Abstract
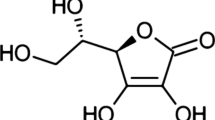
Vitamin C (L-ascorbic acid, AsA) is essential for human health; however, despite our dependency on plants as dietary sources of this nutrient, little is known about its metabolism in crops. Ascorbate protects cells and organelles from oxidative damage by scavenging reactive oxygen species that are produced in response to abiotic and biotic insults, and is also a cofactor of many enzymes, controls cell division, affects cell expansion, and is a modulator of plant senescence. Biosynthesis of AsA in plants is carried out by a complex metabolic network involving D-mannose/L-galactose, D-galacturonate, L-gulose, and myo-inositol as main precursors. The recent cloning of several genes that regulate AsA synthesis and recycling has facilitated the generation of transgenic plants with enhanced AsA levels, and in some cases as much as sixfold increases in AsA relative to wild-type plants have been achieved. In this review, we provide an overview of research revealing three aspects of the biochemistry of AsA that have not been fully covered elsewhere. First, we discuss the main findings of studies on feeding plant tissues with precursors as a proxy to determine which of the AsA biosynthetic pathways are operational in model and crop plants, and discuss these in the context of the forward and reverse genetic studies that support the operation of each pathway. Next, we critically discuss the consequences of elevating AsA content for plant growth, and finally we explore the effect of AsA content on plant performance under environmental stress.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vitamin C
- Ascorbic acid
- Feeding studies
- Abiotic stress
- Stress tolerance
- Phytoremediation
- High throughput phenotyping
- Phenomics
6.1 Introduction
Ascorbate (L-ascorbic acid, AsA, a.k.a. vitamin C) is ubiquitous in plants, and serves a host of different functions. It protects cells and organelles from oxidative damage by scavenging reactive oxygen species (e.g., superoxide and H2O2), which are produced by aerobic metabolic processes such as photosynthesis and respiration or by environmental stresses like salt, drought, cold, and excess light. AsA also participates in the regeneration of vitamin E [17] and acts as a substrate for synthesis of important organic acids (e.g., L-tartaric, L-threonic, L-glyceric, and L-oxalic acids) [24], as well as being a cofactor for enzymes involved in a diverse array of processes including flavonoid and phytohormone biosynthesis and the xanthophyll cycle [23]. There is also growing evidence that AsA participates in the regulation of cell division and elongation [48, 86], modulates flowering time and the onset of senescence [10], acts as a signaling molecule involved in plant response to environmental stresses such as ozone and pathogen attack [17], and regulates cell polarity during embryo development [15]. In short, AsA is crucial to plant health, as illustrated by the fact that no mutant completely devoid of AsA has ever been described. The vtc2/vtc5 double mutant, a line defective in the expression of two genes in the D-mannose (D-Man)/L-galactose (L-Gal) pathway to AsA that is predicted to entirely lack ascorbate in fact suffers from very early growth arrest and is essentially nonviable [27].
Some animal species, including humans, do not synthesize AsA due to the lack of the enzyme catalyzing the last step of the biosynthetic pathway (L-gulono-1,4-lactone oxidase or GLOase, a.k.a. GulLO), and for them it thus is a vitamin. In addition to its essential roles as redox buffer in key organelles such as the mitochondria and the endoplasmic reticulum [70], vitamin C is an indispensable cofactor in the hydroxylation of proline to lysine, and therefore is essential for collagen synthesis and connective tissue integrity. A consequence of this is that vitamin C deficiency can cause scurvy, a condition characterized by hemorrhages, bleeding gums, and impaired wound healing. Vitamin C is also involved in a wide array of other crucial physiological processes in animals, including the synthesis of cytokines [90], modulation of nitric oxide synthase activity [49], oxidative protein folding and endoplasmic reticulum stress [70, 71], cell proliferation and apoptosis [25], activation of the epithelial cystic fibrosis transmembrane conductance regulator chloride channel [32], maintenance of immune homeostasis [101], promotion of iron absorption and mobilization, and tyrosine, folate and xenobiotic metabolism. In humans, diverse studies indicate that vitamin C may decrease the incidence of various diseases, including dementia [74], cancer [59, 60], stroke [104], heart disease [88], atherosclerosis [76], type 2 diabetes [44], and Charcot–Marie–Tooth disease, a hereditary peripheral neuropathy [83]. According to the 2003–2004 National Health and Nutrition Examination Survey, 7.1 % of the US population was vitamin C-deficient, and smokers, low-income people, and the elderly are among those at increased risk [89]. These data provide a clear rationale for enhancing the vitamin C content of food crops.
Despite the critical importance of AsA to plant health and human nutrition, it is only recently that scientists have succeeded in identifying pathways that lead to AsA synthesis in plants (reviewed in [12, 68]). In contrast to animals, which utilize D-glucuronate (D-GlcUA) as a precursor for AsA synthesis, plants rely on at least four alternative routes for AsA synthesis; these routes utilize myo-inositol (MI) [69], L-gulose (L-Gul) [102], D-Man/L-Gal [100], and D-galacturonate (D-GalUA) [1] as main precursors (Fig. 6.1). The recent cloning of several genes that regulate AsA synthesis and recycling has facilitated the generation of transgenic plants with enhanced AsA levels and in some cases as much as sixfold increases in AsA relative to wild-type plants have been achieved (Table 6.1). We and various other groups who discovered these AsA biosynthetic genes have patented their use in metabolic engineering [42] .
Pathways involved in ascorbate biosynthesis and regeneration in plants: The D-mannose/L-galactose (Man/Gal) route, the L-gulose (Gul) shunt, the D-galacturonate (GalU) pathway, and the myo-inositol (MI) route. A purple acid phosphatase with phytase activity (AtPAP15) has been shown to channel phytate to the MI pathway, while VTC4 has been shown to also use L-myo-inositol-1 phosphate and contribute to both myo-inositol and ascorbate metabolisms. The enzymes participating in the Man/Gal route are: Phosphoglucose isomerase (EC 5.3.1.9); phosphomannose isomerase (PMI, EC 5.1.3.1.8); phosphomannose mutase (PMM, EC 5.4.2.8); GDP-mannose pyrophosphorylase (VTC1, EC 2.7.7.13); GDP-mannose-3′,5′-epimerase (GME, EC 5.1.3.18); GDP-galactose phosphorylase (VTC2, EC 2.7.7.B2); L-galactose-1-phosphate phosphatase (VTC4); L-galactose dehydrogenase (GalDH, EC 1.1.1.48); L-galactono-1,4-lactone dehydrogenase (GLDH, EC 1.3.2.3). The enzymes in the GalU pathway are: D-galacturonate reductase (GalUR) and gluconolactonase (EC 3.1.1.17). The enzymes in the MI pathway are: Inositol phosphate phosphatase (EC 3.1.3.25); myo-inositol oxygenase (MIOX, EC 1.13.99.1); glucuronate reductase (GlcUR, EC 1.1.1.19); gluconolactonase (GNL, EC 3.1.1.17), and L-gulono-1,4-lactone oxidase (GLOase, EC 1.1.3.8). The enzymes involved in AsA recycling are: Monodehydroascorbate reductase (MDHAR, EC. 1.6.5.4) and dehydroascorbate reductase (DHAR, EC 1.8.5.1). Where omitted EC number have not been assigned. (Adapted from [68])
In 2004, we reported that myo-inositol oxygenase (MIOX) overexpression in Arabidopsis thaliana leads to plants with 2–3-fold increase in foliar AsA content [69]. During the past 9 years we have worked with multiple generations of these lines and, with the exception of one line where we found gene silencing, we have always detected elevated foliar AsA content [56, 78]. In a study published in 2009, Endres and Tenhaken failed to detect differences in AsA content between a wild-type line from the Arabidopsis Stock Center in Europe and our MIOX4 overexpressors, but did detect lower MI content in those lines [31]. In 2011, a group in Hungary provided evidence and independent verification that our MIOX4 overexpressors have elevated AsA (1.7-fold), display enhanced growth (a phenotype noted by the authors and documented with a photograph in the article), and are tolerant to high light stress compared to the wild type background used for transformation [94]. We further reported that MIOX4 over-expressors continue to have elevated AsA content (1.75-fold) compared to controls not only when grown under normal conditions, but also when subjected to salt, cold, heat, and pyrene stresses [66].
During the past few months, excellent review papers have been published describing in detail novel functions of AsA and its role in plant evolution [33, 36]. Instead of duplicating that effort, in this review we provide an overview of research revealing three aspects of the biochemistry of AsA that have not been fully covered elsewhere. First, we will discuss the main findings of studies on feeding plant tissues with AsA precursors as a proxy to determine which of the AsA biosynthetic pathways are operational in model and crop plants, and discuss these studies in the context of the forward and reverse genetic studies that support the operation of each pathway (Sect. 6.2). Next, we will critically discuss the consequences of elevating AsA content for plant growth (Sect. 6.3). Finally, also the effect of AsA content on plant performance under environmental stress will be reviewed (Sect. 6.4).
6.2 Which Biosynthetic Pathways Leading to Ascorbate Formation are Operational in Plants?
Feeding plant organs, tissues, or cells with AsA precursors has been an invaluable tool that has allowed researchers to gain a better understanding about the plasticity of the metabolic machinery leading to AsA formation in model and crop plants (Table 6.2). This approach has been employed for more than 5 decades in the field. In most cases, the experiment has consisted in the incubation of detached tissues or organs from the plant of interest in an aqueous solution of each of the AsA precursors to be tested. Most feeding experiments have been conducted under continuous light and have lasted between 1 and 24 h. The main goal of these assays has consisted in measuring the in planta AsA content resulting from the uptake, transport, and conversion of the substrate in question into AsA. Table 6.2 presents a summary of the studies in which more than one AsA precursor has been tested. The reader should note that we did not include several other published studies in which only one particular precursor has been fed to the plant model of study.
The results of these feeding studies should not be analyzed in isolation but as one of many tools available to understand AsA metabolism. These studies using “cold chemicals” although valuable are considered less sensitive and accurate than those where radioactively labeled precursors have been used.
Table 6.2 illustrates that without exception the AsA pathway that is operational and predominant in tissues of all the plant species analyzed with this approach is the Man/Gal route (a.k.a. Smirnoff–Wheeler pathway, [100]). Of the intermediates in this route, L-Gal is the substrate that has led to the by far highest increases in AsA, followed by L-galactono-1,4-lactone (L-GalL) with the exception of sweet pepper [9] and papaya [9] where L-GalL seems to be preferred over L-Gal. These studies also indicate that this pathway is clearly controlled by substrate availability.
In addition to the results of the feeding studies listed in Table 6.2, much biochemical and molecular genetic evidence now exists in support of the Man/Gal route. Genes encoding all the proposed biosynthetic enzymes have been identified in higher plants [19, 20, 27, 34, 50, 58, 65, 72, 81, 102]. Two master regulators of this pathway, Arabidopsis AMR1 [106] and AtERF98 [108] have been recently reported as well.
As shown in Table 6.2, there is a significant body of work supporting the operation of alternative routes leading to AsA formation that serve to supplement the synthesis via L-Gal at certain developmental stages and in particular tissues. In the following paragraphs, we discuss the studies supporting the operation of the L-Gul, D-GalUA, and MI pathways.
Only a few of the studies have included L-Gul in the suite of precursors analyzed (Table 6.2). Of those, the one in chestnut rose [3] and the one in wood tobacco (Aboobucker and Lorence, unpublished) provided data supporting the conversion of this substrate into AsA. L-Gul does not seem to be converted into AsA in black currant under the conditions tested [43].
In addition to the feeding studies in which L-Gul has been effectively converted into AsA, molecular evidence supporting the operation of the L-Gul pathway was obtained through the characterization of the Arabidopsis GDP-mannose-3′-5′-epimerase (GME) enzyme [102]. GME is able to also synthesize GDP-L-gulose, and it may be assumed that this substrate can be converted to L-gulono-1,4-lactone (L-GulL) in an analogous way to the conversion of L-Gal to L-GalL and then to AsA. Maruta and collaborators [73] obtained evidence for the operation in tobacco cells of GLOases (a.k.a. GulLOs), enzymes capable of oxidizing L-GulL into AsA. These enzymes also participate in the MI pathway to AsA.
Multiple studies carried out in apples [22], Arabidopsis [21], kiwi [62], and tomato fruits [107] report the conversion of D-GalUA into AsA. However, this substrate does not seem to contribute in a significant way to vitamin C synthesis in all fruits, as indicated by the lack of conversion to AsA in black currant [43], peaches [51], and leaves of wood tobacco (Aboobucker and Lorence, unpublished).
Molecular evidence in support of the operation of the GalUA pathway to AsA was provided by the characterization of an enzyme with D-GalUA reductase activity [1] in strawberry. When overexpressed, this enzyme leads to plants with elevated AsA.
Five feeding studies have included MI in the suite of precursors assayed. Of these articles, one provides convincing evidence (statistically significant differences compared to controls fed with water) of the conversion of MI into AsA in red tomatoes [75]. Inositol does not seem to be effectively converted into the final product in apples [61], Arabidopsis cells [21], black currant [43], or kiwi fruits [62].
Multiple teams including ours have provided molecular evidence for the operation in higher plants of enzymes capable of using MI and other inositol phosphates including phytate for AsA production [69, 93, 105]. Overexpression of AtMIOX4 led to elevated foliar AsA content in Arabidopsis, as demonstrated by us [69] and independently confirmed by Tóth and collaborators [94]. The enzymes that follow MIOX in the proposed MI pathway to AsA are glucuronate reductase (GlcUR), gluconolactonase (GNL), and GLOase (a.k.a. GulLO). Manuscripts with the detailed characterization of at least one member of each of these enzyme families in Arabidopsis are in preparation in our group.
A multitude of studies have obtained data showing the effective conversion of L-GulL into AsA in apples [22], Arabidopsis [21], beans [8], kiwi [62], peas [82], peaches [51], tobacco [53], tomato [75], and wood tobacco (Aboobucker and Lorence, unpublished). This substrate is structurally quite similar to the immediate AsA precursor in the Man/Gal pathway, L-GalL. However, detailed characterization of L-galactono-1,4-lactone dehydrogenase (GLDH), the terminal enzyme in that pathway, shows that plant GLDHs are highly specific for L-GalL (reviewed in [92]). Therefore, conversion of the alternative substrate L-GulL into AsA is most likely catalyzed by GulLO, the terminal enzyme that connects the L-Gul and MI pathways (Fig. 6.1).
6.3 Effects of Ascorbate Content on Plant Growth
Among the new knowledge that has emerged from the detailed characterization of the function of the various enzymes involved in AsA metabolism in plants are the remarkable negative consequences for growth, morphology, and development of lines that are deficient in this key molecule (Table 6.3). These low-AsA lines have been developed either after chemical mutagenesis or via knockout approaches. A common phenotype reported for Arabidopsis, potato, rice, tomato, and tobacco low-AsA mutants is a significant reduction of growth and biomass accumulation of both aerial and root tissues. At the cellular level, this reduction in plant size and biomass is linked in some cases with decreased cell size and in others with lower number of cells. Reduced AsA levels also seem to have a negative impact on the number of flowers, number of tillers, the size of the fruits, and seed yield. On the other hand, a question that remains open is whether elevated AsA has positive effects for plant growth and development.
We reported that Arabidopsis lines with enhanced AsA content overexpressing enzymes that participate in the inositol pathway, MIOX4 [69] and GLOase [87], accumulate more biomass (measured as dry weight of the aerial tissue) and display a longer inflorescence stem and a wider rosette diameter compared to controls growing in soil under similar conditions. These MIOX4 and GLOase overexpressors also showed enhanced growth of both aerial and root tissues when grown in liquid culture [66, 78]. To our knowledge, this is the first study demonstrating such a marked positive effect on plant growth in lines engineered to have elevated AsA concentrations.
We have recently incorporated the use of a Scanalyzer HTS instrument (LemnaTec, Germany), a powerful tool that allows nondestructive, unbiased, and accurate phenotyping of small plants to the characterization of the high AsA lines (MIOX4 and GLOase). For this experiment, seeds of wild type (untransformed A. thaliana var. Columbia, ABRC stock CS-60000) and homozygous MIOX4 and GLOase lines were cleaned and planted on Murashige and Skoog (MS) plates and vernalized for 3 days at 4 °C. Plates were then incubated in a controlled environmental chamber (Conviron, Pembina, ND) under the following conditions: 23 °C, 65 % humidity, 14:10 h photoperiod, and 150 µmol m−2 s−1 light intensity. After a week, seedlings were transferred to the soil (Arabidopsis Growing Medium, Lehle Seeds, Round Rock, TX) and grown in QuickPot 15 trays (HerkuPlast, Germany) until maturity under the above conditions. During their entire life cycle, plants were phenotyped with the Scanalyzer HTS. The acquired images were analyzed as previously described [4] to calculate plant growth (measured as foliar area in cm2). As illustrated in Fig. 6.2, MIOX4 and GLOase overexpressors grew faster and accumulated more biomass than untransformed controls growing under similar conditions.
Arabidopsis high vitamin C lines, MIOX4 and GLOase, display enhanced growth rate and biomass accumulation compared to wild-type controls. Seeds were germinated on MS media and seedlings were transferred to soil and grown under controlled conditions (23 °C, 65 % humidity, 14:10 h photoperiod, and 150 µmol m−2 s−1 light intensity). Images were acquired with a nondestructive high throughput phenotyping system (Scanalyzer HTS, LemnaTec, Germany), and leaf area was measured as previously described [4]. Images shown correspond to plants at the end of the vegetative growth (29 days after germination). Values are means ± standard error (n = 15)
6.4 Effects of Ascorbate Content on Abiotic Stress Tolerance
Table 6.4 summarizes the results obtained by us and others after detailed characterization of model and crop plants with enhanced AsA content in response to various forms of abiotic stress . The AsA enhancement has been achieved in most cases after constitutive expression of biosynthetic and recycling genes; however, in some cases feeding AsA or its precursors has also led to elevated antioxidant content. The big picture that emerges from this summary is that even modest increases in AsA content have led to broad tolerance to common stresses such as salt, cold, ozone, and herbicide treatment. In addition to the overall AsA content, an aspect equally important for the health of the plant tissue is the ratio of reduced to oxidized ascorbate or AsA redox status. In some of these studies, particularly those where DHAR and MDHAR have been overexpressed, the redox status of the plants changed, helping them overcome the stresses they have been subjected to.
Interestingly, exposure to pyrene, a polycyclic aromatic hydrocarbon and a known inducer of oxidative stress in plants, led to stunted growth of the aerial tissue, reduction in the number of root hairs, and inhibition of leaf expansion in wild-type plants, while these symptoms were less severe in the MIOX4 and GLOase overexpressors [46]. These results indicate the potential of high AsA crops as a tool for phytoremediation applications.
As AsA is intertwined in such a large number of networks (photosynthesis, flowering, ROS signaling, cell growth/division, pathogen response) and elevation of AsA levels has been shown to alter the transcription of many genes [55, 84], there could be unexpected, perhaps negative, consequences to significant elevation of AsA in plants beyond the normal physiological level. Additional research is needed in this regard.
6.5 Conclusions and Perspectives
Feeding studies have revealed that the Man/Gal pathway is operational and predominant in all the plant species analyzed to date. Of the intermediates participating in this route, L-Gal and L-GalL are most effective at increasing AsA content. However, without a doubt, these feeding studies also demonstrate the operation of all the alternative pathways that supplement the one using L-Gal in particular tissues and developmental stages. The research here reviewed indicates the potential of engineering elevated AsA content as an effective strategy to develop crops with enhanced biomass and abiotic stress tolerance. Based on our results and the ones of others, we propose that increases between 1.5- and 6-fold in the total AsA content of plant tissues are necessary to have broad tolerance to abiotic stresses in crops. Future generations of these engineered crops are likely to include the combination of the expression of biosynthetic and recycling genes, and/or the expression of regulatory proteins and transcription factors that have been recently shown to modulate multiple genes at once (e.g., [106, 108]). Although the results obtained by us and others show significant promise for the development of plants with enhanced abiotic stress tolerance, an aspect until now not fully explored is the evaluation of the consequences of elevated AsA content in the ability of plants to interact with insects and other herbivores (reviewed in [38]). This is a crucial aspect that must be evaluated at both greenhouse and field levels before these crops can be deployed.
Abbreviations
- L-Gal:
-
= L-Galactose
- L-GalL:
-
= L-Galactono-1,4-lactone
- D-GalUA:
-
= D-Galacturonic acid
- D-Glc:
-
= D-Glucose
- D-GlcUA:
-
= D-Glucuronic acid
- D-GlucL:
-
= D-Glucuronolactone
- L-Gul:
-
= L-Gulose
- L-GulL:
-
= L-Gulono-1,4-lactone
- D-Man:
-
= D-Mannose
- D-Fruc:
-
= D-fructose
- MI:
-
= Myo-inositol
- Suc:
-
= Sucrose
- AMR1:
-
= Ascorbic acid mannose pathway regulator 1
- AsA:
-
= L-Ascorbic acid, ascorbate, vitamin C
- AtERF98:
-
= Arabidopsis ethylene responsive factor 98
- AtPAP15:
-
= Purple acid phosphatase 15 from Arabidopsis thaliana
- DHA:
-
= Dehydroascorbate
- DHAR:
-
= Dehydroascorbate reductase
- GalDH:
-
= L-Galactose dehydrogenase
- GalUR:
-
= D-Galacturonate reductase
- GlcUR:
-
= D-Glucuronate reductase
- GLDH:
-
= L-Galactono-1,4-lactone dehydrogenase
- GLOase:
-
= L-Gulono-1,4-lactone oxidase (a.k.a. GulLO)
- GME:
-
= GDP-mannose-3′,5′-epimerase
- GNL:
-
= Gluconolactonase
- HBK3:
-
= KNOTTED-like homeobox gene from Norway spruce
- MIOX:
-
= myo-Inositol oxygenase
- MDHAR:
-
= Monodehydroascorbate reductase
- PMI:
-
= Phosphomannose isomerase
- PMM:
-
= Phosphomannose mutase
- VTC1:
-
= GDP-mannose pyrophosphorylase
- VTC2:
-
= GDP-galactose phosphorylase
- VTC4:
-
= L-Galactose-1-phosphate phosphatase
References
Agius F, Gonzalez-Lamothe R, Caballero JL, Muñoz-Blanco J, Botella MA, Valpuesta V (2003) Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat Biotechnol 21:177–181
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, Baldet P (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
An HM, Fan WG, Chen LG, Asghar S, Liu QL (2007) Molecular characterization and expression of L-galactono-1,4-lactone dehydrogenase and L-ascorbic acid accumulation during fruit development in Rosa roxburghii. J Hortic Sci Biotech 82:627–635
Arvidsson S, Perez-Rodriguez P, Mueller-Roeber B (2011) A growth phenotyping pipeline for Arabidopsis thaliana integrating image analysis and rosette area modeling for robust quantification of geno-type effects. New Phytologist. doi:10.1111/j.1469-8137.2011.03756.x
Athar HR, Khan A, Ashraf M (2008) Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ Exp Bot 63:224–231
Badejo AA, Tanaka N, Esaka M (2008) Analysis of GDP-D-mannose pyrophosphorylase gene promoter from acerola (Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing the acerola gene. Plant Cell Physiol 49:126–132
Badejo AA, Eltelib HA, Fukunaga K, Fujikawa Y, Esaka M (2009) Increase in ascorbate content of transgenic tobacco plants overexpressing the acerola (Malpighia glabra) phosphomannomutase gene. Plant Cell Physiol 50:423–428
Baig MM, Kelly S, Loewus F (1970) L-Ascorbic acid biosynthesis in higher plants from L-gulono-1,4-lactone and L-galactono-1,4-lactone. Plant Physiol 46:277–280
Barata-Soares AD, Gomez MLPA, Henrique deMC, Lajol FM (2004) Ascorbic acid biosynthesis: a precursor study on plants. Braz J Plant Physiol 16:147–154
Barth C, De Tullio M, Conklin PL (2006) The role of ascorbic acid in the control of flowering time and the onset of senescence. J Exp Bot 57:1657–1665
Belmonte MF, Stasolla C (2009) Altered HBK3 expression affects glutathione and ascorbate metabolism during the early phases of Norway spruce (Picea abies) somatic embryogenesis. Plant Physiol Biochem 47:904–911
Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-Wang K, Andre C, Brewster D, Karunairetnam S, Allan AC, Laing WA (2012) Enhancing ascorbate in fruits and tubers through over-expression of the L-galactose pathway gene GDP-L-galactose phosphorylase. Plant Biotechnol J 10:390–397
Chen Z, Gallie DR (2005) Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance. Plant Physiol 138:1673–1689
Chen Z, Gallie DR (2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142:775–777
Chen Z, Gallie DR (2012) Induction of monozygotic twinning by ascorbic acid in tobacco. PLoS ONE 7. doi:10.1371/journal.pone 0039147
Chen Z, Young TE, Ling J, Chang S-C, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100:3525–3230
Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27:959–970
Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 93:9970–9974
Conklin PL, Norris SN, Wheeler GL, Williams EH, Smirnoff N, Last RL (1999) Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA 96:4198–4203
Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, Isupov M, Littlechild JA, Smirnoff N (2006) Arabidopsis thaliana vtc4 encodes L-galactose-1-P-phosphatase, a plant ascorbic acid biosynthetic enzyme. J Biol Chem 281:15662–15670
Davey MW, Gilot C, Persiau G, Østergaard J, Han Y, Bauw GC, Van Montagu MC (1999) Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiol 121:535–543
Davey MW, Franck C, Keulemans J (2004) Distribution, developmental and stress responses of antioxidant metabolism in Malus. Plant Cell Environ 27:1309–1320
De Tullio MC, Arrigoni O (2004) Hopes, disillusions and more hopes from vitamin C. Cell Molec Life Sci 61:209–219
Debolt S, Melino V, Ford CM (2007) Ascorbate as a biosynthetic precursor in plants. Ann Bot 99:3–8
Dhar-Mascareño M, Cárcamo JM, Golde DW (2005) Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Rad Biol Med 38:1311–1322
Dolatabadian A, Modarres-Sanavy SAM, Sharifi M (2009) Alleviation of water deficit stress effects by foliar application of ascorbic acid on Zea mays L. J Agron Crop Sci 195:347–355
Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N (2007) Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52:673–689
Eltayeb AE, Kawano N, Badawi H, Kaminaka H, Sanekata T, Morishima I, Shibahara T, Inanaga S, Tanaka K (2006) Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiol Plant 127:57–65
Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt, and polyethylene glycol stresses. Planta 225:1255–1264
Eltayeb AE, Yamamoto S, Eltayeb Habora ME, Yin L, Tsujimoto H, Tanaka K (2011) Transgenic potato overexpressing Arabidopsis cytosolic AtDHAR1 showed higher tolerance to herbicide, drought, and salt stresses. Breeding Sci 61:3–10
Endres S, Tenhaken R (2009) Myo-inositol oxygenase controls the level of myo-inositol in Arabidopsis, but does not increase ascorbic acud. Plant Physiol 149:1042–1049
Fischer H, Schwarzer C, Illek B (2004) Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci USA 101:3691–3696
Gallie DR (2013) The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot 64:433–443
Gatzek S, Wheeler GL, Smirnoff N (2002) Antisense supression of L-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate biosynthesis and reveals light modulated L-galactose synthesis. Plant J 30:541–553
Gest N, Garchery C, Gautier H, Jimenez A, Stevens R (2013) Light-dependent regulation of ascorbate in tomato by a monodehydroascorbate reductase localized in peroxisomes and the cytosol. Plant Biotech J 11:344–354
Gest N, Gautier H, Stevens R (2013) Ascorbate as seen through plant evolution: the rise of a successful molecule? J Exp Bot 64:33–53
Gilbert L, Alhagdow M, Nunes-Nesi A, Quemener B, Guillon F, Bouchet B, Faurobert M, Gouble B, Page D, Garcia V, Petit J, Stevens R, Causse M, Fernie AR, Lahaye M, Rothan C, Baldet P (2009) GDP-D-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell wall biosynthesis in tomato. Plant J 60:499–508
Goggin FL, Avila CA, Lorence A (2010) Vitamin C content in plants is modified by insects and influence susceptibility to herbivory. Bioessays 32:777–790
Goo YM, Chun HJ, Kim TW, Lee CH, Ahn MJ, Bae SC, Cho KJ, Chun JA, Chung CH, Lee SW (2008) Expressional characterization of dehydroascorbate reductase cDNA in transgenic potato plants. J Plant Biol 51:35–41
Guo Z, Tan H, Zhu Z, Lu S, Zhou B (2005) Effect of intermediates on ascorbic acid and oxalate biosynthesis of rice in relation to its stress resistance. Plant Physiol Biochem 43:955–962
Hamada AM, Al-Hakimi ABM (2009) Hydroponic treatment with ascorbic acid decreases the effect of salinity injury in two soybean cultivars. Phyton 49:43–62
Hancock R (2009) Recent patents on vitamin C: opportunities for crop improvement and single-step biological manufacture. Rec Patents Food Nutr Agric 1:39
Hancock RD, Walker PG, Pont SDA, Marquis N, Vivera S, Gordon SL, Brennan RM, Viola R (2007) L-Ascorbic acid accumulation in fruit of Ribes nigrum occurs by in situ biosynthesis via the L-galactose pathway. Funct Plant Biol 34:1080–1091
Harding A-H, Wareham NJ, Bingham SA, Khaw K, Luben R, Welch A, Forouhi NG (2008) Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the european prospective investigation of cancer-Norfolk prospective study. Arch Intern Med 168:1493–1499
Haroldsen VM, Chi-Ham CL, Kulkarni S, Lorence A, Bennett AB (2011) Constitutively expressed DHAR and MDHAR influence fruit, but not foliar ascorbate levels in tomato. Plant Physiol Biochem 49:1244–1249
Harris RS (2009) Analysis of the protective effects of ascorbic acid on thrichloroethylene and pyrene phytotoxicity. Master’s Thesis. Arkansas State University, Jonesboro, AR, USA
Hemavathi, Upadhyaya CP, Young KE, Akula N, Kim HS, Heung JJ, Oh OM, Aswath CR, Chun SC, Kim DH, Park SW (2009) Over-expression of strawberry D-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci 177:659–667
Horemans N, Foyer CH, Potters G, Asard H (2000) Ascorbate function and associated transport systems in plants. Plant Physiol Biochem 38:531–540
Huang A, Vita JA, Venema RC, Keaney Jr JF (2000) Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem 275:17399–17406
Imai T, Karita S, Shiratori G, Hattori M, Numome T, Oba K, Hirai M (1998) L-Galactono-ɣ-lactone dehydrogenase from sweet potato: purification and cDNA sequence analysis. Plant Cell Physiol 39:1350–1358
Imai T, Ban Y, Terakami S, Yamamoto T, Moriguchi T (2009) L-ascorbate biosynthesis in peach: cloning of six L-galactose pathway-related genes and their expression during peach fruit development. Physiol Plant 136:139–149
Imai T, Ban Y, Yamamoto T (2012) Ectopic overexpression of peach GDP-D-mannose pyrophosphorylase and GDP-D-mannose-3’,5’-eperimase in transgenic tobacco. Plant Cell Tiss Organ Cult 111:1–13
Jain AK, Nessler CL (2000) Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Mol Breeding 6:73–78
Keller R, Springer F, Renz A, Kossmann J (1999) Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J 19:131–141
Kiddle G, Pastori GM, Bernard S, Pignocchi C, Antoniw J, Verrier PJ, Foyer CH (2003) Effects of leaf ascorbate content on defense and photosynthesis gene expression in Arabidopsis thaliana. Antiox Redox Signal 5:23–32
Kulkarni S (2012) Elevating ascorbate content in tomato and studying the role of jasmonates in modulating ascorbate in Arabidopsis. MS Thesis, Arkansas State University, Jonesboro, AR, USA
Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160:347–353
Laing WA, Wright MA, Cooney J, Bulley SM (2007) The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci USA 104:9534–9539
Lee KW, Lee HJ, Surh YJ, Lee CY (2003) Vitamin C and cancer chemo- prevention: reappraisal. Am J Clin Nutr 78:1074–1078
Levi F, Pasche C, Lucchini F, La Vecchia C (2001) Dietary intake of selected micro- nutrients and breast-cancer risk. Int J Cancer 91:260–263
Li MJ, Ma FW, Zhang M, Pu F (2008) Distribution and metabolism of ascorbic acid in apple fruits (Malus domestica Borkh cv. Gala). Plant Sci 174:606–612
Li M, Ma F, Liang D, Li J, Wang Y (2010) Ascorbate biosynthesis during early fruit development is the main reason for its accumulation in kiwi. PLoS ONE 5. doi: 10.1371/journal.pone.0014281
Li F, Wu QY, Sun YL, Wang LY, Yang XH, Meng QW (2010) Overexpression of chloroplastic monodehydroascorbate reductase enhance tolerance to temperature and methyl viologen-mediated oxidative stress. Physiol Plant 139:421–434
Li Q, Li Y, Li C, Yu X (2012) Enhanced ascorbic acid accumulation through overexpression of dehydroascorbate reductase confers tolerance to methyl viologen and salt stresses in tomato. Czech J Genet Plant Breed 48:74–86
Linster CL, Gomez TA, Christensen KC, Adler LN, Young BD, Brenner C, Clarke SG (2007) Arabidopsis VTC2 encodes a GDP-L-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants. J Biol Chem 282:18879–18885
Lisko KA, Torres R, Harris RS, Belisle M, Jullian B, Vaughan MM, Chevone BI, Mendes P, Nessler CL, Lorence A (2013) Elevating vitamin C content via overexpression of myo-inositol oxygenase and L-gulono-1,4-lactone oxidase in Arabidopsis leads to enhanced biomass and tolerance to abiotic stresses. In Vitro Cel Develop Biol Plant 49(6):643–655
Liu Y, Yu L, Wang R (2011) Level of ascorbic acid in transgenic rice for L-galactono-1,4-lactone dehydrogenase overexpressing or suppressed is associated with plant growth and seed set. Acta Physiol Plant 33:1353–1363
Lorence A, Nessler CL (2007) Pathway engineering of the plant vitamin C metabolic network. In: Verpoorte R, Alferman AW, Johnson TS (eds) Applications of plant metabolic engineering. Springer, Dordrecht
Lorence A, Chevone BI, Mendes P, Nessler CL (2004) myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol 134:1200–1205
Mandl J, Szarka A, Bánhegyi G (2009) Vitamin C: update on physiology and pharmacology. British J Pharmacol 157:1097–1110
Margittai E, Banhegyi G, Kiss A, Nagy G, Mandl J, Schaff Z, Csala M (2005) Scurvy leads to endoplasmic reticulum stress and apoptosis in the liver of guinea pigs. J Nutr 135:2530–2534
Maruta T, Yonemitsu M, Yabuta Y, Tamoi M, Ishikawa T, Shigeoka S (2008) Arabidopsis phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. J Biol Chem 283:28842–28851
Maruta T, Ichikawa Y, Mieda T, Takeda T, Tamoi M, Yabuta Y, Ishikawa T, Shigeoka S (2010) The contribution of Arabidopsis homologs of L-gulono-1,4-lactone oxidase to the biosynthesis of ascorbic acid. Biosci Biotechnol Biochem 74:1494–1497
Masaki KH, Losonczy KG, Izmirlian G, Foley DJ, Ross GW, Petrovitch H, Havlik R, White LR (2000) Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 54:1265–1272
Mellidou I, Keulemans J, Kanellis AK, Davey MW (2012) Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol 12:239
Napoli C, Williams-Ignarro S, de Nigris F, Lerman LO, Rossi L, Guarino C, Mansueto G, Di Tuoro F, Pignalosa O, De Rosa G, Sica V, Ignarro LJ (2004) Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc Natl Acad Sci USA 101:8797–8802
Naqvi S, Zhu CF, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, Christou P (2009) Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci USA 106:7762–7767
Nessler CL, Lorence A, Chevone B, Mendes P (2007) Increase in plant growth rate, biomass accumulation and stress tolerance in plants over-expressing genes of the ascorbic acid-cell wall biosynthetic network. US Patent Application No 11/908, 551
Nunes-Nesi A, Carrari F, Lytovchenko A, Smith AM, Loureiro ME, Ratcliffe AG, Sweetlove LJ, Fernie AR (2005) Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol 137:611–622
Olmos E, Kiddle G, Pellny TK, Kumar S, Foyer CH (2006) Modulation of plant morphology, root architecture, and cell structure by low vitamin C in Arabidopsis thaliana. J Exp Bot 57:1645–1655
Ostergaard J, Persiau G, Davey MW, Bauw G, van Montagu G (1997) Isolation of a cDNA coding for L-galactono-ɣ-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants. J Biol Chem 272:30009–30016
Pallanca JE, Smirnoff N (1999) Ascorbic acid metabolism in pea seedlings. A comparison of D-glucosone, L-sorbosone, and L-galactono-1,4-lactone as ascorbate precursors. Plant Physiol 120:453–461
Passage E, Norreel JC, Noack-Fraissignes P, Sanguedolce V, Pizant J, Thirion X, Robaglia-Schlupp A, Pellissier JF, Fontés M (2004) Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat Med 10:396–401
Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15:939–951
Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH (2005) Ascorbic acid deficiency activates cell death and disease resistance in Arabidopsis thaliana. Plant Physiol 139:1291–1303
Potters G, De Gara L, Asard H, Hoemans N (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40:537–548
Radzio JA, Lorence A, Chevone BI, Nessler CL (2003) L-Gulono-1,4-lactone oxidase expression rescues vitamin C-deficient Arabidopsis (vtc) mutants. Plant Molec Biol 53:837–844
Rinne T, Mutschler E, Wimmer-Greinecker G, Moritz A, Olbrich HG (2000) Vitamins C and E protect isolated cardiomyocytes against oxidative damage. Int J Cardiol 75:275–281
Schleicher RL, Carroll MD, Ford ES, Lacher DA (2009) Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 90:1252–1263
Schwager J, Schulze J (1998) Modulation of interleukin production by ascorbic acid. Vet Immunol Immunopathol 64:45–57
Shalata A, Neumann PM (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduced lipid peroxidation. J Exp Bot 52:2207–2211
Smirnoff N (2011) Vitamin C: the metabolism anf functions of ascorbic acid in plants. Adv Bot Res 59:107–177
Torabinejad J, Donahue JL, Gunesekera BN, Allen-Daniels MJ, Gillaspy GE (2009) VTC4 is a bifunctional enzyme that affects myo-inositol and ascorbate biosynthesis in plants. Plant Physiol 150:951–961
Tóth SZ, Nagy V, Puthur JT, Kovacs L, Garab G (2011) The physiological role of ascorbate as photosystem II electron donor: protection against photoinactivation in heat-stressed leaves. Plant Physiol 156:382–392
Upadhyaya HCP, Akula N, Young KE, Young KE, Chun SC, Kim DH, Park SW (2010) Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol Lett 32:321–330
Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, Yamauchi Y, Nonaka H, Amako K, Yamawaki K, Murata N (2006) Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol 163:1179–1184
Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127:426–435
Wang Z, Xiao Y, Chen W, Tank K, Zhang L (2010) Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. JIPB 52:400–409
Wevar-Oller AL, Agostini E, Milrad SR, Medina MI (2009) In situ and de novo biosynthesis of vitamin C in wild type and transgenic tomato hairy roots: a precursor feeding study. Plant Sci 177:28–34
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369
Wintergerst ES, Maggini S, Hornig DH (2006) Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab 50:85–94
Wolucka BA, Van Montagu M (2003) GDP-mannose-3’-5’-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosyn thesis of vitamin C in plants. J Biol Chem 278:47483–47490
Yin L, Wanf S, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, Takeuchi Y, Tanaka K (2010) Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231:609–621
Yokoyama T, Date C, Kokubo Y, Yoshiike N, Matsumura Y, Tanaka H (2000) Serum vitamin C concentration was inversely associated with subsequent 20-year incidence of stroke in a japanese rural community: the Shibata study. Stroke 31:2287–2294
Zhang W, Gruszewski HA, Chevone BI, Nessler CL (2008) An Arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol 146:431–440
Zhang WY, Lorence A, Gruszewski HA, Chevone B, Nessler CL (2009) AMR1, an Arabidopsis gene that coordinately and negatively regulates the D-mannose/L-galactose ascorbic acid biosynthetic pathway. Plant Physiol 150:942–950
Zhang C, Liu J, Zhang Y, Cai X, Gong P, Zhang J, Wang T, Li H, Ye Z (2011) Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep 30:389–398
Zhang Z, Wang J, Zhang R, Huang R (2012) The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J 71:273–287
Acknowledgments
This study was supported by funds from the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000, and a sub-award to AL from the Arkansas INBRE program [National Center for Research Resources (5P20RR016460-11) and the National Institute of General Medical Sciences (8P20GM103429-11) from the National Institutes of Health]. The Scanalyzer HTS was acquired with funds from the Arkansas Center for Plant-Powered Production (P3) through the RII Arkansas ASSET Initiative (AR EPSCoR), NSF grant # EPS 0701890. KAL and SIA thank the Molecular Biosciences Graduate program at Arkansas State University for a scholarship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lisko, K., Aboobucker, S., Torres, R., Lorence, A. (2014). Engineering Elevated Vitamin C in Plants to Improve their Nutritional Content, Growth, and Tolerance to Abiotic Stress. In: Jetter, R. (eds) Phytochemicals – Biosynthesis, Function and Application. Recent Advances in Phytochemistry, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-319-04045-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-04045-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-04044-8
Online ISBN: 978-3-319-04045-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)