Abstract
The viability of cellulosic ethanol depends on the optimal use of biomass component through the biorefinery concept and this requires the integration of unit operations that are involved in the production of fuel and chemicals. In this regard, enzymes are important tools to improve the efficiency and sustainability of a biorefinery process. Therefore, a comprehensive approach and full understanding of the structure and function relationships that are involved in the enzymatic hydrolysis of lignocellulosic materials is a fundamental step toward the optimization of these bioconversion processes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
The viability of cellulosic ethanol depends on the optimal use of biomass component through the biorefinery concept and this requires the integration of unit operations that are involved in the production of fuel and chemicals. In this regard, enzymes are important tools to improve the efficiency and sustainability of a biorefinery process. Therefore, a comprehensive approach and full understanding of the structure and function relationships that are involved in the enzymatic hydrolysis of lignocellulosic materials is a fundamental step toward the optimization of these bioconversion processes.
Biomass conversion may be performed by chemical or biochemical routes. For many reasons ranging from process efficiency to environmental issues, most of these pathways are ideally performed by biochemical catalysts (enzymes) such as polysaccharide hydrolases (Mussatto et al. 2010). However, the relatively high cost of enzymes and the complexity of carrying out enzymatic hydrolysis in large scale are still limiting the implementation of biorefineries for fuels and chemicals based on lignocellulosic materials. Besides glycoside hydrolases, it is also widely known that other enzymes have an important role in the deconstruction of the plant cell wall. These include oxidases that are involved not only in lignin degradation but also in the chemical modification of carbohydrates. Hence, a full spectrum of enzymes is required to deal with the wide diversity of chemical linkages and chemical environments that are found in the plant cell wall. This chapter attempts to describe the essential role of plant cell wall degrading enzymes in the success of biorefineries, particularly with regard to the use of lignocellulosic materials for fuels and chemicals.
8.2 Plant Cell Wall
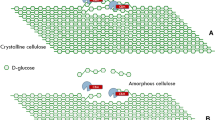
The physical and chemical association of the three main components of the plant cell wall, cellulose, hemicelluloses, and lignin, has been the subject of many reviews that are found in the specialized literature (Higuchi 1985; Matthews et al. 2006; Coughlan and Hazlewood 1993). In short, linear chains of β-(1 → 4)-glucans (cellulose) interact with one another by hydrogen bonding to produce well-organized crystalline regions that are regularly interrupted by less-organized or “amorphous” regions in which these chains are more randomly oriented. These ribbons of polysaccharide chains are embedded in a matrix of hemicelluloses and lignin, whose distribution and close association defines the outstanding physical and chemical properties of this natural composite (Fig. 8.1).
A short review of the chemical and structural properties of the main plant cell wall macromolecular components is presented below. However, important but minor components such as pectic materials were not included in this chapter. Details about this class of compounds may be found in reviews that are already available in the literature (Jayani et al. 2005).
8.3 Cellulose
Cellulose is a linear homopolysaccharide composed of anhydro-d-glucopyranose units joined together by β-(1 → 4) glycosidic linkages (Fig. 8.2). The equatorial orientation of the anomeric hydroxyl of the β-d-glucopyranosyl units confers linearity to the cellulose chains, which interact with one another to produce aggregates of great molecular order whose supramolecular structure is architecturally stabilized by a network of intra- and intermolecular hydrogen bonding (Zhang and Lynd 2004). As a result, adjacent cellulose chains are held together as flat layers, allowing the more hydrophobic faces of the ribbons to stack (Matthews et al. 2006). However, there is a thermodynamic limit beyond which this molecular order is gradually lost, characterizing a transition to less-organized regions in which the cellulose chains are more randomly oriented (amorphous regions). Hence, depending on its “amorphous character,” the whole structure presents cavities or pores that are able to hold relatively large amounts of water by capillarity (Mihranyan et al. 2004).
Cellulose structure. a Cellobiose unit. b Inter- and intramolecular interactions among cellulose chains, with hydrogen bonds (HB) shown in different colors: red for intermolecular HB involving C6 and C3; black for intermolecular HB between C2 and C6; blue for intramolecular HB involving C3 and the hemiacetalic oxygen atom
Cellulose chains may organize themselves in different ways, forming allomorphs that are known as cellulose I, II, III, or IV. The natural cellulose form is the metastable cellulose I, which contains two coexisting phases, I α (triclinic) and I β (monoclinic), and the ratio between them varies depending of its origin, being the type I α commonly found on algae and bacteria, while type I β is primarily found in higher plants. The main difference between celluloses I α and I β lies on the displacements of the sheets relative to one another and cellulose I α can be converted to I β by bending during microfibril formation (Jarvis 2000). For cellulose I α , the chains are regularly displaced from each other in the same direction, whereas for cellulose I β , this displacement is found in alternating directions. This difference leads to different water adsorption profiles as well as different chemical accessibilities for conversion (Matthews et al. 2006).
The other cellulose allomorphs are not natural. Cellulose II is generally obtained either by mercerization or by regeneration of cellulose in organic solvents and ionic liquids (Jhonson 1969; Okano and Sarko 1985). Cellulose III can be produced by treatment with liquid ammonia or in the presence of some amines (e.g., ethylene diamine). This way, cellulose IIII derived from cellulose I while cellulose II leads to cellulose IIIII. Finally, cellulose III can be treated with glycerol at high temperature to produce cellulose IV and, by doing so, cellulose IVI and IVII can be obtained from cellulose IIII and IIIII, respectively (Loeb and Segal 1954; Tsuda and Mukoyama 1957).
For many years, cellulosic materials have been extensively studied as a source for ethanol production. In this case, conversion of lignocellulose to fermentable sugars (mostly glucose and xylose) may be carried out by acid or enzymatic hydrolysis (Caes et al. 2013; Yabushita et al. 2013). However, the use of acid hydrolysis may lead to lower sugar yields due to the use of more drastic reaction conditions, in which an array of both hydrolysis and fermentation inhibitors are usually produced (Ramos 2003). Due to its higher specificity and lower environmental impact the enzymatic hydrolysis of cellulose has received much more attention from the international scientific community as well as from the industry.
The enzymatic conversion of cellulose to glucose is primarily performed by the synergic/concerted action of three main classes of hydrolases, which are usually referred to as the cellulolytic complex or cellulases: endo-β-(1 → 4)-glucanases (EC 3.2.1.4) (EnG), exo-β-(1 → 4)-glucanases (EC 3.2.1.91) (ExG), and β-(1 → 4)-glucosidases (EC 3.2.1.21) (βG). Many EnG and ExG enzymes are able to adsorb on the substrate surface through a carbohydrate-binding module (CBM), which is connected to the catalytic domain by a linker peptide (Notenboom et al. 2001). Several researches have shown that CBMs increase the performance of cellulases and other hydrolases. The role of CBM in hydrolysis was recently shown by Várnai et al. (2013). These authors were able to show that more than 60 % of cellulase genes do not have a CBM or any alternative protein structure linked to them (dockerins) (Várnai et al. 2013). Furthermore, the effect of CBM was more pronounced at low total solids (1 wt%, dry basis), being more important for ExGs than EnGs of Trichoderma reesei. The results suggest that CBMs would not be required at high total solids because these conditions would already promote enough enzyme-to-substrate interactions for hydrolysis to occur.
EnG enzymes have a catalytic domain with a cleft shape active site that is able to break down glycosidic bonds along the cellulose chain, acting mainly at the less-organized “amorphous” regions (Rabinovich et al. 2002). This reaction leads to the formation of two new chain ends triggering off the so-called endo-exo synergism. ExG enzymes have a tunnel-shaped catalytic site through which the cellulose chains must penetrate prior to eliciting its catalytic activity, releasing mostly cellobiose. These enzymes need to adsorb on to the cellulose surface in order to facilitate this process (Beckham et al. 2010). Once captured by cellulase enzymes, the cellulose chain is forced to unglue/unbind from the surface and its gradual solubilization starts processively by ExG enzymes. Finally, cellobiose and other low molecular mass oligomers are converted to glucose by the action of βG enzymes. Figure 8.3 shows a pictorial representation of the enzymatic hydrolysis of cellulose.
T. reesei is the most widely studied organism for the production of cellulases. Wild-type T. reesei strains are able to secret at least four EnGs (TrCel5A, TrCel12A, TrCel7B TrCel45A), two ExGs (TrCel7A, TrCel6A), at least one xyloglucanase (TrCel74A, with EnG activity), and several βGs (Foreman et al. 2013). However, it is known that TrCel7A, TrCel6A, and TrCel5A are the predominant enzymes in the enzymatic pools of T. reesei (Nidetzky and Claeyssens 1994). Therefore, considering that the expression levels of βG by T. reesei are enough for the growing cells but insufficient for industrial applications, enzymes from other fungi such as Aspergillus spp. must be used to supplement this enzyme component. In addition, besides being more tolerant to end-product inhibition, the βG enzymes from Aspergillus spp. are able to act not only on cellooligosaccharides (COS) but also on insoluble COS with an average degree of polymerization of 20 (Sakamoto et al. 1985).
TrCel7B is the major endo-acting enzyme from T. reesei, showing 6–10 % of its total cellulase production (Ståhlberg 1991; Nidetzky and Claeyssens 1994). TrCel7B has been reported as catalytically active on both soluble (modified cellulose such as CMC) and insoluble cellulosic substrates as well as on xylans and glucomannans (Shoemaker et al. 1983). On the other hand, the TrCel5A is not able to act on xylans but it is also active on soluble and insoluble cellulosic substrates including mannans (Henrissat et al. 1985; Macarron et al. 1996; Karlsson et al. 2002). Unlikely the major EGs from T. reesei, minor enzyme components, such as TrCel12A and TrCel45A, can also act on both soluble and insoluble substrates including glucomannans.
Other cellulolytic enzyme systems have been investigated in their performance to hydrolyze cellulosic substrates, such as the proteome of Neurospora crassa (Phillips et al. 2011), Penicillium cellulases (Marjamaa et al. 2013), Cel7A proteins from different thermophilic fungi (Voutilainen et al. 2008) and several EnG enzymes from GH families 5, 6, 7, 9, 12, and 45 (Vlasenko et al. 2010), among others. A thorough description about fungal enzymes that are able to degrade lignocellulosic materials can be found elsewhere (Dashtban et al. 2009).
Cellulolytic enzymes represent one of the most important enzymes for the development of biorefineries. However, ancillary proteins have also been identified as important auxiliary tools to achieve high conversion rates in cellulose saccharification (Arantes and Saddler 2010; Ekwe et al. 2013) such as expansins, swollenins, and lytic polysaccharide monooxygenases (LPMO). Cellulose binding proteins can promote the deagglomeration of the cellulose chains at crystalline regions causing amorphogenesis and this seems to be a critical step toward the development of high accessibilities (Din et al. 1991; Chen et al. 2010). Interestingly, Reese et al. (1950) suggested about 60 years ago that cellulolytic enzymes may require the action of nonhydrolytic proteins in order to promote the disruption of the substrate polymer packing.
The presence of expansins in plant tissues have been originally described by Cosgrove and co-workers (Cosgrove 1999; Cosgrove 2000a, b). Expansins are proteins of 25–27 kDa of molecular mass and their mechanism of action consists on break the noncovalent bonds between cell wall polysaccharides, thereby inducing the plant cell wall extension and swelling (Cosgrove 2000a; Lee et al. 2001). Also, Yuan et al. (2001) proposed that some cellulases such as TrCel12 may have expansin-like properties in addition to its hydrolytic activity.
Likewise expansins, swollenins can also break down the physical interactions among cellulose chains. Jäger et al. (2011) expressed the T. reesei swollenin protein in a recombinant Kluyveromyces lactis strain and studied the effect of this recombinant swollenin on cellulosic substrates. In general, treatment with swollenin led to a decrease in both substrate particle size and crystallinity while increasing the extent of cellulase adsorption on cellulose. As a result, high cellulose hydrolysis rates were obtained. Gourlay et al. (2013) showed that T. reesei swollenin affected especially xylan of pretreated corn stover substrate, enhancing the production of sugars in hydrolysis. More recently, Kang et al. (2013) characterized a novel recombinant swollenin from Penicillium oxalicum with regard to its ability to facilitate cellulose hydrolysis. This new swollenin consists of a family 1 CBM connected to a family 45 endoglucanase-like domain by a linker.
In 2005, Vaaje-Kolstad et al. (2005) identified a novel bacterium able to secret a chitin binding domain (CBP21) that is able to break down chitin while increasing the substrate accessibility to chitin hydrolases. Based in this, CBP21 was classified as a family 33 carbohydrate-binding module (Cantarel et al. 2009). This study revealed that CBP21 cleaves glycosidic bonds in chitin by oxidation, leading to the generation of a terminal gluconic acid residue and a normal nonreducing chain end. These and other authors have also demonstrated that CBP21 is able to increase the accessibility of cellulose to cellulolytic enzymes (Harris et al. 2010; Eijsink et al. 2008; Vaaje-Kolstad et al. 2005) but the mechanism of CBP21 action was only clarified by Vaaje-Kolstad et al. (2010).
New studies with CelS2, a CBM33 protein from Streptomyces coelicolor, showed that it produces aldonic acids on the cellulose surface. Like other oxidative enzymes, CelS2 also depends on the presence of divalent metal ions. Westereng et al. (2011) revealed that these enzymes are copper-dependent monooxygenases. Interestingly, CBM33 was also characterized as a copper-dependent lytic enzyme (Vaaje-Kolstad et al. 2012).
Recently, a new type of fungal protein was discovered and classified as family 61 Glycoside Hydrolases (GH61, LPMO) (Harris et al. 2010; Quinlan et al. 2011; Beeson et al. 2012). Likewise CBP21, this enzyme catalyses the oxidative cleavage of polysaccharides, generating new chain ends while modifying the charge distribution of the cellulosic substrate surface. The activity of these oxidative enzymes depends upon the presence of a divalent metal ions and an electron donor. Also, unlike ExG enzymes, their activity on crystalline cellulose does not require the pull-out of a cellulose chain from the surface of the crystalline matrix (Vaaje-Kolstad et al. 2010). These and other authors have shown that oxidative enzymes such as those belonging to LPMO and which are abundant in fungal genomes increase the rate of conversion of cellulosic materials by enzymatic hydrolysis. Figure 8.4 shows one hypothesis for the action of LPMO. Oxidized cellulose chain ends are partially converted to aldonic acid and this highly solvated-opened structure forces these chains to pull out from the surface, leading to a gradual disaggregation of the cellulose structure and to an increase in the availability of new reaction sites for both ExG and EnG.
Anaerobic microorganisms are also able to produce multi-enzymatic complexes called cellulosomes that are able to deconstruct the structural organization of plant polysaccharides (Fontes and Gilbert 2010). In this system, several types of cellulolytic and hemicellulolytic enzymes are assembled in scaffolding subunits that are connected to the whole cell by protein-to-protein noncovalent interactions involving docking and anchoring protein models that are referred to as docherins and cohesins, respectively (Bayer et al. 1994). Like most fungal cellulases, the cellulosome systems have a CBM in mainly their anchoring protein in order to bind to the cellulose surface (Bayer et al. 1994). Furthermore, recently the cellulosomal enzymes had showed synergistic action on the presence of cellulases (Resch et al. 2013).
8.4 Hemicelluloses
Hemicelluloses are heteropolysaccharides that are strongly associated with cellulose by hydrogen bonds as well as van der Waals forces. Their primary structure ranges from linear to highly branched polymeric chains with varying degrees of substitution which, upon acid hydrolysis, may release different types of monosaccharides such as d-mannose, d-galactose, d-xylose, d-glucose, glucuronic acid, 4-O-methyl-d-glucuronic acid, and l-arabinose addition of l-rhamnose and d-galacturonic acid present in rhamnogalacturonans of pectin materials (Bon et al. 2008). Compared to cellulose, these heteropolysaccharides have lower thermal and chemical stabilities probably due to their lower crystallinity index and lower degree of polymerization, reasons for what they are much more susceptible to both acid and alkaline hydrolysis (Ramos 2003).
The main hemicellulose components of dicotyledonous angiosperms are xylans and these usually correspond to about 20 wt% of plant dry mass (Singh et al. 2003). However, in monocotyledonous plants, xylans are no more than 2 wt% of plant dry mass. The main backbone of these polysaccharides is composed of anhydro-d-xylopyranosyl residues that are linked together by β-(1 → 4) glycosidic bonds in which substituents are usually found such as α-l-arabinofuranosyl, α-d-4-O-methylglucuronosyl and O-acetyl groups (Sunna and Antranikian 1997). In angiosperms, 10 % of the d-xylopyranosyl residues are substituted on C-2 position by O-acetyl (Coughlan and Hazlewood 1993). Figure 8.5a shows a theoretical model of the xylan structure as well as the enzymes involved on its degradation.
Most xylanases are classified in the hydrolase families 10 and 11 (Biely et al. 1997). The main difference between these two families is addressed to their catalytic properties. Therefore, they usually display a greater catalytic versatility, particularly in the hydrolysis of highly substituted xylans. Likewise cellulases, some xylanases have a CBM in their structure, bring either a xylan- or a cellulose binding module (Shareck et al. 1991; Sakka et al. 1993).
Generally, endoxylanases act on xylans releasing mainly xylobiose, xylotriose, and branched xylooligomers up until xylopentaose. In addition, most endoxylanases hydrolyze nonsubstituted xylans more efficiently and their tolerance to the presence of side chain varies from one enzyme to another.
Considering the high degree of substitution of xylans, endo-acting enzymes are dominant to the exo-mode. However, although xylans are mainly composed of β-(1 → 4) linkage, some xylanases are able to hydrolyze β-(1 → 3) linkages. Furthermore, exo-acting enzymes show great affinity for polymeric xylan, however, β-xylosidase rather to act on the xylooligosaccharides. As described earlier, glucomannans may be present as one hemicellulose component of the plant cell wall. They have a primary backbone composed of anhydro-d-mannose and anhydro-d-glucose linked together by β-(1 → 4) glycosidic bonds and this may be furnished by side chain groups such as acetyl and anhydro-d-galactosyl groups. Therefore, like other polysaccharides, different enzymes are required for their total hydrolysis. Figure 8.5b shows the theoretical model of a (galacto)glucomannan fragment as well as the enzymes involved on its degradation.
The main backbone of both glucomannans and galactoglucomannans is hydrolyzed primarily by β-(1 → 4)-endomannanases (EC 3.2.1.78). One of the problems relies on the fact that some mannanases are able to hydrolyze not only the β-(1 → 4) linkage between two mannose residues but also the β-(1 → 4) linkage between glucose and mannose residues (Kusakabe et al. 1988; Tenkanen et al. 1997). Glucomannans are also efficiently hydrolyzed by endoglucanases (Mikkelson et al. 2013).
As the concentration of oligomers builds up as a result of hydrolysis, other enzymes such as β-mannosidase (EC 3.1.1.25) and β-glucosidase assume their role in converting these substrates in the monomeric constituents. These enzymes are able to remove mannose or glucose from the nonreducing end of mannooligomers. Furthermore, T. reesei β-xylosidases and Aspergillus niger β-mannosidases may also catalyze the removal of xylose and mannose units from the chain ends of xylans and mannans, respectively (Margolles-Clark et al. 1996; Ademark et al. 1999). Also, some endoglucanases are able to hydrolyze not only the internal glycosidic linkages of cellulose but also those found in other polysaccharides such as xyloglucans due to the cleft shape of their catalytic domain. In addition, these enzymes are also able to act on mixed β-(1 → 3, 1 → 4)-glucans.
Endo-(1 → 3)-β-d-glucanases are able to catalyze the hydrolysis of β-(1 → 3) linkages; however, these enzymes show limited activity on the mixed glucans. On the other hand, endo-(1 → 3, 4)-β-d-glucanases are able to hydrolyze both (1 → 3) and (1 → 4) β-linkages. Furthermore, some exo-glycosyl hydrolases are able to cleave β-(1 → 3) linkages in glucans by a processive action from the nonreducing end, releasing glucose as its main end-product.
Figure 8.5 shows the average side groups that have been already found in xylans and glucomannans. Therefore, the enzymes required to unfurnish these polysaccharides are clearly different from those involved in the hydrolysis of the main chain. The main enzymes involved in the removal of these side chains are α-glucuronosidases, α-d-galactosidases, α-arabinofuranosidases, acetyl xylan esterases, and ferulic acid esterases. For instance, α-glucuronosidases (EC 3.2.1.139) carry out the partial hydrolysis of heteroxylans releasing both of glucuronic and 4-O-methylglucuronic acid residues.
α-d-Galactosidases has not been as thoroughly studied as other enzymes but their specific activity is critical for the complete hydrolysis of softwood mannans. The role of this enzyme is to catalyze the hydrolysis of α-d-galactosyl side groups that are covalently linked to the O-6 position of the anhydro-d-mannose backbone residues (Puls 1997).
In the case of α-arabinofuranosidases, besides being active on the removal of side chains from xylans, some of these enzymes have been reported as catalytically active in the hydrolysis of pectins, arabinans, and arabinoxylans (Hata et al. 1992; Saha 2000; Ximenes et al. 1996). Besides, these enzymes are particularly important for the deconstruction of the plant cell wall because arabinose units are connected to ferulic acid residues in lignin carbohydrate complexes.
As mentioned earlier, acetyl groups are present in several types of hemicelluloses such as xylans and galactoglucomannans. In hardwood and herbaceous xylans, the level of acetyl groups is much higher than in the case of softwoods. However, acetyl groups can be removed from these polysaccharides by the action of acetyl xylan esterases (AXEs) and, like other enzymes already described in this work, AXEs’ specificity depends on the nature of the substrate and its degree of polymerization. Furthermore, AXEs can also show synergism with other enzymes such as xylanases (Poutanen et al. 1990; Bartolome et al. 1997). For biorefinery processes, the use of AXEs must be carefully planned because the release of acetyl groups from the hemicellulose structure decreases the pH and this may be not favorable to some fermenting microorganisms (de Mancilha and Karim 2003; Martin and Jonsson 2003; Lima et al. 2004).
Non-saccharide side chains can also be found in hemicelluloses, such as in the case of ferulic acid in herbaceous and hardwood xylans. Ferulic acid is normally esterified at the C-2 position of an arabinosyl residue (Fig. 8.5a) and its role is apparently associated to the three-dimensional stability of the polymer network (Mathew and Abraham, 2004). Basically, ferulic acid units may be involved in the crosslinking of adjacent xylan backbones by ether linkages forming diferulate bridges, and may also play an important role in linking hemicelluloses directly to the lignin component (Bartolome et al. 1997) (Fig. 8.1). Ferulic acid esterases (FAEs) are responsible for removing ferulic acid decorations from xylans and some of these enzymes are also effective in releasing coumaric acid from similar chemical environments (Donaghy and McKay 1997). Likewise, some FAEs may differ from each other by the affinity to the substrates that they act upon, either polysaccharides (xylans and pectins) or substituted xylan oligomers (de Vries and Visser 1999). Furthermore, new studies have demonstrated the presence of synergism between xylanases and FAEs, and also an enhanced catalytic activity in FAE/xylanase fusion proteins (Faulds et al. 1995; de Vries et al. 2000; de Vries and Visser 2001; Yu et al. 2003). However, likewise AXEs, the activity of FAEs may lead to the release of aromatic compounds that are inhibitory to fermentation microorganism.
The use of a specific ratio of hollocellulose degrading enzymes, including EnGs, ExGs, βGs, xylanases, β-xylosidases, mannases, and β-mannases, is a critical step toward the completed hydrolysis of lignocellulosic materials and this ratio must be in agreement with the pretreatment technology applied in the process (Várnai et al. 2011). In the case of hemicelluloses, both debranching and depolymeration enzymes are required to improve the extent by which these polysaccharides are hydrolyzed. However, different criteria may apply when the desired products are oligosaccharides with special properties for special uses.
The synergistic action among debranching and depolymeration enzymes with different specificities has already been extensively reported. For instance, the synergism between α-glucuronosidases and endoxylanases in the hydrolysis of wheat xylans led to the highest release of 4-O-methylglucuronic acid (de Vries et al. 2000). Therefore, α-arabinofuranosidases can act synergistically with many different enzymes such as xylanases, acetyl xylan esterases, and ferulic acid esterases (Kroon and Williamson 1996; Coutinho and Henrissat 1999; de Vries et al. 2000; Puls 1997; Bachmann and McCarthy 1991).
The factors affecting the performance of hydrolytic enzymes in biorefinery processes are diverse and originate from enzyme characteristic, process conditions, and substrates. The high catalytic efficiency of individual proteins and optimal ratio of mixture components are the first prerequisites for efficiency. Thermal stability has been shown to be beneficial for enzymes, due to better stability, higher conversion rates, and flexibility in terms of process design (Viikari et al. 2007). Nonproductive adsorption on biomass, especially on lignin reduces the availability of enzymes for hydrolysis, and results in enzyme inactivation especially in high temperature (Rahikainen et al. 2011). Enzyme inhibition has been extensively studied, and it can be caused by several compounds, such as sugars and oligosaccharides, various chemical compounds being often degradation products of biomass, and also by each other on biomass surfaces. The inhibitory environment can be improved by milder pretreatment conditions and by intelligent design of the process. The behavior of enzymes in high dry matter conditions, applied in industrial conditions differ clearly from that in laboratory conditions which are in most cases used for screening and evaluation studies. High dry matter has consequences in the performance of enzymes (e.g., Jørgensen et al. 2007) as well as to fundamental features such as the effect of CBMs in hydrolysis (Várnai et al. 2013).
8.5 Lignin
Lignin is the most abundant polyphenolic compound in nature, reaching 20–30 % of the lignocellulosic biomass produced worldwide (Fengel and Wegener 1989). Its hydrophobic and complex structure is mainly formed by the following units: 4-(3-hydroxyprop-1-enyl)-phenol, 4-(3-hydroxyprop-1-enyl)-2-methoxyphenol and 4-(3-hydroxyprop-1-enyl)-2, 6-dimethoxyphenol. Like hemicelluloses, the lignin type and distribution depends on the plant species and varies from one tissue to another. Besides, the chemical characteristics of isolated lignin depend largely on the method used for extraction.
Unlike cellulose and hemicelluloses, the lignin building blocks or monomeric units are not disposed in order and their crosslink includes ether linkages between aromatic rings and aliphatic chains (β-O-4′ and O-α 4′) and different carbon-to-carbon bonds involving aliphatic chains (β-β′, α-α′, and α-β′), aliphatic chains and aromatic rings (β-5′, β-1′, α-1′, and β-6′), and aromatic rings (5-5′) (Higuchi, 1985). According to Lee (1997), the most important linkages in the lignin structure are the β-1 and β-O-4 types, the latter of which corresponding to more than 50 % of its polyphenolic structure. Figure 8.6 shows the model structure of a lignin fragment derived from P. albis (Higuchi 1985) in close association with a feruloylated arabinoxylan, forming a lignin-carbohydrate complex.
Some microorganisms are able to produce lignin-degrading enzymes such as lignin peroxidase (LiP) and manganese peroxidase (MnP), which are extracellular heme proteins (Shin et al. 2005; Sharma et al. 2011). In a general, LiP catalyses the conversion of aromatic compounds in the presence of H2O2 to their corresponding aldehydes or ketones, and the hydroxylation of benzylic methylene groups. On the other hand, MnP may behave as an oxidase or a peroxidase (Singh et al. 2011). MnP acts by oxidating Mn2+ to Mn3+ with H2O2 in order to convert aromatic compounds to polycyclic aromatic hydrocarbons (Steffen et al. 2002; Shin et al. 2005). Therefore, LiP and MnP are known as primary enzymes for degradation of lignin. Besides the LiP and MnP, laccases are also known to degrade lignin to a certain extent (Youn et al. 1995; Eggert et al. 1997). Several studies have demonstrated the use of laccases in the detoxification of aromatic compounds but its role in lignin degradation has not been well established as yet. Furthermore, it is known that mushrooms can grow on lignocellulosic materials using plant carbohydrates as the carbon source while secreting lignin-degrading enzymes.
In general, oxidative enzymes require the presence of cofactors such as metallic ions and H2O2 and for this reason it is very difficult to carry out a bioprocess with simultaneous use of lignin-degrading enzymes and carbohydrate-degrading enzymes. Therefore, for the biorefinery processes development based on the use of the lignocellulosic materials, these enzymes are mainly useful for the biological pretreatment of the substrate such as in the case of biopulping (Aguiar and Ferraz 2012).
8.6 The Role of Enzymes in the Biorefinery
Apart from improvements in the development viable enzyme technologies for converting biomass to fuels and chemicals, insights on the pretreatment technologies of the lignocellulosic materials also represent a key factor for the industrial biorefinery based on agroindustrial wastes. In other words, pretreatment is crucial for the technical and economic viability of the overall process.
There are several pretreatment technologies already available for separating the main plant cell wall components in different streams: steam explosion with and without the use of an exogenous catalyst (Ramos 2003), dilute acid hydrolysis (Larsson et al. 1999), liquid hot water (Laser et al. 2002; Mosier et al. 2005), wet oxidation (Martin et al. 2007), ammonia fiber expansion (Balan et al. 2009; Chundawat et al. 2010), alkali extraction (Gupta and Lee 2010), alkaline hydrogen peroxide (Xiang and Lee 2000), organosolv extraction (Araque et al. 2008; Obama et al. 2012), and treatment with ionic liquids (Li et al. 2010), among others. When removed, hemicelluloses and lignin can be utilized in direct applications or as precursors for a wide range of industrial chemicals and materials. For instance, lignin can be directly used as a fuel (Menon and Rao 2012) or be converted to many value-added products including activated carbon (Demirbas 2004), binders (Dizhbite et al. 1999), dispersants, emulsifiers, and sequestrants (Suhas and Ribeiro 2007; Adler, 1997), vanillin and polyurethanes (Borges da Silva et al. 2009). Lignin can also be used in blends with polyhydroxyalkanoates (Ghosh et al. 2000) and polylactides and polyglycolides (Doherty et al. 2011), in epoxy resins (Wang et al. 1992) and as antioxidant in asphalts (Pan 2012). By contrast, hemicelluloses such as xylans can be converted to furfural (Montané et al. 2002), hydrogen (Caye et al. 2008), succinic acid (Nghiem, 2005), xylitol (Felipe et al. 1997), and xylooligosaccharides (Vazquez et al. 2000). Finally, apart from its more classical uses, cellulose can be converted to glucose to produce ethanol (Wyman 1994; Sun and Cheng 2002), lactic acid (Hofvendahl and Hahn-Hägerdahl 2000), succinic acid (Wang et al. 2011), and acetic acid (Wang et al. 2013) by fermentation, or used in pharmaceutical applications (Cherian et al. 2011) and as reinforcing agent in nanocomposites (Alves et al. 2013).
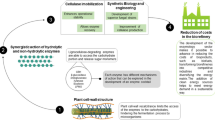
Figure 8.7 shows a simplified scheme for a biorefinery based on lignocellulosic materials. This biorefinery involves a multistep process in which the first step is the pretreatment of the biomass to render its macromolecular components amenable for further processing. The outputs of this process could be used as it is or be converted into chemical building blocks for further processing into polymers, chemicals, fuels, energy, and composite materials.
According to the applied pretreatment technology, different substrates are produced and their chemical composition would require a different enzyme composition for optimal enzymatic hydrolysis. In fact, this is a major challenge for commercial enzymes because none of them can be claimed as universal in their application to substrates with different compositional analysis and physical properties such as degree of crystallinity, degree of polymerization, particle size, available surface area, and pore volume distribution.
Acid pretreatments tend to remove most of the hemicelluloses as water-soluble mono- and oligosaccharides (the so-called hemicellulose hydrolysate), leaving a lignocellulosic material whose hydrolysis would require an enzyme cocktail that is less susceptible to hydrophobic interactions and to the inhibitory effects of aromatic compounds such as phenolic acids derived from lignin. The immediate consequence of this pretreatment option is the possibility of using the hemicellulose hydrolysate for a variety of applications including ethanol production after partial detoxification. Also, by enzymatic hydrolysis of acid-pretreated materials, glucose is obtained as the main product and this could be one important issue for the desired integration of cellulosic ethanol into the currently existing first-generation ethanol producing technologies. Finally, the lignin-rich residue obtained after enzymatic hydrolysis could be used for co-generation or bioelectricity and also for other applications in the fuel and chemical platforms.
Alkaline pretreatments are able to extract the lignin component of plant biomass and depending on the extent of lignin extraction, the resulting fibrous material may be classified as holocellulose. In this case, higher hemicellulase activities would be required in the enzyme cocktail to achieve complete hydrolysis of the delignified cellulosic material. Alternatively, the hemicellulose component could be extracted from these substrates in its poly- or oligomeric form, allowing its use as a polyelectrolyte, sizing agents, food additives, thickeners, films, and as a component of natural composites. Also, the lignin component can be obtained in higher molecular mass and with a lower degree of condensation, opening a venue of possible industrial applications in resins, emulsions, adsorbents, carbon fiber, films, polymers, adhesives, and composites.
With the abundance of biomass wastes, the development of new technologies that will make use of biomass for materials production beyond biofuels represents an important opportunity to fully utilize the resources. Development of efficient techniques to fractionate lignocellulosic biomass into its core components will facilitate research on the production of specific biomass-derived sugars, building block chemicals, and ultimately value-added commodity chemicals while preserving the concept of the biorefinery approach by promoting effective utilization of all feedstock fractions.
References
Ademark P, Lundqvist J, Hagglund P, Tenkanen M, Torto N, Tjerneld F, Stalbrand H (1999) Hydrolytic properties of a beta-mannosidase purified from Aspergillus niger. J Biotechnol 75(2–3):281–289
Adler E (1997) Lignin chemistry: past, present and future. Wood Sci Technol 11(3):169–218
Aguiar A, Ferraz A (2012) Use of additives in the wood biodegradation by the fungus Ceriporiopsis subvermispora: effect in the manganese peroxidase-dependent lipid peroxidation. Quim Nova 35(6):1107–1111
Alves HS, Pires WF, Oliveira N, Pasquini D (2013) Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind Crops Prod 44:427–436
Arantes V, Saddler JN (2010) Access to cellulose limits the efficiency of enzymatic hydrolysis: the role of amorphogenesis. Biotechnol Biofuels 3:4
Araque E, Parra C, Freer J, Contreras D, Rodríguez J, Mendonça R, Baeza J (2008) Evaluation of organosolv pretreatment for the conversion of Pinus radiata D. Don to ethanol. Enzym Microb Technol 43:214–219
Bachmann SL, McCarthy AJ (1991) Purification and cooperative activity of enzymes constituting the xylan-degrading system of Thermomonospora fusca. Appl Environ Microbiol 57(8):2121–2130
Balan V, Sousa LDC, Chundawat SPS, Marshall D, Sharma LN, Chambliss CK, Dale BE (2009) Enzymatic digestibility and pretreatment degradation products of AFEX-treated hardwoods (Populus nigra). Biotechnol Program 25:365–375
Bartolome B, Faulds CB, Kroon PA, Waldron K, Gilbert HJ, Hazlewood G, Williamson G (1997) An Aspergillus niger esterase (ferulic acid esterase III) and a recombinant Pseudomonas fluorescens subsp cellulosa esterase (XylD) release a 5–5′ ferulic dehydrodimer (diferulic acid) from barley and wheat cell walls. Appl Environ Microbiol 63(1):208–212
Bayer EA, Morag E, Lamed R (1994) The cellulosome–a treasure-trove for biotechnology. Trends Biotechnol 12(9):379–386
Beckham GT, Bomble YJ, Matthews JF, Taylor CB, Resch MG, Yarbrough JM, Decker SR, Bu LT, Zhao XC, McCabe C et al (2010) The O-glycosylated linker from the Trichoderma reesei family 7 cellulase is a flexible, disordered protein. Biophys J 99(11):3773–3781
Beeson WT, Phillips CM, Cate JHD, Marletta MA (2012) Oxidative cleavage of cellulose by fungal copper-dependent polysaccharide monooxygenases. J Am Chem Soc 134(2):890–892
Biely P, Vrsanska M, Tenkanen M, Kluepfel D (1997) Endo-beta-1, 4-xylanase families: differences in catalytic properties. J Biotechnol 57(1–3):151–166
Bon EPS, Pereira NJ, Girio F. Enzimas na Produção de Etanol. In: Elba P.S. Bon et al. (eds). Enzimas em Biotecnologia: Produção, Aplicações e Mercado. 1 ed. Rio de Janeiro, Brazil: Editora Interciência Ltda., 2008
Borges da Silva EA, Zabkova M, Araújo JD, Cateto CA, Barreiro MF, Belgacem MN, Rodrigues AE (2009) An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem Eng Res Des 87:1276–1292
Caes BR, Palte MJ, Raines RT (2013) Organocatalytic conversion of cellulose into a platform chemical. Chemical Sci 4(1):196–199
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The carbohydrate-active enzymes database (cazy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Caye DC, Nghiem NP, Terry WH (2008) Biofuels engineering process technology. McGraw-Hill, New York
Chen XA, Ishida N, Todaka N, Nakamura R, Maruyama JI, Takahashi H, Kitamoto K (2010) Promotion of efficient saccharification of crystalline cellulose by Aspergillus fumigatus swo1. Appl Environ Microbiol 76(8):2556–2561
Cherian BM, Lopes A, Ferreira S, Manzine LM, Molina G, Kottaisamy M, Nagarajan ER, Thomas S (2011) Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medicalapplications. Carbohydr Polym 86:1790–1798
Chundawat SPS, Vismeh R, Sharma L, Humpula J, Sousa L, Chambliss CK, Jones AD, Balan V, Dale BE (2010) Multifaceted characterization of cell wall decomposition products formed during ammonia fiber expansion (AFEX) and dilute-acid based pretreatments. Bioresour Technol 101:8429–8438
Cosgrove DJ (1999) Enzymes and other agents that enhance cell wall extensibility. Ann Rev Plant Physiol Plant Mol Biol 50:391–417
Cosgrove DJ (2000a) Loosening of plant cell walls by expansins. Nature 407(6802):321–326
Cosgrove DJ (2000b) New genes and new biological roles for expansins. Curr Opin Plant Biol 3(1):73–78
Coughlan MP, Hazlewood GP (1993) Beta-1, 4-D-Xylan-degrading enzyme-systems: biochemistry, molecular-biology and applications. Biotechnol and Applied Biochemistry 17:259–289
Coutinho PM, Henrissat B (1999) Carbohydrate-active enzymes: an integrated database approach. In: Gilbert HJ, Davies GBH, Svensson B (eds) Recent advances in carbohydrate bioengineering. The Royal Society of Chemistry, Cambridge, p 312
Dashtban M, Schraft H, Qin W (2009) Fungal Bioconversion of Lignocellulosic Residues: Opportunities & Perspectives. Int J Biol Sci 5(6):578–595
de Mancilha IM, Karim MN (2003) Evaluation of ion exchange resins for removal of inhibitory compounds from corn stover hydrolyzate for xylitol fermentation. Biotechnol Prog 19(6):1837–1841
de Vries RP, Kester HCM, Poulsen CH, Benen JAE, Visser J (2000) Synergy between enzymes from Aspergillus involved in the degradation of plant cell wall polysaccharides. Carbohydr Res 327(4):401–410
de Vries RP, Visser J (1999) Regulation of the feruloyl esterase (faeA) gene from Aspergillus niger. Appl Environ Microbiol 65(12):5500–5503
de Vries RP, Visser J (2001) Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev 65(4):497–522
Demirbas A (2004) Adsorption of lead and cadmium ions in aqueous solutions onto modified lignin from alkali glycerol delignification. J Hazard Mater 109(1–3):221–226
Din N, Gilkes NR, Tekant B, Miller RC, Warren AJ, Kilburn DG (1991) Non-hydrolytic disruption of cellulose fibers by the binding domain of a bacterial cellulase. Bio-Technology 9(11):1096–1099
Dizhbite T, Zakis G, Kizima A, Lazareva E, Rossinskaya G, Jurkjane V (1999) Lignin a useful bioresource for the production of sorption-active materials. Bioresour Technol 67(3):221–228
Doherty WO, Mousavioun P, Fellows CM (2011) Value-adding to cellulosic ethanol: Lignin polymers. Ind Crops Prod 33:259–276
Donaghy J, McKay AM (1997) Purification and characterization of a feruloyl esterase from the fungus Penicillium expansum. J Appl Microbiol 83(6):718–726
Eggert C, Temp U, Eriksson KEL (1997) Laccase is essential for lignin degradation by the white-rot fungus Pycnoporus cinnabarinus. FEBS Lett 407(1):89–92
Eijsink VGH, Vaaje-Kolstad G, Varum KM, Horn SJ (2008) Towards new enzymes for biofuels: lessons from chitinase research. Trends Biotechnol 26(5):228–235
Ekwe E, Morgenstern I, Tsang A, Storms R, Powlowski J (2013) Non-hydrolytic cellulose active proteins research progress and potential application in biorefineries. Ind Biotechnol 9(3):123–131
Faulds CB, Williamson G (1995) Release of ferulic acid from wheat bran by a ferulic acid esterase (fae-III) from Aspergillus niger. Appl Microbiol Biotechnol 43(6):1082–1087
Felipe MGA, Vitolo M, Mancilha IM, Silva SS (1997) Fermentation of sugar cane bagasse hemicellulosic hydrolysate for xylitol production: effect of pH. Biomass Bioenergy 13:11–14
Fengel D, Wegener G, Greune A (1989) Studies on the delignification of spruce wood by organosolv pulping using SEM-EDXA and TEM. Wood Sci Technol 23(2):123–130
Fontes CMGA, Gilbert HJ (2010) Cellulosomes: highly efficient nanomachines designed to designed to deconstruct plant cell wall complex carbohydrates. Annu Rev Biochem 79(79):655–681
Foreman PK, Brown D, Dankmeyer L, Dean R, Diener S, Dunn-Coleman NS, Goedegebuur F, Houfek T, England G, Kelley A, Meerman H, Mitchell T, Mitchinson C, Olivares H, Teunissen P, Yao J, Ward M (2013) Transcriptional regulation of biomass-degrading enzymes in the filamentous fungus Trichoderma reesei. J Biol Chem 278(34):31988–31997
Ghosh I, Jain RK, Glasser WG (2000) Multiphase materials with lignin. Part 16. Blends of biodegradable thermoplastics with lignin esters. ACS Symp Ser 742:331 (Lignin: Historical, Biological, and Materials Perspectives)
Gourlay K, Hu J, Arantes V, Andberg M, Saloheimo M, Penttilä M, Saddler J (2013) Swollenin aids in the amorphogenesis step during the enzymatic hydrolysis of pretreated biomass. Bioresour Technol 142:498–503
Gupta R, Lee YY (2010) Investigation of biomass degradation mechanism in pretreatment of switchgrass by aqueous ammonia and sodium hydroxide. Bioresour Technol 101:8185–8191
Harris PV, Welner D, McFarland KC, Re E, Poulsen JCN, Brown K, Salbo R, Ding HS, Vlasenko E, Merino S et al (2010) Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: structure and function of a large enigmatic family. Biochemistry 49(15):3305–3316
Hata K, Tanaka M, Tsumuraya Y, Hashimoto Y (1992) Alpha-L-arabinofuranosidase from radish (Raphanus sativus L) seeds. Plant Physiol 100(1):388–396
Henrissat B, Driguez H, Viet C, Schulein M (1985) Synergism of cellulases from Trichoderma reesei in the degradation of cellulose. Bio-Technology 3(8):722–726
Higuchi T (1985) Lignin structure and morphological distribution in plant cell walls. In: Kirk TK, Higuchi T, Chang H-M (eds) Lignin biodegradation: microbiology, chemistry and potential applications, vol 1. CRC Press, Boca Raton, p 237
Hofvendahl K, Hahn-Hägerdal B (2000) Factors affecting the fermentative lactic acid production from renewable resources. Enzym Microb Technol 26:87–107
Jäger G, Girfoglio M, Dollo F, Rinaldi R, Bongard H, Commandeur U, Fischer R, Spiess AC, Buchs J (2011) How recombinant swollenin from Kluyveromyces lactis affects cellulosic substrates and accelerates their hydrolysis. Biotechnol Biofuels 4:33
Jarvis MC (2000) Interconversion of the I alpha and I beta crystalline forms of cellulose by bending. Carbohydr Res 325(2):150–154
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40(9):2931–2944
Jhonson DL (1969) US patent 3,447,939 (03. 06. 1969). Compounds dissolved in cyclic amine oxides
Jørgensen H, Vibe-Pedersen J, Larsen J, Felby C (2007) Liquefaction of lignocellulose at high-solids concentrations. Biotechnol Bioeng 96(5):862–870
Kang K, Wang SW, Lai GH, Liu G, Xing M (2013) Characterization of a novel swollenin from Penicillium oxalicum in facilitating enzymatic saccharification of cellulose. Bmc Biotechnol 13:42
Karlsson J, Momcilovic D, Wittgren B, Schulein M, Tjerneld F, Brinkmalm G (2002) Enzymatic degradation of carboxymethyl cellulose hydrolyzed by the endoglucanases Cel5A, Cel7B, and Cel45A from Humicola insolens and Cel7B, Cel12A and Cel45Acore from Trichoderma reesei. Biopolymers 63(1):32–40
Kroon PA, Williamson G (1996) Release of ferulic acid from sugar-beet pulp by using arabinanase, arabinofuranosidase and an esterase from Aspergillus niger. Biotechnol Appl Biochem 23:263–267
Kusakabe I, Park GG, Kumita N, Yasui T, Murakami K (1988) Specificity of beta-mannanase from Penicillium purpurogenum for konjac glucomannan. Agric Biol Chem 52(2):519–524
Larsson S, Palmqvist E, Hahn-Hägerdahl B, Tengborg Ch, Stenberg K, Zacchi G, Nilvebrant N (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym Microb Technol 24:151–159
Laser M, Schulman D, Allen SG, Lichwa J, Antal MJ, Lynd LR (2002) A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresour Technol 81:33–44
Lee J (1997) Biological conversion of lignocellulosic biomass to ethanol. J Biotechnol 56(1):1–24
Lee Y, Choi D, Kende H (2001) Expansins: ever-expanding numbers and functions. Curr Opin Plant Biol 4(6):527–532
Li CH, Knierim B, Manisseri B, Arora R, Scheller H, Auer M, Vogel K, Simmons B, Singh S (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101:4900–4906
Lima LHA, Felipe MDD, Vitolo M, Torres FAG (2004) Effect of acetic acid present in bagasse hydrolysate on the activities of xylose reductase and xylitol dehydrogenase in Candida guilliermondii. Appl Microbiol Biotechnol 65(6):734–738
Loeb L, Segal L (1954) Preparation of cotton cellulose-IV from cotton cellulose-III. J Polym Sci 14(73):121–123
Macarron R, Acebal C, Castillon MP, Claeyssens M (1996) Mannanase activity of endoglucanase III from Trichoderma reesei QM9414. Biotechnol Lett 18(5):599–602
Margolles-Clark E, Tenkanen M, Soderlund H, Penttila M (1996) Acetyl xylan esterase from Trichoderma reesei contains an active-site serine residue and a cellulose-binding domain. Eur J Biochem 237(3):553–560
Marjamaa K, Toth K, Bromann PA, Szakacs G, Kruus K (2013) Novel Penicillium cellulases for total hydrolysis of lignocellulosics. Enzym Microb Technol 52(6–7):358–369
Martin C, Jonsson LJ (2003) Comparison of the resistance of industrial and laboratory strains of Saccharomyces and Zygosaccharomyces to lignocellulose-derived fermentation inhibitors. Enzym Microb Technol 32(3–4):386–395
Martin C, Klinke HB, Thomsen AB (2007) Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzyme Microb Tech 40(3):426–432
Mathew S, Abraham TE (2004) Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit Rev Biotechnol 24(2–3):59–83
Matthews JF, Skopec CE, Mason PE, Zuccato P, Torget RW, Sugiyama J, Himmel ME, Brady JW (2006) Computer simulation studies of microcrystalline cellulose I beta. Carbohydr Res 341(1):138–152
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 38(4):522–550
Mihranyan A, Llagostera AP, Karmhag R, Stromme M, Ek R (2004) Moisture sorption by cellulose powders of varying crystallinity. Int J Pharm 269(2):433–442
Mikkelson A, Maaheimo H, Hakala T (2013) Hydrolysis of konjac glucomannan by Trichoderma reesei mannanase and endoglucanases Cel7B and Cel5A for the production of glucomannooligosaccharides. Carbohydr Res 3(372):60–68
Montane D, Salvado J, Torras C, Farriol X (2002) High-temperature dilute-acid hydrolysis of olive stones for furfural production. Biomass Bioenergy 22(4):295–304
Mosier NS, Hendrickson R, Brewer M, Ho N, Sedlak M, Dreshel R, Welch G, Dien BS, Aden A, Ladisch MR (2005) Industrial scale-up of pH-controlled liquid hot water pretreatment of corn fiber for fuel ethanol production. Appl Biochem Biotechnol 125:77–97
Mussatto SI, Dragone G, Guimaraes PMR, Silva JPA, Carneiro LM, Roberto IC, Vicente A, Domingues L, Teixeira JA (2010) Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv 28(6):817–830
Nghiem NP, Donnelly M, Sanville-Millard CY (2005) UTBATTELLE, LLC., University of Chicago, assignees. A method to produce succinic acid from raw hydrolysates, World Intellectual Property Organization patent WO/116227
Nidetzky B, Claeyssens M (1994) Specific Quantification of Trichoderma reesei cellulases in reconstituted mixtures and its application to cellulase-cellulose binding studies. Biotechnol Bioeng 44(8):961–966
Notenboom V, Boraston AB, Chiu P, Freelove ACJ, Kilburn DG, Rose DR (2001) Recognition of cello-oligosaccharides by a family 17 carbohydrate-binding module: An X-ray crystallographic, thermodynamic and mutagenic study. J Mol Biol 314(4):797–806
Obama P, Ricochon G, Muniglia L, Brosse N (2012) Combination of enzymatic hydrolysis and ethanol organosolv pretreatments: effect on lignin structures, delignification yields and cellulose-to-glucose conversion. Bioresour Technol 112:156–163
Okano T, Sarko A (1985) Mercerization of cellulose II. Alkali cellulose intermediates and a possible mercerization mechanism. J Appl Polym Sci 30(1):325–332
Pan T (2012) A first-principles based chemophysical environment for studying lignins as an asphalt antioxidant. Constr Build Mater 36:654–664
Phillips CM, Iavarone AT, Marletta MA (2011) Quantitative proteomic approach for cellulose degradation by Neurospora crassa. J Proteome Res 10(9):4177–4185
Poutanen K, Sundberg M, Korte H, Puls J (1990) Deacetylation of xylans by acetyl esterases of Trichoderma reesei. Appl Microbiol Biotechnol 33(5):506–510
Puls J (1997) Chemistry and biochemistry of hemicelluloses: relationship between hemicellulose structure and enzymes required for hydrolysis. Macromolecular Symposia 120:183–196
Quinlan RJ, Sweeney MD, Lo Leggio L, Otten H, Poulsen JCN, Johansen KS, Krogh KBRM, Jorgensen CI, Tovborg M, Anthonsen A et al (2011) Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc Nat Acad Sci USA 108(37):15079–15084
Rabinovich ML, Melnick MS, Bolobova AV (2002) The structure and mechanism of action of cellulolytic enzymes. Biochemistry-Moscow 67(8):850–871
Rahikainen J, Mikander S, Marjamaa K, Tamminen T, Lappas A, Viikari L, Kruus K (2011) Inhibition of enzymatic hydrolysis by residual lignins from softwood - study of enzyme binding and inactivation on lignin-rich surface. Biotechnol Bioeng 108(12):2823–2834
Ramos LP (2003) The chemistry involved in the steam treatment of lignocellulosic materials. Quim Nova 26(6):863–871
Reese ET, Siu RGH, Levinson HS (1950) The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J Bacteriol 59(4):485–497
Resch MG, Donohoe BS, Baker JO, Decker SR, Bayer EA, Beckhamde GT, Himmel ME (2013) Fungal cellulases and complexed cellulosomal enzymes exhibit synergistic mechanisms in cellulose deconstruction. Energy Environ Sci 6(6):1858–1867
Saha BC (2000) Alpha-L-arabinofuranosidases: biochemistry, molecular biology and application in biotechnology. Biotechnol Adv 18(5):403–423
Sakamoto R, Arai M, Murao S (1985) Enzymic properties of 3 beta-glucosidases from Aspergillus aculeatus No-F-50. Agric Biol Chem 49(5):1283–1290
Sakka K, Yoshikawa K, Kojima Y, Karita S, Ohmiya K, Shimada K (1993) Nucleotide-sequence of the Clostridium stercorarium xyla gene encoding a bifunctional protein with beta-d-xylosidase and alpha-l-arabinofuranosidase activities, and properties of the translated product. Biosci Biotechnol Biochem 57(2):268–272
Shareck F, Roy C, Yaguchi M, Morosoli R, Kluepfel D (1991) sequences of 3 genes specifying xylanases in Streptomyces lividans. Gene 107(1):75–82
Sharma JK, Yadav M, Singh NP, Yadav KDS (2011) Purification and characterisation of lignin peroxidase from Pycnoporus sanguineus MTCC-137. Appl Biochem Microbiol 47(5):532–537
Shin KS, Kim YH, Lim JS (2005) Purification and characterization of manganese peroxidase of the white-rot fungus Irpex lacteus. J Microbiol 43(6):503–509
Shoemaker S, Watt K, Tsitovsky G, Cox R (1983) characterization and properties of cellulases purified from Trichoderma reesei strain-L27. Bio-Technology 1(8):687–690
Singh D, Zeng J, Chen S (2011) Increasing manganese peroxidase productivity of Phanerochaete chrysosporium by optimizing carbon sources and supplementing small molecules. Lett Appl Microbiol 53(1):120–123
Singh AB, Biswas AK, Ramana S (2003) Effect of distillery effluents on plant and soil enzymatic activities and groundnut quality. J Plant Nutr Soil Sci 166(3):345–347
Ståhlberg J (1991) Functional Organization of Cellulases from Trichoderma reesei. Dissertation, Uppsala University, Uppsala, Sweden
Steffen KT, Hofrichter M, Hatakka A (2002) Purification and characterization of manganese peroxidases from the litter-decomposing basidiomycetes Agrocybe praecox and Stropharia coronilla. Enzym Microb Technol 30(4):550–555
Suhas PJM, Ribeiro MML (2007) Lignin from natural adsorbent to activated carbon: a review. Bioresour Technol 98(12):2301–2312
Sun Y, Cheng JY (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83(1):1–11
Sunna A, Antranikian G (1997) Xylanolitic enzymes from fungi and bacteria. Crit Rev Biotechnol 17(1):39–67
Tenkanen M, Makkonen M, Perttula M, Viikari L, Teleman A (1997) Action of Trichoderma reesei mannanase on galactoglucomannan in pine kraft pulp. J Biotechnol 57(1–3):191–204
Tsuda Y, Mukoyama S (1957) The Formation of Cellulose-IV in the Viscose Spinning. B Chem Soc Jpn 30(7):718–720
Vaaje-Kolstad G, Bohle LA, Gaseidnes S, Dalhus B, Bjoras M, Mathiesen G, Eijsink VGH (2012) Characterization of the chitinolytic machinery of Enterococcus faecalis V583 and high-resolution structure of its oxidative CBM33 Enzyme. J Mol Biol 416(2):239–254
Vaaje-Kolstad G, Horn SJ, van Aalten DMF, Synstad B, Eijsink VGH (2005) The non-catalytic chitin-binding protein CBP21 from Serratia marcescens is essential for chitin degradation. J Biol Chem 280(31):28492–28497
Vaaje-Kolstad G, Westereng B, Horn SJ, Liu ZL, Zhai H, Sorlie M, Eijsink VGH (2010) An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330(6001):219–222
Várnai A, Huikko L, Pere J, Siika-aho M, Viikari L (2011) Synergistic action of xylanase and mannanase improves the total hydrolysis of softwood. Bioresour Technol 102(19):9096–9104
Várnai A, Siika-aho M, Viikari L (2013) Carbohydrate-binding modules (CBMs) revisited. Reduced amount of water counterbalances the need for CBMs. Biotechnol Biofuels 6:30
Vazquez MJ, Alonso JL, Dominguez H, Parajo JC (2000) Xylooligosaccharides: manufacture and applications. Trends Food Sci Technol 11(11):387–393
Viikari L, Alapuranen M, Puranen T, Vehmaanperä J, Siika-aho M (2007) Thermostable enzymes in lignocellulose hydrolysis. Adv Biochem Eng Biotechnol 108:121–145
Vlasenko E, Schülein M, Cherry J, Xu F (2010) Substrate specificity of family 5, 6, 7, 9, 12, and 45 endoglucanases. Bioresour Technol 101(7):2405–2411
Voutilainen S, Puranen T, Siika-aho M, Lappalainen A, Alapuranen M, Kallio J, Hooman S, Viikari L, Vehmaanperä J, Koivula A (2008) Cloning, expression, and characterization of novel thermostable family 7 cellobiohydrolases. Biotechnol Bioeng 101(3):515–528
Wang D, Li Q, Yang M, Zhang Y, Su Z, Xing J (2011) Efficient production of succinic acid from corn stalk hydrolysates by a recombinant Escherichia coli with ptsG mutation. Process Biochem 46:365–371
Wang J, Manley RSJ, Feldman D (1992) Synthetic polymer-lignin copolymers and blends. Prog Polym Sci 17:611–646
Wang Z, Yan M, Chen X, Li D, Qin L, Li Z, Yao J, Liang X (2013) Mixed culture of Saccharomyces cerevisiae and Acetobacter pasteurianus for acetic acid production. Biochem Eng J 79:41–45
Westereng B, Ishida T, Vaaje-Kolstad G, Wu M, Eijsink VGH, Igarashi K, Samejima M, Stahlberg J, Horn SJ, Sandgren M (2011) The Putative Endoglucanase PcGH61D from Phanerochaete chrysosporium Is a Metal-Dependent Oxidative Enzyme that Cleaves Cellulose. Plos One Vol. 6 No. 11
Wyman CE (1994) Ethanol from lignocellulosic biomass: technology, economics, and opportunities. Bioresour Technol 50:3–15
Xiang Q, Lee YY (2000) Oxidative cracking of precipitated hardwood lignin by hydrogen peroxide. Appl Biochem Biotechnol 84(6):153–162
Ximenes EFF, Puls J, Coughlan MP (1996) Purification and characterization of two arabinofuranosidases from solid-state cultures of the fungus Penicillium capsulatum. Appl Environ Microbiol 62(1):168–173
Yabushita M, Kobayashia H, Fukuoka A (2013) Catalytic transformation of cellulose into platform chemicals. Appl Catal B: Environ (in press), doi:http://dx.doi.org/10.1016/j.apcatb.2013.01.052. I
Youn HD, Hah YC, Kang SO (1995) Role of laccase in lignin degradation by white-rot fungi. FEMS Microbiol Lett 132(3):183–188
Yu PQ, McKinnon JJ, Maenz DD, Olkowski AA, Racz VJ, Christensen DA (2003) Enzymic release of reducing sugars from oat hulls by cellulase, as influenced by Aspergillus ferulic acid esterase and Trichoderma xylanase. J Agric Food Chem 51(1):218–223
Yuan S, Wu YJ, Cosgrove DJ (2001) A fungal endoglucanase with plant cell wall extension activity. Plant Physiol 127(1):324–333
Zhang YHP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol Bioeng 88(7):797–824
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Silveira, M.H.L., Siika-aho, M., Kruus, K., Garriga, L.M., Ramos, L.P. (2014). The Essential Role of Plant Cell Wall Degrading Enzymes in the Success of Biorefineries: Current Status and Future Challenges. In: da Silva, S., Chandel, A. (eds) Biofuels in Brazil. Springer, Cham. https://doi.org/10.1007/978-3-319-05020-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-05020-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05019-5
Online ISBN: 978-3-319-05020-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)