Abstract
During the work, an analytical review was carried out, including the study of the harmful effects of ash and slag dumps and the experience of their use during disposal. The definition of geopolymers has been compiled, as well as the ways of their application in various construction industry areas. Coal generation waste provided by Severodvinsk CHPP-1 was studied for its chemical composition and content of natural radionuclides, and on the basis of this, a class of radiological hazard of materials was assigned. A composition for the synthesis of porous geopolymer materials has been developed, including aluminosilicate raw materials, an alkaline activator and a foaming agent. Coal generation waste was used as aluminosilicate raw material; alkaline activator—a mixture of caustic soda solution and liquid sodium glass; aluminium powder was used as a blowing agent. As a curing mode, low-temperature curing is used—at room temperature, in an oven, as well as microwave radiation in a microwave oven. The effect of temperature-time regimes on the final macrostructure and technical and operational properties of the synthesized porous geopolymers has been studied.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Recycling
- Curing mode
- Ash and slag waste
- Coal generation waste
- Porous geopolymer
- Microwave radiation
- Aluminosilicate
1 Introduction
At the moment, positions on correcting environmental problems in Russia and the world as a whole are receiving more attention. As one of the rather important problems that has a negative effect, one can single out the accumulation of coal generation waste in landfills, which is formed due to the combustion of solid fuels at thermal power plants. To ensure the stable and reliable operation of the ash and slag removal system and reduce the financial costs for its construction and operation, the site for waste dumps is designed cleanly next to the territory of the thermal power plant and residential development, which means the possible occurrence of negative consequences on the environment and public health in general. In accordance with the “European List of Waste Codes”, as well as the “Federal Classification Catalog of Waste of the Russian Federation”, solid fuel waste belongs to hazard class 5, which means that they are practically harmless. But, ash dumps can carry a potential hazard in environmental pollution through dusting of ash from the surface of ash and slag, as well as leaching of heavy elements and various erosion products into the soil by melt or rain streams. The combination of all this can lead to deterioration of the surface layer of the atmosphere and pollution of water and soil [1, 2].
Currently, according to the report of the Joint Stock Company “System Operator of the Unified Energy System” in the Russian Federation, by January 1, 2022, the number of thermal power plants reached 66.14% of all electricity generating stations in the state, of which 24% use coal for operation. At the moment, approximately 2 billion tons of coal generation waste has accumulated in Russia, occupying more than 230 km2, and the accumulation trend is rising by 20–25 million tons annually. The remaining ash and slag wastes are sent for temporary storage, to ash dumps [3,4,5,6].
In foreign countries, the issue of processing man-made raw materials has more extensive solutions. In most European countries, coal generation waste is used as additives to Portland cement and concrete, the volume of processing of which is much higher than in Russia and is more than 90% [7]. This is achieved through legislative measures in these countries, a high tax on the lease of land for ash dumps and, in addition, the use of various incentives for the use of secondary raw materials in production. In turn, in India, coal generation waste is sent to ash dumps only in a small volume, within 30%, while the rest is sent to the production of various building materials: ceramic bricks; tiles; concrete [8]. In various states of America, coal generation waste in volumes close to 60% are used as a partial replacement for natural raw materials in the cement industry, or in the construction of protective structures and dams. Taking into account all of the above, we can conclude that the search for new ways to dispose of coal generation waste, taking into account the geographical needs and legislation of the country, is a very urgent task.
It can be noted that at the moment, geopolymer materials are an innovative way to dispose of ash and slag waste, due to their unique composition and properties by type: high chemical resistance, high temperature stability, significant mechanical properties, as well as low energy consumption in their production [9,10,11]. Geopolymers binders are obtained by mixing man-made aluminosilicate secondary products and wastes, either natural or synthetic minerals, such as coal generation waste (including fly ash and slag), metakaolin, perlite, cullet, clay and rice husk ash, with aqueous solutions containing chemically active substances, called geopolymers [12,13,14,15]. Currently, such geopolymers are used only in the pilot industry. Nevertheless, the growth of the prospect of industrial production of geopolymers exists under conditions when there are sources of industrial waste that are not involved in any way. Therefore, on the basis of the above aluminosilicate raw materials, it is possible to obtain both geopolymer concretes and highly porous materials that can be used as membranes and membrane substrates, adsorbents and filters, catalysts, and sound and heat insulating materials [16,17,18].
The term “geopolymer” was proposed by the French scientist D. Davidovits. In the 1970s, throughout France, cases of ignition of finishing materials, furniture and appliances, which were made from polymer materials based on organic compounds, became more frequent. As a result of massive fires causing damage to property, the environment was polluted due to the release of toxic substances during combustion. Then a workaround was found, consisting in replacing the used polymeric materials with aluminosilicate polymers, that is, a geopolymers [19].
At the initial stages of work on the technology0 of geopolymers, they were obtained on the basis of heat-treated kaolin, the polymerization of which was carried out in solutions of sodium hydroxide or liquid glass. Due to their higher cost, they have not been replaced by polymers, but they have found a wide range of applications. Further, in the course of scientific research, it was found that industrial wastes, such as ash from thermal power plants, slags and sludge, can be used in the production of geopolymers. The cost of new materials has decreased significantly and has risen in line with the cost of conventional polymers, which made it possible to consider geopolymers as an alternative option [20, 21].
The use of geopolymer materials in construction is due to the fact that they provide resistance to high temperatures, which is achieved due to the three-dimensional structure of the geopolymer network, which allows water molecules bound both physically and chemically to evaporate without problems. Also, the structure of the geopolymer provides water resistance, that is, water molecules do not penetrate the geopolymer matrix, so structures using geopolymer concrete do not require additional protection from water. In this regard, geopolymer materials have good frost resistance. Another unique property of such materials is resistance to aggressive environments, that is, the material has absolute chemical resistance. Consequently, the cost of construction using geopolymer materials will be many times less [22].
The creation of heat-insulating materials from porous geopolymers is very relevant when creating frost-protective layers of roads and asphalt concrete pavement, in particular in places with a climate close to extreme conditions, for example, in Norway or the Arctic zone of the Russian Federation. One of the very painful conditions that are present during the installation of roads in permafrost conditions is considered to be “frost heaving”, that is, defect formation on the road surface when unevenly distributed capillary moisture freezes in the soil layer. Theoretically, porous geopolymers will help solve this problem in harsh climatic conditions by creating moisture drainage deep into the soil, thereby increasing its stability.
It is known that overall porosity and pore size distribution are the most important factors influencing the mechanical properties of porous geopolymers. Therefore, it is so important to study the influence of the temperature-time regime of synthesis on their structure and properties, because with the correct structural design, porous geopolymers can become universal and Eco-conscious materials with a low footprint carbon throughout the entire product life cycle.
2 Experimental Part
2.1 Materials, Sample Synthesis and Methods
To solve the problem, as the main aluminosilicate raw materials, we used coal-fired waste from the Severodvinsk CHPP-1, located in the city of Severodvinsk, Arkhangelsk Region, Russia.
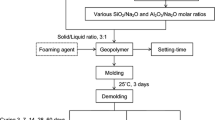
The technology for the synthesis of porous geopolymer materials is shown in Fig. 1. Dried and crushed coal waste was sieved through a sieve with a mesh size of 250 µm [23]. The alkaline activator of aluminosilicate components was prepared in a separate container by preparing a 12 M NaOH solution and mixing it with sodium liquid glass (silicate modulus = 2, water content 56%) manufactured by SilEx, Russia. The alkali used was a chemically pure (main component content of at least 99%) powder NaOH produced by LenReaktiv, Russia. After that, the resulting alkaline activator was poured into a pre-weighed sample of coal generation waste and sent for further mixing to the MSL-1S drum mill manufactured by PromStroyMash LLC, Russia. The mixing of the components was carried out at 120 rpm for 300 s. At the end of mixing, a spherical finely dispersed aluminium powder with a purity of at least 99% and a specific surface area of 140 m2/g produced by Metall Energo Holding, Russia was introduced into the suspension as a foaming agent. After that, the suspension was additionally stirred for 30 s, since a further increase in the mixing time adversely affects the foaming due to the volatilization of gas bubbles and the onset of the reaction of aluminium powder with an alkaline component [24, 25].
Upon completion of mixing, the resulting geopolymer suspension is poured into silicone cubic molds with an edge of 3 cm for further curing in a two-stage mode. The first stage is the same for all samples—curing takes place within 24 h at room temperature (25 ± 2℃) and relative humidity of 65 ± 5% in the room without direct sunlight on the samples. The second stage was carried out with further placement of the samples in an drying oven (LOIP LF 25/350-VS2, Joint-Stock Company “Laboratory Equipment and Devices”, Russia) at temperatures of 60, 70, 80 and 90 ℃ and holding for 8 h. Also for comparison, as additional modes, the results of curing geopolymer materials in a room without direct sunlight on samples at room temperature (25 ± 2 ℃) for 14 days (RT) and using microwave radiation (M) in a Midea C4E AM720C4E-S microwave oven (Midea Group Co., Ltd, China) are given. For 5 min, the sample was exposed to microwave radiation with a power of 700 W at a frequency of 2500 MHz.
At present, microwave radiation is widely used as an innovative method for accelerating heating and increasing the intensity of chemical reactions. This is due to the fact that microwaves are able to quickly penetrate almost any material and release energy, which leads to volumetric heating of the entire material. It also simplifies process control. Microwaves can be especially useful for making geopolymer foams because they have low thermal conductivity, and microwaves can improve heat transfer.
To study the technical-operating and physicochemical properties of the synthesized porous geopolymer materials, the following methods and characteristics were chosen: chemical oxide composition, qualitative X-ray phase analysis, bulk density, kg/m3, ultimate compressive strength, MPa, and thermal conductivity, W/(m·K). Previously, the authors described the measurement techniques used and the formulas in the article [26].
3 Results and Discussions
Like any other raw materials used for various construction industries, coal-fired generation wastes must be examined for the content of natural radionuclides, since it is known that when solid fuels are burned in CHP boilers, they are concentrated and accumulated. The specific activity of radionuclides of radium (226), thorium (232) and potassium (40) in the studied waste is 157 ± 19 Bq/kg, which does not exceed the limit values of 370 Bq/kg. This content does not impose restrictions on the area of use of the studied wastes [24].
The chemical oxide composition of the used aluminosilicate raw materials in the form of waste from coal generation and synthesized porous geopolymer materials is presented in Table 1.
As can be seen from the data obtained, the chemical composition of the synthesized porous geopolymer material differs slightly from the raw material used. There was an increase in the content of silicon dioxide and sodium oxide, in other cases, a decrease in the mass content of oxides can be observed. This is probably due to the fact that the added components, namely, the alkaline activator, partially dissolve oxides, as a result of which they pass into an amorphous structure in the form of a glass phase. An increase in the content of sodium oxide from 3.6% to 7% is also associated with the addition of sodium liquid glass and sodium hydroxide. In both cases, the content of calcium and magnesium oxides does not exceed 5%, which classifies both materials as acidic. “Loss on calcination” in the waste of coal generation are probably represented by “underburnt (unburned fuel)”, and in the synthesized porous geopolymer also underburnt and hydroxocomplex Na[Al(OH)4], which has a decomposition temperature of 800 ℃.
The component composition used for the synthesis of porous geopolymer is presented in Table 2. As mentioned earlier, to research the effect of the temperature–time regime of synthesis on the physicochemical properties and structure of porous geopolymers, 6 modes of two-stage curing were selected: 24 h at room temperature 25 ± 2℃ followed by 8 h in a drying cabinet temperature of 60 ± 1, 70 ± 1, 80 ± 1 and 90 ± 1℃. Also added for comparison is indoor curing at room temperature (25 ± 2℃) without direct sunlight on the samples for 14 days (RT) and in a microwave oven using 700W microwave (M) at 2500 MHz.
The use of aluminium powder as a blowing agent theoretically should increase the strength of the synthesized sample due to the formation of a hydroxocomplex, in contrast to the use of hydrogen peroxide [27]. The foaming mechanism during its use consists in interaction with an alkaline solution, in which hydrogen is released and the Na[Al(OH)4] hydroxocomplex is formed, which can be represented by reaction 1:
X-ray phase analysis has been carried out to identify phases or crystalline peaks of minerals present in porous geopolymers. As mentioned earlier, 6 different low temperature conditions were used in the present study, however only 1 composition. Based on this, the authors carried out an x-ray phase analysis of 3 temperature–time modes: RT, 80 ℃ and M. The obtained x-ray patterns during merged do not differ from each other, since there were no high-temperature heat treatment modes and a change in the component composition, which can affect phase composition. Therefore, in Fig. 2 shows 1 typical X-ray diffraction pattern of the porous geopolymers of composition (80).
According to the data obtained, it should be noted that the X-ray diffraction pattern is characterized by the presence of an amorphous aluminosilicate glass phase, which confirms the presence of a “halo” at shooting angles of 18–34° (2θ). It is formed as a result of solidification of slag in water and the subsequent sharp decrease in its temperature, as a result of which the aluminosilicate melt does not have time to crystallize and an amorphous glass phase is formed. It should also be noted that the presence of an amorphous structure increases the reactivity of the raw material. Porous geopolymers are composed of the same crystalline phases in the form of quartz (ICCD PDF# 82-0512) and mullite, at the sensitivity limit of the instrument (ICCD PDF# 15-0776).
Based on the component composition of the raw mixture and the presented technology, porous geopolymer materials were obtained with the following external structure and physicochemical properties, shown in Fig. 3 and in Table 3.
As can be seen from the data obtained, a change in the curing temperature has an insignificant effect on the density of the geopolymers: rise in temperature for every 10 ℃ reduces the density of the samples by an average of 2.5%. The density difference between 60 and 90 ℃ is 8%. Curing of porous geopolymers at room temperature 25 ± 2 for 14 days also has no significant effect on the density of the samples—it is at the level of 60–70 ℃. However, using microwave as the curing mode increases the average density by 19% compared to the RT composition. Since thermal conductivity depends almost linearly on density and porosity, a similar development of this indicator can be observed.
A completely different trend can be observed when studying the ultimate compressive strength of synthesized samples. An increase in temperature has a significant effect on strength characteristics: so increasing it by 10 ℃, from 60 to 70 ℃, reduces strength by 13%, from 70 to 80 ℃ by 15%, from 80 to 90 ℃ by 4%, which ultimately leads to a 30% deterioration in strength characteristics. Samples with curing mode RT show the worst strength of 0.58 MPa. The use of microwave radiation, on the contrary, significantly increases the ultimate compressive strength: relative to the RT composition, there was an increase in strength by 126%, which is an excellent result. If we compare microwave radiation with thermal conditions (60 ℃), we can see a not so grandiose, but very noticeable increase in tensile strength by 28%, which is also a good result.
The obtained dynamics of strength reduction is probably associated with the following factors: with an increase in temperature, the intensity of water evaporation increases and, as a result, macropores increase in size. This contributes to a decrease in the thickness of the interpore partitions, which ultimately affects the density and ultimate strength of the samples.
Also, due to the increase in the rate of the geopolymerization reaction and the rapid release of water from the samples, the solid/liquid phase ratio changes, which has a huge impact on the viscosity and rheology of the reaction mixture. Due to the rapid increase in viscosity and, as a result, a decrease in the reactivity of the geopolymer mixture, the geopolymerization reaction does not have time to complete before the samples are cured, which has a negative effect on the ultimate strength of porous geopolymers.
With regard to exposure by microwave radiation, presumably, it also allows to increase the temperature of the geopolymer raw material mixture in the shortest possible time, which leads to the adhesion reaction of aluminosilicate groups and the creation of an impermeable film. Further heating above 100 °C results in the formation of steam, which expands the film, causing swelling and pore formation. This continues until the aluminosilicate groups form a rigid brittle bond consisting of sialates. Probably, with further heating for more than 5 min, the pore size increases, which affects the final properties of the obtained samples.
4 Conclusion
Analyzing the above, we can summarize the following results:
-
1.
Despite the maximum density among all temperature–time modes, with the component composition used, the mode using microwave radiation is the most optimal. In this case, the obtained density is 391 ± 16 kg/m3, and the compressive strength is 1.31 ± 0.05 MPa.
-
2.
Among the low-temperature conditions using a drying oven, the best is the curing of porous geopolymers with exposure for 24 h at room temperature, followed by placement in an oven for 8 h at a temperature of 60 ℃. In this case, the obtained density is 332 ± 2 kg/m3, and the ultimate compressive strength is 1.02 ± 0.04 MPa.
-
3.
When the curing temperature rises from 60 to 90 ℃, the density decreases by 8% from 332 to 306 kg/m3, and the compressive strength from 1.02 to 0.73 MPa, which is 30%. The most significant factor influencing the decrease in density and strength is an increase in the size of macropores and a decrease in the thickness of interpore partitions due to more intensive water evaporation. Also, the ratio of solid and liquid phases, which slows down or accelerates the rate of the geopolymerization reaction, which is individual for each chemical composition of the raw material and the component composition of the geopolymer mixture, has a huge impact on the properties obtained.
References
Cherentsova AA, Olesik SM (2013) Evaluation of ash waste as a source of pollution and a source of secondary raw materials. Mining Info Anal Bull (Scient Tech J) S3:230–243
Guidance on classification of waste according to EWC-Stat categories. https://ec.europa.eu/eurostat/documents/342366/351806/Guidance-on-EWCStat-categories-2010.pdf/0e7cd3fc-c05c-47a7-818f-1c2421e55604. Accessed 11 June 2023
State report “On the state and protection of the environment of the Russian Federation in 2020” https://www.mnr.gov.ru/docs/gosudarstvennye_doklady/gosudarstvennyy_doklad_o_sostoyanii_i_ob_okhrane_okruzhayushchey_sredy_rossiyskoy_federatsii_v_2020/?special_version=Y. Accessed 11 June 2023
Khagleev EP (2017) Ash and slag dumps of annual regulation, differentiated flows of ash and slag from coal thermal power plants. News of High Educ Insitu, Energy Prob 19:21–32
Thang VL, Nguyen ZT, Samchenko SV (2019) Addition of additives of ash and slag waste to the properties of sulfoaluminate Portland cement. Vestnik MGSU 14:991–1003
Ivashina MA, Krivoborodov YuR (2017) The use of industrial waste in the technology of sulfoaluminate clinker. Adv Chem Chem Tech 31:22–24
Jin S et al (2021) Comparison and summary of relevant standards for comprehensive utilization of fly ash at home and abroad. IOP Conf Ser: Earth Environ Sci 621(1):012006
Yousuf A et al (2020) Fly ash: production and utilization in India-an overview. J Mater Environ Sci. 11(6):911–921
Novais RM, Buruberri LH, Ascensão G et al (2016) Porous biomass fly ash-based geopolymers with tailored thermal conductivity. J Clean Prod 119:99–107
Hlaváček P, Šmilauer V, Škvára F et al (2015) Inorganic foams made from alkali-activated fly ash: mechanical. Chem Phys Prop, J Eur Ceram Soc 35(2):703–709
Hemra K, Aungkavattana P (2016) Effect of cordierite addition on compressive strength and thermal stability of metakaolin based geopolymer. Adv Powder Technol 27(3):1021–1026
Cyr M, Idir R, Poinot T (2012) Properties of inorganic polymer (geopolymer) mortars made of glass cullet. J Mater Sci 47(6):2782–2797
Vaou V, Panias D (2010) Thermal insulating foamy geopolymers from perlite. Miner Eng 23(14):114–1151
Badanoiu AI, Al Saadi THA, Stoleriu S et al (2015) Preparation and characterization of foamed geopolymers from waste glass and red mud. Constr Build Mater 84:284–293
He J, Jie Y, Zhang J et al (2013) Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem Concr Compos 37:108–118
Bai C, Colombo P (2017) High-porosity geopolymer membrane supports by peroxide route with the addition of egg white as surfactant. Ceram Int 43(2):2267–2273
Minelli M, Medri V, Papa E et al (2016) Geopolymers as solid adsorbent for CO2 capture. Chem Eng Sci 148:267–274
Alzeer MIM, MacKenzie KJD, Keyzers RA (2016) Porous aluminosilicate inorganic polymers (geopolymers): a new class of environmentally benign heterogeneous solid acid catalysts. Appl Catal A: Gen 524:173–181
Davidovits J (2002) 30 years of successes and failures in geopolymer applications. In: Market Trends and Potential Breakthroughs. Geopolymer Conference, October 28–29. Melbourne, Australia
Davidovits J (2011) Geopolymer chemistry and applications, 3rd edn. Institute Geopolymer, France, Saint-Quentin, 614 p
Davidovits J (1989) Geopolymers and geopolymeric materials. J Therm Anal 35:429–441
Alfred J (2013) Engineering properties of a proprietary premixed geopolymer concrete. In: Proceeding Concrete Institute of Australia Biennial Conference, Understanding Concrete, Gold Coast, Australia
Yatsenko EA, Goltsman BM, Klimova LV, Yatsenko LA (2020) Peculiarities of foam glass synthesis from natural silica-containing raw materials. J Therm Anal Calorim 142(1):119–127
Yatsenko EA, Goltsman BM, Trofimov SV et al (2022) Improving the properties of porous geopolymers based on TPP ash and slag waste by adjusting their chemical composition. Materials 15:2587
Lynch JLV et al (2018) Preparation, characterization, and determination of mechanical and thermal stability of natural zeolite-based foamed geopolymers. Constr Build Mater 172:448–456
Yatsenko EA, Goltsman BM, Trofimov SV, Novikov YV, Smoliy VA, Ryabova AV, Klimova LV (2023) Influence of various coal energy wastes and foaming agents on foamed geopolymer materials’ synthesis. Mat 16:264. https://doi.org/10.3390/ma16010264
Yatsenko EA, Ryabova AV, Vilbitskaya NA et al (2021) Ecogeopolymers based on ash and slag waste from thermal power plants—Promising materials for the construction of roads in the Arctic zone of the Russian Federation. Glass Ceram 12:32–37
Acknowledgements
The research was supported by Russian Science Foundation (project No. 21-19-00203), "Efficient temperature-solidificable eco-geopolymers for road construction in the Arctic zone of the Russian Federation based on waste from the local thermal power plants solid fuel combustion" (supervisor Yatsenko E.A.), in the framework of the 2021 competition "Conducting fundamental scientific research and exploratory scientific research by separate scientific groups".
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Yatsenko, E.A., Trofimov, S.V., Chumakov, A.A., Vilbitsky, S.A., Goltsman, N.S. (2024). Evaluation of the Physical and Performance Properties of Porous Polymers Depending on the Curing Mode. In: Radionov, A.A., Ulrikh, D.V., Timofeeva, S.S., Alekhin, V.N., Gasiyarov, V.R. (eds) Proceedings of the 7th International Conference on Construction, Architecture and Technosphere Safety. ICCATS 2023. Lecture Notes in Civil Engineering, vol 400. Springer, Cham. https://doi.org/10.1007/978-3-031-47810-9_47
Download citation
DOI: https://doi.org/10.1007/978-3-031-47810-9_47
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-47809-3
Online ISBN: 978-3-031-47810-9
eBook Packages: EngineeringEngineering (R0)