Summary
In this chapter, the Type I interferons (IFNs), their modes of production, signaling, regulation of sets of effector genes and their roles in host defense will be introduced. During infection by bacteria and other pathogens, a fine balance in the IFN response is required to ensure protection of the host and the avoidance of toxicity or chronic disease. The pattern recognition receptors (PRRs) involved in inducing the interferon response; the Type I interferon receptors and their downstream signal transduction pathways; the interferon-regulated genes; and the Type I interferon regulated immune and antibacterial responses will be discussed. Case studies of IFNβ and IFNε will be presented as examples of broader principles of how regulating IFN expression in particular ways, organ specific requirements and compartmentalization within an organ can yield different results for host defense. The broad impact of the Type I interferon response to infections with bacterial pathogens including Escherichia coli, Salmonella species, Helicobacter pylori, Staphylococcus aureus, group A and B streptococci, Listeria monocytogenes, Mycobacterium tuberculosis, Chlamydia species and Legionella pneumophilia will be highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The interferons (IFNs) are a family of cytokines that function in the host response to environmental stress [1]. The evolution of the IFN response has adapted to perform a wide range of physiological and pathological functions. The IFNs are classified into three types distinguished by amino acid similarity; cognate receptors, through which they signal; and to a lesser extent, the production stimulus and cell. Type I IFNs are a multi-gene family composed of 13 IFNα subtypes, a single IFNβ, IFNε and IFNω, and other species-specific members, produced by most cell types and acting via IFNAR 1 and 2 receptors [2]. Type II IFN has a single member, IFNγ, produced mainly by activated NK and T cells and signaling via IFNGR1 and 2 receptors [3]. Type III or IFNλ has two members, produced by many cell types stimulated by pathogens and acting via IFNL1 and IL10Rβ receptors [4]. This review will focus on type I IFNs, setting the scene for their role in host defence against bacterial infections. IFNs have multiple effects on cells, which include rendering them resistant to viral infection, modulating proliferation, differentiation, survival and migration, as well as other specialized functions [5]. Thus, IFNs can regulate the development and activation of most effector cells of the innate and adaptive immune response. Type I IFNs signal via the JAK/STAT signaling pathway to regulate the expression of genes that encode the effector proteins of the response including antiviral and antibacterial effectors. Their broad effects on a range of target cells, necessitates a fine balance in the IFN response to ensure protection of the host against insult and a return to homeostasis, but avoid potential toxicity or chronic disease. Excessive IFN production contributes to acute septic shock in animal models, and long-term deregulation of type I IFN signaling contributes to the pathogenesis of autoimmune diseases such as systemic lupus erythematosus. Understanding the regulation of type I IFN production and the actions of this family of proteins on cells is necessary to gain insights into their role in the pathogenesis of bacterial infections. In some cases, particularly with extracellular pathogens, IFNs are protective, whereas they increase susceptibility to intracellular pathogens.
Production of Type I IFNs
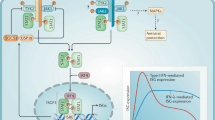
The production of type I IFNs was first described in response to viral infection and remains best characterized in response to these pathogens [6–8]. Nevertheless, it is increasingly evident that type I IFN production is activated by a wide variety of stimuli, including bacteria [9], physiological stimuli [10, 11] and cancer cells [12, 13]. The deluge in information characterizing the pattern recognition receptors (PRRs) that sense “danger” signals has provided considerable explanation of the mechanism whereby type I IFNs are produced [14–16]. Once PRRs bind ligand, they engage intracellular signaling molecules, often specific for the PRR family involved, and then activate kinases that in turn activate a restricted range of transcription factors such as NFκB and the interferon regulatory factors (IRFs) that stimulate the induction of pro-inflammatory cytokines and type I IFNs, respectively. The IRFs are a nine-member family of latent transcription factors involved in type I IFN production (IRF1, 3, 5, 7) and signaling (IRF9), among other functions [17]. As discussed in detail below, IRF3 is activated by many PRRs to induce IFNβ gene expression (in conjunction with NFκB and AP1) but not the expression of IFNαs. On the other hand, IRF7 is also activated by many PRRs, but can activate expression of IFNβ and IFNα subtypes. In addition, IRF5 and IRF1 appear more restricted in their upstream activation pathways and these also activate IFNα gene expression.
TLRs 1, 2, 4, and 6 are cell surface PRRs that sense cell surface or secreted ligands, or pathogen-associated molecular patterns (PAMPs). The TLR4 signaling pathway activated by Gram negative bacterial lipopolysaccharide (LPS) in complex with MD2 and LBP is the best characterized and prototypic PRR signaling pathway. Ligand activated TLR4 engages four TIR domain-containing adaptor molecules: MyD88 and Mal, which activate the NFκB pathway, and TRAM and TRIF, which activate the IRF3 previously phosphorylated upstream by TBK and IKKε [14–16]. This pathway, in conjunction with NFκB, activates expression of IFNβ specifically, since this is the only type I IFN with neighboring IRF3 and NFκB binding elements in its promoter. Escherichia coli and Salmonella are strong activators of TLR4, whereas other bacteria such as Helicobacter pylori produce LPS that only weakly stimulates TLR4, which may explain their relative virulence [9].
TLR2 usually acts as a heterodimer with TLR1 or TLR6 and recognizes different peptidoglycans to activate the NFκB pathway driven pro-inflammatory cytokines via MyD88 and Mal. This signaling pathway is not usually associated with activation of IRFs and IFN production. However, exceptions have been reported [18], including a study involving the commensal Lactobacillus [19], but the details of the pathways remain to be fully elucidated.
TLRs 3, 7, 8, and 9 are endosomal sensors of nucleic acids including dsRNA (TLR3), ssRNA (TLR7/8) and bacterial CpG DNA (TLR9). TLR3 is the only family member that does not utilize MyD88, but signals via TRIF. These endosomal TLRs recruit adaptors and activate TBK and/or IKKε, which in turn activate IRF7 and 3 to drive the induction of IFNαs and IFNβ [16]. TLR9 senses Staphylococcus aureus and activates IFN production via IRF1 [20]. Group A and B streptococci are recognized by TLR7 and activate IFNs via IRF1 [21].
The RIG I-like family of receptors (RLRs) including RIG-I, MDA5 and LGP2 were originally identified as cytosolic sensors of viral 5′-triphosphorylated RNA [22]. Once activated, they are recruited to mitochondria or associated membranes, bind adaptors MAVS/IPS and subsequently activate TBK/IKKε, which phosphorylate IRFs, which themselves translocate to the nucleus and induce expression of IFNα and IFNβ genes [23, 24].
STING was discovered as a molecule that mediated the induction of IFNβ in response to cytosolic DNA from pathogens or necrotic cells [25]. Subsequent studies cast doubt on whether the endoplasmic reticulum-localized STING directly bound DNA (reviewed in [26]). It was found that STING was the receptor for cyclic di-nucleotides such as c-di AMP or c-di GMP which act as PAMPs, for example in macrophages infected with Listeria monocytogenes, after listerolysin O-mediated (LLO) their release from vacuoles, possibly via DDX41 [27–29]. Another STING activating PAMP is cGMP-AMP generated by the IFN-inducible enzyme cGAS, which is important in sensing cytosolic DNA and initiating the innate immune response to pathogens. DNA from Chlamydia muridarum [30], Myocbacterium tuberculosis [31] and Legionella pneumophilia [32] have also been shown to activate STING and induce IFNβ expression.
Cytosolic Sensors
DAI was the first reported cytosolic sensor of DNA from viruses or bacteria, inducing IFN via TBK and IRF3 [33]. DAI senses Streptococcus pneumoniae [34]. Another study showed that DNA-dependent RNA polymerase III converts cytosolic DNA into RNAs that act as PAMPs to activate RIG-I [35]. DNA released into the cytosol during infection with Francisella tularensis is sensed by the AIM2 inflammasome which in turn activates IRF3 and type I IFN production [36, 37]. L. monocytogenes also activates the AIM2 inflammasome [38]. NOD 1 and 2 have been speculated to induce IFN production in response to sensing muramyl dipeptide (MDP) from organisms including M. tuberculosis [39–41].
Thus, the various PRRs constitute a repertoire of sensors, strategically located through evolution, at different subcellular locations to ensure the detection of a pathogen component, be it outside the cell, in endosomes, free in the cytoplasm, associated with organelles or in the nucleus. The various PRR signal transduction pathways activate one of the IRFs (1, 3, 5, or 7) and occasionally NFκB, to bind promoter elements in type I IFN genes. To complement the upstream signaling pathways, the promoters of the 13 IFNα subtypes and IFNβ genes each contain a distinct number and arrangement of transcription factor binding sites to ensure that one or some of these essential cytokines are produced in response to infection with a broad range of pathogens—both viral and bacterial. This promoter diversity is also likely to be important in determining the IFN subtypes produced by different cell types. A thorough investigation of the many transcription factor binding sites in the promoters of the various type I IFN genes is yet to be performed. However, type I IFNs are not only produced by haemopoietic cells as traditionally thought (originally called “leukocyte” IFN), and recent studies have brought attention to their production by epithelial cells as well [42, 43]. Depending on the expression of signaling molecules, different cell types will differ in their pathways, the repertoire of type I IFNs and the amounts they produce. For example, plasmacytoid dendritic cells (DC) express high levels of constitutive IRF7 and therefore rapidly produce high levels of IFNα compared to other cells. Other cell types respond slower because signaling molecules like IRF7 have to be first induced by IFN.
Two decades of studies in mice deficient in Ifnar 1, through which all type I IFNs signal, have demonstrated the crucial role of this family of cytokines in sculpting the response to viral and bacterial infections [44, 45]. Consistent with this scenario, type I IFNs are never all produced, rarely singly (except IFNβ discussed below) and usually in subsets: for example, some IFNαs +/− IFNβ.
In addition to the mammalian cell components, properties of the pathogen also determine the nature of the type I IFN response. For viruses, whether they constitutively harbor RNA or DNA, single or double stranded, determines the cellular PRR response. Pathogens also activate different PRRs depending on their cellular niche. For bacteria, whether they are intracellular or extracellular pathogens and the nature of virulence factors (such as pore-forming toxins) that might be necessary to “release” PAMPs to the responding cellular compartment, will determine the nature of the response.
Type I IFN Signaling:Receptors
All type I IFNs characterized to date transduce signals via interaction with the receptor components, IFNAR1 and IFNAR2. IFNAR2 is the high affinity binding chain and can be differentially spliced to produce a “long” form which transduces signals (IFNAR2c); a truncated transmembrane isoform that contain little or no signaling capacity (IFNAR2b); and a soluble form (IFNAR2a). IFNAR2a and c isoforms are conserved between human and mouse, whereas IFN2b is specific to humans [46–48]. Studies in the murine model have demonstrated that in vitro, soluble IFNAR2a has the capacity to either block signaling or facilitate signaling via a process called trans-signaling whereby soluble receptor binds ligand and presents it to the signaling receptor chain [49]. This process can be a major form of signaling as for IL6, but remains to be determined for the type I IFNs. is [50]. In vivo studies have recently indicated that soluble IFNAR2a does not block responses to IFNβ [51]. The IFNAR 1 chain has been shown to have very low affinity for binding type I IFNs (with one exception, discussed below), but combines with IFNAR2 to generate a high affinity trimeric complex. IFNAR1 is essential for transducing signals for all type I IFNs characterized so far, as determined from numerous studies of IFNAR1 deficient mice. Both receptors appear to be expressed broadly making most cells responsive to IFN, but there have not been extensive studies on the surface expression of receptor components on individual cell types or at different stages of the host responses.
Type I IFN Signaling: Signal Transduction Pathways
Once ligand engages the receptors, the IFNAR1-associated TYK2 and the IFNAR2-associated JAK1 kinases are activated and phosphorylate receptor tyrosine residues [52, 53]. These form docking sites for signal transducers and activators of transcription (STATs), which are themselves phosphorylated, dissociate from the receptors, dimerize and translocate to the nucleus via interaction with importins, and activate the transcription of IFN-regulated genes (IRGs) [54]. Studies have shown that the docking sites for STATs are in the IFNAR2 component of the receptor [52, 55, 56]. The canonical transcription factors of the type I IFN pathway is ISGF3 (composed of a STAT1:STAT2) and IRF9 (also called p48 or ISGF3γ because it is induced by IFNγ). Nevertheless, type I IFNs also activate STAT3, and dimers of STAT3:STAT3 or STAT1:STAT3 can bind GAS sites (interferon-gamma activated sites) in IRGs (sometimes wrongly thought to be IFNγ-specific) [54]. Indeed, type I IFNs can activate all STATs (4, 5, and 6) depending on the cell type. Indeed in PBMCs, STAT5 is the main STAT activated [57, 58].
There are other signaling pathways activated by type I IFNs. Indeed JAK kinases have other substrates [59] and also function to stabilize the IFNAR1 at the plasma membrane [60]. STAT independent signaling was reported for both type I and type II IFNs in STAT-deficient cells using transcriptional profiling [61], but the signaling pathways responsible were not pursued in those studies. Numerous “alternative” type I IFN signaling pathways have been described, including MAPK (p38 and ERK), NFκB and PI3K/AKT pathways [57, 62]. The best characterized of these is the p38 and Erk MAP kinase (MAPK) pathways, which modulate IRG mRNA translation via activation of Mnk kinases [63]. Activation of AKT/mTOR (mammalian target of rapamycin) signaling is also initiated by IFNs, impacting on translation of IRG mRNA [64, 65]. The relative contribution of these and other alternative IFN signaling pathways is likely to be cell and context dependent. For example, type I IFN signaling in T cells has been reported to utilize T cell receptor signaling molecules for antiproliferative activities [66].
Type I IFN Signaling: Interferon Regulated Genes
There have been many studies documenting the nature of IRGs individually for past decades and more recently by transcriptional profiling by microarrays. In an attempt to capture an overview of the response, we have catalogued available microarray datasets of IFN treated cells or organisms; the data is reanalyzed and annotated then placed in a searchable database called the Interferome (v 2.0 http://interferome.its.monash.edu.au) [67, 68]. This represents a tool for identifying a gene as an IRG, or more importantly, for searching a dataset for IRGs. This collection has identified more than 2,000 IRGs (more depending on the statistical cut-off applied) across species, IFNs, and tissue types. These genes encode the effector proteins that mediate the different biological activities of the IFNs. The number of genes in any given condition is usually smaller, often hundreds, and there is considerable difference between different cells or tissues. There are overlaps between type I, II, and III regulated genes and some apparently IFN type-specific genes, although comparisons are often difficult because of differences in experimental conditions [69]. Tools such as the Interferome are important in finding IRG “signatures” associated with disease that might represent modulation of a particular pathway. We have used Interferome and associated tools to identify an IFN signature activated in HIV infected dendritic cells (a gene set regulated by IRF1, despite HIV suppression of IFN production [70]) and another gene signature suppressed in breast cancer metastases (regulated by IRF7; [13]). Interestingly, an IFN signature has been characterized in latent M. tuberculosis infection that appears to correlate with disease pathogenesis and is consistent with studies in animal models showing a role for type I IFN signaling in susceptibility to this pathogen (reviewed in [71]).
The best characterized IRGs are those involved in protecting cells from viral infection; individual ones such as 2′–5′ oligoadenylate synthetase, PKR and Mx proteins, having been well characterized for many years [72]. Recently, elegant, comprehensive screening studies of 350 IFN inducible genes have highlighted new IRGs with direct antiviral activities [72]. The studies may inform similar rationales for characterizing the effector functions (anti-bacterial, immunoregulatory) of the many other IRGs. In broad terms the broad repertoire of antiviral IRGs has the ability to restrict different stages of the viral life cycle and different types of viruses, providing broad protection against infection.
Type I Interferon Regulated Antibacterial Responses
Unlike the antiviral effects of type I IFNs, the effects of IFN on bacterial infections are relatively poorly characterized. In general, type I IFNs are protective against extracellular bacterial infections, yet exacerbate infections with intracellular bacteria. This is at least in part due to the differences in organ and cell specificity, the direct effects of IFNs, the impact on cell survival and indirect actions via regulation of the innate and adaptive immune responses [43, 73, 74]. Examples of direct acting antibacterial IRGs include iNOS, NADPH oxidase, nox-2 [73, 75]; TRAIL [76]; and phospholipidscramblase 1 (PLSCR1) [77]; GTPases [78].
Type I Interferon Regulated Immune Responses
There are many different IRGs, intrinsic or extrinsic to immune cells that can affect the trafficking, development, differentiation, survival, and activity of most innate and adaptive immune cells in response to infections, cancer, and inflammatory diseases (reviewed in [74, 79]). Particular cells and responses have been documented to be important in the response to bacterial infections. TNFα and IFNγ up-regulation by type I IFNs increases protection from S. pneumoniae infection [21]. Repression of type I IFN induced chemokines CXCL10 and CCL5 reduces cells neutrophil infiltration and impairs clearance of Pseudomonas aeruginosa from infected lungs [80, 81]. By contrast, type I IFNs suppress the production of other chemokines such as CCL2, CXCL4 and CXCL9, which recruit monocyte/macrophage and neutrophils [75] leading to exacerbation of infection by C. muridarum. Other IRGs include cytokines that activate or repress immune responses including IL10 [82], IL27, and IL17 [83] and FOXP3 which is important in Treg function [74]. Another IFN induced effect that is important in regulating responses to bacterial infection is the induction of apoptosis in infiltrating cells [84]. It is well known that IFNs can regulate the expression of different cell death pathways including bcl-2 and bcl-X [85] and caspase 11 [86] and that IFNs play a role in mediating necroptosis of Salmonella typhimurium infected macrophages [87].
Cross-Talk, Feedback, and Feed Forward
There have been numerous publications about cross-talk of type I IFNs with other systems. In general terms, many of the receptors and signaling components of other signaling systems are, in fact, IRGs and the positive or negative regulation of these factors underlie the basis of cross-talk [88]. These include other cytokines (reviewed in [5]) likely due to priming of STAT levels [89], TLRs and RLRs [5], and the inflammasome [90, 91]. Indeed, we and others have demonstrated that type I IFNs prime the basal levels of hundreds of IRGs, many of which play central roles in signaling by other systems [42, 92]. Important among these are negative regulators such as SOCS1, which are not only rapidly and strongly IFN inducible but play important roles in dampening responses to type I and type II IFNs, other cytokines and TLRs [93–95]. Indeed neonatal mice deficient in SOCS 1 die from multi-organ inflammation in the absence of SOCS1 suppression of type I [94] and type II IFN signaling [93].
Special Case Study of IFNβ
As discussed above, IFNβ is different from other type I IFNs in being the only one induced by LPS, thus playing a central role in response to bacteria [92, 96]. In addition, the promoter of IFNβ is unusual in having AP1 sites that can be activated by the fos/jun and MAP kinase pathway. This pathway is activated during macrophage development in response to M-CSF and in osteoclast development in response to RANK Ligand [11]. The inhibitory effect of on IFNβ on the proliferation of these myeloid cells may be important in the regulation of pathogen responses. In addition to selective production of IFNβ relative to other type I IFNs, it has a higher binding affinity to receptors and is more potent than the members of the IFNα family in anti-proliferative assays on certain cell types. It is a singularly effective therapeutically in multiple sclerosis [97]. However, until recently, there has been no mechanistic explanation for differential activities of IFNb relative to other type I IFNs..
De Weerd et al. [97] demonstrated that IFNβ but not IFNα formed a complex with IFNAR1 in the absence of IFNAR2. Crystallization of the IFNβ:IFNAR1 complex showed extensive contacts of this IFN with the receptor over a much larger surface area in that crystal structure than any potential IFNα:IFNAR1 [98]. Further studies of Ifnar2 null cells showed that while the binding of IFNβ to IFNAR1 did not induce canonical STAT signaling as expected, there were signals transduced. Approximately 230 genes were induced by this novel IFNβ:IFNAR1 signaling axis by an uncharacterized pathway. Induced genes included several such as TREM1, TREML4, TGM2, and CCL2, which had known roles in the response to sepsis. Using an in vivo murine model of LPS-induced septic shock, it was demonstrated that this unique IFNβ:IFNAR1 signaling axis was important in mediating the previously described IFN-mediated toxicity.
Specifically, this study shows molecular mechanisms whereby IFNβ can transduce specific signals with pathophysiological importance. In general terms it opens the door for discovering previously elusive selective actions of other type I IFNs by differential interaction with IFNAR1 and IFNAR2. Similarly, cells may regulate the response to type I IFNs by differential regulation of the cell surface expression of IFNAR1 and IFNAR2.
Special Case Study of IFNε
Recently, the function of a specialized type I IFN was reported. IFNε was characterized as a type I IFN based on sequence homology, the location of the gene in the type I IFN gene locus on human chromosome 9p (and syntenic murine chromosome 16) and its signaling through IFNAR1 and IFNAR2 [42, 99]. Recombinant IFNe protein induced “classical” IRGs like other type I IFNs and this signaling was abrogated in cells from Ifnar1 or Ifnar2 deficient mice [42]. However, the expression patterns and regulation of this gene showed unique features. Unlike other type I IFNs, it was not pathogen inducible and was constitutively expressed. This constitutive expression was most notable in the female reproductive tract (FRT). Also unlike other type I IFNs, its expression was regulated by hormones: stimulated by estrogen and repressed by progesterone. Accordingly, its expression fluctuated during the female cycle, was dramatically reduced at the time of embryo implantation in the mouse, and was reduced to virtually undetectable in post-menopausal women, when estrogen levels decline. The in vivo functional importance of IFNε was determined in IFNε-deficient mice. These mice were more susceptible to viral infection with HSV and bacterial infection with C. muridarum. The constitutive production of IFNε in the epithelial cells of the endometrium maintained the basal expression of many IRGs including those involved in pathogen defense (Mx, ISG 15, IRGM1) and PRR sensing and primary signaling (IRF7). This priming of the innate immune response by constitutive IFNε ensures protection of the FRT mucosa from early stages of viral and bacterial infection. Furthermore, the absence of IFNε also restricted bacterial clearance, consistent with the continued production of this protective cytokine before and throughout the course of infection since this pathogen did not modulate IFNε expression in vivo. The levels of NK cells, which have been shown to aid clearance of pathogen, correlated with the levels of IFNε: administration of recombinant mu IFNε to IFNε-null mice restored the depleted levels of NK cells and decreased the number of bacteria recovered 3 days post infection. Interestingly, IFNε is the only type I IFN that protects the FRT from Chlamydia infection. Ifnar1 deficient mice show less severe disease; indicating the exacerbation of disease by production of (presumably conventional, α/β) type I IFNs; shown by adoptive transfer experiments to be acting on CD8 T cells driving disease pathogenesis [75]. This is similar to infections with other intracellular bacteria such as L. monocytogenes, F. tularensis, M. tuberculosis, in which disease pathology is exacerbated by type I IFNs (refer above).
Thus, the actions of IFNε in protecting the FRT highlight several general principles that might be applicable to IFN anti-pathogen strategies in general: (1) it is a direct example of how regulating expression in a particular way can achieve specific and functional protection; (2) it shows a specific adaptation of the innate immune response to suit organ-specific requirements of host defense; and (3) it shows how compartmentalization of an IFN response can achieve opposite outcomes—epithelial production of IFNε is protective, whereas mucosal production of conventional IFNs exacerbates disease through their action on immune cells.
Concluding Remarks
The type I IFNs have pleiotropic effects on host defense due to their ability to regulate the parenchymal cells under attack by infectious agents or the innate and adaptive immune cells that traffic to and from the site of infection. While we have made considerable advances in understanding mechanisms of signal transduction via the IFNARs, JAK/STAT and other signal transduction pathways, we are only just beginning to understand the cell context and temporal specificities of type I IFN signaling and responses. This is manifest in the different transcription profiles identified in different cell types responding to IFNs, which represents only a part of the available repertoire of IRGs that encode the effector molecules. Understanding and harnessing the specificity of the response will make inroads into understanding and dealing with the current and emerging threats posed by bacteria and other pathogens.
References
Pestka S, Krause CD, Walter MR (2004) Interferons, interferon-like cytokines, and their receptors. Immunol Rev 202:8–32
de Weerd NA, Samarajiwa SA, Hertzog PJ (2007) Type I interferon receptors: biochemistry and biological functions. J Biol Chem 282:20053–20057
Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75:163–189
Kotenko SV (2011) IFN-lambdas. Curr Opin Immunol 23:583–590
Hertzog PJ, Williams BR (2013) Fine tuning type I interferon responses. Cytokine Growth Factor Rev 24:217–225
Nagano Y, Kojima Y (1954) Pouvoir immunisant du virus vaccinal inactivé par des rayons ultraviolets. Comptes rendus des séances de la Société de biologie et de ses filiales 148:1700–1702
Isaacs A, Lindenmann J (1957) Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 147:258–267
Levy DE, Marie IJ, Durbin JE (2011) Induction and function of type I and III interferon in response to viral infection. Curr Opin Virol 1:476–486
Monroe KM, McWhirter SM, Vance RE (2010) Induction of type I interferons by bacteria. Cell Microbiol 12:881–890
Hamilton JA, Whitty GA, Kola I, Hertzog PJ (1996) Endogenous IFN-alpha beta suppresses colony-stimulating factor (CSF)-1-stimulated macrophage DNA synthesis and mediates inhibitory effects of lipopolysaccharide and TNF-alpha. J Immunol 156:2553–2557
Takayanagi H, Kim S, Taniguchi T (2002) Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis Res 4(Suppl 3):S227–S232
Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, Hertzog P, Smyth MJ (2007) Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol 178:7540–7549
Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, de Weerd NA, Gould J, Argani P, Moller A, Smyth MJ, Anderson RL, Hertzog PJ, Parker BS (2012) Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med 18:1224–1231
Hertzog PJ, O’Neill LA, Hamilton JA (2003) The interferon in TLR signaling: more than just antiviral. Trends Immunol 24:534–539
Noppert SJ, Fitzgerald KA, Hertzog PJ (2007) The role of type I interferons in TLR responses. Immunol Cell Biol 85:446–457
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Tamura T, Yanai H, Savitsky D, Taniguchi T (2008) The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol 26:535–584
Barbalat R, Lau L, Locksley RM, Barton GM (2009) Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 10:1200–1207
Weiss G, Maaetoft-Udsen K, Stifter SA, Hertzog P, Goriely S, Thomsen AR, Paludan SR, Frokiaer H (2012) MyD88 drives the IFN-beta response to Lactobacillus acidophilus in dendritic cells through a mechanism involving IRF1, IRF3, and IRF7. J Immunol 189:2860–2868
Parker D, Prince A (2012) Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J Immunol 189:4040–4046
Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C (2009) Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol 10:587–594
Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S (2005) Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19–28
Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S (2005) IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 6:981–988
Seth RB, Sun L, Ea CK, Chen ZJ (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122:669–682
Ishikawa H, Ma Z, Barber GN (2009) STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–792
Barber GN (2014) STING-dependent cytosolic DNA sensing pathways. Trends Immunol 35:88–93
Woodward JJ, Iavarone AT, Portnoy DA (2010) c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328:1703–1705
Bowie AG (2012) Innate sensing of bacterial cyclic dinucleotides: more than just STING. Nat Immunol 13:1137–1139
Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, Liu ZJ, Modlin RL, Liu YJ, Cheng G (2012) The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol 13:1155–1161
Prantner D, Darville T, Nagarajan UM (2010) Stimulator of IFN gene is critical for induction of IFN-beta during Chlamydia muridarum infection. J Immunol 184:2551–2560
Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS (2012) Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11:469–480
Lippmann J, Muller HC, Naujoks J, Tabeling C, Shin S, Witzenrath M, Hellwig K, Kirschning CJ, Taylor GA, Barchet W, Bauer S, Suttorp N, Roy CR, Opitz B (2011) Dissection of a type I interferon pathway in controlling bacterial intracellular infection in mice. Cell Microbiol 13:1668–1682
Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T (2007) DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501–505
Parker D, Martin FJ, Soong G, Harfenist BS, Aguilar JL, Ratner AJ, Fitzgerald KA, Schindler C, Prince A (2011) Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. MBio 2:e00016-00011
Chiu YH, Macmillan JB, Chen ZJ (2009) RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576–591
Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM (2007) Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med 204:987–994
Henry T, Monack DM (2007) Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell Microbiol 9:2543–2551
Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, Hornung V (2010) Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol 40:1545–1551
Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, Behr MA, Fitzgerald KA, Sassetti CM, Kelliher MA (2009) NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog 5:e1000500
Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Nunez G (2007) RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol 178:2380–2386
Parker D, Planet PJ, Soong G, Narechania A, Prince A (2014) Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. PLoS Pathog 10:e1003951
Fung KY, Mangan NE, Cumming H, Horvat JC, Mayall JR, Stifter SA, De Weerd N, Roisman LC, Rossjohn J, Robertson SA, Schjenken JE, Parker B, Gargett CE, Nguyen HP, Carr DJ, Hansbro PM, Hertzog PJ (2013) Interferon-epsilon protects the female reproductive tract from viral and bacterial infection. Science 339:1088–1092
Parker D, Prince A (2011) Type I interferon response to extracellular bacteria in the airway epithelium. Trends Immunol 32:582–588
Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, Hamilton JA, Whitty G, Bertoncello I, Kola I (1995) A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci U S A 92:11284–11288
Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M (1994) Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921
Novick D, Cohen B, Rubinstein M (1994) The human interferon alpha/beta receptor: characterization and molecular cloning. Cell 77:391–400
Lutfalla G, Holland SJ, Cinato E, Monneron D, Reboul J, Rogers NC, Smith JM, Stark GR, Gardiner K, Mogensen KE et al (1995) Mutant U5A cells are complemented by an interferon-alpha beta receptor subunit generated by alternative processing of a new member of a cytokine receptor gene cluster. EMBO J 14:5100–5108
Owczarek CM, Hwang SY, Holland KA, Gulluyan LM, Tavaria M, Weaver B, Reich NC, Kola I, Hertzog PJ (1997) Cloning and characterization of soluble and transmembrane isoforms of a novel component of the murine type I interferon receptor, IFNAR 2. J Biol Chem 272:23865–23870
Hardy MP, Owczarek CM, Trajanovska S, Liu X, Kola I, Hertzog PJ (2001) The soluble murine type I interferon receptor Ifnar-2 is present in serum, is independently regulated, and has both agonistic and antagonistic properties. Blood 97:473–482
Ruwanpura SM, McLeod L, Brooks GD, Bozinovski S, Vlahos R, Longano A, Bardin PG, Anderson GP, Jenkins BJ (2014) IL-6/Stat3-driven pulmonary inflammation, but not emphysema, is dependent on interleukin-17A in mice. Respirology 19:419–427
Samarajiwa SA, Mangan NE, Hardy MP, Najdovska M, Dubach D, Braniff SJ, Owczarek CM, Hertzog PJ (2014) Soluble IFN receptor potentiates in vivo type I IFN signaling and exacerbates TLR4-mediated septic shock. J Immunol 192:4425–4435
Domanski P, Fish E, Nadeau OW, Witte M, Platanias LC, Yan H, Krolewski J, Pitha P, Colamonici OR (1997) A region of the beta subunit of the interferon alpha receptor different from box 1 interacts with Jak1 and is sufficient to activate the Jak-Stat pathway and induce an antiviral state. J Biol Chem 272:26388–26393
Yan H, Krishnan K, Lim JT, Contillo LG, Krolewski JJ (1996) Molecular characterization of an alpha interferon receptor 1 subunit (IFNaR1) domain required for TYK2 binding and signal transduction. Mol Cell Biol 16:2074–2082
Stark GR, Darnell JE Jr (2012) The JAK-STAT pathway at twenty. Immunity 36:503–514
Piganis RA, De Weerd NA, Gould JA, Schindler CW, Mansell A, Nicholson SE, Hertzog PJ (2011) Suppressor of cytokine signaling (SOCS) 1 inhibits type I interferon (IFN) signaling via the interferon alpha receptor (IFNAR1)-associated tyrosine kinase Tyk2. J Biol Chem 286:33811–33818
Zhao W, Lee C, Piganis R, Plumlee C, de Weerd N, Hertzog PJ, Schindler C (2008) A conserved IFN-alpha receptor tyrosine motif directs the biological response to type I IFNs. J Immunol 180:5483–5489
Platanias LC (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5:375–386
van Boxel-Dezaire AH, Rani MR, Stark GR (2006) Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25:361–372
O’Shea JJ, Plenge R (2012) JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36:542–550
Ragimbeau J, Dondi E, Alcover A, Eid P, Uze G, Pellegrini S (2003) The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J 22:537–547
Ramana CV, Gil MP, Han Y, Ransohoff RM, Schreiber RD, Stark GR (2001) Stat1-independent regulation of gene expression in response to IFN-gamma. Proc Natl Acad Sci U S A 98:6674–6679
Yang CH, Murti A, Pfeffer SR, Kim JG, Donner DB, Pfeffer LM (2001) Interferon alpha/beta promotes cell survival by activating nuclear factor kappa B through phosphatidylinositol 3-kinase and Akt. J Biol Chem 276:13756–13761
Joshi S, Kaur S, Redig AJ, Goldsborough K, David K, Ueda T, Watanabe-Fukunaga R, Baker DP, Fish EN, Fukunaga R, Platanias LC (2009) Type I interferon (IFN)-dependent activation of Mnk1 and its role in the generation of growth inhibitory responses. Proc Natl Acad Sci U S A 106:12097–12102
Kaur S, Sassano A, Dolniak B, Joshi S, Majchrzak-Kita B, Baker DP, Hay N, Fish EN, Platanias LC (2008) Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc Natl Acad Sci U S A 105:4808–4813
Kaur S, Sassano A, Majchrzak-Kita B, Baker DP, Su B, Fish EN, Platanias LC (2012) Regulatory effects of mTORC2 complexes in type I IFN signaling and in the generation of IFN responses. Proc Natl Acad Sci U S A 109:7723–7728
Petricoin EF 3rd, Ito S, Williams BL, Audet S, Stancato LF, Gamero A, Clouse K, Grimley P, Weiss A, Beeler J, Finbloom DS, Shores EW, Abraham R, Larner AC (1997) Antiproliferative action of interferon-alpha requires components of T-cell-receptor signalling. Nature 390:629–632
Samarajiwa SA, Forster S, Auchettl K, Hertzog PJ (2009) INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res 37:D852–D857
Rusinova I, Forster S, Yu S, Kannan A, Masse M, Cumming H, Chapman R, Hertzog PJ (2013) Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res 41:D1040–D1046
Hertzog P, Forster S, Samarajiwa S (2011) Systems biology of interferon responses. J Interferon Cytokine Res 31:5–11
Harman AN, Lai J, Turville S, Samarajiwa S, Gray L, Marsden V, Mercier SK, Jones K, Nasr N, Rustagi A, Cumming H, Donaghy H, Mak J, Gale M Jr, Churchill M, Hertzog P, Cunningham AL (2011) HIV infection of dendritic cells subverts the IFN induction pathway via IRF-1 and inhibits type 1 IFN production. Blood 118:298–308
Berry MP, Blankley S, Graham CM, Bloom CI, O’Garra A (2013) Systems approaches to studying the immune response in tuberculosis. Curr Opin Immunol 25:579–587
Schoggins JW (2014) Interferon-stimulated genes: roles in viral pathogenesis. Curr Opin Virol 6C:40–46
Decker T, Muller M, Stockinger S (2005) The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol 5:675–687
Gonzalez-Navajas JM, Lee J, David M, Raz E (2012) Immunomodulatory functions of type I interferons. Nat Rev Immunol 12:125–135
Nagarajan UM, Prantner D, Sikes JD, Andrews CW Jr, Goodwin AM, Nagarajan S, Darville T (2008) Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect Immun 76:4642–4648
de Almeida LA, Carvalho NB, Oliveira FS, Lacerda TL, Vasconcelos AC, Nogueira L, Bafica A, Silva AM, Oliveira SC (2011) MyD88 and STING signaling pathways are required for IRF3-mediated IFN-beta induction in response to Brucella abortus infection. PLoS One 6:e23135
Lizak M, Yarovinsky TO (2012) Phospholipid scramblase 1 mediates type I interferon-induced protection against staphylococcal alpha-toxin. Cell Host Microbe 11:70–80
Taylor GA, Feng CG, Sher A (2004) p47 GTPases: regulators of immunity to intracellular pathogens. Nat Rev Immunol 4:100–109
Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I (2011) Direct effects of type I interferons on cells of the immune system. Clin Cancer Res 17:2619–2627
Carrigan SO, Junkins R, Yang YJ, Macneil A, Richardson C, Johnston B, Lin TJ (2010) IFN regulatory factor 3 contributes to the host response during Pseudomonas aeruginosa lung infection in mice. J Immunol 185:3602–3609
Power MR, Li B, Yamamoto M, Akira S, Lin TJ (2007) A role of Toll-IL-1 receptor domain-containing adaptor-inducing IFN-beta in the host response to Pseudomonas aeruginosa lung infection in mice. J Immunol 178:3170–3176
Chang EY, Guo B, Doyle SE, Cheng G (2007) Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol 178:6705–6709
Guo B, Chang EY, Cheng G (2008) The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest 118:1680–1690
Carrero JA, Unanue ER (2006) Lymphocyte apoptosis as an immune subversion strategy of microbial pathogens. Trends Immunol 27:497–503
Stephanou A, Brar BK, Knight RA, Latchman DS (2000) Opposing actions of STAT-1 and STAT-3 on the Bcl-2 and Bcl-x promoters. Cell Death Differ 7:329–330
Broz P, Monack DM (2013) Noncanonical inflammasomes: caspase-11 activation and effector mechanisms. PLoS Pathog 9:e1003144
Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S (2012) Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol 13:954–962
Ivashkiv LB, Donlin LT (2014) Regulation of type I interferon responses. Nat Rev Immunol 14:36–49
Gough DJ, Messina NL, Hii L, Gould JA, Sabapathy K, Robertson AP, Trapani JA, Levy DE, Hertzog PJ, Clarke CJ, Johnstone RW (2010) Functional crosstalk between type I and II interferon through the regulated expression of STAT1. PLoS Biol 8:e1000361
Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J (2011) Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34:213–223
Malireddi RK, Kanneganti TD (2013) Role of type I interferons in inflammasome activation, cell death, and disease during microbial infection. Front Cell Infect Microbiol 3:77
Thomas KE, Galligan CL, Newman RD, Fish EN, Vogel SN (2006) Contribution of interferon-beta to the murine macrophage response to the toll-like receptor 4 agonist, lipopolysaccharide. J Biol Chem 281:31119–31130
Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TW, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ (1999) SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98:597–608
Fenner JE, Starr R, Cornish AL, Zhang JG, Metcalf D, Schreiber RD, Sheehan K, Hilton DJ, Alexander WS, Hertzog PJ (2006) Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol 7:33–39
Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O’Neill LA, Hertzog PJ (2006) Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol 7:148–155
Karaghiosoff M, Steinborn R, Kovarik P, Kriegshauser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, Levy D, Decker T, Muller M (2003) Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol 4:471–477
de Weerd NA, Vivian JP, Nguyen TK, Mangan NE, Gould JA, Braniff SJ, Zaker-Tabrizi L, Fung KY, Forster SC, Beddoe T, Reid HH, Rossjohn J, Hertzog PJ (2013) Structural basis of a unique interferon-beta signaling axis mediated via the receptor IFNAR1. Nat Immunol 14:901–907
Thomas C, Moraga I, Levin D, Krutzik PO, Podoplelova Y, Trejo A, Lee C, Yarden G, Vleck SE, Glenn JS, Nolan GP, Piehler J, Schreiber G, Garcia KC (2011) Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell 146:621–632
Hardy MP, Owczarek CM, Jermiin LS, Ejdeback M, Hertzog PJ (2004) Characterization of the type I interferon locus and identification of novel genes. Genomics 84:331–345
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hertzog, P.J. (2014). Production and Action of Type I Interferons in Host Defense. In: Parker, D. (eds) Bacterial Activation of Type I Interferons. Springer, Cham. https://doi.org/10.1007/978-3-319-09498-4_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-09498-4_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09497-7
Online ISBN: 978-3-319-09498-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)