Abstract
Nickel oxide (NiO) thin films are a well-known functional p-type material which can be used for many applications such as electrodes, sensors, and electrochromic devices. This work investigated the effect of different deposition power on the crystal structural, electrical, and optical properties of NiO thin films prepared by RF magnetron sputtering at room temperature in a pure argon atmosphere. The structural and optical properties of NiO thin films were examined by using X-ray diffraction and UV–Vis-NIR spectrum, respectively. The thickness and the composition of NiO thin films were analyzed by glow discharge spectrum, and the thickness increased with increasing deposition power. SEM observations showed that the surface particle sizes of NiO thin films increased with increasing deposition power. The crystal structure varied from amorphous phase to (200) orientation as the deposition power increased from 75 to 150 W. The electrical results showed that the 125 W-deposited NiO thin films had the lowest resistivity and the largest mobility and carrier concentration. The absorption edges in the UV region shifted toward lower optical length as deposition power (or thickness) increased. Findings from GDS analysis indicated that the obtained NiO thin films were Ni-rich non-stoichiometric films, which possessed excellent electronic properties.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
19.1 Introduction
Nickel oxide (NiO) is an attractive material due to its excellent chemical stability, as well as optical, electrical, and magnetic properties. It has also been used as antiferromagnetic material [1], material for electrochromic display devices [2], and functional layer material for chemical sensors [3]. Furthermore, it is considered to be a model semiconductor with p-type conductivity thin films due to its wide band-gap energy range from 3.6 to 4.0 eV [4]. NiO thin films can be fabricated by various deposition techniques, which include spray pyrolysis [5], plasma-enhanced chemical vapor deposition [6], and reactive sputtering [3]. Among these methods, the radio frequency (RF) magnetron sputtering has been most widely used. Many researches [6–8] have developed the influences of sputtering parameters on the properties of NiO thin films. The conduction properties of NiO thin films are primarily determined by holes generated from nickel vacancies, oxygen interstitial atoms, and the use of a dopant. As the substrate temperature is increased, the evaporation ratio of the solution is too swift, resulting in the formation of inferior NiO thin films. Many reference data and previous studies [6–8] show that superior electronic and optical properties of NiO thin films can be obtained by RF magnetron sputtering with sputtering pressure in the range of 10−3–10−2 Torr and in a pure argon atmosphere with a heated substrate. In this study, optimal sputtering parameters, as being suggested in Refs. [6–8], are employed to RF magnetron sputtering NiO target in a pure Ar atmosphere. The effects of sputtering parameters on the electrical and optical properties of NiO thin films, such as the crystalline structure and transmittance were all investigated. The structural and optical properties of NiO thin films were characterized by scanning X-ray diffraction (XRD) patterns, electron microscopy (SEM), UV–visible spectroscopy, and Hall measurement, respectively. The value variations in the optical band gap (Eg) of NiO thin films were also evaluated from the plots of (αhv)2 = c(hv-Eg).
19.2 Experimental Procedure
Nickel oxide (NiO) thin films were deposited on the Corning 1737 glass substrates with a thickness of 0.7 mm by a RF magnetron sputtering system, a NiO ceramic with 99.99 % purity was used a target and deposited in a pure argon atmosphere. The chamber was evacuated to a base pressure below 3 × 10−6 Torr before deposition. Deposition of NiO thin films was performed at a gas pressure of 5 × 10−3 Torr for 20 min, and the deposition power of cathode was changed from 75 to 150 W. The crystal structure of the deposited NiO thin films was identified by X-ray diffraction (XRD) analysis. XRD patterns of NiO thin films were determined with PANalytical, 18 kW Rotating Anode X-ray Generator using monochromatic high-intensity CuKα radiation (λ = 1.5406 Å). The surface morphology of the deposited NiO thin films was analyzed by a Field emission scanning electron microscope (FESEM). The transmittance was measured at normal incidence in visible spectrum using JASCO V-570 spectrometer. The thickness and the composition of NiO films were analyzed by glow discharge spectrum (GDS). The chemical bonding states of oxygen and Ni in NiO thin films were investigated using an X-ray photoelectron spectroscopy (XPS).
19.3 Results and Discussion
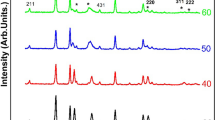
The structure of NiO thin films deposited at various RF powers in pure Ar atmosphere was examined by XRD patterns (not shown here). As 75 W was used as deposition power, no apparent diffraction peak was observed, and NiO thin films showed the amorphous structure. As the deposition power was equal to and more than 100 W, the (200) diffraction peak were observed. The diffraction intensity of (200) peak increased rapidly with increasing deposition power, particularly as the deposition was higher than 100 W. The stronger diffraction peaks indicate that NiO thin films have a highly crystallized and (200)-textured structure.
The concentration profiles of NiO thin films were then analyzed with a GDS system, and the GDS depth profile of NiO thin films was shown in Fig. 19.1. As the deposition power were 75, 100, 125, and 150 W, the thicknesses of NiO thin films were about 36, 47, 56, and 72 nm, respectively. The GDS depth profiles confirm that the thicknesses of NiO thin films increased with deposition power. Figure 19.1 also shows that the deposited NiO thin films had a non-stoichiometric composition, which existed a Ni excess and oxygen vacancy. The results show that Ni-rich in NiO thin films creates vacancies in the normally occupied O sites to preserve overall electrical neutrality in the deposited thin films. Two Ni2+ ions must be converted to Ni3+ for every vacant Ni2+ site formation. Thus, Ni3+ ions could be considered to be positive centers capable of jumping from one Ni2+ site to another. When an electron hops from a Ni2+ to a Ni3+ site, it is as if a positive hole moves through the Ni2+ sites. Thus, NiO with Ni-rich film will reveal as a P-type semiconductor [8]. Applying band model to NiO with excess of oxygen, the Ni3+ sites act as impurity levels above the filled band and the excitation of an electron to these sites leave mobile holes in the band.
Figure 19.2 shows the SEM micrographs of NiO thin films as a function of deposition power. Figure 19.2 clearly shows that the morphology of NiO thin films had a granular structure, with individual grains appearing most clearly as bright spots on the dark field image. The volumes and sizes of the bright spots increased with increasing deposition power. When deposition power of NiO thin films is increased, the mobility of the molecules on the surface is improved, and that has resulted in an increase of the surface grain size and the thickness. Figure 19.2 also shows that the roughness of NiO thin films increased with increasing deposition power.
Figure 19.3 shows the UV absorbed spectra and the calculated energy gaps (Eg) of NiO thin film under various deposition powers. Figure 19.3a shows that the transmittance ratio decreased with increasing deposition power. The decrease of transmittance ratio with increasing deposition power results in increasing the proportion of nickel caused the light to be absorbed, the results in GDS analysis can prove this result. In the past, determination of the optical band gap (Eg) was often necessary to develop the electronic band structure of a thin-film material. However, using extrapolation methods, the Eg values of films can be determined from the absorption edge for direct interband transition, which can be calculated using the relation in Eq. (19.1)
where α is the optical absorption coefficient, c is the constant for direct transition, h is Planck’s constant, and ν is the frequency of the incident photon [9]. The linear dependence of (αhv)2 on hν indicates that NiO thin films are direct transition type semiconductors. The Eg value of 75 W-deposited NiO thin films was 3.38 eV; As the deposition power of NiO thin films increased from 100 to 150 W, the Eg values increased from 3.20 to 3.34 eV. As we know the Eg values of NiO thin film is ranged from 3.6 to 4.0 eV [4] and, except the 75 W-deposited samples, the Eg value obviously increased with deposition power as shown in Fig. 19.3b.
Figure 19.4 shows the results of Hall-effect measurement of NiO thin films at various deposition powers, which indicates the value variations of resistivity, mobility, and carrier concentration were dependent deposition power. The lowest resistivity (2.49 × 10−2 Ω·cm), the largest mobility (3.63 × 101 cm2/V·s), and carrier concentration (6.91 × 1017 cm−3) was obtained at the deposition power of 125 W. The electrical conductivity of undoped NiO thin films has a strong dependence on the microstructural defects existing in NiO crystallites, such as nickel vacancies and interstitial [6–10]. The microstructure, composition, the deposition parameters are the main factors that will affect the electrical properties of NiO thin films. As we know, the variation in carrier concentration of deposited thin films depends on both crystalline microstructures and surface chemical reactions during the sputtering process. The drift velocity and mean free path of the charge carriers were used to determine the value of mobility. Reducing the scattering by the lattice imperfections increases the mobility of carrier with larger grain means less grain boundaries existing, and the scattering effect at the grain boundary is reduced [7]. Nakahata et al. [10] prepared polycrystalline silicon thin films on glass by PECVD and found that the value of Hall mobility depends strongly on the grain size, the texture, the crystallinity, or the fluctuation of grain orientation. For that, we suggest that the observed variation of carrier concentration and Hall mobility are associated with the NiO crystalline microstructure change and point defects.
The Ni 2p3/2 XPS spectra of NiO thin films are shown in Fig. 19.5. The NiO peaks at 853.6 eV and Ni2O3 peak at 855.6 eV, respectively, were revealed in the XPS spectrum, and no significant peak shift was observed in the Ni 2p3/2 spectrum as the deposition power increased. For Ni 2p3/2 electron binding energy, the intensity of Ni2+ bonding state decreased and Ni3+ bonding state increased as deposition power increased from 75 to 125 W and saturated at 125 W. The ratio (fitting area) of Ni3+/Ni2+ increased from 1.83, 7.78, 8.85, and 12.1 as deposition power was 75, 100, 125, and 150 W, respectively. It coincides with the tendency observed in the Ni 2p3/2 peak where the Ni2+ (NiO) bonding state decreases over Ni3+ (Ni2O3) bonding state with increasing deposition power. The past literature had demonstrated that the formation of holes in a NiO thin film is due to an increase in the Ni3+ ions or to the formation of Ni2+ vacancies and the presence of interstitial oxygen atoms [11]. The results in Fig. 19.1 also demonstrate that the formation of holes in NiO thin films is due to the increase of the Ni3+ ions or the formation of the Ni2+ vacancies and the interstitial oxygen atoms. With increase of the hole concentration, the carrier concentration increases, then the resistivity of NiO thin films decrease, as the results shown in Fig. 19.4.
19.4 Conclusions
In this study, the thickness of NiO thin films increased with increasing deposition power. The highest deposition rate and lowest surface roughness were obtained at deposition power of 125 W. XRD spectra indicated that NiO thin films had highly crystalline and (200)-textured structure. For NiO thin films, the lowest resistivity (2.49 × 10−2 Ω·cm), high mobility (3.63 × 101 cm2/V·s), and high carrier concentration (6.91 × 1017 cm−3) were obtained at deposition power of 125 W. The transmittance of NiO thin films decreased with increasing deposition power, the increases of thickness and Ni+3 ions were the reasons. The optical energy band was evaluated from the plots of (αhv)2 = c(hν − Eg), and Eg values were 3.38, 3.20, 3.26, and 3.34 eV, respectively, as the deposition powers were 75, 100, 125, and 150 W.
References
Fujii, E., Tomozawa, A., Torii, H., & Takayama, R. (1996). Preferred orientations of NiO films prepared by plasma-enhanced metalorganic chemical vapor deposition. Japanese Journal of Applied Physics, 35, L328–L330.
Yoshimura, K., Miki, T., & Tanemura, S. (1995). Nickel oxide electrochromic thin films prepared by reactive dc magnetron sputtering. Japanese Journal of Applied Physics, 34, 2440–2446.
Bogner, M., Fuchs, A., Scharnagl, K., Winter, R., Doll, T., & Eisele, I. (1998). Thin (NiO)1-x (Al2O3)x, Al doped and Al coated NiO layers for gas detection with HSGFET. Sensors and Actuators B: Chemical, 47, 145–152.
Sato, H., Minami, T., Takata, S., & Yamada, T. (1993). Transparent conducting p-type NiO thin films prepared by magnetron sputtering. Thin Solid Films, 236, 27–31.
Puspharajah, P., Radhakrishna, S., & Arof, A. K. (1997). Transparent conducting lithium-doped nickel oxide thin films by spray pyrolysis technique. Journal Materials Science, 32, 3001–3006.
Yeh, W. C., & Matsumura, M. (1997). Chemical vapor deposition of Nickel Oxide films from Bis-p- Cyclopentadienyl-Nickel. Japanese Journal of Applied Physics, 36, 6884–6887.
Lu, Y. M., Hwang, W. S., Yang, J. S., & Chuang, H. C. (2002). Properties of nickel oxide thin films deposited by reactive magnetron sputtering. Thin Solid Films, 420–421, 54–61.
Hotovy, I., Huran, J., Spiess, L., Liday, J., Sitter, H., & Hascık, S. (2003). The influence of process parameters and annealing temperature on the physical properties of sputtered NiO thin films. Vacuum, 69, 237–242.
Moss, T. S. (1954). The interpretation of the properties of indium antimonide. Proceedings of the Physical Society, London, Section B, 67, 775–782.
Nakahata, K., Miida, A., Kamiya, T., Fortmann, C. M., & Shimizu, I. (1999). Carrier transport, structure and orientation in polycrystalline silicon on glass. Thin Solid Films, 337, 45–50.
Nandy, S., Saha, B., & Mitra, M. K. (2007). Effect of oxygen partial pressure on the electrical and optical properties of highly (200) oriented p-type Ni1–xO films by DC sputtering. Journal Materials Science, 42, 5766–5772.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this paper
Cite this paper
Wang, F.H., Li, P.J., Diao, C.C., Huang, C.C., Huang, H.H., Yang, C.F. (2014). Properties of the Highly Textured (200) NiO Thin Film Deposited by RF Magnetron Sputtering. In: Juang, J., Chen, CY., Yang, CF. (eds) Proceedings of the 2nd International Conference on Intelligent Technologies and Engineering Systems (ICITES2013). Lecture Notes in Electrical Engineering, vol 293. Springer, Cham. https://doi.org/10.1007/978-3-319-04573-3_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-04573-3_19
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-04572-6
Online ISBN: 978-3-319-04573-3
eBook Packages: EngineeringEngineering (R0)