Abstract
Brazil achieved important success in the implementation of ethanol as a reality renewable energy source after the inception of the National Alcohol Program (PROÁLCOOL) in 1970. Today, ethanol produced from sugarcane replaces almost 50 % of gasoline in Brazil. More than 448 bioethanol production (first-generation ethanol) units are functional, which fulfill the 25 % ethanol blending to gasoline that eventually reduces the import of 550 million oil barrels improving the socioeconomic status and saving foreign exchange reserves. Brazil has more than 80 % of its light vehicles running on bioethanol, reducing greenhouse gas emissions. At present, this demand for ethanol is being met through first-generation (1G) ethanol which is directly produced from sugarcane juice and molasses. However, significant research in bioenergy in the last two decades has shown the possibilities of commercialization of second-generation (2G) ethanol, which can be produced from sugarcane bagasse (SB) and straw (SS), complementing 1G ethanol. Nevertheless, both the residues (SB and SS) are an excellent source for cogeneration of heat and power (CHP) in sugarcane processing units. Process simulation studies have provided additional source of information on the overall use of sugarcane for ethanol production and CHP. For the evaluation of the fullest utilization of sugarcane and its by-products, CTBE (Brazilian Bioethanol Science and Technology Laboratory) has developed the Virtual Sugarcane Biorefinery (VSB), a comprehensive assessment framework to evaluate a sustainability standpoint (economic, environmental, and social), different biorefinery alternatives. This chapter reviews the important insights made into bioethanol production in Brazil. Technical configuration for 1G and 2G ethanol production and sustainability of ethanol (economic and environmental assessment) have also been discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brazilian bioenergy
- Fuel ethanol
- Sugarcane
- Sugarcane residues
- Techno-economic analysis
- Environmental assessment
- Bioelectricity
- Second-generation ethanol
1.1 Introduction
Energy encompasses all the important features of the overall growth of human development. As per the human development index (HDI) set by the United Nations, nearly 4 kW per capita power consumption is required (Dale and Ong 2012). Developed countries reach this HDI by heavy usage of fossil energy; China, India, and other developing countries are approaching their increasing energy needs also with fossil fuels, whereas Brazil is the only exception, depending heavily on renewable energy. In the present scenario, fossil energy is the major source of energy (80 % of the world power consumption) in the form of oil (35.03 %), coal (24.54 %), and gas (20.44 %) (Goldemberg 2007). The use of fossil energy is considered as one of the most important man-made factor impacting on global economy and weather (Vertès et al. 2006). It is a widely accepted fact that fossil energy sources are finite and generally exported from politically unstable nations. Moreover, continuous huge demand of gasoline, low and expensive recovery yields, and oil spills are making the situation worse. The limited sources of fossil fuels in the world may not fulfill the increased demand for gasoline in the future. Already, experts have claimed that “peak-oil” (conferring the maximum rate of oil production) has arrived and the oil production rate after this point must decline (Kerr et al. 2011).
Keeping all the aforementioned points in view, the momentum is shifting toward the implementation of renewable energy sources. Biomass derived fuels have the potential to create a transition in the global economy from fossil fuel to a renewable fuel economy, however, it needs intensive technological and multidisciplinary efforts (Vertès et al. 2006; Yuan et al. 2008; Herrera 2006; Ohlrogge et al. 2009). Among the renewable energy sources (constituting around 13.61 %), biomass derived fuels, particularly “bioethanol” is gaining significant importance due to its inherent properties. Ethanol produced from cane juice (in Brazil) and corn starch (in USA) is already an established energy commodity. Brazil and USA are the two major countries that have successfully implemented bioethanol as an alternative energy source and have shown the signs of global energy commodity making ethanol fully competitive with gasoline and suitable for replication in other countries like India, China, etc. (Goldemberg 2007). Approximately >37.85 billion liters of ethanol (today this figure has more than doubled) is produced globally per year from corn, sugarcane, and sugar beet through fully mature and well-established processes (Rass-Hansen et al. 2007).
1.2 Brazil and Bioethanol
Sugarcane juice derived ethanol has replaced nearly 50 % of gasoline consumption in Brazil. Ethanol production and its implementation have achieved unprecedented success in Brazil as a fuel and gasoline additive. Sugarcane productivity and technical advancements led the ethanol production increase from 0.6 billion liters in 1975/1976 to 24 billion liter in 2012/2013 (Goldemberg 2013; Canilha et al. 2013).
In 2012/2013, it is expected that Brazilian sugar–alcohol mills will process more than 602 million tons of sugarcane, accounting for the production of roughly 39 million tons of sugar and 24 billion liters of ethanol. Experimentally, each ton of processed sugarcane generates approximately 270–280 kg of bagasse and 140 kg of straw (Canilha et al. 2013). Therefore, taking this value into account, it can be estimated that Brazilian mills will produce around 163–169 million tons of sugarcane bagasse and 84 million tons of straw in the 2012/2013 harvest (Canilha et al. 2013). In addition to sugarcane juice derived ethanol (first-generation), the exploration of lignocellulosic residues of sugarcane (bagasse and straw) also has a great potential for ethanol production (Chandel et al. 2012a; Dias et al. 2012a, b). In Brazil, tremendous research efforts are on the way to develop a robust technological setup for the cellulosic ethanol production at commercial scale. Sugarcane is a primary source of renewable energy in Brazil and can be considered as a model feedstock for bioethanol production. The net energy balance (ratio of energy contained in a given volume of ethanol divided by the fossil energy required for its production) for ethanol production from sugarcane is very high (8.2–10) compared with other feedstock sources such as corn (1.3), sugar beet, and wood (approximately 2) (Goldemberg 2008).
The Brazilian National Alcohol Program (PROÁLCOOL) was launched in 1974 to decrease gasoline consumption and thus reduce oil imports. Since then, it has gained significant success and today there is no more government subsidy to the producers (Goldemberg 2008). Nowadays, in the Brazilian automobile sector, more than 90 % of new cars are flex-fueled driven which can run on gasoline as well as on ethanol. Since 1976, ethanol saved 1.51 billion barrels of gasoline correspondingly saving 75 billion US$ (BNDES and CGEE 2008). The successful Brazilian ethanol program can be a learning curve for other countries. Table 1.1 shows the data on ethanol production in various countries and the projected demand per year of ethanol up to 2020/2022.
1.3 Critical Analysis of Technological Routes for Cellulosic Ethanol Production
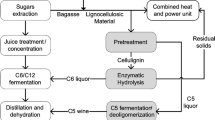
The production of bioethanol from lignocelullosic materials is assumed to present the largest potential among the possible alternatives to increase bioethanol production in the world without compromising food security, even though it is not yet a reality in an industrial scale (Kazi et al. 2010; Dias et al. 2012a). Lignocellulosic materials are abundant and cheap, do not compete with food crops (Ojeda et al. 2011; Dias et al. 2012a), and consequently, have larger potential to be used as feedstock for the production of sustainable biofuels (Dias et al. 2012a). Ethanol production from lignocellulosic biomass usually contains four major unit operations: (1) pretreatment, (2) enzymatic hydrolysis, (3) fermentation of sugars into ethanol, and (4) ethanol recovery.
The pretreatment is perhaps the single most crucial step as it has a large impact on all the other steps in the process (Galbe and Zacchi 2012). It is responsible for removing lignin or hemicellulose from the lignocellulosic material, and allows cellulose accessibility, enhancing the surface area of substrates for improved sugars recovery after enzymatic hydrolysis. An ideal pretreatment must meet the following requirements: minimum chemical requirement, low residence time, low investment cost, high amount of sugars recovery with less degradation of sugars or the ability to subsequently form sugars by hydrolysis, and minimum formation of inhibitory by-products (Kumar et al. 2009; Rocha et al. 2012). The pretreatment process can be categorized into four major categories: physical, physico-chemical, chemical, and biological. Each type of pretreatment has inherent specificity in terms of mechanistic application on cell wall components with the applied conditions. Physicochemical and chemical pretreatment is fast, effective, but nonspecific, and generates hemicellulosic derived inhibitors. Biological pretreatment methods are used for delignification but hampers by slow reaction rates and nonselectivity. The pretreated material needs to be submitted to enzymatic hydrolysis for the utmost sugars recovery (Agbor et al. 2011). The extent of enzymatic hydrolysis depends on lignin removal and the employed hydrolysis conditions. Hemicellulosic hydrolysates obtained after acid catalyzed reactions generally have cell wall derived inhibitors, i.e., furans, furfurals, phenolics, weak acids, among others. These inhibitors affect the efficiency of microorganisms employed in fermentation process leading to poor ethanol yields. It is essential to eliminate these inhibitors prior to microbial fermentation in order to obtain the desired ethanol yields. Several detoxification methods like evaporation, calcium hydroxide overliming, use of membranes, ion-exchange resins, activated charcoal, and enzymatic detoxification using laccases are in practice to overcome these inhibitors (Chandel et al. 2013a). Simultaneous detoxification of hydolysates and fermentation (SDF) is also possible for ethanol production using two different microorganisms (microorganism eliminating inhibitors + microorganism for ethanol production).
There are four configuration processes to produce ethanol from lignocellulosic biomass: separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF), simultaneous saccharification and co-fermentation (SSCF), and consolidated bioprocessing (CBP) (van Zyl et al. 2011). The most common process is separate (or sequential) hydrolysis and fermentation (SHF), where hydrolysis of pretreated lignocellulosic material is done first and the resultant sugar solution is fermented separately into ethanol in different vessel/reactor. SHF is a lengthy process but has shown optimum sugars production followed by their conversion reaching the desired ethanol yields. Both the processes can be carried out employing the most appropriate conditions. After enzymatic hydrolysis, the solid material can be used for cogeneration of heat and electricity. SSF (simultaneous saccharification and fermentation) or SSCF (simultaneous saccharification and co-fermentation) are other configuration processes where the enzymatic hydrolysis of pretreated lignocellulosic material and the fermentation of released sugars or mixture of sugars (pentose + hexose) is carried out simultaneously. SSF/SSCF has shown great advantages over SHF in the terms of reducing processing time and process complexities. However, the temperature difference in both the reactions is of concern (Olofsson et al. 2008). Enzymatic hydrolysis usually shows the best results at 50 °C, while ethanol fermentation is done at 30 °C. Therefore, pre-hydrolysis at 50 °C can be inducted to initiate the hydrolysis for some time followed by the execution of fermentation reaction. To obtain the maximum ethanol yield, thermotolerant yeast or ethanol producers could be more relevant as they can grow and produce ethanol at the hydrolysis temperature (<50 °C). Furthermore, risk of contamination can be avoided using thermo tolerant microorganism in SSF. Another advantage of SSF/SSCF configuration is to avoid the enzyme inhibition by the released glucose as it is simultaneously converted into ethanol by the microorganism. Moreover, capital cost investment and the processing time could be minimized by employing SSF/SSCF (Olofsson et al. 2008). The latest development in process configuration is CBP (consolidated bioprocessing), wherein the enzyme production and hydrolysis of pretreated lignocellulosic material followed by the fermentation of sugars can be performed in a single reactor (Olson et al. 2012). The key difference between CBP and the other strategies of biomass processing is that only one microbial community is employed both for the production of cellulases and fermentation (Cardona and Sánchez 2007).
Realizing the importance of process integration, IBP (integrated bioprocessing) could provide an important breakthrough in developing an economic and sustainable platform for cellulosic ethanol production. IBP includes the microbial assisted pretreatment of biomass followed by enzyme recovery and delignified biomass saccharification coupled with microbial conversion of released sugars into ethanol simultaneously in a single reactor (Chandel et al. 2013b). Nevertheless, ethanol production through IBP has not been tried as yet. In IBP, there is involvement of at least more than one microorganism (one microorganism for biodelignification and another microorganism for ethanol production). The recovered sugars solution from pretreated lignocellulosic biomass can be used for ethanol production via modified fermentation strategies like fed-batch, recycling of immobilized cells in continuous fermentation, and semi-continuous processing. Figure 1.1 shows an overview of the technical configurations required in each processing routes for 2G ethanol production.
Summary of technical routes for ethanol production from biomass under various process configurations. Each box represents the specific reaction performed in sequential order. SHF separate hydrolysis and fermentation, SSF simultaneous saccharification and fermentation, SSCF simultaneous saccharification and co-fermentation, CBP consolidated bioprocessing, IBP integrated bioprocessing
1.4 Process Simulation and Co-products Utilization
Process simulation using computational tools have been increasingly used to evaluate biorefinery configurations, since it allows the integration of process steps, technologies, and routes, thus providing critical information to assess technical feasibility, detecting process bottlenecks and potential advantages. In this context, CTBE (Brazilian Bioethanol Science and Technology Laboratory), one of the national laboratories of the Brazilian Center of Research in Energy and Materials (CNPEM) developed a comprehensive tool—VSB (Virtual Sugarcane Biorefinery)—based on simulation platforms for the evaluation of different technologies through assessment of their sustainability indicators (economical, environmental, and social) (CTBE 2012).
The integration of 1G (from sugarcane juice) and 2G (from bagasse and straw) was assessed using the VSB. The main process configurations and parameters adopted in the construction of the VSB are described in the following sections.
1.4.1 Process Simulation for 1G Ethanol Production
According to BNDES and CGEE (2008), approximately 70 % of the sugarcane processing units in Brazil are annexed plants. First-generation ethanol production from sugarcane takes place in autonomous distilleries or annexed plants. Autonomous distilleries produce only ethanol. In an annexed plant, a fraction of the sugarcane juice is diverted for sugar production and the remaining fraction along with the molasses (residual solution of sugars that came from sucrose crystallization) is used for ethanol production. Usually, the annexed plant operates using half of sugarcane juice for sugar production. Part of the reason for the success of ethanol production in Brazil is the flexibility of annexed plants to produce more ethanol or more sugar, depending on market demands.
The sugarcane processing facility is self-sufficient on its energy consumption: all the thermal and electric energy required for the production process is produced in combined heat and power generation (CHP) systems using bagasse as a fuel. If sugarcane straw is recovered from the field, it may also be used as a fuel to increase energy generation. A scheme of the sugar, ethanol, and electricity production process from sugarcane is shown in Fig. 1.2. In an autonomous distillery, the unit operations related to the sugar production (left side of Fig. 1.2) is not included in the sugarcane mill.
Block-flow diagram of the production of sugar, ethanol, and electricity from sugarcane (CTBE 2012)
1.4.1.1 Process Description and Governing Parameters of the Sugarcane Processing Facility
The basic configuration of an annexed plant (1G) and the related process parameters for ethanol and sugar production have been summarized in this section. The capacity of the sugarcane processing facility has been considered for the processing of 500 metric tons of sugarcane (TC) per hour, during 167 days/year and processing a total of 2 million TC/year.
1.4.1.1.1 Sugarcane Reception and Cleaning
The sugarcane arrives at mills with dirt and other impurities dragged in the harvesting process. Therefore, upon reception in the factory, sugarcane must be cleaned. The efficiency of dirt removal in sugarcane washing is 90 % (BNDES and CGEE 2008). Sugarcane cleaning is usually carried out using wash water, which is recycled to the cleaning process after removal of dirt and other impurities.
The amount of sugar lost during the whole sugarcane washing may be calculated as 25 % of the losses for the mechanically harvested sugarcane washing (3.2 kg/TC) as observed by Rein (2007). However, usually no washing is carried out for mechanically harvested (chopped) sugarcane due to the high sugar losses that would occur. The same authors consider that the average amount of water dragged with sugarcane during washing is 7.5 t/100 TC.
1.4.1.1.2 Sugarcane Processing and Juice Extraction
After cleaning, sugarcane is fed to the cane preparation system, on which a series of equipment (shredder, hammers, etc.) are used to cut open the sugarcane structure and enhance sugar extraction in the following operation. After preparation, sugarcane passes over a magnet that removes eventual metallic particles dragged along prior to entering the mills.
Juice extraction is usually done using crushing mills, where sugarcane juice and bagasse are separated. Water at a rate of 28 wt% of the sugarcane flow (imbibition water) is used to improve sugars recovery. The imbibition water temperature is 50 °C (Ensinas 2008) and the efficiency of sugar extraction in the mills is 96 % (Walter et al. 2008). Sugarcane juice contains water, sucrose, and reducing sugars, in addition to impurities such as minerals, salts, organic acids, dirt, and fiber particles, which must be removed prior to fermentation. A rotary screen is used to remove solid particles from the juice. The fibers obtained in this screen return to the mills for further recovery of sugars, while the juice is sent to juice treatment. Efficiency of dirt and bagasse removal in the screen is around 65 % (Mantelatto 2010).
1.4.1.1.3 Juice Treatment
The aim of the juice treatment process is to separate as much as possible the dissolved and suspended juice impurities without reducing sucrose concentration. It must be done soon after milling to prevent yeasts and enzymes action. Thus, following extraction, the juice undergoes chemical treatment to remove other impurities. This process consists of juice heating from 30 to 70 °C, addition of phosphoric acid or lime followed by a second heating operation, up to 105 °C (Copersucar 1987). Hot juice is flashed to remove dissolved air and after addition of a flocculant polymer, impurities are removed in a settler, where mud and clarified juice are obtained. A filter is used to recover some of the sugars carried along with the mud, and the separated solids are recycled to the process prior to the second heating operation. Bagasse fines, also called bagacillo, and wash water are used in the filter to improve recovery of sugars. The clarified juice is fed to the screens to remove solid particles that were not removed in the clarifier. The clarified juice, at 98 °C, destined for sugar production, contains around 15 wt% solids (Mantelatto 2010) and it is concentrated on a five-stage multiple effect evaporator (MEE) up to 65 wt% solids. In the annexed distillery, a fraction of the syrup, as well as final molasses, are used to concentrate the clarified juice destined for ethanol production up to around 22 wt% solids, which is cooled and fed into the fermenters.
1.4.1.1.4 Sugar Production
The sucrose present in the syrup as sugar crystals is separated from the solution in equipment called vacuum pans and crystallizers, usually operated under vacuum and in fed-batch mode. The syrup is fed into the vacuum pans, where water is removed in a similar way as in the evaporators. The mixture of sugar crystals and molasses (liquid part) inside the equipment is called massecuite. When the amount of material reaches the limit of the vacuum pan (at the end of a batch), the massecuite is transferred to crystallizers and, after an appropriate residence time, it is sent to centrifuges that separate the crystals and the molasses. It is possible to exhaust more the molasses (recuperating more sugar) by repeating the process one or two more times. The washing water temperature (at centrifuges) is 110 °C (Mantelatto 2010).
It is assumed that crystals are separated using the two-boiling system approach, where two types of sugars are produced (Jesus 2004): grade “A” sugar (final product) and grade “B” sugar (intermediate sugar that is produced and recycled inside the process as “B” Magma, a solid–liquid stream rich in sugar crystals). The Brix of the “A” sugar is 99° (Ribeiro 2003) and purity (VVHP—very very high polarization) 99.6 % (Bazico 2010). For the “B” sugar, Brix is 98° (Ribeiro 2003) and purity 88 % (Camargo 1990). The final sugar is dried in a rotary dryer at 100 °C (Camargo 1990) and cooled before shipment.
1.4.1.1.5 Fermentation for Ethanol Production
After the juice treatment, concentrated juice is mixed with molasses and sent for ethanol production in the fermenters. A fed-batch fermentation process with cell recycle is assumed. The temperature of fermentation is 33 °C and conversion of sugars into ethanol is about 89.5 % (Mantelatto 2010), which is slightly lower than the conversion in an autonomous distillery, due to the presence of molasses from sugar production. In this process, yeast cells solution is fed to the fermenters prior to sugarcane juice addition. During fermentation, gases released in the fermenters are collected and sent to an absorption column where the entrained ethanol is recovered using water. After the completion of fermentation reaction, the wine is sent to the centrifuges, where cells are separated from the ethanol solution. Cells obtained in the centrifuges are treated in a separate reactor by the addition of sulphuric acid and water, to decrease bacterial contamination. After this treatment, the cells are recycled to be used in another batch. Some part of the yeast cream, also known as alcohol distillery yeast extract, is removed before being recycled. This product is used mostly as protein source for animal feed. The produced wine is mixed with the alcoholic solution obtained in the absorption process (to recover ethanol from the CO2 stream) and sent for purification. Ethanol content in the wine fed to the distillation columns is 8.5°GL (Mantelatto 2010).
1.4.1.1.6 Distillation
The distillation aims at concentrating the wine until alcoholic content is up near the azeotropic point for the hydrated ethanol production, with ethanol content between 92.6 and 93.8 wt% (92.6 and INPM 93.8°) (Dias 2008). Wine is sent to a series of distillation and rectification columns (Fig. 1.3). Distillation columns comprise two set of columns A, A1 and D, and rectification columns B1 and B, each located one above the other. Wine is preheated in the condenser of column B (heat exchanger E) and by exchanging heat with the bottom of column A (heat exchanger K) before being fed into the top of column A1. Ethanol-rich streams (phlegm) are obtained on top of column A and at the bottom of column D, and then fed to column B-B1. Vinasse is produced at the bottom of column A, containing less than 200 ppm of ethanol, while second grade ethanol is obtained from the top of column D. Hydrated ethanol is produced on top of column B and nearly pure water (phlegmasse) is obtained at the bottom of column B1. Fusel alcohol, containing most of the higher alcohols, is obtained as a side withdrawal in column B.
Simplified scheme of the distillation columns (CTBE 2012)
1.4.1.1.7 Dehydration
The hydrated ethanol must be dehydrated to achieve alcohol content over 99.3 % (mass) to be blended with gasoline. The ethanol dehydration cannot be made by conventional distillation due to the azeotropic nature of ethanol solution (95.6 % mass) at atmospheric pressure. Thus, alternative methods of separation must be used to produce anhydrous ethanol (Dias 2008). The dehydration process for anhydrous ethanol production can be carried out considering azeotropic distillation with cyclohexane or adsorption on molecular sieves. The adsorption on molecular sieves is a separation process with reduced energy consumption and without solvent if compared to the azeotropic distillation. In this process, a zeolite bed is used to adsorb water from hydrated ethanol to produce anhydrous ethanol. Usually three beds are used, one of which is always in regeneration, to remove accumulated water.
1.4.1.1.8 Combined Heat and Power Generation
Traditionally, cogeneration systems (CHP—combined heat and power generation) used in Brazilian sugarcane mills are based on the Rankine cycle (Fig. 1.4). During sugarcane processing, the juice is separated from the fibers, which produces large amounts of bagasse (approximately 140 kg/TC, dry basis). This bagasse is used as a fuel in the cogeneration system to supply thermal, mechanical, and electrical demand for sugar and ethanol production process. Formerly, low efficient boilers (22 kg f/cm2) were used to produce steam and electricity for the plant. However, the restructuring of the electricity sector in Brazil and the incentives for energy production from renewable sources have driven to an increase in investments for the production of surplus electricity in the mills. As a result, more efficient boilers and turbines have been employed in order to produce high pressure steams (65 kg f/cm2), and generate large amounts of electricity. As a consequence, new sugarcane mill projects considering the use of Rankine cycles with steam at higher levels of temperature and pressure, employing extraction-condensing steam turbines and burning all bagasse produced in the mills have been developed. The surplus electricity generated in this new configuration can be sold to the power grid, improving the revenues of the company.
Scheme of back-pressure and extraction-condensing turbines based on Rankine cycle (CTBE 2012)
The amount of electricity produced by the sugarcane processing plant may be increased significantly when sugarcane straw is collected from the field and transported to the factory for further processing. Around 140 kg of straw (dry basis) is produced per ton of sugarcane stalks, but part of the straw is usually left in the field in order to provide for weed and disease control as well as nutrient recycling (Hassuani et al. 2005). However, removal of 50 % of the straw from the fields is considered feasible (Dias et al. 2009; Hassuani et al. 2005; Walter and Ensinas 2010).
Besides being used as a fuel in boilers for the production of steam and electricity, sugarcane lignocellulosic material (bagasse and straw) may also be used as feedstock for second-generation ethanol production. Since it is composed basically of cellulose, hemicellulose, and lignin, it may be converted into fermentable sugars (hexoses and pentoses) through pretreatment and hydrolysis processes. Nevertheless, the amount of surplus lignocellulosic material used as feedstock depends on the energy consumption of the whole production process. In this way, reduction on process steam demand may lead to an increase in the amount of surplus bagasse and straw, which can be employed as feedstock for second-generation ethanol production when lignocellulosic material hydrolysis technologies are available. The residues of the pretreatment and hydrolysis operations (residual cellulose, lignin, and eventually biogas from pentoses biodigestion) may be used as fuels increasing the amount of lignocellulosic material available as feedstock for 2G (Dias et al. 2011, 2012a, b).
Different cogeneration systems were simulated in VSB to represent the integrated 1G and 2G ethanol production processes (Fig. 1.5). The considered alternatives for cogeneration systems as well as the main parameters adopted in the computer simulations are shown in Table 1.2.
Block-flow diagram of the integrated first- and second-generation ethanol production process from sugarcane (CTBE 2012)
1.4.2 Process Simulation for 2G Ethanol Production
Currently, one of the greatest concerns worldwide is the large-scale production of alternative forms of energy, such as biofuels, which could reduce greenhouse gases emissions and improve energy security when compared to their fossil counterparts (Chavez-Rodriguez and Nebra 2010). In this context, bioethanol has received special attention, as it is already produced in large scale and used as automotive fuel (Seabra et al. 2010).
In first-generation plants, sugarcane juice is used for sugar and ethanol production, while sugarcane bagasse is used as a fuel in the boilers, providing heat and power to the plant. However, in the context of expansion of the production and consumption of ethanol, bagasse is considered as a potential feedstock for 2G ethanol, since it does not compete with food crops and is less expensive than conventional agricultural feedstocks (Alvira et al. 2010). In this case, 2G ethanol production processes can be integrated with 1G ethanol plants, sharing part of the infrastructure such as juice concentration, fermentation, distillation, cogeneration, and water cooling systems. Another important residue that may be employed for bioethanol production in the sugarcane industry is sugarcane straw, which includes sugarcane leaves and tops, usually burnt or left in the field (Dias et al. 2011; Macrelli et al. 2012).
For integration of 2G ethanol process with 1G, biomass pretreatment and hydrolysis are usually considered in the processing of lignocellulosic material, since it does not contain monosaccharaides readily available to be fermented. In some cases, fermentation of the pentoses released during the pretreatment step to ethanol can also be carried out; however, conventional microorganisms employed in alcoholic fermentation are not able to ferment pentoses.
Preliminary assessments were carried out using VSB considering two levels of development: current technology—hydrolysis with low yield and low solids loading and biodigestion of C5 liquor—and a second level, potentially available in 2020 (futuristic scenario)—hydrolysis with higher yield and solids loading, C5 fermentation into ethanol, and lower investment and enzyme cost. Process alternatives are shown in Fig. 1.5. Operational conditions and yields are described in subsequent sections.
Due to the high recalcitrance of biomass, pretreatment process is required to increase the accessibility of cellulolytic enzymes toward cellulose (Alvira et al. 2010). Although different types of pretreatments were tested in different conditions over the years, advances are still needed for overall costs to become competitive (Rabelo et al. 2011; Chandel et al. 2010a). In VSB, steam explosion is defined as the pretreatment process where most of the hemicellulose is hydrolyzed into pentoses, with small cellulose loss and no lignin solubilization (Ojeda et al. 2011). The pretreated solids are separated from the pentoses liquor via filtration. In order to allow an increase in hydrolysis yield for the futuristic scenario, an alkaline delignification step of the solid fraction was included after pretreatment, so most of the lignin is removed, decreasing its inhibitory effects on the following enzymatic hydrolysis step (Rocha et al. 2012). Table 1.3 presents main operational conditions and yields for steam explosion pretreatment and alkaline delignification. Cellulose obtained from pretreatment is converted into glucose after saccharification using cellulolytic enzymes. Enzymatic hydrolysis allows the process to be carried out in milder conditions than acid hydrolysis. In addition, enzymes offer the advantage of producing higher yields of sugars with little degradation (Mussatto et al. 2010; Chandel et al. 2012b). Tables 1.4 and 1.5 show the enzymatic hydrolysis operating conditions and sugars yields.
Saccharomyces cerevisiae is one of the traditionally used microorganisms in 1G ethanol production from corn and sugarcane due to its high efficiency in fermenting hexose to ethanol, and superior tolerance to low pH (Zhang et al. 2010) and high ethanol concentration. However, for use in the 2G ethanol production process, microorganisms that can convert C5 sugars into ethanol are limited and generally have low ethanol and inhibitors tolerance and take longer incubation times (Girio et al. 2010). In order to increase sugar yields, efficient conversion and utilization of hemicellulosic sugars has become an important task and an opportunity to reduce ethanol production cost (Alvira et al. 2010).
Alternatively, C5 liquor may be biodigested, producing biogas for use as fuel, increasing the amount of surplus lignocellulosic material. Pentoses biodigestion and fermentation parameters are shown in Table 1.5. Other applications of pentoses include production of xylitol, lactic acid, 2, 3-butanediol, butanol, furfurals, and other valuable products (Chandel et al. 2010b; Girio et al. 2010). However, fermentative production of D-xylitol from hemicellulosic hydrolysate has been considered one of the most beneficial processes to cater to the needs of various commercial sectors (Silva and Chandel 2012) (Table 1.6).
1.4.3 Screening Method Applied to Analysis of Technological Parameters in 1G Ethanol Production Process
Mathematical models are useful tools for development, analysis, and optimization of industrial processes. Models can be defined as a dataset and abstract ideas used to explain a phenomenon of interest and relate the parameters of a given process. A well-adjusted model can predict the parameters behavior so precisely that it becomes a practical and cheap way to obtain information about the process under study. Therefore, if the model is improved, it also improves the description of the reality.
Incorporating mathematical models into computational simulation platforms is not frequently applied to sugarcane-based biorefineries due to its complexity, specificity, variability, interaction with environment, and other inherent characteristics.
In VSB, the simulation for 1G ethanol production is described by variables such as fermentation yield, steam consumption, steam pressure in boiler, among others. The variable values used in this simulation were collected initially from the literature or provided by specialists. In addition, this information was complemented and validated with data from Brazilian sugar and ethanol mills. However, inspite of intense efforts in collecting variable values, this process is a difficult task in the modeling procedure of 1G ethanol production due to its complexity and natural variability.
In this context, screening methods are presented as useful tools to quantify the impact of inputs variations on a given model response (Ruano et al. 2012). Therefore, if a small change in an input variable leads to a large variation in a certain response parameter of the model, this variable is considered important and its determination must be as precise as possible (Cangussu et al. 2003). Assuming that only some input variables contribute significantly to the outcome, screening methods facilitate data collection by limiting the maximal precision to inputs considered most important (Rivera et al. 2013).
Besides being used to obtain information about the degree of importance of each variable, screening methods are frequently used to validate the model itself. This validation reports whether the model follows (or not) an expected behavior. In the simulation for 1G ethanol production in VSB, after screening procedure the ranking of technological parameters can be analyzed to assess if the results agree with what is expected for the current ethanol production in Brazil. Therefore, specialized information (practical knowledge) from professionals is extremely important for analysis and improvements of this model. As a result, screening methods are also mechanisms to detect and adjust model inadequacies (Cangussu et al. 2003). Screening procedure can be performed through design of experiments (DOE) such as central composite design (CCD) (Montgomery 2001) and simulation models.
A study performed in the VSB context illustrates the efficiency and usefulness of CCD as a method to screen the main variables in 1G ethanol production process. Initially, the main input variables of the process were identified as: (i) fermentation yield, (ii) steam consumption, (iii) steam pressure in boiler, (iv) juice extraction yield, (v) residual ethanol concentration in vinasse, and (vi) alcohol content in wine. The influence of these variables on ethanol output and surplus electricity has been studied in the screening procedure. The interpretation of the results was accomplished from the analysis of the model features and the expected behavior of the input variables, bearing in mind the knowledge of the current 1G ethanol production. Therefore, the analysis involved the collaboration among the specialists in the sugarcane sector and CTBE research team.
Variables under study were ranked by CCD with a significance level of 99 %. For Ethanol Output, the input variables juice extraction yield and fermentation yield were shown to be significant. An efficient juice extraction means that a large amount of sugar will be available to the fermentation process without increasing the amount of milled cane. The variable fermentation yield is of significance as it influences directly on the amount of ethanol produced and also in the main dependent variables such as, fermentation time, volume of fermenter, among others.
The variables steam consumption reduction, resulting from energy integration in the production process, and steam pressure in boiler were significant for the surplus electricity parameter. Decreasing the consumption of steam there will be more steam available for the cogeneration process; therefore, more electricity will be produced. The boiler steam pressure is directly related to the electricity cogeneration. More electricity will be produced by the plant with a higher boiler pressure.
The screening procedure was successfully used to identify the relevant variables in 1G ethanol production process. In this procedure, the CCD proved particularly efficient to obtain information about the significance of the input variables. Thus, it was concluded that through screening methods it is possible to understand the behavior of the technological parameters and compare it with the current process.
1.5 Techno-Economic Analysis of Sugarcane-Based Biorefineries
In order to provide a comparison in terms of economic viability, important indicators from Economy Engineering, such as internal rate of return (IRR), production costs of products, beyond others, can be estimated considering a set of scenarios related to different biorefinery alternatives. The principles for the evaluation of economic viability are based on a cash flow projected for each technological scenario to be evaluated, taking into account the investment needed for the project and all expenses and revenues for an expected project lifetime. The main operating expenses (OPEX) and revenues might come from mass and energy balances obtained from computer process simulation. The basis for the monetary values related to the capital expenses (CAPEX) can be obtained from the literature, consulting with engineering companies, experts, and others.
Several studies were carried out at CTBE following the techno-economic and environmental aspects of first- and/or second-generation ethanol production from sugarcane (Dias et al. 2012a, b, 2013a, b; Cavalett et al. 2012; Junqueira et al. 2012). In this section are summarized the most important findings from the previous studies carried out at CTBE related to the techno-economic analysis and environmental impacts of 1G ethanol and 2G ethanol productions from various biorefinery configurations.
1.5.1 1G Ethanol Production Process
Environmental and economic aspects of autonomous distilleries and annexed plants in Brazil were compared by Cavalett et al. (2012). In addition, optimized technologies for autonomous distilleries and annexed plants were considered in the study and benefits of more flexible scenarios for annexed plants were examined. In such configurations, CAPEX proved to be an important issue, since it increases from autonomous to annexed plants and from fixed to flexible plant, having significant impact on the IRR. Another important observation was that annexed plants present higher IRR for both flexible (favoring sugar production) and fixed plants. Although autonomous distillery also presented good results, it is important to take into account that market oscillations can considerably change and flexibility may be decisive for maintaining and even improving the sugarcane biorefinery profitability.
1.5.2 Integrated and Stand-Alone 2G Ethanol Production Processes
Dias et al. (2012c) evaluated different scenarios for integrated and stand-alone 2G ethanol production from sugarcane bagasse and straw. Five scenarios were selected to demonstrate the economic and environmental impacts of 2G ethanol production in comparison to an optimized autonomous 1G ethanol production plant in Brazil. Results showed that the current integrated 1G and 2G ethanol production scenario, characterized by higher investment cost in 2G (due to the fact it will be one of the first plants), higher enzyme cost, lower yield, and lower solids load in the hydrolysis step presents lower IRR in comparison to the optimized 1G ethanol production. However, the integrated 1G and 2G ethanol production considering future scenarios, where target parameters are used for second-generation processes and ethanol can be also produced from C5 sugars, is more attractive to the investor than the optimized 1G ethanol production.
1.5.3 Different Process Configurations for 2G Ethanol Production Process
Junqueira et al. (2012) assessed economic and environmental impacts of different options for the 2G ethanol production process integrated to the 1G sugarcane biorefinery. The study evaluated two pretreatment options (steam explosion and hydrothermal processing), as well as two alternatives for pentose utilization (biodigestion and fermentation to ethanol). A delignification step was also analyzed after both pretreatments. Results showed that hydrothermal pretreatment based on liquid hot water has higher energy consumption than steam explosion; consequently, larger ethanol production is obtained from steam explosion pretreated bagasse. These results are in accordance with economic and environmental analyses, which shows that the process with steam explosion presents the largest IRR and lower life cycle environmental impacts.
Further, Dias et al. (2012b) evaluated different configurations for the 2G ethanol production process (e.g., pretreatment with steam explosion coupled or not with delignification, pentose biodigestion or fermentation to ethanol, solids loading for hydrolysis) in an integrated 1G and 2G ethanol production biorefinery. The results were used to evaluate which process alternatives provide higher ethanol yield, pointing toward the direction in which research should be oriented. Computer simulations of integrated 1G and 2G ethanol production process from sugarcane showed that high hydrolysis yields (that may be achieved using low solids loading on the hydrolysis reactor) do not lead to the best results in terms of overall ethanol production. Because the lignocellulosic material (sugarcane bagasse and straw) used as feedstock in 2G is also used as fuel, low solids loading requires more steam on the concentration step. Therefore, even though that scenario leads to the highest 2G ethanol production per lignocellulosic material processed (around 200 and 400 L/t dry lignocellulosic material (LM) for the processes with pentose biodigestion and fermentation, respectively), lower yields and higher solids loading lead to larger amounts of ethanol produced per ton of sugarcane (up to 122 L/TC for 20 % solids, as opposed to 116 L/TC for the process with pentose fermentation and 5 % solids loading in hydrolysis). This study confirmed the importance of evaluating the whole process to better understand it and to guide further experiments aiming at the viability of 2G ethanol production process.
Dias et al. (2013a) evaluated different cogeneration systems configurations for integrated 1G and 2G ethanol production, as well as different destinations for the pentose (biodigestion or fermentation to ethanol) obtained after pretreatment of the LM. Economic analyses showed that coupling electricity production with 2G ethanol production in the integrated process improves its economic results, even when electricity is produced in relatively low amounts using low efficiency boilers (22 bar boilers). Another interesting finding of the study is that high pressure boilers (82 bar) consume more bagasse than low pressure boilers, thus decreasing final ethanol output. Nevertheless, revenues obtained with the sale of electricity in the processes employing cogeneration systems with high pressure boilers outweigh the losses in ethanol yield and the increased investment of these cogeneration systems. Among the evaluated process configurations, the one with 65 bar boilers presents the lowest environmental impacts in most categories including global warming potential. In the context of C5 use, pentose fermentation allows a large increase in the total ethanol production (40–50 % higher than 1G production) compared to the gains of pentose biodigestion (around 30 %).
1.5.4 Improving 2G Ethanol Production Through Optimization of 1G Plant
Dias et al. (2012a) evaluated some improvements in the 1G ethanol production process, aiming at reducing process steam consumption; maximizing surplus LM; or increasing electricity output. The process improvements analyzed in the study decreased the steam consumption in the 1G ethanol production in an autonomous distillery. Results showed that a considerable increase in the amount of surplus LM can be obtained with the use of efficient cogeneration systems (among other process improvements) and the recovery of 50 % of the straw. Significant increase in ethanol production in optimized autonomous sugarcane distilleries integrated with 2G ethanol production, along with electricity production, can be obtained if high pressure boilers are employed. Gains on ethanol production in an integrated 1G and 2G ethanol production process are possible when efficient, low pressure boilers are employed.
1.5.5 Flexibility on 2G Ethanol and Electricity Production
Dias et al. (2013b) evaluated a flexible biorefinery with the ability of diverting a fraction of the lignocellulosic material (sugarcane bagasse and straw) either for electricity production or as feedstock in 2G ethanol production. The flexible sugarcane biorefinery selling surplus electricity in the spot market when prices are favorable presented better economic returns than the conventional biorefinery using all surplus lignocellulosic material as feedstock for 2G ethanol production. The flexible biorefinery and the plant with maximum ethanol production lead to the highest cut-off in carbon dioxide emissions. However, biorefineries producing more ethanol present higher environmental impacts per unit of ethanol produced than the configuration with maximum electricity production due to the high impacts of chemicals used in the 2G process. The study concluded that even though flexible biorefinery has a high IRR, changes in ethanol prices affect the IRR more significantly compared with increases in electricity spot market prices. Thus, if ethanol prices increase, the fixed biorefinery operating with maximum ethanol production will be more advantageous in economic terms.
1.6 Life Cycle Assessment of Sugarcane-Based Biorefineries
Life cycle assessment (LCA) is a recognized method for determining the environmental impact of a product (good or service) during its entire life cycle, from extraction of raw materials through manufacturing, logistics, use, and final disposal or recycling. In LCA, substantially broader environmental aspects can be covered, ranging from GHG emissions and fossil resource depletion to acidification, toxicity, water, and land-use aspects; hence it is an appropriate tool for quantifying environmental impacts of a product system. The ISO 14040 series provides a technically rigorous framework for carrying out LCAs (ISO 2006a, b). The method consists of four main steps: goal and scope definition, inventory analysis, impact assessment, and interpretation. First, the goal and scope provides the context for the assessment and explains to whom and how the results are to be communicated. This step includes detailing of technical information—such as defining the functional unit, system boundaries, assumptions and limitations of the study, impact categories, and methods used to allocate environmental burdens in cases where there is more than one product or function.
Life cycle inventory (LCI) is the methodological step where an overview is given of the environmental interventions (energy use, resource extraction, or emission to an environmental compartment) caused by or required for processes within the boundaries of the studied system. With its translation of the product system’s environmental flows from the life cycle inventory phase (LCI) into scores that represent their impacts on environment, life cycle impact assessment (LCIA) is essential for the interpretation of the results in relation to the questions posed in the goal definition (Finnveden et al. 2009). The challenge of LCIA is to evaluate the potential impact of the emitted substances by using a procedure that is ideally simple, applicable consistently to all substances, uses a common unit of measure, and gives results that are comparable between impact categories.
A life cycle interpretation is necessary for identifying, quantifying, checking, and evaluating information from the results of the LCI and/or the LCIA. This interpretation should also raise significant environmental issues, including an evaluation of the study considering completeness, sensitivity, and consistency checks; and limitations.
Regarding the possibilities of using different LCIA methods, Cavalett et al. (2013) used seven different LCIA methods for a comparative assessment of ethanol and gasoline in Brazil. The study provided an updated and comprehensive LCI for sugarcane ethanol in Brazil considering the stages of agricultural production, transport, ethanol production, and its final use. Results showed that the use of different LCIA methods can lead to different comparative environmental impacts of ethanol and gasoline, mainly when single-score indicators are applied. A relative convergence in the results of equivalent environmental impact categories using different midpoint LCIA methods was observed. Results of the comparison of the five midpoint LCIA methods showed that ethanol presents better environmental performance than gasoline in important categories such as global warming, fossil depletion, eco-toxicities, and ozone layer depletion but worse environmental performance than gasoline in the categories acidification, eutrophication, photochemical oxidation, and agricultural land use.
Calculated environmental impacts using the LCA methodology presented by Cavalett et al. (2012) indicate that sugarcane biorefinery optimization technologies for 1G ethanol production have a great potential for a significant decrease of the environmental impacts in sugarcane biorefineries (for both autonomous and annexed plants). Ethanol production in annexed plants presented slightly lower environmental impacts in comparison to autonomous distilleries mainly due to allocation rules used in the study. Results also showed that flexibility in annexed plants produce little effect on the environmental impacts when the entire ethanol production chain is considered.
Dias et al. (2012c) observed that a current integrated ethanol production plant (1G and 2G) has potential to decrease the environmental impacts in relation to 1G ethanol production process. The study identified that the use of high amount of sodium hydroxide in the alkaline delignification step has strong influence in the increase of the 2G ethanol environmental impacts. Junqueira et al. (2012) also found that alkaline delignification contributed to higher environmental impacts in the 2G ethanol production process. Also, pentose fermentation should be emphasized in experimental studies instead of biodigestion to produce biogas, since fermentation to ethanol leads to the best technical, economic, and environmental results in the 2G ethanol production process.
Galdos et al. (2013) showed the importance of including black carbon emissions for the calculation of global warming and human health environmental indicators. The results quantitatively demonstrated that the technological trends considering past, present, and future scenarios for ethanol production in Brazil is showing lower environmental impacts. Avoiding the preharvesting burning of LM will decrease the emissions of black carbon and greenhouse gases in the sugarcane production phase. In addition, increase in yield of sugarcane per hectare, and of ethanol per ton of sugarcane, will eventually decrease the environmental impacts per unit of biofuel produced. Results showed that 2G ethanol production plays a key role in increasing the amount of ethanol produced per unit of area. Results also indicated that the Brazilian sugarcane sector presents a trend of using more efficiently the resources per unit of ethanol produced, as well as promoting good management practices that reduce its environmental impacts.
1.7 Conclusions and Future Recommendations
Among the renewable energy sources, use of ethanol as transportation fuel has achieved significant success in countries like Brazil and USA. This review shows the potential of computer-aided process modeling and simulation, life cycle assessment, processing technological routes for biochemical ethanol production (1G + 2G) from sugarcane juice, and lignocellulosic residues of sugarcane.
Brazil is the largest sugarcane producer (625 million tons of sugarcane in 2011) in the world showing the tremendous potential of sugarcane ethanol as sustainable energy source governing the economic, strategic policy, and environmental impacts on the nation. Today, in Brazil, 44 % of energy matrix used is renewable, and 13.5 % of renewable energy is derived only from sugarcane. In the context of the Virtual Sugarcane Biorefinery, from CTBE has extensively worked toward developing rigorous process simulation with the help of in-house derived experimental database to perform mass and energy balances, which is helpful for robust scale-up, and allows a better understanding of economic and environmental impacts. Evaluating several scenarios for 1G and 2 G ethanol production (stand-alone and integrated plants) in the dynamic context of biorefineries using sugarcane as a main energy driver, it was concluded that integrating 2G to 1G ethanol production improves its economic results. Moreover, in the context of C5 use, pentoses fermentation allows a large increase in the total ethanol production (40–50 % higher than 1G production) compared to the gains of pentoses biodigestion (around 30 %). Another interesting finding of the study is that high pressure boilers (82 bar) consume more bagasse than low pressure boilers, thus final ethanol output is small if very high pressure boilers are used. Furthermore, 2G ethanol may favorably compete with bioelectricity production when sugarcane straw is used in addition to the application of improved technologies using low cost enzymes for biomass hydrolysis. In regard to determining environmental impacts by LCA methodology, optimized cellulosic ethanol production technologies could have a great potential for significant decrease in the environmental impacts of present sugarcane biorefineries (autonomous and annexed plants).
Summarizing all the important features, 2G ethanol production in Brazil seems promising in the present scenario, which is a learning example for many countries in order to harness their natural agro resources. This is a fundamental step toward the development of renewable and sustainable sources of energy.
References
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29:675–685
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Bazico (2010) Sugar [Açúcar] (in Portuguese). Available online at www.bazico.com.br/produto/com_acucar.htm
BNDES and CGEE (Coord.), 2008. Sugarcane bioethanol—energy for sustainable development [Bioetanol de cana-de-açúcar – Energia para o Desenvolvimento Sustentável] (in Portuguese). BNDES, Rio de Janeiro
Camargo CA (1990) Energy conservation in the sugar and ethanol industry—handbook of recommendations [Conservação de energia na indústria do açúcar e do álcool – Manual de recomendações] (in Portuguese). IPT, São Paulo
Canilha L, Chandel AK, Milessi TSS, Antunes FAF, Freitas WLC, Felipe MGA, Silva SS. (2013) Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification and ethanol fermentation. J Biomed Biotechnol 2012:1–15. doi:10.1155/2012/989572 (in press)
Cardona CA, Sanchez OJ (2007) Fuel ethanol production: process design trends and integration opportunities. Bioresour Technol 98(12):2415–2457
Cavalett O, Junqueira TL, Dias MOS, Jesus CD, Mantelatto PE, Cunha MP, Franco HCJ, Cardoso TF, Maciel Filho R, Rossell CEV, Bonomi A (2012) Environmental and economic assessment of sugarcane first generation biorefineries in Brazil. Clean Technol Environ Policy 14:399–410
Cavalett O, Chagas MF, Seabra JEA, Bonomi A (2013) Comparative LCA of ethanol versus gasoline in Brazil using different LCIA methods. The Int J Life Cycle Assess. 18: 647–658
Chandel AK, Singh OV, Chandrasekhar G, Rao LV, Narasu ML (2010a) Key-drivers influencing the commercialization of ethanol based biorefineries. J Commer Biotechnol 16:239–257
Chandel AK, Singh OV, Rao LV (2010b) Biotechnological applications of hemicellulosic derived sugars: state-of-the-art. In: Singh OV, Harvey SP (eds) Sustainable biotechnology: renewable resources and new perspectives. Springer, Netherlands, pp 63–81
Chandel AK, Chandrasekhar G, Silva MB, Silva SS (2012a) The realm of cellulases in biorefinery development. Crit Rev Biotechnol 32:187–202
Chandel AK, Silva SS, Carvalho W, Singh OV (2012b) Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. J Chem Technol Biotechnol 87:11–20
Chandel AK, Silva SS, Singh OV (2013a) Detoxification of lignocellulose hydrolysates: Biochemical and metabolic engineering towards white biotechnology. BioEner Res 6:388–401
Chandel AK, Caroline BMG, Strap JL, Silva SS (2013b) Bio-delignification of lignocellulosic substrates: an intrinsic and sustainable pretreatment strategy for clean energy production. Crit Rev Biotechnol. doi:10.3109/07388551.2013.841638 (accepted, in press)
Chavez-Rodriguez MF, Nebra AS (2010) Assessing GHG emissions, ecological footprint, and water linkage for different fuels. Environ Sci Technol 44:9252–9257
Congussu JW, DeCarlo RA, Mathur AP (2003) Using sensitivity analysis to validate a state variable of the software test process. IEEE Trans Softw Eng 29:430–443
Copersucar (1987) Material for the course in Sugar Engineering: sugar production process, part I [Apostila do curso de Engenharia açucareira: Processo de Fabricação de Açúcar, parte I] (in Portuguese). Centro de Tecnologia Copersucar, Piracicaba
CTBE–Brazilian Bioethanol Science and Technology Laboratory (2012) The Virtual Sugarcane Biorefinery (VSB): 2011 Report. Available online at: https://goo.gl/SNZJo. Access: 15.03.2013
Dale BE, Ong RG (2012) Energy, wealth, and human development: why and how biomass pretreatment research must improve. Biotechnol Prog 28:893–898
Dias MOS (2008) Simulation of ethanol production processes from sugar and sugarcane bagasse, aiming process integration and maximization of energy and bagasse surplus [Simulação do processo de produção de etanol a partir do açúcar e do bagaço, visando a integração do processo e a maximização da produção de energia e excedentes do bagaço] (in Portuguese). MSc Dissertation (Chemical Engineering), School of Chemical Engineering, University of Campinas
Dias MOS, Ensinas AV, Nebra SA, Maciel Filho R, Rossell CEV, Maciel MRW (2009) Production of bioethanol and other bio-based materials from sugarcane bagasse: integration to conventional bioethanol production process. Chem Eng Res Des 87:1206–1216
Dias MOS, Cunha MP, Maciel Filho R, Bonomi A, Jesus CDF, Rossell CEV (2011) Simulation of integrated first and second generation bioethanol production from sugarcane: comparison between different biomass pretreatment methods. J Ind Microbiol Biotechnol 38:955–966
Dias MOS, Junqueira TL, Jesus CDF, Rossell CEV, Maciel Filho R, Bonomi A (2012a) Improving second generation ethanol production through optimization of first generation production process from sugarcane. Energy 43:246–252
Dias MOS, Junqueira TL, Rossell CEV, Maciel Filho R, Bonomi A (2012b) Evaluation of process configurations for second generation integrated with first generation bioethanol production from sugarcane. Fuel Proc Technol 109:84–89
Dias MOS, Junqueira TL, Cavalett O, Cunha MP, Jesus CDF, Rossell CEV, Maciel Filho R, Bonomi A (2012c) Integrated versus stand-alone second generation ethanol production from sugarcane bagasse and trash. Bioresour Technol 103:152–161
Dias MOS, Junqueira TL, Cavalett O, Cunha MP, Jesus CDF, Mantelatto PE, Rossell CEV, Maciel Filho R, Bonomi A (2013a) Cogeneration in integrated first and second generation ethanol from sugarcane. Chem Eng Res Des 91:1411–1417
Dias MOS, Junqueira TL, Cavalett O, Pavanello LG, Cunha MP, Jesus CDF, Maciel Filho R, Bonomi A (2013b) Biorefineries for the production of first and second generation ethanol and electricity from sugarcane. Appl Ener 109:72–78
Ensinas AV (2008). Thermal integration and termoeconomic optimization applied to the industrial process of sugar and ethanol from surgarcane [Integração térmica e otimização termoeconômica aplicadas ao processo industrial de produção de açúcar e etanol a partir da cana-de-açúcar] (in Portuguese). Thesis (Ph.D in Mechanical Engineering), School of Mechanical Engineering. University of Campinas
Finnveden G, Hauschild MZ, Ekvall T, Guinee J, Heijungs R, Hellweg S, Koehler A, Pennington D, Suh S (2009) Recent developments in life cycle assessment. J Environ Manage 91:1–21
Galbe M, Zacchi G (2012) Pretreatment: the key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 46:70–78
Galdos M, Cavalett O, Seabra JAE, Nogueira LAH, Bonomi A (2013) Trends in global warming and human health impacts related to Brazilian sugarcane ethanol production considering black carbon emissions. Appl Ener 104:576–582
Girio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Łukasik R (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101:4775–4800
Gnansounou E, Dauriat A (2010) Techno-economic analysis of lignocellulosic ethanol: a review. Bioresour Technol 101:4980–4991
Goldemberg J (2007) Ethanol for a sustainable energy future. Science 315:808–810
Goldemberg J (2008) The Brazilian biofuels industry. Biotechnol Biofuels 1:6
Goldemberg J (2013) Sugarcane ethanol: strategies to a successful program in Brazil. In: Lee JW (ed) Advanced biofuels and bioproducts. doi:10.1007/978-1-4614-3348-4_2, pp 13–20
Hassuani SJ, Leal MRLV, Macedo IC (eds) (2005) Biomass power generation—sugarcane bagasse and trash. PNUD-CTC, Piracicaba
Herrera S (2006) Bonkers about biofuels. Nat Biotechnol 24:755–760
ISO (2006a) ISO 14040—environmental management—life cycle assessment—principles and framework. The International Organization for Standardization
ISO (2006b) ISO 14044—environmental management—life cycle assessment—requirements and guidelines. The International Organization for Standardization
Jesus CDF (2004) Validation of dynamic simulation of evaporation and crystallization steps in sugar production considering industrial plant data (Validação da simulação dinâmica das etapas de evaporação e cristalização da produção de açúcar com dados obtidos em plantas industriais). Ph.D. Thesis in Chemical Engineering, Federal University of São Carlos
Junqueira TL, Dias MOS, Cavalett O, Jesus CDF, Cunha MP, Rossell CEV, Maciel Filho R, Bonomi A (2012) Economic and environmental assessment of integrated 1st and 2nd generation sugarcane bioethanol production evaluating different 2nd generation process alternatives. Comput Aid Chem Eng 30:177–181
Kazi FK, Fortman JA, Anex RP, Hsu DD, Aden A, Dutta A, Kothandaraman G (2010) Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 89:S20–S28
Kerr RA (2011) Energy supplies. Peak oil production may already be here. Science 331:1510–1511
Klein-Marcuschamer D, Simmons BA, Blanch HW (2011) Techno-economic analysis of a lignocellulosic ethanol biorefinery with ionic liquid pre-treatment. Biofuels Bioprod Bioref 5:562–569
Kumar D, Murthy GS (2011) Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol Biofuels 4:27
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Lynd LR, van Zyl WH, McBride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16:577–583
Macrelli S, Mogensen J, Zacchi G (2012) Techno-economic evaluation of 2nd generation bioethanol production from sugar cane bagasse and leaves integrated with the sugar-based ethanol process. Biotechnol Biofuels 5:22
Mantelatto PE (2010) Information about the sugarcane industry. Private communication
Montgomery DC (2001) Design and analysis of experiments, 5th edn. Wiley, New York
Mussatto SI, Dragone G, Guimarães PMR, Silva JPA, Carneiro LM, Roberto IC, Vicente A, Domingues L, Teixeira JA (2010) Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv 28:817–830
OECD/IEA (2010) IEA Statistics Oil Information, Paris. ISBN 978-92-64-08422-3
Ohlrogge J, Allen D, Berguson B, Dellapenna D, Shachar-Hill Y, Stymne S (2009) Energy driving on biomass. Science 324:1019–1020
Ojeda K, Ávila O, Suárez J, Kafarov V (2011) Evaluation of technological alternatives for process integration of sugarcane bagasse for sustainable biofuels production—Part 1. Chem Eng Res Des 89:270–279
Olofsson K, Bertilsson M, Liden G (2008) A short review on SSF—an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels 1:7
Olson DG, McBride JE, Shaw J, Lynd LR (2012) Recent progress in consolidated bioprocessing. Curr Opin Biotechnol 23:396–405
Quintero JA, Moncada J, Cardona CA (2013) Techno-economic analysis of bioethanol production from lignocellulosic residues in Colombia: a process simulation approach. Bioresour Technol 139:300–307
Rabelo SC, Amezquita Fonseca NA, Andrade RR, Maciel Filho R, Costa AC (2011) Ethanol production from enzymatic hydrolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide. Biomass Bioener 35:2600–2607
Rass-Hansen J, Falsig H, Jørgensen B, Christensen CH (2007) Bioethanol: fuel or feedstock? J ChemTech Biotechnol 82:329–333
Rein P (2007) Cane sugar engineering. Verlag Dr Akbert Bartens KG, Berlin
REN21 (2009) Renewables Global Status Report: 2009 Update (Paris: REN21 Secretariat)
Ribeiro P (2003) The sugarcane industry and its automation [A usina de açúcar e sua automação] (in Portuguese), 2ª Ed
Rivera EC, Geraldo VC, Sanghikian N, Junqueira T, Capitani DHD, de Jesus CDF, Maciel Filho R, Bonomi A (2013) A screening design to analyze the influence of technological configurations on techno-economic parameters for autonomous distilleries in Brazil. Chem Eng Trans, AIDIC (submitted)
Rocha GJM, Gonçalves AR, Oliveira BR, Olivares EG, Rossell CEV (2012) Steam explosion pretreatment reproduction and alkaline delignification reactions performed on a pilot scale with sugarcane bagasse for bioethanol production. Ind Crops Prod 35:274–279
Ruano MV, Ribes J, Seco A, Ferrer J (2012) An improved sampling strategy based on trajectory design for design for application of the Morris method to systems with many input factors. Environ Model Soft 37:103–109
Seabra JEA, Tao L, Chum HL, Macedo IC (2010) A techno-economic evaluation of the effects of centralized cellulosic ethanol and co-products refinery options with sugarcane mill clustering. Biomass Bioener 34:1065–1078
Silva SS, Chandel AK (2012) D-xylitol: fermentative production, application and commercialisation. In: Silva SS, Chandel AK (eds) Springer, Heidelberg. ISBN: 978-3-642-31886-3
van Zyl WH, den Haan R, la Grange DC (2011) Developing organisms for consolidated bioprocessing of biomass to ethanol, In: Bernardes MADS (ed) Biofuel production-recent developments and prospects. ISBN: 978-953-307-478-8, InTech, Available from http://www.intechopen.com/books/biofuel-production-recent-developments-andprospects/developingorganisms-for-consolidated-bioprocessing-of-biomass-to-ethanol
Vertès AA, Inui M, Yukaw H (2006) Implementing biofuels on a global scale. Nat Biotechnol 24:761–764
Walter A, Ensinas AV (2010) Combined production of second-generation biofuels and electricity from sugarcane residues. Energy 35:874–879
Walter A, Dolzan P, Quilodrán O, Garcia J, da Silvia C, Piacente F, Segerstedt A (2008). A sustainability analysis of the Brazilian ethanol. A report supported by UK Embassy and DEFRA. Available online at www.unica.com.br
Yuan JS, Tiller KH, Al-Ahmad H, Stewart NR, Stewart CN Jr (2008) Plants to power: bioenergy to fuel the Future. Trends Plant Sci 13:421–429
Zhang X, Shen Y, Shi W, Bao X (2010) Ethanolic co-fermentation with glucose and xylose by the recombinant industrial strain Saccharomyces cerevisiae NAN-127 and the effect of furfural on xylitol production. Bioresour Technol 101:7093–7099
Acknowledgment
The authors are grateful to BIOEN-FAPESP for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Chandel, A.K. et al. (2014). Techno-Economic Analysis of Second-Generation Ethanol in Brazil: Competitive, Complementary Aspects with First-Generation Ethanol. In: da Silva, S., Chandel, A. (eds) Biofuels in Brazil. Springer, Cham. https://doi.org/10.1007/978-3-319-05020-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-05020-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05019-5
Online ISBN: 978-3-319-05020-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)