Abstract
A fraction of tumor cells designated as cancer stem cells (CSC) has been identified in various tumor types. Such cells appear to be capable of initiating and sustaining the growth of a tumor, being responsible for tumor initiation, invasive growth, metastasis, and drug resistance. The isolation of CSC is not easy to achieve due to the need for proving phenotypic and functional features; thus, under many circumstances “putative CSC” is the most appropriate designation. Like normal stem cells, CSC appear to exhibit increased expression of ABC transporters as compared to their nonstem counterparts. Here, the cancer stem cell hypothesis is described with particular reference to the timeline of its development, together with the acquired knowledge on ABC transporters that may be instrumental for therapeutic targeting of CSC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
In all differentiated mammalian normal tissues there are cells that can differentiate in response to environmental stimuli, because they maintain stem cell features [77]. Such cells are abundant in regenerating tissues, but represent a small fraction of the tissue cells in nonregenerating tissues. The existence also in tumors of a cell fraction endowed with self-renewal, differentiating and tumor initiating properties is supported by old and recent studies. Such cells designated as cancer stem cells (CSC) have been proposed to represent a population of cancer cells which initiates and sustains the growth of a tumor, being responsible for tumor initiation, invasive growth, and metastasis [90, 6, 16]. CSC have been identified in different tumor types, but only in a few diseases (e.g., breast and brain tumors) the precise phenotypic and functional features of CSC have been well defined [20]. A better definition of the role of CSC in various tumor types and molecular subtypes will need additional efforts, specifically in establishing refined markers for CSC. Moreover, extensive in vivo work, in particular limiting dilution assays will be required to establish the tumor-initiating capability of the used experimental models. Such approaches are expected to reduce over-interpretation of results that has been a frequent risk in the field of CSC.
Expression of proteins contributing to drug resistance, and in particular of the ATP-binding cassette (ABC) transporters, in putative CSC versus differentiated cancer cells are being regarded as a major feature of CSC that could be pharmacologically targeted in an attempt to improve the efficacy of treatment and to achieve durable responses [22]. It has been reported that normal and tumor stem cells exhibit increased expression of ABC transporters as compared to their nonstem counterparts [5, 103]. Indeed, ABC transporters have been documented to be involved in the regulation of stem cell physiology in studies regarding normal hematopoietic stem cells (HSC, see below).
Here, the cancer stem cell hypothesis is described with particular reference to the timeline of its development, together with the acquired knowledge on ABC transporters that may be instrumental for therapeutic targeting of CSC. The significance of ABC transporters in the biology of CSC is presented by considering the specific features of those transporters that have been implicated in phenotypes related to CSC, such as the side population (SP) phenotype. In addition, the interrelation between ABC transporters and other markers for CSC is examined, as well as the regulation of ABC transporters expressed in CSC or cancer stem-like cells by specific factors or pathways. Moreover, clinical trials involving CSC have been reported. In summary, the available evidence on CSC supports the need for strong basic science efforts before fully translating the knowledge generated in the preclinical context to the clinical setting. Several modulators of ABC transporters seem to have promising therapeutic potential, as shown in preclinical studies. The scientific community is already facing a few clinical observations that may be useful to interpret recurrence and that will be hopefully exploited to define improved therapies to cure cancer patients.
5.2 The Cancer Stem Cell Hypothesis
5.2.1 Rationale and Historical Notes
The CSC hypothesis proposes a hierarchical model for tumors in which cells “at the apex of tumor hierarchy” can be identified. Such a hypothesis is apparently in contrast with the clonal evolution model or stochastic model of tumorigenesis, which is based on increased proliferative potential of the clone with the best fitness among tumor cells. The model of a dominant clone where clonal selection relies on genetic mutation does not appear real, or at least exclusive. In fact, epigenetic mechanisms and also the tumor microenvironment contribute to tumor heterogeneity. In this context, nonmutational mechanisms of drug resistance have been described (i.e., polygenic clinical drug resistance) [39], that may be useful to interpret the real nature of tumor heterogeneity and may be in line with a relevant role for CSC.
The first report that started to build the CSC hypothesis was published in 1937 by Furth and Kahn [89], who documented that a single tumor cell can initiate leukemia in mice. “The transmission of leukemia of mice with a single cell” was subsequently confirmed in several studies [108]. Remarkably, in 1997, Bonnet and Dick reported that human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Such a study shed light on the mechanism underscoring the phenotypic, genotypic, and clinical heterogeneity of acute myeloid leukemia, given the debate about the target cell, within the hematopoietic stem cell hierarchy, that is susceptible to leukemic transformation [8]. The study suggested that normal primitive cells, rather than committed progenitor cells, are the target for leukemic transformation, and that such cells could differentiate in vivo into leukemic blasts, a phenomenon supporting that the leukemic clone is organized as a hierarchy [8]. The first evidence of the existence of CSC in solid tumors was provided in 2003, when the CSC hypothesis was tested starting from considering the phenotypically diverse population of breast cancer cells [1]. In this context, it was found that only a small number of breast cancer cells were able to form new tumors in immune-deficient mice, and tumor initiating cells were distinguished from noninitiating cells based on surface marker expression [1]. Indeed, starting from patient-derived material, tumorigenic cells that were found to be positive for CD44, negative for CD24 or expressing low levels of CD24 and negative for lineage markers could be serially transplanted and generated heterogeneous tumors [1].
5.2.2 Tumor Heterogeneity and Tumor Initiating Capability of CSC
At present, the available evidence supports that a tumor can be regarded as a heterogeneous aberrant tissue, possibly originating from a single cancer stem cell, and maintained by the surrounding niche which contains stromal cells and other components of the microenvironment (i.e., immune cells). Thus, the definition of CSC has been précised over time and expanded to comprise tumor cells capable to regrow the tumor from which they were isolated [115]. In such a view, several laboratories have directed their efforts toward the isolation of CSC from tumor biopsies and from tumor cell lines with the final goal to discover druggable targets expressed by CSC. In principle, CSC are endowed with tumor initiating capability—supported by in vivo testing—and with differentiation properties. In addition, CSC express a set of markers that allow researchers to accomplish their identification and isolation. Using multiple tools, it has been shown that subpopulations of CSC may account for tumor initiation, invasive growth, and dissemination to distant organs [90]. It has been documented that a rare population of CSC is more easily detectable in hematological malignancies than in solid tumors, like for example in leukemia [64]. Indeed, Lapidot and colleagues identified an acute myeloid leukemia cell initiating human acute myeloid leukemia after transplantation into severe combined immune-deficient mice. Such cells that were CD34 positive and CD38 negative, displayed leukemic cell morphology and produced a pattern of dissemination similar to what observed in the original patients [64]. No information about the expression of ABC transporters, in particular of BCRP, was provided in this study for historical reasons. Indeed, the gene coding for BCRP was cloned in 1998 and subsequent studies revealed the expression of ABC transporters (see below).
5.2.3 CSC and the Cell of Origin
A matter of debate in the field of CSC is the relationship between CSC and cell of origin of tumors. It is important to note that a clear distinction between the cancer stem cell and the cell of origin of a tumor has recently been proposed [107]. In this perspective, the cell of origin would be the tumor initiating cell and CSC would be tumor propagating cells. Such distinct cell categories would have different phenotypes. In chronic myeloid leukemia, the cell of origin has been recognized as the hematopoietic stem cell containing the BCR-ABL mutation; however, subsequent genetic lesions in progenitor cells downstream of the hematopoietic stem cell produce leukemia stem cells [107]. Additional evidence has shown that cancer can arise from differentiated cells (e.g. T-cell acute lymphoblastic leukemia) [76]. In particular, the LMO2 oncogene was reported to induce a subset of human T cell acute lymphoblastic leukemias, by promoting the self-renewal of pre-leukemic thymocytes; thus, committed T cells appear to accumulate additional genetic mutations required for leukemic transformation [76]. Moreover, whether in solid tumors the stem or progenitor cells appear to be candidates for tumor initiation, the exact mapping of the cell of origin is far from being completed, and further effort is needed in this field.
5.2.4 CSC and Differentiation
The capability of putative CSC to differentiate can be easily proven in specific tumor types, whereas in others it is not clear which differentiation markers should be considered. In fact, tumors present phenotypic plasticity and dedifferentiation properties that may result in changes of markers and molecular features during disease progression. Some diseases provide examples of clearly assessable differentiation markers (e.g., neuroblastoma, melanoma). In this regard, melanospheres containing tumor initiating cells when cultured in differentiating media for the mesenchymal lineages (adipocytes, chondrocytes, osteocytes) were shown to be capable of differentiating into all cell types [84]. In addition to their differentiation ability, like normal stem cells, CSC must be capable of self-renewal, which is the ability to undergo an unlimited number of replicative cycles, while maintaining the stemness properties. The cancer stem cell produces one cancer stem cell and a cell which differentiates by asymmetric division. This phenomenon allows the maintenance of the pool of CSC (Fig. 5.1).
Schematic representation of cell division of cancer stem cells. Cancer stem cells (CSC) can divide symmetrically producing two daughter cells with the same characteristics of the cancer stem cell of origin, or can divide asymmetrically, thereby generating differentiated cells. Over the different rounds of division, cells with decreased stemness features as compared to the cancer stem cell of origin can be generated. Such cells are designated as pluripotent progenitors. Cells that have lost the stemness phenotype can eventually dedifferentiate and reacquire some stemness features
5.2.5 CSC and Metastasis
Increasing evidence supports the notion that CSC play a role in tumor progression and may be responsible for tumor growth as well as for metastatic spread, a multistep process in which epithelial to mesenchymal transition (EMT) occurs [73]. Of note, the process of EMT—in which cells lose the expression of epithelial markers and acquire a mesenchymal phenotype—has been shown to be able to produce cells with stem cell-like features [74]. The coexistence of a stationary phase population embedded in the epithelial tissue that cannot disseminate (stationary CSC) and a migratory population of mobile cells located at the tumor microenvironment interface (migratory CSC) has been proposed [10]. In keeping with a tight relation between EMT and stemness, when tumor cells undergo EMT, a number of properties and processes (invasiveness, drug resistance, angiogenesis, and metastasis) appear to be increased in parallel, thus generating a tumor with more aggressive characteristics. The acquisition of a drug-resistant phenotype is associated with the expression of ABC transporters (see below).
5.2.6 Tumor Initiating Capability of CSC and Markers
The demonstration of the tumor initiating capability is a critical aspect in the CSC field because in vivo experiments are required and the use of immune-compromised mice is hampered by the need for properly equipped animal facilities as well as by the costs. Besides being capable of self-renewal and asymmetric division, CSC should have tumor initiating capability (Fig. 5.2). However, not all the literature published on CSC contains proofs about the tumor initiating capability of the studied CSC. This is the reason why, the designation of cancer stem-like cells should be preferred in general to that of CSC, and specifically in experimental work not including assessment of the tumor initiating capability. Indeed, CSC are very often identified (a) on the basis of the expression of multiple markers (CD24, CD29, CD44, CD133, ALDH1, etc.) that, however, are not necessarily unique to CSC [53, 56, 103] or (b) on their ability to grow independently of anchorage (i.e., as spheres) in serum-free medium added with growth factors. Of note, such growth factors are recognized as being capable of activating survival pathways, a feature that has rendered complex the set up of comparisons between the growth characteristics of differentiated cells, cultured in serum-containing media, and those of CSC cells cultured in peculiar media, under circumstances often difficult to standardize among different laboratories due to patent issues and/or to complex procedures.
5.3 ATP-Binding Cassette Transporters
Membrane proteins of the ABC super-family have been documented to participate in energy-dependent efflux of a variety of structurally unrelated antitumor agents [26]. Such a phenomenon is known as multidrug resistance and besides over-expression of efflux pumps it can involve other interrelated or independent mechanisms. ABC transporters play a relevant physiological role in protection against xenotoxins. In fact, they decrease the intestinal uptake and tissue penetration representing important physiological barriers (brush border membrane of intestinal cells and epithelium of the blood-brain barrier) and mediate excretion of their substrates [3]. Based on sequence similarity, all ABC transporters can be grouped in seven classes (A–G) and members of at least four classes (A, B, C, G) have been clearly implicated in conferring resistance to antitumor agents [33]. All ABC transporters share an ABC domain, but the organization of the domains is different in various transporters with diverse numbers and location of trans-membrane domains. The structural characteristics may influence folding of the transporter as well as substrate accessibility, thereby regulating transport properties. Among the 49 members of the ABC super-family, different transporters play a major role in multidrug resistance. The first identified transporter for which a contribution to multidrug resistance has been shown is the P-glycoprotein (Pg-p) encoded by the ABCB1 gene [59]. Pg-p is the best known ABC transporter and is a 170 kDa protein which transports neutral, cationic, and hydrophobic compounds, including antitumor agents commonly used in the clinical setting (anthracyclines, camptothecins, epipodophyllotoxins, Vinca alkaloids). The MRP (multidrug resistance-related protein) family, which comprises ten members has been implicated in conferring resistance to several antitumor agents. The first member of the family, MRP1, has shown transport specificity similar to that of Pg-p, but it transports drugs conjugated with glutathione or anionic compounds [17]. BCRP, the second member of the ABCG family, is encoded by ABCG2. It is a 72 kDa protein which transports unmodified drugs and xenobiotics [23]. It is an organic anion pump very efficient in transport of sulfate, glucuronic acid, and glutathione conjugates. ABCB5 codes for a protein of 90 kDa that has been implicated in the efflux of the DNA topoisomerase II inhibitor doxorubicin and more recently in reduced sensitivity to 5-fluorouracil [28, 110].
At the time the above mentioned transporters were identified and cloned, studies regarding the hypothesis on CSC were already ongoing, but the two lines of research were somehow parallel till the SP assay was set up and innovative technical approaches allowed fine molecular studies (Fig. 5.3). The clinical relevance of increased expression of ABC transporters in conferring resistance in patients is still a matter of debate, but it is possible that the ABC transporter super-family plays still not well defined roles in CSC.
5.3.1 Profiling of ABC Transporters
The advent of genome-wide approaches as well as of quantitative methods for examining expression of gene families have allowed researchers to improve the molecular characterization of tumors, with particular focus on drug-resistant tumors. Expression profiling of ABC transporter super-family genes has been carried out both in drug-resistant cell lines and in tumor specimens, including posttreatment tumors [47, 87] to identify genes that are potential regulators of drug resistance or modifiers of progression and/or response. More refined analyses have included an evaluation of specific transporters at the mRNA and protein levels. The concomitant analysis of the transcript and protein appears a wise strategy, if considering the layers of regulation occurring between mRNAs and proteins. In addition to a variable stability of the different mRNA molecules, the regulation by microRNAs (miRNAs) is an important aspect that may explain discrepancies between mRNA and protein levels. In fact, miRNAs, endogenous, noncoding-single stranded RNAs of 19–25 nucleotides that can modulate gene expression, play an important role in regulating different aspect of the biology of CSC (proliferation, differentiation), and are therefore expected to participate in a complex network that also regulates ABC transporters. In general, miRNAs target specific mRNAs, thereby causing their degradation [101]. The levels of several genes of the ABC super-family have been shown to be modulated by miRNAs, which are expected to be involved in CSC biology [33]. Of note, the available literature already supports the complex transcriptional regulation of the ABCG2 transcript that in cellular studies has been reported to be downregulated by various miRNAs such as miR-328, miR519c and miR-520h, miR-487a, miR-181a [33, 71, 66, 58, 97, 109].
An analysis of the expression of ABC transporters has been undertaken also in different models of CSC, as these transporters or at least some of them are considered phenotypic markers of CSC and are regarded as functional regulators. For example, putative prostate stem cells and prostate CSC in benign and malignant tumors have been defined by the expression of BCRP and concomitant lack of the androgen receptor [50]. According to the findings, BCRP may protect prostate CSC from androgen deprivation, hypoxia, or chemotherapy, thus favoring recurrence of prostate cancer [50].
A relevant evidence emerging from the recent literature is the link between the ABC transporter activity and radiation resistance [52]. In this regard, sensitization to radiation was found in pediatric medulloblastoma cells upon treatment with the ABC transporter inhibitors verapamil or reserpine. Of note, radiation tolerant cells displayed stem cell-like behavior (e.g., increased tumorigenic potential). In medulloblastoma specimens, selected ABC transporter super-family members were found to be associated with specific molecular subtypes (high ABCA8 and ABCB4 in Sonic/Hedgehog-driven tumors) [52]. The mechanism for increased ABC transporter expression in radiation resistant cells is not clear, but it is likely that their upregulation results from a stress response, from a pro-survival response, or from activation of regulators of ABC transporters expression. In this regard, it has been shown that both hypoxia and oxidative stress can upregulate or stimulate ABC transporters [54, 95].
In addition, gene expression profiles of normal cells should be taken into account (e.g., melanocyte). In fact, two mRNA isoforms of the ABCB5 gene, ABCB5alfa and ABCB5beta have been shown to be expressed in melanoma, but also in melanocytes, their expression being pigment-cell specific, thereby suggesting their possible involvement in melanogenesis [14]. The expression of ABCB5 and other transporters of the ABC super-family has been linked to the resistance of melanoma cells to structurally unrelated drugs, but also to the resistance of melanocytes to toxic intermediates of melanin metabolism, supporting that the melanogenic pathway may provide therapeutic targets [15].
The profiling of ABC transporters in cancer cells including cancer stem-like cells has been simplified by the availability of quantitative Real time PCR platforms providing standardized assays [37]. However, further effort is needed to set up routine high-throughput analysis for detecting protein levels of the specific transporters.
5.3.2 ABCG2/Breast Cancer Resistance Protein
ABCG2 is a gene included in the super-family of ABC transporters that codes for a protein member of the White subfamily. As briefly mentioned above, the protein is referred to as a breast cancer resistance protein and it acts as a xenobiotic transporter. ABCG2 is a widely studied transporter, which has been characterized in terms of substrate specificity and for its role in drug resistance. Its significance in CSC and in normal stem cells (e.g., placental trophoblasts, neural stem cells, hematopoietic progenitors) physiology is mainly related to its expression in side population cells; indeed, its expression is fundamental for the capability of a cell population to give rise to a side population (see below) [118, 25, 55, 61, 93]. ABCG2 expression in human embryonic stem cells has been debated as conflicting results have been published [83]. However, recent results obtained with sensitive methods indicate that ABCG2 may be regarded as a late stage differentiation marker in cultured human embryonic stem cells.
Leukemic CD34 positive and CD38 negative stem cells are considered relevant to cure acute myeloid leukemia as incomplete eradication of these cells may be responsible for disease relapse. BCRP was found to be expressed by these cells [88]. Inhibition of mitoxantrone extrusion by a specific BCRP inhibitor (the fumitremorgin C analog, KO143) produced increased drug accumulation in cells obtained from different patients, but drug efflux still occurred in the presence of KO143, thereby suggesting that additional transporters including Pg-p and MRP1 are expressed by leukemic stem cells. Consistently, KO143 could not increase chemo-sensitivity of leukemic stem cells. Such a study supports the need for broad-spectrum inhibition of different mechanisms/transporters [88].
Although BCRP was originally identified in breast cancer cells [23], such a transporter plays a role in a variety of tumor types comprising colorectal cancer, brain tumors, etc.
5.3.3 ABCB5
In addition to BCRP, the ABCB5 transporter has been implicated in CSC biology, with particular reference to malignant melanoma; in such a disease ABCB5 has been proposed as a marker of melanoma-initiating cells [94]. Indeed, ABCB5 has been shown to mark CD133-expressing progenitor cells among human epidermal melanocytes, and to positively regulate the propensity of this subpopulation to undergo cell fusion, a process contributing to culture growth and differentiation [29]. ABCB5 has also been involved in doxorubicin efflux transport and it has been already exploited as a therapeutic target by the development of a specific antibody [28].
High ABCB5 expression has been recently associated with progression of oral squamous cell carcinoma and tumor recurrence [44]. Interestingly, double labeling immunofluorescence and immunohistochemistry experiments indicated that ABCB5 was expressed by CD44 positive cells. Unfortunately, in this study, there was no in vitro or in vivo characterization of the stem cell properties of the ABCB5 positive cells. Thus, although the results are statistically sound as ABCB5 was an independent prognostic factor in multivariate analyses, further studies will be needed to establish if such cells are endowed with features of CSC.
ABCB5 positive melanoma cells have been shown to be targeted by parthenolide, a natural sesquiterpene lactone described as an NF-kB inhibitor, endowed with anti-microbial, anti-inflammatory and anticancer effects [19]. Of note, cell survival after treatment exhibited an immunophenotype different from that of control cells. In spite of its limited penetration capacity, parthenolide could target both CSC like cells and bulk tumor cells [19].
Recent evidence suggests that ABCB5 together with CD133 play a critical function in promoting vasculogenic mimicry and the morphogenesis of the perivascular niche in melanoma [63]. In fact, loss of function approaches based on RNA interference could prove that knockdown of CD133 produced an impairment in the cell ability to form vascular mimicry-like channels, a phenomenon associated with the depletion of the ABCB5 positive population [63]. Thus, co-expression of CD133 and ABCB5 in melanoma cells seems to be important for the generation of a vascular-mimicry-dependent perivascular niche, although the specific role of each one of the markers, in particular that of ABCB5, is not clear. Based on the available data, it is uncertain whether the transport capability of ABCB5 is used by CD133/ABCB5 double positive cells to regulate the content of the cellular or extracellular level of molecules which regulate vascular mimicry [63]. This process appears complex and has been shown to involve VEGF-A signaling which stimulates the expression of vascular mimicry associated genes such as CD144, a marker reported to be preferentially expressed by ABCB5 positive cells in colorectal cancer [110]. In this context, ABCB5 appeared to be responsible for resistance to 5-fluorouracil. ABCB5 was expressed only on rare cells within normal intestinal tissue, whereas increased levels of ABCB5 were found in colorectal cancer specimens. The abundance of ABCB5 positive cells appeared increased after treatment in residual disease. Thus, ABCB5 has been proposed as a novel molecular marker of therapy-refractory tumor cells in colorectal cancer patients. Targeting of ABCB5 positive cells is proposed to eradicate such tumors. Moreover, additional evidence is available in melanoma where, by highlighting the role of the immune system in tumor progression, it has been shown that ABCB5 positive melanoma initiating cells induce T regulatory cells via a B7.2-dependent pathway [94].
In keeping with a relevant role for ABCB5 in the biology of CSC and with the drug-resistant phenotype of melanoma cells, melanoma chemotherapy has recently been shown to lead to the selection of ABCB5-expressing cells [12]. In addition, increased expression of the ABCB5 protein from benign nevi to invasive melanoma has been reported in a study in which immunohistochemistry was used [96]. ABCB5 should not be regarded, however, as a transporter playing a role only in melanoma, because an evaluation of its expression in hematological malignances suggested that it may be involved in both the progression and the resistance of acute leukemia [112].
5.3.4 ABCB1/P-Glycoprotein
Breast cancer stem cells have been reported to express high levels of Pg-p [21]. A recent report suggests that the commercially available anti-alcoholism drug disulfiram may be useful in reversing drug resistance of CSC by virtue of its pleiotropic effects on factors expressed by CSC [67]. In fact, disulfiram has been shown to produce persistent inhibition of Pg-p activity by covalent modification of cysteine residues localized in the nucleotide binding domain of the transporter [70]. Besides this effect, disulfiram is capable of inhibiting the activity of ALDH, a marker for CSC [38, 79, 102]. Of note, in a triple negative drug-resistant breast cancer cell line endowed with CSC features (slow cycling, high transporter expression, high levels of embryonic stem cell markers), disulfiram was shown to target CSC characteristics leading to reversal of resistance [67]. This evidence supports the value of drugs that are already available and that may fit with a drug repositioning program.
In addition, although the relevance of Pg-p in conferring resistance in the clinics is still reported as uncertain, likely because in real tumors overexpression is not easily achieved like in cultured cells [9], the clinical relevance of Pg-p might be linked, in principle, to its expression in selected subpopulations of tumors cells present in the tumor (e.g., CSC), that may finally underlie recurrence. Further studies are needed to clarify these aspects.
5.3.5 Multidrug Resistance-Related Protein 1 (MRP1)
MRP1 has also been shown to be increased in SP cells [118], although it does not appear to be a major determinant of the SP phenotype (see below, Sect. 5.4.1). Thus, BCRP appears to be the most relevant determinant of the SP phenotype, but other transporters expressed by SP cells may cooperate with it to efflux drugs, thereby underscoring resistance. Specific inhibitors of each one of the transporters should, therefore, be used to better examine if more than one SP phenotype exists and which transporters are implicated.
5.3.6 ABCA5
Using an approach based on multiple markers, it has been shown that osteosarcoma cell populations enriched for putative CSC are characterized by high ABCA5 expression. Of note, in this study, ABCA5 was proposed as a putative biomarker of CSC [91].
Although ABCA5 is still a poorly understood transporter, its correlation with the differentiation state has been reported in human colon cancer, thereby suggesting a possible role in the CSC biology [82]. Of note, in the same study also ABCB1 was shown to be correlated with the differentiation state, a phenomenon that may be an indicator of common regulation.
5.3.7 Regulation of the Function of Drug Transporters
Increasing evidence supports that cellular survival pathways, in particular the PI3K/Akt pathway, play a role in the biology of CSC. The PI3K/Akt pathway has been shown to be important for maintaining the pluripotency of embryonic stem cells [4]. Indeed, transcriptional analysis and a functional assay have shown that PI3K/Akt together with the MAPK/ERK and NF-kB pathways are down regulated during differentiation of these cells [51]. The PI3K/Akt pathway has been proposed to be required in the maintenance of CSC in the brain, breast, prostate cancer, and glioma [116].
The regulation of CSC by the PI3K/Akt pathway is supported by a variety of studies pointing out (a) a reduced SP cells in the bone marrow of Akt1-null mice, and (b) increased SP cells in the bone marrow of mice following enforced expression of Akt. Moreover, inhibition of the PI3K/Akt pathway has been associated with BCRP internalization, a phenomenon that suggests a regulation of distribution of BCRP by the PI3K/Akt signaling. The precise mechanisms involved are still poorly understood [51]. The relationship between cell survival pathways and ABC transporters is also supported by the association between ABCG2 and HER-2 expression in breast invasive ductal carcinoma [111]. Of note, the Hedgehog pathway had been previously shown to regulate also ABCB1 besides ABCG2, although the molecular determinants of the regulation were less characterized; the pharmacological relevance of the Hedgehog pathway inhibition was proved as its targeting reversed resistance to structurally unrelated antitumor agents [98]. Activated Hedgehog signaling has been implicated in sustaining high ABCG2 expression in diffuse large B cell lymphoma, a disease in which high expression of this transporter was shown to inversely correlate with disease-free survival [99]. A molecular analysis indicated that the ABCG2 promoter contains a binding site for the GLI1 transcription factor; since high ABCG2 and GLI1 expression was found in tumors with lymphnodes involvement, it has been proposed that the stroma microenvironment might regulate ABCG2 and GLI1 [99]. This hypothesis was supported by in vitro assays including coculture experiments in which tumor cells upregulated ABCG2 as a result of stroma cell-induced Hedgehog signaling. In this experimental model, ABCG2 was not the only resistance factor induced by Hedgehog signaling as also anti-apoptotic proteins were upregulated, but ABCG2 was characterized in detail in terms of the transcriptional regulation [99]. An indirect regulation of ABC transporters levels by E2F involving p73 has also been described [2], further corroborating the view of a complex regulatory network acting to favor the survival of CSC.
5.4 The Side Population
ABC transporters are not straightforward markers for CSC, but it has been documented that CSC can express ABC transporters. This characteristic is shared by CSC and normal stem cells. Both normal and CSC can be identified in the so called SP in a dot plot from flow cytometry analysis. In fact, the term “side” refers to the position at the side of the plot. The isolation of SP cells has been carried out and described from different types of normal tissues including the bone marrow and tumors. Indeed, a small fraction of bone marrow cells that can be evidenced by flow cytometry for the ability to efflux the fluorescent dye Hoechst 33342 and are enriched for HSC, has been identified in the hematopoietic compartments of different organisms including humans and in non-hematopoietic tissues [42, 104]. SP cells from murine bone marrow can self-renew and generate both lymphoid and myeloid lineages [42]. Normal HSC express at least two ABC transporters, but the complexity of the ABC transporter family suggests that other members could be present [100]. Studies carried out in mice more than a decade ago have indicated that BCRP, but not MDR1, is responsible for the HSC phenotype [104, 117, 93]. Accordingly, in Abcb1-knockout mice, the SP is not depleted [11]. Expression of the murine orthologue of ABCG2 appears a constant feature of murine stem cells from different sources such as bone marrow, skeletal muscle, primary mammary tissue, and embryos. In murine HSC, Abcg2 is highly expressed and is downregulated during differentiation. The dependency on Abcg2 of the SP phenotype has been clearly defined by gain and loss of function studies [118].
Moreover, flow cytometry approaches applied to the CSC field have allowed researchers to define the existence of a tumor cell fraction that is enriched for drug efflux transporters, specifically ABC transporters. Again, this tumor cell population is functionally defined based on its capability to extrude the specific fluorescent dye Hoechst 33342, an activity that produces a shift of the fluorescence of the cells belonging to this population in a dot plot obtained by flow cytometry analysis. In particular, cells expressing ABC transporters recognizing the fluorescent dye, decrease their fluorescence becoming clearly separated from the rest of the cells. In this kind of assay, verapamil, a calcium channel blocker which binds with high affinity to Pg-p and with less affinity to BCRP, is used, as the SP disappears upon transporter inhibition in the presence of verapamil. Thus, the SP is identifiable as reported in Fig. 5.4. It is important to note that evaluation of the SP is not an easy procedure and should be carried out with a well set up protocol and adequate gating procedures.
Representative plots of side population. The rat C6 glioma cells were used and incubated with Hoechst 33342 (a, b). The so-called side population evidenced in the gate in plot a displays low fluorescence because ABC transporters efflux the dye. If transporters are blocked by verapamil (plot b), the dye is not extruded and the cell fraction which expresses ABC transporters kept inactivated retains the fluorescence of the main cell population. Viable cells were gated and blue (FL-4) and red (FL-5) fluorescence of viable cells are reported. The shown plots are a courtesy of Dr. Emilio Ciusani (Fondazione IRCCS, Istituto Neurologico C. Besta)
The SP assay is routinely carried out in the presence of verapamil, a well known inhibitor of ABCB1. This is surprising as the SP phenotype is thought to be due to the expression of ABCG2. However, verapamil can also inhibit, although less strongly, ABCG2. Indeed, it has been shown that in bone marrow cells, ABCB1 contributes in part to the SP phenotype [118].
A causal link between the expression of ABCG2 and SP has been proposed in a report on leukemia in which over-expression of ABCG2 in Jurkat and HL60 cells was shown to increase the SP; such a phenomenon was concomitant with upregulation of the phosphorylated forms of PI3K and Akt [49]. Conversely, treatment with PI3K or Akt inhibitors downregulated ABCG2 expression, phospho-PI3K, phospho-Akt and SP. Activation of Akt appears to occur via inactivation of PTEN, a lipid phosphatase which has been implicated in preventing leukemogenesis [114]. Thus, ABCG2 regulation by the PI3K/Akt pathway appears a likely phenomenon in leukemia, similarly to what was described in glioma and esophageal cancer [7, 65].
SP cells from bladder cancer were found to be characterized by increased levels of ABCG2 together with phospho-ERK1/2 activation [46]. Accordingly, inhibition of MEK1/2, the upstream regulator of ERK1/2, resulted in inhibition of the SP phenotype. An important finding of this study is the observation that, in tumor specimens, ABCG2 and pERK1/2 were positively correlated and their expression correlated with decreased progression-free survival [46].
The application of the SP approach to the study of CSC has some limitations for subsequent analysis of the tumor initiating capability of the non-SP cell fraction. Such a fraction that in principle should be devoid of tumor initiating capability by virtue of its intrinsic nature, i.e. the lack of stemness, may result devoid of the capability due to the fact that it is treated with a fluorescent dye in the SP assay. In fact, because the dye is a DNA binding agent, it might finally affect the proliferative potential of the non-SP cells simply because it targets the DNA (Fig. 5.5). Again, the use of SP an indicator of stemness should be regarded with caution. Indeed, it has been reported that not all SP populations diplay increased tumorigenic potential as compared to non-SP cells [113].
An important step in the study of the SP has been represented by the isolation of SP cell from biopsies [40]. Using glioblastoma samples grown orthotopically in immune-deficient mice, SP cells of human glioblastoma were found to be stroma-derived and nonneoplastic [40]. Indeed, tumor cells did not exhibit efflux properties which were present in brain-derived endothelial cells and in astrocytes.
In summary, a SP fraction has been demonstrated in different tumor types and the SP has been shown to be endowed with tumor initiating ability. SP cells can divide asymmetrically, generating SP and non-SP cells, and can form spheres when grown in serum-free media. Due to the toxic effects of the dye used in the SP assay, the identification of CSC by the SP assay has major limitations.
5.4.1 Approaches to Modulate the Side Population
Various strategies are being employed at the cellular level to hit the SP in an attempt to discover therapeutic options selective for CSC. Some examples taken from the recent literature are provided below. It has to be considered when examining the literature available on targeting CSC by interference with the SP that, although several of the preclinically tested compounds display an effect on ABC transporters, a direct cause-effect relationship between treatment and ABC transporter inactivation or downregulation cannot always be defined.
A promising approach has exploited the capability of CSC to efflux dyes and to be identified as SP in a high-throughput screening platform in which hit compounds were selected based on decrease of SP after treatment. Thus, in an attempt to hit CSC in breast cancer, in a recent study [41], a combination of an inhibitor of NF-kB (IMD-0354) and nanoparticle-encapsulated doxorubicin has been employed. A reduction in the SP and in ABC transporters (ABCB1, ABCG2) was associated with a decrease of self-renewal genes (Oct4, Sox2, Nanog). The NF-kB inhibitor produced cell death also in non-CSC cells. Of note, targeted delivery to hypoxic cells could be achieved, a feature that allowed the administration of a well tolerated treatment as normal nonhypoxic tissues were spared.
It has been recently reported that low-molecular weight heparin (LMWH), which is approved for anticoagulant therapy, can inhibit survival of lung cancer SP cells, as it decreases their colony forming abilities [81]. Interestingly, it also decreases ABCG2 protein levels by interference with its proteasomal degradation; in fact, LMWH-induced ABCG2 downregulation could be rescued by proteasome inhibition. Treatment with LMWH has been reported to ablate lung cancer cisplatin resistance [81]. However, a clear synergistic interaction between cisplatin and LMWH could not be proven, in keeping with the fact that cisplatin is not a substrate for ABCG2. Thus, it is likely that the combination of cisplatin and LMWH is additive although, in principle, cells downregulating ABCG2 may display reduced fitness when treated with a variety of antitumor agents, even if not substrates, due to the possible transport by ABCG2 of molecules indirectly affecting the cell response to drugs [85]. Among the compounds recently tested on CSC-like models, secalonic acid D, the main toxic metabolite of several strains of Penicillium oxalicum, has shown antiproliferative activity on tumor cells over-expressing ABCB1, ABCC1, and ABCG2 as well as its capability to decrease SP cells in lung cancer cells [48]. Modulation of ABCG2 mRNA levels may occur via epigenetic events induced by pharmacological treatments. For example, the indolamine melatonin which contributes to regulate endocrine functions and has been reported to exhibit cytotoxic and antioxidant effects, appears capable to induce methylation of the ABCG2 promoter [75]. Such a phenomenon—which is prevented by an inhibitor of DNA methylation—has been proposed to underscore the synergism observed between melatonin and antitumor agents in brain tumor stem-like cells [75].
The search for drugs selectively killing CSC has lead to the identification of promising compounds which, however, under most circumstances, are endowed with their activity versus CSC and bulk tumors. Most of the compounds have been tested in preclinical studies, mainly in vitro, and only a fraction of the tested compounds are proposed to act by virtue of their interference with ABC transporters. Among them, salinomycin, a polyether ionophore antibiotic isolated from Streptomyces albus has shown promising results [80]. Such a compound known to be endowed with antibacterial activity was shown to be capable of killing CSC in a murine model of breast cancer [45]. Subsequently, additional studies reported that this biomolecule can kill a variety of human tumor cells [32], thereby providing evidence that it acts both on CSC and the tumor bulk [31]. The drug is already undergoing clinical evaluation [80].
5.5 Markers for CSC Other Than ABC Transporters
Several markers are used to identify CSC in different tumor types, and the available literature suggests unexpected links between some of these markers and ABC transporters. To identify such links, the concomitant expression of ABC transporters and of other markers has to be taken into account. For example, aldehyde dehydrogenase 1 (ALDH1) is a NAD(P)-dependent enzyme which detoxifies endogenous or exogenous aldehydes [38, 56] because it has been implicated in the physiology of normal and CSC, ALDH1 is being used as a marker. Indeed, since the SP allows a functional identification of stem cells, ALDH1 activity has been proposed as a functional marker of potential interest in different tumor types. In 1990, Kastan and colleagues showed that ALDH displays increased activity in human hematopoietic progenitor cells [60]. Since then, a variety of studies have reported the isolation of stem cells from normal and cancer tissues on the basis of ALDH activity [56]. Recent studies have shown that ALDH1 appears to be an appropriate marker for stemness also in human sarcomas [69]. Indeed, the subpopulations characterized by high ALDH1 activity are also endowed with increased proliferation rate, colony forming ability, increased expression of ABC transporters and stemness markers, as well as by reduced sensitivity to antitumor agents as compared to cells with low ALDH1 activity [69].
The concomitant expression of ALDH1 and ABCG2 or other transporters in CSC appears to support the need for the presence in CSC of multiple independent mechanisms for detoxication. Indeed, because stem cells are rare it is reasonable that they try to activate a complex pro-survival response in which different factors can eventually cooperate to improve chances of survival.
A very interesting study has shown that ABC transporters can be transcriptionally regulated by the transcription factor Oct1, which is fundamental in self-renewal [72]. Thus, in drug-resistant cells, there are genes that act as hubs by coregulating multiple processes finally leading to drug resistance. For example, exposure of breast cancer cells to TGF beta or Twist over-expression have been shown to lead to enhanced expression of ABC transporters [105]. Conversely, knockdown of Twist and Zeb, besides reversing EMT, also results in reversal of drug resistance [73]. In addition, a positive correlation between ABCG2 and Oct4 has been reported in cellular models of liver CSC, in which the efflux transporter and the transcription factors involved in self-renewal appeared to be highly expressed in CD90/CD133 positive cells [57].
5.6 Clinical Implications of the CSC Hypothesis
The translation of the CSC hypothesis toward the clinics is far from being accomplished also in view of the skepticism regarding the biology of CSC. There is a wide heterogeneity in the experimental models used for CSC, especially in vitro, where research for appropriate 3D culture systems is still ongoing [13]. In spite of this, some clinical studies may already offer positive results in terms of validation of the CSC hypothesis in the clinical setting. In fact, as recently reviewed [36], enrichment for tumor cells with a CSC phenotype has been reported in minimal residual disease of different tumor types. Thus, the CSC hypothesis may explain why patients cannot be cured in spite of initial responses; some studies support this concept. For example, in breast cancer, residual tumor cells after conventional treatment have been shown to display tumor initiating cell features [18]. In addition, Huff and colleagues have shown that a correlation between clonogenic growth of CSC and clinical outcomes occurs in multiple myeloma [36]. The expression of a stem cell phenotype by minimal residual disease in acute myeloid leukemia has also been documented [34].
The association between breast cancer stem cells and resistance to paclitaxel-epirubicin based chemotherapy has been reported in a case material of primary breast cancer patients [102]. In such a study, breast CSC identified as ALDH1-positive, but not CD44 positive/CD24 negative cells, have been proposed to play a role in resistance to chemotherapy. This study underscores the variability that may result from considering different markers. Although it is likely that ALDH positive cells express ABC transporters, this aspect was not considered in the study.
Many of the ongoing clinical trials in which ABC transporters relevant in the CSC biology, in particular ABCG2, have been taken into account, deal with pharmacokinetics and pharmaco-dynamics issues in an attempt to establish the role of single nucleotide polymorphisms of drug transporters in the efficacy of therapies based on the use of substrates of transporters. Moreover, a certain number of trials focuses on the assessment of the feasibility of isolation and characterization of CSC (NCT01641003), on the set up of reliable drug sensitivity assays (Feasibility Study on Stem Cells Sensitivity Assay, STELLA, NCT01483001), and on the characterization of CSC of different tumor types. A few studies are already directed at evaluating the anti-cancer stem cell activity of treatment, for example by measuring the amount of ALDH1 positive cells before and after treatment (NCT01190345), or in an attempt to target CSC for prevention of relapse (NCT01579812). When the results of these clinical studies will be available, it will be possible to consider the opportunity to translate positive achievements to the routine clinical analysis, following further validation in further studies.
5.7 Discussion
In spite of the technical progress and of the intellectual knowledge acquired in the molecular characterization of tumors and in the processes leading to tumorigenesis, multidrug resistance still represents an obstacle to the cure of cancer. ABC transporters are implicated in multidrug resistance of tumor cells mainly because of their capability to extrude toxic compounds including antitumor agents from cells, but also through indirect mechanisms as recently reviewed by Fletcher and colleagues [26].
If CSC are indeed the tumor cells that maintain the tumor and they express ABC transporters, a successful therapeutic option tempting to cure patients or at least to improve disease-free survival should include drugs targeting transporters of the ABC super-family, in particular ABCG2, because the product of this gene is the most frequently reported transporter in CSC. However, it is evident that the expression of ABC transporters is not an exclusive characteristic of CSC, as their expression in tumors might also be related to the tissue of origin of the tumor. In addition, also normal stem cells express ABC transporters. Thus, a wise therapeutic strategy would need to spare normal cells. Accordingly, selectivity of therapies remains an important issue and there is an effort towards testing of selective approaches at the preclinical level, like for example in a study in which targeting of hypoxic cells was undertaken [41].
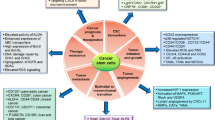
The effects of natural compounds on cancer stem-like cells have been recently reviewed, highlighting the variety of pathways that can be targeted in an attempt to kill CSC [24, 68]. Different pathways including self-renewal pathways, Wnt/β-catenin, Sonic Hedgehog, and Notch signaling are implicated in the biology of CSC and they can favor high self-renewal potential, survival, invasion and the metastatic behavior of CSC and their progeny [78, 106]. Thus, the expression of ABC transporters is only one of the mechanisms by which CSC evade the effects of therapies (Fig. 5.6). Because ABC transporters do not appear to be simply in charge of efflux of drugs, but they participate in a complex molecular network in which other mechanisms, in part even coregulated mechanisms, contribute to cell survival, the inhibition of their function could be regarded as a sort of multi-targeting strategy. Indeed, it has been reported that transcription factors that participate in EMT can positively regulate ABC transporters [72, 92]. Moreover, it has been reported that the PI3K/Akt pathway can modulate the function of transporters through different mechanisms [51]. In addition, in keeping with the wide current interest in metabolic alterations of tumor cells, SP cells have been shown to exhibit increased glycolytic activity than non-SP cells [68].
Multifactorial nature of the drug-resistant phenotype of cancer stem cells. Some characteristics of cancer stem cells (CSC) that can underlie their drug-resistant phenotype are shown. The increased expression of ABC transporters is one of the drug resistance mechanisms of CSC that could produce resistance to drugs of different classes. ABC transporters can be regulated by survival pathways and transcription factors acting in self-renewal can up-regulate ABC transporters
In summary, research on CSC is a fast-moving field, but translation of results in the clinics is still at an early stage. Additional studies are required to establish a precise link between expression of BCRP or other ABC transporters and stem cell-like features and behavior. Prospective studies are required to establish the utility of less characterized transporters as therapeutic targets for CSC. The evidence that protection of CSC against drugs and toxins is mediated by expression of several ABC transporters continues to provide therapeutic opportunities to overcome resistance. However, it is true that a careful consideration of the specific literature should be made when facing the field of CSC, also considering that CSC have been correctly designated as a moving target [27]. With specific reference to the analysis of levels of ABC transporters in addition to modulation of mRNA levels, also protein levels should be considered. Again, not only ABCG2 should be taken into account, given the complexity of the ABC super-family. In an attempt to generate experimental models for studying CSC, enrichment for CSC by therapy has been proposed [30]. However, this approach cannot be exclusive, but it should be complementary to the others reported above. With such caveats and trying to control the complexity of the biology of CSC, it will be easier to establish also the predictive and prognostic significance of CSC, as recently proposed for Non-small Cell Lung Cancer [43, 86]. The complexity of the ABC transporter super-family suggests that it will be a difficult task to clearly define the specific role of ABC transporters in CSC biology and resistance. The role of ABCG2 as well as of a few other transporters has been in part defined, but we are far from effective strategies to target them for modulation of antitumor therapy.
In conclusion, among the transporters involved in CSC biology, ABCG2 appears to play a remarkable role, because it is expressed by SP cells, and its expression is associated with the activation of cell survival pathways and with the expression of self-renewal genes in specific models of CSC. Indeed, BCRP is very likely to play relevant physiological functions because of its expression by normal stem cells and by CSC or cancer stem-like cells. In addition, the less known ABCB5 transporter, besides playing a role in melanoma resistance, may be of relevance also in colon carcinoma and leukemia (see above). It is therefore expected that CSC-related research will provide knowledge useful for the development of novel therapeutic strategies involving targeting of ABC transporters. Even if the contribution of different transporters to drug resistance of CSC remains to be clarified, it is evident that a lot of information about transporters is already available from the in vitro and in vivo preclinical studies carried out using cell cultures or murine models and xenografts. For instance, the mutational status of specific transporters can affect their interaction with substrate and the reversal activity of all modulators may be influenced by the gene status of the transporters. An efficient targeting of CSC will be possibly achieved also considering the complexity of the tumor niche and of all the processes favoring the maintenance and survival of CSC [35, 62].
Abbreviations
- CSC:
-
Cancer stem cells
- ABC:
-
ATP binding cassette
- HSC:
-
Hematopoietic stem cells
- SP:
-
Side population
- BCRP:
-
Breast cancer resistance protein
- EMT:
-
Epithelial to mesenchymal transition
- Pg-p:
-
P-Glycoprotein
- MRP:
-
Multidrug resistance-related protein
- miRNAs:
-
microRNAs
- MRP1:
-
Multidrug resistance-related protein 1
- PI3K:
-
Phosphoinositide-3-kinase
- Akt:
-
V-Akt murine thymoma viral oncogene homolog
- MAPK:
-
Mitogen-activated protein kinases
- ERK:
-
Extracellular signal-regulated kinases
- LMWH:
-
Low molecular weight heparin
- ALDH1:
-
Aldehyde dehydrogenase 1
References
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8.
Alla V, Kowtharapu BS, Engelmann D, Emmrich S, Schmitz U, Steder M, Putzer BM. E2F1 confers anticancer drug resistance by targeting ABC transporter family members and bcl-2 via the p73/DNp73-miR-205 circuitry. Cell Cycle. 2012;11:3067–78.
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–98.
Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, Peters H, Walter T, Stojkovic P, Evans J, Stojkovic M, Lako M. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–913.
Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabro E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106: 16281–6.
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5:899–904.
Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–35.
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7.
Borst P. Cancer drug pan-resistance: pumps, cancer stem cells, quiescence, epithelial to mesenchymal transition, blocked cell death pathways, persisters or what? Open Biol. 2012;2: 120066.
Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5: 744–9.
Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24: 3–12.
Chartrain M, Riond J, Stennevin A, Vandenberghe I, Gomes B, Lamant L, Meyer N, Gairin JE, Guilbaud N, Annereau JP. Melanoma chemotherapy leads to the selection of ABCB5-expressing cells. PLoS One. 2012;7:e36762.
Chen J, Wang J, Chen D, Yang J, Yang C, Zhang Y, Zhang H, Dou J. Evaluation of characteristics of CD44+CD117+ ovarian cancer stem cells in three dimensional basement membrane extract scaffold versus two dimensional monocultures. BMC Cell Biol. 2013;14:7.
Chen KG, Szakacs G, Annereau JP, Rouzaud F, Liang XJ, Valencia JC, Nagineni CN, Hooks JJ, Hearing VJ, Gottesman MM. Principal expression of two mRNA isoforms (ABCB 5alpha and ABCB 5beta) of the ATP-binding cassette transporter gene ABCB 5 in melanoma cells and melanocytes. Pigment Cell Res. 2005;18:102–12.
Chen KG, Valencia JC, Gillet JP, Hearing VJ, Gottesman MM. Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma. Pigment Cell Melanoma Res. 2009;22:740–9.
Clevers H. Stem cells, asymmetric division and cancer. Nat Genet. 2005;37:1027–8.
Cole SP, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM, Deeley RG. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994;54:5902–10.
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–5.
Czyz M, Koprowska K, Sztiller-Sikorska M. Parthenolide reduces the frequency of ABCB5-positive cells and clonogenic capacity of melanoma cells from anchorage independent melanospheres. Cancer Biol Ther. 2013;14:135–45.
Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84.
Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14:3–9.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84.
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–70.
Efferth T. Stem cells, cancer stem-like cells, and natural products. Planta Med. 2012;78: 935–42.
Evseenko DA, Paxton JW, Keelan JA. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos. 2007;35:595–601.
Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–56.
Francipane MG, Chandler J, Lagasse E. Cancer stem cells: a moving target. Curr Pathobiol Rep. 2013;1:111–8.
Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–33.
Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, Sayegh MH, Frank MH. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156–65.
Freitas DP, Teixeira CA, Santos-Silva F, Vasconcelos MH, Almeida GM. Therapy-induced enrichment of putative lung cancer stem-like cells. Int J Cancer. 2014;134(6):1270–8.
Fuchs D, Daniel V, Sadeghi M, Opelz G, Naujokat C. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem Biophys Res Commun. 2010;394:1098–104.
Fuchs D, Heinold A, Opelz G, Daniel V, Naujokat C. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem Biophys Res Commun. 2009;390:743–9.
Gatti L, Cossa G, Beretta GL, Zaffaroni N, Perego P. Novel insights into targeting ATP-binding cassette transporters for antitumor therapy. Curr Med Chem. 2011;18:4237–49.
Gerber JM, Qin L, Kowalski J, Smith BD, Griffin CA, Vala MS, Collector MI, Perkins B, Zahurak M, Matsui W, Gocke CD, Sharkis SJ, Levitsky HI, Jones RJ. Characterization of chronic myeloid leukemia stem cells. Am J Hematol. 2011;86:31–7.
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–17.
Ghiaur G, Gerber J, Jones RJ. Concise review: cancer stem cells and minimal residual disease. Stem Cells. 2012;30:89–93.
Gillet JP, Gottesman MM. Advances in the molecular detection of ABC transporters involved in multidrug resistance in cancer. Curr Pharm Biotechnol. 2011;12:686–92.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67.
Glasspool RM, Teodoridis JM, Brown R. Epigenetics as a mechanism driving polygenic clinical drug resistance. Br J Cancer. 2006;94:1087–92.
Golebiewska A, Bougnaud S, Stieber D, Brons NH, Vallar L, Hertel F, Klink B, Schrock E, Bjerkvig R, Niclou SP. Side population in human glioblastoma is non-tumorigenic and characterizes brain endothelial cells. Brain. 2013;136:1462–75.
Gomez-Cabrero A, Wrasidlo W, Reisfeld RA. IMD-0354 targets breast cancer stem cells: a novel approach for an adjuvant to chemotherapy to prevent multidrug resistance in a murine model. PLoS One. 2013;8:e73607.
Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–45.
Gottschling S, Schnabel PA, Herth FJ, Herpel E. Are we missing the target? Cancer stem cells and drug resistance in non-small cell lung cancer. Cancer Genomics Proteomics. 2012;9: 275–86.
Grimm M, Krimmel M, Polligkeit J, Alexander D, Munz A, Kluba S, Keutel C, Hoffmann J, Reinert S, Hoefert S. ABCB5 expression and cancer stem cell hypothesis in oral squamous cell carcinoma. Eur J Cancer. 2012;48:3186–97.
Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–59.
Hepburn AC, Veeratterapillay R, Williamson SC, El-Sherif A, Sahay N, Thomas HD, Mantilla A, Pickard RS, Robson CN, Heer R. Side population in human non-muscle invasive bladder cancer enriches for cancer stem cells that are maintained by MAPK signalling. PLoS One. 2012;7:e50690.
Hlavac V, Brynychova V, Vaclavikova R, Ehrlichova M, Vrana D, Pecha V, Kozevnikovova R, Trnkova M, Gatek J, Kopperova D, Gut I, Soucek P. The expression profile of ATP-binding cassette transporter genes in breast carcinoma. Pharmacogenomics. 2013;14:515–29.
Hu YP, Tao LY, Wang F, Zhang JY, Liang YJ, Fu LW. Secalonic acid D reduced the percentage of side populations by down-regulating the expression of ABCG2. Biochem Pharmacol. 2013;85:1619–25.
Huang FF, Wu DS, Zhang L, Yu YH, Yuan XY, Li WJ, Chen XP, Zhao XL, Chen FP, Zeng H. Inactivation of PTEN increases ABCG2 expression and the side population through the PI3K/akt pathway in adult acute leukemia. Cancer Lett. 2013;336:96–105.
Huss WJ, Gray DR, Greenberg NM, Mohler JL, Smith GJ. Breast cancer resistance protein-mediated efflux of androgen in putative benign and malignant prostate stem cells. Cancer Res. 2005;65:6640–50.
Imai Y, Yamagishi H, Ono Y, Ueda Y. Versatile inhibitory effects of the flavonoid-derived PI3K/akt inhibitor, LY294002, on ATP-binding cassette transporters that characterize stem cells. Clin Transl Med. 2012;1:24.
Ingram WJ, Crowther LM, Little EB, Freeman R, Harliwong I, Veleva D, Hassall TE, Remke M, Taylor MD, Hallahan AR. ABC transporter activity linked to radiation resistance and molecular subtype in pediatric medulloblastoma. Exp Hematol Oncol. 2013;2:26.
Irollo E, Pirozzi G. CD133: to be or not to be, is this the real question? Am J Transl Res. 2013;5:563–81.
Ishikawa T, Nakagawa H, Hagiya Y, Nonoguchi N, Miyatake S, Kuroiwa T. Key role of human ABC transporter ABCG2 in photodynamic therapy and photodynamic diagnosis. Adv Pharmacol Sci. 2010;2010:587306.
Islam MO, Kanemura Y, Tajria J, Mori H, Kobayashi S, Hara M, Yamasaki M, Okano H, Miyake J. Functional expression of ABCG2 transporter in human neural stem/progenitor cells. Neurosci Res. 2005;52:75–82.
Januchowski R, Wojtowicz K, Zabel M. The role of aldehyde dehydrogenase (ALDH) in cancer drug resistance. Biomed Pharmacother. 2013;67:669–80.
Jia Q, Zhang X, Deng T, Gao J. Positive correlation of Oct4 and ABCG2 to chemotherapeutic resistance in CD90(+)CD133(+) liver cancer stem cells. Cell Reprogram. 2013;15:143–50.
Jiao X, Zhao L, Ma M, Bai X, He M, Yan Y, Wang Y, Chen Q, Zhao X, Zhou M, Cui Z, Zheng Z, Wang E, Wei M. MiR-181a enhances drug sensitivity in mitoxantone-resistant breast cancer cells by targeting breast cancer resistance protein (BCRP/ABCG2). Breast Cancer Res Treat. 2013;139:717–30.
Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V. Amplification of P-glycoprotein in multidrug-resistant mammalian cell lines. Nature. 1985;316:817–9.
Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75:1947–50.
Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–8.
LaBarge MA. The difficulty of targeting cancer stem cell niches. Clin Cancer Res. 2010;16:3121–9.
Lai CY, Schwartz BE, Hsu MY. CD133+ melanoma subpopulations contribute to perivascular niche morphogenesis and tumorigenicity through vasculogenic mimicry. Cancer Res. 2012;72:5111–8.
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8.
Li H, Gao Q, Guo L, Lu SH. The PTEN/PI3K/akt pathway regulates stem-like cells in primary esophageal carcinoma cells. Cancer Biol Ther. 2011;11:950–8.
Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, Yu AM. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328, -519c and -520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol. 2011;81:783–92.
Liu P, Kumar IS, Brown S, Kannappan V, Tawari PE, Tang JZ, Jiang W, Armesilla AL, Darling JL, Wang W. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br J Cancer. 2013;109:1876–85.
Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, Hu Y, Wang P, Ju HQ, Xu RH, Huang P. Metabolic regulation of cancer cell side population by glucose through activation of the akt pathway. Cell Death Differ. 2014;21:124–35.
Lohberger B, Rinner B, Stuendl N, Absenger M, Liegl-Atzwanger B, Walzer SM, Windhager R, Leithner A. Aldehyde dehydrogenase 1, a potential marker for cancer stem cells in human sarcoma. PLoS One. 2012;7:e43664.
Loo TW, Bartlett MC, Clarke DM. Disulfiram metabolites permanently inactivate the human multidrug resistance P-glycoprotein. Mol Pharm. 2004;1:426–33.
Ma MT, He M, Wang Y, Jiao XY, Zhao L, Bai XF, Yu ZJ, Wu HZ, Sun ML, Song ZG, Wei MJ. MiR-487a resensitizes mitoxantrone (MX)-resistant breast cancer cells (MCF-7/MX) to MX by targeting breast cancer resistance protein (BCRP/ABCG2). Cancer Lett. 2013;339:107–15.
Maddox J, Shakya A, South S, Shelton D, Andersen JN, Chidester S, Kang J, Gligorich KM, Jones DA, Spangrude GJ, Welm BE, Tantin D. Transcription factor Oct1 is a somatic and cancer stem cell determinant. PLoS Genet. 2012;8:e1003048.
Mallini P, Lennard T, Kirby J, Meeson A. Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev. 2014;40(3):341–8.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Martin V, Sanchez-Sanchez AM, Herrera F, Gomez-Manzano C, Fueyo J, Alvarez-Vega MA, Antolin I, Rodriguez C. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br J Cancer. 2013;108:2005–12.
McCormack MP, Young LF, Vasudevan S, de Graaf CA, Codrington R, Rabbitts TH, Jane SM, Curtis DJ. The Lmo2 oncogene initiates leukemia in mice by inducing thymocyte self-renewal. Science. 2010;327:879–83.
McCulloch EA, Till JE. Perspectives on the properties of stem cells. Nat Med. 2005;11:1026–8.
Mimeault M, Batra SK. Altered gene products involved in the malignant reprogramming of cancer stem/progenitor cells and multitargeted therapies. Mol Aspects Med. 2013, in press.
Morimoto K, Kim SJ, Tanei T, Shimazu K, Tanji Y, Taguchi T, Tamaki Y, Terada N, Noguchi S. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci. 2009;100:1062–8.
Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol. 2012;2012:950658.
Niu Q, Wang W, Li Y, Ruden DM, Wang F, Li Y, Wang F, Song J, Zheng K. Low molecular weight heparin ablates lung cancer cisplatin-resistance by inducing proteasome-mediated ABCG2 protein degradation. PLoS One. 2012;7:e41035.
Ohtsuki S, Kamoi M, Watanabe Y, Suzuki H, Hori S, Terasaki T. Correlation of induction of ATP binding cassette transporter A5 (ABCA5) and ABCB1 mRNAs with differentiation state of human colon tumor. Biol Pharm Bull. 2007;30:1144–6.
Padmanabhan R, Chen KG, Gillet JP, Handley M, Mallon BS, Hamilton RS, Park K, Varma S, Mehaffey MG, Robey PG, McKay RD, Gottesman MM. Regulation and expression of the ATP-binding cassette transporter ABCG2 in human embryonic stem cells. Stem Cells. 2012;30:2175–87.
Perego M, Tortoreto M, Tragni G, Mariani L, Deho P, Carbone A, Santinami M, Patuzzo R, Mina PD, Villa A, Pratesi G, Cossa G, Perego P, Daidone MG, Alison MR, Parmiani G, Rivoltini L, Castelli C. Heterogeneous phenotype of human melanoma cells with in vitro and in vivo features of tumor-initiating cells. J Invest Dermatol. 2010;130:1877–86.
Perego P, Gatti L, Beretta GL. The ABC of glycosylation. Nat Rev Cancer. 2010;10:523.
Pirozzi G, Tirino V, Camerlingo R, La Rocca A, Martucci N, Scognamiglio G, Franco R, Cantile M, Normanno N, Rocco G. Prognostic value of cancer stem cells, epithelial-mesenchymal transition and circulating tumor cells in lung cancer. Oncol Rep. 2013;29: 1763–8.
Pizzamiglio S, Cossa G, Gatti L, Beretta GL, Corna E, Tinelli S, Verderio P, Perego P. Simultaneous confidence intervals to compare gene expression profiles using ABC transporter TaqMan microfluidic cards. Oncol Rep. 2010;23:853–60.
Raaijmakers MH, de Grouw EP, Heuver LH, van der Reijden BA, Jansen JH, Scheper RJ, Scheffer GL, de Witte TJ, Raymakers RA. Breast cancer resistance protein in drug resistance of primitive CD34+ 38− cells in acute myeloid leukemia. Clin Cancer Res. 2005;11:2436–44.
Raftopoulou M. The needle in the haystack. Nat Cell Biol. 2006;Milestone 6:1.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11.
Saini V, Hose CD, Monks A, Nagashima K, Han B, Newton DL, Millione A, Shah J, Hollingshead MG, Hite KM, Burkett MW, Delosh RM, Silvers TE, Scudiero DA, Shoemaker RH. Identification of CBX3 and ABCA5 as putative biomarkers for tumor stem cells in osteosarcoma. PLoS One. 2012;7:e41401.
Saxena M, Stephens MA, Pathak H, Rangarajan A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011;2:e179.
Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–12.
Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–9.
Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22: 7496–511.
Setia N, Abbas O, Sousa Y, Garb JL, Mahalingam M. Profiling of ABC transporters ABCB5, ABCF2 and nestin-positive stem cells in nevi, in situ and invasive melanoma. Mod Pathol. 2012;25:1169–75.
Shen WW, Zeng Z, Zhu WX, Fu GH. MiR-142-3p functions as a tumor suppressor by targeting CD133, ABCG2, and Lgr5 in colon cancer cells. J Mol Med (Berl). 2013;91:989–1000.
Sims-Mourtada J, Izzo JG, Ajani J, Chao KS. Sonic hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene. 2007;26:5674–9.
Singh RR, Kunkalla K, Qu C, Schlette E, Neelapu SS, Samaniego F, Vega F. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene. 2011;30:4874–86.
Sorrentino BP, McDonagh KT, Woods D, Orlic D. Expression of retroviral vectors containing the human multidrug resistance 1 cDNA in hematopoietic cells of transplanted mice. Blood. 1995;86:491–501.
Sun X, Jiao X, Pestell TG, Fan C, Qin S, Mirabelli E, Ren H, Pestell RG. MicroRNAs and cancer stem cells: the sword and the shield. Oncogene. 2013, doi:10.38/onc2013.492.
Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–41.
Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, La Noce M, Laino L, De Francesco F, Papaccio G. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J. 2013;27:13–24.
Uchida N, Leung FY, Eaves CJ. Liver and marrow of adult mdr-1a/1b(-/-) mice show normal generation, function, and multi-tissue trafficking of primitive hematopoietic cells. Exp Hematol. 2002;30:862–9.
Vesuna F, Lisok A, Kimble B, Raman V. Twist modulates breast cancer stem cells by transcriptional regulation of CD24 expression. Neoplasia. 2009;11:1318–28.
Vidal SJ, Rodriguez-Bravo V, Galsky M, Cordon-Cardo C, Domingo-Domenech J. Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene. 2013, doi:10.38/onc2013.411.
Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–22.
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68.
Wang F, Xue X, Wei J, An Y, Yao J, Cai H, Wu J, Dai C, Qian Z, Xu Z, Miao Y. Hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103:567–74.
Wilson BJ, Schatton T, Zhan Q, Gasser M, Ma J, Saab KR, Schanche R, Waaga-Gasser AM, Gold JS, Huang Q, Murphy GF, Frank MH, Frank NY. ABCB5 identifies a therapy-refractory tumor cell population in colorectal cancer patients. Cancer Res. 2011;71:5307–16.
Xiang L, Su P, Xia S, Liu Z, Wang Y, Gao P, Zhou G. ABCG2 is associated with HER-2 expression, lymph node metastasis and clinical stage in breast invasive ductal carcinoma. Diagn Pathol. 2011;6:90.
Yang M, Li W, Fan D, Yan Y, Zhang X, Zhang Y, Xiong D. Expression of ABCB5 gene in hematological malignances and its significance. Leuk Lymphoma. 2012;53:1211–5.
Zhang H, Xi H, Cai A, Xia Q, Wang XX, Lu C, Zhang Y, Song Z, Wang H, Li Q, Chen L, Guo Z. Not all side population cells contain cancer stem-like cells in human gastric cancer cell lines. Dig Dis Sci. 2013;58:132–9.
Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, Wu H, Li L. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–22.
Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–23.
Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin 3rd E, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–63.
Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 2002;99:12339–44.
Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–34.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Perego, P. (2015). ATP Binding Cassette Transporters in Cancer Stem-Like Cells. In: Efferth, T. (eds) Resistance to Targeted ABC Transporters in Cancer. Resistance to Targeted Anti-Cancer Therapeutics, vol 4. Springer, Cham. https://doi.org/10.1007/978-3-319-09801-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-09801-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09800-5
Online ISBN: 978-3-319-09801-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)