Abstract
With the advances in medicine and surgical treatments and the increase of computational tools and technical aids for supporting surgery, preoperative planning has become even more important and required. This technology relies upon digital tools and helps the clinicians to select the implant, to define its exact location and to establish a task outline for the surgery. Using these computational methods, it is also possible to design medical implants targeting a specific patient or clinical scenario. In this work, a three-step methodology for preoperative planning and design patient-specific implants is presented, namely (i) data acquisition and 3-D reconstruction, (ii) 3-D modelling and (iii) implant design. Two distinct case-studies are considered. The former consisted of a triple fracture of the distal femur, being the second case-study related to a maxillofacial deformity due to cancer removal surgery. For sake of pre-validation of the implant design, plastic prototypes were fabricated by using SL-stereolithography techniques. A tailored design of an implant leads to an enhancement on its biofunctionality, as this novel implant properly fits the extant medical need. Furthermore, the implant customization allows for its correct placement, promoting its fixation and longevity. In cases of osteosynthesis, a personalised design avoids the need of deforming the plates during surgery to fit patient’s anatomy.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Preoperative planning
- Image segmentation
- 3-D reconstruction
- Novel design
- Computer modelling
- Patient-specific implants
- SL-stereolithography
1 Introduction
Preoperative planning offers many benefits for patient, surgeon and remaining members of the surgical team, avoiding delays and misunderstandings. The exercise of thinking throughout the sequential steps of the surgical intervention (i.e. preoperative planning) allows for predicting possible problems and to develop contingency plans for achieving a successful outcome [2]. With the development of imaging and computer technologies, the preoperative planning has become even more present in hospitals and clinical practices. Typically, an orthopaedic surgery of a trauma clinical case is preceded by a 3-D reconstruction of the patient’s anatomy based on CT scans and a preoperative planning [3]. In some cases, additive manufacturing-based technologies (e.g. SL-stereolithography) complements the preoperative planning by combining virtual training with surgical training using physical models [1, 4, 5].
In a broad sense, an orthopaedic surgery involves the implantation of a medical device. These implants can present a standard geometry or a tailored design. Implant customization represents a suitable solution to achieve positive results in patient comfort and adequate strength distribution by designing the implant to fit a patient’s anatomy. Also, the use of patient-specific implants increases the longevity of the implant leading to a deferment or suspension of the surgical revision intervention. The development of an implant with a tailored design is even more important in patients who require orthopaedic reconstruction as a consequence of bone loss (e.g. osteosarcoma [5]) or defect (e.g. trauma [4]). This demand motivates the present study. Thus, a general framework for preoperative planning and tailored design of medical implants is outlined based on digital methods. Two distinct case-studies are considered as demonstrative examples of application.
2 Methods
In this section, a three-step methodology for preoperative planning and tailored design of medical implants is presented, namely (i) data acquisition and 3-D reconstruction, (ii) 3-D modelling and (iii) implant design.
2.1 Data Acquisition and 3-D Reconstruction
Medical imaging techniques (e.g. MRI—Magnetic Resonance Imaging or CT—Computed tomography) are used to acquire patient’s personalized data, which is usually delivered on DICOM file format. Then, a digital software is used for 3-D virtual reconstruction of patient’s anatomy. For this task, MIMICS software (Materialise NV, Leuven, Belgium) is considered. The first step of 3-D virtual reconstruction comprises to import and correctly orient the DICOM image-set. A filter (e.g. discrete Gaussian filter) should be applied to reduce CT noise and artifacts. The next step is the image segmentation, which consists of combining the thresholding tool with the region growing option. Firstly, for the threshold, based on the image grayscale intensities, an interval of histogram level ranges is set to isolate the bone tissue. The region growing feature eliminates noise and separate structures that are not connected. The segmented masks can be edited (edit mask—draw, erase, local thresholding; multiple slice edit; 3-D edit mask; Boolean operations; etc.) in order to correct connections between different bones and isolate bone surfaces. After image segmentation, the 3-D anatomical model is calculated and a virtual reconstruction of the model is accomplished.
Figure 1 illustrates the final result of a segmentation process in MIMICS interface described above. As showed, the software provides three sectional views of the segmented masks (coronal, axial and sagittal) and also, the resultant 3-D model of patient’s anatomy (Fig. 1, lower right corner). At this point, the 3-D virtual model is able to be analysed by the surgeon, who decides if there is any area that need further studies. Then, the STL model is imported to the software of the SL-stereolithography equipment (ViperTM SLA® System, 3D Systems® Corporation, Rock Hill—SC) and an epoxy model of the patient’s anatomy is prototyped.
2.2 3-D Modelling
The 3-D modelling task entails two main steps, an evaluation of the 3-D virtual model and a 3-D manipulation and measuring. Within 3-matic 8.0 software (Materialise NV, Leuven, Belgium), Fix Wizard tool is used to examine and repair the 3-D virtual model in terms of inverted normals, bad edges (such as bad contours, near bad edges and planar holes), possible noise shells, and overlapping/intersecting triangles. Whenever a bone fracture exists, the anatomical position of the bones needed to be verified and realigned (Fig. 2). Afterwards, to determinate the exact dimensions and contours demanded for a proper fitting between the implant and patient’s anatomy, a 3-D measuring is performed. In a broad sense, the 3-D modelling task has a fundamental role in the preoperative planning, since it provides an even more realistic view of the patient’s anatomy and detailed data with relevance for the clinical guidelines of the planned surgery.
2.3 Implant Design
This task comprises the design project of the customized implant and it can be performed in a STL-editor (e.g. 3-matic). Prior to the implant design, the internal structure of the bony tissue, which will surround the implant, is analysed to identify the anatomical regions/points that are more appropriate for cuttings and placement of fixation systems. Furthermore, for sake of manufacturing or other purposes, some modelling adjustments of the implant design can be carried out.
For pre-validation purposes, implant 3-D models are prototyped by using SL-stereolithography techniques. After a careful examination, whenever geometrical imperfections are reported, one of the following actions has to be taken: (i) edit the geometry of the implant to eliminate the detected imperfections; (ii) restart the whole process of implant design. Thus, the implant design is an iterative process that stops with the validation of the implant geometry. The pre-operative planning is concluded when the implant is correctly positioned at the patient bone.
At this point, the orthopaedic surgeon has a digital model of a patient-specific implant, which can be produced by additive manufacturing-based technologies, as well as a preoperative plan with relevant data for the clinical intervention (such as anatomical distances and the positing of the implant and the fixation systems).
3 Demonstrative Examples of Application
3.1 Case-Study 1: Femur Fracture
This case-study consisted of a 46 years old female with a triple fracture of the distal femur, resultant of a trauma accident. CT scans (Brilliance CT 16-slice, Philips Healthcare, Best, Netherlands) are acquired providing specific data of patient’s anatomy and trauma defects. The DICOM image-set comprises a total of 75 slices with 1.9 mm increment and pixel size of 0.487 mm. Using MIMICS 16.0 the DICOM image segmentation is conducted and a 3-D virtual model of patient’s anatomy is accomplished. This provides a visualization of the fractures, i.e. a detailed analysis of the shape and positioning of bone fragments (Fig. 3a).
In the next step, using 3-matic 8.0 software, several operations of 3-D manipulation (such as rotate, move, etc.) were carried out in order to correct the position of the bones, to reduce the fractures and to ensure a correct placement and alignment of bones and its multiple fragments. Figure 3b shows the result of this process.
In order to stabilize the fractures and to keep the bone fragments aligned and in a correct position, a tailored system of implants was developed, namely a surgical plate and a set of screws. The designed plate is based on the Locking Compression Plate (LCP) commercialized by SYNTHES® (Synthes GmbH, Oberdorf, Switzerland) (Fig. 4a). This plate has a combination of locking and compression holes (Combi holes). These holes allow the surgeon to choose between conventional plating techniques, locked plating techniques, or a combination of both. Afterwards, the CAD model of the surgical plate was projected onto patient’s anatomy (Fig. 4b) to ensure an appropriate adjustment of the implant to patient. Within this process, some modelling operations were executed, such as surface fitting, trimming and smoothing. Following, the screws are introduced in order to lock the surgical plate in an anatomical position that will guarantee a correct alignment of the bones fragments and a stabilization of the fractures (Fig. 4c).
Due to the complexity of this case-study (with multiple fractures in the same bone), a pre-validation of the designed implant system is required. Therefore an epoxy model is fabricated by means of SL-stereolithography as Fig. 5 illustrates.
3.2 Case-Study 2: Maxillofacial Deformity
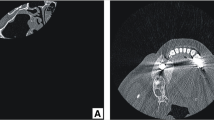
The case-study 2 reports a 68 years old female subject with a maxillofacial deformation resulting from cancer removal. CT scans (Toshiba Aquilion, Toshiba Medical Systems, Japan) are acquired to obtain fundamental information about the deformation and surrounding anatomical structures. The DICOM image-set comprises a total of 215 slices with 1 mm slice thickness and pixel size of 0.351 mm. Similarly to case-study 1, using this CT data, image segmentation process is conducted and a 3-D virtual reconstruction of the patient’s cranium is built. By analysing the model, an absence of part of the zygomatic and superior maxilla bone is observed (Fig. 6a). The 3-D manipulation step is skipped as there are no bone fragments to be repositioned. For sake of comprehension of the patient’s anatomy and malformation, the model is prototyped using SL-stereolithography (Fig. 7a).
Aiming to correct the internal and external appearance/function of the facial bony structure, a customized implant is developed. Within 3-matic 8.0, the reconstruction is performed by means of mirror modelling tools, in which the bone surface profile without defect is mimicked, using the middle sagittal plane as symmetry reference, to fill the missing bone area. An alignment is carried out in order to adapt the position of the mirrored section in the defected area. Finally, due to the importance of the maxilla in the chewing process, a bracket is added to the prosthesis design for supporting a future dental reconstruction (Figs. 6b and 7b).
Taking into account the bone density distribution in the surroundings of the defected area, as well as the prosthesis structural stability, the anatomical points more favourable to support the implant fixation are determined. SL-stereolithography is used for pre-validation of the implant design (Fig. 7).
4 Conclusions
The use of patient-specific implants promotes a correct placement and fixation of the implant and ensure its longevity. Combining digital methods with preoperative planning, a framework for tailored design of medical implants is presented. This approach entails three main tasks: (i) data acquisition and 3-D reconstruction; (ii) 3-D modelling; (iii) implant design. In a broad sense, the proposed methodology offers a set of advantages for orthopaedic surgeons, such as (i) the 3-D visualization of the injured area; (ii) the 3-D measuring and manipulation of bone fragments; (iii) the possibility of testing distinct treatments, i.e. different system of implants and surgical cuts; (iv) the 3-D visualization of the final result.
Within this work, two case-studies are presented resulting in two customized implants. It is important to mention that the project of new implant designs requires a pre-analysis of the internal bony structure to assure the static stability and structural support of the orthopaedic device. In both cases, the developed prosthetic models were prototyped using SL-stereolithography in order to proceed with its fabrication by investment casting in a biocompatible titanium alloy.
As future work, a topology optimization based on finite element methodologies is pointed out. This analysis could be an added value for the presented approach as it may lead to more lightweight products without compromising the structural stability of the prosthesis. Also, investigations on methods for automatizing the design procedure of customized implants are suggested as upcoming developments.
The advantages of the sophisticated design techniques used within the process of implant customization are widely recognised. However, due to its higher time and cost production, the medical community is still regularly using implants with a standard design. Thus, the goal of this work is to reverse this trend by improving and optimizing the process of implant customization in order to ensure high precision, cost-effectiveness and longevity to the orthopaedic implants.
References
Deshmukh TR et al (2012) A novel rapid prototyping and finite element method-based development of the patient-specific temporomandibular joint implant. Comput Methods Biomech Biomed Eng 15363–370
Hak DJ et al (2010) Preoperative planning in orthopedic trauma: benefits and contemporary uses. Orthopedics 33(8):581–584
Lantada AD, Morgado PL (2012) Rapid prototyping for biomedical engineering: current capabilities and challenges. Ann Rev Biomed Eng 14:73–96
Suero EM et al (2010) Use of a virtual 3D software for planning of tibial plateau fracture reconstruction. Injury 41:589–591
Sun SP et al (2009) Full-scale 3D preoperative planning system for calcaneal osteotomy with a multimedia system. J Foot Ankle Surg 48:528–539
Acknowledgments
Authors gratefully acknowledge funding of project Tooling EDGE (Proj.13856) and project SAESCTN-PII&DT/1/2011 co-financed by Programa Operacional Regional do Norte (ON.2—O Novo Norte), under Quadro de Referência Estratégico Nacional (QREN), through Fundo Europeu de Desenvolvimento Regional (FEDER).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Neto, R., Marques, T., Marta, M., Leal, N., Couto, M., Machado, M. (2015). Digital-Based Engineering Tools for Tailored Design of Medical Implants. In: Flores, P., Viadero, F. (eds) New Trends in Mechanism and Machine Science. Mechanisms and Machine Science, vol 24. Springer, Cham. https://doi.org/10.1007/978-3-319-09411-3_77
Download citation
DOI: https://doi.org/10.1007/978-3-319-09411-3_77
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09410-6
Online ISBN: 978-3-319-09411-3
eBook Packages: EngineeringEngineering (R0)