium in transgenic plants. Nat Biotechnol 8:914–919. doi:10.1038/nbt850
Sonoki T, Iimura Y, Kajita S (2012) Phytoremediation of bis-phenol A via secretory fungal peroxidases produced by transgenic plants. In: Ozden Çiftçi Y (ed) Transgenic plants – advances and limitations, PhD. ISBN: 978-953-51-0181-9, In Tech. http://www.intechopen.com/books/transgenic-plants-advances
Sosa Alderete LG, Talano MA, Ibañez SG, Purro S, Agostini E, Medina MI (2009) Establishment of transgenic tobacco hairy roots expressing basic peroxidases and its application for phenol removal. J Biotechnol 139:273–279. doi:10.1016/j.jbiotec.2008.11.008
Sosa Alderete LG, Racagni G, Agostini E, Medina MI (2012) Phospholipid turnover and phospholipase D activity in tobacco hairy roots exposed to phenol. Environ Exp Bot 77:141–145. doi:10.1016/j.envexpbot.2011.11.006
Sudharshan S, Naidu R, Mallavarapu M, Bolan N (2012) DDT remediation in contaminated soils: a review of recent studies. Biodegradation 23:851–863. doi:10.1007/s10532-012-9575-4
Sylvestre M, Macek T, Mackova M (2009) Transgenic plants to improve rhizoremediation of polychlorinated biphenyls (PCBs). Curr Opin Biotechnol 20:242–247. doi:10.1016/j.copbio.2009.01.006
Talano MA, Frontera S, González PS, Medina MI, Agostini E (2010) Removal of 2,4-diclorophenol from aqueous solutions using tobacco hairy root cultures. J Hazard Mater 176:784–791. doi:10.1016/j.jhazmat.2009.11.103
Talano MA, Busso DC, Paisio CE, González PS, Purro SA, Medina MI, Agostini E (2012) Phytoremediation of 2,4-dichlorophenol using wild type and transgenic tobacco plants. Environ Sci Pollut Res 19:2202–2211. doi:10.1007/s11356-011-0724-9
Telke AA, Kagalkar AN, Jagtap UB, Desai NS, Bapat VA, Govindwar SP (2011) Biochemical characterization of laccase from hairy root culture of Brassica juncea L. and role of redox mediators to enhance its potential for the decolorization of textile dyes. Planta 234(6):1137–1149. doi:10.1007/s00425-011-1469-x
Van Aken B, Correa PA, Schnoor JL (2010) Phytoremediation of polychlorinated biphenyls: new trends and promises. Environ Sci Technol 44(8):2767–2776. doi:10.1021/es902514d
Van Dillewijn P, Couselo JL, Corredoira E, Delgado A, Wittich RM, Ballester A, Ramos JL (2008) Bioremediation of 2,4,6-trinitrotoluene by bacterial nitroreductase expressing transgenic aspen. Environ Sci Technol 42:7405–7410
Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, Murphy A, Rea PA, Samuels L, Schulz L, Schulz B, Spalding EJ, Yazaki K, Theodoulou FL (2008) Plant ABC proteins – a unified nomenclature and updated inventory. Trends Plant Sci 13:151–159. doi:10.1016/j.tplants.2008.02.001
Vinayak SA, Jyoti PJ, Vishwas AB (2012) Exploring the phytoremediation potential of cactus (nopalea cochenillifera salm. dyck.) cell cultures for textile dye degradation. Int J Phytoremediation 14: 554–569
Wang S, Mulligan CN (2006) Occurrence of arsenic contamination in Canada: sources, behavior and distribution. Sci Total Environ 366:701–721. doi:10.1016/j.scitotenv.2005.09.005
Wang GD, Li QJ, Luo B, Chen XY (2004) Ex planta phytoremediation of trichlorophenol and phenolic allelochemicals via engineered secretory laccase. Nat Biotechnol 22:893–897. doi:10.1038/nbt982
Wevar Oller AL, Agostini E, Talano MA, Capozucca C, Milrad SR, Tigier HA, Medina MI (2005) Over-expression of a basic peroxidase in transgenic tomato (Lycopersicon esculentum Mill. cv. Pera) hairy roots increases phytoremediation of phenol. Plant Sci 169:1102–1111. doi:10.1016/j.plantsci.2005.07.007
Wojas S, Hennig J, Plaza S, Geisler M, Siemianowski O, Sklodowska A, Ruszczynska A, Bulska E, Antosiewicz DM (2009) Ectopic expression of Arabidopsis ABC transporter MRP7 modifies cadmium root-to-shoot transport and accumulation. Environ Pollut 157:2781–2789. doi:10.1016/j.envpol.2009.04.024
Wojas S, Clemens S, Sklodowska A, Antosiewicz DA (2010) Arsenic response of AtPCS1- and CePCS-expressing plants – effects of external As(V) concentration on As-accumulation pattern and NPT metabolism. J Plant Physiol 167:169–175. doi:10.1016/j.jplph.2009.07.017
Wu Q, Shigaki T, Williams KA, Han JS, Kim CK, Hirschi KD et al (2011) Expression of an Arabidopsis Ca2+/H+ antiporter CAX1 variant in petunia enhances cadmium tolerance and accumulation. J Plant Physiol 168:167–173. doi:10.1016/j.jplph.2010.06.005
Xu Z-S, Lin Y-Q, Xu J, Zhu B, Zhao W, Peng RH, Yao QH (2013) Selective detoxification of phenols by Pichia pastoris and Arabidopsis thaliana heterologously expressing the PtUGT72B1 from Populus trichocarpa. PLoS One 8(6):e66878. doi:10.1371/journal.pone.0066878
Yang H, Nairn J, Ozias-Akins P (2003) Transformation of peanut using a modified bacterial mercuric ion reductase gene driven by an actin promoter from Arabidopsis thaliana. J Plant Physiol 160:945–952, doi 0176-1617/03/160/08-945
Zhang Y, Liu J (2011) Transgenic alfalfa plants co-expressing glutathione S-transferase (GST) and human CYP2E1 show enhanced resistance to mixed contaminates of heavy metals and organic pollutants. J Hazard Mater 189:357–362. doi:10.1016/j.jhazmat.2011.02.042
Zhang WH, Tyerman SD (1999) Inhibition of water channels by HgCl2 in intact wheat root cells. Plant Physiol 120:849–858. doi:10.1104/pp 120.3.849
Zhang D, Xiang T, Li P, Bao L (2011) Transgenic plants of Petunia hybrida harboring the CYP2E1 gene efficiently remove benzene and toluene pollutants and improve resistance to formaldehyde. Genet Mol Biol 34(4):634–639
Zhang Y, Liu J, Zhou Y, Gong T, Wang J, Ge Y (2013) Enhanced phytoremediation of mixed heavy metal (mercury)-organic pollutants (trichloroethylene) with transgenic alfalfa co-expressing glutathione S-transferase and human P450 2E1. J Hazard Mater 260:1100–1107. doi:10.1016/j.jhazmat.2013.06.065
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ibañez, S.G. et al. (2015). Overview and New Insights of Genetically Engineered Plants for Improving Phytoremediation. In: Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L. (eds) Phytoremediation. Springer, Cham. https://doi.org/10.1007/978-3-319-10395-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-10395-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10394-5
Online ISBN: 978-3-319-10395-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)