Abstract
From its introduction until now, more than 20 years ago, stir bar sorptive extraction (SBSE) has been consolidated as sample preparation technique. This chapter revisits the fundamentals of this solventless technique and discusses the different aspects affecting its performance, with special emphasis on working under non-equilibrium conditions. Special attention is focused on its limitations, mainly those derived for the extraction of non-polar compounds, and how researchers try to solve them by resorting to derivatization strategies, by developing new workflows and approaches, and/or by proposing new sorbents and synthetic procedures. Those SBSE-derived extraction techniques and the advantages they present are also described and deeply discussed. An exhaustive revision of those published papers just applying these techniques are not described considering they have been extensively compiled in recent published review articles, but those contributing with some of the above-mentioned developments are commented on.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Stir bar sorptive extraction, abbreviated as SBSE, is a solventless extraction technique invented in 1999, and patented, by Prof. Sandra and co-workers [1]. In its original format, it consists of the partition of analytes between an aqueous sample (or solution) and polydimethylsiloxane (PDMS) (used as sorbent) immobilized on a magnetic-core bar (typically 10–40 mm length) immersed into the aqueous phase, in such a way the bar is stirred by using a laboratory magnetic stirrer. After a defined period of time, the agitation is stopped and the stir bar containing the analytes is taken out and rinsed with deionized water. Afterwards, it is carefully dried with a paper tissue or under a nitrogen stream, and subsequently either it is stirred into an appropriate solvent to back-extract the analytes by liquid desorption (LD), or it is subjected to high temperatures, in case of (semi)volatile and thermally-stable compounds, to desorb them by thermal desorption (TD). LD allows multiple analysis of the extract and it is the preferred option for the subsequent measurement by liquid chromatography (LC), capillary electrophoresis (CE) or inductively coupled plasma (ICP), among others. On the contrary, TD is the preferred option when gas chromatography (GC) is used, and it allows achieving higher sensitivity than with LD, since all the extracted amount is transferred to the measuring instrument. Figure 1 shows a schematic representation of the experimental procedure of SBSE.
From an operational point of view, SBSE is very simple and easy to carry it out, without requiring supervision, so it can be working overnight to compensate the long extraction times often required. Before their use, the stir bar needs to be conditioned by cleaning with suitable solvents (e.g., acetonitrile) or through thermal treatment (e.g., 320 °C) to minimize interferences and memory effects [2].
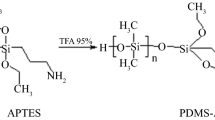
Although nowadays the stir bar is made up of different materials and forms, as it will be discussed later, the original stir bar consisted of three parts, i.e., a magnetic rod that enables the rotating movement, a glass jacket coating the magnetic rod, and a thin layer of PDMS coating the glass jacket where the analytes are really extracted by means of hydrophobic interactions through Van der Waals forces, although hydrogen bonds can also be stablished [3]. Although the intermediate glass jacket could seem unnecessary, it was essential to prevent decomposition of PDMS catalyzed by the metallic rod [4]. These devices have been, and still are, for many years marketed by Gerstel GmbH & Co. KG under the trade name of Twister® [5, 6]. This information will be completed later.
Much has been written about SBSE, as evidenced by the countless review articles that can be found in the bibliography describing the principles and applications of this technique [3, 7,8,9,10,11]. The objective of this chapter is not to repeat once again what has already been published but to revisit the fundamentals and describe them from a more didactic perspective, while describing the evolution of this technique through novel sorbents, instrumental developments, and derived techniques.
2 Fundamentals
SBSE emerged as a way to enhance the extraction efficiency (EE), and thus the sensitivity, achieved by solid-phase microextraction (SPME) (described in this chapter), the unique sorbent-based microextraction technique that existed at that moment.
Both microextraction techniques are based on the partition of the target compound (e.g., A) between the aqueous sample and a small amount of PDMS immobilized in an inert support, either a stir bar in SBSE or a fiber in SPME. The equilibrium constant governing this equilibrium, i.e., the partitioning coefficient (KPDMS/water) can be defined as:
where [A] is the concentration of the compound A either extracted in the PDMS phase or remained in the aqueous phase once the equilibrium is reached, respectively, which in turn can be expressed as the ratio between the mass (m) of the compound A in each phase with respect to the volume (V) of each phase, respectively.
The EE for this compound A is defined as the ratio between the amount extracted of this compound in the PDMS layer (mextracted) with respect to the amount of the same compound that was initially present in the aqueous sample (minitial). After equilibrium is reached, the initial amount is distributed between the PDMS layer and the aqueous solution (mremaining), thus:
Combining both equations, it is easy to see that EE depends on KPDMS/water and volumes ratio. Since the Ko/water values are usually accessible unlike KPDMS/water ones, and PDMS behaves similarly to octanol, it could be assumed that KPDMS/water ~ Ko/water, and thus:
In a partition equilibrium, unlike an adsorption equilibrium where the compounds are adsorbed in the active sites on the surface, the total amount of the extraction phase has a high influence on the EE. According to Eq. 3, for the same compound under the same extraction conditions, the lower the amount of PDMS is, the lower the EE is. This is the reason why EE values are low in SPME, since the extremely thin PDMS-coated fused silica or stainless-steal fibers limit the amount of available PDMS (typically around 0.5 μL for a 100-μm film thickness [1]). On the contrary, the PDMS amount is much higher in SBSE due to the higher surface area of the stir bar, which depending on its length (10–20 mm) and thickness (0.5–1.0 mm) can reach more than 120 μL [5], i.e., more than 240 times compared to SPME. As EE does not depend linearly on VPDMS, the sensitivity does not increase in the same way as VPDMS, as wrongly stated elsewhere, but it does increase notably. The difference between both approaches can be visualized by representing Eq. 3 as in Fig. 2, where, as a practical example, 25 mL of aqueous sample is extracted with SPME (VPDMS = 0.25 and 0.5 μL) or with SBSE (VPDMS = 60 and 120 μL). As it can be seen, the theoretical EE increases with Ko/water, or what is the same, the extraction is more favourable the more non-polar the compound is (i.e., higher Ko/water). As also predicted by Eq. 3, the EE is higher as the amount of PDMS increases, and thus the EE is always superior for SBSE than for SPME. It should be noticed that for moderately non-polar compounds (Ko/water ~ 103–104), which represent most of applications, EE > 70% is achieved by SBSE, whereas it does not reach to 20% for SPME. To a lesser extent, SBSE allows the extraction of polar and medium-polar compounds (Ko/water < 103), which are hardly achievable by SPME. For SPME, quantitative EE values are just achieved for extremely non-polar compounds (Ko/water > 106) and thus quantitative extractions are hardly encountered by employing this microextraction technique.
If we move our attention from the VPDMS to the Vwater, lower theoretical EE but higher theoretical extracted amount (mextracted) are achieved for higher sample volumes maintaining the same PDMS-coated stir bar. This can be seen by plotting EE throughout Eq. 3 and mextracted throughout Eq. 2 for different sample volumes. Figure 3 shows, as a practical example, the extraction of a compound with Ko/water = 104 from a water sample containing 10 ng mL−1 by means of a PDMS-coated stir bar with 60 μL PDMS.
From these results, and taking into account that the desorption is accomplished under the same conditions, large sample volumes are the best option to achieve higher sensitivity, obviously if there are not problems related to sample availability.
Apart from volume ratio, all those experimental variables affecting the Ko/water also affect the EE. Temperature increases the solubility of solutes in a solvent, thus temperature can affect differently the solubility of the target compound in both phases, and then is compound-dependant. However, the increase in solubility is often more accentuated in the aqueous phase and thus it causes a decrease in Ko/water and as a consequence it can be concluded that EE often decreases with temperature. Nevertheless, the effect of the temperature is often ignored and people work at room temperature [9]. In the case of ionisable compounds, such as weak acids and bases, pH plays a key role, since the neutral form is more extractable than the ionic one, thus those values that favour the formation of the neutral form increase the EE. The addition of ion-pair reagents also facilitates the extraction of ionized acids and bases by formation of a neutral adduct. The ionic strength also affects the Ko/w and thus the EE, in such a way, an increase in the salt content forces the solutes to move to the organic phase (‘salting-out effect’). Sometimes, a small amount of polar organic solvent (modifier), such as methanol, is added to the bulk aqueous sample solution to avoid the adsorption of the target compounds onto glass vessels and thus it prevents analyte losses (‘wall-effect’), but it also increases the solubility in the aqueous phase and thus it reduces collaterally the Ko/water and the EE.
Through Eq. 3, researchers can predict the theoretical EE for a specific compound with known Ko/water and for a given volume ratio. However, these theoretical values are rarely achieved experimentally owing mainly to three reasons: on the one hand, it is assumed that KPDMS/water ~ Ko/water, which could be slightly different; on the other hand, for very non-polar compounds, the above mentioned ‘wall-effect’ could be significant; and last but not least, the equilibrium might not have been reached after a defined period of extraction time [1]. Figure 4 shows the results experimentally obtained for the SBSE of a group of semivolatile compounds compared with the predicted theoretical values.
Experimental EE (red squares) compared with theoretical EE (blue line) values obtained for different semivolatile compounds from a 10 mL aqueous sample by using SBSE (VPDMS = 55 μL) (adapted with permission from [1])
Not reaching the equilibrium state is a consequence of the existence, in addition to those variables that affect thermodynamics mentioned above, of other variables involved in the extraction that exert a kinetic control over it. These variables are the extraction time, the stirring rate, the temperature (again) and the surface area of the stir bar.
With the aim to contextualize the discussion on the kinetic control in SPME and SBSE, it should be said that, unlike those microextraction techniques where the sorbent is dispersed (see chapter “Dispersive-Micro-Solid Phase Extraction (d-µSPE)”) [12], the kinetics in both SPME and SBSE are slow. So, longer extraction times are needed to reach the equilibrium state and thus to obtain the maximum thermodynamic EE for a target compound under these conditions. Figure 5 shows a real case obtained in the extraction of polychlorinated biphenyls (PCB) from a water sample [13], where the experimental EE gradually increases with extraction time. This behavior has been reported by other authors.
Experimental evolution of EE with extraction time observed in the extraction of different PCB from water samples (adapted with permission from [13])
In practice, to avoid extending the total analysis time too much, it is usual to shorten the extraction time and to work under non-equilibrium conditions, or if time is not a critical parameter to get the results, the extraction can be left overnight. If we opt by working under non-equilibrium conditions, we should take into account that it does not affect the accuracy of the determination if calibration is conducted with standards extracted under the same working conditions than samples. However, this way of proceeding can jeopardize the precision and sensitivity. Regarding precision, it is affected as a consequence of not working in a plateau, thus the extraction time needs to be strictly controlled. In any case, the precision can be improved by working with surrogates [11]. Regarding sensitivity, it is lowered as a consequence of not achieving the maximum thermodynamic EE. Thus, a compromise situation is usually looked for, so that the selected extraction time is increased if it compensates the increase in the EE, what depends on the region EE (%) vs time in which the system is. To this regard, SBSE, like SPME, is considered as non-exhaustive microextraction technique.
Bearing in mind that extraction occurs under diffusion-controlled conditions, diffusion through the boundary layer between the bulk aqueous solution and PDMS is rate controlling, and this can be enhanced by an efficient stirring. However, a vigorous uncontrolled agitation could physically damage the stir bar and also cause bubble formation, which in both cases negatively affects the EE [8]. Unlike SPME, SBSE integrates the extraction and stirring elements in the same device, which, in addition to simplifying the extraction, reduces the thickness of the boundary layer and thus speeds the diffusion of the extractable compounds from the bulk sample to the stir bar [14, 15].
Apart from the thermodynamic effect that exerts the temperature over the Ko/water discussed before, temperature per se usually accelerates the kinetics, but also diminishes the viscosity of the liquid bulk sample enhancing the mass transfer and thus decrease the time required to reach the equilibrium state. To this regard, as described before, the addition of salt improves the thermodynamics by the ‘salting out’ effect but it could damage the kinetics by increasing the viscosity.
Finally, the higher the surface area of the stir bar exposed to the bulk sample solution, the faster the extraction process is, since the sorbent is more accessible to entrap the target compounds.
With regard to the desorption conditions, we should distinguish between LD or TD, as commented before. In case of LD, a new partitioning equilibrium is now stablished between the PDMS and the desorption solvent (DS), so it is governed by a new partitioning coefficient (KDS/PDMS). In this sense, the nature and volume of this solvent exert a great influence in the thermodynamics of the desorption efficiency (DE), which similarly as discussed early, it could be defined as:
The solvent is chosen from the wide range of options that can be found in the laboratory compatible with the extraction phase. Aqueous solutions buffered at an appropriate pH to cause ionization of the analytes and their back-extraction could also be used, if it is the case. Its compatibility with the subsequent analytical instrument should be considered. To this regard, evaporation and reconstitution in an appropriate solvent could also be implemented despite increasing the total analysis time. Regarding the desorption volume, at least it should totally cover the stir bar to efficiently wet it, whereas, as it can be seen from Eq. 4, the higher the volume, the higher the DE. Nevertheless, as desorption volume increase, the enrichment factor (EF) for a compound A, defined as the ratio of the concentration in the desorption solvent ([A]DS) with respect to the initial concentration in the sample ([A]initial) (Eq. 5), would worsen as a consequence of collateral dilution, thus decreasing the sensitivity of the overall procedure. Nevertheless, at this stage, evaporation and reconstitution in a less amount of this same or another solvent could be carried out.
Here again, the stirring rate and temperature enhance the kinetics [8], but it is usual to work at room temperature conditions.
Regarding TD, it is used in the case of (semi)volatile and thermally-stable compounds, so their boiling points and vapor pressures are the thermodynamic parameters inherently associated to the compounds themselves. For TD, a dedicated unit is required, consisting of two programmable temperature vaporizers (PTV). The first one heats a glass tube containing the stir bar, in such a way the retained compounds are vaporized and transferred to a cold trap (i.e., a quartz tube packed with a sorbent or a series of sorbents of increasing strength) with the aid of the carrier gas (Fig. 6a). There, the compounds are cryofocused to avoid the excessive peak broadening caused if they were transferred directly to the GC instrument. The desorption temperature and the carrier gas flow play a crucial role in this step. After the required desorption time and once the compounds are in the cold trap, the carrier gas flow reverses and the second PTV is ballistically heated to transfer rapidly and efficiently the compounds to the GC instrument (Fig. 6b).
At this point it should be said that SBSE can also operate in headspace (HS) mode rather than in the immersion mode discussed up to now, which is particularly interesting to extract (semi)volatile compounds. In this mode, also known as headspace sorptive extraction (HSSE), the stir bar is held in the HS of the vial by using special devices (Fig. 7), and it remains static in such a way the target compounds are partitioned between the aqueous sample and HS, and then between HS and PDMS. An additional magnetic stir bar immersed into the bulk sample (or solution) is used to facilitate the mass transfer from it to the HS. Compared to the immersion mode, HS-SBSE requires longer extraction times since the kinetics are more limited, whereas it is more selective since non-volatile potentially interfering compounds are avoided. The boiling points and vapor pressures of the target compounds control the thermodynamics, besides the pH, the salinity, and the organic modifier content, as discussed before for the immersion mode.
To conclude with this section, it is obvious to emphasize that the influence of both thermodynamic and kinetic variables needs to be considered in both extraction/desorption steps with the aim of reaching an efficient extraction/desorption in a reasonable time.
3 Main Limitations and Solutions
As said before, PDMS was the unique and commercially available coating material for SBSE for a long time, and therefore it has been the most used and discussed in the literature for a wide range of applications. Despite this, due to the hydrophobic nature of this polymer, SBSE was initially limited to the unspecific extraction of non-polar compounds (generally for those with log Ko/w > 3), so the researchers had to resort to different strategies if the target compounds were of polar nature.
In this sense, a new stir bar with more polar features was later marketed, also by Gerstel GmbH & Co. KG. This alternative stir bar is made up of PDMS and ethylene glycol (EG)-modified silicone (EG/Silicone Twister®), which allows unspecific sorption of non-polar compounds and additional specific binding of polar hydrogen bond donors, such as phenols, demonstrating greater affinity compared to PDMS Twister® [16, 17]. Against, it has been verified that the stability of this coating material is inferior due to the softer nature of the polymer, and for this reason it is covered with an inert supporting grid for mechanical stabilization. Likewise, numerous scratches were observed on the surface of the grid when reused several times. Because of this, its use is recommended either in the HS mode, or immobilized in the aqueous donor phase and being stirred with an additional inert stir bar.
It is worth mentioning that a third stir bar coated with an alternative sorbent was also commercialized for a time by the same company, namely, polyacrylate (PA) with a proportion of polyethylene glycol (PEG) (Acrylate Twister®), but it is no longer available since robustness and applicability were limited [6].
Despite the commercial availability of these less non-polar coatings, their extraction mechanisms are still mainly based on hydrophobic interactions due to the presence, to a greater or lesser extent, of PDMS in its composition, and non-polar and medium polar compounds are by far the most extracted analytes. In fact, even EG/Silicone Twister® was shown to still be a challenge for the extraction of some polar compounds (especially for those with log Ko/w < 2) [18]. In addition, nowadays, multi-residue methods that allow the extraction of the widest possible range of analytes of different polarity is one of the most demanded needs by many analysis laboratories.
For all these reasons, great efforts have been directed to solve these limiting factors. In the following sections, different proposals are detailed and discussed, namely, the derivatization of the analytes, the use of novel workflows, and the fabrication of lab-made stir bars.
3.1 Derivatization
Different derivatization strategies carried out as an alternative to extract polar compounds more efficiently can be found in the literature [19]. In these cases, the polar functional groups of the analytes are converted to less polar derivatives, whose transfer to the PDMS stir bar is feasible. Some examples are alkylation, acylation, or silylation, among others.
In-situ derivatization of the analytes is the most common strategy, which occurs simultaneously with the extraction step in the aqueous donor phase. In this sense, the derivative is first formed within the solution in the presence of the derivatizing agent, which is subsequently extracted by the sorbent phase (Fig. 8a). This procedure reduces the number of steps compared to derivatization prior to extraction, and therefore the whole analysis time. Alternatively, the derivatization can also be on-stir bar, i.e., by previously loading the derivatization agent on the PDMS phase and then the analytes are incorporated into it, thus derivatization and extraction being also simultaneous (Fig. 8b). However, derivatization reactions that can be performed in aqueous solutions are limited, even some of them, such as silylation, does not occur since silylating agents are very sensitive to water and other protic solvents. In addition, derivatizing agents may be a source of interferences and errors.
Apart from these simultaneous derivatization strategies to extract polar analytes on PDMS stir bars, post-extraction derivatization strategies have also been reported to enhance the volatilization of the analytes and thus the chromatographic performance for GC analysis. In-tube derivatization (or in-port derivatization) occurs in the glass TD tube, where a few microliters of the derivatization reagent are added in a capillary tube or glass wool alongside the post-extraction stir bar containing the analytes (desorption and derivatization are simultaneous) (Fig. 8c). However, this on-line derivatization is limited to the fact that the non-derivatized polar compounds have been efficiently extracted on the stir bar, and more so if it is from PDMS phase. If LD is carried out, the derivatization reagent may be added to the desorption solvent after or during the desorption (in-extract derivatization) (Fig. 8d). In this case, the silylation reaction is possible if the solvent is not protic.
3.2 Novel Workflows in SBSE
When multi-residue analyzes are needed for the simultaneous determination of a large number of compounds covering a wide range of polarities, the problem arises when the extraction conditions for the analytes are quite different. For this reason, several alternatives to the conventional SBSE workflow were presented.
Ochiai et al. proposed back in 2006 dual SBSE [20], where two sample aliquots are subjected parallelly to different extractions conditions with separate stir bars, using the optimal conditions for the analytes in each case. After extraction, the stir bars are desorbed together, mainly by TD in the same glass tube (in one or two steps), and consequently only one chromatogram is obtained, reducing overall analysis time (Fig. 9a). It should be noted that what changes in each aliquot are the extraction conditions (pH, derivatization agent, ionic strength, extraction time, etc.), but they should not necessarily be two stir bars with different characteristics.
Although for other purposes, at this point, it should be mentioned that some authors employed the so-called multi-shot SBSE in order to obtain a higher sensitivity. In this methodology, several sample aliquots were extracted under the same extraction conditions using a stir bar per aliquot, and then desorbed together. When the results of this procedure were compared with those obtained using a single stir bar in a sample volume equivalent to the sum of the aliquots, an enhancement in sensitivity was verified [21].
The combination of stir bars with different polarities may expand the range of compounds to be extracted. In sequential SBSE [22], the same sample aliquot is subjected to different extraction conditions, even using different types of stir bars, in a sequential manner (Fig. 9b). It is also possible to modify the extraction modality (immersion and HS) between extractions, which is useful if volatile compounds are determined, as it has been proven that a high temperature may decrease the EE due to the volatilization up to the HS. After extraction, both stir bars are simultaneously desorbed for a single analysis. This workflow usually requires less sample volume than dual SBSE, but as the extractions are not carried out simultaneously, the analysis time increases.
In 2013, Ochiai et al. presented multi SBSE (mSBSE) [23], which enables the extraction of a single sample aliquot using simultaneously both PDMS- and EG-Silicone-coated stir bars, and the simultaneous desorption of both (Fig. 9c). The superior extraction capacity of this workflow to cover a wide polarity range was demonstrated [24]. Although the extraction device can be made in the laboratory [25], Gerstel GmbH & Co. KG commercializes a device under the tradename of Twicester® specifically designed for mSBSE. Up to three stir bars can be used, two of them magnetically positioned with a clip on the inner wall of the vial for HS and the third being stirred in the bottom. This arrangement prevents damage to the EG-Silicone-coated stir bars due to mechanical stirring.
A novel extraction technique that relies on stir bars that have been swollen with solvent was presented in 2016 under the term solvent-assisted SBSE (SA-SBSE) [26] to extend the applicability of conventional SBSE to more polar compounds. In this approach, a small volume of solvent (e.g., ethyl acetate, acetone, acetonitrile, methanol) is added to the conventional PDMS-coated stir bar before the extraction step leading to a swelling of the sorbent phase (Fig. 10a). Thereby, compared to conventional SBSE, the SA-SBSE phase volume is significantly increased (thus reducing the phase ratio), and, at the same time, it modifies its polarity depending on the solvent used, leading to improved extraction not only for polar compounds within the range of log Ko/water values between 1 and 2, but also for non-polar compounds. Gerstel GmbH & Co. KG developed and commercializes a stir bar specifically designed for this approach named as Flex Twister®).
Another alternative to overcome the drawback of poor extraction of polar compounds by conventional SBSE was proposed in the same year by Maslamani et al. [27]. Ice concentration linked with extractive stirrer (ICECLES) is based on the gradual freezing of the aqueous solution during SBSE from the bottom of the vial to the top using a double-walled beaker and a circulating chiller. As the donor phase freezes the analytes are gradually concentrated into the PDMS stir bar that remains in the upper liquid phase (Fig. 10b). It was demonstrated its higher performance for the extraction of polar compounds compared to conventional SBSE [28]. The main drawback was the limited sample volume (up to 10 mL), since it is moved away from the magnetic field as the ice front moves towards the top of the vial.
Recently, the concept of sequential SBSE was extended for a two-step fractionation of compounds with different polarities, by using a combination of mSBSE and SA-mSBSE. This new workflow, termed fractionated SBSE (Fr-SBSE) [29], consists of introducing first a set of three PDMS stir bars in a sample volume to extract the non-polar compounds. Then, after removing these stir bars, three PDMS stir bars swollen with solvent are introduced in the same sample for the extraction of polar compounds (Fig. 11). This extraction procedure provides two fractions with different polarities, which are either thermally desorbed or back-extracted in an organic solvent. A similar but simpler methodology was recently proposed by the same authors, combining SBSE and SA-SBSE with in-situ derivatization [30].
Schematic diagram of Fractionated SBSE (Fr-SBSE) (reproduced with permission from [29])
3.3 Alternative Coatings for SBSE
The development and manufacture of new lab-made coatings has been one of the major aims of researchers working in the field to expand the potential and versatility of SBSE [31]. In this way, there is no dependency on commercial availability, which can limit the application, as previously stated. Beyond the ability to efficiently extract the analytes, the mechanical and chemical stability of the coating are two of the most sought properties when preparing alternative coatings. To obtain an increase of the extraction efficiency, a thick coating layer is preferable.
In the literature, there are different methods for preparing alternative coatings on stir bars with different sorbent materials, which are briefly summarized in the following subsections.
3.3.1 Coating Preparation Methods
The first fabrication approach used for SBSE-stir bars was the sol–gel technology [32]. This approach involves the transformation of a liquid colloidal solution (sol) to a solid matrix (gel). The most typical procedure consists of the hydrolysis of the coating precursors (e.g., methyltrimethoxysilane (MTES)) followed by the polycondensation of the hydroxylated species (i.e., inorganic network growth), incorporation of active organic ligands (e.g., hydroxy-terminated PDMS) into the network, and finally chemical bonding of the coating on the previously treated glass stir bar to generate silanol groups on its surface.
Several other precursors are available and different functional groups (i.e., modifiers) can be easily introduced into the three-dimensional network structure during its growth to provide them with the desired polarity, such as β-cyclodextrin (β-CD) [33], polyaniline (PANI) [34], and other materials detailed in Sect. 3.3.2. Thereby, the inclusion of all these modifiers enhances the extraction of polar compounds compared to PDMS-only stir bars.
These stir bars present a good chemical, thermal and mechanical stability, and thus a long lifetime, since there is a strong chemical bonding between the coating and the glass surface of the stir bar. Additionally, the coating obtained is usually thick and uniform due to the good reproducibility in the preparation. Against, as the typical sol–gel coatings are based on non-polar PDMS, they may still lack selectivity for the most polar compounds, and also the pretreatment of the glass surface may be laborious.
The monolithic fabrication consists of the in-situ polymerization of a monomer and cross-linker mixture in the presence of a porogen solvent and a radical initiator. The polymerization is then thermally- or photo-initiated and lasts for a period of several hours.
Monoliths are porous materials containing a network of interconnected micro-sized pores, and as a result they possess very good permeability and adsorption capacity. Moreover, it is easy to tune the polarity of the resulting monolith by simply selecting the appropriate monomer from a large availability, depending on the chemical properties of the analytes. A combination of various monomers is possible, and the ratio between them and the composition of the porogen affect the rigidity, porosity, and polarity of the resulting monolith.
The fixation of the monolith on the glass surface of the stir bar can be physical or chemical. For the former, the immobilization of the coating is achieved just by simply immersing the glass stir bar in the polymerization mixture inside a mold with the desired dimensions and then starting the polymerization. For the chemical attachment, the coating fabrication involves the pretreatment of the surface of the stir bar by silylation to create double bonds, for example with 3-(trimethoxysilyl)propyl methacrylate (MPS), and subsequent polymer growth on it. Although the physical attachment is significantly easier than the chemical one since the previous step is avoided, the latter presents a higher chemical and mechanical stability due to the chemical bonding.
In a similar way, molecular imprinting technology involves the fabrication of polymers with molecule-specific cavities to recognize a target molecule (i.e., molecularly-imprinted polymer [MIP]), thus enhancing the selectivity of the material. The synthesis of the coating is carried out in the same way as explained above, but in presence of a template molecule (i.e., the target analyte or an analogous compound) in the polymerization mixture, and its subsequent removal at the end by washing steps. The formed cavity complements in size, shape, and chemical environment to the template. The ratio of crosslinker and porogen plays an important role in increasing the recognition capacity of the MIP (i.e., imprinting factor).
On the other hand, the main limitations of MIP fabrication are the need to ensure the complete removal of the template (otherwise it could provide false positives), which lengthens the synthesis time, and its better extraction efficiency in an organic medium rather than in an aqueous one. The latter can be improved with the incorporation of hydrophilic monomers.
Adhesion techniques are efficient alternatives to immobilize the sorbent materials directly on the stir bar surface, either by physical or chemical coating. For the physical coating, different methodologies have been proposed. The first proposal, and one of the most widely used in this context, is to cover the stir bar with an adhesive film (i.e., epoxy glue or a PDMS sol), followed by the attachment of the solid material (e.g., rolling the stir bar in the material), and subsequent incubation and drying [35]. Alternatively, a pretreated stir bar can be placed in an organic solution containing the extraction phase for a period of time. Once removed, the solvent is evaporated, and the coating remains on the surface. Other more sophisticated alternatives are magnetic adhesion [36], if magnetic sorbents are used, or flame deposition [37], among others. On the other hand, chemical adhesion involves the previous modification of the glass stir bar and the subsequent covalent immobilization of the material. Unlike the sol–gel technology, these stir bars are broadly not based on PDMS or the in-situ growth of a three-dimensional network.
Solvent exchange (or immersion precipitation) consists of dissolving or dispersing the material (e.g., a polymer) in a suitable solvent (e.g., formic acid), immerse first the stir bar in the solution to adhere the material onto the surface of the stir bar, and finally immerse the stir bar in water for a period of time to allow diffusion of the solvent out and leaving the film of the material on the surface. The first application of this procedure was reported by Guan et al. [38] who deposited poly(phthalazine ether sulfone ketone) (PPESK) on the stir bar, presenting a good mechanical stability.
3.3.2 Sorbent Materials
As previously indicated, the use of a wide variety of materials as the extraction phase in SBSE has been one of the main focuses of attention of researchers, since it is essential to broaden the applicability of the technique. There are different interesting review articles in the literature that have already addressed this issue [39,40,41,42], so these materials and their applications will only be briefly summarized here. It is important to note at this point that the same material can be immobilized on the stir bar by the different methods described in the previous section. Thus, the selected preparation method will affect the morphology, thickness, and stability of the coating, among others.
Different carbon-based materials, such as graphene oxide (GO) [43], reduced graphene oxide (rGO) [44] or multiwalled carbon nanotubes (MWCNTs) [45, 46], have been shown to be effective in extracting the compounds of interest in SBSE. They present high surface area, thermal and chemical stability, and their ability to have hydrophobic, π − π and/or electrostatic interactions.
Huang et al. [47] were the first to introduce the monolithic materials in SBSE. They prepared an octyl methacrylate (MAOE)-ethylene dimethacrylate (EDMA) monolith for the extraction of non-polar polycyclic aromatic hydrocarbons in water samples and polar steroids in urine samples. Since then, a wide variety of monomers have been used to fabricate monoliths [48, 49]. The selection of the monomers is made based on the properties of the analytes, so that they interact through hydrophobic, hydrophilic, hydrogen bonding and/or electrostatic interactions.
Regarding the use of the selectivity provided by the MIPs, Zhu et al. [50] were the first to report the use of a MIP-based film for SBSE, which was prepared by precipitation of the polymer (nylon-6) containing the template molecule onto the surface of a commercial PDMS-coated stir bar. On the other hand, the first application of a MIP chemically attached to the surface of the stir bar was reported by Xu et al. [51]. Over the years, alternatives have been proposed to avoid the use of expensive or toxic molecules as a template and the problem of possible residual template leakage. In this sense, dummy templates (i.e., molecules similar in shape and interactions to the analytes) have been used [52].
Metal–organic frameworks, widely known as MOFs, are hybrid inorganic–organic microporous crystalline materials with a three-dimensional network by the assembly of metal ions and organic ligands by coordinative bonds. For the last decade, they have been widely used as extraction phases due to their high chemical and thermal stability, large porosity, and huge surface area. Their use in SBSE has not been an exception [53, 54]. Covalent organic frameworks (COFs) are similar to MOFs in chemical structure and properties, but their assembly is between different units by covalent bonds, and they have gained interest as extraction phase for the last years [55,56,57].
Some other sorbent materials have also been used as coatings in SBSE but to a much lesser extent. In this sense, polyurethane foams are polymers produced by the reaction of polyisocyanate with polyols and water in the presence of specific catalyst. These materials offer high chemical stability, flexibility, and the ability to cut them to the desired size. Although this material presented a promising future in SBSE [58], its use has not been exploited in recent years. Layered double hydroxides (LDH) are two-dimensional nanosorbents composed of two layers of divalent and trivalent cations with an anionic interphase [59]. Restricted access materials (RAMs) are biocompatible particles that enable the direct extraction of analytes from biological fluids since are able to fractionate the protein component. However, this material has been scarcely used in SBSE [60]. Immunoaffinity materials such as aptamers have been used since they present a high selectivity degree [61].
In addition, the combination of various materials in the same coating (i.e., hybrid materials) has also been proposed for SBSE purposes. For instance, a novel glycidyl methacrylate (GMA)-based polymer with an amino-modified MOF was recently developed for the first time [62], allowing to incorporate the best features of both materials in the same sorbent.
3.3.3 Stir Bar Geometry and Coating Support
One of the main drawbacks of the stir bars with conventional geometry (i.e., uniform elongated bars) completely covered by the coating material is its direct contact with the bottom of the extraction vessel in immersion mode, which may cause its damage and/or loss due to the high stirring rate. To solve these problems, alternative stir bar geometries with better mechanical resistance than the conventional one have been proposed along the last two decades.
In a first attempt, in 2007, Yu et al. [33] prepared a stir bar from the combination of two glass-coated bars with different diameters placed in parallel, with a long steel wire sealed inside one of the glass bars. One side of the combined stir bar was coated with PDMS/β-CD by sol–gel method, and no coating on the other side. The authors demonstrated that this stirring device was durable to withstand frictional forces at high stirring speed and could be reused at least 100 times with minimum loss in EE.
Two years later, the same authors presented a dumbbell-shaped stir bar to prevent the friction loss [63]. Specifically, a capillary glass bar with an iron wire inside was sealed at both ends in the shape of a spherical bubble (with an internal diameter larger than the glass bar) by alcohol flame (Fig. 12a). Then, the bars were immersed in a sol solution of PDMS/β-CD/divinylbenzene (DVB) to physically adhere the coating. They concluded that the dumbbell-shaped stir bar presented a longer lifetime since it was able to be reused 40 times, while a normal-shaped stir bar was able for 30 times under the same operating conditions.
With a similar setup, Liu et al. [52] proposed in 2016 a MIP-coated glass stir bar that was sleeved by silicone wheels at both edges. In this case, they termed it “barbell-shaped” stir bar (Fig. 12b). In the same way as the dumbbell-shaped, the friction between the coating and the extraction vessel was avoided.
Moreover, although glass-coated stir bars are the most used coating supports in SBSE, other less fragile materials than glass have also been used, with the additional advantage that they can be used directly, avoiding the pretreatment of the surface of the glass stir bars before the immobilization of the extraction material.
Zhang et al. [43] were the first to report a chemically-bonded coating on a stainless-steel wire as jacket-free device for SBSE in 2014. Therein, they modified the wire first with polydopamine and then with GO, resulting in a good stability of the stir bar. Compared to conventional glass-coated stir bar, it avoided the pretreatment, thus saving operational time. Against, the metal rod suffered from corrosion when exposed long time under acidic conditions.
Fan et al. [34] proposed a stainless-steel spring as coating support (Fig. 13a). Its spiral structure presented two advantages. On the one hand, compared to a stainless-steel wire, more extraction phase can be physically fixed on it, which favored the extraction efficiency of the analytes, as demonstrated by the authors. On the other hand, it prevents the friction of the coating with the bottom of the extraction vessel, thus prolonging its lifetime.
An easier-to-prepare dumbbell-shaped stir bar was proposed by Sukree et al. [64], where a stainless-steel net is rolled into a tube, and filled with the sorbent and a metal rod to allow the stirring. Then, the two ends of the tube were closed with Teflon caps with larger diameter than the resulting stainless-steel tube (Fig. 13b). The greater advantage of this stirring device is the possibility of easily changing the sorbent material inside the tube depending on the analytes to be extracted.
In 2018, Zhou et al. [65] applied a etched poly(ether ether ketone) (PEEK) jacket stir bar. As PEEK presents high chemical resistance and smooth surface, it was treated with sulfuric acid before functionalization. Two lollipop-shaped stainless-steel needles prepared by burning polypropylene at one end were inserted into the PEEK tube for the construction of a facile detachable dumbbell-shaped stir bar (Fig. 13c). During the elution, one of the needles was detached in such a way can be easily inserted into a pipette tip. This setup was also recently applied to a polypropylene hollow fiber as the jacket for stir bar [56]. In this case, the porous structure of the bare hollow fiber avoided the tedious etching process with sulfuric acid.
Commercial polytetrafluoroethylene (PTFE) jacketed stir bar has also been employed as coating support for SBSE as it presents affordability and low cost. However, the modification of PTFE can be complicated due to its chemically resistant surface. Zhang et al. [66] immobilized graphene onto the surface previously modified with polydopamine.
Mirzaee et al. [59] proposed in 2020 the in-tube SBSE for the first time. Specifically, they immobilized the extraction material on the inner surface of a small piece of an aluminum tube, which itself participates in the fabrication of the sorbent. As the coating is not in direct contact with the extraction vessel, it prevents its deterioration.
An anodized aluminum wire was electrochemically prepared and used as nanoporous substrate for in-situ growth of a zeolitic imidazole framework (ZIF) by Ghani et al. [67]. The anodized aluminum presents a porous layer on the metal surface, and the stir bar was mechanically stable. Later, this substrate was also used for the in-situ growth of a zeolite imidazolate framework on the surface of a LDH [68].
On the other hand, to avoid interferences from the sample matrix that could affect the lifetime of the stir bar, such as the macromolecules, Mao et al. [69] used a PTFE membrane-protected stir bar, by encapsulating the coated stir bar in a porous membrane. In this way, according to author’s words, the high molecular weight interference compounds would be blocked by the protective porous membrane and the lifetime of the stir bar was prolonged.
In any case, further exploring the suitable support material with porous structure and making a suitable structure design remains one of the goals at SBSE.
4 Novel Developments
The advantages of integrating extraction and stirring in the same unit have propitiated the development of similar formats that share this principle. The most outstanding characteristics of these microextraction devices raised in an increased versatility of both formats, sorbent phases and stirring mode (magnetic or mechanical). The most relevant approaches are detailed in this section, focused on the description of the principle behind the development and its main favourable features rather than in the specific application or analytical figures that can be easily found in the specific reference.
4.1 Magnetically Stirred Units
4.1.1 Stir Membrane Extraction
The use of membranes as active elements for analyte isolation presents several advantages over other configurations. They especially refer to their planar nature, which results in a high surface-to-volume ratio. Moreover, their porous structure permits the flow of the liquid or gaseous samples through them, which improves the kinetics. The incorporation of a membrane in a stirring device synergically combines the benefits of both. Stir membrane extraction (SME) was proposed by Alcudia et al. in 2009 [70]. The device consists of the use of a commercial polypropylene unit as membrane holder (Fig. 14a). It was pierced by a protected iron wire which provided the stirring of the unit under magnetic agitation. Two windows were opened on the plastic holder to allow the flow of the sample through the membrane. This first design was evaluated for the extraction of polycyclic aromatic hydrocarbons from waters. The extraction only required 15 min while elution was accomplished by face-down immersion of the membrane in the desorption solvent therefore, there was no need to remove the membrane from the holder while higher enrichment factors were obtained. The better performance of this configuration over conventional SBSE using PDMS stir bars of different surfaces was also demonstrated by the authors, which was justified by the enhanced transference of the analytes from the sample to the membrane thanks to the stirring and the permeability of the extractant phase.
Different configurations of stir membrane extraction. a Stir membrane extraction. b SME for LPME. c SME for processing limited-volume samples. d SME for the analysis of solid samples. Panels a–c reproduced under Creative Commons Attribution (CC BY) license from reference [15]. Panel d reproduced with permission from [74]
This basic configuration can be adapted to other microextraction modalities. The first modification consists of closing the membrane holder with a plastic cap (Fig. 14b). In this way a small chamber is created over the membrane, which can be filled with an organic (two-phase) [71] or aqueous (three-phase) solution [72]. The versatility of the SME is dramatically increased as, in the first situation, organic compounds are extracted based on the partitioning equilibrium between the sample (aqueous) and the extractant (organic solvent). The second alternative increases the selectivity of the extraction because the transference of the analyte between the two aqueous phases in favored by their intermediate solubilization into the organic phase filling the pores of the membrane (supported liquid membrane) and driven via a pH gradient established between the donor and acceptor aqueous phase. This approach is suitable for the extraction of ionizable polar analytes.
One of the main advantages of miniaturized extraction techniques is the possibility of facing new analytical problems, for example, those involving limited-volume samples such as saliva and related biofluids. In this case, the SME configuration can be modified by increasing the volume of the upper chamber that can be filled with the sample instead of the liquid extractant (Fig. 14c). This system works under the three-phase format and the stirring is accomplished by means of a vortex [73].
Another example of the versatility of the SME is that it also works processing solid samples as indicated in Fig. 14d. An Eppendorf is used as extraction device and the membrane is used to confine the extractant aqueous phase in the cap [74]. The extraction is carried out in the body of the Eppendorf where the sample is in deep contact with the organic extractant. Once isolated from the sample, the target analytes are re-extracted in the aqueous phase by passing through the supported liquid membrane.
Similarly, the polymeric membrane can be substituted by a borosilicate disk [75] or a series of small magnets [76] to broaden the application field through the possibility of using different coatings for analytes extraction.
4.1.2 Stir Cake and Rotating Disk Sorptive Extraction
The substitution of the membrane by a monolithic sorbent resulted in the so-called stir cake sorptive extraction (SCSE) [77]. In this configuration, the sorbent is synthesized inside a home-made plastic holder, which is also fitted with a protected iron wire, responsible for the stirring of the unit under a magnetic field. The advantages of SCSE over SBSE are the higher extraction capacity of the sorbent phase and its longer lifetime (reusability of 300 times versus 60 reuses). Since its proposal in 2011, several sorbents have been prepared and used in this format, including polymeric ionic liquids [78] and organic-phase monoliths [79]. Also, the plastic holder can be modified in terms of size and geometry to contribute to the sustainability of the synthesis, for example, reusing plastic bottle caps [80].
Rotating disk sorptive extraction (RDSE) can also be described in this section as it consists of a PTFE disk (diameter ca. 1.5 cm) with an embedded miniaturized magnet (for rotation by a laboratory magnetic stirrer) and one of its sides coated with the sorbent phase in the form of a thin film [81]. Since the sorbent phase is not in contact with the bottom of the vessel, high stirring speed can be applied without damage. Notwithstanding this, the disk can also be rotated by using a rotary rod connected to an electric stirrer [82]. The authors demonstrated that this last configuration reduces the time needed to reach the extraction equilibrium. This was ascribed to the fact that the movement of the disk reduces the boundary layer, and the transference of the analytes is, therefore, faster. As it is the case with SCSE, the variety of sorbent phases that can be deposited over the disk surface clearly increases the number of different families of compounds that can be extracted. A detailed description of the analytes and samples that can be processed has been recently reviewed [83].
4.1.3 Adsorptive Microextraction
Despite developing novel sorbent phases to increase the versatility of SBSE during the last decades, as described previously, Nogueira et al. proposed in 2010 the decoupling of the sorbent phase from the magnetic stirring unit. The so-called adsorptive microextraction (AµE) was proposed to afford the challenge of extracting polar compounds from waters [84]. For the aim, two different configurations, namely bar adsorptive µ-extraction (BAµE) and multi-spheres adsorptive µ-extraction (MSAµE), were evaluated. Two representative examples are given in Fig. 15. The preparation of BAµE units involved the retention of a powdered sorbent over polypropylene hollow cylindrical substrates by adhesive forces while in MSAµE the spherical particles of sorbent were attached to a threat and covered by the powdered sorbent, which was fixed by thermal curing. Their application in microextraction techniques requires using a conventional Teflon magnetic stirring bar to promote the agitation of the sample and thus the migration of the target analyte to the sorbent phase. As both, BAµE and MSAµE substrates, were lighter than water, they remained below the vortex, under the so-called floating sampling technology.
Schematic representation and images of BAμE (a) and MSAμE (b) during the μ-extraction process (reproduced with permission from [84])
An interesting issue with this configuration is the stability of the coating during the extraction and thus, the potential reusability of the extraction units. In this first study, the authors concluded that the thermal curing confers the sorbent with higher stability in the organic media (direct immersion in pure solvent for 60 min under sonication), temperature (20–50 °C, 3 h, sonication) and pH (1–14, 3 h, sonication). The instability of the adhesive supporting film would be the reason behind this behavior. Nevertheless, both configurations can be used for analyte isolation under standard operational conditions and solvents, although activated carbon and polystyrene-DVB performed better in terms of stability, robustness, and µ-extraction efficiency.
As it was described for SCSE and RDSE, this configuration minimizes the friction with the vessel walls. Moreover, both BAµE and MSAµE require less extraction time and lower agitation speed to reach similar performance than SBSE.
BAµE has evolved following sustainability criteria and in 2018 an eco-friendly alternative was developed [85]. The authors proposed the use of a flexible nylon support of reduced dimensions (7.5 × 1.0 mm) that is coated with the appropriate sorbent. Analyte elution is carried out in a glass vial insert which, on the one hand makes it compatible with automatic instrumental analysis and, on the other hand increases the preconcentration factor that can be achieved and hence, the sensitivity of the analytical method.
A hollow fiber filled with the most convenient organic solvent can also be used as floating extraction unit [86]. The so-called hollow fiber microextraction (HFµE) uses a polypropylene membrane of 10 mm in length which is immersed for a few seconds in the organic solvent of choice, being 20 µL embedded in the pores. Next, the unit is immersed into the sample and the agitation of the vial allows its free floating below the vortex created by the stirring magnetic bar. Once finished, the unit is withdrawn by means of clean tweezes and transferred to a glass insert for liquid desorption with the help of ultrasounds. The whole vial is transferred to an LC autosampler for instrumental analysis. The preparation of this type of extraction unit is quite simple and rapid, using negligible amounts of organic solvent for both, extraction, and elution steps.
The versatility of this miniaturized extraction technique can be improved if two hollow fibers are added to the sample, giving rise to what is known dual-HFµE. In this case, the different nature of the organic solvents broadens the chemical nature of the analytes to be extracted. If it is combined with large volume injection, the sensitivity is dramatically enhanced [87].
4.1.4 Stir Bar Sorptive Dispersive Microextraction
Dispersive microextraction techniques exhibit better performance than non-dispersed miniaturized approaches thanks to the higher contact between the sorbent/solvent phase and the analyte distributed within the sample matrix [12]. However, its main disadvantage is the collection of the extractant phase enriched with the analyte after the extraction step. It usually requires filtration or centrifugation. The inclusion of magnetic materials in these dispersive techniques facilitates the procedure as the extractant is isolated from the sample matrix or eluent by means of an external magnet (see chapter “Dispersive-Micro-Solid Phase Extraction (d-µSPE)”). However, in some cases, the recovery of the solid or liquid extractant phase is not complete or requires and excessive time to occur. In 2014, a new microextraction technique named stir bar sorptive dispersive microextraction (SBSDME) was developed as an elegant combination of SBSE and dispersive microsolid-phase extraction (DμSPE) [88, 89]. The fundamentals of SBSDME are found in the use of a strong permanent magnet over which a thin layer of a magnetic sorbent is deposited. Playing with the stirring rates, the solid phase is retained (lower speeds) or detached (higher speeds) from the magnetic support. Therefore, as indicated in Fig. 16a, in the first step, the magnet coated with the sorbent phase is introduced in the vial containing the sample. Then, the system is stirred at a high-speed provoking the dispersion of the sorbent phase into the liquid sample for a given time. Next, the speed is reduced to zero and the magnetism of the bar retrieves the magnetic sorbent enriched with the analytes without needing an external magnetic field. Finally, the bar is withdrawn from the sample and the target compounds are liquid or thermally desorbed for further instrumental analysis.
Automation of SBSDME has been proposed using a lab-in-syringe manifold on-line connected to a spectrophotometer [90]. The configuration developed all the steps of the process in an on-line fashion: sorbent dispersion, magnetic collection, elution, and detection. The main shortcoming of this configuration is that only 5 mL of sample can be processed. Therefore, to increase the sensitivity of the measurements, the processes was repeated up to eight times using fresh aliquots of sample prior to analytes elution and determination.
Very recently, SBSDME was miniaturized to face the processing of low availability samples [91]. A dedicated device was constructed to hold 400 µL glass vial as sample containers and 3 mm × 2 mm bar shaped magnetic as stirring elements. As can be seen in the Fig. 16b, up to 15 samples can be simultaneously processed. In addition to the low sample volume, the amounts of sorbent and eluent are also reduced thus contributing to the sustainability of the sample preparation.
4.1.5 Solvent Bar Microextraction
Solvent bar microextraction (SBME) was proposed in 2004 by Jiang and Kee-Lee [92]. It consists of the confinement of few microliters of octanol into the lumen of a hollow fiber membrane followed by sealing of both ends. The resulting solvent bar was then added to a liquid sample and stirred by means of an additional magnet. As a main advantage, its use in “dirty” samples (e.g., soil slurries) can be highlighted as the hollow fiber acts as a filter of the particulate matter that can eventually be found dispersed in the matrix.
Since its proposal, several configurations have been developed, including the two-phase and three-phase modes. In the first case, the organic solvent fills the lumen and the pores of the hollow fiber while in the tree-phase the organic medium impregnates the pores and separate two aqueous phases, thus acting as a liquid membrane.
The basis arrangement of SBME has been adapted to increase its performance [93]. For example, one of both ends can remain unreached for compatibility with volatile organic solvents, which are easily evaporated during the seal of the second fiber end [94]. Efficiency of the extraction can be improved by keeping the fiber at the bottom of the sample vessel either using a pipette-tip [95] or a stainless-steel wire [96] Also, a dual solvent-stir bar microextraction has been designed where a stainless-steel bar with four fixing positions was used to hold two SBME [97]. The magnetic bar can also be externally stuck to the hollow fiber although this alternative considerably reduces the surface area available for analytes diffusion [98]. All these approaches are represented in Fig. 17.
Modifications of the conventional SBME setup: a “Cone” SBME; b solvent stir bar SBME; c magnetic bar SBME; d dual solvent stir bar micro-extraction; e magnetic support SBME (reproduced with permission from [93])
4.1.6 Capsuled Microextraction
Extraction phases can also be stirred in the sample solution without the need of a plastic holder. Unlike SME and related techniques, the magnetic bar is attached to the sorbent element in different ways, thus allowing the device to spin itself for analytes diffusion. Georgiadis et al. coined in 2019 the term microextraction capsules (MECs) to describe a device consisting of a built-in magnet, a cellulose fiber substrate coated with a sol–gel organic–inorganic sorbent and porous membrane [99] As it can be seen in Fig. 18, the magnet is introduced in a polypropylene membrane which is joined to the extractant phase protected by a polypropylene membrane. The built-in magnet avoids the need for an additional, independent magnetic bar, while the porous polypropylene membrane allows sample permeation, protecting the sorbent from impurities coming from the matrix, thus extending its reusability. The high porosity of the capsule facilitates analytes diffusion for both isolation and elution steps. Also, it provides many interaction chemistries (polar, non-polar, anion-exchange of mixed mode) which also extend its applicability to a wide variety of analytes.
Preparation of a capsule phase microextraction unit (adapted with permission from [99])
4.1.7 Flat-Shaped Self-Rotating Devices
The use of planar substrates presents several advantages in microextraction. Among them, the larger superficial area, thanks to the fact that the two-sorbent sides are available for extraction, can be highlighted. If they are used under self-rotating configuration, the need for a holder is obviated as well as the need for an additional magnetic bar. These facts make this approach more environmental and user-friendly and, at the same time, reduces costs and time.
Two flat elements have been proposed. Kerman et al. synthesized an iron mesh screen that was electrochemically coated with polypyrrole. The device can rotate in the presence of an external magnetic field and the presence of apertures in the design increases the sorptive phase available for analyte interaction, which promotes the diffusion of the sample through the extractant phase (in comparison to bar or plates) [100]. To demonstrate the advantages of this configuration over other geometries, the authors compared the efficiency of the mesh screen, cylindrical and solid-plate layouts (see Fig. 19a) under standard extraction conditions (temperature 30 °C, stirring rate 1000 rpm, extraction time 30 min and no salt addition). Quantitative extractions were obtained for the mesh screen, followed by the solid plate coated with the same polymeric phase (ca. 55%) and cylindrical geometry performed the worst (ca. 25%). The authors attributed this enhanced performance to the extra stream pathways provided by the open structure of the mesh that facilitate the sample diffusion through the sorbent phase. The cylindrical (rod wound on the support) and the solid plate allow only part of the sorbent to the exposed to interaction with the analyte.
Fabric phase sorptive extraction (FPSE) consists of using a natural or synthetic fabric substrate which is subsequently modified with a hybrid organic–inorganic polymeric phase [101]. The stability of the thin film coating and its porous structure results in extremely high extraction efficiency. The integration of a stirring element allows the FP to freely rotate in the sample [102]. According to the authors’ description, the magnet-integrated-FPSE (MI-FPSE) was constructed using two circular membranes (r = 0.75 cm) integrated with a metallic magnetic stir bar (see Fig. 19b). As it was the case with the previously described approach, this MI-FPSE is easy to handle with better reproducibility, faster extraction equilibrium and shorter extraction times. As an upgrade regarding other alternatives, FPs with different interaction chemistries can be used to fabricate the MI-FPSE thus broadening the range of polarity of the analytes that can be simultaneously extracted.
4.2 Mechanically Stirred Devices
One of the limitations of the magnetically stirred devices and even the self-rotating layouts is their difficulty in being adapted to perform on-site extraction. If the target compounds are on-site extracted, the sampling logistics are reduced, and the analytes integrity is increased during transportation and storage. In these strategies, only the extractant phase containing the analytes is delivered to the laboratory and the absence of the aqueous matrix eliminates undesired secondary reactions. The only requirement is the stability of the analytes on the extraction unit during the storage. All the devices that are described below share the simplicity of installation, removal, and replacement of the extraction units. They also avoid coating deterioration due to the friction with the bottom of the sample vessels.
In a first approach a home-mase portable electric stirrer was coupled to an SBSE, working under the off/on-site modes [103]. The bar was fixed to the stirrer through a magnetic stir rod welded onto the bottom of the mini-electro motor (stirring speed 2000–6000 rpm). The stir bar used was lab-made by coating a glass-coated iron stir bar with a thin film of PDMS. The portable SBSE can work under the HS, direct immersion, or continuous flow modes.
Qin et al. demonstrated the advantages of using a PDMS thin film coupled to an electric drill for on-site extraction in waters [104]. Interestingly, the authors compared the performance of the planar substrate with a SPME fiber. As expected, the PDMS thin film resulted in a better efficiency thanks to the most favorable surface-to-volume ratio and larger extractant phase (ca. 100 times higher).
Borosilicate disks can also be used as planar support of the sorbent phase. They exhibit a higher mechanical stability while being easily functionalized. Roldán-Pijuán et al. modified these disks with oxidized single-walled carbon nanohorns (o-SWNHs) [105]. The o-SWNHs disks were fixed to a screw of a portable drill to develop on-site extractions. The rotation of the disk homogenized a defined volume of sample around it and therefore the extraction can be considered almost independent of sample volume. The methodology was robust and highly reproducible among different synthesized o-SWNHs disks.
Despite the high reproducibility reported for the laboratory-made extraction units, the use of standardized and commercial elements helps to increase this analytical properly and is less time-consuming. Casado-Carmona et al. presented a portable stir membrane device that can be used with commercial nylon membranes to carry out the on-site extraction of target compounds from environmental water samples [106]. A countersunk pot magnet permits the attachment of the nylon membrane using a metallic washer. The system is coupled to a wireless electric drill using a screw of variable length (depending on the sampling site requirements) and a nut. The membrane can be easily removed after each extraction for analyte elution and quantification.
This configuration can be simplified by substituting the membrane by a magnetic paper thanks to the minimization of the diffusion boundary layer. The flat support was prepared by immersion of a piece of paper in a dispersion containing nylon-6 (dissolved) and magnetic nanoparticles (dispersed). The magnetic paper is directly attached to the magnet, avoiding the need of metal washers. In addition, an improved blade is fixed over the magnet to promote mass transference [107].
Commercial particulate particles with different interaction chemistries are widely used in environmental analysis thanks to their high efficiency compared to polymeric sorbents. This material can also be used in the previously described device combining the advantages of a flat extraction unit with the integrated mechanical stirring [108]. The preparation of the sorbent phase consists of the deposition of hydrophilic-lipophilic balance (HLB) particles over a magnetic tape. It was then fixed to a screw by means of a countersunk pot magnet, as previously described. A small blade was added to facilitate the analyte diffusion. The device was integrated into a glass bottle’s cap fitted with a small electric motor. A portable power supply was used to facilitate the portability of the system. The extraction unit can be adapted to the sample volume that would be eventually needed to reach a given sensitivity level by changing the volume of the bottle used for the extraction. The authors used an internal standard to compensate the influence of the ionic strength of the sample on the analytical signal.
Open-sources technologies such Arduino can be used to automate these devices. Also, several sensors (temperature, conductivity) can also be added to enrich the sample information. Moreover, the planar sorbent phase can be attached to the stirring element by means of an alligator clip [109]. This configuration, shown in Fig. 20, maximized the surface available for analyte interaction. In this proposal, mixed mode anion exchange (MAX) particles to avoid pH adjustment were used for analytes isolation and they were achieved to the support by means of a double sided adhesive tape.
Photograph resembling the elements used to construct the on-site extraction device integrating temperature and conductivity probes (adapted with permission from reference [109])
Using several extraction units simultaneously can increase the amount of analyte extracted or, if they are of different nature, expand the variety of compounds (hydrophobic, hydrophilic, charged) that can be isolated in a single extraction step. Makkiniang et al. developed a portable and miniaturized apparatus that can hold up to 6 miniaturized multi-stirred microextractors [110]. A monolithic polymeric phase containing carboxylated MWCNTs was prepared, and the rods were connected to the motor by using pipette tips of different volumes (see Fig. 21a). The low cost of the extraction units allows the simultaneous use of several units, therefore, a higher sample throughput is achieved.
Solvent bars can also be used in mechanical stirrer devices, using an electronic motor [111]. In this case, four hollow fibers were arranged in a cubic-like configuration between two polymeric disks which are connected to the motor (see Fig. 21b). The higher the number of hollow fibers, the better the extraction efficiency. In the case that only one hollow fiber was needed to reach the desired sensitivity, the device can be used to obtain replicate values of the analysis in a single step.
5 Future Remarks
This chapter provides a general overview of SBSE technique from a broad perspective since related techniques and new materials have been outlined. Initially, SBSE was proposed as the simple integration of the sorptive phase into a stirring bar, a common element in any microextraction technique to enhance mass transference. In the last few years, SBSE has experienced a remarkable evolution driven by resolving its initial shortcomings.
Compared to in-fiber SPME, the SBSE coatings are thicker, thus increasing the potential extraction capacity of the technique. However, thicker coatings restrict the extraction kinetics, which are, sometimes only partially, compensated by the efficient stirring of the solution. New porous materials, such as monoliths and membranes, have been proposed to boost the contact area between the sorptive phase and the analytes. In most cases, the extraction units needed to be completely redesigned to deploy the new materials giving rise to new microextraction modalities.
PDMS was extensively used as the coating in the first SBSE approaches. This material has demonstrated an efficient extraction capacity. However, its non-hydrophobic nature somewhat limits the applicability of SBSE to the extraction of non-polar compounds. Developing new coatings covering a wider range of polarities has been vital to widening the technique's versatility. The development of new LPME modalities based on integrating the solvent into the stirring element can be highlighted as a milestone in this evolution.
We foresee some trends for the evolution of SBSE and related techniques in the next years, including:
-
(a)
The development of new commercial coatings covering a wider range of polarities.
-
(b)
The improvement of the portability of the technique allowing the development of on-site extraction procedures.
-
(c)
The evaluation of the direct coupling of the extraction devices with instrumental techniques for the sake of simplification.
-
(d)
The implementation of open technologies, including 3D printing, improving the affordability and versatility of the technique.
Abbreviations
- AµE:
-
Adsorptive microextraction
- BAµE:
-
Bar adsorptive µ-extraction
- CD:
-
Cyclodextrin
- CE:
-
Capillary electrophoresis
- COF:
-
Covalent organic framework
- DE:
-
Desorption efficiency
- DμSPE:
-
Dispersive microsolid-phase extraction
- DS:
-
Desorption solvent
- DVB:
-
Divinylbenzene
- EDMA:
-
Ethylene dimethacrylate
- EE:
-
Extraction efficiency
- EF:
-
Enrichment factor
- EG:
-
Ethylene glycol
- FPSE:
-
Fabric phase sorptive extraction
- GC:
-
Gas chromatography
- GO:
-
Graphene oxide
- HFµE:
-
Hollow fiber microextraction
- HLB:
-
Hydrophilic-lipophilic balance
- HS:
-
Headspace
- HSSE:
-
Headspace sorptive extraction
- ICECLES:
-
Ice concentration linked with extractive stirrer
- ICP:
-
Inductive coupled plasma
- LC:
-
Liquid chromatography
- LD:
-
Liquid desorption
- LDH:
-
Layered double hydroxide
- MAOE:
-
Octyl methacrylate
- MAX:
-
Mixed mode anion exchange
- MECs:
-
Microextraction capsules
- MI-FPSE:
-
Magnet-integrated-FPSE
- MIP:
-
Molecularly-imprinted polymer
- MOF:
-
Metal-organic framework
- MPS:
-
3-(Trimethoxysilyl)propyl methacrylate
- MSAµE:
-
Multi-spheres adsorptive µ-extraction
- MTES:
-
Methyltrimethoxysilane
- MWCNTs:
-
Multiwalled carbon nanotubes
- o-SWNHs:
-
Oxidized single-walled carbon nanohorns
- PA:
-
Polyacrylate
- PANI:
-
Polyaniline
- PCB:
-
Polychlorinated biphenyl
- PDMS:
-
Polydimethylpolysiloxane
- PEEK:
-
Poly(ether ether ketone)
- PEG:
-
Polyethylene glycol
- PPESK:
-
Poly(phthalazine ether sulfone ketone)
- PTFE:
-
Polytetrafluoroethylene
- PTV:
-
Programmable temperature vaporizer
- RAM:
-
Restricted access material
- RDSE:
-
Rotating disk sorptive extraction
- rGO:
-
Reduced graphene oxide
- SA-SBSE:
-
Solvent-assisted stir bar sorptive extraction
- SBME:
-
Solvent bar microextraction
- SBSDME:
-
Stir bar sorptive dispersive microextraction
- SBSE:
-
Stir bar sorptive extraction
- SCSE:
-
Stir cake sorptive extraction
- SME:
-
Stir membrane extraction
- SPME:
-
Solid-phase microextraction
- TD:
-
Thermal desorption
- ZIF:
-
Zeolitic imidazole framework
References
Baltussen E, Sandra P, David F, Cramers C (1999) Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: theory and principles. J Microcolumn Sep 11:737–747. https://doi.org/10.1002/(SICI)1520-667X(1999)11:10<737::AID-MCS7>3.0.CO;2-4
Florêncio Nogueira JM (2017) Stir bar sorptive extraction. Compr Anal Chem 76:463–481. https://doi.org/10.1016/bs.coac.2017.01.006
Nogueira JMF (2015) Stir-bar sorptive extraction: 15 years making sample preparation more environment-friendly. TrAC—Trends Anal Chem 71:214–223. https://doi.org/10.1016/j.trac.2015.05.002
David F, Tienpont B, Sandra P (2003) Stir-bar sorptive extraction of trace organic compounds from aqueous matrices. LC GC North Am 21:108–118
David F, Ochiai N, Sandra P (2019) Two decades of stir bar sorptive extraction: a retrospective and future outlook. TrAC—Trends Anal Chem 112:102–111. https://doi.org/10.1016/j.trac.2018.12.006
David F, Sandra P (2007) Stir bar sorptive extraction for trace analysis. J Chromatogr A 1152:54–69. https://doi.org/10.1016/j.chroma.2007.01.032
Prieto A, Basauri O, Rodil R et al (2010) Stir-bar sorptive extraction: a view on method optimisation, novel applications, limitations and potential solutions. J Chromatogr A 1217:2642–2666. https://doi.org/10.1016/j.chroma.2009.12.051
Zuloaga O, Etxebarria N, González-Gaya B, et al (2019) Stir-bar sorptive extraction. In: Poole C (ed) Handbooks in separation science, 1st edn. Elsevier, pp 493–530
Hasan CK, Ghiasvand A, Lewis TW et al (2020) Recent advances in stir-bar sorptive extraction: coatings, technical improvements, and applications. Anal Chim Acta 1139:222–240. https://doi.org/10.1016/j.aca.2020.08.021
Camino-Sánchez FJ, Rodríguez-Gómez R, Zafra-Gómez A et al (2014) Stir bar sorptive extraction: recent applications, limitations and future trends. Talanta 130:388–399. https://doi.org/10.1016/j.talanta.2014.07.022
Chisvert A, Cárdenas S, Lucena R (2019) Dispersive micro-solid phase extraction. TrAC—Trends Anal Chem 112:226–233. https://doi.org/10.1016/j.trac.2018.12.005
Camino-Sánchez FJ, Zafra-Gómez A, Cantarero-Malagón S, Vílchez JL (2012) Validation of a method for the analysis of 77 priority persistent organic pollutants in river water by stir bar sorptive extraction in compliance with the European Water Framework Directive. Talanta 89:322–334. https://doi.org/10.1016/j.talanta.2011.12.037
Lucena R (2012) Extraction and stirring integrated techniques: examples and recent advances. Anal Bioanal Chem 403:2213–2223. https://doi.org/10.1007/s00216-012-5826-9
Cárdenas S, Lucena R (2017) Recent advances in extraction and stirring integrated techniques. Separations 4:6. https://doi.org/10.3390/separations4010006
Sgorbini B, Cagliero C, Cordero C et al (2012) New medium-to-high polarity twister coatings for liquid and vapour phase sorptive extraction of matrices of vegetable origin. J Chromatogr A 1265:39–45. https://doi.org/10.1016/j.chroma.2012.09.097
Serrano de la Hoz K, Salinas MR, Ferrandino A (2016) Different coatings for the HS-SBSE grape volatile analysis in model solution: preliminary results. Food Chem 212:814–820. https://doi.org/10.1016/j.foodchem.2016.06.047
Gilart N, Miralles N, Marcé RM et al (2013) Novel coatings for stir bar sorptive extraction to determine pharmaceuticals and personal care products in environmental waters by liquid chromatography and tandem mass spectrometry. Anal Chim Acta 774:51–60. https://doi.org/10.1016/j.aca.2013.03.010
Bizkarguenaga E, Iparragirre A, Navarro P et al (2013) In-port derivatization after sorptive extractions. J Chromatogr A 1296:36–46. https://doi.org/10.1016/j.chroma.2013.03.058
Ochiai N, Sasamoto K, Kanda H, Nakamura S (2006) Fast screening of pesticide multiresidues in aqueous samples by dual stir bar sorptive extraction-thermal desorption-low thermal mass gas chromatography-mass spectrometry. J Chromatogr A 1130:83–90. https://doi.org/10.1016/j.chroma.2006.06.032
Kawaguchi M, Ishii Y, Sakui N et al (2004) Stir bar sorptive extraction with in situ derivatization and thermal desorption-gas chromatography-mass spectrometry in the multi-shot mode for determination of estrogens in river water samples. J Chromatogr A 1049:1–8. https://doi.org/10.1016/j.chroma.2004.08.013
Ubeda C, Callejón RM, Troncoso AM et al (2016) Volatile profile characterisation of Chilean sparkling wines produced by traditional and Charmat methods via sequential stir bar sorptive extraction. Food Chem 207:261–271. https://doi.org/10.1016/j.foodchem.2016.03.117
Ochiai N, Sasamoto K, Ieda T et al (2013) Multi-stir bar sorptive extraction for analysis of odor compounds in aqueous samples. J Chromatogr A 1315:70–79. https://doi.org/10.1016/j.chroma.2013.09.070
Berrou K, Dunyach-Remy C, Lavigne JP et al (2019) Multiple stir bar sorptive extraction combined with gas chromatography-mass spectrometry analysis for a tentative identification of bacterial volatile and/or semi-volatile metabolites. Talanta 195:245–250. https://doi.org/10.1016/j.talanta.2018.11.042
Vyviurska O, Thai HA, Garančovská D et al (2022) Enhanced multi-stir bar sorptive extraction for wine analysis: alteration in headspace mode. Food Res Int 158:111510. https://doi.org/10.1016/j.foodres.2022.111510
Ochiai N, Sasamoto K, David F, Sandra P (2016) Solvent-assisted stir bar sorptive extraction by using swollen polydimethylsiloxane for enhanced recovery of polar solutes in aqueous samples: application to aroma compounds in beer and pesticides in wine. J Chromatogr A 1455:45–56. https://doi.org/10.1016/j.chroma.2016.05.085
Maslamani N, Manandhar E, Geremia DK, Logue BA (2016) ICE concentration linked with extractive stirrer (ICECLES). Anal Chim Acta 941:41–48. https://doi.org/10.1016/j.aca.2016.09.003
Skaggs CS, Alluhayb AH, Logue BA (2020) Comparison of the extraction efficiency of ice concentration linked with extractive stirrer, stir bar sorptive extraction, and solid-phase microextraction for pesticides from drinking water. J Chromatogr A 1622:461102. https://doi.org/10.1016/j.chroma.2020.461102
Ochiai N, Sasamoto K, Sasaki T et al (2020) Fractionated stir bar sorptive extraction using conventional and solvent-assisted approaches for enhanced identification capabilities of aroma compounds in beverages. J Chromatogr A 1628:461475. https://doi.org/10.1016/j.chroma.2020.461475
Ochiai N, Sasamoto K, David F, Sandra P (2022) Dual stir bar sorptive extraction using conventional and solvent-assisted approaches including in-situ derivatization followed by thermal desorption and gas chromatography–tandem mass spectrometry for analysis of polyfunctional thiols in white wine sample. Adv Sample Prep 3:100034. https://doi.org/10.1016/j.sampre.2022.100034
Gilart N, Marcé RM, Borrull F, Fontanals N (2014) New coatings for stir-bar sorptive extraction of polar emerging organic contaminants. TrAC - Trends Anal Chem 54:11–23. https://doi.org/10.1016/j.trac.2013.10.010
Liu W, Wang H, Guan Y (2004) Preparation of stir bars for sorptive extraction using sol-gel technology. J Chromatogr A 1045:15–22. https://doi.org/10.1016/j.chroma.2004.06.036
Yu C, Hu B (2007) Novel combined stir bar sorptive extraction coupled with ultrasonic assisted extraction for the determination of brominated flame retardants in environmental samples using high performance liquid chromatography. J Chromatogr A 1160:71–80. https://doi.org/10.1016/j.chroma.2007.05.042
Fan W, He M, You L et al (2020) Spiral stir bar sorptive extraction with polyaniline-polydimethylsiloxane sol-gel packings for the analysis of trace estrogens in environmental water and animal-derived food samples. J Sep Sci 43:1137–1144. https://doi.org/10.1002/jssc.201900819
Mao X, Fan W, He M et al (2015) C18-coated stir bar sorptive extraction combined with HPLC-ICP-MS for the speciation of butyltins in environmental samples. J Anal At Spectrom 30:162–171. https://doi.org/10.1039/c4ja00294f
Díaz-Álvarez M, Turiel E, Martín-Esteban A (2019) Molecularly imprinted polymer monolith containing magnetic nanoparticles for the stir-bar sorptive extraction of thiabendazole and carbendazim from orange samples. Anal Chim Acta 1045:117–122. https://doi.org/10.1016/j.aca.2018.09.001
Feng J, Sun M, Bu Y, Luo C (2016) Development of a carbon-nanoparticle-coated stirrer for stir bar sorptive extraction by a simple carbon deposition in flame. J Sep Sci 39:918–922. https://doi.org/10.1002/jssc.201501008
Guan W, Wang Y, Xu F, Guan Y (2008) Poly(phthalazine ether sulfone ketone) as novel stationary phase for stir bar sorptive extraction of organochlorine compounds and organophosphorus pesticides. J Chromatogr A 1177:28–35. https://doi.org/10.1016/j.chroma.2007.10.077
Hashemi SH, Kaykhaii M (2021) Nanoparticle coatings for stir bar sorptive extraction, synthesis, characterization and application. Talanta 221:121568. https://doi.org/10.1016/j.talanta.2020.121568
Maciel EVS, de Toffoli AL, Neto ES et al (2019) New materials in sample preparation: Recent advances and future trends. TrAC—Trends Anal Chem 119:115633. https://doi.org/10.1016/j.trac.2019.115633
He M, Chen B, Hu B (2014) Recent developments in stir bar sorptive extraction microextraction techniques. Anal Bioanal Chem 406:2001–2026. https://doi.org/10.1007/s00216-013-7395-y
He M, Wang Y, Zhang Q et al (2021) Stir bar sorptive extraction and its application. J Chromatogr A 1637:461810. https://doi.org/10.1016/j.chroma.2020.461810
Zhang W, Zhang Z, Zhang J et al (2014) Covalent immobilization of graphene onto stainless steel wire for jacket-free stir bar sorptive extraction. J Chromatogr A 1351:12–20. https://doi.org/10.1016/j.chroma.2014.05.038
Zhang Q, You L, Chen B et al (2021) Reduced graphene oxide coated nickel foam for stir bar sorptive extraction of benzotriazole ultraviolet absorbents from environmental water. Talanta 231:122332. https://doi.org/10.1016/j.talanta.2021.122332
Hu C, Chen B, He M, Hu B (2013) Amino modified multi-walled carbon nanotubes/polydimethylsiloxane coated stir bar sorptive extraction coupled to high performance liquid chromatography-ultraviolet detection for the determination of phenols in environmental samples. J Chromatogr A 1300:165–172. https://doi.org/10.1016/j.chroma.2013.05.004
Zou N, Yuan C, Liu S et al (2016) Coupling of multi-walled carbon nanotubes/polydimethylsiloxane coated stir bar sorptive extraction with pulse glow discharge-ion mobility spectrometry for analysis of triazine herbicides in water and soil samples. J Chromatogr A 1457:14–21. https://doi.org/10.1016/j.chroma.2016.06.043
Huang X, Yuan D (2007) Preparation of stir bars for sorptive extraction based on monolithic material. J Chromatogr A 1154:152–157. https://doi.org/10.1016/j.chroma.2007.03.132
Yao X, Zhou Z, He M et al (2018) One-pot polymerization of monolith coated stir bar for high efficient sorptive extraction of perfluoroalkyl acids from environmental water samples followed by high performance liquid chromatography-electrospray tandem mass spectrometry detection. J Chromatogr A 1553:7–15. https://doi.org/10.1016/j.chroma.2018.04.014
You L, He M, Chen B, Hu B (2017) One-pot synthesis of zeolitic imidazolate framework-8/poly (methyl methacrylate-ethyleneglycol dimethacrylate) monolith coating for stir bar sorptive extraction of phytohormones from fruit samples followed by high performance liquid chromatography-ultravi. J Chromatogr A 1524:57–65. https://doi.org/10.1016/j.chroma.2017.10.001
Zhu X, Cai J, Yang J et al (2006) Films coated with molecular imprinted polymers for the selective stir bar sorption extraction of monocrotophos. J Chromatogr A 1131:37–44. https://doi.org/10.1016/j.chroma.2006.07.041
Xu Z, Hu Y, Hu Y, Li G (2010) Investigation of ractopamine molecularly imprinted stir bar sorptive extraction and its application for trace analysis of β2-agonists in complex samples. J Chromatogr A 1217:3612–3618. https://doi.org/10.1016/j.chroma.2010.03.046
Liu R, Feng F, Chen G et al (2016) Barbell-shaped stir bar sorptive extraction using dummy template molecularly imprinted polymer coatings for analysis of bisphenol A in water. Anal Bioanal Chem 408:5329–5335. https://doi.org/10.1007/s00216-016-9628-3
Wang C, Zhou W, Liao X et al (2018) Covalent immobilization of metal organic frameworks onto chemical resistant poly(ether ether ketone) jacket for stir bar extraction. Anal Chim Acta 1025:124–133. https://doi.org/10.1016/j.aca.2018.04.056
Heidarbeigi M, Saraji M, Jafari MT (2021) In situ growth of copper-based metal-organic framework on a helical shape copper wire as a sorbent in stir-bar sorptive extraction of fenthion followed by corona discharge ion mobility spectrometry. J Chromatogr A 1651:462279. https://doi.org/10.1016/j.chroma.2021.462279
Zang L, He M, Wu Z et al (2021) Imine-linked covalent organic frameworks coated stir bar sorptive extraction of non-steroidal anti-inflammatory drugs from environmental water followed by high performance liquid chromatography-ultraviolet detection. J Chromatogr A 1659:462647. https://doi.org/10.1016/j.chroma.2021.462647
Liu Z, Zhou W, Hong Y et al (2022) Covalent organic framework-V modified porous polypropylene hollow fiber with detachable dumbbell-shaped structure for stir bar sorptive extraction of benzophenones. J Chromatogr A 1664:462798. https://doi.org/10.1016/j.chroma.2021.462798
Wang J, Feng J, Sun M et al (2023) Sulfonic acid-functionalized covalent organic frameworks as the coating for stir bar sorptive extraction of fluoroquinolones in milk samples. Microchim Acta 190:1–9. https://doi.org/10.1007/s00604-022-05534-9
Neng NR, Pinto ML, Pires J et al (2007) Development, optimisation and application of polyurethane foams as new polymeric phases for stir bar sorptive extraction. J Chromatogr A 1171:8–14. https://doi.org/10.1016/j.chroma.2007.09.033
Mirzaee MT, Seidi S, Razeghi Y et al (2020) In-tube stir bar sorptive extraction based on 3-aminopropyl triethoxysilane surface-modified Ce-doped ZnAl layered double hydroxide thin film for determination of nonsteroidal anti-inflammatory drugs in saliva samples. Microchim Acta 187:528. https://doi.org/10.1007/s00604-020-04489-z
Lambert JP, Mullett WM, Kwong E, Lubda D (2005) Stir bar sorptive extraction based on restricted access material for the direct extraction of caffeine and metabolites in biological fluids. J Chromatogr A 1075:43–49. https://doi.org/10.1016/j.chroma.2005.03.119
Wang M, Zeng J, Wang J et al (2021) Dual-mode aptasensor for simultaneous detection of multiple food-borne pathogenic bacteria based on colorimetry and microfluidic chip using stir bar sorptive extraction. Microchim Acta 188:244. https://doi.org/10.1007/s00604-021-04902-1
Zatrochová S, Martínez-Pérez-Cejuela H, Catalá-Icardo M et al (2022) Development of hybrid monoliths incorporating metal–organic frameworks for stir bar sorptive extraction coupled with liquid chromatography for determination of estrogen endocrine disruptors in water and human urine samples. Microchim Acta 189:92. https://doi.org/10.1007/s00604-022-05208-6
Yu C, Yao Z, Hu B (2009) Preparation of polydimethylsiloxane/β-cyclodextrin/divinylbenzene coated “dumbbell-shaped” stir bar and its application to the analysis of polycyclic aromatic hydrocarbons and polycyclic aromatic sulfur heterocycles compounds in lake water and soil by hig. Anal Chim Acta 641:75–82. https://doi.org/10.1016/j.aca.2009.03.031
Sukree W, Sooksawat D, Kanatharana P et al (2020) A miniature stainless steel net dumbbell-shaped stir-bar for the extraction of phthalate esters in instant noodle and rice soup samples. J Environ Sci Heal—Part B Pestic Food Contam Agric Wastes 55:60–68. https://doi.org/10.1080/03601234.2019.1659053
Zhou W, Wang C, Wang X, Chen Z (2018) Etched poly(ether ether ketone) jacket stir bar with detachable dumbbell-shaped structure for stir bar sorptive extraction. J Chromatogr A 1553:43–50. https://doi.org/10.1016/j.chroma.2018.04.022
Zhang Z, Mwadini MA, Chen Z (2016) Polytetrafluoroethylene-jacketed stirrer modified with graphene oxide and polydopamine for the efficient extraction of polycyclic aromatic hydrocarbons. J Sep Sci 39:4011–4018. https://doi.org/10.1002/jssc.201600716
Ghani M, Ghoreishi SM, Azamati M (2018) In-situ growth of zeolitic imidazole framework-67 on nanoporous anodized aluminum bar as stir-bar sorptive extraction sorbent for determining caffeine. J Chromatogr A 1577:15–23. https://doi.org/10.1016/j.chroma.2018.09.049
Khoobi A, Salavati-Niasari M, Ghani M et al (2019) Multivariate optimization methods for in-situ growth of LDH/ZIF-8 nanocrystals on anodized aluminium substrate as a nanosorbent for stir bar sorptive extraction in biological and food samples. Food Chem 288:39–46. https://doi.org/10.1016/j.foodchem.2019.02.118
Mao X, He M, Chen B, Hu B (2016) Membrane protected C18 coated stir bar sorptive extraction combined with high performance liquid chromatography-ultraviolet detection for the determination of non-steroidal anti-inflammatory drugs in water samples. J Chromatogr A 1472:27–34. https://doi.org/10.1016/j.chroma.2016.10.051
Alcudia-León MC, Lucena R, Cárdenas S, Valcárcel M (2009) Stir membrane extraction: a useful approach for liquid sample pretreatment. Anal Chem 81:8957–8961. https://doi.org/10.1021/ac9016192
Alcudia-León MC, Lucena R, Cárdenas S, Valcárcel M (2011) Stir membrane liquid-liquid microextraction. J Chromatogr A 1218:869–874. https://doi.org/10.1016/j.chroma.2010.12.075
Alcudia-León MC, Lucena R, Cárdenas S, Valcárcel M (2011) Determination of phenols in waters by stir membrane liquid-liquid-liquid microextraction coupled to liquid chromatography with ultraviolet detection. J Chromatogr A 1218:2176–2181. https://doi.org/10.1016/j.chroma.2011.02.033
Roldán-Pijuán M, Alcudia-León MC, Lucena R et al (2013) Stir-membrane liquid microextraction for the determination of paracetamol in human saliva samples. Bioanalysis 5:307–315. https://doi.org/10.4155/bio.12.312
Rodríguez-Gómez R, Roldán-Pijuán M, Lucena R et al (2014) Stir-membrane solid-liquid-liquid microextraction for the determination of parabens in human breast milk samples by ultra high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1354:26–33. https://doi.org/10.1016/j.chroma.2014.05.071
Roldán-Pijuán M, Lucena R, Alcudia-León MC et al (2013) Stir octadecyl-modified borosilicate disk for the liquid phase microextraction of triazine herbicides from environmental waters. J Chromatogr A 1307:58–65. https://doi.org/10.1016/j.chroma.2013.07.086
Alcudia-León MC, Lucena R, Cárdenas S, Valcárcel M (2013) Magnetically confined hydrophobic nanoparticles for the microextraction of endocrine-disrupting phenols from environmental waters. Anal Bioanal Chem 405:2729–2734. https://doi.org/10.1007/s00216-012-6683-2
Huang X, Chen L, Lin F, Yuan D (2011) Novel extraction approach for liquid samples: stir cake sorptive extraction using monolith. J Sep Sci 34:2145–2151. https://doi.org/10.1002/jssc.201100283
Huang X, Chen L, Yuan D, Bi S (2012) Preparation of a new polymeric ionic liquid-based monolith for stir cake sorptive extraction and its application in the extraction of inorganic anions. J Chromatogr A 1248:67–73. https://doi.org/10.1016/j.chroma.2012.06.004
Lin F, Nong S, Huang X, Yuan D (2013) Sensitive determination of organic acid preservatives in juices and soft drinks treated by monolith-based stir cake sorptive extraction and liquid chromatography analysis. Anal Bioanal Chem 405:2077–2081. https://doi.org/10.1007/s00216-012-6646-7
Oliva-Lamarca Y, Fresco-Cala B, Cárdenas S (2019) Synthesis, characterization, and application of chemically interconnected carbon nanotube monolithic sorbents by photopolymerization in polypropylene caps. Anal Bioanal Chem 411:3291–3299. https://doi.org/10.1007/s00216-019-01795-1
Richter P, Leiva C, Choque C et al (2009) Rotating-disk sorptive extraction of nonylphenol from water samples. J Chromatogr A 1216:8598–8602. https://doi.org/10.1016/j.chroma.2009.10.044
Jachero L, Ahumada I, Richter P (2014) Rotating-disk sorptive extraction: Effect of the rotation mode of the extraction device on mass transfer efficiency. Anal Bioanal Chem 406:2987–2992. https://doi.org/10.1007/s00216-014-7693-z
Richter P, Arismendi D, Becerra-Herrera M (2021) The fundamentals, chemistries and applications of rotating-disk sorptive extraction. TrAC—Trends Anal Chem 137:116209. https://doi.org/10.1016/j.trac.2021.116209
Neng NR, Silva ARM, Nogueira JMF (2010) Adsorptive micro-extraction techniques—novel analytical tools for trace levels of polar solutes in aqueous media. J Chromatogr A 1217:7303–7310. https://doi.org/10.1016/j.chroma.2010.09.048
Ide AH, Nogueira JMF (2018) New-generation bar adsorptive microextraction (BAμE) devices for a better eco-user-friendly analytical approach—application for the determination of antidepressant pharmaceuticals in biological fluids. J Pharm Biomed Anal 153:126–134. https://doi.org/10.1016/j.jpba.2018.02.001
Ide AH, Nogueira JMF (2018) Hollow fiber microextraction: a new hybrid microextraction technique for trace analysis. Anal Bioanal Chem 410:2911–2920. https://doi.org/10.1007/s00216-018-0971-4
Ide AH, Nogueira JMF (2020) Dual-hollow fiber microextraction (dual-HFμE)—application for monitoring trace levels of organochlorine pesticides in real matrices. Int J Environ Anal Chem 100:1402–1414. https://doi.org/10.1080/03067319.2019.1655006
Benedé JL, Chisvert A, Giokas DL, Salvador A (2014) Development of stir bar sorptive-dispersive microextraction mediated by magnetic nanoparticles and its analytical application to the determination of hydrophobic organic compounds in aqueous media. J Chromatogr A 1362:25–33. https://doi.org/10.1016/j.chroma.2014.08.024
Vállez-Gomis V, Grau J, Benedé JL et al (2021) Fundamentals and applications of stir bar sorptive dispersive microextraction: a tutorial review. Anal Chim Acta 1153:338271. https://doi.org/10.1016/j.aca.2021.338271
Maya F, Palomino Cabello C, Estela JM et al (2015) Automatic in-syringe dispersive microsolid phase extraction using magnetic metal-organic frameworks. Anal Chem 87:7545–7549. https://doi.org/10.1021/acs.analchem.5b01993
Azorín C, Benedé JL, Chisvert A (2023) New challenges in sample preparation: miniaturized stir bar sorptive dispersive microextraction as a high-throughput and feasible approach for low-availability sample preparation. Anal Chim Acta 1238:340627. https://doi.org/10.1016/j.aca.2022.340627
Jiang X, Lee HK (2004) Solvent bar microextraction. Anal Chem 76:5591–5596. https://doi.org/10.1021/ac040069f
López-López JA, Mendiguchía C, Pinto JJ, Moreno C (2019) Application of solvent-bar micro-extraction for the determination of organic and inorganic compounds. TrAC—Trends Anal Chem 110:57–65. https://doi.org/10.1016/j.trac.2018.10.034
López-López JA, Ogalla-Chozas E, Lara-Martín PA, Pintado-Herrera MG (2017) Solvent bar micro-extraction (SBME) based determination of PAHs in seawater samples. Sci Total Environ 598:58–63. https://doi.org/10.1016/j.scitotenv.2017.04.125
Liu W, Zhang L, Fan L et al (2012) An improved hollow fiber solvent-stir bar microextraction for the preconcentration of anabolic steroids in biological matrix with determination by gas chromatography-mass spectrometry. J Chromatogr A 1233:1–7. https://doi.org/10.1016/j.chroma.2012.01.064
Correa L, Fiscal JA, Ceballos S et al (2015) Hollow-fiber solvent bar microextraction with gas chromatography and electron capture detection determination of disinfection byproducts in water samples. J Sep Sci 38:3945–3953. https://doi.org/10.1002/jssc.201500324
Yu C, Liu Q, Lan L, Hu B (2008) Comparison of dual solvent-stir bars microextraction and U-shaped hollow fiber-liquid phase microextraction for the analysis of Sudan dyes in food samples by high-performance liquid chromatography-ultraviolet/mass spectrometry. J Chromatogr A 1188:124–131. https://doi.org/10.1016/j.chroma.2008.02.065
Fashi A, Salarian AA, Zamani A (2018) Solvent-stir bar microextraction system using pure tris-(2-ethylhexyl) phosphate as supported liquid membrane: a new and efficient design for the extraction of malondialdehyde from biological fluids. Talanta 182:299–305. https://doi.org/10.1016/j.talanta.2018.02.002
Georgiadis DE, Tsalbouris A, Kabir A et al (2019) Novel capsule phase microextraction in combination with high performance liquid chromatography with diode array detection for rapid monitoring of sulfonamide drugs in milk. J Sep Sci 42:1440–1450. https://doi.org/10.1002/jssc.201801283
Kermani M, Jafari MT, Saraji M (2021) Self-rotating stir mesh screen sorptive extraction for analyzing chlorpyrifos by ion mobility spectrometry. Anal Methods 13:2631–2644. https://doi.org/10.1039/d1ay00595b
Kabir A, Mesa R, Jurmain J, Furton KG (2017) Fabric phase sorptive extraction explained. Separations 4:21. https://doi.org/10.3390/separations4020021
Alampanos V, Kabir A, Furton K, Samanidou V (2021) Magnet integrated fabric phase sorptive extraction of selected endocrine disrupting chemicals from human urine followed by high-performance liquid chromatography—photodiode array analysis. J Chromatogr A 1654:462459. https://doi.org/10.1016/j.chroma.2021.462459
Mao X, Hu B, He M, Fan W (2012) Stir bar sorptive extraction approaches with a home-made portable electric stirrer for the analysis of polycyclic aromatic hydrocarbon compounds in environmental water. J Chromatogr A 1260:16–24. https://doi.org/10.1016/j.chroma.2012.08.062
Qin Z, Bragg L, Ouyang G et al (2009) Solid-phase microextraction under controlled agitation conditions for rapid on-site sampling of organic pollutants in water. J Chromatogr A 1216:6979–6985. https://doi.org/10.1016/j.chroma.2009.08.052
Roldán-Pijuán M, Lucena R, Cárdenas S, Valcárcel M (2014) Micro-solid phase extraction based on oxidized single-walled carbon nanohorns immobilized on a stir borosilicate disk: application to the preconcentration of the endocrine disruptor benzophenone-3. Microchem J 115:87–94. https://doi.org/10.1016/j.microc.2014.02.015
Casado-Carmona FA, Alcudia-León M del C, Lucena R, Cárdenas S (2019) Portable stir membrane device for on-site environmental sampling and extraction. J Chromatogr A 1606:360359. https://doi.org/10.1016/j.chroma.2019.07.013
Casado-Carmona FA, Lucena R, Cárdenas S (2021) Magnetic paper-based sorptive phase for enhanced mass transference in stir membrane environmental samplers. Talanta 228:122217. https://doi.org/10.1016/j.talanta.2021.122217
Casado-Carmona FA, Jiménez-Soto JM, Lucena R, Cárdenas S (2022) Portable stirring device for the on-site extraction of environmental waters using magnetic hydrophilic-lipophilic balance tape. Anal Chim Acta 1189:339186. https://doi.org/10.1016/j.aca.2021.339186
Casado-Carmona FA, Lucena R, Cárdenas S (2023) Lab in a bottle, open-source technologies for the design of affordable environmental samplers integrating on-site extraction. J Environ Chem Eng 11:109713. https://doi.org/10.1016/j.jece.2023.109713
Makkliang F, Kanatharana P, Thavarungkul P, Thammakhet-Buranachai C (2018) A miniaturized monolith-MWCNTs-COOH multi-stir-rod microextractor device for trace parabens determination in cosmetic and personal care products. Talanta 184:429–436. https://doi.org/10.1016/j.talanta.2018.03.024
Badiee H, Zanjanchi MA, Zamani A, Fashi A (2019) A four-hollow fibers geometry of revolving solvent bar microextraction setup for the enrichment of trace ammonia. Talanta 199:170–177. https://doi.org/10.1016/j.talanta.2019.02.028
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Benedé, J.L., Lucena, R., Cárdenas, S., Chisvert, A. (2024). Stir Bar Sorptive Extraction. In: Rodríguez-Delgado, M.Á., Socas-Rodríguez, B., Herrera-Herrera, A.V. (eds) Microextraction Techniques. Integrated Analytical Systems. Springer, Cham. https://doi.org/10.1007/978-3-031-50527-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-50527-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50526-3
Online ISBN: 978-3-031-50527-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)