Abstract
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants, and some are known to be carcinogenic. PAH content of soils from three test sites including a refinery, a dismantled oil depot and a petrol filling station was analysed. Except dibenz(a,h)anthracene, 15 other PAHs were detected. Total PAH concentrations were in the order refinery > petrol filling station > oil depot. PAH levels were higher in the upper layer of soils at all sites. Three-ring and four-ring PAHs were found to be dominant at both depths. In greenhouse study, Cymbopogon jwarancusa and Helianthus annuus were screened for their ability to phytoremediate PAHs. Soil amendments to enhance their potential were also evaluated. Many C. jwarancusa treatments succumbed. In all vegetated treatments, the decline in TPAH levels was not only higher but also more rapid than the unplanted control. Total PAH degradation ranged from 95 to 99 % in C. jwarancusa at 240 DAT (days after treatment) and 75–84 % in H. annuus at 120 DAT. The final reduction of total PAHs in the unplanted control T0 was about 73 %. Rhizodegradation seemed to be the main mechanism of phytoremediation involved. Individual PAH degradation trends differed as did the efficacy of different amendments. Physiological parameters stabilised within 30–60 days of study.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Phytoremediation
- Rhizodegradation
- PAHs
- Benzo[a]pyrene
- Soil contamination
- Carcinogenicity
- Sunflower
- Lemongrass
1 Introduction
Accumulation and persistence of toxic materials in the environment is a major concern. Anthropogenic activities have been steadily altering the natural biogeochemical cycles of the environment ever since the industrial revolution. Every compartment of the biosphere, today, is reeling under the ill effects of the rapid pace of industrialisation, urbanisation and intensive agricultural activities. Soil is one of the natural resources being over-exploited globally due to increased industrial, agricultural and other human activities. Soil contamination, both diffuse and localised, can lead to severe damage of soil functions as well as contamination of surface and groundwater. Urban soils are increasingly acting as a sink for a wide range of contaminants due to the rapid pace of development. Various organics are generated as by-products from various industries (petroleum, pulp and paper, chemical industries etc.), which may be released into the environment due to negligence or accidents. Toxic aromatics and their chlorinated derivatives which are difficult to biodegrade are of primary concern. These are generated from petroleum and petrochemicals, e.g. polycyclic aromatic hydrocarbons, polychlorinated biphenyls (PCBs), pesticides and herbicides, etc. Fossil fuels are still the major energy source for most industries. Metals, radionuclides and other inorganic contaminants are also very prevalent.
1.1 Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs), which occur naturally in crude oil and are a part of its aromatic fraction, are mutagenic and carcinogenic contaminants that are widely present in air, water, soils and sediments. PAHs are a large group of organic compounds with two or more fused aromatic rings. There are more than 100 different PAHs which are introduced into the environment mainly via natural and anthropogenic sources. The contribution from natural sources of PAHs is limited, being restricted to spontaneous forest and prairie fires and volcanic emissions (Bourotte et al. 2005). Anthropogenic PAHs are mainly formed as a result of pyrolytic processes such as petroleum refining, chemical manufacturing, air blowing for asphalt, emissions from coal, oil, gas and wood refuse incineration for cooking and heating, biomass burning, power generation and vehicle emissions (Nadal et al. 2004; Durand et al. 2004).
Once they enter the soil, they accumulate in horizons rich in organic matter where they are likely to be retained for many years due to their persistence and hydrophobicity. Sediments and soils are therefore considered the main sinks for PAHs in the environment, and PAHs with four or more aromatic rings are persistent environmental pollutants (Chen et al. 2004). Comparatively the more toxic components of petroleum, PAHs have been placed on the United States Environmental Protection Agency (USEPA) priority pollutant list. The structures of the 16 PAHs currently on the USEPA priority pollutant list are shown in Fig. 15.1. Selected physicochemical properties are given in Table 15.1.
Colourless, white or pale yellow-green solids, PAHs are planar, relatively inert and volatile in nature. They are hydrophobic compounds and their persistence in the environment is mainly due to lower water solubility and electrochemical stability. Their low solubilities in water are expected from their nonpolar character. These decrease dramatically in going from the two- and three-ring compounds to five-ring B(a)P (Mackay and Shiu 1992). Evidence suggests that the lipophilicity, environmental persistence and genotoxicity of PAHs increase as the molecular size of the PAHs increases up to four or five fused benzene rings.
Most of the 16 USEPA priority PAHs included in the present investigation have no known use except as research chemicals. Some, however, find use in myriad ways like naphthalene in ‘moth balls’; anthracene in synthetic fibre and dye production, scintillation counter crystals and organic semiconductor research; as the chemotherapeutic agent amsacrine; acenaphthene in the manufacture of dyes, pharmaceuticals and plastics; and as an insecticide and fungicide. Fluorene is used as a chemical intermediate in many chemical processes, in the formation of polyradicals for resins and in the manufacture of dyestuffs. Phenanthrene is used in the manufacture of dyestuffs and explosives and in biological research (Holloway et al. 1987; Wadler et al. 1986; Windholz 1983).
More than 200 compounds have tested positive as possible carcinogens. Among these, 25 % have been found tumorigenic, and about 30 % of these were PAHs. Lamb and Kaplan (1980) reported that BaP is a definite carcinogen with an LD50 of 24 μg. PAHs being highly lipid soluble are absorbed by the lungs and gut of mammals. At sufficient dose levels, laboratory studies show that some PAHs cause adverse health effects including cancer and reproductive difficulties in animals. These are mutagenic and carcinogenic environmental contaminants that are widely present in the air, water and aquatic system, soils and sediments (Zhang et al. 2004). The ubiquitous nature of these ‘chemicals of potential environmental concern’ is evident from the fact that these 16 priority PAHs pollutants are found in urban airsheds throughout the world (USEPA 1988). It is believed that there is no threshold or safe level for mutagenic compounds; hence, exposure to these PAHs at any level provides the risk of toxic effects.
The United States Environment Protection Agency has classified B[a]P as a probable human carcinogen in Group B2 (USEPA 2002). A value of 7.3 mg−1 kg−1 day−1 has been defined as the oral cancer slope factor for B[a]P (USEPA 1999). Besides B[a]P, only six other PAHs have been considered to be carcinogenic. All these—benzo[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, chrysene, dibenz[a,h]anthracene and indeno[1,2,3-c,d]pyrene—have also been included in Group B2 (USEPA 2002). In turn, apart from B[a]P, the International Agency for Research on Cancer (IARC 1987) considers benzo[a]anthracene and dibenz(a,h)anthracene as probable carcinogenic agents (Group 2A), while the remaining are only classified as possible carcinogens (Group 2B) or even not classifiable (Group 3).
2 Case Study
The levels of toxic contaminants in urban soils have been monitored and well documented in developed countries of North America and Europe, but there is an acute paucity of data on soil pollution due to petroleum products and its remediation in developing countries. Phytoremediation is especially suited for countries where such labour, expertise and cost-saving techniques are vital due to constraints of funds for other methods. The mechanisms and synergies of interactions between plants, microbes, soils, contaminants and the environment must be better understood in order to predict the outcome of in situ phytoremediation applications. Variations in climatic and edaphic conditions must also be taken into consideration. It therefore appears logical that studies are taken up to generate feasibility data about such remediation methods in the Indian context especially with regard to sites that appear to be at risk of contamination by petroleum products.
Agra (27°10′N, 78°05′E, 169 m.s.l.) is located in Uttar Pradesh in the north central part of India. It is roughly 200 km southeast of the national capital, New Delhi. Bounded by the Thar desert of Rajasthan on its southeast, west and northwest peripheries, it is a semiarid area (Fig. 15.2).
The world-renowned Mughal monument, the Taj Mahal is situated in Agra, and therefore the area is recognised as a sensitive zone, and a trapezoid area of about 10,400 sq. km. around the Taj, known as Taj Trapezium Zone (TTZ), has been identified by the government where new industries are not permitted and the relevance of existing industries has also been considered for further reducing the total pollution load (CPCB report: CUPS/7/1981-82). The study area is a part of Indo-Gangetic alluvium of Quaternary age and is made up of recent unconsolidated fluviatile formations comprising sand, silt, clay and kankar with occasional beds of gravel.
2.1 Experimental Design
Three test sites potentially polluted with PAHs were selected in and around Agra. The Mathura Refinery was designated site I as a petroleum refining site. It lies within the sensitive Taj Trapezium Zone (TTZ) along the National Highway-2, about 50 km away from Agra. The present refining capacity of this refinery is 8.00 million tonnes per annum. In 2005, it became the first refinery in India to produce auto fuels that adhere to 100 % of the Euro-3 rules. Site II is the erstwhile Idgah oil depot [IOCL] dismantled less than a decade ago and happens to be in the middle of a densely populated residential colony. This represented a petroleum storage/transportation site. An interstate bus station is just 600 m away from the site. Site III is a petrol filling station in the heart of the city that faces heavy traffic including heavy diesel vehicles (at night, when these are allowed within city limits). It lies on the road connecting three national highways. Thus, it represented the pollution profile due to petroleum fuel consumption. At each site, soil samples were collected from two depths, i.e. 0–10 and 10–20 cm (depths A and B, respectively) using a core sampler. Five random cores were collected from each site to obtain five samples for each depth. Two-way analysis of variance (ANOVA) was utilised to assess statistical significance between mean PAH contents at sites.
2.2 PAHs in Soils
It has been estimated that soils contain the vast majority (>90 %) of the total environmental burden of polycyclic aromatic hydrocarbons (Wild and Jones 1995; Ribes et al. 2002), and the atmosphere is their main transport vector (Drooge et al. 2002). PAHs with four or more rings persist for a long time in the environment in general and in soils and sediments in particular. The PAH concentrations in the soil samples are presented in Table 15.2. The fact that the topsoil from site I had the highest total PAH concentration followed by sites III and II can be easily noted. This was expected since site I is in the vicinity of the Mathura Refinery, hence, faces emissions from petroleum refining processes. Besides, it is along the busy National Highway-2, an important freight corridor connecting New Delhi to Kolkata, and also faces traffic especially heavy vehicles that run on diesel. It is also important to note that the PAH levels were not very different from those at site III which is a petrol/diesel station in a busy locality with high traffic density and experiences considerable quantities of traffic emissions including those from diesel vehicles. Site II registered lower levels as it has not been in use for nearly a decade since the oil depot here was dismantled and shifted elsewhere. Also, being located within a densely populated residential area, it does not face much traffic of the heavier kind unlike sites I and III. The results obtained indicate that PAH concentrations are linked to land use.
Zhang et al. (2006) analysed 16 EPA priority polycyclic aromatic hydrocarbons (PAHs) in surface soil (0–10 cm) samples collected from rural and urban areas of Hong Kong. Total PAH concentrations were in the range of 7–410 μg kg−1 (dry wt.), with higher concentrations in urban soils compared to rural soils. The three predominant PAHs were fluoranthene, naphthalene and pyrene in rural soils, while fluoranthene, naphthalene and benzo(b+k)fluoranthene dominated the PAHs of urban soils. The workers concluded that though light-duty diesel vehicles represent only 6.5 % of the total vehicular fleet of the metropolitan region of Sao Paulo, they are a significant source of PAHs. Zohair et al. (2006) reported that PAH burden was dominated by the low molecular weight compounds acenaphthene/fluorene ranging from 1.6 ± 0.3 to 9.84 ± 2.9 μg kg−1 and phenanthrene in the range 2.07 ± 0.3 to 8.28 ± 1.3 μg kg−1. These compounds are more water soluble and more volatile than the relatively high molecular weight PAHs.
Out of the 16 USEPA priority PAHs, only dibenz(a,h)anthracene was not detected in the soils from selected sites. On computing two-way analysis of variance (ANOVA), it was observed that for Nap, Acy, Flu, Fla and Chy, the difference in the mean values among the different sites was greater than would be expected by chance after allowing for effects of differences in depth and vice versa. In other words, there was a statistically significant difference in mean concentrations among the sites and depths, independently. The effect of different sites depended on the depth from which the soil sample was collected for Ace, Anth, Pyr, BbF and IP + BghiP. In other words, there was a statistically significant interaction between sites and depth of soil samples. In the case of BaA, BkF and BaP, there was a statistically significant difference in mean concentrations among the sites independent of depth. The difference among depths was insignificant. In the case of Phen, the difference was not significant statistically. The same was the case for depths.
The average TPAH (total PAH) concentrations measured in soils at the two designated depths (A and B) at the studied sites are presented in Fig. 15.3.
Sites I and III showed higher PAH levels at both depths studied. Depth-based variation in the concentrations of individual PAHs in soil at the sites is evident from Fig. 15.4. It is also clear that much variation exists in individual PAH concentrations. Control values showed negligible depth-based variations, and as such, mean values have been used.
The trends of PAH concentrations were different for the two depths; PAH levels were higher in upper layer of soils at all sites. At site I, the top three PAHs were chrysene > indeno(1,2,3-cd)pyrene + benzo(g,h,i)perylene > fluoranthene at depth A and fluoranthene > chrysene > indeno(1,2,3-cd)pyrene +benzo(g,h,i)perylene at depth B. At site II, the trend was acenaphthene > fluorene > chrysene depth A and chrysene > fluorene > fluoranthene for depth B, whereas at site III, it was benzo(b)fluoranthene > chrysene > fluoranthene for depth A and indeno(1,2,3-cd)pyrene + benzo(g,h,i)perylene > chrysene> anthracene for depth B. Chrysene and fluoranthene were thus the predominant compounds. Zhang et al. (2006a) also reported that fluoranthene was among the three dominant PAHs in soils from both rural and urban areas in Hong Kong, although higher concentrations can also be attributed to a host of sources other than direct contamination due to petroleum spill/leakage, e.g. industrial-oil burning, wood combustion and emissions from diesel-powered vehicles (Ravindra et al. 2001).
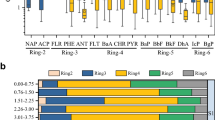
Figure 15.5 shows the relative contribution of 2-, 3-, 4-, 5- and 6-ring PAHs in the upper and lower layers (designated depths A and B) of soils of different locations investigated in this study. The distribution patterns do not show much variation with control except for site II. If the percentage of PAHs based on the number of benzene rings is considered, 3-ring and 4-ring PAHs were found to be dominant at both depths in soils at all sites.
The concentration of PAHs in the environment varies widely depending on the level of industrial development and contamination due to petroleum products. PAH concentrations range from a low of 5 μg g−1 soil in an undeveloped area to 1.79 × 106 μg g−1 at an oil refinery (Cerniglia 1992). Nam et al. (2003) determined the content and type of polycyclic aromatic hydrocarbons (PAHs) in soils from paddy fields and upland areas in South Korea. The overall distribution of PAH was found to be closely related to the pollution sources, the size of city and the type of industry. The PAH content ranged from 23.3 to 2,384 μg kg−1 with an average of 236 μg kg−1. The highest concentrations were found in soils near iron processing plants. The concentration of PAH decreased in the order fluoranthene > benzo(b)fluoranthene > pyrene. Agarwal et al. (2006) studied the distribution, concentration trends and possible sources of PAHs in bank sediment of river Yamuna in Delhi, India. The levels of 16 priority polycyclic aromatic hydrocarbons (PAHs) were analysed during pre-monsoon, monsoon and post-monsoon seasons in the sediment fraction <53 μm. The sum of 16 PAH compounds ranged from 4.50 to 23.53 μg g−1 with a mean concentration of 10.15 ± 4.32 μg g−1 (dry wt.). From the predominance of 2–4-ring PAHs, the authors have suggested relatively recent local sources of PAHs in the study area. Source apportionment based on molecular indices also illustrates pyrogenic source fingerprint of PAHs.
Table 15.3 shows a comparison of worldwide PAH concentrations with those observed in the present study. The total PAH concentration in upper soil near the refinery along National Highway-2, i.e. site I (12.33 μg g−1), is much less than the concentrations found in Belgium (300 μg g−1), Novi Sad (47.87 μg g−1) and Nigeria (45.9 μg g−1). This is commendable. The Mathura Refinery is the first in Asia and the third in the world to receive the coveted ISO-14001 certification for Environment Management System in 1996. A full-fledged environment protection (EP) cell having qualified engineers is at place to deal with all environmental issues. A well-equipped pollution control lab has also been established to monitor environmental performance on day-to-day basis.
The lab is approved by the Ministry of Environment & Forests (MoEF) and accredited by the National Accreditation Board for Testing and Calibration Laboratories (NABL). The picture of PAH contamination was similar at site III, a petrol/diesel station. The values at sites I and III are also similar to those at industrial and roadside sites (13.7 and 12.9 μg g−1, respectively) reported from Agra itself by Masih and Taneja (2006). In the case of site II, a dismantled oil depot, the total PAH concentration (6.77 μg g−1 in upper soil) was about half that of sites I and III. This was far below that of refinery locations as seen above. However, this site lies within a densely populated residential zone, and in this context, the levels are higher than several residential localities worldwide as seen in Table 15.3. It is to be noted that the level of contamination was lower than that reported from another residential area (9.37 μg g−1) in the same city (Masih and Taneja 2006).
2.3 Toxicity Analysis of PAHs
The environmental occurrence of PAHs has been associated with adverse effects on public health (Fang et al. 2004). A significant health concern resulting from exposure to PAHs is their potential for carcinogenicity, which is chemical-structure dependent. Four- to six-ring PAHs have been shown to be carcinogenic in laboratory animals (DHHS 1995). As mentioned earlier, International Agency for Research on Cancer has classified PAHs according to carcinogenicity (IARC 1987). These PAHs [benzo(a)pyrene, benzo(a)anthracene, dibenz(a,h)anthracene, benzo(b)fluoranthene, benzo(k)fluoranthene and indeno(1,2,3-cd)pyrene] are of special concern to human health. BaP is a five-ring (C20H12) compound, which is mutagenic for human cells in culture (Osborne et al. 1987) and carcinogenic in whole animal assays (Cerna et al. 2000). It is to be noted that five-ring compounds including benzo(a)pyrene contribute 10–20 % of the TPAH at depth A and 11–14 % at depth B. Percentage carcinogenic content of PAHs (based on IARC 1987) in soil was ~32 % at both depths at site I and 33 % and 33 % at depth A and B, respectively at site III. The proportion was lower at site II (24 % and 28 % at depth A and B, respectively) and control site (~28 %). Percentage carcinogenic content of total PAHs in soil was ~32 % at both depths at site I and 33 % and 33 % at depth A and B, respectively at site III. The proportion was lower at site II (24 % and 28 % at depth A and B, respectively) and control site (~28 %) (Fig. 15.5).
In light of the fact that several PAHs are known human carcinogens, the carcinogenic potencies of PAHs in soils of the three test sites were also determined. In principle, the carcinogenic potency of a given PAH compound is assessed on the basis of its BaPeq (BaP equivalent) concentration. The calculation of BaPeq concentration for a given compound is determined by its toxic equivalency factor (TEF), which represents the relative carcinogenic potency of the given PAH compound, using BaP as a reference compound to adjust its original concentration. The TEF for BaP is one, which is the highest among PAHs. The Nisbet and LaGoy scale was adopted for the present study, and the BaPeq of each PAH was determined by multiplying the concentration of each PAH with its toxic equivalency factor (TEF). In order to calculate the carcinogenic potencies associated with the total PAH exposures from soil, the sum of each BaPeq, i.e. Σ BaPeq, was used as a surrogate indicator. The above method has the main advantage of being relatively easy to apply in the environments affected by human sources; however, it may underestimate risks since limited PAHs are considered (WHO/IPCS 1998). Listed in Table 15.4 are the TEFs for individual PAHs suggested by Nisbet and LaGoy (1992) along with the TEF-adjusted concentrations of PAHs in soils. All these values are below 1 μg g−1 indicating that at the present levels, soil PAH exposure in Agra is not carcinogenic. It should be noted that the above inference is based on the exposure of soil to each individual PAH compound not for TPAHs.
3 Remediation
3.1 Conventional Techniques
Conventional remediation methods employed to clean contaminated soils include natural attenuation, engineering techniques and bioremediation. Each of these technologies has specific benefits and limitations (EPA 1997). Natural attenuation relies entirely on natural processes with no human intervention. These may be physical, chemical and biological in nature such as dilution, dispersion and adsorption of the contaminants (USEPA 1996a) as well as unassisted growth of plants and microbial communities that break down contaminants. However, only a fraction of sites offer such naturally occurring conditions where contaminants are degraded quickly enough to prevent their spreading (Committee on In Situ Bioremediation 1993). Engineering techniques are primarily physical, chemical and mechanical in nature and may involve ex situ and in situ processes. Immobilisation and extraction by physicochemical methods can be expensive; in effect, limiting their suitability for areas where rapid complete decontamination is the prerequisite (Bio-Wise 2000). The most important concern is the prohibitive costs of these techniques. Bioremediation employs a number of bacteria, protozoa and fungi to degrade contaminants into less toxic or nontoxic compounds (USEPA 1996b). The involvement of bacteria, e.g. Pseudomonas, Arthrobacter, Alcaligenes and Corynebacterium, and soil fungi, e.g. Aspergillus ochraceus, Cunninghamella elegans and Phanerochaete chrysosporium, has been documented in the degradation of petroleum hydrocarbons (Bossert and Bartha 1984; Sutherland 1992). It can be applied both ex situ and in situ. Bioremediation does not involve the use of plants and generally employs more invasive engineering techniques than phytoremediation.
3.2 Phytoremediation
The global emphasis at present is to use natural materials and methods as far as possible to combat a variety of problems. The same has been extended to soil remediation programmes. It is known that plants can cause profound alteration of surrounding soil by simple life processes like water and mineral uptake, decay, etc. Phytoremediation may be defined as ‘the engineered use of green plants to remove, contain or render harmless environmental contaminants like heavy metals, trace elements, organic compounds and radioactive compounds in soil or water’ (Hinchman et al. 1998). It has been derived from other fields such as agronomy, forestry, chemical and agricultural engineering, microbiology, etc. Since its inception, it has developed into an independent field of study and a widely applicable technology (Tsao 2003).
Phytoremediation has many benefits. It leaves the topsoil in usable condition, and it is aesthetically pleasing. It requires minimal equipment and less energy inputs as plants do most of the work using solar energy. Thus, it is an eco-friendly process. The plants used can later be harvested, processed and disposed off in an environmentally sound manner. It is also potentially cheaper—approximately 90 %—than conventional treatments (Hinchman et al. 1998), which are primarily chemical based and pursue an energy-intensive approach. This approach is hence emerging as an innovative tool with a great potential to decontaminate soil and water and thus achieving a sustainable development status (Desouza et al. 1999). Up to 25 % of the remediation agenda of the United States Environmental Protection Agency (USEPA) included phytoremediation of metals and radionuclides as a thrust area during 2000.
3.3 Mechanisms of Phytoremediation
There are three primary mechanisms by which plants and microorganisms remediate contaminated soil and groundwater. These mechanisms include degradation, containment as well as transfer of contaminants from soil to the atmosphere. In the case of oil contamination, the mechanism primarily involved is rhizosphere degradation (Merkl et al. 2004).
In phytodegradation, plants and microbes aid, directly or indirectly, in the degradation of organic contaminants into products like alcohols, acids, carbon dioxide and water. These are generally less toxic and less persistent in the environment than the parent compounds (Eweis et al. 1998). Though plants and microorganisms can degrade petroleum hydrocarbons independently of one another, it is the interaction between plants and microorganisms, i.e. the rhizosphere effect, which is the primary mechanism responsible for petrochemical degradation in phytoremediation. Soil redox conditions, organic content, moisture and other soil properties are manipulated by the activity of plant roots. The rhizosphere is the region of soil closest to the roots of the plants and hence under direct influence of the root system. The remediation process wherein contaminants are degraded/transformed by microbes in the rhizosphere is referred to as rhizodegradation. Critical components of the rhizosphere, in addition to a variety of free-living microorganisms, include root exudates that provide carbohydrates to ‘feed’ the microorganisms, organic acids that make the ions of nutrients and contaminants more mobile in the soil and enzymes that have important natural functions including degradation of organic contaminants with nitro groups or halogens (Hinchman et al. 1998). Root exudates of sugars, alcohols and acids can amount to 10–20 % of plant photosynthesis annually (Schnoor et al. 1995), and due to these exudates, microbial population and activities are 5–100 times greater in the rhizosphere than in bulk soil, i.e. soil not in contact with plant roots (Gunther et al. 1996). This plant-induced enhancement of microbial population is termed as the rhizosphere effect (Atlas and Bartha 1998) and is believed to enhance degradation of organic contaminants in the rhizosphere.
Containment involves using plants to reduce or eliminate the bioavailability of both organic and inorganic contaminants to other biota. It includes (1) accumulation of contaminants within the plants, known as phytoextraction, (2) adsorption of the contaminants on the root surface and (3) binding of contaminants with soil organic matter or humus by plant enzymes. These two processes are referred to as phytostabilisation. When plant roots accumulate or adsorb contaminants from the waste water, this is known as rhizofiltration. Contaminants are not necessarily degraded when they are contained. Various studies have documented that plants accumulate petroleum hydrocarbons to a small degree in their roots and shoots as well as adsorb these compounds onto the surface of roots (Edwards 1988; Durmishidze 1977; Ferro et al. 1997; Duxbury et al. 1997; Wild and Jones 1992; Anderson et al. 1993). Plants may also transfer volatile petroleum constituents from the soil to the atmosphere (Wiltse et al. 1998; Watkins et al. 1994; Kroening et al. 2001). This process is known as phytovolatilisation.
4 Greenhouse Study
In order to evaluate the extent to which plants can be used as phytoremediators either singly or with supplementation using different amendments, studies were also conducted to determine the ability of two plants Cymbopogon jwarancusa (lemongrass) and Helianthus annuus (sunflower) to degrade/contain PAHs in soil. The former is an aromatic oil-yielding grass with enormous potential for resurrection. The latter is an oil-yielding plant previously documented in phytoremediation studies.
4.1 Experimental Design
To assess the potential of selected species to phytoremediate PAHs from soil, pots were prepared with garden soil. Each pot could hold 4 kg of soil and was prepared with a layer of crocks and gravel about 1.5 in. deep at the bottom. The soil had a pH of 8 and electrical conductivity of 0.54 dS m−1 with an organic carbon content of 0.64 %. The bulk density was 1.45 g cm−3. Available nitrogen, phosphate and potash were 112.9, 17.5 and 393 kg ha−1, respectively.
In order to compare the raw potential of all species, the treatment designated T1 did not have any enhancing amendments. Uniform seedlings of each species were transplanted from nursery bed to pots, and the enhancement treatments were applied. The sets were allowed to mature for 3 weeks after which diesel were added to each pot near the stem at 10 % w/w. Each treatment was set up with nine replicates, three to be taken apart at each test date. Unplanted, unamended pots were set up as controls to compare degradation of diesel components without plants. All pots were kept in a random block design and watered as and when required in such a way as to prevent loss of contaminants by leaching. Any leachates obtained were added to the pots at next watering. The PAH content was measured in five random pots at day 1, and the average was taken as initial PAH concentration. Subsequently, three replicates of each treatment were taken apart at 60, 120 and 240 days after treatment to obtain a successive picture of PAH degradation. At these days, PAH content of soil, root and shoot as well as physiological parameters were measured. A composite sample was prepared from the three replicates for PAH analysis.
Uncontaminated, unamended plants were maintained as controls to compare chlorophyll and proline content at the testing dates. In addition to the regular testing dates, these parameters were also measured at 10, 20 and 30 DAT to monitor stress symptoms, if any, at the initial stages of the study. Growth parameters, i.e. shoot and root length and biomass (dry), were measured at regular testing dates. Since H. annuus is a seasonal, the final testing date for all parameters was 120 DAT for this plant. The various treatment pots were set up as follows:
T0 | Unvegetated pot + 10 % diesel w/w (control) |
T1 | Plant + 10 % diesel w/w |
T2 | Plant + 10 % diesel w/w + vesicular-arbuscular mycorrhiza (VAM) |
T3 | Plant + 10 % diesel w/w + Azospirillum |
T4 | Plant + 10 % diesel w/w + phosphorus-solubilising bacteria (PSB) |
T5 | Plant + 10 % diesel w/w + Pseudomonas putida |
T6 | Plant + 10 % diesel w/w + Trichoderma viride |
T7 | Plant + 10 % diesel w/w + biosurfactant |
T8 | Plant + 10 % diesel w/w + Azospirillum + PSB |
T9 | Plant + 10 % diesel w/w + maleic acid |
4.2 Phytoremediation of PAHs
Initial concentrations of individual PAHs ranged from undetectable to 32.42 μg g−1. Initial total PAH (TPAH) content in treatments was 99.77 μg g−1. Low molecular weight PAHs were dominant with concentrations ranging from 1.85 to 32.42 μg g−1; ΣLPAH was 84.58 μg g−1, which is 85 % of the TPAH content. ΣHPAH was 15.19 μg g−1. The co-eluents BghiP and IP were undetectable.
Different treatments had varying effects on PAH-reducing capacity of different plants. In all vegetated treatments, the decline in TPAH levels was not only higher but also more rapid than the unplanted control, more so in T2 (VAM), T5 (Pseudomonas), T7 (biosurfactant) and T8 (Azospirillum + PSB). Figure 15.6 summarises the PAH concentrations in treatments at initial and final days of study clearly. It is to be noted that C. jwarancusa did not survive the dose of diesel in the case of T1, T3, T4, T6 and T8.
The decreasing trends of PAH concentrations in C. jwarancusa and H. annuus treatments as compared to the unvegetated control demonstrate the fact that contaminated soils can be remediated more rapidly using plants. At successive days of analysis, treatments with plants had lower levels of TPAH compared to unplanted control. Total PAH degradation ranged from 95 to 99 % in C. jwarancusa at 240 DAT (days after treatment) and 75 to 84 % in H. annuus at 120 DAT. The final reduction of total PAHs in the unplanted control T0 was about 73 %. The PAH degradation in soil of H. annuus treatments is lower as being a seasonal it could only be monitored for 4 months. Similar results where vegetated treatments consistently showed a higher and faster decline in PAH concentrations have been reported by many other workers in studies ranging from laboratory and greenhouse experiments to pilot and field-scale demonstrations (Pradhan et al. 1998; Reynolds et al. 1999; Spriggs et al. 2005; Lin 2004; Widdowson et al. 2007).
The behaviour of organic compounds in soils and plants is generally dependent upon whether they are hydrophobic or hydrophilic. To measure this, the log of their octanol-to-water partition coefficient or ‘log K ow’ is often used. Chemicals that are highly water soluble are hydrophilic compounds with a log K ow < 0.5 while hydrophobic chemicals have a log K ow > 3.0 (Kömives and Gullner 2000). Compounds with very low log K ow values are hydrophilic and soluble in the polar soil solution. Over time, hydrophobic organic compounds can become sequestered in soil micropores or tightly bound to soil particles. The log K ow values of PAHs are between 3.5 and 7.66, indicating their moderate to highly hydrophobic behaviour. Higher molecular weight PAHs with more than three benzene rings are less water soluble, have little vaporisation and are more hydrophobic than the smaller PAHs (Maliszewka-Kordybach 1999). The smaller ring PAHs may be volatilised from soil or rapidly biodegraded within several months of application especially if the soil is moist, leaving behind the larger PAHs that are more persistent and resistant to degradation (Hawthorne and Grabanski 2000). In this context, different trends of degradation obtained for individual PAHs were expected. It was not surprising to see that LPAHs were degraded quite efficiently. However BkF, BaP and DbA which happen to be 5–6-ring HPAHs were also degraded to below detection levels by all test plants but not in the unplanted control. In the light of the reported recalcitrance of these high molecular weight PAHs (Li et al. 2010), their degradation below detection level is remarkable. However, (Juhasz et al. 1997) found that for soils contaminated with mixtures of PAHs, the high molecular weight PAHs might be degraded more rapidly in the presence of low molecular weight PAHs, which may serve as carbon sources for soil microbes. This is a phenomenon known as co-metabolism (Cunningham and Berti 1993).
The parameter found to have the most influence on PAH retention in soil is the amount of organic matter present, which controls soil sorption affinity towards PAHs (Maliszewka-Kordybach 1999). A high organic carbon content (>5 %) in soil usually leads to strong adsorption and, therefore, low availability, while a moderate organic carbon content (1–5 %) may lead to limited availability (Otten et al. 1997). Uptake of organic contaminants is greatest in soils with low organic matter content, as this provides a strong sorptive surface. The organic carbon content of the soil used in this study was below 1 %, so this certainly enhanced the availability of the PAHs in the treatment pots and their subsequent degradation.
The effect of the agronomic treatments applied to enhance the phytoremedial potential was seen to be plant specific. T7 and T8 were the top two treatments consistently at each successive sampling date (60, 120 and 240 DAT) followed by T2, though the other treatments showed different efficiencies at these dates. Enhancement of phytoremedial potential of plants by surfactants as well as N and P addition has been studied and reported by workers previously. In a study using nutrients with sophorose lipids blended with PAH-contaminated soil, many PAHs were significantly removed (Kosaric 2001). In another study, the addition of a surfactant accelerated initial PAH dissipation but did not attain final PAH concentrations below those obtained with non-mycorrhizal plants (Joner et al. 2001).
Radwan and Dashti (2005) showed that co-inoculation of Vicia faba (broad beans) plant roots in oily sand with nodule-forming rhizobia (Rhizobium leguminosarum) and plant-growth-promoting rhizobacteria (PGPR) (Pseudomonas aeruginosa and Serratia liquefaciens) enhances the phytoremediation potential of this plant for oily desert sand through improving plant growth and nitrogen fixation. The addition of Pseudomonas seemed to protect C. jwarancusa (lemongrass) from succumbing to the diesel content in its growth matrix. This could be attributed to the metabolic activities of the bacterium in the rhizosphere which have degraded or transformed PAHs the fastest. When PAHs become tightly adsorbed to the soil and less available for microbial degradation, a bacterial strategy is to release a biosurfactant. These are small detergent-like molecules with a hydrophilic head and lipophilic tail. They form spherical or lamellar micelles with cores where hydrophobic compounds become solubilised leading to transfer of PAH from solid to liquid phase (Volkering et al. 1997). As a result, it can be reasonably inferred that the root-PAH interaction was decreased in this case. Since the treatment T1 (diesel only) set up to monitor the raw potential of the plant did not survive, the effect of Pseudomonas could not be compared with it. In H. annuus, it showed no distinguishable difference in total PAH reduction when compared to control T1 though initially (60 DAT) it showed better results.
The other two treatments that showed some enhancement of total PAH reduction were VAM and Azospirillum (T2 and T3, respectively). The beneficial effects of these treatments have also been reported. (Joner et al. 2001; Dominguez-Rosado and Pichtel 2004; Rivera-Espinoza and Dendooven 2004).
4.3 Toxicity Analysis of PAHs
Initially, the Σ BaPeq in various treatments was 1.83 μg g−1. At 240 DAT (Table 15.5), it was still 1.38 μg g−1 in T0. At the same day, it ranged from 0.04 to 0.12 in C. jwarancusa treatments. Even in H. annuus where the study lasted 120 days, the values ranged from 0.26 to 0.38 μg g−1. All Σ BaPeq values of vegetated treatments are below 1 μg g−1 indicating that in all vegetated treatments, soil PAH exposure was not carcinogenic. It should be noted that the above inference is based on the exposure of soil to each individual PAH compound not for TPAHs.
4.4 Rhizodegradation v/s Phytoaccumulation
Although plants and microorganisms can degrade petroleum hydrocarbons independently of one another, it is the interaction between plants and microorganisms which is the primary mechanism responsible for petrochemical degradation in phytoremediation. The remediation process wherein contaminants are degraded/transformed by microbes in the rhizosphere is referred to as rhizodegradation. Critical components of the rhizosphere, in addition to a variety of free-living microorganisms, include root exudates that provide carbohydrates to ‘feed’ the microorganisms, organic acids that make the ions of nutrients and contaminants more mobile in the soil and enzymes that have important natural functions including degradation of organic contaminants with nitro groups or halogens (Hinchman et al. 1998). Root exudates of sugars, alcohols and acids can amount to 10–20 % of plant photosynthesis annually (Schnoor et al. 1995), and due to these exudates, microbial population and activities are 5–100 times greater in the rhizosphere than in bulk soil, i.e. soil not in contact with plant roots (Gunther et al. 1996). This plant-induced enhancement of microbial population is termed as the rhizosphere effect (Atlas and Bartha 1998). Plants may also beneficially affect PAH-degrading communities by influencing other important factors, including soil aeration, moisture levels and bioavailability.
Studies have indicated that stimulation of microbial activity in the rhizosphere of plants can stimulate biodegradation of various toxic organic compounds (Liste and Alexander 2000a, b; Daane et al. 2001). In the rhizosphere, soil redox conditions, organic content, moisture and other soil properties are manipulated by the activity of plant roots. Rhizodegradation is responsible for the enhanced removal of petroleum hydrocarbons from soil by deep-rooted trees and other annual species (Kim et al. 2006; Margesin et al. 2007; Teng et al. 2010). In fact, microbial activity or rhizodegradation has been deemed the most influential and significant cause of PAH removal from soil (Cerniglia 1997).
Dehydrogenase activity, which indicates the biological oxidation processes in soils and other systems, was used as an indicator of overall microbial activity. Measurement of dehydrogenase activity is related to the presence of viable microorganisms (Ceccanti et al. 2006; Vivas et al. 2008). Compared with control soils, microbial activity was higher in all treated soils (Fig. 15.7). Soil DHA was observed in the order 60 DAT > 120 DAT > 240 DAT in all plants. Correlation coefficients in Table 15.6 show significant positive correlation between decrease in PAH content of soil and microbial activity at the three test dates. Microbial activity was high in treatments with low PAH content.
The differential behaviour in stimulation of microbial activity in the rhizosphere exhibited by the test plants was to be expected as many factors are involved in such synergies. It has been shown that the structure of microbial communities inhabiting the rhizosphere can be affected by root architecture, root age and plant age (Nicol et al. 2003), but the complex interaction between soil type, plant species and root zone location probably is the main factor (Marschner et al. 2001).
In addition to their role in supporting rhizospheric degradative activities, plants may possess a limited capacity to transport some of the more mobile pollutants into roots and shoots via fine roots. In those situations where uptake does occur (i.e. only limited microbial activity in the rhizosphere), there is good evidence that the pollutant may be metabolised. Various vegetables (Lactuca sativa, Solanum tuberosum, Daucus carota, Brassica oleracea) and plants like Plantago major and Spartina alterniflora (a salt-marsh species) and grasses grown in PAH-polluted environments were shown to take up and bioaccumulate these compounds (Bakker et al. 2000; Fismes et al. 2002; Watts et al. 2006). In Spartina fragilis , the uptake and bioaccumulation of PAHs from oiled sediments to the plant shoot, wherein only roots were exposed to pollutant, has been demonstrated (Meudec et al. 2006).
Uptake of ΣLPAH was far more (1.5–2 times) than that of ΣHPAH (Tables 15.7 and 15.8). In C. jwarancusa, it was nearly equal in shoots. More efficient uptake of HPAHs was observed in roots v/s shoots. The actual accumulation of PAHs did not vary much between the three dates, i.e. the uptake was not related to PAH concentration of soil.
Thus, better accumulation characteristics were obtained at 240 DAT, when PAH content was lowest. In C. jwarancusa, the presence of VAM (T2) seemed to favour the accumulation of HPAHs over LPAHs in roots at all days. Accumulation patterns of PAHs also indicate that the treatments T2 (VAM), T3 (Azospirillum) and T7 (biosurfactant) in general enhanced the mobilisation of PAHs in the rhizosphere or otherwise improved their uptake by roots of the test plants. The beneficial effects of these treatments have been reported (Gamal 2005; Joner et al. 2001; Bossert and Bartha 1984).
To assess the phytoaccumulation potential of plants, some factors were employed based on simple ratios of contaminant concentration in plant parts and growth matrix. Bioabsorption coefficient (BAC) is the ratio of PAH content in shoot to soil, translocation factor (TF) is the ratio of PAH content in shoot to root, and root concentration factor (RCF) is the ratio of PAH content in root to soil. Plants exhibiting TF and BAC values greater than 1 are generally considered promising phytoextractors of metals (Fitz and Wenzel 2002). Here, we extend the same logic in the context of PAHs to see if the uptake is as low as reported. The RCF describes the capability of roots to accumulate contaminants from culture/growth medium (Briggs et al. 1983; Polder et al. 1995).
Due to their low solubility in water and tendency to bind to soil particles, PAHs are generally not absorbed by plant roots in large quantities. A lack of PAH accumulation in shoot was exhibited by BAC values of all treatments except for T2 (VAM) in C. jwarancusa (ΣHPAH) at 240 DAT. RCF values at 240 DAT indicate some potential for accumulation in roots in few treatments of C. jwarancusa (Tables 15.8, 15.9 and 15.10). However, it is to be noted that this could be simply due to the low concentrations of PAHs in soils at that date as the PAH concentrations in plants were not seen to vary much at the various testing dates. TF values of all species clearly indicate limited potential in translocation of PAHs from root to soil as no value is above 1.
TPAH accumulation in plants and percentage decrease in PAH content at the testing dates did not show much correlation (P < 0.05) (Table 15.6). In light of other accumulation characteristics, especially the consideration that the actual uptake values did not change much during the study irrespective of the TPAH concentrations in soil, we can safely conclude that accumulation does not a play a major role in the phytoremediative potential of these plants. On the other hand, significant positive correlation between decrease in PAH content of soil and microbial activity was obtained at the three test dates (Table 15.6). In other words, microbial activity was high in treatments in which more PAH degradation had been achieved. This indicates that rhizodegradation is in fact the major pathway for phytoremediation of PAHs in the soil, at least for the four species assessed here. In fact, rhizodegradation has been deemed the most influential and significant mechanism in phytoremediation of PAH-contaminated soil.
4.5 Physiology and Growth
PAHs are known to affect germination and growth as well as physiological processes such as photosynthesis or mineral uptake, inducing a gradual deterioration of plant metabolism and the disturbance of their development. For instance, benzo(a)pyrene affects plant photosynthesis and respiration by a chlorophyll breakdown and an enzymatic inhibition of the electron transport, respectively (Huang et al. 1996; Marwood et al. 2001). Meudec et al. (2007) investigated the relationship between heavy fuel oil phytotoxicity and PAH contamination in Salicornia fragilis in an artificial experiment. They hypothesised that the observed symptoms of chlorosis, yellowing and/or degeneration of tissues may have been the result of chemical stress at the tissue and cellular level. This view is supported by Alkio et al. (2005) who reported chlorosis and necrosis because of a localised H2O2 production, oxidative stress and cell death in Arabidopsis thaliana exposed to phenanthrene.
Li et al. (2008) used phenanthrene and pyrene in soil to investigate physiological and biochemical responses of rice (Oryza sativa L.) to PAH stress, in the presence or absence of a PAH-degrading bacteria (Acinetobacter sp.). A number of parameters including biomass and water, chlorophyll and chlorophyll a/b ratio, electrolyte leakage, activities of superoxide dismutase (SOD) and peroxidase and soluble carbohydrate and soluble protein contents were monitored. Results show that rice plants have good resistance and tolerance to lower levels of PAHs stress, while adding high levels of PAHs to soils resulted in adverse effects on rice plants such as a reduction in biomass and damage to photosynthetic function. Inoculation with PAH-degrading bacteria promoted growth and photosynthesis of rice.
Chlorophyll ‘a’ content in all test plants was found to be higher than chlorophyll ‘b’ across all treatments. Total chlorophyll content trends were more varied. C. jwarancusa was most susceptible to diesel toxicity; most plants succumbed to diesel contamination. In these, broad bands of chlorosis followed by necrosis were observed prior to death. The treatments T2 (VAM), T5 (Pseudomonas), T7 (biosurfactant) and T9 (Maleic acid) survived. The efficacy of T5 and T7 was more marked. In H. annuus, the trends of chl ‘a’ and chl ‘b’ as well as total chlorophyll content in leaves were not very different as far as effect of diesel contamination in the growth matrix is concerned. T5 (Pseudomonas) and T3 (Azospirillum) seemed to be the most effective in overcoming PAH toxicity.
It has been reported that plants exposed to sublethal doses of used engine oil had low chlorophyll content (Odjegba and Sadiq 2002; Odjegba and Atebe 2007), and this could have a direct effect on the carbon-fixing efficiency of the plant. Rosso et al. (2005) exposed S. virginica to sediments polluted by two types of crude oils and reported reductions in growth and photosynthesis.
All surviving treatments of C. jwarancusa (Fig. 15.8) exhibited greater proline accumulation in the shoots till 60 DAT. The addition of diesel to the growth matrix was seen to promote proline accumulation in H. annuus too (Fig. 15.9). The magnitude of accumulation was many times more than the control. There was a gradual decline in proline accumulation as the study progressed, and by 120 DAT, all plants exhibited values similar to control.
Maximum accumulation of proline was noticeable in T7 (biosurfactant) in both species with T9 (Maleic acid) a close second. This could be due to the increased bioavailability of pollutants in the rhizosphere which magnified the stress on the plants. The treatment T5 (Pseudomonas) which showed most rapid degradation of PAHs in the soil also registered the lowest levels of proline in the plants. This combined with the higher accumulation seen in increased bioavailability treatments substantiates the hypothesis that proline accumulation in plants was actually in response to contaminants in the rhizosphere.
Detailed studies have shown the accumulation of free proline in plant, viz. Crinum asiaticum, Phaseolus vulgaris, Eruca sativa, Helianthus annuus and Cajanus cajan due to metals like Pb, Cu, Co, Cd, Zn and Hg (Varun et al. 2011; Zengin and Munzuroglu 2005; Faheed 2005). Methionine, proline and phenylalanine were found to be the amino acids. Proline and methionine have been found to indicate stress in PAH-treated spruce seedlings (Berteigne et al. 1989). Needle and root growth were also inhibited by the presence of PAHs in the growth matrix.
The addition of diesel to the growth matrix (T1) was seen to inhibit growth in terms of shoot and root length (Table 15.11). More variations in these parameters among the various treatments were visible as the study progressed. Some amendments were able to provide resistance/protect plants from diesel contamination. A gradual stabilising trend was established as the study progressed.
Biomass was negatively affected to varying degrees when diesel was added to the growth matrix without any soil amendment. Both stimulatory (Wieczorek et al. 2001; Maliszewska-Kordybach and Smreczak 2000) and inhibitory effects of PAHs in the growth matrix (Wieczorek et al. 2001; Henner et al. 1999) on stem and root length, their biomass and dry matter have been observed. Smith et al. (2006) observed growth reduction but no significant effect on germination rate of seven oleaginous and grass species after 12 weeks in the presence of PAHs in soils. A similar behaviour—no effect on germination but biomass reduction—was described by Sverdrup et al. (2007) working with L. perenna, T. pretense and Brassica alba and by Besalatpour et al. (2008) with another group of species.
Some amendments were more potent in providing resistance and/or protecting plants from diesel contamination as compared to treatment T1 where only diesel was applied. In general, the treatments T2 (VAM), T3 (Azospirillum), T4 (PSB), T5 (Pseudomonas), T7 (biosurfactant) and T8 (Azospirillum + PSB) seemed to be more protective of plant growth in terms of root length, shoot length as well as total biomass.
Vesicular-arbuscular mycorrhiza (VAM) are known to improve plant growth and health by improving mineral nutrition and increasing resistance or tolerance to biotic and abiotic stresses. PAH-contaminated soils are more or less hydrophobic, and thus plant growth may be limited by water uptake and access to mineral nutrients dissolved in inaccessible soil water. Mycorrhiza-inoculated plants show better tolerance of water stress. In addition, indirect effects of mycorrhizae such as modified root architecture (Hooker and Atkinson 1996), improved membrane integrity (Graham et al. 1981) or enhanced production of oxidative enzymes (Salzer et al. 1999) may improve the performance of inoculated plants in the presence of organic compounds.
Gamal (2005) demonstrated that AM inoculation increased the total biomass of wheat, mung bean and eggplant grown in soil spiked with PAHs. The chlorophyll content of mycorrhiza-inoculated plants also increased in spiked as well as unspiked treatments. Similarly, inoculants used as phytostimulators (Azospirillum) or as biological control agents of fungi (Pseudomonas and Trichoderma) have shown beneficial effects on plant growth and health. Modifications of the microbial community structure in the rhizosphere have also been reported subsequent to such amendments (Mar Vázquez et al. 2000).
5 Conclusions
The PAH profile at test sites indicates that levels were much below many contaminated sites worldwide. Yet, these concentrations need to be monitored in the future so that timely interventions may be made. Based on the greenhouse study, it may be concluded that the plant-contaminant-microbe synergy in growth matrices is highly individualistic. Not only the metabolic activities but also the growth of plants can be modified by the presence of pollutants in the rhizosphere as well as by soil amendments and microbiota that are protective/growth promoting in nature. This needs to be extensively studied to gain insight about individual species and their tolerance of soil contamination for phytoremedial strategies to be effective.
References
Agarwal T, Khillare PS, Shridhar V (2006) PAHs contamination in bank sediment of the Yamuna River, Delhi, India. Environ Monit Assess 123(1–3):151–166
Alkio M, Tabuchi TM, Wang X, Colon-Carmona A (2005) Stress responses to polycyclic aromatic hydrocarbons in Arabidopsis include growth inhibition and hypersensitive response-like symptoms. J Exp Bot 56(421):2983–2994
Anderson TA, Guthrie EA, Walton BT (1993) Bioremediation in the rhizosphere. Environ Sci Technol 27(13):2630–2636
Atlas RM, Bartha R (1998) Microbial ecology: fundamentals and applications. Benjamin/Cummings, Don Mills, ON
Bakker MI, Casado B, Koerselman JW, Tolls J, Kolloffel C (2000) Polycyclic aromatic hydrocarbons in soil and plant samples from the vicinity of an oil refinery. Sci Total Environ 263:91–100
Berteigne M, Rose C, Gérard J, Dizengremel P (1989) Effects of polyaromatic hydrocarbons on the forest ecosystem and woody plants. Ann Sci For 46(Suppl):561–564
Besalatpour A, Khoshgoftarmanesh AH, Hajabbasi MA, Afyuni M (2008) Germination and growth of selected plants in a petroleum contaminated calcareous soil. Soil Sediment Contam 17(6):665–676
BIO-WISE (2000) Contaminated land remediation: a review of biological technology, London Dept. of Trade and Industry, Govt. of UK
Bossert I, Bartha R (1984) The fate of petroleum in soil ecosystems. In: Atlas RM (ed) Petroleum microbiology. MacMillan, New York
Bourotte C, Forti MC, Taniguchi S, Bicego M, Lotufo P (2005) A wintertime study of PAHs in fine and coarse aerosols in Sao Paulo city, Brazil. Atmos Environ 39:3799–3811
Briggs GG, Bromilow RH, Evans AA, Willams M (1983) Relationship between lipophilicity and the distribution of non-ionized chemicals in barley shoot following uptake by the root. Pestic Sci 14:492–500
Capuano F, Cavalchi B, Martinelli G, Pecchini G, Renna E, Scaroni I, Bertacchi M, Bigliardi G (2005) Environmental prospection for PCDD/PCDF, PAH, PCB and heavy metals around the incinerator power plant of Reggio Emilia town (Northern Italy) and surrounding main roads. Chemosphere 58:1563–1569
Ceccanti B, Masciandaro G, Garcia C, Macci C, Doni S (2006) Soil bioremediation: combination of earthworms and compost for the ecological remediation of a hydrocarbon polluted soil. Water Air Soil Pollut 177:383–397
Cerna M, Pochmanova D, Pastorkova A, Bene I, Lenicek J, Topinka J, Binkova B (2000) Genotoxicity of urban air pollutants in the Czech Republic Part I. Bacterial mutagenic potencies of organic compounds adsorbed on PM10 particulates. Mutat Res 469:71–82
Cerniglia CE (1992) Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3(2–3):351–368
Cerniglia CE (1997) Fungal metabolism of polycyclic aromatic hydrocarbons: past, present and future applications in bioremediation. J Indus Microbiol Biotechnol 19:324–333
Chen B, Xuan X, Zhu L, Wang J, Gao Y, Yang K, Shen X, Lou B (2004) Distributions of polycyclic aromatic hydrocarbons in surface waters, sediments and soils of Hangzhou City, China. Water Res 38:3558–3568
Chen L, Ran Y, Xing B, Mai B, He J, Wei X, Fu J, Sheng G (2005) Contents and sources of polycyclic aromatic hydrocarbons and organochlorine pesticides in vegetable soils of Guangzhou, China. Chemosphere 60:879–890
Committee on In Situ Bioremediation, Water Science and Technology Board, Commission on Engineering and Technical Systems, and National Research Council (1993) In situ bioremediation: when does it work? National Academy Press, Washington, DC
Crépineau C, Rychen G, Feidt C, Le Roux Y, Lichtfouse E, Laurent F (2003) Contamination of pastures by polycyclic aromatic hydrocarbons (PAHs) in the vicinity of a highway. J Agric Food Chem 51:4841–4845
Cunningham SD, Berti WR (1993) Remediation of contaminated soils with green plants: an overview. In Vitro Cell Dev Biol Plant 29(4):207–212
Daane LL, Harjono I, Zylstra GJ, Maggblom MM (2001) Isolation and characterization of polycyclic aromatic hydrocarbon degrading bacteria associated with the rhizobia of salt marsh plants. Appl Environ Microbiol 67:2683–2691
Desouza MP, Huang CP, Chee N, Terry N (1999) Rhizosphere bacteria enhance the accumulation of Selenium and Mercury in wetland plants. Planta 209(2):259–263
DHHS (1995) Agency for toxic substances and disease registry. Toxicological profile for polyaromatic hydrocarbons, U.S. Department of Health & Human Services
Dominguez-Rosado E, Pichtel J (2004) Phytoremediation of soil contaminated with used motor oil: II. Greenhouse studies. Environ Eng Sci 21(2):169–180
Drooge BL, Grimalt JO, Torres-Gracia CJ, Cuevas E (2002) Semivolatile organochlorine compounds in the free troposphere of the Northeastern Atlantic. Environ Sci Technol 36:1155–1161
Duke O, Albert IO (2007) Spatial variation and distribution of polycyclic aromatic hydrocarbons in soil. Bull Chem Soc Ethiop 21(3):331–340
Durand C, Ruban V, Ambles A, Oudot J (2004) Characterization of the organic matter of sludge: determination of lipids, hydrocarbons and PAHs from road retention/infiltration ponds in France. Environ Pollut 132:375–384
Durmishidze SV (1977) Metabolism of certain air-polluting organic compounds in plants (review). Appl Biochem Microbiol 13(6):646–653 (Transl. 1978)
Duxbury CL, Dixon DG, Greenberg BM (1997) Effects of simulated solar radiation on the bioaccumulation of polycyclic aromatic hydrocarbons by the duckweed Lemna gibba. Environ Toxicol Chem 16:1739–1748
Edwards NT (1988) Assimilation and metabolism of polycyclic aromatic hydrocarbons by vegetation – an approach to this controversial issue and suggestions for future research. In: Polycyclic aromatic hydrocarbons: a decade of progress – 10th International symposium, Columbus, OH, Battelle Press
EPA (1997) Electro kinetic laboratory and field processes applicable to radioactive and hazardous mixed waste in soil and groundwater. EPA 402/R-97/006. Washington, DC
Eweis JB, Ergas SJ, Chang DPY, Schroeder ED (1998) Bioremediation principles. McGraw-Hill, Toronto
Faheed FA (2005) Effect of lead stress on the growth and metabolism of Eruca sativa M. seedlings. Acta Agron Hung 53(3):319–327
Fang GC, Yang IL, Chen MH (2004) PAHs in the ambient air of suburban and industrial regions of central Taiwan. Chemosphere 54:443–452
Ferro A, Kennedy J, Doucette W, Nelson S, Jauregui G, McFarland B, Bugbee B (1997) Fate of benzene in soils planted with alfalfa: uptake, volatilization, and degradation. In: Kruger EL, Anderson TA, Coats JR (eds) Phytoremediation of soil and water contaminants, ACS Symposium Series 664. American Chemical Society, Washington, DC, pp 223–237
Fismes J, Perrin-Ganier C, Empereur-Bissonnet P, Morel JL (2002) Soil to root transfer and translocation of polycyclic aromatic hydrocarbons by vegetables grown on industrial contaminated soils. J Environ Qual 31:1649–1656
Fitz WJ, Wenzel WW (2002) Arsenic transformation in the soil–rhizosphere–plant system, fundamentals and potential application of phytoremediation. J Biotechnol 99:259–278
Gamal HR (2005) Role of arbuscular mycorrhizal fungi in phytoremediation of soil rhizosphere spiked with poly aromatic hydrocarbons. Microbiology 33(1):41–50
Graham J, Leonard R, Menge JA (1981) Membrane-mediated decrease in root exudation responsible for phosphorus-inhibition of vesicular-arbuscular mycorrhiza formation. Plant Physiol 68:6488552
Gunther T, Dornberger U, Fritsche W (1996) Effects of ryegrass on biodegradation of hydrocarbons in soil. Chemosphere 33(2):203–215
Harvey RG (1997) Polycyclic aromatic hydrocarbons. Wiley-VCH, New York
Hawthorne SB, Grabanski CB (2000) Vaporization of polycyclic aromatic hydrocarbons (PAHs) from sediments at ambient conditions. Environ Sci Technol 34(20):4348–4353
Henner P, Schiavon M, Druelle V, Lichtfouse E (1999) Phytotoxicity of ancient gaswork soils. Effect of polycyclic aromatic hydrocarbons (PAHs) on plant germination. Org Geochem 30:963–969
Hinchman RR, Negri MC, Gatliff EE (1998) Phytoremediation: using green plants to clean up contaminated soil, groundwater, and wastewater. Submitted to the U.S. Department of Energy, Assistant Secretary for Energy Efficient and Renewable Energy under Contract W-31-109-Eng-38.
Holloway MP, Biaglow MC, McCoy EC, Anders M, Rosenkranz HS, Howard PC (1987) Photochemical instability of 1-nitropyrene, 3-nitrofluoranthene, 1,8-dinitropyrene and their parent polycyclic aromatic hydrocarbons. Mutat Res 187(4):199–207
Hooker JE, Atkinson D (1996) Arbuscular mycorrhizal fungi-induced alternation to tree-root architecture and longevity. J Plant Nutr Soil Sci 159:229–234
Huang XD, Zeiler LF, Dixon DG, Greenberg BM (1996) Photoinduced toxicity of PAHs to the foliar regions of Brassica napus (Canola) and Cucumbis sativus (Cucumber) in simulated solar radiation. Ecotoxicol Environ Saf 35:190–197
IARC (International Agency for Research on Cancer) (1987) IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, supplement 7. IARC, Lyons
Joner EJ, Johansen A, Loibner AP, de la Cruz MA, Szolar OH, Portal JM, Leyval C (2001) Rhizosphere effects on microbial community structure and dissipation and toxicity of polycyclic aromatic hydrocarbons (PAHs) in spiked soil. Environ Sci Technol 35(13):2773–2777
Juhasz AL, Britz ML, Stanley GA (1997) Degradation of benzo[a]pyrene, dibenzo[a,h]anthracene and coronene by Burkholderia cepacia. Wat Sci Technol 36(10):45–51
Kim J, Kang SH, Min KA, Cho KS, Lee IS (2006) Rhizosphere microbial activity during phytoremediation of diesel-contaminated soil. J Environ Sci Health A Tox Hazard Subst Environ Eng 41(11):2503–2516
Kömives T, Gullner G (2000) Phytoremediation. In: Wilkinson RE (ed) Plant-environment interactions. Marcel Dekker, New York
Kosaric N (2001) Biosurfactants for soil bioremediation. Food Technol Biotechnol 39(4):295–304
Kroening SJ, Leung DW, Greenfield LG, Galilee C (2001) Losses of diesel oil by volatilisation and effects of diesel oil on seed germination and seedling growth. Environ Technol 22(9):1113–1117
Lamb SI, Kaplan IR (1980) Organic compounds in urban atmosphere. J Air Pollut Control Assoc 30:1098–1115
Li JH, Gao Y, Wu SC, Cheung KC, Wang XR, Wong MH (2008) Physiological and biochemical responses of rice (Oryza Sativa L.) to phenanthrene and pyrene. Int J Phytoremediation 10(2):106–118
Li J, Shang X, Zhao Z, Tanguay R, Dong Q, Huang C (2010) Polycyclic aromatic hydrocarbons in water, sediment, soil and plants of the Aojiang River waterway in Wenzhou, China. J Hazard Mater 173:75–81
Lin H (2004) The study of phytoremediation of oil spill contaminated wetland soil. oai: NSYSU: etd-0721104-155933. http://etd.lib.nsysu.edu.tw/ETD-db/ETD-search/view_etd?URN=etdetd-0721104-155933
Liste HH, Alexander M (2000a) Plant-promoted pyrene degradation in soil. Chemosphere 40:7–10
Liste HH, Alexander M (2000b) Accumulation of phenanthrene and pyrene in rhizosphere soil. Chemosphere 40:11–14
Loening K, Merrit J (1990) Polynuclear aromatic hydrocarbons: nomenclature guide, 1st edn. Battelle, Columbus, OH
Mackay D, Shiu WY (1992) Illustrated handbook of physical-chemical properties and environmental fate of organic chemicals, Vol. II – Polynuclear aromatic hydrocarbons and polychlorinated dioxins and dibenzofurans. Lewis, Chelsea
Maliszewska-Kordybach B (1996) Polycyclic aromatic hydrocarbons in agricultural soils in Poland: preliminary proposals for criteria to evaluate the level of soil contamination. Appl Geochem 11:121–127
Maliszewka-Kordybach B (1999) Persistent organic contaminants in the environment: PAHs as a case study. In: Bioavailability of organic xenobiotics in the environment, (eds.) Baveye PH, Block JC, Goncharuk VV, pp3-34. Kluwer Publ. Netherlands
Maliszewska-Kordybach B, Smreczak B (2000) Ecotoxicological activity of soils polluted with polycyclic aromatic hydrocarbons (PAHs) – effect on plants. Environ Technol 21(10):1099–1110
Mar Vázquez M, César S, Azcón R, Barea JM (2000) Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Environ Sci Pollut Res 10(4):235–244
Margesin R, Hämmerle M, Tscherko D (2007) Microbial activity and community composition during bioremediation of diesel-oil-contaminated incubation soil: effects of hydrocarbon concentration, fertilizers, time. Microb Ecol 53(2):259–269
Marschner P, Yang PH, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol Biochem 33:1437–1445
Marwood CA, Solomon KR, Greenberg BW (2001) Chlorophyll fluorescence as a bioindicator of effects on growth in aquatic macrophytes from mixtures of PAHs. Environ Toxicol Chem 20:890–898
Masih A, Taneja A (2006) Polycyclic aromatic hydrocarbons (PAHs) concentrations and related carcinogenic potencies in soil at a semi-arid region of India. Chemosphere 65:449–456
Merkl NR, Schultze K, Infante C (2004) Phytoremediation in the tropics – the effect of crude oil on the growth of tropical plants. Bioremediat J 8(3–4):177–184
Meudec A, Dussauze J, Deslandes E, Poupart N (2006) Evidence for bioaccumulation of PAHs into internal shoot tissues a halophytic plant artificially exposed to petroleum polluted-sediments. Chemosphere 65:474–481
Meudec A, Poupart N, Dussauze J, Deslandes E (2007) Relationship between heavy fuel oil phytotoxicity and polycyclic aromatic hydrocarbon contamination in Salicornia fragilis. Sci Tot Environ 381:146–156
Miller MM, Wasik SP, Huang GL, Shiu WY, Mackay D (1985) Relationships between octanol–water partition coefficient and aqueous solubility. Environ Sci Technol 19(6):522–529
Motelay-Massei A, Ollivon D, Garban B, Teil MJ, Blanchard M, Chevreuil M (2004) Distribution and spatial trends of PAHs and PCBs in soils in the Seine River basin, France. Chemosphere 55:555–565
Nadal M, Schuhmacher M, Domingo JL (2004) Levels of PAHs in soil and vegetation samples from Tarragona County, Spain. Environ Pollut 132(1):1–11
Nam JJ, Song BH, Eom KC, Lee SH, Smith A (2003) Distribution of polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in South Korea. Chemosphere 50:1281–1289
Nicol GW, Glover LA, Prosser JI (2003) Spatial analysis of archaeal community structure in grassland soil. Appl Environ Microbiol 69:7420–7429
Nisbet IC, LaGoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300
Odjegba VJ, Atebe JO (2007) The effect of used engine oil on carbohydrate, mineral content and nitrate reductase activity of leafy vegetable (Amaranthus hybridus L.). J Appl Sci Environ Manag 11(2):191–196
Odjegba VJ, Sadiq AO (2002) Effects of spent engine oil on the growth parameters, chlorophyll and protein levels of Amaranthus hybridus L. Environmentalist 22:23–28
Omar NYMJ, Abas MRB, Ketuly KA, Tahir NM (2002) Concentrations of PAHs in atmospheric particles (PM10) and roadside soil particles collected in Kuala Lumpur, Malaysia. Atmos Environ 36:247–254
Osborne MR, Crosby NT (1987) Benzopyrenes, Cambridge, UK; Cambridge University Press
Otten A, Alphenaar A, Pijls C, Spuij F, de Wit H (1997) In situ soil remediation. Kluwer, Boston, MA
Polder MD, Hulzebos EM, Jager DT (1995) Validation of models on uptake of organic chemicals by plant roots. Environ Toxicol Chem 14(9):1615–1623
Pradhan SP, Conrad JR, Paterek JR, Srivastava VJ (1998) Potential of phytoremediation for treatment of polycyclic hydrocarbons in soil at MGP sites. J Soil Contam 7(4):467–480
Radwan SS, Dashti N (2005) Enhancing the growth of Vicia faba plants by microbial inoculation to improve their phytoremediation potential for oily desert areas. Int J Phytoremediation 7(1):19–32
Ravindra K, Mittal AK, Van Grieken R (2001) Health risk assessment of urban suspended particulate matter with special reference to polycyclic aromatic hydrocarbons: a review. Rev Environ Health 16:169–189
Reynolds CM, Wolf DC, Gentry TJ, Perry LB, Pidgeon CS, Koenen BA, Rogers HB, Beyrouty CA (1999) Plant enhancement of indigenous soil micro-organisms: a low-cost treatment of contaminated soils. Polar Rec 35:33–40
Ribes S, Grimalt JO, Torres CJ, Cuevas E (2002) Temperature and organic matter dependence of the distribution of organochlorine compounds in mountain soils from the subtropical Atlantic (Teide, Tenerife island). Environ Sci Technol 36:1879–1885
Rivera-Espinoza Y, Dendooven L (2004) Dynamics of carbon, nitrogen and hydrocarbons in diesel-contaminated soil amended with biosolids and maize. Chemosphere 54(3):379–386
Rogge WF, Hildemann LM, Mazurek MA (1993) Sources of the fine organic aerosol 3. Road dust, tire debris, and organometallic brake lining dust: roads as sources and sinks. Environ Sci Technol 27:1892–1904
Rosso PH, Pushnik JC, Lay M, Ustin SL (2005) Reflectance properties and physiological responses of Salicornia virginica to heavy metal and petroleum contamination. Environ Pollut 137:241–252
Salzer P, Corbiere H, Boller T (1999) Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus mosseae. Planta 208:319–325
Schnoor JL, Licht LA, McCutcheon SC, Wolfe NL, Carreira LH (1995) Phytoremediation of organic and nutrient contaminants. Environ Sci Technol 29(7):318–323
Siewniak M (1975) Studies on air and soil pollution in the surroundings of petrochemical complex in Plock. Zeszyty Naukowe Szkoly Glownej Gospodarstwa Wiejskiego Akademii Rolniczej – Warszawa 53:1 (in Polish)
Skrbic B, Miljevic N (2002) An evaluation of residues at an oil refinery site following fires. J Environ Sci Health A Tox Hazard Subst Environ Eng 37(6):1029–1039
Smith MJ, Flowers TH, Duncan HJ, Alder J (2006) Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues. Environ Pollut 141:519–525
Spriggs T, Banks MK, Schwab P (2005) Phytoremediation of Polycyclic Aromatic Hydrocarbons in Manufactured Gas Plant-Impacted Soil. J Environ Qual 34:1755–1762
Sutherland JB (1992) Detoxification of polycyclic aromatic hydrocarbons by fungi. J Ind Microbiol 9:53–62
Sverdrup LE, Hagen SB, Krogh PH, van Gestel CAM (2007) Benzo[a]pyrene shows low toxicity to three species of terrestrial plants, two soil invertebrates, and soil-nitrifying bacteria. Ecotoxicol Environ Saf 66:362–368
Tang L, Tang XY, Zhu YG, Zheng MH, Miao QL (2005) Contamination of polycyclic aromatic hydrocarbons (PAHs) in urban soils in Beijing, China. Environ Int 31:822–828
Tebaay RH, Welp G, Brummer GW (1993) Gehalt an Polycyclischen Aromatischen Kohlenwasserstoffen (PAK) und deren Verteilungsmuster in unterschiedlivh belasteten Boden. Z Pflanzernernaehr, Bodenkd 156:1–10
Teng Y, Luo Y, Sun X, Tu C, Xu L, Liu W, Li Z, Christie P (2010) Influence of arbuscular mycorrhiza and Rhizobium on phytoremediation by alfalfa of an agricultural soil contaminated with weathered PCBs: a field study. Int J Phytoremediation 12(5):516–533
Trapido M (1999) Polycyclic aromatic hydrocarbons in Estonian soil: contamination and profiles. Environ Pollut 105:67–74
Tsao DT (2003) Advances in biochemical engineering/biotechnology Vol. 78, Phytoremediation. Springer, Berlin
USEPA (1988) Second supplement to compendium of methods for the determination of toxic organic compounds in ambient air, atmospheric research and exposure assessment laboratory. Research Triangle Park, NC, EPA 600/4-89-018, pp 97
USEPA (1996a) A citizen’s guide to natural attenuation – technology fact sheet. Office of Solid Waste and Emergency Response. EPA 542-F-96-015
USEPA (1996b) A citizen’s guide to bioremediation – technology fact sheet. Office of Solid Waste and Emergency Response. EPA 542-F-96-007
USEPA (1999) Integrated risk information system (IRIS). National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC
USEPA (2002) Polycyclic organic matter. US Environmental Protection Agency. Available at http://www.epa.gov/ttn/atw/hlthef/polycycl.html
Varun M, D’Souza R, Kumar D, Paul MS (2011) Bioassay as monitoring system for lead phytoremediation through Crinum asiaticum L. Environ Monit Assess 178:373–381
Vivas A, Moreno B, del Val C, Benitez E (2008) Metabolic and bacterial diversity in soils historically contaminated by heavy metals and hydrocarbons. J Environ Monit 10:1287–1296
Volkering F, Breure AM, Rulkens WH (1997) Microbiological aspects of surfactant use for biological soil remediation. Biodegradation 8:401–417
Voutsa D, Terzi H, Muller L, Samara C, Kouimtzis T (2004) Profile analysis of organic micropollutants in the environment of a coal burning area, NW Greece. Chemosphere 55:595–604
Wadler S, Fuks JZ, Wiemik PH (1986) Phase I and II agents in cancer therapy: Part I. Anthracyclines and related compounds. J Clin Pharmacol 26:491–509
Wang XJ, Zheng Y, Liu RM, Li BG, Cao J, Tao S (2003) Medium scale spatial structures of Polycyclic Aromatic Hydrocarbons in the topsoil of Tianjin area. J Environ Sci Health B 38:327–335
Watkins JW, Sorensen DL, Sims RC (1994) Volatilization and mineralization of naphthalene in soil-grass microcosms. In: Anderson T, Coats JR (eds) Bioremediation through rhizosphere Technology, ACS Symposium Series 563. American Chemical Society, Washington, DC, pp 123–131
Watts AW, Ballestero TP, Gardner KH (2006) Uptake of polycyclic aromatic hydrocarbons (PAHs) in salt marsh plants Spartina alterniflora grown in contaminated sediments. Chemosphere 62:1253–1260
Weiss P, Riss A, Gschmeidler E (1994) Investigation of heavy metal, PAH, PCB patterns and PCDD/F profiles of soil samples from an industrialized urban area with multivariate statistical methods. Chemosphere 29:2223–2236
WHO/IPCS (1998) Environmental health criteria 202, selected non-heterocyclic PAHs. WHO, Geneva
Widdowson M, Marr L, Novak J (2007) Mechanisms for phytoremediation of PAH compounds: a long-term field investigation. Geophys Res Abstr 9:10373
Wieczorek JK, Wieczorek ZJ, Olszewski J, Badyga B, Smoczyñska K, Smoczyñski SS (2001) Effect of high anthracene concentration in the soil on its accumulation and growth of pea plants. Nat Sci 8:135–143
Wilcke W, Muller S (1999) Polycyclic aromatic hydrocarbons in hydromorphic soils of the tropical metropolis Bangkok. Geoderma 91:297–309
Wilcke W, Lillienfein J, do Carmo Lima S (1999) Contamination of highly weathered urban soils in Uberlandia, Brazil. J Plant Nutr Soil Sci 162:539–548
Wild SR, Jones KC (1992) Polynuclear aromatic hydrocarbon uptake by carrots grown in sludge-amended soil. J Environ Qual 21:217–225
Wild SR, Jones KC (1995) Polynuclear aromatic hydrocarbons in the UK environment: a preliminary source inventory and budget. Environ Pollut 88:91–108
Wiltse CC, Rooney WL, Chen Z, Schwab AP, Banks MK (1998) Greenhouse evaluation of agronomic and crude oil phytoremediation potential among alfalfa genotypes. J Environ Qual 27:169–173
Windholz M (1983) The Merck index, 10th edn. Merck, Rahway, NJ
Wong F, Harner T, Liu QT, Diamond ML (2004) Using experimental and forest soils to investigate the uptake of polycyclic aromatic hydrocarbons (PAHs) along an urban-rural gradient. Environ Pollut 129:387–398
Yang SYN, Connell DW, Hawker DW, Kayal SI (1991) Polycyclic aromatic hydrocarbons in air, soil, and vegetation in the vicinity of an urban roadway. Sci Total Environ 102:229–240
Yang Y, Zhang XX, Korenaga T (2002) Distribution of polynuclear aromatic hydrocarbons (PAHs) in the soil of Tokushima, Japan. Water Air Soil Pollut 138:51–60
Zengin FK, Munzuroglu O (2005) Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Cracov Bot 47(2):157–164
Zhang Z, Huang J, Yu G, Hong H (2004) Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui River of Beijing, China. Environ Pollut 130:349–361
Zhang X, Cheng S, Zhu C, Sun S (2006) Microbial PAH-degradation in soil: degradation pathways and contributing factors. Pedosphere 16(5):5655–5665
Zohair A, Salim AB, Soyibo AA, Beck AA (2006) Residues of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and organochlorine pesticides in organically-farmed vegetables. Chemosphere 63:541–553
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
D’Souza, R., Varun, M., Lakhani, A., Singla, V., Paul, M.S. (2015). PAH Contamination of Urban Soils and Phytoremediation. In: Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L. (eds) Phytoremediation. Springer, Cham. https://doi.org/10.1007/978-3-319-10395-2_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-10395-2_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10394-5
Online ISBN: 978-3-319-10395-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)