Abstract
This chapter examines the science of geochemistry, as the core, background discipline of biogeochemistry. Biogeochemistry is based on geochemistry; in that, it is the framework for those aspects of biology that link to the chemical bases of earth materials, which are defined as within the study of geochemistry. Therefore, the justification for this chapter is that a fuller understanding of biogeochemistry requires a study of geochemistry and the possibilities for its linkage with the biological sciences. Geochemistry is the study of the interfaces of geology and chemistry, using the methodology of the chemical sciences to investigate the composition of earth materials and the occurrence and movements of chemical elements and compounds within the earth system. Geochemistry is consequently an extremely wide subject, as it examines all aspects of the chemistry of earth materials. However, few studies have explored the links between geochemistry and the biological sciences, as the basis for biogeochemical studies. This chapter examines the relevant topics of chemistry, the content of geochemistry, and the developments that have forged a closer link with biogeochemistry. The branches of chemistry, namely, analytical, inorganic, organic, physical chemistry, and biochemistry, are examined, as is their relationship with geochemistry. The branches of geochemistry include organic geochemistry, inorganic geochemistry, isotope geochemistry, aqueous geochemistry, cosmochemistry, trace-element geochemistry, igneous rock geochemistry, metamorphic rock geochemistry, photogeochemistry, and low-temperature or environmental geochemistry. The literary evidence indicates that developments in chemistry, biology, geology, and even archaeology and astronomy (the last two linked to isotope geochemistry and cosmochemistry) have benefited geochemistry as a discipline, and in combination, these have contributed to the advancement of biogeochemistry. Variable issues in biogeochemistry are principally the concern of some branches of geochemistry, such as carbon, inorganic and marine chemistry, and organic, inorganic, isotope, and aqueous geochemistry. This examination contributes to knowledge on the interfaces between the biological, chemical, and geological sciences.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
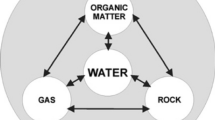
Geochemistry, the science of the chemistry of the solid Earth, and its interactions with biological and biochemical topics are arguably core backgrounds to biogeochemistry; hence, a description of geochemistry is a necessary prelude to a detailed study of biogeochemistry (Campbell, 2020). Here, a short description of geochemical links to biogeochemistry is presented, followed by a detailed set of definitions of geochemistry. As geochemistry refers to the chemistry of the Earth, Hellweger (2008: 386) argues that “the field of biogeochemistry deals with the effect of biological organisms on the chemistry of the Earth. Since there are numerous living organisms, all of which affect the chemistry of their environment in multiple ways, biogeochemistry is a large subject area covering many processes.” The noted authority Hallberg (2009: 435) adds that “biogeochemistry tries to combine biology and geochemistry to achieve a deeper understanding of the whole complex of chemical transformations occurring in nature, in the continuous interactions between its biotic and abiotic components.” Biogeochemistry is argued to straddle three disciplines (Hallberg, 2009: 436), namely biology, geology, and chemistry, and as the “root of the term biogeochemistry is chemistry and above all chemistry related to geoscience: geochemistry,” it is noted that “adding the prefix bio to geochemistry implies that biogeochemistry is concerned with the activities of living organisms in the migration, distribution, dissipation, and concentration of chemical elements” (Hallberg, 2009: 436).
Commenting on geochemistry’s important, developing role in recent scientific development, White (2017) writes that “geochemistry has grown over the last 50 or 60 years to touch virtually every aspect of earth science. The contributions of geochemistry to this advance have been simply enormous. Much of this progress has come from innovation in analytical techniques and the ability to assess the nature of natural materials with evermore precision and on evermore finer scales.” Bianchi (2021: 154) gives a copious account of the development and relevance of geochemistry to the development of a complex model of biological, geological, and chemical interactions (biogeochemistry) at the global and smaller scales, arguing that geology still played a role in the evolution of biogeochemistry in the twentieth and twenty-first centuries, including topics such as global sedimentary modeling, plate tectonics theory, carbon and climate change, and increasingly global chemical cycling (see also Gaillardet & Galy, 2008). Bianchi (2021, 155) further argues that geochemistry evolved out of the writing mostly of Clarke and Washington (1924) on the chemical composition of the Earth’s crust, Goldschmidt (1954), and Strunz (1941). It is argued that “these important chemical developments now allow in part, for perspectives on how biological compounds interact with minerals in nature and how organic matter is chemically altered after being buried for millennia – we now see the emergence of yet another new discipline inherently linked with biogeochemistry, organic geochemistry” (Bianchi, 2021, 155; see also Kvenvolden, 2006).
The development of organic chemistry, and related topics in geochemistry also played a role in the development of biogeochemistry (Bianchi, 2021). This development acknowledged the importance of organic compounds in previously inorganic-focused analyses. Organic geochemistry redirected the focus to biochemical issues, such as botanical physiology and productivity, and climate change, and links to organic residues such as crude oil and other carbon compounds in the Earth’s crust. Early writings in this regard included those of Treibs (1936) (considered by some as the father of organic geochemistry) and of Trask and Patnode (1942), which in some cases “introduced the broader geological linkages between bulk organic material in source sedimentary rock and source beds of petroleum” and examined links between organic geochemistry and biogeochemistry (Bianchi, 2021). These studies developed the notion that some fossils and fossil organic molecules can be used as chemical biomarkers to provide a window to the past ecosystems (Bianchi, 2021, 155; see also Peters et al., 2005; Kvenvolden, 2006; Gaines et al., 2009; Bianchi & Canuel, 2011; Greenwalt et al., 2013; Killops & Killops, 2013; Briggs & Summons, 2014; Whiteside & Grice, 2016). Bianchi (2021: 155) further elaborates that “the application of novel chemical biomarkers/proxies, both organic and inorganic, in paleoreconstruction has been particularly useful in understanding major biogeochemical shifts in Earth’s 4-billion-year history, which are central in making predictions about future changes in global biogeochemical cycles” (see also Lenton & Daines, 2017).
Geochemistry is defined as the study of the Earth using the techniques of chemistry, which may also be used for studies linked to the relevant biological sciences, and hence biogeochemistry (Campbell, 2020). Geochemistry, as a complex discipline, has been defined and described by numerous authorities with sometimes variable foci, since its development in the late nineteenth century. These definitions point to geochemistry as principally a chemical science, which uses the techniques of the chemical sciences for the study, analyses, and understanding of Earth studies. Victor Goldschmidt (1888–1947) is the oft-cited “father” of geochemistry, especially due to his systematization of the “behavior of the chemical elements in an Earth Sciences context via his classification of them according to their preferred host phases on silicate Earth (Lithophile, siderophile, chalcophile and atmophile)” (Mather, 2013). Mather (2013) further notes that geochemistry is “a broad and fascinating subject both in terms of the topics it addresses, and the techniques employed.” Lastovicka (2009) writes that “Goldschmidt’s definition of this scientific discipline from the 1930s is reasonably applicable even now,” which was “the distribution and amounts of the chemical elements in minerals, ores, soils, waters, and the atmosphere, and the circulation of the elements in nature, on the basis of the properties of their atoms and ions.”
White (2017) argues that based on the name “geochemistry,” this science is “a marriage between chemistry and geology, or more broadly geosciences or earth science, and it is arguably a subdiscipline of both. A better explanation of geochemistry is that it is applies chemistry and chemical principles to understanding the Earth and its cosmic environment and to using that understanding to better the human condition.” Britannica (2014) defines geochemistry as the “scientific discipline that deals with the relative abundance, distribution, and migration of the Earth’s chemical elements and their isotopes.” Inamuddin et al. (2021: xiii) give a much broader definition: geochemistry is “a branch of earth science” and note that “since it is a field of study that uses the tools and principles of chemistry to explain the mechanisms in geologic environments, it often focuses on determining processes that control the abundance and composition of minerals and their distribution in the earth’s crust…” and the subject also “plays a vital role in environmental soil and water systems in identifying and modulating environmental problems, and in studying the composition, structure, and processes of the Earth.” Yale University (2022) gives a similar definition as “the branch of Earth Science that applies chemical principles to deepen an understanding of the Earth system and systems of other planets” and its practitioners “consider Earth composed of discrete spheres – rocks, fluids, gases and biology – that exchange matter and energy over a range of time scales.”
Sarala (2015) writes that “geochemistry can be broadly defined as the science concerned with all geological studies involving chemical change. It includes the study of the distribution of elements in minerals, rocks, and soils along with the interaction between these earth materials.” Mather (2013) points out that geochemistry “utilizes the tools and principles of chemistry to explain the mechanisms regulating the workings – past and present – of the Earth. The reach of geochemistry is broad and extends to the study of the formation of the Earth and the Solar System as well as topics such as the origins and evolution of the Earth’s crust, oceans, and atmosphere.” Lastovicka (2009) defines geochemistry as dealing with “the distribution and cycling of the chemical elements, and their isotopes, throughout nature. It is of great importance to understanding the Earth and planets, and their origin and nature” and as eventually evolving from “a substantially descriptive to a highly quantitative and predictive discipline.”
Trueman et al. (2019: 271) look further at the geochemical research methods, defining geochemistry as the study of “the chemistry of natural earth materials and the chemical processes operating within and upon the Earth, both now and in the past,” with geochemical analyses “carried out on any natural sample such as air, volcanic gas, water, dust, soil, sediment, rock, or biological hard tissues (especially ancient biological tissues) and also on anthropogenic materials such as industrial effluent and sewage sludge. Geochemical analyses, therefore, involve a wide range of materials and analytes of interest and may be performed for industrial, environmental, or academic reasons.” They further argue that “all of the naturally occurring elements in the periodic table are important for one geochemical investigation or another.” The application of geochemical research techniques may focus on the “analysis of the inorganic constituents of the materials most closely associated with geology – rocks and rock forming minerals” or may be broader, including “the chemical analysis of soils, waters, biological tissues, and organic geochemistry” (Trueman et al., 2019: 271).
Another set of definitions deals with the development and components of geochemistry as a discipline. Currently, geochemistry has developed into a well-established discipline, with numerous subdisciplines (Campbell, 2020). Mather (2013) determines the subdisciplines of geochemistry as distinguishable from each other variably, with considerable overlaps between them. The criteria for the division include “analytical geochemistry in contrast to theoretical studies, the technique being employed or the types of measurements an area is concerned with (e.g., isotope geochemistry); the type of sample (e.g., igneous geochemistry and organic and petroleum geochemistry); the class of scientific questions being addressed (e.g., cosmochemistry); the pressure-temperature regime that a certain subset of geochemists are engaged with (e.g., low-temperature geochemistry).” Geochemistry is also increasingly linked to other, related sciences (Campbell, 2020). For example, Lastovicka (2009) argues that geochemistry as an interdisciplinary subject links with the earth sciences of geology, geophysics, astronomy, planetary sciences and atmospheric science, and the more basic sciences of physics, chemistry, biology, and material sciences. Geochemical research methods also have important practical functions, including “the search for deposits of various raw materials like oil, gas, and various metallic ores” and “direct environmental applications” (Lastovicka, 2009). These complexities may intrude into the relationship between geochemistry and biogeochemistry, with both fields being extremely complex and variously linked to other disciplines (Campbell, 2020).
Lastovicka (2009) further notes that “the study of geochemistry is of great importance to the Earth and planetary sciences, on both purely scientific and more immediate practical grounds, because chemical processes are fundamental to understanding how the planetary bodies formed and evolved at all scales, from atomic to solar system.” This vital background means that “geochemistry plays a central role in understanding a diverse set of scientific questions, such as the formation and differentiation of the Earth and planets, the origin and evolution of life, the controls on global climate and climate change, and the formation and management of natural resources” (Lastovicka, 2009). Scott (2014) concurs: “Geochemistry plays an essential role in our understanding of processes that produce economic concentrations of minerals whether by hydrothermal, magmatic, metamorphic, hydraulic (both surficial and subterranean) or weathering agents, or a combination of these. Geochemistry also contributes importantly to exploration.”
The rest of this chapter continues the trend of the main thesis of this book, that an understanding of biogeochemistry requires an in-depth study of the associated sciences that contribute to the theoretical developments, methodologies and research technologies of biogeochemistry. The relevant developments in geochemistry include those within the fields of organic, inorganic, aqueous, trace-element and isotope geochemistry, which may link with biogeochemistry through chemical cycling, and some of the more solid earth subdisciplines such as igneous and metamorphic rock geochemistry. Cosmogeochemistry and photo-geochemistry are also important developing areas.
Links of Geochemistry with Biogeochemistry
The main link between geochemistry and biogeochemistry appears to be the extent of connections between geochemistry and biological topics, which add the “bio” prefix to the name of the science. Both disciplines retain similar structures. Indiana University (2022) notes that “geochemistry and biogeochemistry are inherently multidisciplinary, positioned at the intersection of biology, geology, and chemistry, and typically combining field investigations and sampling with laboratory analyses and experimentation to yield empirical data that can constrain computational models.” Recent, cutting-edge developments concerning the links between the two disciplines concern the addition of biological links to geochemistry, from which the foundations of biogeochemistry emerge.
Sahai et al. (2016: 389) note that the link between geochemistry and biogeochemistry is mostly concerned with two multidisciplinary subjects, but the addition of biological studies may include the origin of life to the Earth science perspective. Notably, “paradigm-changing discoveries about stellar and planetary evolution, the survival of organic molecules and microorganisms under extreme conditions, and geochemical environments on early Earth and other planets are sparking a synergistic dialogue between geoscientists, chemists, and biologists to understand how life originated” (Sahai et al., 2016: 389). It is argued that a principal approach is to “explain the non enzymatic synthesis of biologically relevant organic molecules under geologically plausible conditions,” “overcome the rigid conceptual dichotomy of the “RNA world” versus the “metabolism-first” hypotheses” and “develop high-throughput analytical systems to sample the myriad possible combinations of environmental conditions to find those that could initiate life” (Sahai et al., 2016: 389). Hence, the links between geochemistry and biogeochemistry may include the study of developments with organic chemistry, and the organic molecules and other links with possible origins of life, this adding the “bio” to geochemistry. Sahai et al. (2016: 389) remark, concerning the issue of the journal Elements that it is vital to “highlight the roles of minerals and geochemical environments in the emergence of protocells, the cell-like entities that might have preceded the Last Universal Common Ancestor.”
Bianchi (2021: 141) notes, concerning the development of biogeochemistry with its links with geochemistry, that “the development of organic geochemistry as a discipline, allowed for new roots to develop in the evolution of biogeochemistry through linkages between short and long-term carbon cycles.” Mather (2013) adds points on the importance of organic geochemistry to the links with biogeochemistry, as “organic geochemistry studies the distribution, composition, and fate of organic matter in the geosphere on both bulk and molecular levels, combining aspects of geology, chemistry, and biology. This is often concerned with biogeochemical cycles, with the carbon cycle probably being one of the most fundamental, without which life could not exist.” Sinha (2013) explores the area between organic geochemistry and biogeochemistry, as the former is “the study of the organic compounds found in geologic materials and meteorites, including those of problematic biological origin” and the latter is “the study of the behaviour of inorganic chemical elements in biological systems of geologic scope.” The subject areas classified within biogeochemistry and organic geochemistry include ore deposit, petroleum and life origins, coal chemistry, the composition of primitive atmospheres, biogeochemical prospecting for mineral deposits, the chemistry of natural waters, and soil formation. As “almost all geologic processes that occur at Earth’s surface are affected by biological activity” (Sinha, 2013), these links underscore the role of organic geochemistry and of geochemistry in general for the understanding and development of biogeochemistry.
Subfields of Geochemistry
Subfields of geochemistry are listed in Table 2.1. These are differentiated by their methodologies, and element, isotope, and regional focus (Langmuir, 1997; Dickin, 2005; Killops & Killops, 2013; Mather, 2013; Schlesinger & Bernhardt, 2013; Rass et al., 2014; Doane, 2017; White, 2020; Organic Geochemistry, 2022). Each of these subfields share a common interest: the distribution, change and formation of chemical elements and compounds on the Earth’s surface (land, soil, water, rock) and sometimes that of other space bodies. Such chemical changes may be due to volcanism, deposition, erosion, heat, or even light. With such a broad range of factors for composition and change, each subfield may be related to other fields, such as geophysics and atmospheric physics, hydrology, biology, sedimentology, and stratigraphy, as well as the subfields of chemistry, including physical, organic, and inorganic chemistry (Housecroft & Sharpe, 2008; Atkins & de Paula, 2009; Burrows et al., 2009; White, 2020). Each of these subfields will be examined, including examples of some of the recent, cutting-edge research on related topics, based on published articles in refereed journals and critical book chapters.
Recent studies of geochemistry generally focus on ever more detailed examination and analyses of the composition of Earth surface materials, using increasingly sophisticated methods. This can be seen by an examination of the articles published in the leading geochemically oriented journals. As noted by Lu et al. (2019: 682) “academic journals are important carriers of scientific activities, important academic communication media, and channels in the process of scientific development, and an important research unit in the structure and classification system of scientific knowledge.” Leading journals include: Geochemistry, Geochemistry International, Organic Geochemistry, Applied Geochemistry, Results in Geochemistry, Geochimica et Cosmochimica Acta, Geochemical Journal, Geochemical Exploration, International Journal of Coal Geology, Lithos, Journal of Asian Earth Sciences: X, Journal of Palaeogeography, Journal of South American Earth Sciences, Engineering Geology, Geoscience Frontiers, Physics and Chemistry of the Earth, Parts A/B/C, and Marine and Petroleum Geology.
Others are Chemical Geology, Journal of Volcanology and Geothermal Research, Precambrian Research, Journal of Geodynamics, Oceanologia, Continental Shelf Research, Contributions to Mineralogy and Petrology, Journal of Geophysical Research, American Mineralogist, Bulletin of Volcanology, Precambrian Research, Earth and Planetary Science Letters, Journal of Volcanology and Geothermal Research, Geophysics, AAPG Bulletin, Solid Earth, Journal of Geosciences, Environmental Chemistry, Geological Journal, Journal of the Geological Society, Australian Journal of Earth Sciences, Tellus Series B: Chemical and Physical Meteorology, Nonlinear Processes in Geophysics, Applied Clay Science, Environmental Geochemistry, Physics and Chemistry of Minerals and Lithosphere. Tables 2.2, 2.3, 2.4, 2.5, 2.6, and 2.7 present the main research foci of these journals, topics ranging from geochemistry to related sciences.
Some recent, cutting-edge studies on the various branches of geochemistry are listed in the sections below. Geochemical research tends to advance with attention to greater detail and specialization, simultaneously with more studies linking geochemistry to developments in related or linked sciences (transdisciplinary, multidisciplinary, interdisciplinary, methodology), such as geophysics and related physics subdisciplines, branches of chemistry (including environmental chemistry and even pollution), space sciences, oceanography, biology and fossil studies, and computer sciences. In these blossoming relations, geochemistry is gradually spanning the entire range of the earth and environmental sciences, and with this development a possibly broader engagement with biogeochemistry (Geochemical Society, 2022; Geoscience Australia, 2022; Imperial College, 2022). Each section below comments on several such recent studies, mainly in the topic listed in the heading. These studies are taken from some of the journals listed above and indicate some of the directions of geochemistry.
Organic Geochemistry
Organic geochemistry is the subdiscipline of geochemistry that focuses on organic (carbon bearing) compounds found in geologic environments. Research methods are mostly focused on the study of organic, carbon-based compounds in earth materials, using geochemical techniques. Volkman (1998) defines organic geochemistry as “the study of the origins and fates of organic matter in the geosphere, and it encompasses studies of coals, oils, sediments and natural waters” and that “much of organic geochemistry can be classified into general themes” on organic matter. The Geochemical Society defines organic geochemistry as encompassing diverse research areas such as “biogeochemistry, aspects of climate change studies, petroleum geochemistry, aspects of archaeology, and studies of extraterrestrial organic matter” and documents that there is “potential for large, more-than incremental, advances which the diversity of organic geochemical approaches can collectively yield when applied to complex natural systems” (Geochemical Society, 2022). The definition of organic geochemistry is mirrored in the work of the Society’s Organic Geochemistry Division, which is stated as encouraging and fostering “studies on the origin, nature, geochemical significance, and behavior during diagenesis and catagenesis of naturally occurring organic substances in the Earth, and also studies of extraterrestrial organic matter” (Geochemical Society, 2022). Utrecht University (2022) describes the work of organic geochemists as using a molecular approach to examine and study the origin and development of organic matter in the bio- and geosphere with the focus of research in that university being mostly on land-ocean organic carbon movement, paleoclimate reconstructions, proxy development, and ecosystem processes and the “the relationships between the occurrence, distribution, and/or isotopic composition of specific molecules, so-called biomarkers, and environmental parameters in modern systems.”
Organic geochemistry may also examine evidence of environmental change in the Earth’s crust. For example, Imperial College (2022) describe their Organic Geochemistry Research (ICOG) program as including research on the evidence for mass extinctions in rock chemistries, especially the big five mass extinctions at the end Ordovician, Late Devonian, end Permian, end Triassic, and end Cretaceous, examining the organic remains of the organisms that existed during these events, which “are entombed in rocks and can be extracted and analysed using organic geochemical methods. Interpreting these molecular fossils allows us to reconstruct the environments in which these organisms prevailed and thereby understand the causes and consequences of the extinction events” (Imperial College, 2022). Other research focuses on environmental change, using organic material such as plant spores, which contain pigments that protect their genetic materials from mutation by UV light. The analysis of the pigment contents of spores collected over long periods, it is possible to determine the trends in the amount of UV penetrating to the Earth’s surface (Imperial College, 2022). Rock matrices deposited in layers at regular intervals, can also be examined, and the detailed study of these layers and the existing diagnostic molecules enables the abstraction of data that documents long term environmental change (Imperial College, 2022).
For practical results, organic geochemistry may also examine sources of petroleum in the Earth’s crust, examining sources, compositional changes, and movement of such petroleum sources. Petroleum geochemistry has become one of the most important foci of organic geochemistry, with the main issues being the development of ever more sophisticated and detailed method for the detection of organic compounds and oil being strata in ground materials. Imperial College (2022), describes their Organic Geochemistry Research (ICOG) program as also including Petroleum Studies, arguing that “recently, the demand for oil is focusing scientific attention on unconventional hydrocarbon deposits. Imperial College Organic Geochemistry activities involve research into both conventional and unconventional petroleum deposits.” This is echoed by Mather (2013), who writes that “organic geochemistry studies the distribution, composition, and fate of organic matter in the geosphere on both bulk and molecular levels, combining aspects of geology, chemistry, and biology… Given the origins of fossil fuels such as crude oil, natural gas, or coal via the incorporation into sediments and burial of biomass from decayed organisms (followed by complex chemical reactions under the influence of temperature, catalysis, and, in shallow sediment layers, microbial activity), organic geochemistry is intimately associated with petroleum geochemistry.”
Philp and Mansuy (1997: 749) point out that petroleum geochemistry is an important discipline for several areas of exploration and production for fossil fuels. Additionally, there have been recent developments alongside other developments in analytical chemistry, including gas chromatography and gas chromatography−mass spectrometry. An important development is the use of new techniques for the detection of usually trace amounts of biomarker compounds, which are compounds in oil with source rock extracts, the analysis of which can provide information on the origin of the oils in the Earth’s strata. Philp and Mansuy (1997: 749) argue that at the time of their publication in the mid-90s, major developments in geochemistry included the evolution of methods for reservoir studies (e.g., the use of high-resolution gas chromatography to study reservoir continuity, and high-temperature gas chromatography to study wax deposits), new methods in exploration and biomarker geochemistry (e.g., pyrolysis techniques to study insoluble organic matter in source rocks or asphaltenes in oils), techniques which enable “a far more detailed and comprehensive picture on the origin of fossil fuels than could ever have been imagined a mere two decades ago” (Philp & Mansuy, 1997, 749).
Mukhopadhyay et al. (1995: 86) developed the argument that “the major unresolved issues on hydrocarbon generation, migration, and entrapment, include: (a) the distribution of source rocks in various stratigraphic intervals; (b) variations in maturity for both source rocks and crude oils and condensates; (c) the relation between hydrocarbon generation and over pressuring; and (d) possible oil-oil and oil-condensate source rock correlation.” Here the issues concern studies that examined and described some potential source rocks in several stratigraphic intervals, the geochemical properties of some oils and condensates, and some possible oil-oil and oil-source rock correlations (Mukhopadhyay et al., 1995, 86). Related studies examine the possibly ways to study and predict oil bearing sediments and the formation of such varied physical forms. For example, Zou (2017) writes about “unconventional petroleum geology,” of which an example is the theory of kerogen thermal degradation. This was initially based on earlier research, such as that of Bray and Van Tuyl (1961) who found that the carbon number distributions of n-alkanes or normal alkanes (alkanes are hydrocarbons in which the carbon atoms are held together by single bonds, with the general formula CnH2n+2) and further research increased knowledge on “the diagenetic evolution mechanism of organic matter and its relationship with the formation of oil” (Zou, 2017). Phillippi (1965) is cited as one of the founders of such studies, as the research findings indicated that sedimentary organic matter can only be transformed into hydrocarbons if it is at a particular burial depth and temperature. Later studies which examined the links between the generation of petroleum and temperature and time, such as those of Connan (1974) and Tissot and Welte (1978) “established a petroleum generation model that quantitatively determines the hydrocarbon-generating capacity of source rocks” this supporting the theory of kerogen thermal degradation (Zou 2017).
This theory assumes that deposited organic matter changes into kerogen after biochemical and polycondensation processes (Zou 2017). The shrinkage of the kerogen to a greater depth slowly generates oil through thermal degradation. Such organic matter includes bitumen, which is soluble in organic solvents, and kerogen which is insoluble in organic solvents. The character and hydrocarbon potential of kerogen can be measured using these parameters and using theoretical and practical analysis may evaluate the sources and amounts of hydrocarbon development. This study of hydrocarbon generation is linked to that of the concept of petroleum migration, the latter being important for the field of petroleum geology. Petroleum became a topic of primary interest in petroleum geology. Zou (2017) cites some formative literature that explored the relationships of the primary migration of petroleum, including those of Magara (1978) who looked at mudstone compaction and other primary migration issues, Barker (1972) who examined the role of aquathermal pressure, Burst (1969) who investigated the dehydration of clay minerals, Leithauser et al. (1982) who examined hydrocarbon diffusions and Dickey (1975) which looked at the primary migration phase of oil. Zou (2017) argues that these studies “led to the general identification of key scientific issues such as the phase, dynamics, and pathways of the primary migration of petroleum.”
Other oil-based geochemical studies include that of Boreham et al. (2021), which examines the monoalkene contents of a case study of Australian oils. A wide range of oils were selected for study, and the authors argue that “thirteen Australian oils and one condensate, covering oil reservoir ages from Mesoproterozoic to Early Cretaceous, show monoalkene contents varying from 0.01 to 22.3 wt% of the whole liquid” (Boreham et al., 2021). The methodology was based on radiolysis of saturated hydrocarbons (radiolysis refers to the molecular damage to a substance by ionizing radiation), which is stated as the “most likely process leading to oils with high alkene contents.” Alkanes and alkenes are referred to in this study. Alkanes have single bonds between carbon atoms and are called saturated hydrocarbons. Alkenes have at least one carbon-carbon double bond and are unsaturated hydrocarbons. Boreham et al. (2021) found that the main radiolytic component was an unresolved complex mixture (UCM), with most of the resolved alkene compounds being positional isomers (the basic carbon skeleton remaining the same, but the important groups moved on the skeleton) of n-alkenes), with minor components being methyl branched and cyclohexyl alkenes.
Importantly, the authors note that “the oil with the longest reservoir residence time shows the highest content of internal n-alkenes relative to terminal 1-alkenes as well as the highest trans/cis ratio, suggesting the extended time has resulted in rearrangement to near thermodynamic equilibrium of the congruent monoalkenes” and the “internal n-alkene isomers have a trans configuration dominant over the cis isomer” (Boreham et al., 2021). Here cis isomer is part of Cis–trans isomerism, “where Cis and trans isomers occur when an alkene has two different atoms or groups of atoms attached to each double-bonded carbon atom” (Ouellette & Rawn, 2019). Boreham et al. (2021) found that the “relative proportion of alkene mimics the relative abundance of n-alkanes, suggesting that radiolytic C–C bond cleavage is suppressed when the alkene/alkane ratio is elevated and that the preferred pathway of n-alkane radiolysis favors the production of terminal monoalkenes: and the radiolysis of the alkane UCM with the networking of n-alkane-derived radiolysis products contribute to the higher relative proportion of the alkene UCM, and conclude that the similarity of the carbon and hydrogen isotopic ratios of the n-alkanes and n-alkenes supports a parent–daughter relationship, and radiolytic n-alkenes have a similar distribution to that of the n-alkanes; and additionally an unresolved complex mixture dominates the unresolved complex mixture, there is a similarity of the carbon and hydrogen isotope values of n-alkenes and n-alkanes; and a long term oil residence results in a near-equilibrium n-alkene isomer distribution (Boreham et al., 2021).
Another study by Curiale and Frolov et al. (1998) examined the origin and occurrence of alkenes in petroleum, and primary and secondary sources for the alkene content in oils. The primary sources of alkenes were from the primary migrating phase, expelled from the source rock. The secondary sources of alkenes are migration-contamination, usually contamination from lower maturity organic matter, from the migration pathway or disseminated within the reservoir, and alteration within or near the trapped fluid, and the “latter process can involve either thermal pyrolysis or low temperature radiolytic dehydrogenation of saturated hydrocarbons (Curiale & Frolov, 1998). An igneous intrusion near the hydrocarbons in the reservoir or within an organic matter-rich rock may cause transient high temperature thermal degradation of the oil and condensate, hence producing an alkene-rich migrating fluid (Curiale & Frolov, 1998). The authors conclude that “enrichments of radiogenic elements (U, Th and K) within reservoir rock minerals can result in the radiolytic-generated alkenes making more than 10 wt% of the altered petroleum liquid” (Curiale & Frolov, 1998; see also Frolov et al., 1998).
Apart from oil and petroleum studies, organic geochemistry considers other aspects of organic materials in atmosphere, lithosphere and ocean. An important issue concerns marine dissolved organic matter (DOM), which is a major bioactive reservoir of carbon in the ocean. This reservoir is estimated at a stock of 700 Pg C in the global ocean, which is similar in size to the stock of carbon resident in atmospheric CO2 (Hansell & Carlson, 2014 xxi). Hansell and Carlson (2014, xxi) note that with the development of studies of the elemental cycles (carbon, nitrogen, and phosphorus), searching research has been conducted on such organic matter accumulation, with certain central questions: “can we accurately, with community wide consistency, measure the concentrations of dissolved organic matter in the ocean; what are the distributions of the dissolved organic C/N/P pools and what processes controls these distributions; what are the rates, biogeographical locations, and controls on elemental cycling through the pools; what are the biological and physicochemical sources and sinks; what is the composition of the pools and what does this tell us about elemental cycling? Finally, do we understand DOM in elemental cycling well enough to accurately represent the processes in numerical models?” Answering such questions has become important studies on DOM.
Catalá et al. (2021, 7225) note that marine-dissolved organic matter (DOM) is a huge and mostly unexplored molecular space, mostly resident in the ocean for millennia. This huge reservoir is very diverse one for the most diverse molecular mixtures yet documented, and comprises millions of individual compounds. Research has been conducted on the role of dissolved organic matter in the processes of biogeochemical cycles, climate dynamics, microorganisms, and the molecular composition of the DOM. Catalá et al. (2021, 7225) nevertheless note that the study of DOM bioactivities is still undeveloped, mostly due to the technical challenges of analyzing such chemically complex material. The current solution is to develop more advanced technologies, for the analysis of DOM bioactivities. The newer screening technologies are slowly enabling more detailed studies, especially the accelerated identification of bioactivities for small molecules from natural products and seek to replace the “laborious chemical fractionation” with the “application of untargeted metabolomics and multiplexed high-throughput molecular-phenotypic screening techniques that are providing first insights on previously undetectable DOM bioactivities” (Catalá et al., 2021, 7225).
In another study, Pötz et al. (2021) note that organic matter held to be an important reductant in some sediment-hosted base metal deposits, but the exact nature of the interactions between OM and hydrothermal fluids is debatable, and the interconnected reactions that develop over the long term, geological timescales are also currently being studied. Their study examined organonitrogen, -sulfur, and -oxygen (NSO) compounds, the justification of this focus being that these compounds are the most affected by organic–inorganic interactions with mineral surfaces, metals, and aqueous fluids (Pötz et al., 2021). The study methodology used ultra-high resolution mass spectrometry (FT–ICR–MS). According to Qin et al. (2021, 347) “FT-ICR MS measures the mass-charge ratio and abundance of all ions at the same time. FT-ICR allows detection of very low ion concentrations due to its ability to amplify signals. This way, the method achieves high sensitivity as well as resolution.” In the study by Pötz et al. (2021) the findings indicated a generally homogenous deposition of type II marine organic matter, hydrothermal issues, losses of extractable organic matter and especially long-chain n-alkanes and enrichment in oxygenated compounds and increased molecular oxygen content. Zakaria et al. (2018, 345) refer to n-Alkanes as “saturated hydrocarbons…, hydrogen and carbon with solely single bonds” with the formula CnH2n+2 showing the maximum bonds between carbon and hydrogen atoms. The authors argue that “the main source of global occurrence of n-alkanes is petroleum; however, they also occur naturally. n-Alkanes can be detected in riverine, estuarine, and marine sediments as well as aquatic organisms” Zakaria et al. (2018, 345).” Pötz et al. (2021) conclude that “FT–ICR–MS, combined with determination of stable hydrogen isotope compositions, can provide valuable information about interaction time scales and the origin of mineralizing hydrothermal fluids.”
Other geochemical studies include micro-assessments of chemical compound materials in earth or water systems, sometimes with applications to microbiology. For example, O’Beirne et al. (2022) write about the “characterization of diverse bacteriohopanepolyols in a permanently stratified, hyper-euxinic lake.” The authors argue that these bacteriohopanepolyols (BHPs) are a diverse class of bacterial lipids that have potential status as biomarkers of some specific microbes, microbial processes, and environmental conditions (O’Beirne et al., 2022). The subject study of Mahoney Lake in Canada was of a water and sediment environment characterized by hyper-euxinic (usually with no oxygen and higher levels of hydrogen sulfide H2S) and meromictic (layers of water that do not intermix) conditions (see Wetzel, 2001; Meyer & Kump, 2008). The findings of the study indicated that there were clearly distinct BHP distributions in the oxic mixolimnion (mixolimnion refers to the upper layer of a meromictic lake, above the chemocline, mixed by the wind, with free circulation and low density, oxic refers to oxygen present), the chemocline (this is the zone in a meromictic lake, that transitions between the upper mixolimnion and lower monimolimnion layers, with a change from aerobic to anaerobic conditions, and monimolimnion refers to the lower layer of a meromictic lake, below the chemocline, with dense static water) (O’Beirne et al., 2022).
The findings also indicated that Bacteriohopanetetrol (BHT) and unsaturated BHT are the main BHPs in the oxic mixolimnion and the chemocline, a novel BHP occurs in the euxinic monimolimnion. Additionally, composite BHPs occurred in the euxinic monimolimnion and sediments, showing production by anaerobic bacteria. Bacteriohopanepolyols (BHPs) were produced by anaerobic bacteria and methylated bacteriohopanepolyols are produced from low oxygen and high osmolarity (osmolarity is the number of particles per liter of solution) (O’Beirne et al., 2022). The authors also found that “BHTs are produced within the euxinic sediments in response to low oxygen and high osmotic concentrations, as opposed to being diagnostic biomarkers of cyanobacteria and aerobic metabolisms” (O’Beirne et al., 2022).
Another study by Cooke et al. (2009: 1151), on bacteriohopanepolyols in soils, focuses on a group of BHPs, including adenosylhopane and related compounds, which enable the transport of terrestrial organic matter (terrOM) to the marine environment. The case study was of Russian (estuary surface sediments) and American rivers (river sediments). Using the BHP signatures and high performance liquid chromatography–tandem mass spectrometry (HPLC–MSn), the study found 15 different BHPs from several different bacteria, and a significant mound of OM from terrestrial sources and suggested that increases in terrestrial OM or increased preservation of OM resulted from of shorter periods of permafrost thawing (Cooke et al., 2009: 1151). In addition to variation between the rivers (great differences between the North American rivers in BHP input and higher soil OM contribution, and variations between the Russian rivers in “soil-marker” BHPs and tetrafunctionalized BHPs), “aminobacteriohopanepentol, an indicator of aerobic methane oxidation, was observed in all the sediments, with the source being either the marine environment or methane producing terrestrial environments” (Cooke et al., 2009: 1151).
Organic geochemistry may be closely linked to biogeochemistry, as carbon has strong biological links. Rafferty (2013) describes the relationship between biogeochemistry and organic geochemistry: biogeochemistry is the “study of the behaviour of inorganic chemical elements in biological systems of geologic scope as opposed to organic geochemistry, which is the study of the organic compounds found in geologic materials and meteorites, including those of problematic biological origin.” Topics within both biogeochemistry and organic geochemistry are listed as petroleum and life origins, biogeochemical prospecting for mineral deposits, some ore deposit origins, natural water chemistry, soil formation, and coal chemistry (Rafferty, 2013). Organic geochemical studies may apply to biogeochemistry. For example, a study of the organosulfur compound dimethylsulfide (DMS) by Hopkins et al. (2023: 361) titled “The biogeochemistry of marine dimethylsulfide” argued that “advances in molecular genetics and large-scale biogeochemical measurements have revealed the global prevalence of DMS-related processes, including in previously overlooked environments and organisms, such as sediment-dwelling bacteria.” The study examined the production and cycling of marine DMS and its entry into the atmosphere and noted that the study of dimethylsulfide in the atmosphere, which may be based on crudely defined biological parameters, including total chlorophyll, require more advanced study of the biogeochemical processes that control DMS production (Hopkins et al., 2023: 361).
Inorganic Geochemistry
Definitions of inorganic geochemistry usually emphasize the role of carbon in organic geochemistry and the lack of carbon in studies in inorganic geochemistry. For example, Geoscience Australia (2022) notes that “inorganic chemistry is concerned with the properties of all the elements in the periodic table and their compounds, with the exception of organic compounds (compounds containing C-H bonds). Inorganic chemistry investigates the characteristics of substances that are not organic, such as nonliving matter and minerals found in the Earth’s crust.” Inorganic geochemistry is linked to inorganic chemistry: the American Chemical Society (ACS) (2022) defines inorganic chemistry as concerned “with the properties and behavior of inorganic compounds, which include metals, minerals, and organometallic compounds. While organic chemistry is defined as the study of carbon-containing compounds, inorganic chemistry is the study of the remaining (i.e., not carbon-containing) subset of compounds. But there can be overlap between the two fields. For example, organometallic compounds usually contain a metal or metalloid bonded directly to carbon.”
Several authors have written concerning the foci of inorganic geochemistry, including the main inorganic elements and minerals, and the techniques for their study. For example, Kumar et al. (2021, 149), write about highly sophisticated analytical methods, including powder X-ray diffraction, scanning electron microscopy, electron probe micro-analysis and other methods, which can be used for the study of inorganic or geological minerals. Elements and minerals studied with these systems include iron (Fe), oxygen (O), silicon (Si), magnesium (mg), quartz (SiO2), feldspar (KAlSi3O8 – NaAlSi3O8 – CaAl2Si2O8), among others), amphiboles (SiO4 tetrahedra, containing ions e.g., iron and/or magnesium, etc.), pyroxene (generally the formula ABSi2O6), olivine (Mg2+, (Fe2+) 2SiO 4), kaolinite (Al2Si2O5(OH)4), bentonite ((Na,Ca)0.33(Al,Mg)2Si4O10(OH)2·(H2O)n), also sodium bentonite (Al2H2Na2O13Si4), calcium montmorillonite ((Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O), vermiculite ((Mg,Fe2+,Fe3+)3 [(Al,Si)4 O10](OH)2·4H2O), and biotite (K(Mg,Fe)3(AlSi3O10) (F,OH)2).
Geochemistry (2022) reports that the major elements in Earth’s mantle and crust are, in order of abundance: oxygen (44%), magnesium (23%), silicon (21%), iron (8%), calcium (2.5%), and aluminum (2.4%). Iron is one of the main elements described in studies of inorganic geochemistry. For example, Williamson (1998) notes that “iron commonly exists in one of three oxidation states: Fe0 (elemental iron), Fe2+ (ferrous iron), and Fe3+ (ferric iron). It is the fourth most abundant element in the Earth’s crust, exceeded only by oxygen, silicon and aluminum, but is believed to be the most abundant in the Earth as a whole.” It exists at a crustal average of 7–8%, in ultramafic 9.6%, basalt 8.6%, granite 2.2%, limestone 0.33%, and sandstone 0.98% (Taylor & McLennan, 1985; Faure, 1991).
Inorganic geochemistry may also be concerned with oil exploration and analysis. For example, Ivanov et al. (2021) examined the inorganic geochemistry of crude oils of northern Eurasia using mass-spectroscopy with inductively coupled plasma (ICP-MS), which enabled the study of the microelement composition of crude oil and its derivatives “with the limit of detection (LOD) at the ppt level” taking an example from West Siberian and Tatarstan Romashinskoye oilfields. The findings using the ICP-MS method indicated variations in microelements in the oil reservoirs. Fifty rare, rare earth, and other microelements were detected, and the studied crudes were found to have a specific microelement composition. It was argued that the main geochemical feature of the crude oil was the extremely low content of many trace elements. This study of the inorganic geochemistry of West-Siberian and Tatarstan crude oils “using the most modern equipment shows that crude oils (at least from these two provinces, and, apparently, others) have an extremely specific microelement composition with no comparisons” (Ivanov et al., 2021).
The authors note that “according to modern concepts…, the analysis of the distribution of rare and rare earth elements in crude oils makes it possible to identify the sources and characteristics of naphthide-forming fluids (Naphthalene is an organic compound with formula C10H8, and naphthide is a compound of naphthalene or its radical with a metallic element, mercuric naphthide) (Ivanov et al., 2021). It was concluded that there was a specific microelement composition to the studied crudes, and based on the elevated contents of transit elements and platinoids, the study made a conclusion about the “ultrabasic” geochemical–metallogenic specialization of studied petroleum systems and the assumption about its origin was proposed” (Ivanov et al., 2021; see also Marakushev et al., 2004; Ivanov et al., 2008; Gottikh et al., 2009).
In another example of the application of inorganic geochemistry methods to petroleum sources, Ratcliffe et al. (2012, 4) give a case study of oil extraction from shale (the commonest sedimentary rock), arguing that as shale continues to be the most actively explored and developed hydrocarbon plays in North America, their study “demonstrates how inorganic whole-rock geochemical data can be used to help with the development of shale plays.” The authors further note that the elemental data which is utilized for chemostratigraphy may be used for modeling mineralogy and total organic carbon, and also to assess paleoredox facies and derive information on the formation brittleness, which are important pieces of information for the exploitation of the shale resource plays (Ratcliffe et al., 2012, 4). Here chemostratigraphy may be defined as “the study of the inorganic/organic chemical variations within the sedimentary sequences, either based on the elemental or isotopic composition of the rock. It provides a useful tool for unconventional resource exploration and development” (Slatt et al., 2021).
Ratcliffe et al. (2012: 4) also argue that recently in the United States, shale has become an important oil source, but “the fine grained, macro-scale homogeneity of many shale plays currently being exploited has negated some of the more traditional approaches to reservoir characterization and stratigraphic correlation, resulting in the search for new methodologies that enable better understanding of shale reservoirs.” In this study, the technique used was the application of inorganic whole-rock geochemical data to shale resource plays. Features measured by this chemostratigraphy technique, which help to understand shale reservoirs, include changes in element concentrations through time and using those to model changes with respect to geological events, such as paleoclimate, and relative rock brittleness (see also Pearce et al., 2005; Ratcliffe et al., 2006, 2007, 2010; Tribovillard et al., 2006, 2008; Turgeon & Brumsack, 2006; Negri et al., 2009; Jenkyns, 2010; Hildred et al., 2010; Wright et al., 2010). Ratcliffe et al. (2012: 9) conclude that although until relatively recently, stratigraphic studies were the main objective for deriving whole rock inorganic geochemical data for the petroleum industry, it is now more obvious that these same datasets may be used to assess bulk mineralogy and total organic carbon (TOC) (both semiquantitatively), temporal and lateral variation in paleoredox conditions, changes in rock brittleness, and changes in mineralogical composition for industrial purposes.
Other studies of inorganic geochemistry focus on elements or compounds in the earth’s crust, either in solid materials or water. For example, Smith et al. (2009, 2348) examine the occurrence of ammonium (NH4+) and nitrogen processing in water from coal-bed natural gas (CBNG) production wells, in a case study of the Powder River Basin, Wyoming, USA. The findings of this study indicated that dissolved ammonium concentrations ranged from 95 to 527 μM, ammonium concentrations decreased with the transport distance (with later increases in nitrite and nitrate concentrations) and the removal efficiency, or uptake, of total dissolved inorganic nitrogen also differed between the channel types. The study also found that the uptake of dissolved inorganic nitrogen was greater in channels in gentle-sloped, vegetated topography, than in the steeper, less vegetated channels. There were also high correlations between dissolved inorganic nitrogen and diel (diel refers to 24 hours; daily diel alternation between night and day) patterns of incident light and dissolved oxygen concentration. Other findings were that in a larger main channel with several discharge inputs (n = 13), dissolved inorganic nitrogen (DIN) concentrations were > 300 μM, with pH > 8.5, after 5 km of transport. Ammonium comprised 25–30% of the large-channel DIN, and ammonium concentrations were relatively constant over with time, and there was only a weak diel pattern (Smith et al., 2009, 2348). The authors conclude from the findings that CBNG discharge may sometimes be an important DIN source in western watersheds, and that the net oxidation and/or removal depends upon the amount of contact with sediment and biomass, the type of drainage channel, and the time of day (Smith et al., 2009: 2348).
In another study, Sowder et al. (2010, 1604) studied trace metal occurrence in Coalbed natural gas (CBNG)-produced water, which can accumulate in produced water retention ponds, taking a case study of the Powder River Basin (PRB) of Wyoming. The objective of the study was to ascertain the groundwater pollution potential and to explain the factor for the high Arsenic (As) concentrations (Sowder et al., 2010: 1604). The parameters were the infiltration characteristics, subsurface hydrology, the fall and pond water quality, the isotope signatures, and the trace metal balances. The findings indicated that there was little or no infiltration of pond water, and there was no measurable contamination of the shallow groundwater. The high pond As concentrations were caused by semicontinuous inputs of CBNG-produced water with low As concentrations (0.20–0.48 microg L(−1)), which were affected by the low pond water volumes during low rainfall. Sowder et al. (2010: 1604) concluded that “because of reduced infiltration and high evaporation rates, As became concentrated over time” and “reduced infiltration was most likely caused by the high sodium concentration and high sodium adsorption ratio of the CBNG-produced water, which disrupt soil structure.”
In another study, Soldatova et al. (2021: 441) examined nitrogen compound behavior in a shallow groundwater-soil system, which is an important aspect of the nitrogen cycle within agricultural landscapes, and a factor for groundwater chemical composition, with a case study from an agricultural landscape in the Poyang Lake area (Jiangxi Province, China). The methodology involved soil and groundwater samples taken before and after harvesting (spring and fall)., examining the data on the chemical and microbiological composition of the soils and groundwater, and isotopic data on dissolved nitrate, to determine the entry of nitrogen into the studied system. The findings indicated that nitrogen entered through organic nitrogen compounds (mostly through early fertilization), which change into nitrate due to soil nitrifiers before entering the aquifer, where there may be denitrification. The authors conclude that in the case study, the reducing conditions, and the formation of clay minerals from the aqueous solution, may create a geochemical barrier for the accumulation of nitrogen compounds, which may stop the transformation of ammonium to nitrate and providing its sorption (sorption refers to the removal of a compound from solution by solid phase constituents). They also note that “bacterial diversity in the shallow groundwater has a strong relation with the amount of nitrate in the system, whereas in the soil, it is connected with sampling depth” (Soldatova et al., 2021: 441).
Links between biogeochemistry and inorganic geochemistry largely concern the presence of inorganic elements and compounds such as those of iron, oxygen, silicon, magnesium, and mercury in the environment and in organisms, especially in fresh and saltwater (Campbell, 2020). For example, Morel and Price (2003) detail planktonic uptake of metals, which contributes to low concentrations of these metals in surface seawater. The study found that microorganisms release complexing agents and catalyze redox reactions that may change the bioavailability of trace metals and increase cycling in the upper water column, this contributing to the taking up of the micronutrients. It is also argued that the low availability of some metals contributes to photosynthesis rates and the transformation and uptake of nutrients such as nitrogen. The authors conclude that “the extremely low concentrations of several essential metals are both the cause and the result of ultraefficient uptake systems in the plankton and of widespread replacement of metals by one another for various biochemical functions” (Morel & Price, 2003: 944).
Another study examines the role of the sulfur biogeochemical cycle in mercury methylation in marine sediments (Wang et al., 2022). The basis of the study is that “estuaries are sinks for mercury, in which the most toxic mercury form, neurotoxic methylmercury (MeHg), is produced by mercury methylators and accumulates in estuarine sediments” (Wang et al., 2022). In association, sulfate-reducing bacteria may trigger the microbial sulfur cycle and may act as the mercury methylator. The study examined sulfur and mercury speciation in sediments from a case study of 70 estuaries distributed globally. The findings indicated abundant mercury and sulfur species, with mercury levels risky to aquatic ecological systems, and significant correlations between sulfur and MeHg concentrations, with the porewater sulfate concentration positively correlated to MeHg production. The sulfur cycle contributed to MeHg formation by activating mercury methylator activities and reducing the bioavailability of mercury, which may increase or inhibit MeHg formation at variable sulfur speciation concentrations. The authors concluded that the findings suggest “sulfur biogeochemical cycle plays an important role in mercury methylation in estuarine sediments, and the effect of the sulfur cycle on mercury methylation deserves to be further explored in future research.”
In another study, Nóbrega et al. (2013: 7393) also examined the role of iron and sulfur, which are “key elements in the biogeochemistry of estuarine soils, in which Fe and sulfate reduction (SR) pathways are important for organic matter decomposition.” Taking a case study of the Brazilian coastal mangrove forest with strong weather variations and shrimp farms, the objective was to assess the impacts of shrimp farm effluents, mostly wastewater on iron and sulfur geochemistry in mangrove soils. A comparison was of two mangrove forest soils from a mangrove forest receiving wastewater from shrimp ponds and a control site). The findings indicated a decrease in pyritic iron and degree of pyritization in the soils of the first case study, possibly linked to the anaerobic oxidation of pyrite and nitrate reduction, or the dominance of denitrification over sulfate reduction. The lower TOC contents in the first site suggested that underground decomposition increased in reaction to eutrophication. Seasonal variations impacted the semiarid mangrove soils, as during the drier period, soils had oxidizing conditions with losses of reduced and oxidized iron, which is environmentally significant as iron is biolimiting for marine primary production. The authors conclude that the findings indicate “that both factors (seasonal weather variations and shrimp effluents) play important roles in the geochemical processes that occur in these soils and, thus, may affect their functioning and maintenance” (Nóbrega et al., 2013: 7393).
Isotope Geochemistry
Isotopes are defined as atoms of the same element that have different numbers of neutrons, but the same numbers of electrons and protons, this translating into different physical characteristics. Isotope geology is the study and application of isotopes to geological processes and the time scales of these processes (Kendall et al., 1995; Kendall & Caldwell, 1998). Isotope geochemistry is defined by Allaby (2008) as the “study of the abundance ratios of isotopes (both stable and radioactive) of major and trace elements in rocks (e.g., Rb/Sr, Pb/U, etc.), to elucidate a number of geologic problems and processes. These include the age relationships of rocks, and the age of the Earth itself (see geochronology; isotopic dating; and radiometric dating); paleotemperatures and geothermometry (see oxygen-isotope analysis; and geothermometer); and the provenance of natural waters, ore-forming fluids, and magmas (see isotope fractionation; SMOW-standard mean ocean water; d/h ratio; oxygen–isotope ratio; and stable-isotope studies).”
Isotope geochemistry may be divided into stable isotope geochemistry and radiogenic or radioactive isotope geochemistry. Li et al. (2019) describe isotopes as divided into stable isotopes and radioactive isotopes, where stable isotopes refers to the isotopes whose nuclear remain stable through time, as opposed to radioactive isotopes that decay into daughter isotopes of different elements.” Mamedov (2021: 6) compares the stable and unstable types, notes that “in contrast to unstable (radioactive) isotopes or isotopes produced from the decay of another element (radiogenic), stable-isotope geochemistry uses isotopes whose abundances do not change with time.” Yadav et al. (2021, 93) point out that “stable isotopes have shown very versatile applications in various fields. Vallero (2014, 139) notes that “stable isotopes do not undergo natural radioactive decay, whereas radioactive isotopes involve spontaneous radioactive decay, as their nuclei disintegrate.”
Additionally, the stable isotopes of light elements, including hydrogen, carbon, nitrogen, oxygen, and sulfur have been used in earth science research from the 1950s, and have consequently been described as traditional stable isotopes. The stable isotopes of other elements (note: most of them are metals such as Mg, Cu, Fe, Zn, Hg) have been described as nontraditional stable isotopes, because they have mostly received attention since the beginning of the 2000s (Li et al., 2019). Yadav et al. (2021: 93) have also written that in the case of geochemistry, two types of stable isotopes have been used, namely the traditional stable isotopes including C, N, O, S, and H, and nontraditional stable isotopes such as Zn, Cu, Fe, Pd, Cd, and other metals. Yadev et al. (2021: 93) also contend that traditional isotopes have been used in studies as geothermometers, tracers in hydrological, biological systems, ore deposits, hydrothermal systems, among others, while nontraditional isotopes have increasingly been used in several fields, including the tracing of toxic environmental pollutants like Cr and Hg, application in mining (Yadav et al. (2021: 93).
Traditional stable isotopes are referred to as “those of carbon, hydrogen, nitrogen, oxygen, and sulfur, elements with relatively low atomic masses that play a significant role in geological and biological processes” and the basis of traditional stable isotope geochemistry, which is “based on using small atomic mass differences between isotopes of the same element to probe earth system processes. Isotopes fractionate themselves between different phases or molecules in ways that are related to temperature and reaction mechanism” (Stern & Wieman, 2021,100). Nontraditional isotopes are described by Hu and Teng (2021, 114) as “isotope systems other than those of H, C, N, O, and S”; these authors also point out that “the field of stable isotope geochemistry has advanced rapidly with the developing capability to make high-precision measurements using multi-collector inductively-coupled-plasma mass spectrometry (MC-ICPMS) at high mass-resolution.”
ChemEurope (2022, see also Dickin, 2005) describes radioactive isotopes as the parents to the radiogenic daughter isotopes, because a radiogenic nuclide is one that is produced by a process of radioactive decay. Vallero (2014, 139) maintains that the radioactive decay of the radioactive isotopes contributes to the formation of new isotopes or elements, and that the stable product of an element’s radioactive decay is termed a radiogenic isotope. The example is cited of lead (Pb), which has four naturally occurring isotopes, with varying masses (204Pb, 206Pb, 207Pb, 208Pb), of which only the first listed is stable. The isotopes 206Pb and 207Pb are classified as “daughter” (or progeny) products from the radioactive decay of uranium (U), while 208Pb is a product from thorium (Th) decay (Vallero, 2014, 139). ChemEurope (2022, see also Dickin, 2005) also cites lead as “perhaps the best example of a radiogenic substance, as it is produced from the radioactive decay of uranium and thorium. Specifically, Pb-206 is formed from U-238, Pb-207 from U-235, and Pb-208 from Th-232.” Other adiogenic substances include argon-40, which is formed from radioactive potassium, and nitrogen-14, formed by the decay of carbon-14, radon and helium (ChemEurope (2022).
Radiogenic isotope geochemistry is important for current scientific research on the chronology of rock-forming events, as isotope geochemistry is related to the relative and absolute concentration of elements and their isotopes in Earth (Haldar, 2018). ChemEurope (2022) also states that radiogenic nuclides (more commonly referred to as “radiogenic isotopes”) are among the most important tools in the Earth sciences, with two main applications: (1) “in comparison with the quantity of the radioactive ‘parent isotope’ in a system, the quantity of the radiogenic ‘daughter product’ is used as a radiometric dating tool (e.g., uranium-lead geochronology)” and (2) “in comparison with the quantity of a nonradiogenic isotope of the same element, the quantity of the radiogenic isotope is used as an isotopic tracer (e.g., 206Pb/204Pb).” Vallero (2014: 139) also adds that “radiogenic isotopes are useful in determining the relative age of materials. The length of time necessary for the original number of atoms of a radioactive element in a rock to be reduced by half (radioactive half-life) can range from a few seconds to billions (109) of years.”
Radiogenic isotopes may also be used for studying certain geological formations. For example, Cooke et al. (2014: 364) argue that radiogenic isotopes (mentioning U–Pb, Sm–Nd, Rb–Sr, and Lu–Hf isotopes), “are used extensively to investigate the igneous and hydrothermal evolution of porphyry deposits and the potential sources of contained metals.” The authors note that the U–Pb (uranium-lead) isotopic system is one of the most used tools in the study of porphyry deposits (these are deposits of minerals of copper, molybdenum, gold, or other metals, within small veinlets within hydrothermally altered igneous formations), as a geochronologic tool and a isotopic tracer for the evaluation of magma and metal sources in reservoirs, as lead is a common element in some rock-forming silicate minerals and sulfide minerals in porphyry deposits (Cooke et al., 2014 364). It is further argued that lead isotopes are useful for the tracking of metal sources and determination of magmatic and other ore-forming processes. Isotopic evolution histories can be determined by Th/U (Thorium-Uranium, where both uranium and thorium have unstable isotopes) and U/Pb ratios, which reveal geological history (see also, e.g., Chiaradia & Fontbote, 2002; Kamenov et al., 2002; MacFarlane et al., 1990; Wooden et al., 1988).
In the Arizonan case study of copper deposits, Cooke et al. (2014: 364) write that certain distinct Paleoproterozoic basement terranes impart distinguishable Pb isotopic characteristics to the Mesozoic and Cenozoic igneous rocks which intruded into the region and to related hydrothermal systems. The lead isotopic characteristics show the igneous processes, where low Pb concentration magmas which are derived from the mantle assimilate crustal material as they move upward into the shallower crust. As the Paleoproterozoic crystalline crust and the contemporaneous underlying mantle have isotopic compositions which are different from the younger mantle-derived magma, (because of the growth of radiogenic daughter isotopes), the assimilation of a small amount of the “ancient crystalline crust can change the Pb isotopic composition of any magma and derived porphyry Cu hydrothermal system to values reflecting the values of the crustal column” (Cooke et al., 2014: 364; see also Bouse et al., 1999; Titley, 2001; Wooden et al., 1988).
Radiogenic isotopes may also be used for marine geochemistry. For example, Roy-Barman and Jeandel (2016: 129) describe radioactive and radiogenic isotopes as chronometers and source tracers in the ocean, because a radioactive isotope of an element has a constant probability per unit time (the time constant) to decay into a radiogenic isotope of another element. The authors argue that long-lived isotopes continually decay though the life of the Earth; therefore, the abundance of the radiogenic isotopes produced may vary according to the geological history of the rock that contains them and provides the source signatures when they are released into the ocean (Roy-Barman & Jeandel, 2016: 129). The long-lived isotopes are exemplified by 87Rb, 147Sm, 238U, and 235U (rubidium is Rb, samarium is Sm, U is uranium), and produced radiogenic isotopes include 87Sr, 143Nd, 206Pb, and 207Pb (Strontium is Sr, Neodymium is Nd, lead is Pb). The authors also hold that the short-lived isotopes are produced by spallation reactions (note that spallation refers to a reaction when a target is bombarded by very high-energy particles) in the atmosphere (10Be, 14C), during the U and Th radioactive decay chains (230Th, 222Rn, 226Ra) and by anthropogenic nuclear activity (14C, 226Pu). These isotopes are chronometers used to determine rates (sedimentation rate with 230Th, circulation rate with 14C and 3H).” Here, Be refers to beryllium, C refers to carbon, Rn refers to Radon, Ra refers to radium, and Pu refers to plutonium, all these elements having radioactive isotopes.
In practical geological studies, applications of isotope geochemistry include temperature determination and dating. Britannica (2014) writes about “the enrichment or impoverishment of certain isotopic species that results from the influence of differences in mass of molecules containing different isotopes. Measurements of the proportions of various isotopic species can be used as a form of geologic thermometer.” Crucially, one method concerns the ratio of oxygen-16 to oxygen-18 in calcium carbonate secreted by marine organisms from solutions of calcium carbonate in the oceans. This is related to the ocean temperature; therefore, measurement of the proportions of oxygen-16 with respect to oxygen-18 in calcareous shells of marine fossils can enable estimations of the ocean temperature during habitation by these fossil species. One application is the estimation of the ocean temperatures during the ice ages, using the isotopic composition of fossils in sea floor sediment (Britannica, 2014).
Another important application of isotopic geochemistry is radiometric age dating. These radiometric dating methods are based on the timed rate of radioactive isotope decay which may produce a daughter isotope. Such radiometric measures are “based on the ratio of the proportion of parent to daughter isotopes, others on the proportion of parent remaining, and still others on the proportion of daughter isotopes with respect to each other” (Britannica, 2014). Cited examples include uranium-238, which decays to a natural lead isotope, lead-206. Time measurements may be based on minerals containing uranium-238, measuring the proportions of lead-206 and uranium-238; as “the older the specimen, the greater the proportion of lead-206 with respect to uranium-238” (Britannica, 2014). Another dating method is based on the decay of potassium-40 to form argon-40, and the isotope of uranium (uranium-235), which decays to form lead-207, and thorium-232, which eventually decays to lead-208. These decay periods are extremely long: the half-life uranium-238 is about 4,510,000,000 years and that of uranium-235 is about 713,000,000 years (Britannica, 2014). For shorter periods, carbon-14, which is a radioactive isotope of carbon (unlike carbon-12 and carbon-13, which are stable isotopes) has a half-life of 5570 years. As carbon-14 is part of all living material, linked to atmospheric carbon dioxide, with its short half-life it can be used for dating recent biological materials and events such as the recent ice ages (Britannica, 2014).
Devriendt (2016) cites the case of the oxygen isotope ratio (18O/16O) of inorganic and biogenic carbonate minerals, which is used to measure past temperatures, and sea level, ice volume and hydrologic changes. Devriendt (2016) argues that temperature and temperature-independent factors may be important for the carbonate-water oxygen isotope fractionation (αc/w), but “uncertainties surrounding the reasons for αc/w variations complicate paleoenvironmental reconstructions based on carbonate 18O/16O” (Devriendt, 2016). Devriendt (2016) argues that an incisive approach is a “new general model of oxygen isotope fractionations in the CaCO3-DIC-H2O system that quantifies DIC-H2O and CaCO3-DIC fractionation as a function of temperature, pH, salinity, calcite or aragonite saturation state (Ω), DIC residence time in solution and the activity of the enzyme carbonic anhydrase.” This model in the study is used with supporting data “to explore the cause of αc/w variations for inorganic calcite, ostracod calcite and coral aragonite” (Devriendt 2016). “Isotopic fractionation is defined as the relative partitioning of the heavier and lighter isotopes between two coexisting phases in a natural system” (Tiwari et al., 2015). Tiwari et al. (2015) note two types of isotopic fractionation processes, namely, “equilibrium” and “kinetic” where Equilibrium fractionation is where “an exchange of isotopes takes place, which affects the equilibrium constant…, isotopes can move to and fro and equilibrium is attained when the isotopic ratios do not change any more with time” and Kinetic fractionation is “associated with incomplete and unidirectional processes..,” affecting the rate constant of the reaction., with no isotopic equilibrium attained (Tiwari et al., 2015).
Isotope geochemistry is linked to isotope biogeochemistry, the latter referring to the methods described above in biologically related studies (Kendall et al., 2014). For example, Paytan et al. (2020) note that “sulfur isotope biogeochemistry has broad applications to geological, biological, and environmental studies.” Stricker (2023) also points out that “biogeochemical cycling is a cornerstone of ecosystem function and structure. Much has been learned about element cycles in a variety of systems using standard geochemical techniques.” In a study by Sanders Jr et al. (2011), linked aquatic food webs and groundwater nutrients, in northern Lake Huron. Samples were collected from groundwater vents and analyzed using 13C, 15N, and 34S isotopes, and the findings indicated that dissolved inorganic carbon (DIC) in the groundwater was depleted in 13C. The aqueous sulfate was enriched in 34S, with the mean variation between the groundwater and reference sites were −3.9‰ and +12.0‰, respectively. Benthic primary producers, macroinvertebrates, and benthivorous fish were found to have lower δ13C values in groundwater environments. Benthivorous fish were depleted (−2.5‰) in δ34S at the groundwater sites, when compared to the reference sites. The δ15N values were similar between groundwater and reference sites, and the pelagic ecosystem plankton and planktivorous and piscivorous fish were similar in both δ13C and δ15N. The authors concluded that the findings indicated benthic metazoan communities near groundwater vents related to benthic primary production from groundwater, but planktivorous and piscivorous communities which are not directly linked with the benthos are not reliant on groundwater nutrients.
Aqueous Geochemistry
Aqueous geochemistry is concerned with the chemical interactions of water (H2O) and rocks and may therefore be an offshoot of aqueous chemistry, groundwater chemistry, hydrochemistry, or water chemistry (Sen, 2015). As biogeochemistry is strongly concerned with chemical cycling between the hydrosphere, lithosphere, biosphere, and atmosphere, this is an extremely important part of the relationship between geochemistry and biogeochemistry. Nordstrom (1997) defines this subject as “the application of chemistry to reactions between rock and natural water” and further argues that this involves the perspectives of physical, analytical, inorganic and organic chemistry, with examples of studied processes including “the dissolution and precipitation of minerals, adsorption and desorption of ions, oxidation-reduction or redox reactions, gas uptake or production, transformations involving organic matter, complexation and chelation, evaporation, ion exchange, and anthropogenic changes” which may be applied to various aspects of the hydrosphere (e.g., rain, riverine, and marine environments) (Nordstrom, 1997).
White (2010: 231) takes the example of springs, which in this context differ from other water sources in that “springs result from a concentration of groundwater flow paths, so that water issues from a single location instead of diffuse flow into surface streams.” The chemistry of spring waters consequently results from the interaction of the groundwater with the aquifer host rock and any possible chemical constituents introduced from surface sources, with little difference between spring water chemistry and groundwater chemistry. This author defines the approach to the study of aqueous chemistry as examining the composition, thermodynamic properties, mineral solubilities, and interactions of the host rock’s minerals, from which the water chemistry can be predicted. Common ions in spring water include the cations (positively charged ions) Ca2+, Mg2+, Na+, and K+, and the anions (negatively charged ions) \( HC{O}_{3^{-}} \), \( {\mathrm{SO}}_4^{2-} \), and Cl−. White (2010: 231) points out that these ions are the main indicators for the classification of spring water. There are three main groups in such classifications, these being bicarbonate waters, where Ca and Mg may be the dominant cations; sulfate waters, where Mg levels may be greater than those of Ca, and there are also alkali ions; and chloride waters, where alkali ions may be the dominant ions. In such classifications, the dominant chemical composition of the spring generally depends on the chemistry of the host aquifer rock from which it emerges (White, 2010: 231).
Important concepts of aqueous geochemistry include chemical equilibrium and mineral saturation. Minerals differ in their reaction to water, with some minerals dissolving in the water, and breaking up into their component ions, while other minerals may react chemically, with the formation of soluble ions and sometimes insoluble residues (White, 2010: 231). Examples are cited of halite (NaCl), gypsum (CaSO4 2H2O), and calcite (CaCO3), for which the author notes there is a “certain equilibrium concentration, defined thermodynamically by the concentration at which the free energies of the species on both sides of the dissolution reaction balance exactly or kinetically where the rate of dissolution is equal to the rate of precipitation.” This ideal equilibrium concentration may not be fully present in the real world, arguably because “the concentrations of dissolved species are often not at equilibrium with the minerals of the host rock. This is particularly true in carbonate rocks, where the nonequilibrium is a useful interpretive tool” (White, 2010: 231). Calculations of mineral solubilities nevertheless may represent a reference for the evaluation of spring water chemistry, as the chemical levels predicted from equilibrium calculations may be compared with water chemical samples, using chemical analysis (White, 2010: 231). Literature relevant to these issues are listed by the author (Hem, 1985; Morel & Hering, 1993; Stumm & Morgan, 1996; Drever, 1997; Langmuir, 1997; Parkhurst & Appelo, 2008).
Aqueous biogeochemistry adds biological themes to aqueous geochemistry, when the elements, compounds and processes that exist in the geochemistry of water intrude into or relate to organisms. One important process is biomineralization, which concerns the precipitation of minerals by living organisms (Hendry et al., 2018). Nature Portfolio (2023) defines biomineralization as the “process by which living organisms produce minerals” which “often lead to the hardening or stiffening of the mineralized materials” and the “formation of silicates in algae and diatoms, carbonates in invertebrates, and calcium phosphates and carbonates in the hard tissues of vertebrates.” Examples of biomineralization include the formation of iron oxide by brown algae, which is affected by environmental conditions, and other processes which may be dependent on the metabolism of the organisms under indirect genetic control and the cellular processes that enable biomineral formation. There is also “biologically controlled biomineralization” which “requires direct genetic control, generates characteristically patterned structures, and involves selective uptake and pre-concentration of mineral ions” (Hendry et al., 2018). Biomineralization may also concern iron oxide, strontium sulfate, calcareous compounds, and silica (SiO2·nH2O), the last being described as the “taxonomically widespread biomineral” and “present in all eukaryotic supergroups” (Hendry et al., 2018). Hendry et al. (2018) also note that “the most extreme degree of silicification is evident in the diatoms, where almost all species have an obligate requirement for silicon to complete cell wall formation and cell division” and “biogeochemically and ecologically, diatoms are believed to be the most important silicifiers in modern marine ecosystems.”
Cosmochemistry
Cosmochemistry has been variously defined, but the consensus is that it studies the chemical composition of the physical constituents of space, including gases, solids and even liquids. Numerous books and research articles have examined topics related to cosmochemistry, such as those of Wasserburg and Hayden (1955), Schmus and Wood (1967), Black and Pepin (1969), Lee et al. (1977), Hoyle and Wickramasinghe (1981), Burke (1986), McKay et al. (1996), Lodders and Fegley Jr. (1998), Railsback (2003), Davis (2004), Lauretta and Killgore (2005) and Lauretta and McSween Jr. (2006). These studies present increasingly more sophisticated analyses of space materials, from the perspectives of chemistry, physics, and astronomy, and document the development of related research methods.
Gounelle (2011: 380) defines cosmochemistry as the study of the formation and evolution of the Solar System and its individual components through the analysis of extraterrestrial samples in the laboratory.” MacPherson and Thiemens (2011: 19130) add that “cosmochemistry is the chemical analysis of extraterrestrial materials. This term generally is taken to mean laboratory analysis, which is the cosmochemistry gold standard because of the ability for repeated analysis under highly controlled conditions using the most advanced instrumentation unhindered by limitations in power, space, or environment.” Further, “recent advances in the state of the art of cosmochemistry…, range from instrumental analysis of meteorites to theoretical–computational and astronomical observations” (MacPherson & Thiemens, 2011: 19130).
McSween and Huss, G. (2010: 1) define cosmochemistry as “the study of the chemical composition of the universe and the processes that produced those compositions” primarily on the immediate solar system, including the compositions of all the bodies in the Solar System, namely the Sun, planets and their satellites, the asteroids and comets, meteorites, and interplanetary dust particles (IDPs). Cosmochemists study the composition, chemistry and formation chronology of these bodies, using laboratory measurements of samples and/or remote-sensing techniques (McSween Jr & Huss, 2010). The science of cosmochemistry has important relationships with other sciences, especially with geochemistry, and astronomy, astrophysics, and geology (McSween Jr & Huss, 2010: 1). This view is supported by Makishima (2017), who notes that there are important links between cosmochemistry and other Astro sciences, including astronomy, astrobiology, astrogeology, and astrophysics.
McSween Jr and Huss (2010) point out that “traditionally, cosmochemistry has been treated as a branch of geochemistry – usually defined as the study of the chemical composition of the Earth,” with geochemistry principally focused on the Earth (hence the prefix “geo”), with generally little coverage of cosmochemistry in geochemistry textbooks. McSween Jr and Huss (2010) further mention that “the line between geochemistry and cosmochemistry has always been somewhat fuzzy. The most prominent technical journal in this discipline, Geochimica et Cosmochimica Acta, has carried both names since its inception in 1950.” Also mentioned is the rising field of planetary geochemistry, which is still has the “geo” prefix, despite the focus being the non-Earth solar bodies. McSween Jr and Huss (2010) argue that for the inclusion of solar bodies in the study of geochemistry “a broader and more appropriate definition of geochemistry might be the study of element and isotope behavior during geologic processes, such as occur on and within the Earth and other planets, moons, and planetesimals. Using this definition, we will include planetary geochemistry as an essential part of our treatment of cosmochemistry.”
Another important issue with cosmochemistry concerns the source of research materials, which may be derived from direct sourcing in space or from falling meteors. Initially, cosmochemistry studies were dependent on the chance discovery of meteorites (from observed falls or chance discovery on the ground). However, in 1969, Japanese explorers in Antarctica led by Masaru Yoshida found meteorites on bare ice, which influenced the formation of meteorite-collecting expeditions to Antarctica, and meteorite-collecting in the southwestern United States, Western Australia, and North Africa. The meteorites so discovered varied, including carbonaceous chondrites (containing carbon-bearing compounds, with primarily organic molecules), but disputes arose as to whether the carbon organic molecules were from space or Earth surface contamination (McSween Jr & Huss, 2010). Currently, smaller meteorites may be recovered when they fall on bare rock, sand, or ice, while larger meteorites may leave craters or other damage evidence on the Earth’s surface.
Research methods in cosmochemistry have also developed. Davis (2014) argues that “cosmochemistry has made tremendous progress since the 1960s, largely because of improvements in analytical technology” with an important boost experienced by the Apollo space program, which acquired physical samples from the moon, which influenced increased investments in analytical laboratories during the 1960s and 1970s. Developments included “advanced analytical instrumentation with isotopic precision (thermal ionization and gas source mass spectrometry), precise microbeam chemical analysis (electron probe microanalysis), and sensitive trace-element analysis (neutron activation analysis)” and the fall of and recovery of the Allende (Chihuahua, Mexico, 1969) and Murchison (Murchison, Australia in 1969) meteorites, which influenced more advanced research on cosmochemistry (Davis, 2014). Koeberl (2007: 1) writes about the geochemistry and cosmochemistry of impact craters and processes, which is argued to be “a rapidly developing research area that encompasses such wide-ranging topics as the simple chemical characterization of the various rock types involved (target rocks, impact breccias, melt rocks, etc.), the identification of extraterrestrial components in impact ejecta, the determination of the impactor (projectile) composition, and the determination of the causes of environmental changes from chemo-lithostratigraphic analyses.”
Due to the lack of evidence of life in space, there are few connections between cosmochemistry and biogeochemistry. However, Callahan et al. (2011:13995) describe possibilities of chemical substances recorded on meteorites, which may indicate extraterrestrial sources for compounds detected on the Earth’s surface. The basis of this argument is that organisms depend on nucleic acids, namely ribonucleic acid (RNA) and deoxyribonucleic acid (DNA), which encode genetic information using pyrimidine and purine nucleobases. It is speculated that carbon rich meteorites may have been a source of organic compounds during the development of life on Earth. The authors note that “so far, the few nucleobases reported in meteorites are biologically common and lacked the structural diversity typical of other indigenous meteoritic organics” (Callahan et al., 2011:13995). Carbonaceous chondrites described rare meteorites, which may contain organic compounds, including compounds important for biology (see also Sephton, 2002; Schmitt-Kopplin et al., 2010). The study by Callahan et al. (2011:13995) investigated nucleobases and nucleobase analogs in formic acid extracts of meteorites, using liquid chromatography–mass spectrometry, and concluded that the purines in meteorites were consistent with some products of ammonium cyanide chemistry, which might be a mechanism for their synthesis in the asteroids. The authors conclude that as “meteorites provide a record of the chemical processes that occurred in the solar system before life began on Earth” the “discovery of new nucleobase analogs in meteorites also expands the prebiotic molecular inventory available for constructing the first genetic molecules” (Callahan et al., 2011:13995).
Trace-Element Geochemistry
Trace elements are an important focus of geochemical research, and are also examined within the context of biogeochemistry, when trace elements occur in living organisms. A trace element is defined as one with a concentration of less than 1000 ppm or 0.1% of a rock’s composition. White (2013: 259) notes that “though trace elements, by definition, constitute only a small fraction of a system of interest, they provide geochemical and geological information out of proportion to their abundance” and “trace element geochemistry has been of enormous use in understanding the evolution of the Earth.” Kennedy (1998: 221) define trace elements (TE) as those elements that occur in a mineral in very small amounts, to the extent that they are excluded from the mineral’s chemical formula, and they may also be defined as “elements that occur at less than 0.1 wt% in the Earth’s crust; however, this is not true of all trace elements” (see Brownlow, 1979). Kennedy (1998: 221) argues that “early interest in trace element geochemistry focused on factors contributing to the occurrence of trace elements in the crystal lattice of various minerals” citing Goldschmidt (1937). Rubidium (Rb) is cited as an example of a common trace element in orthoclase, a feldspar mineral (KAlSi3O8). Other common trace elements (including rare earth elements (REEs), which may be used in research) are cited, as barium (Ba), Strontium (Sr), zirconium (Zr), chromium (Cr), vanadium (V), rubidium (Rb), zinc (Zn), nickel (Ni), copper (Cu), cobalt (Co), yttrium (Y), niobium (Nb), lithium (Li), scandium (Sc), gallium (Ga), lead (Pb), thorium (Th), hafnium (Hf), uranium (U), lanthanum (La), cerium (Ce), neodymium (Nd), praseodymium (Pr), gadolinium (Gd), dysprosium (Dy), erbium (Er), ytterbium (Yb), europium (Eu), holmium (Ho), terbium (Tb), thulium (Tm), and lutetium (Lu) (Kennedy, 1998; see also Wedepohl, 1995).
Recent research on trace elements has underscored their importance for geochemical analyses, but definitions analyses may be problematic. For example, White (2013: 260) writes about the difficulty of definition, and notes that for igneous, sedimentary, and metamorphic rocks, a possible definition is that trace elements are those elements that “are not stoichiometric constituents of phases in the system of interest” which is a “a bit fuzzy” because “a trace element in one system is not one in another.” The example is cited of potassium, which may be a trace element in mid-ocean ridge basalts but is not a trace element in granites. White (2013: 260) further notes that for most silicate rocks, oxygen (O), silicon (Si), aluminum (Al), sodium (Na), magnesium (Mg), calcium (Ca), and iron (Fe) are “major elements,” while hydrogen (H), carbon (C), sulfur (S), potassium (K), phosphorus (P), titanium (Ti), chromium (Cr), and manganese (Mn) “are sometimes ‘major elements’ in the sense that they can be stoichiometric constituents of phases. These are often referred to as ‘minor elements’” and “all the remaining elements are always trace elements” (White, 2013: 260).
For identification and assessment of trace elements in geological formations, increasingly sophisticated methods may be employed. For example, Torró et al., 2022 presents a study of the trace-element occurrence in sphalerite (a sulfide mineral with the formula (Zn,Fe)S) and chalcopyrite (a copper iron sulfide mineral, which is the commonest copper ore mineral, with the formula CuFeS2) from four volcanogenic massive sulfide (VMS) deposits. VMS deposits are metal sulfide ore deposits, mostly copper-zinc formations linked to volcanic hydrothermal occurrences in submarine environments. Torró et al. (2022) defines VMS deposits as features which “are exhalative and/or replacive accumulations of sulfide minerals formed at or near the seafloor where uprising hydrothermal fluids driven and in part generated by synchronous magmatism are entrained with cold seawater or porewaters.” The research method was laser ablation and inductively coupled plasma mass spectrometry.
The general findings of this study by Torró et al. (2022) were that for the studied sulfide deposits, from the surface downward, for sphalerite there was a progressive increase in the trace elements In, Cu, Mn, and Se, and a decline in Ge (Germanium). It is argued that this distribution pattern correlates with increasing crystallization temperatures and/or volatile magmatic influx near the lower portion of the mineralization (Torró et al., 2022). For chalcopyrite, the distribution of the trace elements was “rather uneven except for a sustained enrichment in Se toward the basal portion of the sulfide body” (Torró et al., 2022). The author argues that the preservation of such trends despite extensive recrystallization during thermal metamorphism in parts of the study area massive sulfide mineralization suggests that it is a closed metamorphic system and that element interdiffusion was largely local (Torró et al., 2022).
The findings also indicated that in sphalerite, Fe, Mn, Cd, Hg, Ag, Sb, Se, In, Ge, and Ga are lattice-bound, but Sn, Tl, Bi, and Pb are at least partly mineral micro-inclusions. Here lattice-bound refers to the regular arrangement of atoms/molecules within a crystal. Frye (1981) defines a lattice as “a three-dimensional array of points, each of which has an identical surrounding of neighboring points… Lattices extending infinitely in three dimensions are used in crystallography to describe the structural patterns found in all crystalline materials.” Stoll et al. (2021) define micro-inclusions as small particles, for example micrometer-sized (μm, one millionth of a meter), that occur in a substance. Torró et al. (2022) also found variations in the contents trace elements are observed in sphalerite grains from deposits. There were also substitutions between the elements, with a strong negative correlation between iron and zinc.
For chalcopyrite (a copper–iron sulfide mineral with chemical formula CuFeS2), Zn, Ag, Sn, Cd, Se, In, Ga, and Ge were found to be lattice-bound, and Mo, Au, Tl, Sb, Pb, and Bi may be micro-inclusions (Torró et al., 2022). Compared with sphalerite, chalcopyrite differed in having lower amounts of In and Ga, and higher concentrations of Ge. Zinc had the highest concentrations of the other trace elements in the examined chalcopyrite, with lower of amounts of silver, selenium, tin, and cadmium. The authors noted that “general positive correlation trends between Zn, Cd, In, Ge, and Ga in chalcopyrite suggest varied coupled substitution mechanisms of Fe and Cu with fluctuating valences due to covalent bonding” (Torró et al., 2022).
Another study using trace elements for geological assessments is that of Dudás et al. (2021), which examined trace and major and trace-element data for the Permian-Triassic boundary (PTB) at Meishan in China, using strata samples covering the last 75,000 years of the Permian (a 47 million year geologic period and stratigraphic system from 298.9 million BP to 251.9 million years BP) and the first 335,000 years of the Triassic Period (another 50.6 million year geological period, lasting from the end of the Permian period, 251.902 million years BP, to 201.36 million years BP) (Dudás et al., 2021).This covers the Permian–Triassic Extinction Event (about 251.9 million years BP), when the majority of the Earth’s living species were rendered extinct, possibly due to the environmental impacts of rising temperatures. The study ranks layer beds as from before the extinction event or interval just before the PTB (beds 22 to 24e), the extinction event beginning (bed 24f) to after the extinction event and PTB (beds 28 to 34) (Dudás et al., 2021).
The main rock change documented was from carbonate dominated strata in the Permian to mudstone and marl in the Triassic, which the authors argue indicated “an increase of siliciclastic input and MgO in and above the extinction interval… and silica diagenesis in carbonates below the extinction horizon” with evidence that “siliciclastic input dominates trace element distributions in the Triassic” (Dudás et al., 2021). During the extinction interval, there were increases in As, Mo, U and transition metals, and enrichment of V, Cr, Co, Ni, Cu, Zn, Pb, and Ba. Below the strata representing the extinction interval there was enrichment of V, Cr, Co, Ni, Cu, Zn, Pb, and Ba within a layer diagenetic silicification. The authors conclude that “trace elements thus reflect siliciclastic input, diagenetic redistribution, and responses to redox conditions” and such trace element patterns “suggest either a change in provenance of the detrital component, or a change in the proportion of mechanical to chemical weathering that is coincident with the beginning of the extinction” period (Dudás et al., 2021).
Considering the behavior of the trace elements in the layer samples, Dudás et al. (2021) report anomalous behavior from Ba, Zr, and Zn, with little variation from Ba, despite variations in biological activity and redox conditions. Zinc concentrations decline in the extinction horizon, possibly due to changes in phytoplankton productivity. Zr mobility is suggested, as the enrichment factor for Zr is found to be variable in the carbonates below the extinction layers. Dudás et al. (2021) conclude that a “transition from carbonate-dominated sedimentation in beds 24e and below” (below the extinction levels), “to dolomite or ankerite in beds 24f and above” (the extinction levels and above), strong correlations between trace elements with Al2O3 (Sc, Ga, Rb, Ba, Th) within the siliciclastic fraction of the Meishan rocks, and “trace element data suggest that there was a change in either the provenance of the detrital fraction, or in the proportion of mechanical to chemical weathering coincidentally with the extinction beginning at the top bed 24e,” which may not be linked to volcanism of the period (Dudás et al., 2021). Note that detrital refers to rock particles derived from the physical breakdown of pre-existing rocks, due to weathering and erosion (see Marshak, 2009).
Trace-element geochemistry has also been applied to archaeological studies, contributing to the field of geoarchaeology, a research field using the methods of the sciences of geomorphology, hydrology, sedimentology and pedology, the research methods of remote sensing, geophysics, geochemistry, and chemical, radiometric, magnetic, biological methods, including geochronology, and exploration geophysics (Elias, 2021). Geoarchaeology’s research points include site date and formation, ancient environmental characteristics, and human decisions about site location. Although older geoarchaeological research was mostly on “climate reconstruction, paleodiet analysis, or artifact conservation,” recent studies have acknowledged the importance of archeological site sediments, and more advanced dating and remote sensing techniques for site identification and surveying archeological sites (Elias, 2021).
Elias (2021) documents the work of Jenkins (1989) on trace-element geochemical applications to archaeology, which is described as “a foundational paper on this topic,” which highlighted the “great potential use of trace element geochemistry in archeological research” and also “highlighted the problem of being able to differentiate between anthropogenic and naturally occurring trace element anomalies, given the inherent geochemical diversity of sediments as well as the superimposed chemical alterations resulting from subsequent pedogenic processes in archeological sites” (Elias, 2021). Trace-element analysis has been useful in the chemical fingerprinting of flints/cherts and obsidian, and the origins of artifacts, including glazed ceramic ware, and charcoal analysis. The study by Jenkins (1989) illustrated the dating of charcoal using trace-element analyses. Elias (2021) notes that the results of this study points to the fact that charcoal accumulates certain trace elements significantly and selectively, and this concentration provides for greater sensitivity than bulk analysis including the nearby soil.
Jenkins (1989: 57) noted that people “can leave a geochemical imprint on an archaeological site in several ways” based on “a selective enrichment of elements in his body tissues which, upon death and burial, may lead to detectable anomalies,” with phosphorus being the most obvious, and other possibilities being metals such as tin (Sn), silver (Ag), gold (Au), copper (Cu), lead (Pb) and zinc (Zn) which may have been used by ancient cultures, and may be measured in sites. Jenkins (1989, 57) also states that charcoal is among the commonest finds during excavations, and it can adsorb and concentrate metals from percolating solutions progressively from its burial time. Careful analysis may therefore derive an important historical record. Therefore, “providing care is taken to interpret results in their particular geochemical and pedochemical context, trace element analysis may thus offer a useful insight into the history of man’s activities in an archaeological site” (Jenkins, 1989, 57).
Elias (2021) also describes the development of trace-element analyses of charcoal, including the recently designed Fourier transform infrared spectroscopy (FTIR), which can analyze samples more than 100 times smaller than those of the techniques described by Jenkins (1989). Elias (2021) also argues that most trace-element studies have focused on copper (Cu), iron (Fe), manganese (Mn), lead (Pb), and zinc (Zn), as some of these metals can remain stable in soils for long periods, because their ions are adsorbed and precipitated onto clay surfaces, and can form stable compounds, including insoluble oxides, sulfates, or carbonates. Examples include the study of metallic elements (e.g., hematite Fe2O3 and cinnabar HgS) in pre-Columbian Mayan sites for pigments and paints, which may indicate locations for pigment processing, and craft workshops. Study methods include trace metal extraction and inductively coupled plasma mass spectrometry (ICP/MS) or atomic emission spectroscopy (ICP-AES) analyses of soil and floor samples at the Guatemalan archeological sites in Cancuén, Piedras Negras, and Aguateca (Elias, 2021; see also Terry, 2017).
Trace-element geochemistry may also be linked to cosmochemistry. For example, a study by Ollila et al. (2013: 255) examined the findings of the ChemCam instrument package on the Mars Rover, Curiosity, which “provides new capabilities to probe the abundances of certain trace elements in the rocks and soils on Mars using the laser-induced breakdown spectroscopy technique.” The study focused on detecting and quantifying lithium (Li), barium (Ba), rubidium (Rb) and strontium (Sr). The findings indicated that Li was detected for the first time on Mars, with low concentrations (<15 ppm). There were local areas with higher concentrations of Li, up to ~60 ppm. Bathurst_Inlet (a rock on the surface of Aeolis Palus, between Peace Vallis and Aeolis Mons in Gale crater) is documented as a fine-grained bedrock with some evidence of a decrease in Li and other alkalis with depth, possibly indicating past low-level aqueous alteration drawing alkalis to the surface. Ba (with a concentration of ~1000 ppm) was found in the Akaitcho sand ripple and may correlate with Si, Al, Na, and K, which the authors argue may indicate a “feldspathic composition” Ollila et al. (2013: 255). Rb and Sr were found in concentrations of >100 ppm and >1000 ppm, respectively. The authors note that these areas also tended to have high concentrations of Si and alkali, these combinations being consistent with a feldspar composition. Ollila et al. (2013: 255) conclude that “together, these trace-element observations provide possible evidence of magma differentiation and aqueous alteration.”
Trace-element biogeochemistry is derived from the issues, methods, and findings of trace-element geochemistry, which may examine trace elements in inanimate matter, and also in organisms and living tissue (Winkel & Sunderland, 2022). The International Conference on the Biogeochemistry of Trace Elements (ICOBTE) (2023) writes that “trace elements (TEs) play an important role in the biogeochemical cycle of the whole environment. In various ecosystems TEs are enriched, while the sources can be either natural (geogenic) or anthropogenic (human-induced). Natural processes releasing TEs into the biosphere include mineral weathering, volcano eruptions, and natural erosion.” In addition to the natural issues of the solid earth mentioned earlier, trace-element biogeochemistry may include chemical artificially introduced and/or increased by human activities. The relevant activities and inputs may include the externalities of industrial production, including mining, electroplating, smelting, wastewater, aerosol deposition, sewage, and fertilizers (ICOBTE, 2023).
Winkel and Sunderland (2022) elaborate on the themed issue of the journal Environmental Science: Processes & Impacts by the Royal Society of Chemistry, which aims to document advances in trace-element biogeochemistry research, where trace elements are defined as including metals, metalloids, and nonmetals. They note that this examines “a wide range of biogeochemical processes and environmental impacts of toxic and essential TEs,” with a strong focus on research across phase- and compartment interfaces and coupled biogeochemical cycles between TEs and/or major elements.” Key issues currently emerging for trace-element biogeochemistry include changes in materials science, electronics and computing, and “global mining and use of TEs has increased massively over the last decades and there has been strong global demand for the production and use of rare earth elements and platinum-group elements” (Winkel & Sunderland, 2022). The authors therefore argue that new issues include elements important for battery technology, electric vehicles, renewable energy, a low-carbon economy, and the need for less environmental contamination in biota, air, water, and human environments. The authors also mention the three main topics of the themed issue as, firstly, the “biotic processes involved in cycling of toxic and essential trace elements in soils and marine and brackish waters,” secondly the “sediments and soils and their interfaces to aquatic systems (e.g., groundwater, river water, and the hyporheic zone and lake systems), and thirdly, the “TE distributions at large geographical scales in soils, sediments and porewater, lakes, oceans and in the atmosphere” (Winkel & Sunderland, 2022).
Igneous and Metamorphic Rock Geochemistry
The geochemistry of both igneous and metamorphic rocks largely concerns the elements and compounds that occur in the processes, solid materials and sinks within which these chemicals exist. As igneous rocks are created by magmatism, and metamorphic rocks are usually pre-existing igneous, sedimentary, and metamorphic rocks that have undergone more heating or pressure, there may be differences in the geochemistry of igneous and metamorphic rocks, and the possible links with biogeochemistry (Campbell, 2020). This section looks at the geochemistry of igneous and metamorphic rocks and takes silicon as an example of an element that cycles though the igneous and metamorphic geochemical cycles, and is vital to global biogeochemical cycles, hence illustrating the links between geochemistry and biogeochemistry, the latter when organisms are involved in the cycling.
Igneous rock geochemistry concerns the chemistry of igneous rocks, which are rocks that result from volcanic activity (Campbell, 2020). Kudo and Jahns (2022) define igneous rocks as “any of various crystalline or glassy rocks formed by the cooling and solidification of molten earth material… formed from the solidification of magma, which is a hot (600 to 1,300 °C, or 1,100 to 2,400 °F) molten or partially molten rock material.” Hence, the immense heating of volcanic activity comprises an essential aspect of the development of igneous rock. Mather (2013) states that in “igneous geochemistry… processes generally occur at much higher temperatures and pressures” than in other fields such as low temperature, earth surface geochemistry. Kudo and Jahns (2022) argue that most igneous are composed of silicate minerals (compounds including silicon (Si) and oxygen (O), and in some cases carbonates). The example is cited of a sodium carbonate lava (Na2CO3), which included 0.05 wt% of silica (silicon dioxide, SiO2) from the Ol Doinyo Lengai volcano in Tanzania. Generally, the low levels of silica and in soda (Na2O) and potash (K2O) correlate with increased occurrences of magnesium oxide (MgO) and iron oxides (FeO, Fe2O3, and Fe3O4). High levels of silica also correlate with high levels of calcium oxide (CaO) and alumina (Al2O3).
Kudo and Jahns (2022) also write that as silica relates to several other chemical compounds in rocks, the silica content is the feature that is often used to classify igneous rocks. These classifications include “silicic or felsic, rocks having more than 66 percent silica; intermediate, rocks with 55 to 66 percent silica; and subsilicic, rocks containing less than 55 percent silica. The latter may be further divided into two groups: mafic, rocks with 45 to 55 percent silica and ultramafic, those containing less than 45 percent” (Kudo & Jahns, 2022). As the subsilicic rocks are enriched in iron (Fe) and magnesium (Mg), they may be called femic, a term derived from ferrous iron and magnesium. The silicic rocks are called sialic (a combination of silica and aluminum) or salic (also from silica and aluminum). Kudo and Jahns (2022) write that the terms mafic (from magnesium and ferrous iron) and felsic (feldspar and silica) may be used interchangeably with femic and sialic.
Silicate minerals may form from the inclusion of silica from the cooling and crystallization of magma, and the combination of silica with other cationic oxides. Examples include the combination of SiO2 and MgO to form MgSiO3 (magnesium-rich pyroxene, enstatite), the combination of two moles of SiO2 with one mole each of CaO and Al2O3 to create CaAl2Si2O8 (calcium-rich plagioclase, anorthite). Note that pyroxenes are the commonest group of rock-forming ferromagnesian silicates. Kudo and Jahns (2022) point out that there are cases where there are insufficient silica levels in the magma to create pyroxene; hence, compounds requiring less silica may be created (in subsilicic rocks), in addition to pyroxene, such as Mg2SiO4, magnesium-olivine (forsterite), and Mg2SiO4, in addition to pyroxene. Such subsilicic rocks contain silicate minerals such as magnesium-olivine, sodium-nepheline (NaAlSiO4), leucite (KAlSi2O6), which may partially substitute for enstatite, albite (NaAlSi3O8), and orthoclase feldspar (KAlSi3O8) which require more silica, and there may be no quartz. The first three minerals are termed undersaturated in silica and are found in undersaturated subsilicic rocks. There are also cases where there is excess silica in the magma; hence, the excess silica after the formation silicate minerals (which were formed though combination of silica and oxides) may form into quartz (SiO2) or related minerals, and magnesium-pyroxenes (see Simmons, 2014 for a discussion of pyroxenes). Where rocks have excess silica, the rocks are termed silicic and supersaturated rocks, containing quartz and magnesium-pyroxene, considered saturated minerals (Kudo & Jahns, 2022).
Due to this variation into excess or lower silica content, igneous rocks can generally be divided into two groups. These are the felsic (derived from feldspar and silica) and mafic (derived from magnesium and ferrous iron). Feldspars usually contain sodium, calcium, potassium, orbarium, with the formulas KAlSi3O8 – NaAlSi3O8 – CaAl2Si2O8 (based on these formulae, Orthoclase or Orthoclase feldspar, Plagioclase or Plagioclase feldspar, Anorthite, respectively). Ferrous iron refers to Iron (II) (Fe2+) (contrasted with iron (III) salts, or Fe3+) (Weiss et al., 2003; Anderson & Anderson, 2010). Felsic minerals include quartz, tridymite, cristobalite, feldspars (plagioclase and alkali feldspar), feldspathoids (nepheline and leucite), muscovite, and corundum. They lack iron and magnesium, hence are usually light colored or leucocratic. Mafic minerals include olivine, pyroxenes, amphiboles, and biotites, which are dark colored or melanocratic. Due to this contrast in color, felsic rocks are usually classified as having a color index of less than 50, and mafic rocks with a color index over 50, and ultramafic rocks with a color index above 90. These numbers refer only to the rock mineralogical content but may not represent chemical terms. Kudo and Jahns (2022) cite the examples of a mineral plagioclase rock, which would be felsic, but chemically would be a subsilicic mafic rock; and a pyroxene igneous rock with a silica content of around 50%, which would be a mineralogically ultramafic rock, but a chemically igneous rock. During the magmatic formation of these rocks, supersaturated minerals (such as quartz) are separated from undersaturated minerals (such as feldspathoids like leucite and nepheline) or magnesium-rich olivine. Other minerals termed accessory minerals are also formed and exist in igneous rocks in minor amounts include monazite, allanite, apatite, garnets, ilmenite, magnetite, titanite, spinel, and zircon (Kudo & Jahns, 2022).
The geochemistry of igneous rocks is relevant to environmental change. For example, Bataille et al. (2017) argue that the chemical weathering of silicates transfers elements from the continental crust to ocean waters and hence affects some biogeochemical cycles. Chemical weathering of silicates transfers calcium (Ca) and magnesium (Mg) to ocean waters and regulates atmospheric carbon dioxide levels and temperature at the surface by impacting on the rate of marine carbonate precipitation. Bataille et al. (2017) further remark that there is insufficient knowledge of the impact of the composition of igneous rocks on the content of chemical weathering products, and the consequent impacts on ocean waters and atmospheric change. The authors use the strontium isotope ratio in seawater [(87Sr/86Sr) seawater] as a proxy for chemical weathering and test the sensitivity of (87Sr/86Sr) seawater changes to the strontium isotopic composition (87Sr/86Sr) in igneous rocks which were generated over time.
The use of the Strontium (87Sr/86Sr) isotope is important as a versatile application. For example, Salifu et al. (2018: 42) state that “the 87Sr/86Sr ratio is not fractionated by these processes, which refer to the fact that “interpretation of geochemical data based primarily on elemental concentrations often leads to ambiguous results due to multiple potential sources including mineral weathering, atmospheric input, biological cycling, mineral precipitation and exchange processes.” Salifu et al. (2018: 43) also argue that “the Strontium (87Sr/86Sr) isotope ratio is an important and powerful investigative tool which has been extensively and successfully utilized in petrogenesis, mineral weathering and acidity in soils, discriminating between atmospherically-derived Sr and those of mineral weathering inputs as well as differentiating between carbonate and silicate weathering sources” (the authors also cite Graustein & Armstrong, 1983; Åberg et al., 1989; Jacks et al., 1989; Miller et al., 1993; Nakai et al., 1993; Bullen et al., 1996; Stewart et al., 2001; Jacobson et al., 2002; Chamberlain et al., 2005; Tipper et al., 2006; Shand et al., 2007; Jin et al., 2011; Subίas et al., 2015; Kozlik et al., 2016).
Bataille et al. (2017) found that the 87Sr/86Sr ratio in igneous rocks was correlated to the epsilon hafnium (εHf) of their hosted zircon grains and used the detrital zircon record to reconstruct the evolution of the 87Sr/86Sr ratio in the zircon-bearing igneous rocks. The findings indicated that the reconstructed 87Sr/86Sr variations in igneous rocks were correlated with the (87Sr/86Sr) seawater variations over the last 1000 million years, which suggested a direct controlling link of the isotopic composition of silicic magmatism on (87Sr/86Sr) seawater variations. The study therefore showed that the correlation between 87Sr/86Sr ratio in igneous rocks and seawater decreases over long periods, which may indicate changes in the chemical weathering rates which are associated with paleogeographic, climatic, or tectonic events, and there is the strong argument that “for most of the last 1000 million years, the (87Sr/86Sr)seawater variations are responding to changes in the isotopic composition of silicic magmatism rather than to changes in the global chemical weathering rate” and that “the (87Sr/86Sr)seawater variations are of limited utility to reconstruct changes in the global chemical weathering rate in deep times” (Bataille et al., 2017). The suggestion is that when the global isotopic composition of silicic igneous rocks increases, the fast cycling and later rock weathering contributes to an increase in the (87Sr/86Sr)seawater ratio within a relatively short time period (<20 My)” (Bataille et al., 2017).
Bataille et al. (2017) conclude that their analysis represents “a new method to reconstruct the evolution of the strontium isotopic composition of silicic igneous rocks through time” as the (87Sr/86Sr)i-zig variations exerted dominant control of (87Sr/86Sr)seawater evolution over the last 1000 My, and (87Sr/86Sr)seawater variations were interpreted as tracking the relative proportion of evolved versus less evolved magmas in the continental crust, rather than changes in the chemical weathering rate of radiogenic continental surfaces (Bataille et al., 2017). The changes in the global isotopic composition of silicic magmas may be related to continental mountain-building events, and subduction phases. It is concluded that other (87Sr/86Sr)seawater variations could result from variations in the relative Sr flux from isotopically distinct sources which may be linked to “specific paleogeographic configurations, mountain-building events, emplacement of LIPs, or climate variations” and “the (87Sr/86Sr)seawater variations are of limited utility to reconstruct the long-term chemical weathering rate” (Bataille et al., 2017).
Metamorphic (from the root word metamorphosis, meaning change) rock geochemistry concerns the chemical composition and change, mostly of the metamorphic process where pre-existing igneous, sedimentary, or metamorphic rocks undergo extreme heating (without reaching the melting stage, which would convert the rock to igneous rock), pressure or other change and create new, metamorphic rocks (United States Geological Survey, 2022). The United States Geological Survey (2022) states that “the process of metamorphism does not melt the rocks, but instead transforms them into denser, more compact rocks. New minerals are created either by rearrangement of mineral components or by reactions with fluids that enter the rocks. Pressure or temperature can even change previously metamorphosed rocks into new types… metamorphic rocks do not get hot enough to melt, or they would become igneous rocks.” Examples of metamorphic rocks include gneiss (from the igneous rock granite), slate (from the sedimentary rock shale), marble (from the sedimentary rock limestone), greenschist, amphibolite, or eclogite (from the igneous rock basalt), and schist (from mudstone/shale, or some igneous rocks, the result of greater heating and pressure, and with coarser, larger crystals than slate – i.e., a greater degree of schistosity) (University of Auckland, 2005).
The United States Geological Survey (2022) distinguishes two types of metamorphic rocks. Foliated metamorphic rocks, such as granite gneiss and biotite schist, which are banded or foliated, where foliated refers to the parallel arrangement of some mineral grains, giving the rock a striped appearance, as the pressure forced the elongate minerals in the rock into aligned, platy or sheet-like structure in the direction of the pressure that reflects the direction that pressure was applied. Nonfoliated metamorphic rocks have nonplaty or nonsheet-like structure and may result from the metamorphism of rocks such as limestone, composed of minerals that will never be elongated regardless of pressure, or contact metamorphism, which results from heating (e.g., a hot, igneous intrusion into pre-existing rock, without pressure (United States Geological Survey (2022).
Research on metamorphic rock geochemistry contributes to knowledge on a variety of topics on geodynamics. For example, Tang et al. (2007: 48) present of geochemical studies of metamorphism for the understanding of the geodynamics of continental movement. Using the case study of the Jiaobei terrane northeast of the Dabie-Sulu orogenic belt in China, the authors argue that the “tectonic affinity of tectono-lithological units close to ultrahigh-pressure metamorphic belt is a key issue for understanding the geodynamics of continental collision” (Tang et al., 2007: 48). The methodology was based on the use of data from LA-ICPMS zircon U–Pb dating, whole-rock elements and Nd–Sr isotopes, and mineral O isotopes for the study area. The two Triassic collided continents were the North and the South China Blocks. Protolith (the original, unmetamorphosed rock from which the metamorphic rock is formed) ages for TTG gneiss, amphibolite and mafic granulite were ∼2.7, ∼2.5 and ∼2.4 Ga (Ga (for giga annum or billions of years), respectively, and regional metamorphism occurred at ∼1.76 Ga (Tang et al., 2007: 48). Here TTG refers to tonalite–trondhjemite–granodiorite (TTG) rocks, which are intrusive rocks with granitic composition (quartz and feldspar), with only a little potassium feldspar (see Winter, 2013).
The findings indicated that the protolith of the TTG gneiss was generated by the partial melting of mantle-derived rocks at the bottom of the crust, the protolith of the amphibolite could be a product of arc-like magmatism, and the protolith of the mafic granulite was derived from a depleted mantle source. The findings indicated that both protoliths were contaminated by supracrustal materials, the protoliths of paragneiss and schist were largely derived from supracrustal sources, but protolith of amphibolite was of mantle-derived signature (Tang et al., 2007: 48). Further findings were that the Jiaobei metamorphic rocks preserved their original mantle-like O isotope compositions, unlike the ultra-high-pressure metamorphism (UHP) metaigneous rocks in the Dabie-Sulu orogenic belt that did not and showed 18O-depletion (18O isotopes). Additionally, the characteristics of the geological events recorded in the metamorphic rocks from the Jiaobei terrane are more like those from the North China Block than the South China Block. The authors therefore argue that “the Jiaobei terrane is concluded to have tectonic affinity to the former but behave like a micro-continent during the Triassic continental collision” and that the “∼1.76 Ga regional metamorphism in the Jiaobei terrane is likely related to reworking of the arc-continent collisional orogen in the periphery of the North China Block rather than the ∼1.85 Ga collision event between the eastern and western North China Blocks” (Tang et al., 2007: 48). It is also argued that the findings support the established assumption that the suture boundary between the North and South China Blocks in the Sulu orogen is along the Wulian-Yantai fault and that tectonic mingling along the Wulian-Yantai fault maybe linked to subduction erosion during the continental collision (Tang et al., 2007: 48).
Links between biogeochemistry, and igneous and metamorphic geochemistry may be based on specific chemical elements that are relevant to metamorphic rocks and organisms. For example, Aston (1983) describes silicon as occupying a dominant place in biogeochemistry, as it is an essential nutrient and found in animal skeletons. For example, Tréguer et al. (2021: 1269) point out that “the element silicon (Si) is required for the growth of silicified organisms in marine environments, such as diatoms. These organisms consume vast amounts of Si together with N, P, and C, connecting the biogeochemical cycles of these elements. Thus, understanding the Si cycle in the ocean is critical for understanding wider issues such as carbon sequestration by the ocean’s biological pump.” Silicon “often combines with oxygen or other elements to form silicates which are regarded as the largest class of rock-forming materials” (Geetha Thanuja et al., 2022). Silica is added to soil and taken up by plants after weathering from metamorphic rocks, and “weathering is a complex function of rainfall, runoff, lithology, temperature, topography, and vegetation” (Conley, 2002: 68).
Another study is that of Sutton et al. (2018) which acknowledges the crucial role of silicon in the regulation of primary productivity and carbon cycling in the oceans and continents. The study acknowledges the beginnings of the global Si cycle with the chemical weathering of silicate minerals, which may be transformed and redistributed into waterbodies, soils, and organisms, and into mineral form again. Through these “transformations, Si interacts with numerous other major (e.g., C, N) and minor (e.g., Al, Ge, Zn) elements and, in turn, influences their biogeochemical cycles” (Sutton et al., 2018). The study focuses on the analytical methods that are used to study the sources, sinks, and fluxes of the silicon cycle though the Earth’s spheres, these including the elemental and stable isotope ratio data for silicon, zinc, germanium, and other elements (Sutton et al., 2018). The authors note an emphasis on the geochemistry (e.g., Al/Si, Ge/Si, Zn/Si, δ13C, δ15N, δ18O, δ30Si) of dissolved and biogenic Si, present case studies, such as the Silicic Acid Leakage Hypothesis, and investigate the issues associated with the development of these environmental proxies for the global silicon cycle (Sutton et al., 2018). Silicon is a particularly relevant element for research methods that transcend geochemistry and biogeochemistry, as its sources, sinks, and processes are widely distributed in the terrestrial, freshwater, and marine systems, and “exists in major pools in dissolved and solid forms in all reservoirs: extra-terrestrial, continental (e.g., soil, vegetation, hydrothermal), freshwater (e.g., rivers, lakes, groundwater, organisms, sediment), atmospheric (e.g., aerosols), and oceanic (e.g., water column, organisms, sediment and pore-waters, oceanic crust, hydrothermal)” (Sutton et al., 2018).
Sutton et al. (2018) argue that “the chemical weathering of silicate minerals and the eventual cycling of weathered products (clays, dissolved Si) provide the starting point of Si biogeochemistry and its interaction with other elemental cycles such as carbon. Silicate weathering represents an important sink of atmospheric CO2 over geological time scales.” The main transformation processes are the weathering of silicon minerals from rocks, the formation of secondary minerals and dissolved silicon, and the uptake of dissolved Si by organisms, which results in the biomineralization of biogenic silicon, and the remineralization of silicon.” The organisms that use dissolved silicon include diatoms, silicoflagellates, radiolarians and sponges, and in some cases plants, which may create biogenic silica (BSiO2) (Sutton et al., 2018). Moriceau et al. (2009: 1381) describe biogenic silica is a key factor in biogeochemistry, with a study based on the role of biogenic silica in the degradation of diatom organic carbon, and the role of organic compounds in biogenic silica dissolution. Sutton et al. (2018) conclude that “the bio-geochemical analyses used to study the global biogeochemical cycling of Si (e.g., stable isotopes and associated trace elements), are emerging as useful tools to examine the influence that silicifying organisms have on the different reservoirs of Si (e.g., atmospheric, terrestrial, freshwater, and marine).” These analyses have been conducted by several tools, including low temperature bio-geochemical tools, which have garnered information on silicon cycling and environmental interactions, including the role of silicifying organisms in other (e.g., nitrogen and carbon) biogeochemical cycles (Sutton et al., 2018).
Photogeochemistry
Photogeochemistry refers to the impact of sunlight on the chemistry of the Earth. Doane (2017) defines photogeochemistry as the “photochemistry of Earth-abundant minerals in shaping biogeochemistry, and this can be extended to the entire interface between photochemistry and geochemistry to include any chemical reaction induced by sunlight among naturally occurring substances” (see also Schrauzer et al., 1983; Kim et al., 2011; Falkowski, 2015). Doane (2017) also notes that “photogeochemistry describes photochemical reactions on Earth that are not facilitated by living organisms. The reactions that comprise photosynthesis in plants and other organisms, for example, are not included, since the physiochemical context for these reactions is installed by the organism and must be maintained in order for the reactions to continue (the photoreactions cease if the organism dies).” A qualification is that “if a certain substance is produced by an organism, and the organism dies but the substance remains (e.g., plant residue or biogenic mineral precipitates), photoreactions involving this substance still contribute to photogeochemistry” (Doane, 2017). Photogeochemistry therefore represents a combined relationship between photochemistry and surface geochemistry. Doane (2017) argues along this line, in that “by overlaying photochemistry and surface geochemistry, complementary approaches can be adopted to identify natural photochemical reactions and discern their significance in the environment… principles of photochemistry can be readily merged with geochemistry” (Doane, 2017).
Regarding the position of photogeochemistry in Earth scientific research, Kim et al. (2011: 10073) argue that “photochemical reactions of minerals are underappreciated processes that can make or break chemical bonds” and “that the photochemistry of Earth-abundant minerals with wide band gaps would have potentially played a critical role in shaping the biogeochemical evolution of early Earth.” Their case study of the photooxidation of siderite (FeCO3) by UV radiation to produce hydrogen gas and iron oxides via a two-photon reaction, suggests that the photooxidation of siderite could be a source of molecular hydrogen for the earlier half of Earth’s history, which could have resulted in the formation of iron oxides during anoxic conditions, from the Archean to possibly the early phases of the Great Oxidation Event, which could have provided a process for the oxidation of the atmosphere through hydrogen losses to space, while providing a reductant for microbial metabolism (Kim et al., 2011: 10073).
Photogeochemistry is important for biogeochemistry, as some research indicates close linkages (Kim et al., 2011; Doane, 2017). Doane (2017) argued that photogeochemistry may parallel biogeochemistry, with some previous findings based on biological research, in efforts to find “analogous photochemical reactions”; some studies finding analogous, photochemical counterparts to biochemical reactions included in the photochemical disproportionation of acetic acid, which is similar to acetoclastic methanogenesis. The latter is a form of methane production, largely through the action of two methanogenic microbial genera (Methanosaeta and Methanosarcina) (Kraeutler & Bard, 1978; Miyoshi et al., 1991; Kurade et al., 2019). There are also studies of light-induced depletion of O2 through catalytic cycles including organic matter and iron, which is similar to microorganism consumption of O2 (Miles & Brezonik, 1981; Doane, 2017).
Environmental or Low-Temperature Geochemistry
Environmental or low-temperature geochemistry is concerned with the chemical processes occurring in the earth’s surficial environments at natural surficial temperatures, and as such may be closely connected to biogeochemistry (Forbes, 2018). White (2017) even defines biogeochemistry as “a subfield of low-temperature geochemistry that focuses on the interaction between life and its environment.” Mather (2013) notes that “environmental geochemistry refers to the chemical makeup of rocks, soil, water, air, and organisms near the surface of the earth and the chemical reactions that take place among them. It deals not only with natural processes, but also with interactions between humans and their environment.” The Scripps Institution of Oceanography (2022) states that this field observes the chemical processes affecting minerals, water, and air at or just below the earth’s surface, typically below about 200 °C. Forbes (2018) describes included topics as “acid-base equilibrium, reduction-oxidation reactions, and solubility,” within a broad field, and major areas of study including “mineral precipitation, chemical weathering, soil chemistry, sedimentary processes and diagenesis, biogeochemical cycles, and contaminant transport.” Mather (2013) notes that in low-temperature geochemistry, the environment is generally cooler than in fields such as igneous geochemistry, as the latter may concern volcanism and tectonic pressure and friction. Important topics include environmental geochemistry, diagenesis of sediments and geochemical exploration for mineral deposits (Mather, 2013). University of Michigan (2022) also points out that low-temperature geochemistry research includes “geochemistry of sediments, minerals, waters, fossils, soils, and organic matter occurring at temperatures commonly found at or near the Earth’s surface” and topics may focus on “noble gas content in groundwater, organic carbon content of modern Arctic permafrost and runoff, trace-element behavior in modern and fossil soils and marine carbonates, and many more.”
Recent research results in low-temperature geochemistry are both pure science-based and more related to technological applications in resource extraction. For example, Tranter (2015) describes the development of a wide range of geochemical topics over the recent decades, including the rock cycle, rock-water interactions, and chemical processes (see also Garrels & Christ, 1965; Garrels & Mackenzie, 1971); multiple geochemical cycles on the Earth’s surface (see also Holland, 1978, 1984); the modeling of early diagenesis (physical, chemical and/or biological change of sediments into sedimentary rock at relatively low temperatures and pressures, changing the original mineralogy and texture) (see also Berner, 1980); links between atmospheric CO2 concentrations and geological timescale (millions of years) carbonate and silicate weathering cycles (see also Berner et al., 1983); the oceans as CO2 reservoirs regulating atmospheric CO2 levels over glacial-interglacial timescales (alternating cold and warm periods over thousands of years) (see also Broecker & Peng, 1982; Broecker, 2002); fresh and saline water compositional variations (see also Drever, 1997); and the geochemical bases of rock, water and gas interactions (see also Stumm & Morgan, 1996; Langmuir, 1997).
Tranter (2015) notes that these low-temperature geochemistry studies are examples of early developments in the field, and more important contributions are being promoted, citing the example of recent advances in biogeochemical processes in the cryosphere, where “cold environments are now thought of as a biome, rather than as abiotic systems” (see Priscu & Christner, 2004; Anesio & Laybourn-Parry, 2012), and more importantly within geochemistry as “potential fertilizers of the polar oceans” (Tranter, 2015, see also Raiswell et al., 2006; Wadham et al., 2013). Glaciation as a field of study has also advanced, acknowledging the glacial agency in physical erosion, mostly abrasion, and fracture/traction which create transported, suspended, and deposited sediments (see Hallet et al., 1996; Knight, 1999). Tranter (2015) points out the role of glacial erosion in producing eroded bedrock, which liberates trace reactive components of these rocks, including sulfides and carbonates, and mineral phosphates such as apatite which enter water, creating the high rates of P weathering in glaciated areas (see also Föllmi et al., 2009; Tranter & Wadham, 2013). Tranter (2015) writes that crucially, current understandings acknowledge “most geochemical reactions, apart from hydrolysis, are microbially mediated… This was a step change for the discipline less than 20 years ago since glacier beds were formerly thought to be abiotic” (Sharp et al., 1999; Tranter et al., 2002; Montross et al., 2013).
Other examples of such low-temperature geochemistry include studies of microbial life, and linked sulfide oxidation and organic matter oxidation, which are processes that reduce the Oxidation/Reduction Potential (Eh) of waters distant from inputs of oxygenated surface melt, and REDOX reactions which may restrict the reaction types, linked to the oxidation of sulfides and organic matter, with oxidizing agents including \( {\mathrm{NO}}_3^{-} \), Fe(III), Mn(IV), and \( {\mathrm{SO}}_4^{2-} \) (Tranter, 2015). Tranter (2015) argues that “mass balance constraints are also imposed – reaction extents are ultimately decided by the sulfide and organic matter content of the basal debris and available oxidants.” Tranter (2015) also cites links between geochemical activity (which would be dependent on sulfides, O2, and organic matter) under thick ice sheets overlying crystalline bedrock, as therefore, high geochemical activity is likely to be linked to areas near “legacy organic matter found in overridden soils and sedimentary rocks” rather than the rare or absent recent, rapidly changing (labile) organic matter and O2 inputs (see Skidmore et al., 2000; Wadham et al., 2010). As the source of organic matter from legacy sources would be limited, the linked microbial systems would be rare and declining, with the erosion of the sedimentary rock, but Tranter (2015) argues that “the production of H2 by glacial crushing removes this gloomy fate” as it creates the possibility of microbial communities on crystalline bedrocks supported by the energy generated by physical comminution (reduction into smaller particles) of bedrock, this increasing the likelihood subglacial lakes under thick Antarctic ice have microbial life. In research, silicate rocks like those of subglacial environments in Arctic and Antarctica were crushed and wetted in an inert, laboratory atmosphere, with hydrogen (H2) production resulting at 0 °C, like methanogenesis rates in Greenland and Canadian glacier studies (Tranter, 2015; see also Siegert et al., 2001; Boyd et al., 2010; Telling et al., 2015).
Geochemical Techniques
Geochemical research techniques are complex, due to the wide range of environmental contexts and materials explored, as can be seen from the research foci and methods of the branches of geochemistry described earlier. These may also link to biogeochemical research (Campbell, 2020). These mostly focus on the study of chemical elements and compounds, and their movements within inanimate environments, using methods that may also be used in biogeochemical studies incorporating biological environments. An example is the research at the interface between Environmental Geochemistry and Biogeochemistry, as pursued at the Department of Geology and Environmental Science at the University of Pittsburgh. This research looks at geochemical processes between biological and geological at several scales, using geochemical and isotopic tracers for chemical constituents, and the modeling of processes through soil, plant, and hydrologic systems. The Department notes that “the application of a wide variety of isotopic methods is a fundamental strength of our department’s research… our understanding of present-day biogeochemical cycling is extended to the near and distant past through analysis of unique geochemical signals in lacustrine and marine depositional systems, providing constraints on ancient environments and processes” (Department of Geology and Environmental Science, 2023).
Another practical application of such geochemical methods is on mineral (including oil) exploration, which may also be used for biogeochemical studies. Rose (1998) notes that “geochemical exploration is any method of mineral or petroleum exploration that utilizes systematic measurements of one or more chemical properties of a naturally occurring material. The materials analyzed most commonly are rock, soil, stream and lake sediment, natural waters, vegetation, and soil air. Ores of metals such as Cu, Ni and Au are the most common targets of geochemical exploration, but nonmetallic commodities (diamonds, talc, and fluorite) and deposits of oil and gas are also sought by geochemical exploration.” Also, as noted earlier, geochemical research may focus on any of the naturally occurring elements in the Earth, and on the inorganic constituents of rocks and rock forming minerals, as well as organic chemistry, and soils, waters, and biological tissues (Trueman et al., 2019).
Geochemical prospecting for minerals, examining chemical constituents is a major area where the techniques of the geochemical sciences have been applied. For example, Hawkes (1957: 226) notes that “geochemical prospecting for minerals, as denned by common usage, includes any method of mineral exploration based on systematic measurement of one or more chemical properties of a naturally occurring material. The chemical property measured is most commonly the trace content of some element or group of elements; the naturally occurring material may be rock, soil, gossan (refers to highly oxidized, weathered or decomposed rock), glacial debris, vegetation, stream sediment, or water.” This is based on the definition of geochemistry, which paraphrases as “the determination of the relative and absolute abundance of the elements…in the earth” and “the study of the distribution and migration of the individual elements in the various parts of the earth…with the object of discovering the principles governing this distribution and migration” (Hawkes, 1957).
Sarala (2015: 711) refers to geochemical exploration methods as “mainly based on observations of anomalous concentrations of major or trace elements that are derived from a core part of a mineral deposit itself or a wider halo surrounding the ore body”…in this process the method is the “use of chemical properties of naturally occurring substances (including rocks, glacial debris, soils, stream sediments, waters, vegetation, and air) to find economic deposits of metals, minerals, and hydrocarbons.” Further, a geochemical survey can be scaled at continental, regional, targeting, and local scales and have several purposes and consequent designs, including the evaluation of the areal elemental and mineralogical concentrations, the geochemical or mineralogical anomalies within a potential mineralized area, or mineral exploration in a known mineralized area (see also McMartin & McClenaghan, 2001; McClenaghan et al., 2013).
Winterburn et al. (2017: 495) argue that although “geochemistry continues to be a major component of mineral exploration and evaluation programs on a global basis” recent developments have factored a decline in the cutting-edge research on geochemical methods, and “geochemical exploration has required less innovation as traditional techniques have continued to be used with success in shallowly covered or outcropping terrains.” In terms of the application of geochemical techniques to mineral exploration, Winterburn et al. (2017, 495) point to the fact that developments in geochemical methods lie within four main areas, namely the study of metal mobility and mechanisms, fast geochemical analyses, effective data use, and innovation in the laboratory-based techniques (see also Winterburn et al., 2020).
SGS (2022) lists some tasks that may be included in their top class geochemical services, including sample preparation. These focus on the classic geochemical foci of chemical ions, elements and compounds, and trace-element analysis. Mentioned techniques include Mobile Metal Ion (MMITM) analysis, Mobile Metal Ion (MMI) orientation surveys, ultratrace (an ultratrace refers to a chemical element that may be less than one microgram per gram of a given organism but is important for its metabolism) and trace-element analysis by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) and ICP-MS. As noted by Neikov and Yefimov (2019), “the ICP-AES is an analytical technique based on the principles of atomic spectroscopy for the determination of more than 70 elements with detection limits in the parts per billion to parts per million range.” This would enable the detection of elements in biogeochemical analyses. Inductively coupled plasma mass spectrometry (ICP-MS) is a mass spectrometry method, and the sample is ionized by an inductively coupled plasma. The atomization of the sample creates atomic and small polyatomic ions, which may be detected, this allowing the detection of low concentrations of metals and some nonmetals in liquid samples, and different isotopes of the same element (Lee, 2018). Wilschefski and Baxter (2019) describe inductively coupled plasma mass spectrometry (ICP-MS) as an analytical technique that can measure elements at trace levels in biological fluids, which is superseding older techniques including atomic absorption and atomic emission.
Other methods listed by SGS (2022) include X-ray fluorescence, metal speciation studies, carbon and sulfur analysis and reference material services. X-ray fluorescence (XRF) refers to the emission of fluorescent X-rays from materials made unstable by contact with X-rays, with applications to the chemical analysis of metals and other substances and for research in geochemistry (Van Grieken & Markowicz, 2002; Beckhoff et al., 2006; Pessanha et al., 2019). Intertek (2022) notes that “metals speciation testing provides… precise analysis of metal species such as mercury, arsenic, sulfur, and lead in crude oil, chemicals, and many other products, as well as aqueous samples such as seawater.”
Applied to biogeochemical research, such methods may be used to the interfaces of biological and inanimate environments, and the presence and movements of ions, elements, and compounds. One biogeochemically-based study is that of Reid and Hill (2010:105), which uses vegetation-based studies for mineral exploration, in this case gold. The authors describe this technique as testing the “ability of plants to show signatures of mineralisation as well as the optimum scale of sampling for first-pass mineral exploration surveys.” The study is based in the semiarid Tanami Gold Province in northern Australia, which includes many plant species. The Snappy gum (Eucalyptus brevifolia F.Muell.) had a distribution and sulfur and zinc signature, which indicated an underlying geological stratum that contained gold mineralization. Soft spinifex (Triodia pungens R.Br. 1810), a species of grass native to northwestern Australia, had gold, arsenic, zinc, sulfur, and cerium, which may indicate underground gold mineralization. The authors point out that in the study area, mineralization occurs at the contact between granite and dolerite, and biogeochemical signatures from snappy gum and dogwood (Acacia coriacea) showed higher levels of gold, arsenic, zinc, sulfur, and cerium. Therefore, the biogeochemical methods can be used to locate mineralization, substrate differences, supported by more information on the local soil, hydrology, and geological formations. The effectiveness of the biogeochemical method depended on the coverage of an area (Reid & Hill, 2010:105).
Another important geochemical method is geochemical mapping (Demetriades, 2021: 267). This is defined as a method “to document the spatial distribution of chemical elements and compounds, and physicochemical properties across the Earth’s surface” (Demetriades, 2021, 267). The functions of geochemical mapping include mineral exploration, forest assessment, medical research, the location of contaminated areas, and even the mapping of the geochemical environment in urban areas. Demetriades (2021: 267) notes that geochemical mapping initially developed in the Union of Soviet Socialist Republics (USSR) in the 1930s for mineral exploration, a function that eventually developed internationally. Demetriades (2021, 267) argues that “this expansion in the usage of geochemical data has been facilitated by the production of national multielement and multipurpose geochemical atlases compiled at different sample densities and various map scales depending on project objectives. The end-product is always an interpretation of the spatial distribution of chemical elements and compounds, and the processes that control or influence this spatial variation.” Systematic geochemical mapping is therefore the most effective method for recording the spatial variation of chemical elements, which can be applied to surface and shallow strata (Demetriades, 2021: 267).
Applied to biogeochemistry, geochemical mapping is termed biogeochemical mapping, with a greater focus on vegetation and soil constituents related to vegetation (Higueras et al., 2019). Higueras et al. (2019) mention soil health measurement as an important application of biogeochemical mapping, with soil health defined as “the continued capacity of the soil to function as a vital living ecosystem that sustains plants, animals and humans” and “an approach to the consideration of soil as a living being.” Rinot et al. (2019: 1484) note that “soil health index should reflect soil ability to provide ecosystem services (ES)” and “the relationship between soil attributes and ES should be quantified” and “The soil health (SH) concept has been introduced due to an evolving understanding that soil is not just a growing medium for crops but that it provides a foundation for other essential ecosystem services (ES).” Laishram et al. (2012: 20) present a definition of soil health and/or quality, which states that “degradation or deterioration in soil health or quality implies loss of the vital functions of soil” these functions being those that provide “physical support, water and essential nutrients required for growth of terrestrial plants,” the regulation of environmental water flow, and the “elimination of the harmful effects of contaminants by means of physical, chemical and biological processes.”
Geochemical applications are also complex and varied. For example, petroleum geochemistry, as an aspect of organic geochemistry has emerged as an important application in current applied technology and industry. Petroleum geochemistry is defined as “the science and application of chemical concepts to understand the origin of petroleum – natural gas, condensate, and crude oil – and its occurrence and fate on the earth’s surface and within its crust” (Curiale, 2017). Kvenvolden (2006: 1) argues that “organic geochemistry had its origin in the early part of the twentieth century when organic chemists and geologists realized that detailed information on the organic materials in sediments and rocks was scientifically interesting and of practical importance.” It is also noted that “organic geochemistry is now a widely recognized geoscience in which organic chemistry has contributed significantly not only to geology (i.e., petroleum geochemistry, molecular stratigraphy) and biology (i.e., biogeochemistry), but also to other disciplines, such as chemical oceanography, environmental science, hydrology, biochemical ecology, archaeology, and cosmochemistry.”
Dembicki Jr. (2017) lists some of the developments of petroleum geochemistry over the past century. While acknowledging that “petroleum geochemistry is a relatively young science, tracing its roots back to the 1934 discovery of chlorophyll-like structures in crude oil by Albert Treibs (1934, 1936), major developments have occurred, including the discovery of the organic origins of crude oil, the establishment of professional societies and research conferences on organic geochemistry, the development of analytical tools, including gas chromatography and mass spectrometry, and of the concept of biological maker compounds, and studies of the links between chemical fossils and oil sources (see also Breger, 1963; Eglinton & Calvin, 1967; Durand, 2003). Other developments in the late twentieth century included the study of the hydrocarbon generation process and the oil window concept, the thermal maturity of sediments and the kerogen composition (a solid, insoluble organic matter in sedimentary rocks, see Vandenbroucke & Largeau, 2007), and the Rock-Eval instrument which used as a standardized pyrolysis method for source-rock characterization and evaluation in petroleum geochemistry, and new treatises on petroleum geology (Dembicki Jr., 2017; see also Tissot & Welte, 1978; Espitalié et al., 1977). Studies included foci on hydrocarbon fingerprinting, forensic geochemistry, and gas chromatography (Hunt, 1979). Later developments included advances and understandings in pyrolysis techniques, biomarker applications, petroleum migration, basin modeling, and the introduction of personal computers for use in geochemical studies (Dembicki Jr., 2017; see also Lopatin, 1971; Waples, 1980; Sluijk & Parker, 1984).
Instructive scientific publications during the late twentieth century included those of Sluijk and Parker (1984) on the value of petroleum geochemistry in exploration, Kaufman et al. (1990) on reservoir continuity, and production problem analysis, Magoon and Dow (1994) on the concept of petroleum systems and the associated role of petroleum geochemistry Passey et al. (2010) on understanding source rocks and reservoirs, and Dembicki Jr. (2014) on the fluid properties and phase behavior for successful oil exploitation. Dembicki Jr. (2017) concludes the study of the historical development of geochemical methods by stating that “whatever problems the future holds in petroleum exploration and production, innovative applications of the concept and methods of petroleum geochemistry will continue to contribute to solutions” (Kvenvolden, 2002, 2006; Hunt et al., 2002; Durand, 2003; Dow, 2014).
The methods of petroleum geochemistry may be applied to biogeochemistry. For example, Head et al. (2014) describe a study of “microbiological, geochemical, and biogeochemical data” that contributes to knowledge of processes regulating deep life in petroleum reservoir ecosystems and of biotic and abiotic factors that determine the biodegradation of petroleum in situ, as this phenomenon may oil exploration and production.” This study proposes that reservoir formation water salinity affects the occurrence of biodegraded heavy oil reservoirs. The authors note that in the study of microbial activity in the ground, the important factors are “temperature, pH, salinity, water activity, radiation and availability of resources such as carbon and energy sources, electron acceptors and inorganic nutrients” and for in-reservoir crude oil biodegradation the important factors are “temperature, salinity, and inorganic nutrient availability” (Head et al., 2014). It is also noted that nutrient availability is major control for the degradation of crude oil in aerobic environments, as such degradation depends on hydrocarbon-degrading organisms, with hydrocarbon conversion to biomass for growth requiring nitrogen and phosphorus. Microbial biomass has also been found to correlate with organic carbon content (Head et al., 2014).
Conclusions
This chapter has examined the relationship between the highly multidisciplinary and related sciences of geochemistry and biogeochemistry, taking the former as the background of the latter. Geochemistry, as the science that examines the Earth and sometimes other solar bodies from the approach of the chemical sciences, is plainly one of the roots for the more recently developed discipline of biogeochemistry, which adds more foci on biological roles in chemical cycling. As the evolution of life is based on a chemical foundation, mostly the carbon link with water and living tissue, the relationship between these two sciences sits on the most interesting science issue: the origin and continuing development of life, on a chemical foundation. This chapter has skimmed over the main topics of the extremely broad and deep science of geochemistry, looking at subdisciplines including organic geochemistry, inorganic geochemistry, isotope geochemistry, aqueous geochemistry, cosmochemistry, trace-element geochemistry, igneous rock geochemistry, metamorphic rock geochemistry, photo-geochemistry and low-temperature or environmental geochemistry. More mention will be made in later chapters of the main points of this chapter, to explore further the links between biogeochemistry and the supporting Earth sciences.
Change history
28 February 2024
A correction has been published.
References
Åberg, G., Jacks, G., & Hamilton, P. J. (1989). Weathering rates and 87 Sr/86 Sr ratios: An isotopic approach. Journal of Hydrology, 109(1), 65–78.
Allaby, M. (2008). A dictionary of earth sciences (3rd ed.). Oxford University Press. https://doi.org/10.1093/acref/9780199211944.001.0001
American Association of Petroleum Geologists. (2022). Journals. Retrieved from https://www.aapg.org/publications/journals/bulletin
American Chemical Society. (2022). Inorganic Chemistry. Retrieved from https://www.acs.org/content/acs/en/careers/chemical-sciences/areas/inorganic-chemistry.html
American Geophysical Union. (2022). Journal of Geophysical Research. Retrieved from https://agupubs.onlinelibrary.wiley.com/journal/21562202
American Mineralogist. (2022). American Mineralogist. Mineralogical Society of America. Retrieved from http://www.minsocam.org/MSA/AmMin/Scope_and_Mission_2final.htm
Anderson, R. S., & Anderson, S. P. (2010). Geomorphology: The mechanics and chemistry of landscapes. Cambridge University Press.
Anesio, A. M., & Laybourn-Parry, J. (2012). Glaciers and ice sheets as a biome. Trends in Ecology & Evolution, 27, 219–225. https://doi.org/10.1016/j.tree.2011.09.012
Aston, S. R. (1983). Silicon geochemistry and biogeochemistry. Academic.
Atkins, P., & de Paula, J. (2009). Elements of physical chemistry (5th ed.). Oxford University Press.
Barker, C. (1972). Aquathermal pressuring—Role of temperature in development of abnormal-pressure zones. AAPG Bulletin, 56, 2068–2071.
Bataille, C. P., Willis, A., Yang, X., & Liu, X. M. (2017). Continental igneous rock composition: A major control of past global chemical weathering. Science Advances, 3(3), e1602183. https://doi.org/10.1126/sciadv.1602183
Beckhoff, B., Kanngießer, B., Langhoff, N., Wedell, R., & Wolff, H. (2006). Handbook of practical X-ray fluorescence analysis. Springer.
Berner, R. A. (1980). Early diagenesis. A theoretical approach. Princeton University Press.
Berner, R. A., Lasaga, A. C., & Garrels, R. M. (1983). The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. American Journal of Science, 283, 641–683. https://doi.org/10.2475/ajs.283.7.641
Bianchi, T. S. (2021). The evolution of biogeochemistry: Revisited. Biogeochemistry, 154, 141–181. https://doi.org/10.1007/s10533-020-00708-0
Bianchi, T. S., & Canuel, E. A. (2011). Chemical biomarkers in aquatic ecosystems. Princeton University Press.
Black, D. C., & Pepin, R. O. (1969). Trapped neon in meteorites II. Earth and Planetary Science Letters, 6, 395–405.
Boreham, C., Jinadasa, N., Sohn, J., Hong, Z., & Blake, C. (2021). Characterisation of radiogenic monoalkenes in Australian oils and condensate. Organic Geochemistry, 163, 104332. https://doi.org/10.1016/j.orggeochem.2021.104332
Bouse, R. M., Ruiz, J., Titley, S. R., Tosdal, R. M., & Wooden, J. L. (1999). Lead isotope compositions of Late Cretaceous and Early Tertiary igneous rocks and sulfide minerals in Arizona: Implications for the sources of plutons and metals in porphyry copper deposits. Economic Geology, 94(2), 211–244. https://doi.org/10.2113/gsecongeo.94.2.211
Boyd, E. S., Skidmore, M., Mitchell, A. C., Bakermans, C., & Peters, J. W. (2010). Methanogenesis in subglacial sediments. Environmental Microbiology Reports, 2, 685–692. https://doi.org/10.1111/j.1758-2229.2010.00162.x
Bray, L. A., & Van Tuyl, H. H. (1961). Laboratory development of a carrier-precipitation process for the recovery of strontium from purex wastes. General Electric Co. Hanford Atomic Products Operation.
Breger, I. A. (1963). Organic geochemistry. Pergamon, Macmillan.
Briggs, D. E. G., & Summons, R. E. (2014). Ancient biomolecules: Their origins, fossilization, and role in revealing the history of life. BioEssays, 36, 482–490.
Britannica. (2014). Geochemistry. Encyclopedia Britannica. https://www.britannica.com/science/geochemistry
Broecker, W. S. (2002). The glacial world according to wally. Eldigio Press.
Broecker, W. S., & Peng, T.-H. (1982). Tracers in the sea. Eldigio Press.
Brownlow, A. H. (1979). Geochemistry. Prentice-Hall, Inc.
Bullen, T., White, A., Blum, A., Harden, J., & Schulz, M. (1996). Chemical weathering of a soil chronosequence on granitoid alluvium: II. Mineralogic and isotopic constraints on the behaviour of strontium. Geochimica et Cosmochimica Acta, 61(2), 291–306.
Burke, J. G. (1986). Cosmic debris: Meteorites in history. University of California Press.
Burrows, A., Holman, J., Parsons, A., Pilling, G., & Price, G. (2009). Chemistry3. Oxford University Press.
Burst, J. F. (1969). Diagenesis of Gulf Coast clayey sediments and its possible relation to petroleum migration. Bulletin of the American Association of Petroleum Geologists, 53(l), 73–93.
Callahan, M. P., Smith, K. E., Cleaves, H. J., 2nd, Ruzicka, J., Stern, J. C., Glavin, D. P., House, C. H., & Dworkin, J. P. (2011). Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proceedings of the National Academy of Sciences of the United States of America, 108(34), 13995–13998. https://doi.org/10.1073/pnas.1106493108
Campbell, M. (2020). The physical geography of El Salvador: A geosphysical and ecological approach. Nova Science Publishers.
Catalá, T. S., Shorte, S., & Dittmar, T. (2021). Marine dissolved organic matter: A vast and unexplored molecular space. Applied Microbiology and Biotechnology, 105, 7225–7239. https://doi.org/10.1007/s00253-021-11489-3
Chamberlain, C. P., Waldbauer, J. R., & Jacobson, A. D. (2005). Strontium, hydrothermal systems, and steady-state chemical weathering in active mountain belts. Earth and Planetary Science Letters, 238(3), 351–366.
ChemEurope. (2022). Radiogenic. Retrieved from https://www.chemeurope.com/en/encyclopedia/Radiogenic.html
Chiaradia, M., & Fontbote, L. (2002). Lead isotope systematics of Late Cretaceous – Tertiary Andean arc magmas and associated ores between 8°N and 40°S: Evidence for latitudinal mantle heterogeneity beneath the Andes. Tera Nova, 14(5), 337–342. https://doi.org/10.1046/j.1365-3121.2002.00426.x
Clarke, F. W., & Washington, H. S. (1924). The composition of the Earth’s crust. United States Geological Survey Professional Paper, 127, 117.
Commonwealth Scientific and Industrial Research Organisation (CSIRO). (2022). Environmental Chemistry. Retrieved from https://www.publish.csiro.au/en/AbouttheJournal
Conley, D. J. (2002). Terrestrial ecosystems and the global biogeochemical silica cycle. Global Biogeochemical Cycles, 16(4), 68-1–68-7. https://doi.org/10.1029/2002GB001894
Connan, J. (1974). Time-temperature relation in oil genesis: Geologic notes. AAPG Bulletin, 58, 2516–2521. https://doi.org/10.1306/83D91BEB-16C7-11D7-8645000102C1865D
Cooke, M., Dongen, B., Talbot, H., Semiletov, I., Shakhova, N., Guo, L., & Gustafsson, Ö. (2009). Bacteriohopanepolyol biomarker composition of organic matter exported to the Arctic Ocean by seven of the major Arctic rivers. Organic Geochemistry, 40, 1151–1159. https://doi.org/10.1016/j.orggeochem.2009.07.014
Cooke, D. R., Hollings, P., Wilkinson, J. J., & Tosdal, R. M. (2014). Geochemistry of porphyry deposits. In H. D. Holland & K. K. Turekian (Eds.), Treatise on geochemistry (Vol. 13, 2nd ed., pp. 357–381). https://doi.org/10.1016/B978-0-08-095975-7.01116-5
Curiale, J. A. (2017). Petroleum geochemistry. In R. Sorkhabi (Ed.), Encyclopedia of petroleum geoscience (Encyclopedia of earth sciences series). Springer. https://doi.org/10.1007/978-3-319-02330-4_2-1
Curiale, J. A., & Frolov, E. B. (1998). Occurrence and origin of olefins in crude oils. A critical review. Organic Geochemistry, 29, 397–408.
Czech Geological Society. (2022). Journal of Geosciences. Retrieved from http://www.jgeosci.org/scope
Davis, A. M. (2004). Treatise on geochemistry, volume 1: Meteorites, comets, and planets. Elsevier Pergamon.
Davis, A. M. (2014). Meteorites and cosmochemical processes. In H. D. Holland & K. K. Turekian (Eds.), Treatise on geochemistry (2nd ed., pp. 1–52). NL Elsevier.
Dembicki, H., Jr. (2014). Challenges to black oil production from shales: AAPG Search and Discovery Article #80355.
Dembicki, H., Jr. (2017). Practical petroleum geochemistry for exploration and production. Elsevier Science. https://doi.org/10.1016/C2014-0-03244-3
Demetriades, A. (2021). Geochemical mapping. In D. Alderton & S. A. Elias (Eds.), Encyclopedia of geology (2nd ed., pp. 267–280). https://doi.org/10.1016/B978-0-08-102908-4.00059-X
Department of Geology and Environmental Science. (2023). Environmental geochemistry and biogeochemistry. Retrieved from https://www.geology.pitt.edu/research-area/environmental-geochemistry-and-biogeochemistry
Devriendt, L. S. J. (2016). Controls on the oxygen isotope ratio of inorganic and biogenic calcium carbonates. Unpublished PhD Thesis, School of Earth and Environmental Sciences, University of Wollongong. https://ro.uow.edu.au/theses/4901
Dickey, P. A. (1975). Possible migration of oil from source rock in oil phase. American Association of Petroleum Geologists Bulletin, 59, 337–345.
Dickin, A. P. (2005). Radiogenic isotope geology. Cambridge University Press.
Doane, T. A. (2017). A survey of photogeochemistry. Geochemical Transactions, 18(1). https://doi.org/10.1186/s12932-017-0039-y
Dow, W. (2014). Musings on the history of petroleum geochemistry - from my perch. Retrieved from https://www.searchanddiscovery.com/documents/2014/80375dow/ ndx_dow.pdf
Drever, J. I. (1997). The geochemistry of natural water surface and groundwater environments (3rd ed.). Prentice Hall.
Dudás, F. Ö., Zhang, H., Shen, S., & Bowring, A. (2021). Major and trace element geochemistry of the Permian-Triassic boundary section at Meishan, South China. Frontiers in Earth Science. https://doi.org/10.3389/feart.2021.637102
Durand, B. (2003). A history of organic geochemistry. Oil & Gas Science and Technology – - Revue d’IFP Energies nouvelles, 58(2), 203–231.
Eglinton, G., & Calvin, M. (1967). Chemical fossils. Scientific American, 16(1), 32–43. http://www.jstor.org/stable/24931372
Elias, S. A. (2021). Geoarchaeology. In D. Alderton & S. A. Elias (Eds.), Encyclopedia of geology (2nd ed., pp. 538–553). Elsevier. https://doi.org/10.1016/B978-0-12-409548-9.12467-4
Espitalié, J., Laporte, J. L., Madec, M., Marquis, F., Leplat, P., Paulet, J., & Boutefeu, A. (1977). Rapid method for characterizing source rocks their oil potential and their degree of development. Journal of the French Petroleum Institute, 32, 23–42.
European Geosciences Union. (2022a). Nonlinear processes in geophysics. Retrieved from https://www.nonlinear-processes-in-geophysics.net/about/aims_and_scope.html
European Geosciences Union. (2022b). Solid earth. Retrieved from https://www.solid-earth.net/about/aims_and_scope.html
Falkowski, P. G. (2015). From light to life. Origins of Life and Evolution of Biospheres, 45, 347–350.
Faure, G. (1991). Principles and application of inorganic geochemistry. A comprehensive textbook for geology students. Macmillan.
Föllmi, K. B., Hosein, R., Arn, K., & Steinmann, P. (2009). Weathering and the mobility of phosphorus in the catchments and forefields of the Rhône and Oberaar glaciers, Central Switzerland: Implications for the global phosphorus cycle on glacial-interglacial timescales. Geochimica et Cosmochimica Acta, 73, 2252–2282. https://doi.org/10.1016/j.gca.2009.01.017
Forbes, T. Z. (2018). Low-temperature geochemistry. In W. M. White (Ed.), Encyclopedia of geochemistry (Encyclopedia of earth sciences series). Springer. https://doi.org/10.1007/978-3-319-39312-4_23
Frolov, E. B., Smirnov, M. B., Melikhov, V. A., & Vanyukova, N. A. (1998). Olefins of radiogenic origin in crude oils. Organic Geochemistry, 29, 409–420.
Frye, K. (1981). Lattice. In Mineralogy (Encyclopedia of earth science). Springer. https://doi.org/10.1007/0-387-30720-6_65
Gaillardet, J., & Galy, A. (2008). Himalaya: Carbon sink or source? Science, 320(5884), 1727–1728.
Gaines, S. M., Eglinton, G., & Rullkotter, K. (2009). Echoes of life. What fossils reveal about earth history. Oxford University Press.
Garrels, R. M., & Christ, C. L. (1965). Solution, minerals and equilibria. Freeman Cooper.
Garrels, R. M., & Mackenzie, F. T. (1971). Evolution of sedimentary rocks. Norton.
Geetha Thanuja, K., Reddy Kiran Kalyan, V. S., Karthikeyan, S., & Anthoniraj, S. (2022). Microbial transformation of silicon in soil. In C. J. Hurst (Ed.), Microbial metabolism of metals and metalloids (Advances in environmental microbiology) (Vol. 10). Springer. https://doi.org/10.1007/978-3-030-97185-4_15
Geochemical Journal. (2022). Geochemical Journal. Retrieved from https://geochemical-journal.jp
Geochemical Society. (2022). Organic geochemistry division. Retrieved from https://www.geochemsoc.org/programs/organicgeochemistrydivisio
Geochemistry. (2022). Retrieved from https://www.encyclopedia.com/environment/energy-government-and-defense-magazines/geochemistry
Geological Society of London. (2012). Journal of the Geological Society. Retrieved from https://www.geolsoc.org.uk/jgs
Geoscience Australia. (2022). Geochemistry. Retrieved from https://www.ga.gov.au/scientific-topics/disciplines/geochemistry#:~:text=Inorganic%20Geochemistry,-Inorganic%20chemistry%20is&text=Inorganic%20chemistry%20investigates%20the%20characteristics,found%20in%20the%20Earth's%20crust
GeoScienceWorld. (2022). Lithosphere. Retrieved from https://pubs.geoscienceworld.org/lithosphere
Goldschmidt, V. M. (1937). The principles of distribution of chemical elements in minerals and rocks. Journal of the Chemical Society, 655–672.
Goldschmidt, V. M. (1954). Geochemistry. Clarendon Press.
Gottikh, R. P., Vinokurov, S. F., & Pisotskii, B. I. (2009). Rare-earth elements as geochemical criteria of endogenous sources of microelements contained in oil. Doklady Earth Sciences, 425, 325–329.
Gounelle, M. (2011). Cosmochemistry. In M. Gargaud, R. Amils, J. C. Quintanilla, H. J. Cleaves, W. M. Irvine, D. L. Pinti, & M. Viso (Eds.), Encyclopedia of astrobiology (pp. 380–387). Springer. https://doi.org/10.1007/978-3-642-11274-4_1747
Graustein, W. C., & Armstrong, R. L. (1983). The use of strontium-87/strontium-86 ratios to measure atmospheric transport into forested watersheds. Science, 219(4582), 289–292. https://doi.org/10.1126/science.219.4582.289
Greenwalt, D. E., Goreva, Y. S., Siljestrom, S. M., Rose, T., & Harbachet, R. E. (2013). Hemoglobin-derived porphyrins preserved in a Middle Eocene blood-engorged mosquito. Proceedings of the National Academy of Sciences USA, 110, 18496–18500.
Haldar, S. K. (2018). Exploration geochemistry. In S. K. Haldar (Ed.), Mineral exploration: Principles and applications (2nd ed., pp. 85–101). https://doi.org/10.1016/B978-0-12-814022-2.00005-8
Hallberg, R. O. (2009). Biogeochemistry. In V. Cilek (Ed.), Earth system: History and natural variability - Volume IV (pp. 435–446). Developed under the Auspices of the UNESCO, Eolss Publishers.
Hallet, B., Hunter, L., & Bogen, J. (1996). Rates of erosion and sediment evacuation by glaciers: A review of field data and their implications. Global and Planetary Change, 12(1–4), 213–235. https://doi.org/10.1016/0921-8181(95)00021-6
Hansell, D. A., & Carlson, C. A. (Eds.). (2014). Biogeochemistry of marine dissolved organic matter. Academic. https://doi.org/10.1016/C2012-0-02714-7
Hawkes, H. E. (1957). Principles of geochemical prospecting. USGS Numbered Series, 1000, 225–355. https://doi.org/10.3133/b1000F
Head, I. M., Gray, N. D., & Larter, S. R. (2014). Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management. Frontiers in Microbiology, 5, 566. https://doi.org/10.3389/fmicb.2014.00566
Hellweger, F. L. (2008). Biogeochemical models. In Encyclopedia of ecology (pp. 386–396). Elsevier B.V.
Hem, J. D. (1985). Study and interpretation of the chemical characteristics of natural water (3rd ed.). US Geological Survey Water-Supply Paper 2254, University of Virginia.
Hendry, K. R., Marron, A. O., Vincent, F., Conley, D. J., Gehlen, M., Ibarbalz, F. M., Quéguiner, B., & Bowler, C. (2018). Competition between silicifiers and non-silicifiers in the past and present ocean and its evolutionary impacts. Frontiers in Marine Science, 5, 22. https://doi.org/10.3389/fmars.2018.00022
Higueras, P., Campos, J. A., Esbrí, J. M., García-Noguero, E. M., & Elmayel, I. (2019). Biogeochemical mapping: A new tool to assess the soil quality and health. In D. Doronzo, E. Schingaro, J. Armstrong-Altrin, & B. Zoheir (Eds.), Petrogenesis and exploration of the Earth’s interior. CAJG 2018 (Advances in science, technology & innovation). Springer. https://doi.org/10.1007/978-3-030-01575-6_1
Hildred, G. V., Ratcliffe, K. T., Wright, A. M., Zaitlin, B. A., & Wray, D. S. (2010). Chemostratigraphic applications to low-accommodation fluvial incised-valley settings; an example from the Lower Mannville Formation of Alberta, Canada. Journal of Sedimentary Research, 80, 1032–1045.
Holland, H. D. (1978). The chemistry of the atmosphere and oceans. Wiley.
Holland, H. D. (1984). The chemical evolution of the atmosphere and oceans. Princeton University Press.
Hopkins, F. E., Archer, S. D., Bell, T. G., Suntharalingam, P., & Todd, J. D. (2023). The biogeochemistry of marine dimethylsulfide. Nature Reviews Earth & Environment, 4, 361–376. https://doi.org/10.1038/s43017-023-00428-7
Housecroft, C. E., & Sharpe, A. G. (2008). Inorganic chemistry (3rd ed.). Pearson Education.
Hoyle, F., & Wickramasinghe, C. (1981). Where microbes boldly went. New Scientist, 91, 412–415.
Hu, Y., & Teng, F. Z. (2021). Non-traditional stable isotope geochemistry. In D. In Alderton & S. A. Elias (Eds.), Encyclopedia of geology (2nd ed., pp. 114–124). Academic.
Hunt, J. M. (1979). Petroleum geochemistry and geology. W.H. Freeman and Company.
Hunt, J. M., Philp, R. P., & Kvenvolden, K. A. (2002). Early developments in petroleum geochemistry. Organic Geochemistry, 33(9), 1025–1052. https://doi.org/10.1016/S0146-6380(02)00056-6
Imperial College. (2022). Organic Geochemistry Research (ICOG). Retrieved from https://www.imperial.ac.uk/earth-science/research/research-groups/icog/research/
Inamuddin, Ahamed, I. M., & Lichtfouse, E. (2021). Water pollution and remediation: Heavy metals environmental chemistry for a sustainable world. https://doi.org/10.1007/978-3-030-52421-0
Indiana University. (2022). Department of Earth and Atmospheric Sciences: Geochemistry and biogeochemistry. Retrieved from https://earth.indiana.edu/research/research-areas/geochemistry-and-biogeochemistry/index.html#:~:text=Geochemistry%20and%20Biogeochemistry%20are%20inherently,that%20can%20constrain%20computational%20models
Intertek. (2022). Metals speciation analysis and testing: Metal speciation analysis. Retrieved from https://www.intertek.com/chemicals/metals-speciation/
Ivanov, K. S., Kucherov, V. G., & Fedorov, Y. N. (2008). On the question of the deep origin of oil STATE, trends and problems of development of oil and gas potential of Western Siberia. In Materials of the international academic conference (pp. 160–173). ZapSibNIIGG.
Ivanov, K. S., Erokhin, Y. V., & Kudryavtsev, D. A. (2021). Inorganic geochemistry of crude oils of northern Eurasia after ICP-MS data as clear evidence for their deep origin. Energies, 15(1), 48. https://doi.org/10.3390/en15010048
Jacks, G., Åberg, G., & Hamilton, P. J. (1989). Calcium budgets for catchments as interpreted by strontium isotopes. Hydrology Research, 20(2), 85–96.
Jacobson, A. D., Blum, J. D., Chamberlain, C. P., Poage, M. A., & Sloan, V. F. (2002). Ca/Sr and Sr isotope systematics of a Himalayan glacial chronosequence: Carbonate versus silicate weathering rates as a function of landscape surface age. Geochimica et Cosmochimica Acta, 66(1), 13–27.
Jenkins, D. A. (1989). Trace element geochemistry in archaeological sites. Environmental Geochemistry and Health, 11, 57–62. https://doi.org/10.1007/BF0178299
Jenkyns, H. C. (2010). Geochemistry of oceanic anoxic events. Geochemistry Geophysics Geosystems, 11, 1–30.
Jin, Z., You, C., Yu, J., Wu, L., Zhang, F., & Liu, H. (2011). Seasonal contributions of catchment weathering and eolian dust to river water chemistry, northeastern Tibetan plateau: Chemical and Sr isotopic constraints. Journal of Geophysical Research: Earth Surface, 116(F4).
John Wiley & Sons Ltd. (2022). Geological Journal. Retrieved from https://onlinelibrary.wiley.com/page/journal/10991034/homepage/productinformation.html
Kamenov, G., MacFarlane, A. W., & Riciputi, L. (2002). Sources of lead in the San Cristobal, Pulacayo, and Potosí mining districts, Bolivia, and a reevaluation of regional ore lead isotope provinces. Economic Geology, 97(3), 573–592. https://doi.org/10.2113/gsecongeo.97.3.573
Kaufman, Y. J., Tucker, C. J., & Fung, I. (1990). Remote sensing of biomass burning in the tropics. Journal of Geophysical Research, 95, 9927–9939. https://doi.org/10.1029/JD095iD07p09927
Kendall, C., & Caldwell, E. A. (1998). Fundamentals of isotope geochemistry. In C. Kendall & J. J. McDonnell (Eds.), Isotope tracers in catchment hydrology (pp. 51–86). Elsevier Science.
Kendall, C., Sklash, M. G., & Bullen, T. D. (1995). Isotope tracers of water and solute sources in catchments. In S. T. Trudgill (Ed.), Solute modelling in catchment systems (pp. 261–303). Wiley.
Kendall, C., Doctor, D., & Young, M. (2014). Environmental isotope applications in hydrologic studies. In Treatise on geochemistry (Vol. 7, 2nd ed., pp. 273–327). https://doi.org/10.1016/B978-0-08-095975-7.00510-6
Kennedy, M. E. (1998). Elements: Trace. In Geochemistry. Encyclopedia of earth science (pp. 221–223). Springer. https://doi.org/10.1007/1-4020-4496-8_1_11
Killops, S. D., & Killops, V. J. (2013). Introduction to organic geochemistry (2nd ed.). Wiley-Blackwell.
Kim, J. D., Yee, N., Nanda, V., & Falkowski, P. G. (2011). Anoxic photochemical oxidation of siderite generates molecular hydrogen and iron oxides. Proceedings of the National Academy of Sciences of the United States of America, 110(25), 10073–10077. https://doi.org/10.1073/pnas.1308958110
Knight, P. G. (1999). Glaciers. Stanley Thornes (Publishers) Ltd.
Koeberl, C. (2007). 1.28 - The geochemistry and cosmochemistry of impacts. In H. D. Holland & K. K. Turekian (Eds.), Treatise on geochemistry (Vol. 1, pp. 1–52). https://doi.org/10.1016/B978-008043751-4/00228-55
Kozlik, M., Gerdes, A., & Raith, J. G. (2016). Strontium isotope systematics of sheelite and apatite from the felbertal tungsten deposit, Austria–results of in-situ LA-MC-ICP-MS analysis. Mineralogy and Petrology, 110(1), 11–27.
Kraeutler, B., & Bard, A. J. (1978). Heterogeneous photocatalytic synthesis of methane from acetic acid-new Kolbe reaction pathway. Journal of the American Chemical Society, 100(7), 2239–2240. https://doi.org/10.1021/ja00475a049
Kudo, A. M., & Jahns, R. H. (2022). Igneous rock. Encyclopedia Britannica. https://www.britannica.com/science/igneous-rock
Kumar, S. P., Mandlimath, T. R., & Ramesh, M. (2021). Inorganic geochemistry. In Inamuddin, M. I. Ahamed, R. Boddula, & T. Altalhi (Eds.), Geochemistry: Concepts and applications (pp. 149–160). Wiley; Scrivener Publishing. https://doi.org/10.1002/9781119710134.ch9
Kurade, M. B., Saha, S., Salama, E. S., Patil, S. M., Govindwar, S. P., & Jeon, B. H. (2019). Acetoclastic methanogenesis led by Methanosarcina in anaerobic co-digestion of fats, oil and grease for enhanced production of methane. Bioresource Technology, 272, 351–359. https://doi.org/10.1016/j.biortech.2018.10.047
Kvenvolden, K. A. (2002). Methane hydrate in the global organic carbon cycle. Terra Nova, 14(5), 302–306. https://doi.org/10.1046/j.1365-3121.2002.00414.x
Kvenvolden, K. A. (2006). Organic geochemistry – A retrospective of its first 70 years. Organic Geochemistry, 37(1), 1–11. https://doi.org/10.1016/j.orggeochem.2005.09.001
Laishram, J., Saxena, K. G., Maikhuri, R. K., & Rao, K. S. (2012). Soil quality and soil health: A review. International Journal of Ecology and Environmental Sciences, 38(1), 19–37.
Langmuir, D. (1997). Aqueous environmental geochemistry. Prentice Hall.
Lastovicka, J. (2009). Geophysics and geochemistry. Retrieved from https://www.eolss.net/ebooklib/bookinfo/geophysics-geochemistry.aspx#
Lauretta, D. S., & Killgore, M. (2005). A color atlas of meteorites in thin section. Golden Retriever Press.
Lauretta, D. S., & McSween, H. Y., Jr. (Eds.). (2006). Meteorites and the early solar system II. University of Arizona Press.
Lee, H.-C. (2018). Review of inductively coupled plasmas: Nano-applications and bistable hysteresis physics. Applied Physics Reviews, 5(1), 011108.
Lee, T., Papanastassiou, D. A., & Wasserburg, G. J. (1977). Aluminum-26 in the early solar system: Fossil or fuel? Astrophysical Journal Letters, 211, L107–L110.
Leithauser, P., Schaefer, R. G., & Yukler, A. (1982). Role of diffusion in primary migration of hydrocarbons. American Association of Petroleum Geologists Bulletin, 66, 408–429.
Lenton, T. M., & Daines, S. J. (2017). Biogeochemical transformations in the history of the ocean. Annual Review of Marine Science, 9, 31–58.
Li, W., Gou, W., Li, W., Zhang, T., Yu, B., Liu, Q., & Shi, J. (2019). Environmental applications of metal stable isotopes: Silver, mercury and zinc. Environmental Pollution, 252(Part B), 1344–1356. https://doi.org/10.1016/j.envpol.2019.06.037
Lodders, K., & Fegley, B., Jr. (1998). The planetary scientist’s companion. Oxford University Press.
Lopatin, N. V. (1971). Temperature and time as factors of coalification. Izvestiya Akademii Nauk SSSR, Seriya gelogicheskaya (in Russian), 3, 95–106.
Lu, X., Ma, C., & Wang, S. (2019). Classifying the geology journals by editorial board interlocks. Procedia Computer Science, 162, 682–687. https://doi.org/10.1016/j.procs.2019.12.038
MacFarlane, A. W., Marcet, P., LeHuray, A. P., & Petersen, U. (1990). Lead isotope provinces of the Central Andes inferred from ores and crustal rocks. Economic Geology, 85(8), 1857–1880. https://doi.org/10.2113/gsecongeo.85.8.1857
MacPherson, G. J., & Thiemens, M. H. (2011). Cosmochemistry: Understanding the solar system through analysis of extraterrestrial materials. PNAS, 108(48), 19130–19134. https://doi.org/10.1073/pnas.1111493108
Magara, K. (1978). Compaction and fluid migration: Practical petroleum geology fundamentals of numerical reservoir simulation (Developments in petroleum science 9). Elsevier Scientific Pub. Co.
Magoon, L. B., & Dow, W. G. (1994). The petroleum system. In L. B. Magoon & W. G. Dow (Eds.), The petroleum system—From source to trap (Vol. 60, pp. 3–24). AAPG Memoire.
Makishima, A. (2017). Origins of the earth, moon, and life. An interdisciplinary approach. Elsevier.
Mamedov, L. (2021). Stable isotope geochemistry. Journal of Science and Geosciences, 9(2), 6–7.
Marakushev, A. A., Pisotskii, B. I., Paneyakh, N. A., & Gottikh, R. P. (2004). Geochemical features of oil and the origin of oil fields. Doklady Earth Sciences, 399, 1120–1124.
Marshak, S. (2009). Essentials of geology (3rd ed.). W. W. Norton & Company.
Mather, T. A. (2013). Reference module in earth systems and environmental sciences || Geochemistry. https://doi.org/10.1016/B978-0-12-409548-9.05929-7
McClenaghan, M. B., Plouffe, A., McMartin, I., Campbell, J., Spirito, W., Paulen, R., Garrett, R., & Hall, G. (2013). Till sampling and geochemical analytical protocols used by the Geological Survey of Canada. Geochemistry Exploration Environment Analysis, 13(4), 285–301. https://doi.org/10.1144/geochem2011-083
McKay, D. S., Gibson, E. K., Jr., Thomas-Keprta, K. L., Vali, H., Romanek, C. S., Clemett, S. J., Chillier, X. D., Maechling, C. R., & Zare, R. N. (1996). Search for past life on Mars: Possible relic biogenic activity in Martian meteorite ALH84001. Science, 273(5277), 924–930. https://doi.org/10.1126/science.273.5277.924
McMartin, I., & McClenaghan, M. B. (2001). Till geochemistry and sampling techniques in glaciated shield terrain: A review. Geological Society London Special Publications 185(1):19–43. https://doi.org/10.1144/GSL.SP.2001.185.01.02. In M. B. McClenaghan, P. T. Bobrowsky, G. E. M. Hall, & S. J. Cook (Eds.), Drift exploration in glaciated terrain (pp. 19–43). Geological Society of London. https://doi.org/10.1144/GSL.SP.2001.185
McSween, H., Jr., & Huss, G. (2010). Introduction to cosmochemistry. In H. McSween Jr. & G. Huss (Eds.), Cosmochemistry (pp. 1–28). Cambridge University Press. https://doi.org/10.1017/CBO9780511804502.002
Meyer, K. M., & Kump, L. R. (2008). Oceanic euxinia in earth history: Causes and consequences. Annual Review of Earth and Planetary Sciences, 36(1), 251–288.
Miles, C. J., & Brezonik, P. L. (1981). Oxygen consumption in humic-colored waters by a photochemical ferrous-ferric catalytic cycle. Environmental Science & Technology, 15(9), 1089–1095. https://doi.org/10.1021/es00091a010
Miller, E. K., Blum, J. D., & Friedland, A. J. (1993). Determination of soil exchangeable-cation loss and weathering rates using Sr isotopes. Nature, 362(6419), 438–441.
Miyoshi, H., Mori, H., & Yoneyama, H. (1991). Light-induced decomposition of saturated carboxylic acids on iron oxide incorporated clay suspended in aqueous solutions. Langmuir, 7(3), 503–507. https://doi.org/10.1021/la00051a015
Montross, S. N., Skidmore, M., Tranter, M., Kivimäki, A.-L., & Parkes, R. J. (2013). A microbial driver of chemical weathering in glaciated systems. Geology, 41, 215–218. https://doi.org/10.1130/G33572.1
Morel, F. M. M., & Hering, J. G. (1993). Principles and applications of aquatic chemistry. Wiley.
Morel, F. M. M., & Price, N. M. (2003). The biogeochemical cycles of trace metals in the oceans. Science, 300(5621), 944–947. https://doi.org/10.1126/science.1083545
Moriceau, B., Goutx, M., Guigue, C., Lee, C., Armstrong, R., Duflos, M., Tamburini, C., Charrière, B., & Ragueneau, O. (2009). Si–C interactions during degradation of the diatom Skeletonema marinoi. Deep Sea Research Part II: Topical Studies in Oceanography, 56(18), 1381–1395. https://doi.org/10.1016/j.dsr2.2008.11.026
Mukhopadhyay, P. K., Wade, J. A., & Kruge, M. A. (1995). Organic facies and maturation of Jurassic/cretaceous rocks, and possible oil-source rock correlation based on pyrolysis of asphaltenes, Scotian Basin, Canada. Organic Geochemistry, 22, 85–104. https://doi.org/10.1016/0146-6380(95)90010-1
Nakai, S., Halliday, A. N., & Rea, D. K. (1993). Provenance of dust in the Pacific Ocean. https://doi.org/10.1016/0012-821X(93)90012-X.
Nature Portfolio. (2023). Biomineralization articles from across Nature Portfolio. Retrieved from https://www.nature.com/subjects/biomineralization
Negri, A., Ferretti, A., Wagner, T., & Meyers, P. A. (2009). Organic-carbon-rich sediments through the Phanerozoic: Processes, progress, and perspectives. Palaeogeography, Palaeoclimatology, Palaeoecology, 273, 302–328.
Neikov, O. D., & Yefimov, N. A. (2019). Chapter 1 - Powder characterization and testing. In O. D. Neikov, S. S. Naboychenko, & N. A. Yefimov (Eds.), Handbook of non-ferrous metal powders (Technologies and applications) (2nd ed., pp. 3–62). Elsevier. https://doi.org/10.1016/C2014-0-03938-X
Nóbrega, G. N., Ferreira, T. O., Romero, R. E., Marques, A. G., & Otero, X. L. (2013). Iron and sulfur geochemistry in semi-arid mangrove soils (Ceará, Brazil) in relation to seasonal changes and shrimp farming effluents. Environmental Monitoring and Assessment, 185(9), 7393–7407. https://doi.org/10.1007/s10661-013-3108-
Nordstrom, D. K. (1997). Some fundamentals of aqueous geochemistry. In G. S. Plumlee, M. J. Logsdon, & L. F. Filipek (Eds.), The environmental geochemistry of mineral deposits: Part A: Processes, techniques, and health issues part B: Case studies and research topics (Vol. 6). Society of Economic Geologists, Inc. https://doi.org/10.5382/Rev.06
O’Beirne, M., Sparkes, R., Hamilton, T., Dongen, B., Gilhooly, W., III, & Werne, J. (2022). Characterization of diverse bacteriohopanepolyols in a permanently stratified, hyper-euxinic lake. Organic Geochemistry, 168, 104431. https://doi.org/10.1016/j.orggeochem.2022.104431
Ollila, A., Newsom, H., Clark, B., Wiens, R., Cousin, A., Blank, J., Mangold, N., Sautter, V., Maurice, S., Clegg, S., Gasnault, O., Forni, O., Tokar, R., Lewin, E., Dyar, M., Lasue, J., Anderson, R., McLennan, S., Bridges, J., & Team, M.S.L. (2013). Trace element geochemistry (Li, Ba, Sr, and Rb) using curiosity’s ChemCam: Early results for gale crater from Bradbury landing site to rocknest. Journal of Geophysical Research: Planets, 119(1), 255–285. https://doi.org/10.1002/2013JE004517
Organic Geochemistry. (2022). Organic geochemistry. Retrieved from https://www.journals.elsevier.com/organic-geochemistry
Ouellette, R. J., & Rawn, J. D. (2019). Organic chemistry: Structure, mechanism, synthesis (2nd ed.). Elsevier. https://doi.org/10.1016/C2016-0-04004-4
Parkhurst, D. L., & Appelo, C. A. J. (2008). PHREEQC interactive 2.15.0. United States Geological Survey.
Passey, Q. R., Bohacs, K. M., Esch, W. L., Klimentidis, R., & Sinha, S. (2010). From oil-prone source rock to gas-producing shale reservoir – Geologic and petrophysical characterization of unconventional shale-gas reservoirs. In International oil and gas conference and exhibition in China, Beijing, China. https://doi.org/10.2118/131350-MS
Paytan, A., Yao, W., & Faul, K., & Gray, E. T. (2020). Sulfur isotope. Stratigraphy, 1, 259–278. https://doi.org/10.1016/B978-0-12-824360-2.00009-7
Pearce, T. J., Wray, D. S., Ratcliffe, K. T., Wright, D. K., & Moscariello, A. (2005). Chemostratigraphy of the upper carboniferous schooner formation, Southern North Sea. In J. D. Collinson, D. J. Evans, D. W. Holliday, & N. S. Jones (Eds.), Carboniferous hydrocarbon geology: The southern North Sea and surrounding onshore areas (Occasional publications series 7) (pp. 147–164). Yorkshire Geological Society.
Pessanha, S., Queralt, I., Carvalho, M. L., & Sampaio, J. M. (2019). Determination of gold leaf thickness using X-ray fluorescence spectrometry: Accuracy comparison using analytical methodology and Monte Carlo simulations. Applied Radiation and Isotopes, 152, 6–10.
Peters, K. E., Walters, C. C., & Moldowan, J. M. (2005). The biomarker guide (2nd ed.). Cambridge University Press.
Phillippi, G. T. (1965). On the depth, time and mechanism of petroleum generation. Geochimica et Cosmochimica Acta, 29, 1021–1104.
Philp, R. P., & Mansuy, L. (1997). Petroleum geochemistry: Concepts, applications, and results. Energy & Fuels, 11(4), 749–760. https://doi.org/10.1021/ef960174v
Pötz, S., Liu, Y., Vieth-Hillebrand, A., Yang, S., Magnall, J. M., Gleeson, S. A., & Schulz, H.-M. (2021). The Kupferschiefer in Spremberg, East Germany: Signals of organic matter alteration from hydrogen isotopes and Nso compounds - conference proceedings. In 30th international meeting on organic geochemistry (IMOG 2021) (Online 2021). https://doi.org/10.3997/2214-4609.202134210
Priscu, J. C., & Christner, B. C. (2004). Earth’s icy biosphere. In A. T. Bull (Ed.), Microbial diversity and prospecting (pp. 130–145). ASM Press.
Qin, Z., Liu, H. M., Ma, Y. X., & Wang, X. D. (2021). Chapter 9: Developments in extraction, purification, and structural elucidation of proanthocyanidins (2000–2019). Studies in Natural Products Chemistry, 68, 347–391. https://doi.org/10.1016/B978-0-12-819485-0.00008-6
Rafferty, J. P. (2013). Biogeochemistry. Retrieved from https://www.britannica.com/science/biogeochemistry
Railsback, L. B. (2003). An earth scientist’s periodic table of the elements and their ions. Geology, 31, 737–740.
Raiswell, R., Tranter, M., Benning, L. G., Siegert, M., De’Ath, R., Huybrechts, P., & Payne, T. (2006). Contributions from glacially derived sediment to the global iron (oxyhydr)oxide cycle: Implications for iron delivery to the oceans. Geochimica et Cosmochimica Acta, 70(11), 2765–2780. https://doi.org/10.1016/j.gca.2005.12.027
Rass, I. T., Aranovich, L. Y., Korpechkov, D. I., & Kozlovskii, V. M. (2014). Geochemistry of metamorphic processes in mafic rocks of the Krasnaya Guba area, Belomorian Mobile Belt. Geochemistry International, 52(8), 670–686. https://doi.org/10.1134/S0016702914060068
Ratcliffe, K. T., Hughes, A. D., Lawton, D. E., Wray, D. S., Bessa, F., Pearce, T. J., & Martin, J. (2006). A regional chemostratigraphically defined correlation framework for the late Triassic TAG-I in Blocks 402 and 405a, Algeria. Petroleum Geoscience, 12, 3–12.
Ratcliffe, K. T., Morton, A., Ritcey, D., & Evenchick, C. E. (2007). Whole rock geochemistry and heavy mineral analysis as exploration tools in the Bowser and Sustut Basins, British Colombia, Canada. Journal of Canadian Petroleum Geology, 55, 320–237.
Ratcliffe, K. T., Wright, A. M., Montgomery, P., Palfrey, A., Vonk, A., Vermeulen, J., & Barrett, M. (2010). Application of chemostratigraphy to the Mungaroo formation, the gorgon field, offshore Northwest Australia. Australian Petroleum Production and Exploration Association Journal, 50, 371–385.
Ratcliffe, K. T., Wright, A. M., & Schmidt, K. (2012). Application of inorganic whole-rock geochemistry to shale resource plays: An example from the Eagle Ford Shale Formation, Texas. Sedimentary Record, 10(2), 4–9. https://doi.org/10.2110/sedred.2012.2.4
Reid, N., & Hill, S. (2010). Biogeochemical sampling for mineral exploration in arid terrains: Tanami Gold Province, Australia. Journal of Geochemical Exploration, 104(3), 105–117. https://doi.org/10.1016/j.gexplo.2010.01.004
Rinot, O., Levy, G. J., Steinberger, Y., Svoray, T., & Eshel, G. (2019). Soil health assessment: A critical review of current methodologies and a proposed new approach. The Science of the Total Environment, 648, 1484–1491. https://doi.org/10.1016/j.scitotenv.2018.08.259
Rose, A. W. (1998). Geochemical exploration. In Geochemistry. Encyclopedia of earth science. Springer. https://doi.org/10.1007/1-4020-4496-8_137
Roy-Barman, M., & Jeandel, C. (2016). Radioactive and radiogenic isotopes. In M. Roy-Barman & C. Jeandel (Eds.), Marine geochemistry: Ocean circulation, carbon cycle and climate change (pp. 129–161). Oxford University Press. https://doi.org/10.1093/acprof:oso/9780198787495.003.0004
Sahai, N., Kaddour, H., & Dalai, P. (2016). The transition from geochemistry to biogeochemistry. Elements, 12(6), 389–394. https://doi.org/10.2113/gselements.12.6.389
Salifu, M., Aiglsperger, T., Hällström, L., Martinsson, O., Billström, K., Ingri, J., Dold, B., & Alakangas, L. (2018). Strontium (87Sr/86Sr) isotopes: A tracer for geochemical processes in mineralogically-complex mine wastes. Applied Geochemistry, 99, 42–54. https://doi.org/10.1016/j.apgeochem.2018.10.022
Sanders, T. G., Jr., Biddanda, B. A., Stricker, C. A., & Nold, S. C. (2011). Benthic macroinvertebrate and fish communities in Lake Huron are linked to submerged groundwater vents. Aquatic Biology, 12, 1–11. https://doi.org/10.3354/ab00318
Sarala, P. (2015). Chapter 10.1: Surficial geochemical exploration methods. In W. D. Maier, R. Lahtinen, & H. O’Brien (Eds.), Mineral deposits of Finland (pp. 711–731). https://doi.org/10.1016/B978-0-12-410438-9.00027-3
Schlesinger, W. H., & Bernhardt, E. S. (2013). Biogeochemistry: An analysis of global change (Third ed.). Academic.
Schmitt-Kopplin, P., Gabelica, Z., Gougeon, R. D., Fekete, A., Kanawati, B., Harir, M., Gebefuegi, I., Eckel, G., & Hertkorn, N. (2010). High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proceedings of the National Academy of Sciences of the United States of America, 107(7), 2763–2768. https://doi.org/10.1073/pnas.0912157107
Schmus, W. R., & Wood, J. A. (1967). A chemical–petrologic classification for the chondritic meteorites. Geochimica et Cosmochimica Acta, 31, 747–765. https://doi.org/10.1016/S0016-7037(67)80030-9
Schrauzer, G. N., Strampach, N., Hui, L. N., Palmer, N. R., & Salehi, J. (1983). Nitrogen photoreduction on desert sands under sterile conditions. Proceedings of the National Academy of Sciences of the United States of America, 80, 3873–3876.
Science Direct. (2022a). Organic geochemistry. Retrieved from https://www.sciencedirect.com/journal/organic-geochemistry/about/aims-and-scope
Science Direct. (2022b). Applied clay science. Retrieved from https://www.sciencedirect.com/journal/applied-clay-science
Science Direct. (2022c). Applied geochemistry. Retrieved from https://www.sciencedirect.com/journal/applied-geochemistry/about/aims-and-scope
Science Direct. (2022d). Applied geochemistry. Retrieved from https://www.sciencedirect.com/journal/applied-geochemistry
Science Direct. (2022e). Chemical geology. Retrieved from https://www.sciencedirect.com/journal/chemical-geology
Science Direct. (2022f). Continental shelf research. Retrieved from https://www.science direct.com/journal/continental-shelf-research
Science Direct. (2022g). Earth and planetary science letters. Retrieved from https://www.sciencedirect.com/journal/earth-and-planetary-science-letters/about/aims-and-scope
Science Direct. (2022h). Engineering geology. Retrieved from https://www.sciencedirect.com/journal/engineering-geology/about/aims-and-scope
Science Direct. (2022i). Geochemistry. Retrieved from https://www.sciencedirect.com/journal/geochemistry/about/aims-and-scope
Science Direct. (2022j). Geochimica et Cosmochimica Acta. Retrieved from https://www.sciencedirect.com/journal/geochimica-et-cosmochimica-acta/about/aims-and-scope
Science Direct. (2022k). Geoscience Frontiers. Retrieved from https://www.sciencedirect.com/journal/geoscience-frontiers/about/aims-and-scope
Science Direct. (2022l). International Journal of Coal Geology. Retrieved from https://www.sciencedirect.com/journal/international-journal-of-coal-geology/about/aims-and-scope
Science Direct. (2022m). Journal of Asian Earth Sciences: X. Retrieved from https://www.sciencedirect.com/journal/journal-of-asian-earth-sciences-x/about/aims-and-scope
Science Direct. (2022n). Journal of Geochemical Exploration. Retrieved from https://www.sciencedirect.com/journal/journal-of-geochemical-exploration/about/aims-and-scope
Science Direct. (2022o). Journal of Geodynamics. Retrieved from https://www.science direct.com/journal/journal-of-geodynamics/about/aims-and-scope
Science Direct. (2022p). Journal of Palaeogeography. Retrieved from https://www.sciencedirect.com/journal/journal-of-palaeogeography/about/aims-and-scope
Science Direct. (2022q). Journal of South American Earth Sciences. Retrieved from https://www.sciencedirect.com/journal/journal-of-south-american-earth-sciences
Science Direct. (2022r). Journal of Volcanology and Geothermal Research. Retrieved from https://www.sciencedirect.com/journal/journal-of-volcanology-and-geothermal-research
Science Direct. (2022s). Lithos. Retrieved from https://www.sciencedirect.com/journal/lithos/about/aims-and-scope
Science Direct. (2022t). Marine and petroleum geology. Retrieved from https://www.sciencedirect.com/journal/marine-and-petroleum-geology/about/aims-and-scope
Science Direct. (2022u). Physics and Chemistry of the Earth, Parts A/B/C. Retrieved from https://www.sciencedirect.com/journal/physics-and-chemistry-of-the-earth-parts-a-b-c/about/aims-and-scope
Science Direct. (2022v). Precambrian Research. Retrieved from https://www.science direct.com/journal/precambrian-research/about/aims-and-scope
Science Direct. (2022w). Results in Geochemistry. Retrieved from https://www.sciencedirect.com/journal/results-in-geochemistry/about/aims-and-scope
Scott, S. D. (2014). Volume Editor’s introduction. In K. Turekian & H. Holland (Eds.), Treatise on geochemistry (Vol. 13, 2nd ed., pp. xxiii–xxv).
Scripps Institution of Oceanography. (2022). Earth low temperature geochemistry. Retrieved https://scripps.ucsd.edu/research/topics/low-temperature-geochemistry
Sen, Z. (2015). Practical and applied hydrogeology. Elsevier. https://doi.org/10.1016/C2013-0-14020-2
Sephton, M. A. (2002). Organic compounds in carbonaceous meteorites. Natural Product Reports, 19(3), 292–311. https://doi.org/10.1039/b103775g
SGS. (2022). Mining geochemistry. Retrieved from https://www.sgs.ca/en/mining/analytical-services/geochemistry
Shand, P., Darbyshire, D. F., Gooddy, D., & Haria, A. H. (2007). 87Sr/86Sr as an indicator of 87Sr/86Sr ratios flow paths and weathering rates in the Plynlimon experimental catchments, Wales, UK. Chemical Geology, 236(3), 247–265.
Sharp, M., Parkes, J., Cragg, B., Fairchild, I. J., Lamb, H., & Tranter, M. (1999). Widespread bacterial populations at glacier beds and their relationship to rock weathering and carbon cycling. Geology, 27(2), 107–110. https://doi.org/10.1130/0091-7613(1999)027<0107:WBPAGB>2.3.CO;2
Siegert, M. J., Ellis-Evans, J. C., Tranter, M., Mayer, C., Petit, J.-R., Salamatin, A., & Priscu, J. C. (2001). Physical, chemical, and biological processes in Lake Vostok and other Antarctic subglacial lakes. Nature, 414, 603–609. https://doi.org/10.1038/414603a
Simmons, W. B. (2014). Pyroxene. Encyclopedia Britannica. https://www.britannica.com/science/pyroxene
Sinha, S. (2013). Biogeochemistry. Retrieved from https://www.britannica.com/science/biogeochemistry#ref1068660
Skidmore, M. L., Foght, J. M., & Sharp, M. J. (2000). Microbial life beneath a high arctic glacier. Applied and Environmental Microbiology, 66(8), 3214–3220. https://doi.org/10.1128/AEM.66.8.3214-3220.2000
Slatt, R. M., Zhang, J., Torres-Parada, E., Brito, R., Duarte, D., & Milad, B. (2021). Unconventional petroleum resources. In D. Alderton & S. A. Elias (Eds.), Encyclopedia of geology (pp. 783–807). Academic. https://doi.org/10.1016/B978-0-08-102908-4.00023-0
Sluijk, D., & Parker, J. R. (1984). Comparison of predrilling predictions with post drilling outcomes. AAPG Bulletin, 68(4), 528. https://doi.org/10.1306/AD4611E2-16F7-11D7-8645000102C1865D
Smith, R. L., Repert, D. A., & Hart, C. P. (2009). Geochemistry of inorganic nitrogen in waters released from coal-bed natural gas production wells in the Powder River Basin, Wyoming. Environmental Science & Technology, 43(7), 2348–2354. https://doi.org/10.1021/es802478p
Soldatova, E., Dong, Y., Li, J., Liu, Y., Zan, J., Boeckx, P., & Sun, Z. (2021). Nitrogen transformation and pathways in the shallow groundwater-soil system within agricultural landscapes. Environmental Geochemistry and Health, 43(1), 441–459. https://doi.org/10.1007/s10653-020-00733-w
Sowder, J. T., Kelleners, T. J., & Reddy, K. J. (2010). The origin and fate of arsenic in coalbed natural gas-produced water ponds. Journal of Environmental Quality, 39(5), 1604–1615. https://doi.org/10.2134/jeq2009.0428
Springer Nature. (2022a). Bulletin of Volcanology. Retrieved from https://www.springer.com/journal/445/aims-and-scope
Springer Nature. (2022b). Contributions to mineralogy and petrology. Retrieved from https://www.springer.com/journal/410#:~:text=Contributions%20to%20Mineralogy%20and%20Petrology%20is%20an%20international%20journal%20that,metamorphic%20petrology%2C%20geochemistry%20and%20mineralogy
Springer Nature. (2022c). Geochemistry International. Retrieved from https://www.springer.com/journal/11476
Springer Nature. (2022d). Physics and Chemistry of Minerals. Retrieved from https://www.springer.com/journal/269
Stern, J. C., & Wieman, S. T. (2021). Traditional stable isotope geochemistry. In D. Alderton & S. A. Elias (Eds.), Encyclopedia of geology (2nd ed., pp. 100–113). Academic.
Stewart, B. W., Capo, R. C., & Chadwick, O. A. (2001). Effects of rainfall on weathering rate, base cation provenance, and Sr isotope composition of Hawaiian soils. Geochimica et Cosmochimica Acta, 65(7), 1087–1099.
Stoll, N., Eichler, J., Hörhold, M., Erhardt, T., Jensen, C., & Weikusat, I. (2021). Microstructure, micro-inclusions, and mineralogy along the EGRIP ice core – Part 1: Localisation of inclusions and deformation patterns. The Cryosphere, 15, 5717–5737. https://doi.org/10.5194/tc-15-5717-2021
Stricker, C. (2023). Biogeochemistry: Stable isotope applications. Retrieved from https://www.usgs.gov/centers/fort-collins-science-center/science/biogeochemistry-stable-isotope-applications#overview
Strunz, H. (1941). Mineralogische Tabellen. Akademische Verlagsgesellschaft.
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry, chemical equilibria and rates in natural waters (3rd ed.). Wiley.
Subίas, I., Fanlo, I., & Billström, K. (2015). Ore-forming timing of polymetallic-fluorite low temperature veins from central Pyrenees: A Pb, Nd and Sr isotope perspective. Ore Geology Reviews, 70, 241–251.
Sutton, J. N., André, L., Cardinal, D., Conley, D. J., de Souza, G. F., Dean, J., Dodd, J., Ehlert, C., Ellwood, M. J., Frings, P. J., Grasse, P., Hendry, K., Leng, M. J., Michalopoulos, P., Panizzo, V. N., & Swann, G. E. A. (2018). A review of the stable isotope biogeochemistry of the global silicon cycle and its associated trace elements. Frontiers of Earth Sciences, 5, 112. https://doi.org/10.3389/feart.2017.00112
Tang, J., Zheng, Y., Wu, Y. B., Gong, B., & Liu, X. (2007). Geochronology and geochemistry of metamorphic rocks in the Jiaobei terrane: Constraints on its tectonic affinity in the Sulu orogen. Precambrian Research, 152, 48–82. https://doi.org/10.1016/j.precamres.2006.09.001
Taylor and Francis Online. (2022a). Australian Journal of Earth Sciences. Retrieved from https://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=taje20
Taylor and Francis Online. (2022b). Tellus B: Chemical and physical meteorology. Reterived from https://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=zelb20
Taylor, S. R., & McLennan, S. M. (1985). The continental crust: Its composition and evolution. Blackwell Scientific Publishers.
Telling, J., Boyd, E. S., Bone, N., Jones, E. L., Tranter, M., Macfarlane, J. W., Martin, P. G., Wadham, J. L., Lamarche-Gagnon, G., Skidmore, M. L., Hamilton, T. L., Hill, E., Jackson, M., & Hodgson, D. A. (2015). Rock comminution as a source of hydrogen for subglacial ecosystems. Nature Geoscience, 8, 851–855. https://doi.org/10.1038/ngeo2533
Terry, R. E. (2017). Field geochemistry. In A. S. Gilbert (Ed.), Encyclopedia of geoarchaeology (Encyclopedia of earth sciences series). Springer. https://doi.org/10.1007/978-1-4020-4409-0_165
The International Conference on the Biogeochemistry of Trace Elements (ICOBTE). (2023). Retrieved from https://icobte-ichmet-2023.com/frontend/index.php
Tipper, E. T., Bickle, M. J., Galy, A., West, A. J., Pomiès, C., & Chapman, H. J. (2006). The short term climatic sensitivity of carbonate and silicate weathering fluxes: Insight from seasonal variations in river chemistry. Geochimica et Cosmochimica Acta, 70(11), 2737–2754. https://doi.org/10.1016/j.gca.2006.03.005
Tissot, B. P., & Welte, D. H. (1978). Petroleum formation and occurrence. Springer. https://doi.org/10.1007/978-3-642-96446-6
Titley, S. R. (2001). Crustal affinities of metallogenesis in the American Southwest. Economic Geology, 96(6), 1323–1342. https://doi.org/10.2113/gsecongeo.96.6.1323
Tiwari, M., Singh, A. K., & Sinha, D. K. (2015). Stable isotopes: Tools for understanding past climatic conditions and their applications in chemostratigraphy. In M. Ramkumar (Ed.), Chemostratigraphy: Concepts, techniques, and applications (pp. 65–92). https://doi.org/10.1016/C2013-0-09872-6
Torró, L., Benites, D., Vallance, J., Laurent, O., Ortiz Benavente, B. A., Chelle-Michou, C., Proenza, J., & Fontboté, L. (2022). Trace element geochemistry of sphalerite and chalcopyrite in arc-hosted VMS deposits. Journal of Geochemical Exploration, 232, 106882. https://doi.org/10.1016/j.gexplo.2021.106882
Tranter, M. (2015). Grand challenge for low temperature and pressure geochemistry—Sparks in the dark, on Earth, Mars, and throughout the Galaxy. Frontiers in Earth Science, 3, 69. https://doi.org/10.3389/feart.2015.00069
Tranter, M., & Wadham, J. L. (2013). Geochemical weathering in glacial and proglacial environments. In J. I. Drever (Ed.), Treatise on geochemistry (Vol. 5, Surface and ground water, weathering, and soils, 2nd ed., pp. 153–174). Elsevier.
Tranter, M., Sharp, M. J., Lamb, H. R., Brown, G. H., Hubbard, B. P., & Willis, I. C. (2002). Geochemical weathering at the bed of Haut Glacier d’Arolla, Switzerland - A new model. Hydrological Processes, 16, 959–993. https://doi.org/10.1002/hyp.309
Trask, P., & Patnode, D. (1942). Source beds of petroleum. American Association of Petroleum Geologists.
Tréguer, P. J., Sutton, J. N., Brzezinski, M., Charette, M. A., Devries, T., Dutkiewicz, S., Ehlert, C., Hawkings, J., Leynaert, A., Liu, S. M., Llopis Monferrer, N., López-Acosta, M., Maldonado, M., Rahman, S., Ran, L., & Rouxel, O. (2021). Reviews and syntheses: The biogeochemical cycle of silicon in the modern ocean. Biogeosciences, 18, 1269–1289. https://doi.org/10.5194/bg-18-1269-2021
Treibs, A. E. (1934). Chlorophyll- und Häminderivate in bituminösen Gesteinen, Erdölen, Erdwachsen und Asphalten. Ein Beitrag zur Entstehung des Erdöls, 510(1), 42–62.
Treibs, A. E. (1936). Chlorophyll- und Ha¨minderivate in organischen Mineralstoffen. Angewandte Chemie, 49, 682–686.
Tribovillard, N., Algeo, T. J., Lyons, T., & Riboulleau, A. (2006). Trace metals as paleoredox and paleoproductivity proxies: An update. Chemical Geology, 232, 12–32.
Tribovillard, N., Bout-Roumazeilles, V., Algeo, T., Lyons, T. W., Sionneau, T., Montero-Serrano, J. C., Riboulleau, A., & Baudin, F. (2008). Paleodepositional conditions in the Orca Basin as inferred from organic matter and trace metal contents. Marine Geology, 254, 62–72.
Trueman, C., Rodgers, K., McLellan, I., & Hursthouse, A. (2019). Geochemistry: Inorganic. In P. Worsfold, C. Poole, A. Townshend, & M. Miró (Eds.), Encyclopedia of analytical science: Reference module in chemistry, molecular sciences and chemical engineering (3rd ed., pp. 271–282). Elsevier Limited. https://doi.org/10.1016/B978-0-12-409547-2.14362-8
Turgeon, S., & Brumsack, H.-J. (2006). Anoxic vs. dysoxic events reflected in sediment geochemistry during the Cenomanian–Turonian Boundary Event (Cretaceous) in the Umbria–Marche Basin of central Italy. Chemical Geology, 234, 321–339.
United States Geological Survey. (2022). What are metamorphic rocks? Retrieved from https://www.usgs.gov/faqs/what-are-metamorphic-rocks
University of Auckland. (2005). Geology: Rocks and minerals. https://rocksminerals.flexiblelearning.auckland.ac.nz/rocks/schist.html
University of Michigan. (2022). Low temperature geochemistry. Retrieved from https://lsa.umich.edu/earth/research/geochemistry.html
Utrecht University. (2022). Organic geochemistry. Retrieved from https://www.uu.nl/en/research/department-of-earth-sciences/organic-geochemistry
Vallero, D. (2014). Inherent properties of air pollutants. In D. Vallero (Ed.), Fundamentals of air pollution (pp. 139–195). Academic. https://doi.org/10.1016/B978-0-12-401733-7.00006-2
Van Grieken, R. E., & Markowicz, A. A. (2002). Handbook of X-ray spectrometry (2nd ed.). Marcel Dekker.
Vandenbroucke, M., & Largeau, C. (2007). Kerogen origin, evolution and structure. Organic Geochemistry, 38(5), 719–833. https://doi.org/10.1016/j.orggeochem.2007.01.001
Volkman, J. K. (1998). Organic geochemistry. In Geochemistry (Encyclopedia of earth science). Springer. https://doi.org/10.1007/1-4020-4496-8_226
Wadham, J. L., Tranter, M., Skidmore, M., Hodson, A. J., Priscu, J., Lyons, W. B., Sharp, M., Wynn, P., & Jackson, M. (2010). Biogeochemical weathering under ice: Size matters. Global Biogeochemical Cycles, 24, GB3025. https://doi.org/10.1029/2009GB003688
Wadham, J. L., De’ath, R., Monteiro, F., Tranter, M., Ridgwell, A., Raiswell, R., & Tulaczyk, S. (2013). The potential role of the Antarctic Ice Sheet in global biogeochemical cycles. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 104(1), 55–67. https://doi.org/10.1017/S1755691013000108
Wang, J., Dai, J., Chen, G., & Jiang, F. (2022). Role of sulfur biogeochemical cycle in mercury methylation in estuarine sediments: A review. Journal of Hazardous Materials, 423(Pt A), 126964. https://doi.org/10.1016/j.jhazmat.2021.126964
Waples, D. W. (1980). Time and temperature in petroleum generation-application of Lopatin’s technique to petroleum exploration. AAPG Bulletin, 64, 916–926.
Wasserburg, G. J., & Hayden, R. J. (1955). Age of meteorites by the 40Ar–40K method. Physics Review, 97, 86–87. https://doi.org/10.1103/PhysRev.97.86
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta, 59, 1217–1232. https://doi.org/10.1016/0016-7037(95)00038-2
Weiss, J. V., Emerson, D., Backer, S. M., & Megonigal, P. (2003). Enumeration of Fe(II)-oxidizing and Fe(III)-reducing bacteria in the root zone of wetland plants: Implications for a rhizosphere iron cycle. Biogeochemistry, 64, 77–96. https://doi.org/10.1023/A:1024953027726
Wetzel, R. G. (2001). Limnology: Lake and river ecosystems (Third ed.). Academic.
White, W. B. (2010). Chapter 6: Springwater geochemistry. In N. Kresic & Z. Stevanovic (Eds.), Groundwater hydrology of springs: Engineering, theory, management, and sustainability (pp. 231–268). Butterworth-Heinemann. https://doi.org/10.1016/C2009-0-19145-6
White, W. M. (2013). Geochemistry. Wiley-Blackwell.
White, W. M. (2017). Geochemistry. In W. White (Ed.), Encyclopedia of geochemistry(Encyclopedia of earth sciences series). Springer. https://doi.org/10.1007/978-3-319-39193-9_294-1
White, W. M. (2020). Geochemistry. Wiley.
Whiteside, J. H., & Grice, K. (2016). Biomarker records associated with mass extinction events. Annual Review of Earth and Planetary Sciences, 44, 581–516.
Williamson, M. A. (1998). Iron. In Geochemistry (Encyclopedia of earth science). Springer. https://doi.org/10.1007/1-4020-4496-8_172
Wilschefski, S. C., & Baxter, M. R. (2019). Inductively coupled plasma mass spectrometry: Introduction to analytical aspects. The Clinical Biochemist. Reviews, 40(3), 115–133. https://doi.org/10.33176/AACB-19-00024
Winkel, L. H. E., & Sunderland, E. M. (2022). Introduction to the biogeochemistry of the trace elements themed issue. Environmental Science: Processes & Impacts, 24, 1277–1278. https://doi.org/10.1039/D2EM90031A
Winter, J. D. (2013). Principles of igneous and metamorphic petrology. Pearson Education.
Winterburn, P. A., Noble, R. R. P., & Lawie, D. (2017). Advances in exploration geochemistry, 2007 to 2017 and beyond. Geochemistry. Paper, 34, 495–505.
Winterburn, P. A., Noble, R. R. P., & Lawie, D. (2020). Advances in exploration geochemistry, 2007 to 2017 and beyond. Geochemistry: Exploration, Environment, Analysis, 20(2), 157–166. https://doi.org/10.1144/geochem2019-030
Wooden, J. L., Stacey, J. S., Howard, K. A., Doe, B. R., & Miller, D. M. (1988). Pb isotopic evidence for the formation of Proterozoic crust in the southwestern United States. In Metamorphism and crustal evolution of the western United States (pp. 69–86). Prentice-Hall.
Wright, A. M., Ratcliffe, K. T., Zaitlin, B. A., & Wray, D. S. (2010). The application of chemostratigraphic techniques to distinguish compound incised valleys in low-accommodation incised-valley systems in a foreland-basin setting: An example from the Lower Cretaceous Mannville Group and Basal Colorado Sandstone (Colorado Group), Western Canadian Sedimentary Basin. In K. T. Ratcliffe & B. A. Zaitlin (Eds.), Application of modern stratigraphic techniques: Theory and case histories (Vol. 94, pp. 93–109). SEPM Special Publication.
Yadav, P. K., Mauraya, A. K., Kochar, C., Taneja, L., & Swarupa Tripathy, S. (2021). Isotope geochemistry. In Inamuddin, M. I. Ahamed, R. Boddula, & T. Altalhi (Eds.), Geochemistry: Concepts and applications (pp. 93–109). Wiley; Scrivener Publishing.
Yale University. (2022). The Department of Earth & Planetary Sciences. Retrieved from https://earth.yale.edu/geochemistry
Zakaria, M., Bong, C. W., & Vaezzadeh, V. (2018). Fingerprinting of petroleum hydrocarbons in Malaysia using environmental forensic techniques. In S. A. Stout & Z. Wang (Eds.), Oil spill environmental forensics case studies (pp. 345–372). Butterworth-Heinemann.
Zou, C. (2017). Unconventional petroleum geology. Retrieved from Unconventional Petroleum Geology. Retrieved from https://www.sciencedirect.com/book/9780128122341/unconventional-petroleum-geology
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Campbell, M.O. (2023). Geochemistry as the Core of Biogeochemistry. In: Biogeochemistry and the Environment. Springer, Cham. https://doi.org/10.1007/978-3-031-47017-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-47017-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-47016-5
Online ISBN: 978-3-031-47017-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)