Abstract
The development of calibration procedures, as well as the certification of readily available reference materials (RMs) and the improvement of their metrological characteristics, are topical issues for thermal analysis methods. In the course of the work, the necessity of conducting a study in order to expand the range of CRMs for temperature and enthalpy of phase transitions is substantiated. The substantiation of the choice of the measurement procedure and starting materials is given. The conditions for conducting experiments are described in detail, and the choice of these conditions is justified. The developed CRMs passed the metrological examination and were included in the Register of approved types of reference materials FIF EUM as a set of certified reference materials for temperature and specific enthalpy of phase transitions (set SOTSF-2) GSO 11890-2022/GSO 11896-2022. The practical significance of the obtained results is as follows: certified reference materials allow expanding the possibility of establishing and monitoring the stability of the calibration characteristics of thermal analysis installations and measuring instruments; certification of measurement procedures (methods) and accuracy control of the measurement results of the phase transition temperature of metals, metal salts, metal oxides, and polymeric materials, organic and inorganic substances.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Reference material
- Thermal analysis
- Phase transition temperature
- Specific enthalpy of phase transition
- State primary standard
Introduction
Currently, thermal analysis (TA) refers to a group of methods in which a physical property of a substance is measured as a function of temperature or time while the substance is subjected to a temperature controlled program [1]. The temperature program can include heating, cooling at a constant rate, exposure at a constant temperature (isotherm), and a combination of these modes or a temperature modulation mode with amplitude and frequency setting.
One of the most advanced, highly sensitive and, therefore, the most commonly used methods for phase analysis of heterogeneous systems are differential thermal analysis (DTA) and differential scanning calorimetry (DSC). They make it possible to determine the temperature and enthalpy of transitions, the heat capacity and its dependence on thermodynamic parameters, as well as the kinetic characteristics of physical and chemical processes under conditions of a linear temperature variation. Instruments that implement these methods are widely used, quite simple in design, and easy to operate. However, DSC/DTA instruments, unlike adiabatic calorimeters, are not absolute measuring instruments. The values of the amount of released or absorbed heat and heat flow rates are measured under dynamic conditions, and not under heat equilibrium conditions. Therefore, the measurement results of DSC/DTA instruments are influenced by numerous parameters associated with the measured sample, instrument, and operator. The main influencing parameters include: the mass and geometry of the sample, its state of aggregation and thermophysical properties, the type of the measured effect, the magnitude of the measured effect, the dimensions of the crucible and the material from which it is made, the thermal resistance between the calorimeter, the crucible, and the sample, the response time of the heating/cooling device, the design and material of the measuring cell, the position of the sample in the crucible and the crucible in the calorimeter, the type of purge gas, its pressure, and purge rate, the procedure for collecting and processing experimental data. Nevertheless, errors in measuring the phase transition temperature from 0.01 to 0.5 °C, the enthalpy changes of phase transitions from 0.5 to 1.0%, and heat capacity from 1 to 2% [2] can be achieved in the temperature range from 200 to 800 K subject to careful calibration of DSC/DTA instruments and accurate measurements. In addition, the measurement accuracy of thermophysical properties performed using DSC/DTA devices can be affected by changes occurring in the device—the drift of electronic parameters and degradation of thermally sensitive elements (thermocouples and thermal resistances). The dynamics of these changes primarily depends on the intensity of MI operation and measurement conditions: heating rate, atmosphere, and sample holder material. Therefore, a fundamental requirement for every thermoanalytical study is the pre-calibration of the respective instrument, which should be performed using uniform and reliable procedures. Calibration is understood as the procedure for establishing the relationship between the value of a quantity determined by the measuring instrument and its true value. The developments of calibration procedures, as well as the certification of readily available reference materials (RMs) and the improvement of their metrological characteristics, are topical issues for thermal analysis methods.
Theoretical Background
The range of reference materials used should correspond to the operating ranges of measuring instruments, as well as the materials used for the manufacture of measuring cells and crucibles. Since the development and implementation of the ISO-9000 standard [3], ensuring the comparability and traceability of thermal analytical data has become a fundamental requirement.
One of the first calibration procedures for DSC/DTA instruments were those described in [4,5,6]. But none of them acted as an instrument-independent procedure. In order to unify the calibration methods that existed at that time and reduce the magnitude of systematic measurement errors, the German Society for Thermal AnalysisFootnote 1 formed a working group “Calibration of scanning calorimeters” (1987), whose goal was to develop scientifically based, instrument-independent, and generally accepted calibration procedures, the application of which would significantly reduce the systematic errors of the values measured by DSC/DTA. The results of the working group were published in [7,8,9,10,11,12,13] and were adopted by the International Confederation for Thermal Analysis and Calorimetry (ICTAC)Footnote 2 as a recommendation to its members (2000).
Since DSC/DTA instruments are used to determine the temperature and heat of phase transitions, as well as the rate of heat flow (when determining the temperature dependence of the heat capacity of a substance or studying the kinetics of a reaction), it becomes necessary to calibrate MI using three scales: temperature, enthalpy, and heat flow.
The temperature scale is calibrated by comparing the known phase transition (melting/crystallization) temperatures of RMs with the corresponding temperatures measured at the appropriate heating rate. Then a correction curve is constructed in the given temperature range. Depending on the range to be covered and the shape of the calibration curve, at least three RMs are required to be measured—even if the calibration is linear, a third point is needed to confirm this. Since temperature sensors are most often located inside the device and not in the sample, the need for extrapolation to a zero heating rate requires measuring RMs at three or more heating rates [2].
Melting (crystallization) or a phase transition in a solid appears on the DSC curve as an endo- or exothermic peak; therefore, the enthalpy calibration procedure makes it possible to relate the peak area obtained for the RM to the enthalpy change known for it and obtain peak area to enthalpy conversion factor. Since experience has shown that this factor is a function of temperature and temperature scan rate (or any other of the experimental parameters that should be kept constant throughout the measurement series), the measurements of several enthalpy reference materials are required to cover the entire temperature range of the DSC/DTA instrument.
The heat flow rate in the DSC experiment is related to the heat capacity of the sample; and if any reaction occurs in the sample, it is related to the “apparent” heat capacity. The ideal RM for heat flow calibration should be thermodynamically inert (have no phase transformations) over a wide temperature range. From this point of view, α-alumina (polycrystalline corundum or monocrystalline leucosapphire) is a universal RM. With its use, experimental data on heat capacity for other well-studied substances can be reproduced with an accuracy of ± 1%, and in some cases even better [14].
The recommendations of the GEFTA working group for calibrating DSC/DTA instruments and performing subsequent measurements are formulated as follows [15]:
-
Experimental conditions for calibration and sample measurements should differ as little as possible. Therefore, a valid calibration procedure should specify in detail how to establish reproducible environmental conditions and how to check for non-linearities;
-
Any asymmetry in heat flows, temperature gradients, and measurement effects should be avoided. It is necessary to create quasi-steady-state conditions. However, a detailed study of the dependence of the measured values on the parameters of the sample and instrument is mandatory to eliminate systematic errors;
-
Calibrations already performed by the manufacturer of DSC/DTA instruments should be carefully checked;
-
Calibrations should be checked at regular intervals according to the required accuracy. It also provides information on any long-term systematic changes to the measuring system.
The recommended substances by GEFTA and ICTAC for use as RMs cover the temperature range from 120 to 1350 °C and mainly refer to materials that determine the fixed points of the International Temperature Scale 1990 (ITS-90) [15] (Table 1).
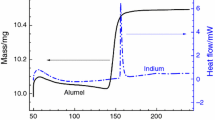
The GEFTA and ICTAC recommendations also describe the procedure for processing experimental data obtained from measurements of RMs on a calibrated DSC/DTA instrument, including a method for determining the characteristic temperatures of endothermic (exothermic) peaks of phase transformations. Figure 1 shows the determination of the characteristic temperatures in the case of an endothermic peak (for example, metal melting) [2].
Determination of characteristic DSC/DTA peak temperatures, where: Ti is the initial temperature: the first visible deviation of the DSC/DTA curve from the interpolated baseline; Te is the extrapolated peak start temperature: the temperature at which the tangent at the inflection point of the rising slope of the peak crosses the interpolated baseline. The temperature Te is taken as the melting or phase transition temperature; Tp is the peak temperature: the temperature corresponding to the largest distance between the DSC/DTA curve and the interpolated baseline; Tc is the extrapolated peak end temperature: the temperature at which the tangent at the inflection point of the downward slope of the peak crosses the interpolated baseline; Tf is the final temperature: the last visible deviation of the DSC/DTA curve from the interpolated baseline
It is more convenient to determine the values of temperatures Ti, Tf from the first derivative of the DSC/DTA curve, where the moment of deviation of the DSC/DTA signal from the interpolated baseline is determined much better.
Using the example of UNIIM, a competent manufacturer of CRMs for thermophysical properties (SOTS), 6 types of CRMs for temperature and heat of phase transitions were developed based on potassium chloride, as well as gallium, indium, tin, zinc, antimony (set SOTSF, Table 2). The development and certification of CRMs were carried out in accordance with the State verification schedule for means of measuring specific enthalpy and specific heat-conguction of solid frames in the range of temperature from 700 to 1800 K [16] using the National primary special standard GET 67-2013 for the units of specific enthalpy and specific heat of solids at temperatures in the 700–1800 K range [17].
GSO 2312-82/2316-82 Reference materials of the approved type of temperatures and heats of phase transitions (set SOTSF). Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/19/items/393963. Accessed 10 October 2022 (In Russ.).
GSO 1363-78 Reference materials of thermodynamic properties (potassium chloride) (SOTS-5). Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/19/items/394230. Accessed 10 October 2022 (In Russ.).
As shown in Table 2, the temperature range provided by the available GSOs is from ~ 300 to ~ 1050 K, and the range of specific heat of fusion is from ~ 28 to ~ 59 kJ/kg.
At the same time, TA instruments supplied to the Russian market by leading manufacturers of thermoanalytical equipment, such as “Netzsch-Geratebau GmbH” (Germany), “TA Instruments” and “PerkinElmer, Inc” (USA), “Setaram Instrumentation” (France), and “Mettler-Toledo AG” (Switzerland) cover a much wider range of temperatures and heats of phase transitions: from ~ 90 to ~ 1920 K and from 10 to 1000 kJ/kg, respectively. Moreover, recently a number of high temperature instruments have appeared on the market, the upper limits of the operating range of which are 2273 K (DSC 404 F1/F3 Pegasus, STA 449 F1 Jupiter, Netzsch, Germany), 2673 K (STA 449 F3 Jupiter, Netzsch, Germany, SETSYS Evolution TGA-DTA/DSC, Setaram Instrumentation, France), and even 3073 K (DIL 402 Expedis HT, Netzsch, Germany).
The analysis showed that the available list of GSOs for temperatures and heats of phase transitions do not fully provide the measurement ranges of the corresponding thermophysical quantities. In addition, Table 3 indicates the existing limitations on the compatibility of GSO materials with the materials of crucibles used in measurements [8].
Table 3 shows that, for example, GSO 2312-82/2316-82 can be used for graduation, calibration, and temperature verification of thermal analyzers in the case of measurements in non-metallic (ceramic, glass, etc.) crucibles. At the same time, only GSO 2313-82 (In) and GSO 2314-82 (Sn) can be used for graduation, calibration, and verification by specific heat of fusion. There is only GSO 1363-78 based on potassium chloride for use with metal crucibles (platinum, steel, etc.), which is clearly not enough for graduation, calibration, and verification of thermal analysis MI.
Thus, the study of determining the temperature and heat of phase transitions for the metrological support of MI of higher temperature and traceability to the basic units of physical quantities is of particular relevance.
Therefore, the purpose of this work is the development and certification of new RMs for temperature and heat of phase transitions for thermal analysis instruments, primarily for DSC/DTA instruments.
Materials and Methods
Reagents
The commercially available Netzsch RMs used for calibration of thermal analysis instruments were selected as candidate RMs (Table 4).
This list of 24 substances can be divided into three groups: the first is for the low temperature range (from − 87 to 122 °C) and consists mainly of organic compounds; the second and third are for the high temperature range and consist of salts (for metal crucibles) and metals (for ceramic crucibles).
The main objective in selecting a candidate RM for the development and certification of new GSO for temperature and heat of phase transitions from those given in Table 4 was to expand the temperature ranges provided by new RMs for both metal and non-metal crucibles. Therefore, the GSO set, later called SOTSF-2, included samples of four high-purity metals (wt%): Bi (99.999%), Al (99.999%), Ag (99.99%), Au (99.999%), as well as three samples of high-purity metal salts: Ag2SO4 (99.999%), CsCl (99.999%), BaCO3 (99.98%).
Thus, the temperature range provided by the GSO was supposed to be expanded to 1337.2 K with a total amount of RMs of 10 pieces (6—SOTSF, 4—SOTSF-2) for non-metal crucibles. According to the enthalpy of phase transitions, the upper limit of the range will be 397 J/g with a total number of RMs of 6 pieces (2—SOTSF, 4—SOTSF-2). Similarly, the lower and upper limits of the temperature range provided by the GSO should be 699.4 K and 1081 K with a total number of reference materials of 4 pieces (GSO 1363-78, 3—SOTSF-2) for metal crucibles. According to the enthalpy of phase transitions, the range is from 17.2 to 357.29 J/g with a total amount of RMs of 4 pieces (GSO 1363-78, 3—SOTSF-2).
Equipment
All phase transformation temperature studies were performed on a thermal analyzer STA 449 F5 Jupiter (NETZSCH, Germany), which implements the method of simultaneous thermal analysis (STA), which combines the method of differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA), from the State Primary Standard of units of mass fraction, mass (molar) concentration of water in solid and liquid substances and materials GET 173-2017.Footnote 4
The mass of the starting material (candidate RM) was measured on a laboratory balance of special accuracy class I (Sartorius, Germany) with a weighing resolution of 10 μg and an expanded uncertainty of 80 μg. The mass of the crucible and the weight of the crucible with a sample were obtained in ten parallel measurements. The sample mass was determined by the mass differences. The establishment of certified values of the specific enthalpy of phase transformations of new GSO was carried out by direct measurements on the National primary special standard GET 67-2013 for the units of specific enthalpy and specific heat of solids at temperatures in the 700–1800 K range.Footnote 5 GET 67-2013 is an isothermal mixing calorimeter designed to measure specific enthalpy and specific heat, as well as to study phase transformations in solid and liquid materials in a wide temperature range (from room temperature to 1800 K).
A description of the GET 67-2013 design and methods for measuring the specific enthalpy and specific heat capacity are given in [17]. The theory of calorimetric measurements by the mixing method is considered in detail in [18]. The derivation of the equation for measuring the specific enthalpy of phase transformations using an isothermal mixing calorimeter is described in [19].
Research Methods and Procedure
The establishment of certified values of the temperature of phase transformations of new GSO was performed using a certified measurement method FR.1.31.2021.40481.Footnote 6 The indicators of the measurement method are presented in Table 5.
The establishment of certified values of the specific enthalpy of phase transformations of new GSO was performed in accordance with the operating manual and technical documentation for GET 67-2013.
To reduce the impact of factors affecting the accuracy, the measurements were carried out in accordance with the following procedure:
-
in accordance with the requirements of ASTM E 967, high-purity Al2O3 crucibles were used for metal measurements in order to avoid interaction of the test material with the crucible material. High-purity metal salts were measured in platinum crucibles. Closed crucibles were used to reduce the influence of sample color on its heating rate, as well as a more uniform heat distribution;
-
it is recommended to use a gas with low thermal conductivity, therefore, the measurements were carried out in N2 atmosphere with a purity of 99.99% at a flow rate of 40 ml/min;
-
the measurements were carried out using a DSC/TG sample holder with an S-type thermocouple and a sensitivity of 1.2 µV/mW;
-
good contact between the material and the bottom of the crucible is essential to increase the heat transfer and, therefore, the measurement accuracy. In this regard, the starting material was placed in the crucible so as to completely cover the bottom for the case of measuring samples of metal salts; then the compaction was carried out using special equipment, which is a common technique in sample preparation [20].
-
samples weighing no more than 20 mg were used to reduce the effect of sample weight on the measurement result;
-
the thermal analyzer was calibrated using GSO 2312-82/2316-82 and GSO 1363-78 (set SOTSF), the accuracy indicators of which correspond to the working standards of the 1st category according to the state temperature verification scheme (GOST 8.558-2009 [21]);
-
a heating rate of 10 °C/min was used during the study of RMs, since high heating rates lead to a shift in heat effects to higher temperatures. This heating rate value is recommended for use in verification methods for TA MI.
Results and Discussion
The DSC curves for some materials obtained in determining the certified values of the phase transition temperature of CRMs are shown in Figs. 2, 3, 4 and 5.
The DSC curves presented in Figs. 2, 3, 4 and 5 were processed in accordance with the requirements of the MP; two or three sample weights were used for each material. It follows from the obtained data that the standard deviation of the measurement results does not exceed 0.01 °C, which meets the requirements of the MP in the entire temperature range.
When determining the certified value of RMs in accordance with GOST ISO Guide 35-2015 [22], studies were carried out, and the contributions to the uncertainty due to heterogeneity of the starting materials, short- and long-term stabilities of materials were estimated.
The obtained values of the metrological characteristics are as follows: the phase transition temperature (TPhT) and specific enthalpy of phase transition (HPhT) of the investigated batch of CRMs are presented in Table 6.
GSO 11890-2022/11896-2022 Reference materials of the temperature and specific enthalpy of phase transitions of metals and metal salts (SET RM SOTSF-2). Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/19/items/1398408. Accessed 10 October 2022 (In Russ.).
It should be noted that the melting temperatures of metals coincide within the error of the measurement method used by us with fixed points of the ITS-90 [23]. The HPhT values for metals are close to the data given in [24], as well as in a number of other sources [2, 8, 12, 15]. Similarly the values of the phase transition temperature are consistent with our results better than the values of the enthalpy of phase transformations [25] for metal salts.
The existing differences can be primarily due to differences in the chemical composition of micro-impurities in the composition of the substances researched in different studies, as well as some differences in sample preparation and measurements, which is completely difficult to avoid even with the most careful implementation of the GTEFTA and ICTAC recommendations [15].
Taking into account the obtained results for other materials, the correctness of the chosen method and experimental conditions, it can be concluded that the obtained values are representative.
The traceability of the certified values of the phase transition temperature is ensured to the temperature unit (°C) reproduced by the State Primary Standard of temperature unit in the range from 0 to 3200 °C GET 34-2020,Footnote 8 and provided through the use of reference materials of In, Sn, Zn, Al, Ag, and Au, which are the fixed points of the International Temperature Scale (ITS–90). The procedure described above can be recommended for determining the characteristics of other high-purity metals and their salts, as well as for certification of RMs based on them.
Conclusion
The purpose of this work was the development and certification of new certified reference materials for phase transition temperature based on high-purity metals and metal salts traceable to SI unit “temperature” °C for thermal analysis measuring instruments, primarily for DSC/DTA instruments.
In the course of experimental studies by differential scanning calorimetry using the thermal analyzer STA 449 F5 Jupiter from GET 173-2017, CRM certified values were determined. The procedure was carried out in accordance with GOST ISO Guide 35-2015; the contributions to the uncertainty due to heterogeneity of the starting materials were evaluated; the short- and long-term stabilities of the materials were studied.
The obtained metrological characteristics of the investigated batch of CRMs are as follows: the phase transition temperature (TPhT) and specific enthalpy of phase transition (HPhT) of the investigated batch of CRMs. The certified TPhT values for the developed CRMs based on high-purity metals (Bi, Al, Ag, Au) are consistent with the temperatures of the ITS-90 fixed points with an accuracy of no worse than ± 0.01 °C. The certified HPhT values for CRMs based on high-purity Bi, Al, Ag, Au are consistent with the results given in [2, 10, 12, 13, 15, 25] in the range from 0.3 to 1.3%.
Similarly, there is also a fairly good agreement between the TPhT and HPhT certified values and the reference data for CRMs based on high-purity metal salts (Ag2SO4, CsCl, BaCO3). For example, the obtained TPhT value for BaCO3 practically coincides, and the HPhT value differs by 2.7% from the results obtained in [25].
The developed CRMs passed the metrological examination and were included in the Register of approved types of reference materials FIF EUMFootnote 9 as a set of certified reference materials for temperature and specific enthalpy of phase transitions (set SOTSF-2) GSO 11890-2022/GSO 11896-2022.
The practical significance of the obtained results is as follows: certified reference materials allow expanding the possibility of establishing and monitoring the stability of the calibration characteristics of thermal analysis installations and measuring instruments; certification of measurement procedures (methods) and accuracy control of the measurement results of the phase transition temperature of metals, metal salts, metal oxides, and polymeric materials, organic and inorganic substances.
Notes
- 1.
Gesellschaft fur Thermische Analyse (GEFTA). Available via GEFTA. https://www.gefta.org/index.php. Accessed 10 October 2022.
- 2.
International Confederation for Thermal Analysis and Calorimetry (ICTAC). Available via ICTAC. http://www.ictac.org/index.html. Accessed 10 October 2022.
- 3.
GSO 2312-82/2316-82 Reference materials of the approved type of temperatures and heats of phase transitions (set SOTSF). Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/19/items/393963. Accessed 10 October 2022 (In Russ.).
GSO 1363-78 Reference materials of thermodynamic properties (potassium chloride) (SOTS-5). Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/19/items/394230. Accessed 10 October 2022 (In Russ.).
- 4.
GET 173-2017 State primary standard of units of mass fraction, mass (molar) concentration of water in solid and liquid substances and materials: UNIIM—Affiliated Branch of the D. I. Mendeleyev Institute for Metrology. Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/12/items/397857. Accessed 10 October 2022 (In Russ.).
- 5.
GET 67-2013 State primary special standard of units of specific enthalpy and specific heat capacity of solids in the temperature range from 700 to 1800 K: UNIIM—Affiliated Branch of the D. I. Mendeleyev Institute for Metrology. Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/12/items/397945. Accessed 10 October 2022 (In Russ.).
- 6.
FR.1.31.2021.40481 Method for Measuring Phase Transition Temperatures of High-Purity Metals and Inorganic Compounds by Differential Scanning Calorimetry:: UNIIM—Affiliated Branch of the D. I. Mendeleyev Institute for Metrology. Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/16/items/1391509. Accessed 10 October 2022 (In Russ.).
- 7.
GSO 11890-2022/11896-2022 Reference materials of the temperature and specific enthalpy of phase transitions of metals and metal salts (SET RM SOTSF-2). Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/19/items/1398408. Accessed 10 October 2022 (In Russ.).
- 8.
GET 34-2020 State primary standard of temperature unit in the range from 0 to 3200 °C: D. I. Mendeleyev Institute for Metrology. Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/12/items/1385580. Accessed 10 October 2022 (In Russ.).
- 9.
Federal Information Fund for Ensuring the Uniformity of Measurements. Available via FIF EUM. https://fgis.gost.ru/fundmetrology/registry/19. Accessed 10 October 2022 (In Russ.).
Abbreviations
- TA:
-
Thermal analysis
- DTA:
-
Differential thermal analysis
- TGA:
-
Thermal gravimetric analysis
- DSC:
-
Differential scanning calorimetry
- STA:
-
Simultaneous thermal analysis
- MP:
-
Measurement procedure
- MI:
-
Measuring instrument
- RMS:
-
Root mean square
- CRM:
-
Certified reference material
References
ASTM E 473-16 (2019) Standard terminology relating to thermal analysis and rheology. https://doi.org/10.1520/E0473-16
Gatta GD, Richardson MJ, Sarge SM, Stölen S (2006) Standards, calibration, and guidelines in microcalorimetry. Part 2. Calibration standards for differential scanning calorimetry (IUPAC Technical Report). Pure Appl Chem 78(7):1455–1476. https://doi.org/10.1351/pac200678071455
ISO 9000:2015 Quality management systems—fundamentals and vocabulary. Available via ISO. https://www.iso.org/ru/standard/45481.html. Accessed 10 Oct 2022
Hill JO (1991) For better thermal analysis and calorimetry (Ed.). International confederation for thermal analysis, 3rd ed.
DIN 51004:1992 (1994) Thermal analysis; determination of melting temperatures of crystalline materials by differential thermal analysis. Deutsches Institut for Normung, Beuth-Verlag, Berlin, p 4
DIN 51007:1994 (1994) Thermal analysis; differential thermal analysis; principles. Deutsches Institut for Normung, Beuth-Verlag, Berlin, p 11
Höhne GWH, Cammenga HK, Eysel W, Gmelin E, Hemminger W (1990) Die temperaturkalibrierung dynamischer kalorimeter. PTB-Mitteilungen 100(1):25–31
Höhne GWH, Cammenga HK, Eysel W, Gmelin E, Hemminger W (1990) The temperature calibration of scanning calorimeters. Thermochica Acta 160(1):1–12. https://doi.org/10.1016/0040-6031(90)80235-Q
Cammenga HK, Eysel W, Gmelin E, Hemminger W, Höhne GWH, Sarge SM (1992) Die temperaturkalibrierung dynamischer Kalorimeter II. Kalibriersubstanzen. PTB-Mitteilungen 102(1):13–18
Cammenga HK, Eysel W, Gmelin E, Hemminger W, Höhne GWH, Sarge SM (1993) The temperature calibration of scanning Kalorimetrs. Part 2. Calibration substances. Thermochica Acta 219:333–342. https://doi.org/10.1016/0040-6031(93)80510-H
Sarge SM, Gmelin E, Höhne GWH, Cammenga HK, Hemminger W, Eysel W (1993) Die kalorische kalibrierung dynamischer Kalorimeter. PTB-Mitteilungen 103:491–512
Sarge SM, Gmelin E, Höhne GWH, Cammenga HK, Hemminger W, Eysel W (1994) The caloric calibration of scanning calorimeters. Thermochica Acta 247(2):129–168. https://doi.org/10.1016/0040-6031(94)80118-5
Sarge SM, Hemminger W, Gmelin E, Höhne GWH, Cammenga HK, Eysel W (1997) Metrologically based procedure for the temperature, heat and heat flow rate calibration of DSC. J Therm Anal 49:1125–1134. https://doi.org/10.1007/BF01996802
Donald GA, Richard WC (2000) Thermodynamic properties of the NaCl+H2O system. 4. Heat capacities of H2O and NaCl(aq) in cold-stable and supercooled states. J Phys Chem B 104(35):8563–8584. https://doi.org/10.1021/jp0003914
Gmelin E, Sarge SM (1995) Calibration of differential scanning calorimeters. Pure Appl Chem 67(11):1789–1800. https://doi.org/10.1351/pac199567111789
GOST R 8.872-2014 (2014) State system for ensuring the uniformity of measurements. State verification schedule for means of measuring specific enthalpy and specific heat-conguction of solid frames in the range of temperature from 700 to 1800 K. Standartinform, Moscow, 8 p (in Russian)
Kazantsev VV, Cherepanov VI, Sennikova VN, Averkiev MV (2015) National primary special standard GET 67-2013 for the units of specific enthalpy and specific heat of solids at temperatures in the 700–1800 K range. Meas Techn 58(2):121–126. https://doi.org/10.1007/s11018-015-0673-7
Popov MM (1954) Thermometry and calorimetry, 2nd ed. Moscow University publ, Moscow, p 943 (in Russian)
Zharskiy IM, Novikov GI (1988) Physical research methods in inorganic chemistry: textbook for chemical and chemical-technological universities. Vysshaya shkola, Moscow, p 271 (in Russian)
Kosova DA, Provotorov DI, Kuzovchikov SV, Uspenskaya IA (2020) Thermal analysis study of phase transformations of magnesium and calcium methanesulfonates. Russ J Inorganic Chem 65(5):752–757. https://doi.org/10.31857/S0044457X20050128
GOST 8.558-2009 (2019) State system for ensuring the uniformity of measurements. State verification schedule for means measuring temperature. Standartinform, Moscow, p 8 (in Russian)
GOST ISO Guide 35-2015 (2017) Reference materials. General and statistical principles for certification. Standartinform, Moscow, p 62 (in Russian)
Preston-Thomas H (1990) The international temperature scale of 1990 (ITS-90). Metrologia 27(1):3–10. https://doi.org/10.1088/0026-1394/27/1/002
Sarge SM, Höhne GWH, Cammenga HK, Eysel W, Gmelin E (2000) Temperature, heat and heat flow rate calibration of scanning calorimeters in the cooling mode. Thermochica Acta 361(1–2):1–20. https://doi.org/10.1016/S0040-6031(00)00543-8
Earnest CM, Miller ET (2017) An assessment of barium and strontium carbonates as temperature and enthalpy standards. J Therm Anal Calorim 130:2277–2282. https://doi.org/10.1007/s10973-017-6539-2
Acknowledgements
The research did not receive financial support in the form of a grant from any organization in the public, commercial or non-profit sector. All measurements were performed using the equipment of the D. I. Mendeleyev Institute for Metrology.
Contribution of the Authors
Nepomiluev A. M.—development of the research concept, conducting research work;
Shipitsyn A. P.—development of a methodology/procedure, implementation of a formal analysis, writing a draft version of the article, conducting research work;
Tyurnina A. E.—manuscript revision and editing.
Conflict of Interest
The article was prepared on the basis of a report presented at the V International Scientific Conference “Reference Materials in Measurement and Technology” (Yekaterinburg, September 13–16, 2022). The article was admitted for publication after the abstract was revised, the article was formalized, and the review procedure was carried out.
The version in the Russian language is published in the journal “Measurement Standards. Reference Materials” 2023;19(1):17–27. (In Russ.) https://doi.org/10.20915/2077-1177-2023-19-1-17-27.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 D. I. Mendeleyev Institute for Metrology
About this paper
Cite this paper
Nepomiluev, A.M., Shipitsyn, A.P., Tyurnina, A.E. (2024). Study of Certified Reference Materials for Temperature and Specific Enthalpy of Phase Transitions of Metals and Metal Salts. In: Sobina, E.P., et al. Reference Materials in Measurement and Technology . RMMT 2022. Springer, Cham. https://doi.org/10.1007/978-3-031-49200-6_21
Download citation
DOI: https://doi.org/10.1007/978-3-031-49200-6_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-49199-3
Online ISBN: 978-3-031-49200-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)