Abstract
The majority of proximal humerus fractures are amenable to nonoperative treatment. Most patients are elderly sustain low energy injuries. Nearly all of these patients will have an acceptable result with nonoperative management. A thorough history and physical examination should be performed with each patient to evaluate for concomitant extremity fractures as well as neurovascular injury. Standard sling immobilization with early and progressive mobilization will provide the most acceptable clinical outcome. Rarely complications such as stiffness, osteonecrosis, malunion, and nonunion can occur with nonoperative management of proximal humerus fractures. Very few high level outcome studies exist examining surgical and nonsurgical management of proximal humerus fractures, but nonoperative treatment of the majority of these fractures can provide acceptable pain relief, patient satisfaction, and function in most individuals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Proximal humerus fractures are common but debilitating injuries, which result in significant dysfunction for the patient and both diagnostic and treatment challenges for the physician. Knowledge of the complex bone and soft tissue anatomy of the shoulder is paramount in successful treatment of proximal humerus fractures. Proximal humerus fractures account for 5 % of all fractures, and they are third in frequency among the most common types of fractures [1–3]. In general, there is a unimodal distribution of these injuries. The vast majority are low energy fractures occurring in elderly individuals with more high energy and complex fractures in younger patients happening less frequently [4–6]. Incidence does tend to increase with age, and elderly individuals who sustain these fractures are more commonly female, over the age of 60, and have a history of osteoporosis [3, 7, 8]. Nearly ¾ of proximal humerus fractures occur in patients older than 60 who have fallen from a standing height [2, 4]. The majority of proximal humerus fractures in this demographic are relatively non-displaced and can be treated successfully without surgery [9]. Risk factors for proximal humerus fractures include elderly patients, low bone mineral density, impaired vision and balance, no history of hormone replacement therapy, smoking, >3 chronic illnesses, and previous fragility fracture [4, 10, 11]. Younger patients sustain proximal humerus fractures as a result of motor vehicle accidents, seizures, electric shock, and fall from greater than a standing height [12]. These injuries tend to involve more significant bony and soft tissue disruption and accordingly are treated with surgical intervention [2, 11].
Regardless of the age of the patient or mechanism of injury, restoration of pain-free functional range of motion remains the primary treatment goal of these injuries [13]. Some difficulty in clinical assessment and classification of proximal humerus fractures has resulted in a lack of standardization over treatment protocols [9]. Numerous factors contribute to post injury functional outcomes; therefore, a large debate exists over appropriate treatment [14, 15]. In addition, a lack of high-level evidence with regards to treatment and outcomes after proximal humerus fractures despite the relative frequency of the injury has resulted in a lack of consensus based protocol driven treatment [14, 16, 17]. Recent advances in technology have provided new treatment options without substantiation over historical options [9]. There currently exists several dilemmas such as when to perform surgery and which surgery is the most appropriate method of treatment, which have yet to be definitively determined. High-level outcome based studies are currently being performed to help answer questions but uncertainty still remains [18]. Regardless of treatment selected, early active mobilization has led to improved outcomes [19, 20].
Anatomy
Several anatomic characteristics must be considered when deciding appropriate treatment of proximal humerus fractures. The shoulder is an unconstrained ball-and-socket articulation, which relies on both a complex bone and soft tissue anatomy for stability and function. It has more inherent motion than any major joint in the body, therefore any injury disrupting the bone and soft tissue restraints can lead to both instability and dysfunction. Moderate loads to the glenohumeral joint are offset by dynamic restraints such as the deltoid and rotator cuff, whereas, larger loads are counteracted by the capsulolabral structures and the bone [12].
Bone anatomy of the proximal humerus can be subdivided into four main parts based off classification of typical injury patterns [21]. The proximal humerus consists of the humeral head, lesser tuberosity, greater tuberosity, and shaft fragments. The head fragment is spherical in shape and has an average diameter of 46 mm (37–57 mm) [22]. The height of the head is 8 mm superior to the greater tuberosity with an offset 3 mm posterior and 7 mm medial to the shaft [23, 24]. The head has an average of 20° retroversion with a high anatomical variance (6.7° anteversion–47.5° retroversion), and it is inclined 130° with respect to the shaft [23, 25]. The anatomical neck separates the head and tuberosities and serves as a site of attachment for the capsular structures. Injury at this location portends to a poor prognosis as it disrupts the entire blood supply to the head [26]. Bone quality of the proximal humerus can be predicted by the cortical thickness of the proximal diaphysis [27]. The subchondral bone underlying the articular surface is the densest, and there is a particular decrease in density in the humeral head when moving from superior to inferior and from posterior to anterior [27–30].
The tuberosity fragments serves as anatomical attachment sites of the rotator cuff. These soft tissue attachments lead to displacement through predictable force vectors. Greater displacement of fragments leads to greater soft tissue disruption and loss of blood supply [3]. The supraspinatus, infraspinatus, and teres minor tendons all attach on separate facets of the greater tuberosity. These attachments result in the typical posterior and a superior displacement seen with fractures of the greater tuberosity. The lesser tuberosity serves as a site of attachment for the subscapularis tendon and results in medial displacement of the fractured lesser tuberosity fragment [3]. The bone of the tuberosities tends to be denser at the rotator cuff insertion site, and the tendons are usually stronger than the bones at the sites of attachments [31]. The bicipital groove separates the greater and lesser tuberosities, and the distal groove the slightly internally rotated with respect to the proximal portion of the groove [32]. Fractures between the tuberosities typically occur posterior to the bicipital groove [33]. The tuberosities are separated from the shaft fragment via the surgical neck, which is an indistinct region of metaphyseal bone below the tuberosities and above the shaft. Fractures of the tuberosity dysfunctions the attached rotator cuff muscles, and malunion secondary to displacement can lead to subacromial and subcoracoid impingement [34, 35].
The proximal humerus articulates with both the glenoid and coracoacromial arch. The head articulates with the glenoid, which is a convex structure shaped like an inverted pear. The capsulolabral structure attaches both and can be disrupted with injuries to the proximal humerus [12]. The coracoacromial arch is made up of the acromion, coracoacromial ligament, and coracoid. This rigid bony and ligamentous structure imparts stability on the shoulder, and fracture and subsequent displacement can disrupt normal gliding between the arch and proximal humerus causing impingement and dysfunction [26]. In addition, the subdeltoid and subacromial bursa can become thickened, fibrotic, and scarred as a result of fracture causing adhesions and limits of motion. Early motion is theorized to limit these adhesions [20].
The proximal humerus has an extensive vascular network (Fig. 2.1). The anterior and posterior humeral circumflex arteries, creating a vascular leash, surround the proximal humerus. The anterior humeral circumflex artery arises from the axillary artery at the inferior border of the subscapularis, and it provides the majority of the vascular inflow for the humeral head along with its interosseous branch the arcuate artery [36–38]. More significant soft tissue displacement and higher energy fractures are associated with increasing vascular disruption to the humeral head. Injury to the arcuate artery in proximal humerus fractures is associated with AVN, but extraosseous collateral circulation can perfuse the humeral head despite arcuate artery injury [39–41]. The posterior humeral circumflex artery travels with the axillary nerve posteriorly to supply the posterior rotator cuff and posterior capsule. In some proximal humerus fractures, the posterior humeral circumflex artery and its branches to the posterior capsule can maintain humeral head perfusion alone [41]. Comminution of the medial metaphysis with an articular segment of less than 1 cm has been associated with avascular necrosis after proximal humerus fracture due to disruption of the anterior and posterior humeral circumflex vessels [22, 41]. Severe vascular injury can be seen in 5–6 % of proximal humerus fractures [12]. The axillary artery is most commonly injured and is seen in patients with comorbid conditions [42].
With their close proximity to the proximal humerus, neurologic structures are at risk for injury after proximal humerus fracture. Neurologic injuries generally occur secondary to traction but can happen secondary to blunt trauma as well [43]. The axillary nerve is most commonly injured. It has a distance of 6.1 cm from the superior aspect of the proximal humerus and 1.7 cm from the surgical neck [43, 44]. Injury can occur in any of the three branches to the deltoid, teres minor, or the superior lateral cutaneous nerve. Suprascapular neuropathy can occur secondary to traction at either the exit from the upper trunk of the brachial plexus or under the transverse scapular ligament [43, 45]. Musculocutaneous nerve injury is uncommon but can occur with blunt trauma as it enters the conjoint tendon 3.1–8.2 cm from the tip of the coracoid [46, 47]. In addition, there is a high association of brachial plexopathy with axillary artery injuries [42].
Epidemiology
Proximal humerus fractures are an age related phenomenon that can only be expected to rise with an increasingly aging population [48]. As stated previously, proximal humerus fractures represent the third most common fracture related injury in patients over the age of 60, and they represent 5 % of all injuries to the extremities [1, 3, 37, 49]. Incidence of the injury seems to increase with age, and females are more likely to sustain proximal humerus fractures in comparison to males [3]. Population studies have shown that as many as 70–80 % of all proximal humerus fractures occur in women [4, 50–52]. These injuries are less common in Japanese populations than Europeans or white Americans [53, 54]. In addition, white Americans sustain proximal humerus fractures at a greater frequency than black Americans [55]. The incidence of proximal humerus fracture is 63–105 fractures per 100,000 populations per year [4, 51, 52, 55, 56]. The prevalence of the injury is expected to continue to rise in conjunction with the shifting population demographics [8]. There is a unimodal elderly distribution curve of the injury with a low incidence under the age of 40 and a sharp increase thereafter [50]. The majority of proximal humerus fractures occur in the elderly who have a history of osteoporosis and sustain low energy injuries. In patients over the age of 60, 97 % of proximal humerus fractures are secondary to a fall with a direct blow to the shoulder [4, 57]. Due to this shifting demographic, the incidence of osteoporotic fractures is expected to triple over the next three decades [58]. Long-term Finnish studies have confirmed the correlation of increasing incidence of proximal humerus fractures with age [59]. Women who are over the age of 60 have an 8 % lifetime risk of proximal humerus fracture [60]. The correlation of osteoporosis with proximal humerus fractures can complicate both fracture treatment and patient management of post fracture complications. Risk factors for sustaining a proximal humerus fracture include osteoporosis and frequent falls [61–63]. In prospective and consecutive osteoporosis screening only 13 % of 239 hospitalized fracture treatment patients had a normal bone density [64]. In addition, history of poor balance and impaired vision has been correlated with an increase in fracture risk [9]. In contrary to the high-energy high-displacement injuries seen in younger patients, nearly 80–85 % of all proximal humerus fractures are minimally displaced, and therefore can be treated safely without surgery [21]. If surgery is indicated, osteoporosis complicates surgical management. Fixation failure is likely with decreasing bone mineral density, and osteoporosis can compromise both functional and radiographic outcomes associated with fracture healing [65]. The majority of proximal humerus fractures are fragility fractures, and the greatest risk factor for future fragility fracture is a history of previous fragility fracture [9]. To prevent any future complications, osteoporosis treatment should be part of the global care given to any patient who sustains a proximal humerus fracture [3].
Etiology
While the majority of proximal humerus fractures arise secondary to low energy injuries, mechanism of injury is directly correlated with the age of the patient. The injury occurs the most frequently in the elderly population, and most injuries occur as a result of a fall onto an outstretched hand from a standing height in patients over the age of 60 (Fig. 2.2) [5, 8, 59]. Nearly ¾ of proximal humerus fractures occur after a low energy domestic fall [4, 51, 52, 55]. Younger patients without osteoporosis generally sustain a proximal humerus fracture after motor vehicle accidents, falls from greater than a standing height, seizures, or electric shock [2, 66, 67].
The biomechanics of the fracture and general bone quality of the patient tend to produce varying injuries in the proximal humerus. In general, fractures occur as either a direct blow to the shoulder of from indirect force transfer from a fall onto an outstretched hand [3, 9, 12]. The impact drives the proximal humerus into the glenoid resulting in significant energy transfer to the proximal humerus. The glenoid bone is generally harder and denser than the proximal humerus, and therefore acts as an “anvil” on which the proximal humerus is impacted [68]. The combination of the direction of the blow to the humerus, quality of bone in the proximal humerus, as well as the pull of the soft tissues produces the various types of fracture patterns [9].
Medical comorbidities both increase the risk for fracture and type of fracture sustained. Proximal humerus fractures are seen in a greater frequency in patients with a depleted neuromuscular response [69–71]. It has been suggested that patients who sustain proximal humerus fractures are frailer than those who sustain distal radius fractures [72]. The proximal humerus is injured more frequently in patients with decreased neuromuscular response who cannot raise their arm quickly enough to break a fall [73, 74]. Risks factors such as delayed reaction time; cognitive impairment, neuromuscular disorder, impaired balance, and intoxication are all associated with proximal humerus fracture [75]. Middle-aged patients who sustain proximal humerus fractures are physiologically older with a higher incidence of medical comorbidities, alcohol, tobacco, and drug usage [76, 77]. Early menopause is the most common physical aging comorbidity associated with proximal humerus fractures [9]. In addition to osteoporosis, pathologic fracture from either primary malignancy or metastatic disease can occur secondary to minimal trauma [5].
Clinical Evaluation
During initial evaluation of proximal humerus fractures, a complete history and physical must be performed. The history should initially determine whether the injury is a high or low energy injury and proceed accordingly. With a high-energy injury after a motor vehicle accident, fall from greater than standing height, or similar injury, ATLS protocol should be initiated. Chest injuries associated with high-energy proximal humerus fractures can include pneumothorax, rib fracture, and hemothorax [9]. Cervical spine injuries are commonly associated with significant shoulder fractures secondary to high-energy trauma [9]. Rare case reports of intrathoracic and retroperitoneal proximal humerus fracture dislocation exist [78, 79].
A thorough history should include mechanism, pre injury level of function, occupation, hand dominance, history of malignancy, history of previous fragility fractures, and rehabilitation potential. The presence concomitant extremity injuries should be assessed. Patients with proximal humerus fractures can present with injuries to the hip, elbow, wrist, and hand, and any tenderness or pain in those areas should be thoroughly addressed. Conversely, patients with more distal extremity fractures with pain in the shoulder should be evaluated for proximal humerus fracture.
A complete physical examination should assess the entire upper extremity and focus on other areas of concern. The skin envelope is robust around the shoulder, and open fractures are exceedingly rare [9]. Occasionally, a significantly displaced humeral shaft in slim individuals can create pressure necrosis on the skin. The rate of skin compromise is approximately 0.2 % and commonly associated with significantly displaced two part surgical neck fractures [80]. Mechanism for skin penetration involves blunt trauma to the shoulder, and can either occur by initial penetration or delayed opening secondary to skin tenting [80]. Significant ecchymosis occurs often in a delayed fashion, and due to gravity, tracks down fascial planes. As a result, swelling and bruising can be pronounced at the elbow. Anterior and posterior fracture dislocations can cause an increased swelling and fullness in the anterior and posterior aspects of the shoulder respectively (Fig. 2.3) [67]. Very severe swelling can occasionally be associated with vascular injury, but nearly all proximal humerus fractures have some degree of swelling associated with the injury [42]. For more subtle injuries, specific palpation of the proximal humerus should be performed. Non-displaced and minimally displaced fractures of the greater tuberosity are overlooked in nearly 53 % of all initial examinations [81]. A thorough neurovascular examination should include inspection of the distal circulation, axillary nerve, as well as distal neurologic status. A thorough secondary survey for other extremity injuries and head, neck, chest, and facial trauma should be performed.
Neurologic injuries are common after significant proximal humerus fractures and often overlooked [82]. The axillary nerve arises from the C5 and C6 nerve roots and in the axilla splits from the brachial plexus via the posterior cord. The axillary nerve carries three branches, a sensory branch supplying the skin overlying the lateral deltoid, and two motor branches to the deltoid and teres minor, respectively. The axillary nerve travels around the inferior aspect of the subscapularis and posteriorly along the surgical neck. It travels through the quadrangular space with the posterior humeral circumflex artery. Incidence of neurologic injury with proximal humerus fracture ranges between 6.2 % and 67 % with axillary nerve injuries being the most common [83]. The axillary nerve is susceptible to a tethering type injury with significant displacement of the surgical neck and particularly in anterior fracture-dislocations [43]. Brachial plexopathy can occur via direct blow from displaced fragments, and multiple nerves are at risk during treatment for proximal humerus fractures [43, 45, 82]. A complete neurologic evaluation can be difficult due to pain and guarding secondary to fracture, but a full axillary nerve and peripheral nerve exam should be performed with each injury. Both the brachial plexus and peripheral nerves are at risk during operative treatment, and risk facture such as cervical spine disease, low BMI, diabetes mellitus, and delay of operative treatment for more than 14 days are associated with an increased incidence of nerve dysfunction [83].
A large vascular leash surrounds the proximal humerus, but major vascular injury is only rarely associated with proximal humerus fractures [84]. Even when a vascular injury occurs, a rich collateral circulation exists in the upper extremity, so obvious signs such as expansile hematoma, pulsatile external bleeding, unexplained hypotension, and plexus injury should raise suspicion of vascular injury [9]. Significant medial displacement of either the head or the shaft can result in an axillary artery injury [42, 43]. Vascular injury should be assumed with a four-part proximal humerus fracture with axillary dislocation of the head. When fracture is associated with dislocation, risk of blood vessel injury increases 30 % [85]. Distal circulation should be assessed in all patients with proximal humerus fractures.
Soft tissue injury associated with proximal humerus fracture is commonly encountered, and it should be expected if injuries do not follow typical clinical course [86]. Superficial muscle perforation can occur after significant displacement of fracture fragments. With fractures of the greater or lesser tuberosity, the rotator cuff is essentially defunctioned, and rotator cuff dysfunction should be expected [9]. A complete rotator cuff examination cannot usually be performed in an acute setting due to pain and swelling, but rotator cuff function should be followed throughout the typical clinical course to ensure adequate function. Due to the age of most patients who sustain proximal humerus fractures, previous rotator cuff disease is likely, and certainly a new rotator cuff tear can occur in conjunction with proximal humerus fractures [86]. With less severe bony injuries that do not follow the typical healing course, labral pathology should be suspected as well. Case reports of isolated SLAP lesions and combined SLAP and rotator cuff injuries after non-displaced proximal humerus fractures exist [87]. In this report, patients continued to have shoulder pain and dysfunction despite appropriate bony healing that resolved with arthroscopic repair of rotator cuff and labral injuries [87].
A complete radiologic evaluation should be included in every clinical evaluation of proximal humerus fractures. A trauma series should include an AP and lateral taken in the scapular plane along with an axillary lateral. Due to the anatomic positioning of the glenoid in relation to the thorax, the standard anteroposterior radiograph taken in most emergency departments is generally unsatisfactory to assess shoulder anatomy. In general, most AP radiographs taken of the shoulder are mainly views of the upper quadrant and have significant overlap of anatomic structures such as the coracoid, glenoid, humeral head, and scapula. The surgeon must be able to specify to the radiology technician appropriate methods to obtain a true AP of the shoulder. The patient’s affected shoulder should be placed against the X-ray plate and the opposite shoulder is tilted approximately 40° towards the beam [9]. This positioning will ensure a direct view through the glenohumeral joint. To obtain an appropriate scapular lateral, the anterior shoulder is placed on the X-ray plate with the unaffected shoulder tilted forward 40°. The beam is placed posteriorly and directed along the scapular spine [12]. An axillary lateral view is paramount to assess anterior or posterior displacement of the humeral head in relation to the glenoid. To obtain the view the arm must be abducted as close to 90° as possible. The cassette is placed on the superior aspect of the shoulder and the beam directed from inferior perpendicular to the cassette [12]. Due to the nature of the injury, abduction of the shoulder can usually not be achieved after proximal humerus fracture. Therefore, a modified axillary view or Velpeau view can be substituted for an axillary lateral [12, 88]. The view allows the patient to remain in the sling; therefore, it is much less painful for patients. The view is obtained by leaning the patients over a table on which the cassette lies. The beam is then directed from superior to inferior. In cases where a more subtle greater tuberosity fracture of Hill Sachs lesion is suspected, internal and external rotation views can be obtained to further evaluate the anatomy of the humeral head [12]. A complete X-ray examination can provide information with regards to the typical displacement seen after proximal humerus fracture. The internal rotation views, axillary or scapular lateral will show typical greater tuberosity posterior displacement due to the pull of the infraspinatus and supraspinatus [3]. Both the anteroposterior and lateral views showcase the medial displacement of the lesser tuberosity and shaft consistently produced by the pectoralis major and subscapularis muscles [89]. Head displacement is typically variable and related to remaining soft tissue attachments. The axillary lateral view is needed to evaluate for humeral head dislocation [3]. Posterior dislocation of the humeral head associated with proximal humerus fractures is typically missed without an appropriate axillary lateral view [13].
Computed tomography analysis of proximal humerus fractures can provide enhanced bony detail and greater understanding of fracture patterns and displacement (Fig. 2.4a, b). CT provides an enhanced understanding of tuberosity displacement, fracture comminution, impaction, humeral head involvement, and glenoid articular surface injury [5, 67]. Both two-dimensional and three-dimensional images can be obtained through software programs at most institutions to provide even greater detail of complex fracture patterns [90]. CT scan with 3D reconstructions has been shown to provide the highest interobserver agreement with regard to classification and treatment recommendations among upper-extremity specialists [91].
Magnetic Resonance Imaging provides very little benefit to the initial evaluation of proximal humerus fractures. If pathologic fracture is suspected, an MRI can aid in staging of the disease prior to treatment [12]. If either rotator cuff or labral injury is suspected after bony healing, MRI can be helpful in assessing for these injuries [87]. In the acute setting, bleeding from the fracture and soft tissue swelling can make the use of MRI difficult in assessing soft tissue injury after a fresh proximal humerus fracture [9].
Clinical Decision Making
When deciding the appropriate treatment method for proximal humerus fractures, the surgeon must have a clear understanding of the primary goals for treatment. The goal of treatment of proximal humerus should be to minimize pain and maximize shoulder function [33]. Achieving the goal is paramount regardless if it is through surgical or nonsurgical means. Multiple factors play into treatment decision making, but all surgeons should strive for complication free healing to produce a pain free, mobile, stable, and functional shoulder [9].
Factors related to the patients, surgeon, and injury all determine appropriate treatment methods. When deciding between operative and nonoperative treatment, patient characteristics such as age, mental status, substance abuse, medical comorbidities, osteoporosis, rehabilitation potential, functional expectations, and limited life expectations should all effect treatment methods [9, 12, 13]. In general, a lower demand individual with significant medical comorbidities is more appropriately treated nonsurgically with the goal of establishing early functional pain-free motion [19]. Older patients tend to have worse functional outcomes after treatment for proximal humerus fractures [92]. This trend has been attributed to such factors as fragility, cognitive deficits, rotator cuff injuries, osteoporosis, and poor rehabilitation potential [63, 70, 93, 94]. Patient factors have been proven to effect treatment related outcomes after both surgical and nonsurgical management. Complications such as infection, nonunion, osteonecrosis, fixation failure, and compliance with rehabilitation can all be related to medical comorbidities [9]. Specifically, alcohol abuse increases the risk of noncompliance and nonunion, and tobacco usage increased a patients risk of nonunion [77, 95]. Osteoporosis is associated with increased rates of comminution, defects due to impaction, and loss of fixation and reduction after surgical management [3].
Injury related factors influencing treatment decision include fracture type, displacement, soft tissue injury, and concomitant injuries. The majority of all proximal humerus fractures are minimally or non-displaced, and therefore can be successfully managed without surgery [5, 96]. Approximately only 20 % of proximal humerus fractures are either comminuted or displaced sufficiently that they require operative intervention [5, 97]. Fracture type alone has been seen as a limited predictor of overall outcome [5, 97]. Bone quality, comminution, displacement, rotator cuff status and vascular risk can all be related to varied outcomes [9, 12]. Treatment controversy exists when considering injury related factors alone, and traditional guidelines for treatment proposed by Neer are not the gold standard according to current evidence based medicine [21, 98–101]. Near functional normality can only be expected after simple injuries to the proximal humerus [20, 92, 102–104]. For patients with more severe and complex proximal humerus fractures proper counseling prior to either nonoperative or operative treatment is paramount to establish patient expectations and goal prior to proceeding with treatment [96, 105]. Investigation of outcomes after displaced four part proximal humerus fractures show that both operative and nonoperative treatment can achieve similar outcomes, although several limitation exist when comparing studies of different patient selection criteria, procedures, and outcome measures in small patient populations [101]. Proper patient selection is the most important factor in achieving a good outcome with treatment [33].
Surgeon expertise, comfort, and experience influences appropriate treatment of proximal humerus fractures as well. With modern advances in orthopedic technology, the surgeon has an armamentarium of options to treat fractures of the proximal humerus. From nonoperative treatment to limited percutaneous fixation, open reduction internal fixation with standard plating, locked plating techniques, intramedullary nails, suture fixation, various bone grafting options, and arthroplasty, the surgeon has multiple options to address various injuries to the proximal humerus [18, 52, 106–110]. Each option has various advantages and disadvantages associated with treatment, and the surgeon must be familiar with each prior to proceeding with treatment and determining which method is the most appropriate for each individual patient. Overall, results after nonoperative treatment will be superior to a poorly performed operative procedure regardless of the method of fixation [9].
General indications for surgical management are open fractures, significant displacement, and segmental injuries in patients who are healthy enough for surgery [14, 18, 70, 80, 111]. Nonoperative treatment is indicated in simple and non-displaced proximal humerus fractures, but can be utilized effectively in more complex injuries and patients unfit for surgery [20, 92, 101–104]. Most current treatment recommendations are based off of expert opinion and low powered studies [112]. Until larger and higher level comparative studies are performed, treatment of proximal humerus fractures likely will depend on surgeon experience and preference [3].
Nonoperative Treatment
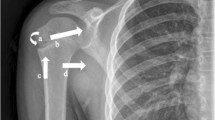
As stated previously, the majority of proximal humerus fractures are stable fracture patterns and very amenable to nonoperative treatment [5, 96]. Relatively non-displaced two and three-part fractures rely on surrounding soft tissue restraints for both healing and stability. The rotator cuff, periosteum and surrounding joint capsule provide and internal sling for the fracture fragments and resist any further displacement of fracture fragments [3]. Minimal tuberosity displacement with shaft impaction into the shaft reduces the risk of nonunion [9]. Absolute stability is difficult to determine on an initial examination. X-ray characteristics such as minimal comminution, three or less fragments, absence of significant tuberosity displacement, cortical contact, relative impaction of the stem into the head, and no history of dislocation suggest relative stability of the fracture fragments [2, 21, 91, 92, 113]. On physical examination, gentle rotation of the elbow and forearm can be performed with simultaneous palpation of the humeral head. Fracture stability is implied if the fragments appear to move as a unit [12]. Despite appropriate X-ray and examination findings, late displacement of fragments can occur, therefore, serial X-ray examinations over the first 2–3 weeks post injury are recommended to ensure late displacement does not occur [114]. The appearance of slight inferior subluxation of the glenohumeral joint in conjunction with a proximal humerus fracture or “pseudosubluxation” is not an indicator of an unstable fragment [58, 115]. Factors such as deltoid atony, deltoid inhibition, neuropraxia, hemarthrosis, and rotator cuff dysfunction all contribute to the appearance of mild inferior subluxation [58, 115]. This finding is common after proximal humerus fracture and tends to be self-resolving during the typical healing course (Fig. 2.5a, b).
Unlike fractures in the humeral shaft, closed reduction and functional bracing is a rare option for treatment. Fractures of the humeral shaft can be effectively immobilized with a fracture brace [116]. Sarmiento showed high healing rates and acceptable functional outcomes after nonoperative treatment with functional bracing of the humeral shaft [117]. The surrounding soft tissue envelope and ability to control fragments proximal and distal to the fracture site allow successful treatment of humeral shafts [117]. Unfortunately, the proximal humerus has multiple complex deforming forces, which cannot be neutralized with a brace, and control of the bone proximal to the fracture fragments is impossible with an external brace [13]. For historical purposes, airplane splints and shoulder spica casting with the arm placed in abduction and forward elevation can neutralize some deforming forces of the proximal humeral shaft, but this method of treatment is poorly tolerated and not currently indicated in treatment of proximal humerus fractures [13].
Rarely, an unstable two-part proximal humerus fracture can become stable with closed reduction [118]. Tuberosity displacement is difficult to reduce without operative fixation, but shaft displacement can potentially be managed with reduction [13]. A displaced surgical neck fracture usually results in medial and anterior displacement of the humeral shaft secondary to the pull of the pectoralis major muscle [89]. The reduction maneuver for a proximal humeral shaft involves longitudinal traction with adduction and a posterior directed force on the humeral shaft [119, 120]. This maneuver attempts to neutralize the pectoralis and align the head and shaft. After alignment, the shaft must be impacted into the head to achieve a stable position. If a stable reduction is achieved, nonoperative treatment can achieve an acceptable outcome [118].
Bracing options include a standard sling, shoulder spica cast, hanging arm cast, and airplane splint. A standard sling provides adequate immobilization for all proximal humerus fractures treated nonoperatively [121]. A sling allows slight gravity distraction to the bone ends to aide in initial pain relief [97]. Hanging arm casts provide no advantage over a standard sling, and excessive distraction of the bone ends by a hanging arm casts can promote to nonunion, and other methods of immobilization are poorly tolerated [96, 105, 122].
Initial pain control after the injury is difficult, but a combination of oral medications, topical modalities, and sling immobilization provides adequate pain control over the first several days after injury. Some patients will have difficulty with sleeping in a bed after proximal humerus fractures, therefore, sleeping in a sitting position in a recliner should be recommended for individuals after proximal humerus fracture. Most patients can be managed as an outpatient with these injuries, but frail elderly individuals who live alone occasionally will require hospital admission. More incapacitated patients can benefit from hospital admission for both pain control and rehabilitation to aide with activities of daily living after discharge [9].
Early protection with gradual mobilization is the primary tenant of nonoperative treatment of proximal humerus fractures [20, 105, 113, 123, 124]. Absolute sling immobilization should only be performed over the first 7–10 days post injury [19, 20, 125]. Excessive immobilization has not been shown to improve outcomes [20]. Prolonged immobilization can result in increases in pain and decrease in ultimate range of motion and function [19, 20, 125].
The functional recovery improves when physical therapy is instituted as close to the injury as possible [19, 20, 125]. Both the timing and type of exercises performed after proximal humerus fracture will contribute to a successful outcome [20]. Distal extremity exercises should be started immediately after the injury, and shoulder range of motion should be initiated within 10 days of the injury if pain allows [19]. Koval et al. showed improved outcomes when physical therapy was instituted within 14 days of the injury [20]. Exercises instituted by the 14-day mark resulted in decreased rates of stiffness and improved function and ability to perform activities of daily living. It is important to remember close radiographic follow-up to ensure no further angulation or displacement of fracture fragments after initiation of physiotherapy [114]. Some form of therapy, whether a supervised or structured home program, should continue until maximal functional recovery, which can take up to a year after injury [20, 105]. Even minimal therapy is better than a complete absence of treatment [126]. A single therapy session with subsequent performance of exercises at home can be as effective as a supervised therapy program [126]. More involved therapy modalities such as hydrotherapy or pulsed electrotherapy does not seem to improve outcomes [5, 127].
Successful Rehabilitation should include a standard therapy protocol of exercises that maintain motion and increase strength as fracture healing allows. Active elbow, wrist, and hand exercises should be initiated immediately after injury. Shoulder pendulum exercises should be initiated immediately, and attempts at assisted shoulder flexion, abduction, and rotation should begin at 1-week post injury [125]. Isometric deltoid and cuff exercises should be initiated at 3 weeks, and progressive strengthening and stretching can usually be initiated between 6 and 12 weeks [20].
When examining functional outcomes after non-displaced proximal humerus fractures, Koval et al. proposed a protocol, which improved outcomes if initiated within 2 weeks after injury [20]. They utilized sling for initial pain relief, then at 1 week everyone was instructed on range of motion exercises and referred to physical therapy. Therapy consisted of biweekly visits where active hand, elbow, and wrist exercises were performed in conjunction with passive shoulder motion. Initially, shoulder exercises were performed in the supine position and included forward elevation, external rotation, and internal rotation to the chest. The sling was continued for 4–6 weeks, and exercises were performed four times daily at home. Once clinical fracture union was confirmed, the sling was discontinued. Active range of motion and deltoid and rotator cuff isometric strengthening was added. Active exercises were initiated in the supine position and progressed to the seated position. As range of motion improved, active resistance deltoid and rotator cuff exercises were begun. Three months after the injury, an aggressive stretching and strengthening program was continued until final outcome was achieved [20].
Outcomes
In the absence of complications, most elderly patients with stable proximal humerus fractures will have a functional pain-free shoulder [9]. Functional improvement can occur up to 2 years after the injury, but rapid improvement are made in the first 6 months and near full improvement occurs at 1 year [128–130]. Patients should be counseled that their shoulder would most likely never be completely normal after a proximal humerus fracture [9]. Most patients can expect minor aches with vigorous activity, but most should be able to perform activities of daily living [121]. Fortunately, functional expectations in elderly individuals are diminished in comparison to younger patients, thus a less than satisfactory result for a young patient can be a completely acceptable result for an elderly individual [105, 113, 131]. Even with decreased outcome scores, elderly patients perception of outcome and quality of life can be acceptable [92]. Court-Brown reported a series of 125 valgus-impacted fractures treated nonoperatively. One year after injury, 80 % of the primarily elderly patients have a good to excellent outcome, despite residual deficits in strength and range of motion [92].
Anatomic classifications utilized to determine treatment provide prediction of outcomes. Nonsurgical treatment of comminuted four part fractures has yielded poor outcome results [14]. Despite poor constant scores, patient satisfaction levels remained high at 10-year follow-up [132]. In a prospective cohort study, Caceres et al. examined nonoperative treatment in both displaced and non-displaced proximal humerus fractures [132]. While healing occurred in most patients, constant scores worsened with worsening severity of fracture [132]. Functional outcomes improved progressively from four part to three part and subsequently two part fractures. Pain outcomes worsened with three and four part fractures in relation to two part injuries, and individuals who were under the age of 75 and had non-displaced injuries had improved functional outcomes [132]. The authors concluded that nonoperative treatment of proximal humerus fractures in elderly can provide pain relied with limited functional outcome, but this did not seem to effect quality-of-life perception. Patients with more severe and displaced fractures should be counseled of the possibility of inferior outcomes [132].
Recently, patterns of displacement have been correlated to outcome in nonoperatively treated proximal humerus fractures [133]. Radiographic and CT studies were used to classify patterns of displacement into posteriormedial (varus) impaction, lateral (valgus) impaction, isolated greater tuberosity, and anteriomedial impaction. Factors such as head orientation, impaction of the surgical neck, and displacement of the tuberosity correlated strongly with outcome [133]. Lateral impaction fractures had a worse outcome than other patterns. As both posteriormedial and greater tuberosity displacement increased, outcome worsened [133]. Overlap of the greater tuberosity was associated with a worse outcome if it overlapped the posterior articular surface [133]. In varus, or posteriormedial, impaction, outcome worsened as the articular surface displaced inferiorly and increased the distance from the acromion. Functional outcome is difficult to assess, and many variables contribute to a successful patient outcome [133].
Standard radiographic measurements can be used to predict functional outcomes. Humeral head angulation on initial radiographs correlates with ultimate functional outcome [134]. Angulation of the humeral head on both a standard AP projection and scapular lateral view had a significant association with Constant-Murley outcome. The optimum predictive angulation was a Y view of 55° of angulation at the time of fracture. Initial and 1 week Y view measurements were the most important predictors of the decreased functional outcome at a median of 2.2 years follow-up [134].
Very little high level evidence exists assessing outcomes after proximal humerus fractures [14, 16, 17, 135]. A lack of consistently successful surgical techniques and common complications has resulted in a preference for nonoperative treatment over surgery [13]. Also, there are few significant comparison studies of operative and nonoperative treatment. In a prospective randomized study, Zyto et al. could find no functional differences between patients with three- and four-part fractures treated with tension band fixation verses conservatively [18]. Retrospective studies of elderly populations with three-part and valgus impacted fractures show favorable results regardless of surgical versus nonsurgical treatment [15, 92]. Meta-analysis of three- and four-part fractures revealed that patients treated conservatively had more pain and worse range of motion than those treated with either fixation or arthroplasty [14]. Overall, conflicting results exist with some studies favoring operative intervention with others failing to show a large benefit for more displaced and unstable fractures [136]. A prospective case series examined nonoperative treatment of multiple fracture types in 160 patients [121]. The injuries included 75 one-part, 60 two-part, 23 three-part, and 2 four-part and head splitting fractures. After 1 year, the difference in constant score was 8.2; DASH was 10.2 with the uninjured shoulder. Risk of nonunion was 7.0 %. Nine patients underwent surgery (four fixation, five arthroscopic subacromial decompression). The authors suggest that these results make it difficult to demonstrate significant benefit of surgery over nonoperative treatment for proximal humerus fractures [121].
Complications
Major complications following nonoperative treatment of proximal humerus fractures include osteonecrosis, nonunion, stiffness, and rotator cuff dysfunction [20, 41, 104, 137]. Although relatively rare with nonoperative treatment, complications adversely effect outcomes and often require additional intervention [62, 105, 106, 138]. Both patient and injury related factors can increase complication risk with proximal humerus fracture. Increased age, osteoporosis, medical comorbidity, worsening fracture comminution and displacement, and increasing soft tissue injury are all associated with increased risk of complications with proximal humerus fractures [9]. Complication rates are extremely low with nonoperative treatment of non-displaced and minimally displaced proximal humerus fractures, but complication rates increase with conservative treatment of proximal humerus fractures in elderly patients and in displaced multipart fractures [62, 105, 113, 138].
Osteonecrosis occurs after a loss of blood supply to the subchondral bone with subsequent collapse, irregularity of the articular surface, and clinical symptoms of pain and stiffness [39, 41, 139, 140]. With increasing comminution and displacement, an increased injury to the soft tissues and subsequently the vascular supply to the humeral head is expected [39–41]. Hertel et al. assessed risk factors for development of aseptic necrosis after proximal humerus fracture. They found that humeral head ischemia increased after anatomic neck fracture, metaphyseal head extension of less than 8 mm, and medial head disruption of more than 2 mm [41]. The combination of these three factors had a 97 % positive predictive value for humeral head ischemia (Fig. 2.6) [41]. Gerber noted that outcomes with osteonecrosis improved with anatomic fracture alignment [139]. Osteonecrosis was much better tolerated in patents whose fractures healed with anatomic alignment over those with some degree of malunion [139]. Extent of head involvement can affect outcome. Osteonecrosis is a rare occurrence after three-part fracture, and when it does occur usually involves only a portion of the humeral head and causes little discomfort [139].
With a robust soft tissue envelope, fracture healing rates are high in the proximal humerus, but nonunion can occur and result in persistent pain and dysfunction [99, 137]. Total incidence of nonunion with proximal humerus fracture is 1.1 %. These rates increase if metaphyseal comminution is present (8 %) and with significant displacement of the surgical neck (10 %) [137]. Most fractures that fail to unite, regardless of classification, have metaphyseal comminution and loss of cortical contact [137]. Patient factors increase nonunion risk with patients with osteoporosis, medical comorbidities, drug treatment, smoking history, and alcohol abuse being most at risk to develop a nonunion [95]. Preinjury stiffness secondary to degenerative joint disease or inflammatory causes can predispose nonunion [141]. In addition, inappropriate immobilization with a hanging arm cast or overzealous mobilization can result in nonunion [99].
With nonoperative treatment of proximal humerus fractures with displacement, some form of malunion occurs with each fracture. This common complication can frustrate the patient and present a treatment dilemma for the surgeon [142–145]. While slight malunion of the head and shaft is well tolerate, tuberosity malunion can cause stiffness, pain, and loss of function [144–148]. Biomechanical data shows a 5 mm superior malunion of the greater tuberosity increases the deltoid force for abduction by 16 % [149]. With posterior and superior displacement of the greater tuberosity, deltoid force for abduction increases 29 % [149]. While tuberosity malunion in elderly individuals with low functional expectations does not usually adversely effect outcomes, the secondary impingement and functional compromise caused by tuberosity malunion in younger patients is poorly tolerated [9].
Some degree of stiffness is nearly expected after all proximal humerus fractures [12]. The cause of stiffness is multifactorial after proximal humerus fractures [145]. Adhesions can form secondary to the trauma of the injury and after immobilization. Absolute immobility greater than 2 weeks after injury with nonoperative treatment leads to increasing rates of stiffness [20]. Hodgson showed that immobilization longer than 3 weeks prolonged recovery from 1 to 2 years [19, 125]. Factors such as capsular contracture, malunion, complex regional pain syndrome, impingement, rotator cuff dysfunction, delayed rehabilitation, and non-compliance with rehabilitation all contribute to the development of stiffness [9]. Ultimately, early mobilization is the best method to prevent stiffness in the proximal humerus fracture treated without surgery [19, 20, 125].
References
DeFranco MJ, Brems JJ, Williams Jr GR, Iannotti JP. Evaluation and management of valgus impacted four-part proximal humerus fractures. Clin Orthop Relat Res. 2006;442:109–14.
Green A, Norris T. Proximal humerus fractures and fracture-dislocations. In: Jupiter J, editor. Skeletal trauma. 3rd ed. Philadelphia: Saunders; 2003. p. 1532–624.
Hartmann A, Resch H. Treatment of proximal humerus fractures. In: Galatz L, editor. Orthopaedic knowledge update shoulder and elbow 3. Rosemont: American Academy of Orthopaedic Surgeons; 2008. p. 405–22.
Court-Brown C, Garg A, McQueen M. The epidemiology of proximal humerus fractures. Acta Orthop Scand. 2001;72:365–71.
Lobo MJ, Levine WN. Classification and closed treatment of proximal humerus fractures. In: Wirth MA, editor. Proximal humerus fractures. Chicago: American Academy of Orthopaedic Surgeons; 2005. p. 1–13.
Salem MI. Bilateral anterior fracture-dislocation of the shoulder joints due to sever electric shock. Injury. 1983;14:361–3.
Doetsch AM, Faber J, Lynnerup N, et al. The effect of calcium and vitamin D3 supplementation on the healing of the proximal humerus fracture: a randomized placebo controlled study. Calcif Tissue Int. 2004;75:183–8.
Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop Relat Res. 2006;442:87–92.
Robinson CM. Proximal humerus fractures. In: Bucholz RW, Court-Brown CM, Heckman JD, Tornetta P, editors. Fractures in adults. Philadelphia: Lippincott Williams & Wilkins; 2010. p. 1039–105.
Huopio J, Kroger H, Honkanen R, Saarikoski S, Alahava E. Risk factors for perimenopausal fractures: a prospective study. Osteoporos Int. 2000;11:219–27.
Lin J, Hou S-M, Hang Y-S. Locked nailing for displaced surgical neck fractures of the humerus. J Trauma. 1998;45:1051–7.
Bohsali KI, Wirth MA. Fractures of the proximal humerus. In: Rockwood CA, Matsen FA, Wirth MA, Lippitt SB, editors. The shoulder. 4th ed. Philadelphia: Saunders Elsevier; 2009. p. 295–332.
Schmidt A. Proximal humeral fractures and shoulder dislocations. In: Stannard JP, Schmidt AH, Kregor PJ, editors. Surgical treatment of orthopaedic trauma. New York: Thieme; 2007. p. 238–62.
Misra A, Kapur R, Maffulli N. Complex proximal humeral fractures in adults: a systematic review of management. Injury. 2001;32:363–72.
Zyto K, Kronberg M, Brostrom L-A. Shoulder function after displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 1995;4:331–6.
Handoll HH, Gibson JN, Madhok R. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2003;12, CD000434.
Lanting B, MacDermid J, Drosdowech D, Faber KJ. Proximal humeral fractures: a systematic review of treatment modalities. J Shoulder Elbow Surg. 2008;17:42–54.
Zyto K, Ahrengart L, Sperber A, Tornkvist H. Treatment of displaced proximal humeral fractures in elderly patients. J Bone Joint Surg Br. 1997;79:412–7.
Hodgson S. Proximal humerus fracture rehabilitation. Clin Orthop Relat Res. 2006;442:131–8.
Koval KF, Gallagher MA, Marsicano JG, Cuomo F, McShinawy A, Zuckerman JD. Functional outcome after minimally displaced fractures of the proximal part of the humerus. J Bone Joint Surg Am. 1997;79:203–7.
Neer II CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52:1077–89.
Resch H, Beck E, Bayley I. Reconstruction of the valgus-impacted humeral head fracture. J Shoulder Elbow Surg. 1995;4:73–80.
Boileau P, Walch G. The three-dimensional geometry of the proximal humerus: implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79:857–65.
Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82:159–1602.
Iannotti JP, Gabriel JP, Schneck DL, Evans BG, Misra S. The normal glenohumeral relationships: an anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74:491–500.
Matsen III FA, Clinton J, Rockwood CA, Wirth MA, Lippitt SB. Glenohumeral arthritis and its management. In: Rockwood CA, Matsen FA, Wirth MA, Lippitt SB, editors. The shoulder. 4th ed. Philadelphia: Saunders Elsevier; 2009. p. 1089–247.
Tingart MJ, Apreleva M, von Stechow D, Zurakowski D, Warner JJ. The cortical thickness of the proximal humeral diaphysis predicts bone mineral density of the proximal humerus. J Bone Joint Surg Br. 2003;85B:611–7.
Hepp P, Lill H, Bail H, Korner J, Niederhagan M, Haas NP, et al. Where should implants be anchored in the humeral head? Clin Orthop Relat Res. 2003;(415):139–47.
Tingart MJ, Bouxsein ML, Zurakowski D, Warner JP, Apreleva M. Three-dimensional distribution of bone density in the proximal humerus. Calcif Tissue Int. 2003;73:531–6.
Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15:620–4.
Hawkins RJ, Bell RH, Gurr K. The three-part fracture of the proximal part of the humerus: operative treatment. J Bone Joint Surg Am. 1986;68:1410–4.
Itamura J, Dietrick T, Roidis N, et al. Analysis of the bicipital groove as a landmark for humeral head replacement. J Shoulder Elbow Surg. 2002;11:322–6.
Parnes N, Jupiter JB. Fixed-angle locking plating of displaced proximal humerus fractures. In: Sperling JW, editor. Shoulder and elbow 2: instructional course lectures. Rosemont: American Academy of Orthopaedic Surgeons; 2010. p. 163–76.
Gerber C, Terrier F, Ganz R. The role of the coracoid process in the chronic impingement syndrome. J Bone Joint Surg Br. 1985;67B:703–8.
Neer CS. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70–7.
Brooks CH, Revell WJ, Heatley FW. Vascularity of the humeral head after proximal humeral fractures: an anatomical cadaver study. J Bone Joint Surg Br. 1993;75:132–6.
Duparc F, Muller JM, Freger P. Arterial blood supply of the proximal humeral epiphysis. Surg Radiol Anat. 2001;23:185–90.
Gerber C, Scheneeberger AG, Vihn TS. The arterial vascularization of the humeral head: an anatomical study. J Bone Joint Surg Am. 1990;72:1486–94.
Bastian JD, Hertel R. Initial post fracture humeral head ischemia does not predict development of necrosis. J Shoulder Elbow Surg. 2008;17:2–8.
Gerber C, Lambert SM, Hoogewoud HM. Absence of avascular necrosis of the humeral head after posttraumatic rupture of the anterior and posterior humeral circumflex arteries. A case report. J Bone Joint Surg Am. 1996;78A:1256–9.
Hertel R, Hempfing A, Steihler M, Leunig M. Predictors of humeral head ischemia after intracapsular fracture of the proximal humerus. J Shoulder Elbow Surg. 2004;13:427–33.
McLaughlin JA, Light R, Lustrin I. Axillary artery injury as a complication of proximal humerus fractures. J Shoulder Elbow Surg. 1998;7:292–4.
Visser CP, Coene LN, Brand R, et al. Nerve lesions in the proximal humerus. J Shoulder Elbow Surg. 2001;10:421–7.
Bono CM, Grossman MG, Hochwald N, Tornetta P III. Radial and axillary nerves. Anatomic considerations for humeral fixation. Clin Orthop Relat Res. 2000;(373):259–64.
Visser CP, Tavy DL, Coene LN, Brand R. Electromyographic findings in shoulder dislocations and fractures of the proximal humerus: comparison with clinical neurological examination. Clin Neurol Neurosurg. 1999;101:86–91.
Flatow EL, Bigliani LU, April EW. An anatomic study of the musculocutaneous nerve and its relationship to the coracoid process. Clin Orthop Relat Res. 1989;244:166–71.
Flatow EL, Cuomo F, Maday MG, et al. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73:1213–8.
Kannus P, Palvanen M, Niemi, S, Parkkari J, Jarvienen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051–2.
Livesley PJ, Mugglestone A, Whitton J. Electrotherapy and the management of minimally displaced fracture of the neck of the humerus. Injury. 1992;23:323–7.
Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37:691–7.
Kristiansen B, Barfod G, Bredesen J, et al. Epidemiology of proximal humeral fractures. Acta Orthop Scand. 1987;58:75–7.
Lind T, Kroner K, Jensen J. The epidemiology of fractures of the proximal humerus. Arch Orthop Trauma Surg. 1989;108:285–7.
Baron JA, Barrett JA, Karagas MR. The epidemiology of peripheral fractures. Bone. 1996;183(Suppl):209S–13.
Hagino H, Yamamoto K, Ohshiro H, et al. Changing incidence of hip, distal radius, and proximal humerus fractures in Tottori Prefecture, Japan. Bone. 1999;24:265–70.
Rose S, Melton J, Morrey B, et al. Epidemiological features of humeral fractures. Clin Orthop Relat Res. 1982;168:24–30.
Horak J, Nilsson BE. Epidemiology of fracture of the upper end of the humerus. Clin Orthop Relat Res. 1975;(112):250–3.
Palvanen M, Kannus P, Parkkari J. Injury mechanisms of osteoporotic upper limb fractures. J Bone Miner Res. 2000;15:S539.
Pritchett JW. Inferior subluxation of the humeral head after trauma or surgery. J Shoulder Elbow Surg. 1997;6:356–9.
Kannus P, Palvenen M, Niemi S, et al. Osteoporotic fractures of the proximal humerus in elderly Finnish persons: sharp increase in 1970–1998 and alarming projections for the new millennium. Acta Orthop Scand. 2000;71:465–70.
Lauritzen JB, Schwarz P, Lund B, McNair P, Transbol I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3:127–32.
Chu SP, Kelsey JL, Keegan TH, et al. Risk factors for proximal humerus fracture. Am J Epidemiol. 2004;160:360–7.
Olsson C, Petersson CJ. Clinical importance of comorbidity in patients with a proximal humerus fracture. Clin Orthop Relat Res. 2006;442:93–9.
Schwartz AV, Nevitt MC, Brown Jr BW, Delsey JL. Increased falling as a risk factor for fracture among older women: the study of osteoporotic fractures. Am J Epidemiol. 2005;161:180–5.
Astrand J, Thorngren KG, Tagil M. One fracture is enough! Experience with a prospective and consecutive osteoporosis screening program with 239 fracture patients. Acta Orthop. 2006;77:3–8.
Sadowski C, Riand N, Stern R, Hoffmeyer P. Fixation of fractures of the proximal humerus with the PlantTan humerus fixator plate: early experience with a new implant. J Shoulder Elbow Surg. 2003;12:148–51.
Desai KB, Ribbans WJ, Taylor GJ. Incidence of five common fracture types of the greater tuberosity of the proximal humerus. Injury. 1996;27:97–100.
Hartsock LA, Estes WJ, Murray CA, Friedman RJ. Shoulder hemiarthroplasty for proximal humeral fractures. Orthop Clin North Am. 1998;29(3):467–75.
Edlson G, Kelly I, Vigder F, et al. A three-dimensional classification for fractures of the proximal humerus. J Bone Joint Surg Br. 2004;86:413–25.
Hessman M, Baumgaertel G, Gehling H, et al. Plate fixation of proximal humeral fractures with indirect reduction: surgical technique and results utilizing three shoulder scores. Injury. 1999;30:453–62.
Palvanen M, Kannus P, Parkkari J, et al. The injury mechanism of osteoporotic upper extremity fracture among older adults: a controlled study of 287 consecutive patients and their 108 controls. Osteoporos Int. 2000;1:822–31.
Sabick MB, Hay JG, Goel VK, et al. Active responses decrease impact forces at the hip and shoulder in falls to the side. J Biomech. 1999;32:993–8.
Kelsey JL, Browner WS, Seeley DG, et al. Risk factors for fractures of the distal forearm and proximal humerus. The Study of Osteoporotic Fractures Research Group. Am J Epidemiol. 1992;135:477–89.
Olsson C, Nordquist A, Petersson CJ. Long-term outcome of a proximal humerus fracture predicted after 1 year: a 13-year prospective population-based follow-up study of 47 patients. Acta Orthop. 2005;76:397–402.
Shortt NL, Robinson CM. Mortality after low-energy fractures in patients aged at least 45 years old. J Orthop Trauma. 2005;19:396–400.
Lee SH, Dargent-Molina P, Breart G. Risk factors for fractures of the proximal humerus: results from the EPIDOS prospective study. J Bone Miner Res. 2002;17:817–25.
Nguyen TV, Center JR, Sambrook PN, et al. Risk factors for proximal humerus, forearm, and wrist fractures in elderly men and women: the Dubbo Osteoporosis Epidemiology Study. Am J Epidemiol. 2001;153:587–95.
Nordqvist A, Petersson CJ. Shoulder injuries common in alcoholics. An analysis of 413 injuries. Acta Orthop Scand. 1996;4:271–80.
Kozak TK, Skirving AP. Fracture dislocation with associated humeral shaft fracture. Injury. 1995;26:129–30.
O’Donnell TM, McKenna JV, Kenny P, et al. Concomitant injuries to the ipsilateral shoulder in patients with a fracture of the diaphysis of the humerus. J Bone Joint Surg Br. 2008;90B:61–5.
Robinson CM, Stone OD, Murray IR. Proximal humeral fractures due to blunt trauma producing skin compromise. J Bone Joint Surg Br. 2011;93(12):1632–7.
Ogawa K, Yoshida A, Ikegami H. Isolated fractures of the greater tuberosity of the humerus: solutions to recognizing a frequently overlooked fracture. J Trauma. 2003;54:713–7.
de Laat EA, Visser CP, Coene LN, et al. Nerve lesions in primary shoulder dislocations and humeral neck fractures. A prospective clinical and EMG study. J Bone Joint Surg Br. 1994;76B:381–3.
Warrender WJ, Oppenheimer S, Abboud JA. Nerve monitoring during proximal humeral fracture fixation: what have we learned. Clin Orthop Relat Res. 2011;469(9):2631–7.
Chervu A, Quinones Baldrich WJ. Vascular complications in orthopedic surgery. Clin Orthop Relat Res. 1988;(235):275–88.
Zuckerman JD. Fractures of the proximal humerus: diagnosis and management. In: Iannotti JP, editor. Disorders of the shoulder: diagnosis and management. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 639–85.
Gallo RA, Sciulli R, Daffner RH, et al. Defining the relationship between rotator cuff injury and proximal humerus fractures. Clin Orthop Relat Res. 2007;458:70–7.
Kendall CB, Tanner SL, Tolan SJ. SLAP tear associated with a minimally displaced proximal humerus fracture. Arthroscopy. 2007;23(12):1362e1–3.
Wallace WA, Hellier M. Improving radiographs of the injured shoulder. Radiography. 1983;49:229–33.
Neer CS. Four-segment classification of proximal humeral fractures: purpose and reliable use. J Shoulder Elbow Surg. 2002;11:389–400.
Jurik AG, Albrechtsen J. The use of computed tomography with two- and three-dimensional reconstructions in the diagnosis of three- and four-pare fractures of the proximal humerus. Clin Radiol. 1994;49:800–4.
Foroohar A, Tosti R, Richmond JM, Gaughan JP, Ilyas AM. Classification and treatment of proximal humerus fractures: inter-observer reliability and agreement across imaging modalities and experience. J Orthop Surg Res. 2011;6:38.
Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus: the results of nonoperative treatment. J Bone Joint Surg Br. 2002;84:504–8.
Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–7.
Olsson C, Nordqvist A, Petersson CJ. Increased fragility in patients with fracture of the proximal humerus: a case control study. Bone. 2004;34:1072–7.
Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46–50.
Rasmussen S, Hvass I, Dalsgaard J, et al. Displaced proximal humeral fractures: results of conservative treatment. Injury. 1992;23:41–3.
DePalma AF, Cautilli RA. Fractures of the upper end of the humerus. Clin Orthop Relat Res. 1961;20:73–93.
Brien H, Noftall F, MacMaster S, Cummings T, Landells C, Rockwood P. Neer's classification system: a critical appraisal. J Trauma. 1995;38(2):257–60.
Neer II CS. Displaced proximal humeral fractures. II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am. 1970;52:1077–89.
Siebenrock DA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75:1751–5.
Tingart M, Bathis H, Bouillon B, Tiling T. The displaced proximal humeral fracture: is there evidence for therapeutic concepts? Chirurg. 2001;72:1284–91.
Court-Brown C, Garg A, McQueen MM. The translated two-part fracture of the proximal humerus. Epidemiology and outcome in the older patient. J Bone Joint Surg Br. 2001;83:799–804.
Court-Brown CM, McQueen MM. The impacted varus A2.2 proximal humeral fracture: prediction of outcome and results of nonoperative treatment in 99 patients. Acta Orthop Scand. 2004;75:736–40.
Gaebler C, McQueen MM, Court-Brown CM. Minimally displaced proximal humeral fractures: epidemiology and outcome in 507 cases. Acta Orthop Scand. 2003;74:580–5.
Leyshon RL. Closed treatment of fractures of the proximal humerus. Acta Orthop Scand. 1984;55:48–51.
Bosch U, Skutek M, Fremerey R, Tscherne H. Outcome after primary and secondary hemiarthroplasty in elderly patients with fractures of the proximal humerus. J Shoulder Elbow Surg. 1998;7:479–84.
Chudik SC, Weinhold P, Dahners LE. Fixed-angle plate fixation in simulated fractures of the proximal humerus: a biomechanical study of a new device. J Shoulder Elbow Surg. 2003;12:578–88.
Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89A:1700–9.
Doursounian L, Grimberg J, Cazeau C, et al. A new internal fixation technique for fractures of the proximal humerus-the bilboquet device: a report on 26 cases. J Shoulder Elbow Surg. 2000;9:279–88.
Koval KJ, Blair B, Takei R, Kummer FJ, Zuckerman JD. Surgical neck fractures of the proximal humerus: a laboratory evaluation of ten fixation techniques. J Trauma. 1996;40:778–83.
Moda S, Chadha N, Sangwan S, et al. Open reduction and internal fixation of proximal humeral fractures and fracture dislocations. J Bone Joint Surg Br. 1990;72B:1050–2.
Handoll HH, Ollivere BJ. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2010;12, CD000434.
Young TB, Wallace WA. Conservative treatment of fractures and fracture-dislocations of the upper end of the humerus. J Bone Joint Surg Br. 1985;67B:373–7.
Koval KJ, Zuckerman JD. Orthopaedic challenges in the again population: trauma treatment and related clinical issues. Instr Course Lect. 1997;46:423–30.
Yosipovitch Z, Goldberg I. Inferior subluxation of the humeral head after injury to the shoulder. A brief note. J Bone Joint Surg Am. 1989;71A:751–3.
Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59:596–601.
Sarmiento A, Zagorski JB, Zynch GA, Latta LL, Capps CA. Functional bracing for the treatment of fractures of the humeral diaphysis. J Bone Joint Surg Am. 2000;82(4):478–86.
Hepp P, Voigt C, Josten C. The conservative treatment of proximal humeral fractures. In: Lill H, editor. Die proximal Humerusfraktur. Stuttgart: Thieme; 2006. p. 40–5.
Iannotti JP, Ramsey ML, Williams GR, et al. Nonprosthetic management of proximal humerus fractures. J Bone Joint Surg Am. 2003;85:1578–93.
Jaberg H, Warner JJ, Jakob RP. Percutaneous stabilization of unstable fractures of the humerus. J Bone Joint Surg Am. 1992;74:508–15.
Hanson B, Neibenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612–21.
Svend-Hansen H. Displaced proximal humeral fractures. A review of 49 patients. Acta Orthop Scand. 1974;45:359–64.
Clifford PC. Fractures of the neck of the humerus: a review of the late results. Injury. 1980;12:91–5.
Kristiansen B, Angermann P, Larsen TK. Functional results following fractures of the proximal humerus. A controlled clinical study comparing two periods of immobilization. Arch Orthop Trauma Surg. 1989;108:339–41.
Hodgson SA, Mawson SJ, Stanley D. Rehabilitation after two-part fractures of the neck of the humerus. J Bone Joint Surg Br. 2003;85B:419–22.
Bertoft ES, Lundh I, Ringqvist I. Physiotherapy after fracture of the proximal end of the humerus. Comparison between two methods. Scand J Rehabil Med. 1984;16:11–6.
Revay S, Dahlstrom M, Dalen N. Water exercise versus instruction for self-training following a shoulder fracture. Int J Rehabil Res. 1992;15:327–33.
Robinson CM, Aderinto J. Posterior shoulder dislocations and fracture-dislocations. J Bone Joint Surg Am. 2005;87A:639–50.
Robinson CM, Page RS. Severely impacted valgus proximal humeral fractures. Results of operative treatment. J Bone Joint Surg Am. 2003;85A:1647–55.
Robinson CM, Teoh KH, Baker A, et al. Fractures of the lesser tuberosity of the humerus in the adult: epidemiology and functional outcome after operative treatment. J Bone Joint Surg Am. 2009;91(3):512–20.
Mills HJ, Horne G. Fractures of the proximal humerus in adults. J Trauma. 1985;25:801–5.
Torrens C, Corrales M, Vila G, Santana F, Caceres E. Functional and quality-of-life results of displaced and nondisplaced proximal humeral fractures treated conservatively. J Orthop Trauma. 2011;25(10):581–7.
Foruria AM, de Gracia MM, Larson DR, Munuera L, Sanchez-Sotelo J. The pattern of the fracture and displacement of the fragments predicts the outcome after proximal humerus fracture. J Bone Joint Surg Br. 2011;93(3):378–86.
Poeze M, Lenssen AF, Van Empel JM, Verbruggen JP. Conservative management of proximal humeral fractures: can poor functional outcome be related to standard transscapular radiographic evaluation? J Shoulder Elbow Surg. 2010;19(2):273–81.
Bhandari M, Matthys G, McKee MD. Four-part fractures of the proximal humerus. J Orthop Trauma. 2004;18:126–7.
Kristiansen B, Kofoed H. Transcutaneous reduction and external fixation of displaced fractures of the proximal humerus. J Bone Joint Surg. 1988;70B:821–4.
Court-Brown CM, McQueen MM. Nonunions of the proximal humerus: their prevalence and functional outcome. J Trauma. 2008;64:1517–21.
Serin E, Karatosun V, Balci C, et al. Two-prong splint in the treatment of proximal humeral fracture. Arch Orthop Trauma Surg. 1999;119:368–70.
Gerber C, Hersche O, Berberat C. The clinical significance of post-traumatic avascular necrosis of the humeral head. J Shoulder Elbow Surg. 1998;7:586–90.
Aj W, Roolker W, Patt TW, et al. Open reduction and internal fixation of three- and four-part fractures of the proximal part of the humerus. J Bone Joint Surg Am. 2002;84A:1919–25.
Rooney PJ, Cockshott WP. Pseudarthrosis following proximal humeral fractures: a possible mechanism. Skeletal Radiol. 1986;15:21–4.
Boileau P, Krishnan SG, Tinsi L, et al. Tuberosity malposition and migration: reason for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11:401–12.
Dines DM, et al. Posttraumatic changes of the proximal humerus: malunion, nonunion, and osteonecrosis: treatment with modular hemiarthroplasty or total shoulder arthroplasty. J Shoulder Elbow Surg. 1993;2:11–21.
Siegel JA, Dines DM. Proximal humerus malunions. Orthop Clin North Am. 2000;31:35–50.
Wirth MA. Late sequelae of proximal humerus fractures. In: With MA, editor. Proximal humerus fractures. Chicago: American Academy of Orthopaedic Surgeons; 2005. p. 49–55.
Beredjiklian PK, Iannotti JP. Treatment of proximal humerus fracture malunion with arthroplasty. Instr Course Lect. 1998;47:135–40.
Green A, Izzi Jr J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12:641–9.
Hinov V, Wilson F, Adams G. Arthroscopically treated proximal humeral fracture malunion. Arthroscopy. 2002;18:1020–3.
Bono CM, Renard R, Levine RG, Levy AS. Effect of displacement of fractures of the greater tuberosity on the mechanics of the shoulder. J Bone Joint Surg Br. 2001;83:1056–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Twiss, T. (2015). Nonoperative Treatment of Proximal Humerus Fractures. In: Crosby, L., Neviaser, R. (eds) Proximal Humerus Fractures. Springer, Cham. https://doi.org/10.1007/978-3-319-08951-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-08951-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-08950-8
Online ISBN: 978-3-319-08951-5
eBook Packages: MedicineMedicine (R0)