Abstract
Matrix solid phase dispersion (MSPD) is an extremely simple, fast and effective technique of sample preparation, in which sample destruction, homogenization and extraction takes place simultaneously in a single step of the MSPD procedure. This technique has been known for nearly 30 years. At that time, new types of sorbents, abrasive materials and extraction fluids were introduced to the MSPD procedure, and the process itself began to be assisted by ultrasounds, vortexing or microwaves. The result of these improvements is far-reaching miniaturization accompanied by greater isolation efficiency per unit of time achieved using more ecological and economical analytical procedures. Due to its flexibility and versatility, the MSPD technique is currently being implemented in various research laboratories for the isolation of endo- and exogenous compounds, including hazardous or prohibited compounds, volatile and non-volatile, present in various concentrations not only in solid but also in semi-solid and viscous samples, which can be generally grouped into environmental, biological, pharmaceuticals, food and everyday products samples. This chapter outlines the various analytical challenges where MSPD is useful and the sorbents that are currently being used to meet these challenges, with particular emphasis on new research areas where the MSPD process has come into use.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sample preparation
- Sorptive extraction
- Miniaturized sample preparation method
- Solventless extraction
- SSDM
- MSPD
1 Introduction
The term miniaturization is the key word of the modern world, a determinant of trends and directions of activities in many areas of our lives, including analytical chemistry and the related sample preparation process [1]. Behind miniaturization are the analytical capabilities of modern systems, especially chromatographic ones, which allow for the determination of compounds at increasingly lower concentration levels in the minimum amount of sample needed for a single and accurate analysis. In addition, miniaturization is supported by the desire to meet the challenges of modern analytics and innovative research areas, and the need to overcome the problems of classic methods of sample preparation, and especially making them more economical and ecological methods. This is all the more important as sample preparation is still a critical step in any analytical process [2,3,4,5]. Sample preparation usually consists of several stages, so it is not surprising that it is still one of the most laborious and time-consuming stages of any analytical procedure. In addition, this stage is extremely prone to errors, which often cannot be corrected at the later stages of the analytical procedure, because it is the properly targeted stage of sample preparation that guarantees the method of analysis independent of any changes in the sample matrix as well as accurate and indisputable results. Therefore, the correct preparation of the sample is not only the key to the success of the analysis, but also improves it, contributing to the increase in the number of analyses and the reduction of both labour time and costs.
Extraction is one of the most commonly used methods to prepare a sample for analysis. It owes its popularity to the ability to achieve all the objectives of the sample preparation step, as it allows for complete isolation of the analyte from complex and complicated matrices, concentration of the analyte, removal of accompanying interfering substances and replacement of the matrix with a solvent compatible with the target analytical technique. In addition, various physicochemical properties of analytes and matrices do not limit the area of its application and, as a result, it is used to isolate volatile and non-volatile compounds from solid, liquid and gaseous matrices [5]. The current trend in the use of the extraction method is focused on miniaturization in the broad sense, miniaturization understood as the use of scaled-down extraction systems capable of processing very small sample volumes using (if any) significantly reduced volumes of organic solvents, and simplification of analytical procedures by combining several stages of sample preparation or analytical procedure into one, while eliminating, importantly, the loss/degradation of sample components [1, 5, 6].
In the last decade, microextraction techniques of sorption extraction gained popularity, effectively displacing the classical methods of solvent extraction. One of the first such techniques is solid-phase microextraction (SPME). One of the newest is the supported liquid extraction (SLE) technique, touted as the best kept secret in sample preparation. Currently the block of miniaturized sorption extraction techniques includes stir bar sorptive extraction (SBSE), solid phase dynamic extraction (SPDE), microextraction in packed syringe (MEPS), microextraction by packed sorbent (MEPS), the combination of liquid–liquid extraction and dispersive solid phase extraction known as Quick Easy Cheap Effective Raged and Safe (simply QuECHERS) [1]. An important item in the aforementioned block of techniques is the matrix solid-phase dispersion (MSPD) technique, known for over 30 years. This is one of the least equipment-demanding techniques for the isolation of compounds from solids, with efficiency equal to advanced extraction techniques such as pressure liquid extraction (PLE). Due to its simplicity, the MSPD procedure can be performed by anyone, which makes it one of the most attractive and more frequently used methods of extraction applied in various areas of research, often going beyond the framework of chemical analytics. The validity of these statements is confirmed by the number of review papers dedicated to the progress and applications of the MSPD technique that have been published in the recent period [4, 6,7,8,9,10].
2 Fundamentals
2.1 General Information About MSPD
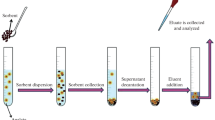
The MSPD technique, the stages of which are shown in Fig. 1, was introduced by Baker in the 1990s. This is a simpler version of the solid phase extraction (SPE) process. It involves grinding the sample with a solid abrasive material to obtain a semi-dry and homogeneous material with a specific structure. The abrasive material is most often a sorbent, which not only releases the analyte from the matrix but also increases the selectivity of the extraction. However, the sorbent can be replaced with another solid material, such as sand, to obtain a cheaper version of the process with the same isolation efficiency [9]. Homogenization is carried out in a mortar with a pestle made of glass, agate or quartz, and the addition of a small amount of solvent increases the dispersion of the sample components in the space of the abrasive material. The mixture obtained by grinding is quantitatively transferred to a syringe barrel (SPE column) with sintered paper at the bottom, pressed to ensure the best possible contact surface with the eluent, and then eluted dropwise under reduced pressure. Finally, the obtained extract is subjected to an analytical procedure.
The efficiency of the MSPD process depends on several factors, which can be easily optimized by selecting the appropriate type of abrasive, specifying the ratio of the mass of the sample to the abrasive, mixing time, composition of the eluent and/or its volume. Most often, due to the limited sorption capacity of the typically used sorbents, a four-fold excess of the sorbent mass in relation to the mass (amount) of the sample is used, with 0.5 g of the sample being typically used. Depending on the degree of hardness of the sample matrix, the homogenization time varies from 5 to 10 (15) min in the case of harder matrices. Taking into account that the elution stage is governed by the principles of frontal analysis and the first drops of the extract are the richest in compounds, only a small amount of extractant is needed for quantitative elution. As a result, MSPD allows you to reduce the consumption of organic solvent. Moreover, it promotes the concentration of compounds. An important feature of this extraction technique is that it does not require special equipment, and by combining sample breaking, extraction and purification in one step, it reduces sample preparation time. These attributes explain why MSPD is recognized as a very simple, cheap and quick sample preparation procedure that can be easily implemented in any research laboratory [9]. In addition, a wide range of different, more or less selective, ad(ab)sorptive abrasive materials suitable for use in the MSPD procedure makes it a technique with a great application potential.
To date, various MSPD materials have been introduced and employed such as e.g. silica- or carbon-based materials, nanoparticles, molecularly imprinted polymers, molecular sieves or ionic liquids [4, 6,7,8,9,10]. For example, Wianowska et al. which is discussed in more detail later in this chapter, applied sand as abrasive material for the extraction of volatile and non-volatile compounds from plants and herbs [11,12,13,14]. Sowa et al. showed that aniline deposited on silica gel particles successfully isolate triterpenes [15]. Yet, conventionally applied dispersion MSPD sorbents are silica gel, florisil and alumina. These are inorganic sorbents working, to use the typical chromatographic term, in the normal phase (NP) mode. For this reason, they are briefly referred to as normal phase materials (NP sorbents) capable of retaining polar analytes from less polar liquids by adsorption. Since this phenomenon is associated with access to functional groups present on the surface of the adsorbent, the extraction efficiency determined by the sorption capacity, a parameter characteristic of sorption processes, is often low and insufficient for effective concentration of the analytes. In order to increase the effectiveness of isolation, sorbents with a chemically bonded phase are used, which show absorptive properties in interactions with polar and non-polar components of the sample, while increasing the selectivity of the extraction process. One of the most commonly used sorbents of this type is silica gel with a chemically bound octadecyl phase (C18 sorbent). This material, in contrast to the previously mentioned group of inorganic sorbents, is used in the reversed phase (RP) system, enabling the extraction of non-polar (medium-polar) analytes.
As for the basic characteristics of the liquids used for elution of compounds from the MSPD blend, due to the destructive nature of the process, their choice mainly depends on the properties of the sorbent used and the target analytical technique. In general, non-polar eluting liquids are used when working with NP sorbents, while more polar liquids are used when working with RP sorbents. In the latter, eluents typically used include methanol, acetonitrile or acetone and mixtures of these solvents with water. Doping the organic solvent with water on the one hand allows to create a more selective eluting mixture, but is also a way to further reduce the consumption of organic, i.e. toxic liquids.
3 Novel Developments
The simplicity, effectiveness and versatility of MSPD makes it a technique worth working on, making it even better. In general, these efforts are focused on the use of new sorption or abrasive materials as alternatives to the commonly used silica-based materials in tandem with safer and environmentally friendly elution liquids and the development of more effective, faster and simpler MSPD procedures [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. In the latter case, the efforts go in two directions. Firstly, to increase the effectiveness of isolation from difficult matrices (hard or swellable), the MSPD process is supported by the application of additional force or energy source [28, 34, 36,37,38,39,40,41,42,43]. Secondly, to increase the selectivity of the extraction process, especially when using an abrasive material with inert properties, the MSPD process is combined with other extraction techniques [25, 26, 44,45,46]. Examples of the use of various MSPD materials, under specific and characteristic for them conditions, together with the overall analytical performance of the analytical method are presented in Table 1.
3.1 New Sorption Materials
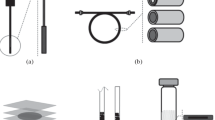
In the MSPD procedures, due to greater analytical possibilities, sorbents with a chemically bound phase are more willingly used. As mentioned, these sorbents, due to their significant sorption capacities, allow for more effective concentration of the analyte in the extract. In addition, owing to the access to a wide range of commercially available materials with different chemical properties of the functional groups forming the bonded phases, it is possible to select the one that will interact more selectively with the analyte. However, polar silica gel is most often used as a support of polar or non-polar functional groups. Thus, as a result of grinding the sample matrix with the RP sorbent, both polar and non-polar components of the sample interact with the support and the chemically bonded phase, and the expected high extraction selectivity is lost. Hence, the recently observed tendency to increase the sensitivity and selectivity of the analysis, especially of natural samples in the pre-analytical stage, is based on the use of very selective new materials. Providing a high selectivity of the extraction process, these materials have an extraordinary enrichment ability, which allows to reduce the consumption of solid and liquid reagents. Moreover, they make it possible to conduct g the MSPD process in a more miniaturized version. New MSPD materials, mostly adsorptive, with good chemical and mechanical properties are discussed below and illustrated in Fig. 2. However, it should be mentioned that this group also includes absorbents that have been known for a long time, but their usefulness in the MSPD process has been confirmed in recent years (e.g. polyvinylpolypyrrolidone) [16] and those that have recently been developed by combining well-defined polymers with inorganic substrates to create polymer-inorganic hybrids such as as SiO2/polyvinylimidazole hybrid polymer [17] with much better absorption capacity.
One of a rapidly developing technique for the preparation of functional polymers having specific molecular recognition properties is molecular imprinting. Thus, molecularly imprinted polymers (MIPs) are new selective sorbents for the MSPD prcedures of organic compounds in complex natural matrices. Their selectivity mimics the interactions between natural receptors in antibody-antigen interactions. It is based on the concept of matching a three-dimensional structure of a sorbent to the structure of an analyte molecule. To achieve this matching, functionalized monomers are polymerized around a template analyte molecule, creating a highly cross-linked three-dimensional network polymer with affinity only for the target molecule used in the imprinting procedure.
MIPs preparation is not simple. The most commonly reported issues include incomplete analyte template removal, non-uniform distribution, and poor site availability. However, the increased stability and resistance to a wide range of pH values, temperatures and solvent types compensate for the high price of materials available on the market. However, these materials can be prepared in the laboratory. An example of the synthesis and use of a MIP sorbent in the analysis of polyphenols, with the limit of detection (LOD) method established at 0.25 µg/mL, is available in [18]. The basic features of this and other representative MSPD procedure are summarized in Table 1. Another interesting article of the use of molecularly imprinted matrix solid-phase dispersion (MI-MSPD) in the plants analysis, showing the full spectrum of the possibilities of these materials, is presented in [19] on the example of the analysis of phytohormones from the auxin group in orange samples. According to the quoted paper, the only parameter that requires optimization is the sample to sorbent mass ratio. Under the optimal conditions, LOD is in the range of 1.2–2.4 ng/g.
The MI-MSPD procedures have also been used in the LC analysis of insecticides [20] and the GC analysis of phosphorothioate pesticides [21] in food samples. In the latter case, owing to the use of new molecularly imprinted polymer nanomicrospheres, which were synthesized using a typical structural analogue of tolclophos-methyl as a template by surface-graft polymerization on nanosilica, the detection limit of the method was obtained in the range of 0.012–0.026 ng/g and the recoveries ranged from 85.4 to 105.6% with RSD ≤ 9.6%.
In a different approach to the preparation of composite materials, owing to the use of the embedding method, not only the problems encountered in the synthesis of MIPs were eliminated, but also sorbents with exceptional enrichment capacity were obtained. In this context, special attention should be paid to sorbents in which a metal–organic framework (MOF) was used as a support for synthetic composite materials MOF-MIP.
The MOF materials are highly porous coordination polymers in which the three-dimensional structures of organic linkers with metal ions are formed through coordination bonds. There are few applications of these new sorbents in the context of microextraction of organic compounds. An exception is the article by Zhang et al. [22] which reports the synthesis of MOF (marked as MOF808) for accurate and sensitive analysis of ginsenosides in Panax ginseng C.A. Meyer root by means of MSPD and UPLC coupled to a quadrupole time-of-flight MS detector. In compliance with the article, under the optimal conditions, using only 20 mg of sorbent during 60 s grinding, analytical recoveries ranging from 87.04 to 103.78% are obtained, with RSD below 5% and LOD in the range from 0.087 to 0.114 μg/mL (see Table 1). According to the authors, the proposed MOF-assisted MSPD procedure, compared to the traditional extraction method and other published procedures, is characterized by higher extraction efficiency, simpler operation and provides a purer extract with less use of organic reagents.
Molecular sieves are another example of selective dispersion materials used in MSPD. These extremely selective materials are crystalline metal aluminosilicates composed of interconnected three-dimensional networks of tetrahedral oxides. Currently, more than 200 different molecular sieve frameworks have been described in the literature [9]. However, only two have been used in MSPD so far. These are sieves marked as SBA-15 and TS-1, the latter of which has a three-dimensional channel system with linear and zigzag locations. Both are attractive materials for MSPD due to their uniform and adjustable pores size, their large volumes, well-defined channels giving a high surface area to volume ratio, easy surface functionalization and hydrothermal stability. Their use allows for significant miniaturization of the sample preparation process for chromatographic analysis. For example, in [23], the SBA-15 molecular sieve was used to prepare a sample of orange fruit peels for the analysis of flavonoids using the UPLC-UV technique. Optimal extraction conditions boiled down to dispersing 25 mg of pre-ground sample in 25 mg of sorbent and then eluting the target compounds with only 0.5 mL of methanol, giving LOD of 0.02–0.03 µg/mL. In turn, in [24], the TS-1 mesoporous molecular sieve was used to prepare a sample of Schisandrae Chinensis Fructus for the analysis of lignans by microemulsion electrokinetic chromatography (MEEKC). The developed method, adapted to the preparation of a very small amount of sample (25 mg) with the use of an equally small amount of sorbent (50 mg) and low consumption of elution solvent, in accordance with the principles of green chemistry, showed good precision with the limit of quantification (LOQ) below 2.77 μg/mL. Compared to conventional MSPD procedures, the proposed methodology turned out to be extremely efficient, which was reflected in the modification of the MSPD name to the micro-MSPD version.
One of the newer research trends, already mentioned in the context of Sowa's research, is the use of sorbents obtained by modifying the silica gel surface with specialized liquids. These specialized liquids used in MSPD are mainly ionic liquids [25,26,27,28]. Nevertheless, the literature also describes the use of, e.g., poly(N-vinylimidazole) and deep eutectic solvents (DESs) for this purpose [29].
Ionic liquids (ILs) are non-molecular solvents that are organic and inorganic salts with the melting point below 100 °C. They are characterized by unique properties such as adjustable viscosity, miscibility with water and organic solvents, and low vapor pressure associated with high thermal stability. Moreover, they can be immobilized in the micropores of silica gel to obtain the silica-supported ionic liquid (S-SIL). The ILs ILs then lose their liquid state, but retain their beneficial properties. Since the S-SIL material has many micropores filled with ILs, S-SIL-based extraction improves the mass transfer rate and achieves a high level of recovery while reducing IL consumption. These features explain why S-SIL is now so often used as a dispersion adsorbent in the plant MSPD process. An example of the use of S-SIL materials in the MSPD process is the work [27] in which a methodology for the determination of phenolic compounds in a difficult material such as propolis was proposed. In the work, its authors showed that, compared to the classic ultrasonically assisted extraction (UAE) and extraction in the Soxhlet apparatus, their method allows for lower consumption of the sample and organic solvents and a shorter extraction time. In terms of the method performance, the limits of detection and quantification were in the range of 5.8–22.2 ng/mL and 19.2–74.0 ng/mL, respectively. The recoveries ranged from 65.51 to 92.32%, with RSDs lower than 8.95%.
As mentioned, the factor determining the success of the MSPD extraction is the selectivity and sorption capacity of the sorbents used in the process. In this respect, cyclodextrins (CDs) are an interesting material enabling the isolation of racemic mixtures, as well as configurational and constitutional isomers. The structure of these chiral materials has a characteristic truncated-cone shape. It is based on cyclic oligosaccharides built of D-glucose units linked by α(1,4)-glucosidic bonds. These materials, by selectively binding various molecules in their hydrophobic cavities, can form supramolecular host–guest complexes with high molecular recognition potential and excellent adsorption properties. One of the most commonly used CDs materials is β-CD containing seven glucose units in its structure. For example, in [30] this material was used for MSPD microextraction of various phenolic isomers from the honeysuckle flowers (Lonicera japonica Thunb.) before further analysis by UPLC-UV-Q-TOF/MS and NMR to determine and characterize the exact structure isolated compounds. In this method, 25 mg of samples were homogenized with 75 mg of β-CD using 0.5 mL of methanol–water mixture (80:20, v/v) as elution solvent, obtaining an LOD ranging from 1.62 to 3.33 ng/mL and recovery in the range of 87–105%. In [31], a mixture of β-CD and primary and secondary amine (PSA) sorbents was used for the isolation and quantification of ibuprofen enantiomers from human breast milk, combining a vortex-assisted MSPD and direct chiral liquid chromatography (CLC) with UV detection. The presence of the chiral β-CD sector was found to promote a variety of interactions resulting in good analytical performance o. In addition to the secondary interactions, hydrogen bonding or dipole–dipole interactions with the hydroxyl groups, the specific shape of β-CD and the appropriate size of the cavity, enabling the formation of inclusion complexes with enantiomers, determine the greater adsorption of the latter. Under optimal conditions (see Table 1), the proposed method provided good repeatability and accuracy, with RSDs of 6.4% and 8.3% for intra-day and inter-day precision, respectively, and recoveries in the range of 71.0–88.2%.
In this review of newer sorbents, it is also worth noting the possibilities of using chitin and chitosan as an alternative abrasive and adsorption materials in the miniaturized MSPD extraction process. Chitosan is produced as a result of deacetylation of one of the most common polysaccharides, i.e. chitin. It is mainly obtained from the hard outer skeleton of marine animals and insect cuticles. This material attracts attention with its special properties, which includ good biocompatibility, biodegradability and adsorption capacity guaranteed by a large surface area. In addition this material is characterized by, non-toxicity, renewability, and hydrophobicity [32]. It has many free hydroxyl and amino groups in its structure, allowing forming the hydrogen bonds and participation in electrostatic and ion-exchange interactions. For example, [33] describes the use of medium-molecular chitosan prior to the UPLC-Q-TOF/MS analysis of natural compounds (phenols) contained in plum fruits at various concentration levels. Optimized MSPD parameters were determined by choosing the amount of chitosan (25 mg), grinding time (60 s), and methanol:water (6:4, v:v) mixture as the eluting solvent. The method showed LOD in the range of 69.6–358.4 ng/g, and recoveries exceeded 80%. In [34], chitin and chitosan were used to extract various pharmaceuticals from fish samples using MSPD-LC–MS/MS. However, recoveries were low compared to other tested materials, with the best results being obtained with diatomaceous earth. Under optimal conditions, recoveries ranged from 58 to 128%, with RSD below 15% and LOQ values for all analytes ranging from 5 to 1000 ng/g. According to the authors, the reason for the unsatisfactory results was the irreversible sorption of the target compounds on both materials, because chitin and chitosan contain basic nitrogen centers, which gives these materials a greater ability to adsorb the analytes. To confirm the validity of the conclusion, the authors cited a higher extraction efficiency on chitin, which, compared to chitosan, contains less alkaline nitrogen centers.
To conclude the review of newer sorption materials that have been used in MSPD, it is worth mentioning nanoparticles. In general, two types of nanoparticles find application in the new MSPD procedures. The first type is titanium dioxide nanoparticles which, for example, Shen et al. [35] applied for the selective extraction, visualization and analysis of phospholipids from olive fruit and oil by a matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF/MS). The advantage of these materials is chemical stability in a wide range of pH and good adsorption capacity. The second type is magnetic nanoparticles of graphene oxide, used e.g. in [36], which allow for a significant simplification of the MSPD procedure and the possibility of their reuse, which makes this procedure even more ecological (see Table 1).
Nevertheless, over the last decade, not only materials with adsorption or absorption capabilities have been developed. As mentioned earlier, attention was also paid to sand in an alternative approach to the MSPD process, called the sea sand disruption method (SSDM). The rationale for using this inert material was to further simplify the MSPD process, reduce its cost, and make it more environmentally friendly. Currently, there are many examples of analytical procedures indicating the usefulness of this approach in the effective isolation of both essential oils (EOs), non-volatile analytes, and those that are not necessarily easy to recover from plant matrices [12, 49,50,51]. For example in [13, 14] it was shown that the optimal SSDM conditions for extracting EOs from conifer needles are determined by the sand to plant mass ratio of 24:1 with a volume of 3 mL of 1,4-dioxane used as the eluting solvent. The use of lower mass ratios, including the 4:1 ratio most often used in the conventional MSPD processes with a sorbent, results not only in lower efficiency of EOs components, but also proves to be difficult in the practical application. The blending step requires much more physical effort. Moreover, the bed of the homogenized material is less permeable to the eluting solvent.
At this point, however, it is worth emphasizing that the SSDM approach is eagerly used to release natural compoundsfrom hard matrices [9, 49, 50]. Another example of the use of this technique is the isolation of thermally unstable compounds, which is more effective compared to the amounts released by high-temperature extraction techniques [51,52,53,54]. This issue will be briefly discussed later. Nevertheless in order to balance the advantages of the SSDM process, it should be noted that the use of sand as an abrasive material leads to a reduction in its selectivity, which may be a problem when using less selective detectors such as UV [9, 44]. Therefore, the currently observed trend is the development of more complex procedures in which the efficiency of homogenization of the SSDM process, which goes hand in hand with the ability to release compounds even from strong interactions with other components of the matrix, is combined with the selectivity of other extraction techniques, especially sorptive extraction, e.g. in processes such as ion pair—solid-phase extraction (IP-SPE) [44].
3.2 Assisted MSPD Extraction
As mentioned, the MSPD process can be adjusted to improve the extraction efficiency. This usually involves selecting the appropriate type of sorbent, the composition of the eluent and/or its volume, as well as determining the sample to sorbent mass ratio and the mixing time. Occasionally, modifiers such as chelators, salts, acids, bases, and co-sorbents are added during the blending to increase the MSPD efficiency. A new trend in MSPD of very hard/difficult materials is the use of assisted methods of dispersion by means of ultrasounds, microwaves or vortex mixing. This not only shortens the extraction time, but also reduces the consumption of organic solvents and the amount of sample needed to fully isolation of trace compounds.
In vortexed-assisted MSPD (VA-MSPD), vortex mixing is used to further improve the isolation of compounds from solid biological matrices and save time. The procedure is carried out in two different ways. In the first one, after destruction/dispersion of the sample with a sorbent in a mortar, the resulting homogeneous mixture is quantitatively transferred to a centrifuge tube and then a solvent is added to the sample to obtain a suspension. The content of the tube is then vortexed for several minutes to increase the contact surface (usually from 1 to 3 min, see Table 1) and then centrifuged to recover the extract ready for analysis [34, 37]. In the second, the step of blending the sample in a mortar is omitted. A pre-prepared sample, e.g. by freezing, is dispersed by 2 min vortexing in a mixture of solids, then the blend is transferred to a SPE column filled with a co-sorbent at the bottom and finally eluted with a solvent by gravity or after applying a low vacuum [38, 39]. An interesting way of combining the vortexed-assisted MSPD approach with a non-toxic ionic liquid was proposed by Du et al. in [28] in the procedure called ionic liquid-based vortex-forced matrix solid phase dispersion (IL-VF-MSPD). In the cited paper, 1-dodecyl-3-methyl-1H-imidazolium bisulfate was applied as the ionic liquid used to elute 5-hydroxymethylfurfurol and iridoid glycosides from 20 mg of Fructus Corni. Silica (20 mg) was used as a dispersant and vortex mixing (3 min) was applied to contact the solid phase with 6 mL of the IL which, after centrifugation, was injected into the UPLC system (5 µL) achieving a limit of quantification (LOQ) in the range of 0.02–0.08 μg/mL. The recoveries were in the range of 95.2–103% (RSD < 5.0%).
In ultrasound-assisted MSPD (UA-MSPD), the sample homogenization or solvent elution step is amplified by ultrasounds. In the first case, a syringe cylinder filled with sorbent and sample, closed on both sides, is periodically sonicated in an ultrasonic bath [40]. In the second, a previously homogenized sample placed in a sealed vessel and flooded with the eluting solvent is subjected to ultrasound [41]. The use of the phenomenon of cavitation accompanying the passage of an acoustic wave through the extraction system is conducive in the first case to increasing the degree of sample homogenization and facilitating the release of compounds from the solid matrix, and in the second to increasing the mass transfer while reducing the volume of solvents used. Generally compared to the usual MSPD process, UA-MSPD shortens the extraction time and increases its efficiency even up to 25% [42].
The approach to enhance the MSPD efficiency with the use of microwaves is slightly different. In this strategy, microwaves are generally used after homogenization to facilitate elution of the compounds. For example, Zhang et al. [43] used microwave-assisted MSPD (MA-MSPD) coupled with LC-MS/MS to accurately determine two common and health-hazardous insecticides in rice samples. In this method, a 2 g sample of rice was homogenized with 4 g of basic alumina and, after addition of solvent, exposed to microwave radiation in a 600 W microwave oven to give a limit of detection (LOD) of 0.8 ng/g (the recoveries were in the range of 88.6–96.5% with RSD values of 2.6 and 8.1% for intra- and inter-day precision respectively).
The block of assisted MSPD techniques also includes a procedure in which the previously mentioned magnetic nanoparticles of graphene oxide are used. In this method, unlike those previously presented, it is possible to reuse of the sorbent. In addition, the extraction time is reduced by eliminating the need to fill the SPE column. The approach is known as magnetically assisted MSPD (MA-MSPD). It was introduced by Fotouhi et al. [36] for the determination of parabens in breast milk. In the developed method, a modified magnetic nanosorbent in the form of poly(indole-thiophene) magnetic graphene oxide in the amount of 50 mg was mixed with 200 µL of milk and 550 mg of Na2SO4. The mixture was transferred to a beaker containing 5.0 mL of distilled water, mechanically stirred for several minutes, and then the nanosorbent was separated from the suspension with a strong magnet and immersed in methanol (1.0 mL) to desorb the analytes from the sorbent, after which the sorbent was again removed from the eluate with a magnet, allowing it to be reused.
3.3 Coupling MSPD with Other Isolation Techniques
As mentioned in the Introduction, one of the areas of contemporary research activity is the development of efficient and sensitive methods that not only allow drawing quantitative conclusions from very small amounts of samples, but also lead to savings in reagents. And all this is due to the miniaturization of extraction systems. Above are presented those of the newly developed MSPD procedures in which the demands of selectivity, efficiency and miniaturization are intertwined into one due to the use of non-standard dispersion materials and those in which the efficiency of extraction is supported by the energy of microwaves, ultrasounds or the effect of vortex mixing. A characteristic feature of the newly developed MSPD procedures is also the combination of the MSPD process with other isolation techniques, in particular liquid–liquid microextraction techniques such as dispersion liquid–liquid microextraction (DLLME) and homogeneous liquid–liquid microextraction (HLLME). Another trend of changing MSPD selectivity, especially when using non-selective dispersion materials, is based on combining MSPD with backward extraction and/or extraction proceeding with the formation of neutral ion pairs of the analyte. These and other ways to change selectivity in the MSPD process will be outlined below.
DLLME and HLLME represent a new approach to liquid–liquid extraction (LLE) where the extraction efficiency depends on the contact area between the two liquid phases. In the former, the amount of organic solvent (extractant) is kept to a minimum, and in order to guarantee an appropriate contact surface, a slightly larger amount of another less toxic solvent is introduced that has an affinity for both the aqueous and organic phases. The liquid introduced into the extraction system is the so-called dispersant increasing the contact surface between the phases. The difference of the second approach consists in the use of alternative extraction solvents miscible with the water phase in an unlimited way. In HLLME, ILs or DESs are used as extractans. The general concept of ILs is presented above. So when it comes to DESs, they generally consist of two cheap components capable of self-association, often through hydrogen bonding interactions, to form an eutectic mixture with a melting point lower than the melting point of each individual component [45]. These eutectic solvents are soluble in water when they are made up of hydrophilic components, resulting in a homogeneous extraction system that guarantees the highest contact surface area between the phases. To convert this system into a two-phase system characteristic of LLE, an aprotic solvent is added to induce phase separation.
The combination of DLLME with the MSPD process was first presented by Yan et al. [46]. It is also worth emphasizing that these authors were the first to attempt to use molecularly imprinted microspheres (MIM) as an MSPD sorbent for the selective extraction and determination of Sudan dyes in egg yolks by the MIM-MSPD-DLLME-HPLC-UV method. MIM was synthesized by aqueous suspension polymerization using phenylamine-naphthol as a dummy template. In the developed method, briefly, 0.1 g portions of yolk were dispersed with 0.2 g MIM and eluted with 3 mL of acetone/acetic acid (95:5, v/v) which was used as the dispersing liquid in DLLME for further purification and enrichment of analytes prior to HPLC separation. The developed method combined the high selectivity of MIM, the excellent MSPD dispersion of complex solid samples and the high enrichment factor (over 18–20-fold) obtained by further purification using the DLLME technique.
Wang et al. [25] proposed combining diatomaceous earth with the surface-deposited IL (1-hexyl-3-methylimidazolium tetrafluoroborate [C6MIM][BF4]), used in amounts of 1.5 g and 0.12 mL, respectively, with HLLME for the analysis of illegal dyes (chrysoidins, safranins O, auramines O and rhodamines B) in spice samples by the IL-based MSPD-HLLME procedure. It should be clarified that [C6MIM][BF4] acted as the extraction solvent. In turn, the ion-pairing agent, [NH4]PF6, was used in HLLME method for further purification of the extract. For this purpose, the target analytes were eluted with 6 mL of water. Then, 1.0 mL of 2.0 M of [NH4]PF6 was added to the eluate (the molar ratio of [C6MIM][BF4] to [NH4]PF6 was 1:4) to facilitate the separation of the newly formed [C6MIM][PF6] phase. Finally, acetonitrile was added to dilute the extract and an aliquot was analyzed by UPLC-UV with LOD of 6.7–26.8 µg/kg.
Another interesting and clever method combining the high selectivity of ILs deposited on the surface of the sorbent with excellent dispersion of the MSPD process of solid samples is the method of ionic liquid-matrix solid-phase dispersion-solvent flotation (IL-MSPD-SF) developed by Zhan et al. [26] for the determination of acetanilide herbicides in rice samples. The proposed method resulted in high recoveries (89.4–108.7%) with RSD < 7.1%. LOQs were in the range from 38.0 to 84.7 µg/kg.
A simple, economic, and eco-friendly method able to detect triazole fungicides in tomato samples using a DES-based MSPD extraction followed by liquid–liquid back-extraction was porposed by Gallo et al. [55]. The developed method enables the MSPD extraction with alumina as a dispersant sorbent by replacing the organic solvents with a DES as extraction solvent during the MSPD blend elution. Choline chloride-ethylene glycol in a molar ratio of 1:2 (n/n) and ethyl acetate was used as a deep eutectic-organic solvent. Back extraction of analytes from the DES solution into ethyl acetate allows sample concentration overcoming the limited DES low vapor pressure, improving the method sensitivity. The LOQ was in the range of 5–11 ng/g and recoveries varied from 61 to 116%.
4 Main Application
By providing a complete fractionation of the sample matrix components and the ability to selectively elution of compounds from the sample, MSPD is widely used to isolate a variety of endogenous and exogenous compounds from solid, semisolid or viscous materials. A brief overview of MSPD applications in various analytical areas is presented in the aforementioned Table 1. A broader insight into the possibilities and contemporary applications of MSPD, along with a statistical analysis of the results of searching for information in available databases (Scopus, Web of Science) can be found in the review articles [6,7,8,9,10]. An updated view of the percentage use of MSPD by research areas, reflecting the thematic specificity of journals publishing the articles devoted to MSPD, together with the involvement of MSPD in the preparation of different samples types is presented in Fig. 3 a and b, respectively. This comprehensive search was conducted using keywords (“matrix solid-phase dispersion”, “matrix solid-phase disruption”, “MSPD”, “SSDM” and “plants”, “foods”, “animals”, “environmental samples”, “fishes”, tissues”, “soils”, “pharmaceuticals’, and “cosmetics”. The search was limited to the English language. In addition, the abstracts were pre-screened before studying the whole documents.
These data show that, apart from the typical field of MSPD application in chemical analysis of compounds, accounting for 35% of all applications, MSPD is widely used in the area of biochemical and molecular biology analyses (20% of applications). In addition, this method is also applied in the field of agricultural and environmental sciences, engineering, pharmacology, toxicology, pharmaceutics, and medicine. The percentage of MSPD use for the preparation of different sample types shown in Fig. 3b proves that the main area of MSPD applications relates to the preparation of processed food samples. Further places in the frequency of using this method are taken by the preparation of pre-unprocessed animal and plant tissue samples. Undoubtedly, the method is the least frequently used for the preparation of pharmaceutical and cosmetics samples [31, 34, 36, 56]. The use of MSPD for the preparation of environmental samples for pollutant analysis occupies an intermediate place. However, it should be emphasized that the analysis of compounds whose presence is in admissible or whose concentrations are limited to very low levels, especially pesticides and drugs residues, is the main area of MSPD application in the analysis of food and animal tissues. In addition, numerous papers have recently been published on the use of MSPD for multi-residue screening analyses in plant and animal tissues [16, 42, 56,57,58]. Yet, in plants research, the use of MSPD to analyze the main and characteristic components of a given plant comes to the fore. One of the trends in the use of MSPD in plant research is the analysis of unstable compounds that either can be degraded/transformed using conventional extraction methods, especially those that are applied at elevated temperature, or are formed during these processes not being a result of cellular metabolism [11, 49, 50, 59]. There is also great interest in the use of the solvent-free MSPD method to analyze the composition of plant essential oils [13, 14].
The versatility and flexibility of adapting the MSPD technique to solving various analytical problems is presented below, highlighting two areas of application of this technique, i.e. in the analysis of secondary plant metabolites and the determination of hazardous (potentially hazardous) substances in various types of natural and artificial matrices.
4.1 Analysis of Secondary Plant Metabolites
The term “secondary plant metabolites” identifies those compounds that are not directly involved in the normal growth and development of the plant. As already noted, their analysis is one of the main application areas of MSPD [11, 13, 14, 19, 23, 24, 33, 40, 49, 50, 59,60,61,62,63,64]. This is understandable, because the importance of these compounds for human health and many industries attracts the attention of researchers from many different fields. Nevertheless, the popularity of this topic is also supported by the number of currently known secondary plant metabolites (over 50,000). Among them, the group of polyphenolic compounds is the most numerous. These compounds are also the most widespread compounds found in nature. Due to their moderate polarity and large molecular sizes, they are routinely analyzed in the reversed phase LC systems. Therefore, the MSPD procedures typically use the C18-bounded silica sorbent and water-organic mixtures. Non-modified silica or Florisil, i.e. sorbents characteristic of the NP systems, are much less frequently used [40, 61, 62]. Among the new selective sorbents, MIPs, molecular sieves, titanium dioxide nanoparticles, cyclodextrins, chitosan or silica-supported ILs are most often used in these applications [18, 24, 27, 30, 33, 35]. In another approach, consistent with the principles of green chemistry, sand is used instead of the MSPD sorbent in order to increase the effectiveness of plant tissue disruption while reducing the cost of analysis [11,12,13,14, 49, 50].
As stated, a typical MSPD extraction of secondary plant metabolites proceeds in the reversed phase system. The best example of possibilities of the application of the RP systems is the paper by Deng et al. [47] where a modified MSPD procedure for profiling of plant hormones from the gibberellins group in a single leaf is presented. The modification concerned not only the reduction of the sample quantity (<1 mg) but also the amount of sorbent used (2 mg) in a single analysis and the way in which the MSPD process was realized. In this approach the grinding step, extraction and purification were performed in one microcentrifuge tube without any sample transfer step, resulting in an obvious decrease in the sample loss and an increase in the sensitivity (LOD was established on the attomol level).
In the context of the use of MSPD in the analysis of plant constituents, it is worth paying attention to those applications that relate to the extraction of essential oils (EOs). Due to the MSPD conditions, i.e. grinding in an open mortar, the suitability for EOs extraction can be considered as less attractive due to the high volatility of essential oil components. In addition, the analysis of the EOs composition is not easy and its result depends on the method of their isolation as shown in [53, 54, 65]. The Dawidowicz's research team was one of the first to demonstrate the usefulness of the MSPD process in the chromatographic analysis of EOs composition [13, 14]. Similarly, comparing the total amount and composition of EOs from various species of herbs and needles of coniferous trees with the amounts obtained by the steam distillation, recognized as the standard method of obtaining EOs and one of the most effective extraction techniques, i.e. pressure liquid extraction (PLE), they demonstrated that the efficiency of the MSPD process is equivalent to that obtained by both of the above-mentioned methods. Thus, MSPD is suitable for the isolation of these compounds, even if the C18 sorbent is replaced with sand, as mentioned earlier. In addition, they found that the MSPD method provides the most representative profile of all essential oil components because no heat is applied. Therefore, this environmentally friendly method was proposed by them as the main extraction procedure for the differentiation of essential oil components in plants for scientific and industrial purposes. In [66], the researchers proposed a different method of extracting essential oils using the MSPD technique with the solventless blending step, making the process even more environmentally friendly. The results presented in the cited article showed that when using the C18 sorbent in the MSPD process of volatile compounds, the use of a solvent at the grinding stage (the so-called dispersing liquid) is redundant, because the sorption capacity of the octadecyl brush is sufficient for the quantitative retention of isolated compounds. By studying various plants, the authors proved that the proposed method does not depend on the composition of essential oils and the volatility of individual components of the mixture under study. Then they showed that the extraction efficiency of the simplified MSPD method is equivalent to the conventional MSPD method and the PLE technique, which is a much more complex and technically advanced method of extracting plant components.
Concluding the review of the MSPD application in the analysis of secondary plant metabolites, it is worth bringing the previously mentioned topic of using sand in the MSPD procedure. Wianowska [12] successfully used it in the SSDM procedure for the analysis of quercetin in onions, apples and tea, revealing that only in the case of onions the SSDM results are not comparable with those obtained using PLE. This discrepancy became the basis for further studies, which showed that the instability of quercetin glycosidic derivatives under the PLE conditions and their degradation to quercetin aglycone is responsible for the overestimation of the amount of quercetin in onions by the PLE technique [5, 51]. The conclusion about the instability of phenolic compounds under the high-temperature extraction conditions leading to an overestimation of the extraction efficiency of some compounds, and thus to their incorrect quantitative estimation in plant materials, was independently confirmed by the results presented in [11, 49, 50, 67]. Two variants of the SSDM procedure were used in these experiments, with and without dispersant liquid, and both variants revealed comparable amounts of compounds. On their basis, a general conclusion was drawn that SSDM does not cause any transformations and/or degradation processes of secondary metabolites. Thus, the use of SSDM/MSPD in plant analysis not only allows to determine the actual concentration of individual compounds in plants, but also to determine which derivatives `are native plant components and what is their concentration level. Moreover using the MSPD process carried out under the conditions where the relationship between the reciprocal of the analyte efficiency and the mass ratio of the sorbent to the plant is linear, it is possible to estimate the actual content of a compound in a plant sample [63]. To appreciate the importance of this simple method of assessing the actual content of a compound, it should be added that there are few materials certified for the content of organic compounds, and hundreds of thousands of different organic compounds are known.
4.2 Analysis of Hazardous Substances
Apart from the analysis of plant components, the second important area of application of the MSPD technique is the analysis of hazardous substances, not only exogenous but also endogenous. The role of MSPD in this research area cannot be overlooked, if only for the reason that the MSPD technique was introduced to facilitate this type of analysis.
One of the priority groups of hazardous substances that are increasingly appearing in various elements of the environment all over the world, and especially in its bloodstream, i.e. the aquatic environment, is a group of organic pollutants known as endocrine disrupting chemicals (EDCs). These substances are not only ubiquitous but also permanent. These include many families of compounds that can cause disorders in the human endocrine system even at low doses. As a result, they are toxic and raise concerns about the potential negative effects not only on humans but also on wildlife, especially as they undergo bioaccumulation. The threats posed by these pollutants make it necessary to constantly monitor them. Therefore, new methods of their sensitive and selective analysis are being developed. An example of one such method using the MSPD technique is provided in the article [68], which presents the MSPD procedure in tandem with LC-MS/MS for the simultaneous analysis for 45 contaminants, including antibiotics, non-steroidal anti-inflammatory drugs, β-blockers, antidepressants, antimicrobials and preservatives in sewage sludge. Vela-Soria et al. [69] developed an accurate, selective and sensitive MSPD-UHPLC-MS/MS method for the simultaneous determination of 10 EDCs, including parabens and benzophenone-UV filters, in human placental tissue samples with a LOQ in the range of 0.2–0.4 ng/g and a non-precision of 5.4–12.8%. The main advantage of both examples of the above-mentioned methods is the possibility of comprehensive determination of many compounds in complex matrices, and owing to the use of MSPD, sample preparation is easier and faster to perform compared to other commonly used methods.
Casado et al. [70] developed a method combining MSPD sample preparation (using a mixture of sorbents, a strong cation exchanger and PSA) with LC-MS/MS for a more selective and sensitive analysis of azole antifungal drugs (absolute recoveries ranged from 70 to 118%, and the LOQs of the method ranged from 5 to 8 ng/g). These substances, apart from non-steroidal anti-inflammatory drugs, are another group of compounds recognized as emerging environmental pollutants as a result of their widespread use and relatively high stability during biological treatments in sewage treatment plants or during chlorination and disinfection of treated sewage with UV rays, leading to their ineffective removal. In addition, another manifestation of the toxic effects of these pharmaceuticals is their inhibitory effect on certain enzymes.
As mentioned, an important area of research is the analysis of drug residues in food. In [56], MSPD with UPLC-MS/MS was used to extract and determine the residues of 10 steroid hormones in food matrices, achieving a LOD of 10 ng/kg with recovery of hormones estimated for chicken, pork, beef and sausage ranging from 77 to 99% with RSDs less than 10%.
An example of substances prohibited for use in food products in any concentration due to their allergenic and/or asthmatic effects are synthetic dyes. Nevertheless, despite the ban, it happens that these substances are present in food products. The usefulness of MSPD in the analysis of Sudan dyes has already been cited earlier [46]. Similarly, the use of MSPD for the isolation of four artificial colorants from chili spice samples was mentioned [25]. As the tested dyes are water-soluble polar compounds, they were eluted from the MSPD blend using water instead of an organic solvent, which allowed the authors of this procedure to call the method an organic solvent-free MSPD procedure. In turn, in [71], a miniaturized version of the MSPD was developed for the rapid and simultaneous determination of nine regulated water-soluble dyes in personal care and decorative products using the Florisil sorbent.
Analysis of agrochemicals such as herbicides, insecticides, pesticides and fungicides is another extremely important research area with significant MSPD activity. For years, these substances have been commonly and often, unfortunately, incorrectly used to protect crops. The consequence is a negative impact on the entire ecosystem. These compounds get into the food chain, contaminate soil and surface waters. The knowledge of the risks resulting from the excessive use of agrochemicals has led to changes in the applicable maximum residue levels (MRLs) and forced a revision of the analytical procedures used so far. Nevertheless, because the skillful use of these compounds increases the quantity and quality of crops, new plant protection products are still being developed. In [72], a simple method for the determination of metrafenone is presented, which is a new type of fungicide with the MRL level established in the European Union from 0.01 to 2.00 mg/kg. The key for clean-up of the proposed method was the use of an appropriate combination of dispersant and elution solvent, respectively a 1:1 mixture of alumina with silica gel and dichloromethane. As a result, the LOD was obtained at the level of 2 µg/kg with recovery >96% and RSD < 10%. In [73], the MSPD procedure with Florisil was proposed for the analysis of one of the most mobile, both in the aquatic and terrestrial environments, herbicides characterized by high toxicity to aquatic plants, i.e. penoxsulam. In the cited paper, the results obtained by the MSPD technique were compared with those obtained by classical LLE, showing their convergence, although considering the amount of time and effort involved, the authors found the MSPD extraction to be superior. Another interesting example of using MSPD in the analysis of hazardous compounds is the work developed by Medina-Dzul et al. [48] describing the MSPD-GC/MS analytical procedure for the simultaneous isolation and quantification of organophosphorus compounds in beeswax.
Summing up the review of the newly developed MSPD extraction methods, it is worth emphasizing its applicability in the analysis of microcystins (MCs) causing the worldwide problem of “blooms”. MCs are a class of toxins produced by some freshwater cyanobacteria, mainly Microcystis aeruginosa, which are chemically covalently bound cyclic heptapeptides. More than 90 different MCs are known. Of these, the most common, toxic and the most studied isomeric form is MC marked as MC-LR. Qian et al. [60] developed a selective and sensitive method for the determination of MC-LR against other common MCs in vegetables, based on MSPD with a mixture of graphitized carbon black (GCB) and PSA, followed by HPLC-MS, setting the limits of detection of the proposed method at 13.0 μg/kg.
In conclusion, the versatility and flexibility of the MSPD process makes the technique applicable in the isolation of a wide variety of different compounds from hard or sticky and waxy matrices. However, the chemical nature of the typical analytes and thus the eluting solvents used in MSPD suggests that the technique is mainly used for the preparation of samples analyzed by liquid chromatography. Nevertheless, due to the availability of new selective sorbents, MSPD is increasingly used to prepare samples analyzed by gas chromatography [13, 14, 40, 41, 58, 64, 66]. MSPD's routine application area includes study of plants [11, 13, 14, 19, 23, 24, 33, 40, 49, 50, 59,60,61,62,63,64], meat [34, 37, 38, 56, 57, 69, 74], soils [73, 75], juices [19, 42], milk [58], oils [35] and breast milk [31, 36, 76]. New application of the MSPD method in very specific areas of analysis including forensic research are the examination of cosmetics [71], sediment [70, 72], human placenta [69, 74], molasses [77], bee products [27, 48] and even human hair [78].
5 Conclusions and Future Trends
One of the main challenges facing the analyst is to guarantee a sensitive and selective analysis of volatile and non-volatile compounds present at low concentrations levels in small amounts of complex matrices of solid, semi-solid and liquid samples. The access to modern extremely sensitive and selective chromatographic systems means that the responsibility for the quality of the analysis results rests at the sample preparation stage. At this stage, various more or less advanced extraction techniques are most often used. In the face of the growing need to reduce the cost of analyses and make them more environmentally friendly, the MSPD method enjoys the interest and attention of researchers from various fields of analytics.
The MSPD method is extremely simple, therefore it is fast and, what is important, cheap. It does not require special equipment and can be performed by anyone and anywhere. It allows you to carry out the entire sample preparation procedure in one stage of homogenization, extraction, purification and concentration, significantly reducing the time of sample preparation. Its effectiveness in isolating compounds is at least comparable to that of more sophisticated extraction techniques and therefore represents a simple and cheap alternative to them. However, by eliminating the need for high temperature to increase the efficiency of the extraction process, the MSPD reveals unique benefits and potential in the analysis of unstable compounds.
This chapter summarizes the recent achievements of the MSPD. These include the development of new adsorbents and compatible pro-environmental elution liquids as well as new more ecological and miniaturized procedures. These procedures allow to meet a variety of analytical challenges, which is a characteristic feature distinguishing the MSPD technique from other currently available sample preparation techniques. Since there is still room for improvement, this chapter identifies new tools to improve MSPD performance and selectivity by combining it with other extraction techniques, especially microextraction, and to further reduce sample preparation costs and organic solvent consumption by replacing the sorbent by sand and dissemination of the use of the solvent-free method even in the analysis of volatile compounds.
Abbreviations
- β-CD:
-
β-Cyclodextrin
- C18:
-
Chemically bound octadecyl phase
- CDs:
-
Cyclodextrins
- CLC:
-
Chiral liquid chromatography
- DESs:
-
Deep eutectic solvents
- DLLME:
-
Dispersive liquid–liquid microextraction
- EDCs:
-
Endocrine disrupting chemicals
- EOs:
-
Essential oils
- FID:
-
Flame ionization detector
- GC:
-
Gas chromatography
- GCB:
-
Graphitized carbon black
- HLLME:
-
Homogeneous liquid–liquid microextraction
- HPLC:
-
High performance liquid chromatography
- ILs:
-
Ionic liquids
- IL-VF-MSPD:
-
Ionic liquid based vortex-forced matrix solid phase dispersion
- IP-SPE:
-
Ion pair—solid-phase extraction
- LC:
-
Liquid chromatography
- LLE:
-
Liquid–liquid extraction
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- MALDI:
-
Matrix assisted laser desorption ionization
- MA-MSPD:
-
Microwave-assisted matrix solid phase extraction
- MCs:
-
Microcystins
- MEEKC:
-
Microemulsion electrokinetic chromatography
- MEPS:
-
Microextraction in packed syringe or microextraction by packed sorbent
- MI:
-
Molecularly imprinted
- MIM:
-
Molecularly imprinted microsphere
- MI-MSPD:
-
Molecularly imprinted matrix solid-phase dispersion
- MIPs:
-
Molecularly-imprinted polymers
- MOF:
-
Metal–organic framework
- MS:
-
Mass spectrometry
- MSPD:
-
Matrix solid-phase dispersion
- NMR:
-
Nuclear Magnetic Resonance
- NP:
-
Normal phase
- PLE:
-
Pressurized liquid extraction
- PSA:
-
Primary and secondary amine
- Q:
-
Quadrupole
- QuECHERS:
-
Quick Easy Cheap Effective Raged and Safe
- RP:
-
Reversed phase
- RSD:
-
Relative standard deviation
- SBSE:
-
Stir bar sorptive extraction
- SF:
-
Solvent flotation
- SLE:
-
Supported liquid extraction
- SPDE:
-
Solid phase dynamic extraction
- SPE:
-
Solid-phase extraction
- SPME:
-
Solid-phase microextraction
- SSDM:
-
Sea sand disruption method
- S-SIL:
-
Silica-supported ionic liquid
- q-TOF:
-
Time of flight
- UAE:
-
Ultrasonically assisted extraction
- UA-MSPD:
-
Ultrasound-assisted matrix solid phase dispersion
- UV:
-
Ultraviolet detection
- UPLC:
-
Ultra-performance liquid chromatography
- VA-MSPD:
-
Vortexed-assisted matrix solid phase dispersion
References
Wianowska D, Gil M, Olszowy M (2020) Miniaturized methods of sample preparation. In: Hussain Ch (ed) Handbook on miniaturization in analytical chemistry: application of nanotechnology. Elsevier, pp 99–125. https://doi.org/10.1016/b978-0-12-819763-9.00005-2
Martín-Pozo L, de Alarcón-Gómez B, Rodríguez-Gómez R, García-Córcoles MT, Çipa M, Zafra-Gómez A (2019) Analytical methods for the determination of emerging contaminants in sewage sludge samples. A review. Talanta 192:508–533. https://doi.org/10.1016/J.TALANTA.2018.09.056
Wianowska D, Gil M (2019) Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem Rev 18:273–302. https://doi.org/10.1007/s11101-018-9592-y
Hoff RB, Pizzolato TM (2018) Combining extraction and purification steps in sample preparation for environmental matrices: a review of matrix solid phase dispersion (MSPD) and pressurized liquid extraction (PLE) applications. TrAC—Trends Anal Chem 109:83–96. https://doi.org/10.1016/J.TRAC.2018.10.002
Wianowska D, Gil M (2019) Critical approach to PLE technique application in the analysis of secondary metabolites in plants. TrAC—Trends Anal Chem 114:314–325. https://doi.org/10.1016/j.trac.2019.03.018
Shi M-Z, Yu Y-L, Zhu S-C, Yang J, Cao J (2022) Latest development of matrix solid phase dispersion extraction and microextraction for natural products from 2015–2021. Sep Purif Rev. https://doi.org/10.1080/15422119.2022.2094274
Tu H, Chen W (2018) A review on the recent progress in matrix solid phase dispersion. Molecules 23(11):2767. https://doi.org/10.3390/molecules23112767
Capriotti AL, Cavaliere C, Foglia P, Samperi R, Stampachiacchiere S, Ventura S, Laganà A (2015) Recent advances and developments in matrix solid-phase dispersion. TrAC—Trends Anal Chem 71:186–193. https://doi.org/10.1016/j.trac.2015.03.012
Wianowska D, Gil M (2019) New insights into the application of MSPD in various fields of analytical chemistry. TrAC—Trends Anal Chem 112:29–51. https://doi.org/10.1016/j.trac.2018.12.028
El-Deen AK (2023) An overview of recent advances and applications of matrix solid-phase dispersion. Sep Purif Rev. https://doi.org/10.1080/15422119.2023.2172734
Wianowska D, Typek R, Dawidowicz AL (2015) How to eliminate the formation of chlorogenic acids artefacts during plants analysis? Sea sand disruption method (SSDM) in the HPLC analysis of chlorogenic acids and their native derivatives in plants. Phytochemistry 117:489–499. https://doi.org/10.1016/j.phytochem.2015.07.006
Wianowska D (2015) Application of sea sand disruption method for HPLC determination of quercetin in plants. J Liq Chromatogr Relat Technol 38:1037–1043. https://doi.org/10.1080/10826076.2015.1012520
Dawidowicz AL, Czapczyńska NB, Wianowska D (2013) Relevance of the sea sand disruption method (ssdm) for the biometrical differentiation of the essential-oil composition from conifers. Chem Biodivers 10:241–250. https://doi.org/10.1002/cbdv.201200001
Dawidowicz AL, Wianowska D, Rado E (2011) Matrix solid-phase dispersion with sand in chromatographic analysis of essential oils in herbs. Phytochem Anal 22:51–58. https://doi.org/10.1002/pca.1250
Sowa I, Wójciak-Kosior M, Strzemski M, Sawicki J, Staniak M, Dresler S, Szwerc W, Mołdoch J, Latalski M (2018) Silica modified with polyaniline as a potential sorbent for matrix solid phase dispersion (MSPD) and dispersive solid phase extraction (d-SPE) of plant samples. Materials 11(4):467. https://doi.org/10.3390/ma11040467
Cao Y, Tang H, Chen D, Li L (2015) A novel method based on MSPD for simultaneous determination of 16 pesticide residues in tea by LC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci 998–999:72–79. https://doi.org/10.1016/j.jchromb.2015.06.013
Medina-Dzul K, Carrera-Figueiras C, Pérez-Padilla Y, Vilchis-Nestor RA, López-Téllez G, Sánchez M, Muñoz-Rodríguez D (2015) SiO2/polyvinylimidazole hybrid polymer as a sorbent for extraction by matrix solid-phase dispersion (MSPD): synthesis, characterization, and evaluation. J Polym Res 22. https://doi.org/10.1007/s10965-015-0677-7
Hong Y, Chen L (2013) Extraction of quercetin from Herba Lysimachiae by molecularly imprinted-matrix solid phase dispersion. J Chromatogr B Anal Technol Biomed Life Sci 941:38–44. https://doi.org/10.1016/j.jchromb.2013.10.002
Yan H, Wang F, Wang H, Yang G (2012) Miniaturized molecularly imprinted matrix solid-phase dispersion coupled with high performance liquid chromatography for rapid determination of auxins in orange samples. J Chromatogr A 1256:1–8. https://doi.org/10.1016/j.chroma.2012.07.037
Chen L, Li B (2012) Determination of imidacloprid in rice by molecularly imprinted-matrix solid-phase dispersion with liquid chromatography tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 897:32–36. https://doi.org/10.1016/j.jchromb.2012.04.004
Zhou M, Hu N, Shu S, Wang M (2015) Molecularly imprinted nanomicrospheres as matrix solid-phase dispersant combined with gas chromatography for determination of four phosphorothioate pesticides in carrot and yacon. J Anal Methods Chem 2015. https://doi.org/10.1155/2015/385167
Zhang R, Xu N, Wang Y, Liu X, Wang S, Cao J (2020) Metal–organic framework assisted matrix solid-phase dispersion microextraction of saponins using response surface methodology. Electrophoresis 41(15):1354–1363. https://doi.org/10.1002/elps.202000042
Cao W, Hu SS, Ye LH, Cao J, Pang XQ, Xu JJ (2016) Trace matrix solid phase dispersion using a molecular sieve as the sorbent for the determination of flavonoids in fruit peels by ultra-performance liquid chromatography. Food Chem 190:474–480. https://doi.org/10.1016/j.foodchem.2015.05.133
Chu C, Wei M, Wang S, Zheng L, He Z, Cao J, Yan J (2017) Micro-matrix solid-phase dispersion coupled with MEEKC for quantitative analysis of lignans in Schisandrae Chinensis Fructus using molecular sieve TS-1 as a sorbent. J Chromatogr B Anal Technol Biomed Life Sci 1063:174–179. https://doi.org/10.1016/j.jchromb.2017.08.024
Wang Z, Zhang L, Li N, Lei L, Shao M, Yang X, Song Y, Yu A, Zhang H, Qiu F (2014) Ionic liquid-based matrix solid-phase dispersion coupled with homogeneous liquid-liquid microextraction of synthetic dyes in condiments. J Chromatogr A 1348:52–62. https://doi.org/10.1016/j.chroma.2014.04.086
Zhang L, Wang C, Li Z, Zhao C, Zhang H, Zhang D (2018) Extraction of acetanilides in rice using ionic liquid-based matrix solid phase dispersion-solvent flotation. Food Chem 245:1190–1195. https://doi.org/10.1016/j.foodchem.2017.11.029
Wang Z, Sun R, Wang Y, Li N, Lei L, Yang X, Yu A, Qiu F, Zhang H (2014) Determination of phenolic acids and flavonoids in raw propolis by silica-supported ionic liquid-based matrix solid phase dispersion extraction high performance liquid chromatography-diode array detection. J Chromatogr B Anal Technol Biomed Life Sci 969:205–212. https://doi.org/10.1016/j.jchromb.2014.08.022
Du K, Li J, Bai Y, An M, mei Gao X, xu Chang Y (2018) A green ionic liquid-based vortex-forced MSPD method for the simultaneous determination of 5-HMF and iridoid glycosides from Fructus Corni by ultra-high performance liquid chromatography. Food Chem 244:190–196. https://doi.org/10.1016/j.foodchem.2017.10.057
Gutiérrez-Solís MC, Muñoz-Rodríguez D, Medina-Peralta S, Carrera-Figueiras C, Ávila-Ortega A (2013) Matrix solid-phase dispersion extraction of organophosphorus pesticide using SiO2-poly(N-vinylimidazole). IOP Conf Ser Mater Sci Eng 45:5–9. https://doi.org/10.1088/1757-899X/45/1/012022
Xu JJ, Cao J, Peng LQ, Cao W, Zhu QZ, Zhang QY (2016) Characterization and determination of isomers in plants using trace matrix solid phase dispersion via ultrahigh performance liquid chromatography coupled with an ultraviolet detector and quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A 1436:64–72. https://doi.org/10.1016/j.chroma.2016.01.046
León-González ME, Rosales-Conrado N (2017) Determination of ibuprofen enantiomers in breast milk using vortex-assisted matrix solid-phase dispersion and direct chiral liquid chromatography. J Chromatogr A 1514:88–94. https://doi.org/10.1016/j.chroma.2017.07.072
Cao W, Hu S-S, Ye L-H, Cao J, Xu J-J, Pang X-Q (2015) Trace-chitosan-wrapped multi-walled carbon nanotubes as a new sorbent in dispersive micro solid-phase extraction to determine phenolic compounds. J Chromatogr A 1390:13–21. https://doi.org/10.1016/j.chroma.2015.02.060
Peng LQ, Li Q, xu Chang Y, An M, Yang R, Tan Z, Hao J, Cao J, Xu J, Hu SS (2016) Determination of natural phenols in olive fruits by chitosan assisted matrix solid-phase dispersion microextraction and ultrahigh performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A 1456:68–76. https://doi.org/10.1016/j.chroma.2016.06.011
Hertzog GI, Soares KL, Caldas SS, Primel EG (2015) Study of vortex-assisted MSPD and LC-MS/MS using alternative solid supports for pharmaceutical extraction from marketed fish. Anal Bioanal Chem 407:4793–4803. https://doi.org/10.1007/s00216-015-8685-3
Shen Q, Dong W, Yang M, Baibado JT, Wang Y, Alqouqa I, Cheung HY (2013) Lipidomic study of olive fruit and oil using TiO2 nanoparticle based matrix solid-phase dispersion and MALDI-TOF/MS. Food Res Int 54:2054–2061. https://doi.org/10.1016/j.foodres.2013.10.001
Fotouhi M, Seidi S, Shanehsaz M, Naseri MT (2017) Magnetically assisted matrix solid phase dispersion for extraction of parabens from breast milks. J Chromatogr A 1504:17–26. https://doi.org/10.1016/j.chroma.2017.05.009
Escarrone ALV, Caldas SS, Soares BM, Martins SE, Primel EG, Maia Nery LE (2014) A vortex-assisted MSPD method for triclosan extraction from fish tissues with determination by LC-MS/MS. Anal Methods 6:8306–8313. https://doi.org/10.1039/c4ay01518e
Chen JM, Yang CC, Chung WH, Ding WH (2016) Vortex-homogenized matrix solid-phase dispersion coupled with gas chromatography-electron-capture negative-ion mass spectrometry to determine halogenated phenolic compounds in seafood. RSC Adv 6:96510–96517. https://doi.org/10.1039/c6ra20680h
Chen YH, Chang CY, Ding WH (2016) Vortex-homogenized matrix solid-phase dispersion for the extraction of short chain chlorinated paraffins from indoor dust samples. J Chromatogr A 1472:129–133. https://doi.org/10.1016/j.chroma.2016.10.048
Albero B, Sánchez-Brunete C, Miguel E, Tadeo JL (2017) Application of matrix solid-phase dispersion followed by GC-MS/MS to the analysis of emerging contaminants in vegetables. Food Chem 217:660–667. https://doi.org/10.1016/j.foodchem.2016.09.017
Aznar R, Albero B, Sánchez-Brunete C, Miguel E, Martín-Girela I, Tadeo JL (2017) Simultaneous determination of multiclass emerging contaminants in aquatic plants by ultrasound-assisted matrix solid-phase dispersion and GC-MS. Environ Sci Pollut Res 24:7911–7920. https://doi.org/10.1007/s11356-016-6327-8
Barfi B, Asghari A, Rajabi M, Barfi A, Saeidi I (2013) Simplified miniaturized ultrasound-assisted matrix solid phase dispersion extraction and high performance liquid chromatographic determination of seven flavonoids in citrus fruit juice and human fluid samples: hesperetin and naringenin as biomarkers. J Chromatogr A 1311:30–40. https://doi.org/10.1016/j.chroma.2013.08.078
Zhang P, Ding J, Hou J, Zhao L, Chen Y, Ding L (2017) Dynamic microwave assisted extraction coupled with matrix solid phase dispersion for the determination of chlorfenapyr and abamectin in rice by LC-MS/MS. Microchem J 133:404–411. https://doi.org/10.1016/j.microc.2017.04.006
Wianowska D (2022) Combination of sea sand disruption method and ion-pair solid-phase extraction for effective isolation and purification of chlorogenic acid from plants prior to the HPLC determination. Molecules 27(12):5601. https://doi.org/10.3390/molecules27175601
Lomba L, Ribate P, Sangüesa E, Concha J, Garralaga P, Errazquin D, García CB, Giner B (2021) Deep eutectic solvents: are they safe? Appl Sci 11(21):10061. https://doi.org/10.3390/app112110061
Yan H, J.Qiao J, Wang H, Yang G, Row KH (2011) Molecularly imprinted solid-phase extraction combined with ultrasound-assisted dispersive liquid–liquid microextraction for the determination of four Sudan dyes in sausage samples. Analyst 136:2629–2634. https://doi.org/10.1039/c0an00951b
Deng T, Wu D, Duan C, Yan X, Du Y, Zou J, Guan Y (2017) Spatial profiling of Gibberellins in a single leaf based on microscale matrix solid-phase dispersion and precolumn derivatization coupled with ultraperformance liquid chromatography-tandem mass spectrometry. Anal Chem 89:9537–9543. https://doi.org/10.1021/acs.analchem.7b02589
Medina-Dzul K, Medina-Peralta S, Carrera-Figueiras C, Sánchez M, Muñoz-Rodríguez D (2017) Matrix solid-phase dispersion extraction of organophosphorous pesticides from beeswax. Int J Environ Anal Chem 97:831–840. https://doi.org/10.1080/03067319.2017.1363195
Wianowska D, Dawidowicz AL, Bernacik K, Typek R (2017) Determining the true content of quercetin and its derivatives in plants employing SSDM and LC-MS analysis. Eur Food Res Technol 243:27–40. https://doi.org/10.1007/s00217-016-2719-8
Typek R, Dawidowicz AL, Wianowska D, Bernacik K, Stankevič M, Gil M (2019) Formation of aqueous and alcoholic adducts of curcumin during its extraction. Food Chem 276:101–109. https://doi.org/10.1016/j.foodchem.2018.10.006
Wianowska D, Dawidowicz AL (2016) Effect of water content in extraction mixture on the pressurized liquid extraction efficiency—stability of quercetin 4′-glucoside during extraction from onions. J AOAC Int 99:744–749. https://doi.org/10.5740/jaoacint.16-0019
Teixeira DM, Patão RF, Coelho AV, da Costa CT (2006) Comparison between sample disruption methods and solid–liquid extraction (SLE) to extract phenolic compounds from Ficus carica leaves. J Chromatogr A 1103:22–28. https://doi.org/10.1016/J.CHROMA.2005.11.047
Wianowska D (2014) The influence of purge times on the yields of essential oil components extracted from plants by pressurized liquid extraction. J AOAC Int 97:1310–1316. https://doi.org/10.5740/jaoacint.13-318
Dawidowicz AL, Czapczyńska NB, Wianowska D (2012) The loss of essential oil components induced by the Purge Time in the Pressurized Liquid Extraction (PLE) procedure of Cupressus sempervirens. Talanta 94:140–145. https://doi.org/10.1016/j.talanta.2012.03.008
Gallo V, Della Posta S, Gentili A, Gherardi M, De Gara L, Fanali Ch (2023) Back-extraction applied to green matrix solid-phase dispersion for fungicides determination in tomatoes. Sep Sci Plus 2200140. https://doi.org/10.1002/sscp.202200140
Fan YB, Yin YM, Bin Jiang W, Chen YP, Yang JW, Wu J, Xie MX (2014) Simultaneous determination of ten steroid hormones in animal origin food by matrix solid-phase dispersion and liquid chromatography-electrospray tandem mass spectrometry. Food Chem 142:170–177. https://doi.org/10.1016/j.foodchem.2013.06.104
Li H, Sun N, Zhang J, Liang S, Sun H (2014) Development of a matrix solid phase dispersion-high performance liquid chromatography-tandem mass spectrometric method for multiresidue analysis of 25 synthetic colorants in meat products. Anal Methods 6:537–547. https://doi.org/10.1039/c3ay41664j
Cai H, Ji S, Zhang J, Shang G, Tao G, Peng C, Chen G, Hou R, Zhang L, Wan X (2017) Determination of 11 photoinitiators and their migration into tea and milk by gas chromatography-tandem mass spectrometry (MSPD-GC-MS/MS). Anal Methods 9:2957–2963. https://doi.org/10.1039/c7ay00156h
Wianowska D, Typek R, Dawidowicz AL (2015) Chlorogenic acid stability in pressurized liquid extraction conditions. J AOAC Int 98:415–421. https://doi.org/10.5740/jaoacint.14-200
Qian ZY, Li ZG, Ma J, ting Gong J, Xian QM (2017) Analysis of trace microcystins in vegetables using matrix solid-phase dispersion followed by high performance liquid chromatography triple-quadrupole mass spectrometry detection. Talanta 173:101–106. https://doi.org/10.1016/j.talanta.2017.05.079
Gao Y, Sun Y, Wang Y, Zhang J, Xu B, Zhang H, Song D (2013) A practical and rapid method for the simultaneous isolation, purification and quantification of geniposide from the fruit of Gardenia jasminoides Ellis by MSPD extraction and UFLC analysis. Anal Methods 5:4112–4118. https://doi.org/10.1039/c3ay40638e
Han Y, Wen J, Zhou T, Fan G (2015) Chemical fingerprinting of Gardenia jasminoides Ellis by HPLC-DAD-ESI-MS combined with chemometrics methods quality evaluation. Food Chem 188:648–657. https://doi.org/10.1016/j.foodchem.2015.05.039
Dawidowicz AL, Wianowska D (2009) Application of the MSPD technique for the HPLC analysis of rutin in Sambucus nigra L.: the linear correlation of the matrix solid-phase dispersion process. J Chromatogr Sci 47:914–918
Rashidipour M, Heydari R, Feizbakhsh A, Hashemi P (2015) Rapid monitoring of carvacrol in plants and herbal medicines using matrix solid-phase dispersion and gas chromatography flame ionisation detector. Nat Prod Res 29:621–627. https://doi.org/10.1080/14786419.2014.980247
Mardarowicz M, Wianowska D, Dawidowicz AL, Sawicki R (2004) Comparison of terpene composition in Engelmann Spruce (Picea engelmannii) using hydrodistillation, SPME and PLE. Zeitschrift Für Naturforsch C 59:641–648. https://doi.org/10.1515/znc-2004-9-1006
Wianowska D, Dawidowicz AL (2016) Can matrix solid phase dispersion (MSPD) be more simplified? Application of solventless MSPD sample preparation method for GC-MS and GC-FID analysis of plant essential oil components. Talanta 151:179–182. https://doi.org/10.1016/J.TALANTA.2016.01.019
Wianowska D (2014) Hydrolytical instability of hydroxyanthraquinone glycosides in pressurized liquid extraction. Anal Bioanal Chem 406:3219–3227. https://doi.org/10.1007/s00216-014-7744-5
Li M, Sun Q, Li Y, Lv M, Lin L, Wu Y, Ashfaq M, ping Yu C (2016) Simultaneous analysis of 45 pharmaceuticals and personal care products in sludge by matrix solid-phase dispersion and liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 408:4953–4964. https://doi.org/10.1007/s00216-016-9590-0
Vela-Soria F, Rodríguez I, Ballesteros O, Zafra-Gómez A, Ballesteros L, Cela R, Navalón A (2014) Simplified matrix solid phase dispersion procedure for the determination of parabens and benzophenone-ultraviolet filters in human placental tissue samples. J Chromatogr A 1371:39–47. https://doi.org/10.1016/j.chroma.2014.10.063
Casado J, Castro G, Rodríguez J, Ramil M, Cela R (2015) Selective extraction of antimycotic drugs from sludge samples using matrix solid-phase dispersion followed by on-line clean-up. Anal Bioanal Chem 407:907–917. https://doi.org/10.1007/s00216-014-8167-z
Guerra E, Celeiro M, Lamas JP, Llompart M, Garcia-Jares C (2015) Determination of dyes in cosmetic products by micro-matrix solid phase dispersion and liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A 1415:27–37. https://doi.org/10.1016/j.chroma.2015.08.054
Li J, Li Y, Xu D, Zhang J, Wang Y, Luo C (2017) Determination of metrafenone in vegetables by matrix solid-phase dispersion and HPLC-UV method. Food Chem 214:77–81. https://doi.org/10.1016/j.foodchem.2016.07.061
Kaur P, Kaur K, Bhullar MS (2014) Quantification of penoxsulam in soil and rice samples by matrix solid phase extraction and liquid-liquid extraction followed by HPLC-UV method. Environ Monit Assess 186:7555–7563. https://doi.org/10.1007/s10661-014-3947-7
Villaverde-de-Sáa E, Rodil R, Quintana JB, Cela R (2016) Matrix solid-phase dispersion combined to liquid chromatography–tandem mass spectrometry for the determination of paraben preservatives in mollusks. J Chromatogr A 1459:57–66. https://doi.org/10.1016/j.chroma.2016.06.070
Iparraguirre A, Rodil R, Quintana JB, Bizkarguenaga E, Prieto A, Zuloaga O, Cela R, Fernández LA (2014) Matrix solid-phase dispersion of polybrominated diphenyl ethers and their hydroxylated and methoxylated analogues in lettuce, carrot and soil. J Chromatogr A 1360:57–65. https://doi.org/10.1016/j.chroma.2014.07.079
Samanidou VF, Frysali MA, Papadoyannis IN (2014) Matrix solid phase dispersion for the extraction of bisphenol a from human breast milk prior to HPLC analysis. J Liq Chromatogr Relat Technol 37:247–258. https://doi.org/10.1080/10826076.2012.745133
Kamal YT, Alam P, Alqasoumi SI, Foudah AI, Alqarni MH, Yusufoglu HS (2018) Investigation of antioxidant compounds in commercial pomegranate molasses products using matrix-solid phase dispersion extraction coupled with HPLC. Saudi Pharm J 26:839–844. https://doi.org/10.1016/j.jsps.2018.03.015
Argente-García A, Moliner-Martínez Y, Campíns-Falcó P, Verdú-Andrés J, Herráez-Hernández J (2016) Determination of amphetamines in hair by integrating sample disruption, clean-up and solid phase derivatization. J Chromatogr A 1447:47–56. https://doi.org/10.1016/j.chroma.2016.04.036
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors have declared no conflict of interest.
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wianowska, D., Olszowy-Tomczyk, M. (2024). Matrix Solid-Phase Disperion. In: Rodríguez-Delgado, M.Á., Socas-Rodríguez, B., Herrera-Herrera, A.V. (eds) Microextraction Techniques. Integrated Analytical Systems. Springer, Cham. https://doi.org/10.1007/978-3-031-50527-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-50527-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50526-3
Online ISBN: 978-3-031-50527-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)