Abstract
This chapter provides a background to the science of biogeochemistry, examining its history, content, and relationship with other disciplines. This supports the novel approach of this book, which is to examine biogeochemistry from the perspective of its relationship with other disciplines, and thus illustrates its importance in current scientific research in the biological, environmental, and earth sciences. Biogeochemistry is an extremely broad, yet detailed subject, with a multidisciplinary, cross-disciplinary, and interdisciplinary focus. Although it is fundamentally a biological science, it is deeply rooted in the study of chemical elements and compounds and their spatial and temporal movements through global, regional, and local physical spaces. Its name, including the prefixes “geo” and “bio,” implies a concern with the earth sciences (e.g., geography, geology, geophysics, and geochemistry), the life sciences (e.g., biology, biogeography, biochemistry, and biophysics), and pure and environmental chemistry. With the word “chemistry” as the main stem word, biogeochemistry is conceived as a branch or an allied discipline of the chemical sciences. Currently, biogeochemistry has developed into a vast field of study, including studies of biological, chemical, and geological aspects of the environment, their interactions, and the chemical cycling between these spheres. This chapter examines the historical development of biogeochemistry and the chemical cycling of the main chemical elements. Biogeochemistry developed from the basic, earth, and environmental sciences during the nineteenth and twentieth centuries, and later included topics such as atmospheric chemistry, carbon cycling, climate change and weathering, biological sciences, and links between climate and the solid earth. Chemical elements studied included carbon (C), nitrogen (N), phosphorous (P), potassium (K), oxygen (O), iron (Fe), calcium (Ca), selenium (Se), sulfur (S), and mercury (Hg). Each of these elements is examined as a component of compounds that comprise and move through the lithosphere, atmosphere, hydrosphere, and biosphere. For example, common compounds that contain carbon include diamond, graphite, Carbon-14 (pure), calcium carbonate (CaCO3), and dolomite (CaMg(CO3)2). Compounds containing oxygen include carbon monoxide (CO), carbon dioxide (CO2), calcium hydroxide (Ca (OH)2, and calcium oxide (CaO), while those with nitrogen include ammonium (NH+4), nitrite (NO−2), nitrate (NO−3), nitrous oxide (N2O), and nitric oxide (NO). In recent decades, biogeochemistry has evolved into a complex discipline beyond the chemical bases of its development, including the broader issues of global chemical cycling, and the interfacial relations between the biosphere, lithosphere, hydrosphere, and atmosphere. This breadth is shared with the related sciences. This chapter, therefore, explores the rudiments of these relationships, for the greater detail of the later chapters.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Biogeochemistry is a broad, yet deep, discipline, with a regional and global focus, serving as the backbone of some other large-scale spatial sciences like biogeography and oceanography, and is strongly linked to its background subjects including geochemistry, environmental chemistry, and ecology (Butcher et al., 1992; Libes, 1992; Dobrovolsky, 1994; Malyuga, 1995; Schlesinger, 1997; Kabata-Pendias & Pendias, 1999; Bashkin & Howart, 2002; Campbell, 2017, 2018; Schlesinger & Bernhardt, 2020). With this breadth, the definitions of the science of biogeochemistry may vary (Campbell, 2020). The only common terms in the definition are usually based on the biological, geological and chemical bases of the subject (bio, geo, chemistry), and the global focus (e.g., global chemical cycling), with varying focus on these components. The study of chemical element movements through the Earth’s solid, water, and air systems (the lithosphere, hydrosphere, and atmosphere, respectively) has come to dominate some research developments (Butcher et al., 1992; Libes, 1992; Dobrovolsky, 1994; Malyuga, 1995; Campbell, 2017, 2018; Schlesinger & Bernhardt, 2020). Britannica (2020) refers to the term biogeochemical “as a contraction that refers to the consideration of the biological, geological, and chemical aspects of each cycle” and biogeochemical cycling as “any of the natural pathways by which essential elements of living matter are circulated.” Zavarzin (2008) writes that the biogeochemical cycles “represent the major chemical machinery on the Earth.” These chemical movements are also fundamental to the understanding of modern physical geography, environmental science, and ecology (Campbell, 2018).
Citing the location of biogeochemistry between the physical/chemical sciences and the life sciences, up to the global level, Cutter (2005) notes that biogeochemistry can be defined “as the mutual interactions (two-way) between the biology and chemistry of the Earth system, and as such is clearly an important component of the broader discipline of geobiology.” Karl and Schlesinger (2014) give an extremely wide definition, as biogeochemistry refers to “the holistic study of the Earth system,” and biogeochemical research includes “theoretical, observational, experimental, and modeling components, and now spans from molecular studies to the largest scales in space and time that affect our planet. Therefore, as a scientific discipline, biogeochemistry is both integrative and transdisciplinary, and it is attracting a new and diverse spectrum of investigators each year.” According to this definition, an important distinguishing point is the focus on the interactions of living organisms within the lithosphere, atmosphere, and hydrosphere. There is also an acknowledgment of the increasing relevance of biogeochemistry to the current global climate changes and anthropogenic impacts (Karl & Schlesinger, 2014). It is further argued that “while there are no rigid intellectual boundaries between biogeochemistry and other fields that also seek a fundamental understanding of our universe, a distinguishing characteristic is the interaction of living organisms within the crust of the Earth and its atmosphere and hydrosphere” (Karl and Schlesinger, 2014).
Schlesinger (2004) also gives a very broad definition, mostly from chemical perspectives, but inclusive of other sciences, as basically, “the chemistry of the arena of life – that is Earth’s biogeochemistry – will be at the center of how well we do, and all biogeochemists should strive to articulate that message clearly and forcefully to the public and to leaders of society, who must know our message to do their job well.” Biogeochemistry is defined as a recently developed discipline, while also being an integrative subject that attempts an understanding of the functioning of the planet Earth, which is a closed chemical system supportive of life for possibly over 3.5 billion years. The interdisciplinary nature of biogeochemistry is important, as the subject is studied by different specialists; “for example, it is pursued by physicists who want to understand what determines Earth’s climate, also by molecular biologists who want to understand what controls the gene expression for certain biochemical pathways, and by geologists who want to understand what controls the breakdown of rocks and the composition of the oceans through geologic time” (Schlesinger, 2004). It is further noted that “biogeochemistry is often described as the chemistry of the surface of the Earth, where the imprint of life is pervasive. Biogeochemistry is at the heart of global-change biology – trying to predict the human impact on the surface chemistry of the planet” (Schlesinger, 2021a).
Recent developments in biogeochemistry have engaged with dynamic changes in global, regional, and local ecological, biogeographical, and environmental issues, and according to Karl and Schlesinger (2014), there have been “collaborations among individuals who do not normally interact, where the whole of the coordinated effort is much greater than the sum of its parts,” the reduction of the “rigid intellectual boundaries between biogeochemistry and other fields,” new sections in biogeosciences in the Ecological Society of America and the American Geophysical Union, new professional journals, and increased links between biogeochemistry and geochemistry (Karl & Schlesinger, 2014). The authors also defend the “bio” in biogeochemistry, as “biota – ranging from bacteria to higher plants” relating to “the level of CO2 in the atmosphere, the amount of precipitation that falls on land, the content of nitrogen and phosphorus in rivers, and the silicon that is deposited in ocean sediments,” which therefore indicates that “life’s diversity – the bio in biogeochemistry – performs a great service to us, which we must understand better if we are to preserve its function” (Karl & Schlesinger, 2014).
Academic institutions have also cited definitions that support their research, and relate to current realities, which are important as such definitions may reflect the rapidly changing world of scientific work and student education in the twenty-first century. The Scripps Institution of Oceanography (2022) states that biogeochemistry studies the “chemical interactions between living things and the natural environment, ranging from how organisms incorporate and respond to elements in their environment to the alterations biological systems make to the chemical environment of the Earth.” The Department of Marine Sciences (2022) of the University of Georgia also prioritizes the biological aspect of the discipline, as “biogeochemistry examines the role of biological processes in mediating the chemical and geological dynamics of the Earth’s atmosphere, hydrosphere, and lithosphere. It is an inherently interdisciplinary science… The integrative nature of this work requires the application of tools from a wide range of disciplines such as microbiology, geochemistry, ecology, hydrology, mathematics, and physics.” This integration between the life and physical sciences was acknowledged by the Woods Hole Oceanographic Institution (2022), which offers a broader definition: biogeochemistry “explores the physical, chemical, biological, and geological processes and reactions that govern the composition of and changes to the natural environment… the cycles of crucial elements, such as carbon and nitrogen, and their interactions with other substances and organisms as they move through Earth’s atmosphere, hydrosphere (water and ice), biosphere (life), and lithosphere (rock).” Additionally, biogeochemistry “focuses especially on the diverse and interlinked chemical cycles that are either driven by or have an impact on biological activity, in particular carbon, nitrogen, sulfur, and phosphorus” (Woods Hole Oceanographic Institution, 2022).”
Historically, biogeochemistry traces its roots to the developments of the basic, earth, and environmental sciences, mostly during the nineteenth and twentieth centuries (Bianchi, 2021) (Table 1.1). For example, Laane and Middleburg (2011) describe the concept of biogeochemistry as about one hundred years old, being originated by the Russian scientist Vernadsky in 1926. Bianchi (2021, see also Gorham, 1991; Butcher et al., 1992; Libes, 1992; Dobrovolsky, 1994; Malyuga, 1995; Schlesinger, 1997; Schlesinger & Bernhardt, 2020) notes that biogeochemistry evolved with new methodologies and understandings of earth science topics such as atmospheric chemistry, carbon cycling, and climate change and weathering, and biological (including microbial) links between climate and the solid earth, with links to ecology, evolution and biogeography. Unlike Ecosystem Science and Earth System Science, “the roots of biogeochemistry are… not… as centrally linked with ecology and geology, respectively” (Bianchi, 2021). Twentieth-century developments included increased knowledge of carbon cycling, agricultural technology, human demographic change, and environmental events such as eutrophication. By the twenty-first century, molecular technologies, genetics, geomatics, and Earth system models had also made important contributions (Bianchi, 2021) (Table 1.1).
At the beginning of the twentieth century, there was a pronounced tendency for scientific disciplines to divide into variably related, specialized subdisciplines. The biosphere concept, originated by the Austrian geologist Eduard Suess in his work Die Entstehung der Alpen (1875), was further developed by Vernadsky, who by contrast, sought to create a trend toward increased integration of the biological and physical sciences. Bianchi (2021) notes that biogeochemistry was “born from multidisciplinary interactions between biological, geological, and chemical sciences early in the 19–20th centuries,” and “has continued to expand its scope in the twenty-first century on scales that range from microbiological/‘omics approaches (genomics, transcriptomics, proteomics, and metabolomics) to global elemental flux transfers in Earth System models” (see also Steffen et al., 2020), and that biogeochemistry enabled clustering of geobiology and geochemistry. Other researchers have published related pieces on the development of biogeochemistry within the earth, environmental, and even socio-economic sciences (Campbell, 2017, 2018).
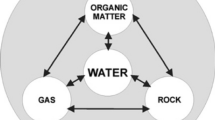
Laane and Middelburg (2011) note that eventually, biogeochemistry gained much greater attention, “especially because the footprint of man on his own environment is increasing and integrated information and knowledge is necessary to understand man’s impact,” and “an enormous explosion has been observed in the number of scientific papers containing the keyword biogeochemistry,” making the examination of the content and changing trends of biogeochemistry a historically timely endeavor (Laane & Middleburg, 2011). These studies may involve chemical as well as biological foundations. Numerous chemical elements may be studied, and these may form complex compounds (Tables 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 1.10, 1.11, and 1.12). These tables show some of the chemical elements and the compounds that may be formed between these compounds. Further, it is noted that “elements within biogeochemical cycles flow in various forms from the nonliving (abiotic) components of the biosphere to the living (biotic) components and back” (Britannica (2020). See Figs. 1.1, 1.2, 1.3, 1.4, and 1.5 for examples of the components of the Earth’s system.
Biogeochemical cycles transform energy and matter into forms usable for ecosystem functioning and relate to the movement of matter between the Earth’s main reservoirs (the atmosphere, the terrestrial biosphere, the oceans, and the solid soil and rock geosphere) (Brusseau et al., 2019). The study of biogeochemical cycles assumes the existence of two main components. These are “a reservoir (nutrient) pool – a larger, slow-moving, usually abiotic portion- and an exchange (cycling) pool – a smaller but more-active portion concerned with the rapid exchange between the biotic and abiotic aspects of an ecosystem” (Britannica, 2020). The biogeochemical cycles can be defined as gaseous, in which the reservoir is either the air or the oceans (through evaporation), or sedimentary, where the reservoir is the Earth’s crust. The gaseous cycles include those of nitrogen, oxygen, carbon, and water, while the sedimentary cycles comprise those of iron, calcium, phosphorus, sulfur, and other more-earthbound elements (Britannica, 2020). These biogeochemical cycles are crucial for biological systems and influence global, regional, and local biogeography (Campbell, 2018). The commonest elements for organic molecules, which are the basis of life forms are carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur (Table 1.2). Boero et al. (2019) add the importance of biogeochemical studies for the “understanding of primary producers, these often termed ‘nutrients’… and hence biogeochemical cycling may be an indication of ecosystem functioning and ecological processes” (Boero et al., 2019) and a measure of ecological processes (Campbell, 2022).
Biogeochemical studies, as noted in several definitions of the research focus (e.g., Karl & Schlesinger, 2014), may also examine the impact of biological organisms on the Earth’s chemistry, a complex subject due to the vast number of biological species which may impact variously on the chemical environment, increasing the size of the biogeochemical subject area (Hellweger, 2008; Campbell, 2017, 2018; Schlesinger & Bernhardt, 2020). Tartowski and Howarth (2013) support this definition, stating that biogeochemistry is the “discipline which studies biotic controls on the chemistry of the environment and geochemical controls on the structure and function of ecosystems.” Woods Hole Oceanographic Institution (2022) points to the importance of biological phenomena, as “biogeochemistry is the study of the nearly limitless “transactions” that drive the entire planetary system, including life on Earth. Understanding these fundamental processes provides crucial insights into how life formed, has evolved, is sustained, and is threatened on our planet, and how the various chemical cycles govern and regulate Earth’s climate and environment.”
Table 1.2 shows the common chemical elements and some of the compounds created when these elements combine with other elements. Tables 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 1.10, 1.11, and 1.12 show more details on the combinations of elements into compounds. These tables are provided to illustrate the chemical elements and compounds that compose the materials of biogeochemical cycling. The models in Figs. 1.6, 1.7, 1.8, 1.9, 1.10, 1.11, 1.12, 1.13, 1.14, 1.15, and 1.16 show simplified examples of the biogeochemical cycles. All the cycles include stages in the lithosphere, biosphere, hydrosphere, and atmosphere. All the compounds described may be formed naturally or through human scientific action, but frequently occur in the environment and may have variable impacts on plant and animal ecologies. Some compounds are relatively insoluble solids, while others may be dissolved into water or gaseous form, especially those containing nitrogen, oxygen, and carbon (Campbell, 2022).
The phosphorous cycle. This is one of the most important cycles. Kvakić et al. (2020) note that “phosphorus (P) is the second most important nutrient after nitrogen (N) and can greatly diminish plant productivity if P supply is not adequate. Plants respond to soil P availability by adjusting root biomass to maintain uptake and productivity due to P use”
Links Between Biogeochemistry and Other Sciences
Biogeochemistry is strongly linked to numerous environmental and earth sciences, as it is scaled from the study of microbial ecologies and chemical elements to the characteristics of vast Earth systems (Figs. 1.17, 1.18, 1.19, and 1.20). At the most basic level, the primary science is chemistry (Campbell, 2017, 2018). The basic components of biogeochemistry include the atmospheric, solid earth, biological and water sciences. Branches of biogeochemistry may link with associated disciplines, or even disciplines less related, but relevant to chemistry or even biology, such as urban planning and pollution. The spatial, global perspective of biogeochemistry also links it with the geographical sciences, including oceanography (Figs. 1.26, 1.27, 1.28, 1.29, 1.30, and 1.31).
Relationship between biogeochemistry and atmospheric sciences, based on changing emissions. The chemical compounds change the chemical composition of the air and increase the aerosols, contributing to atmospheric change. (Source: Denman et al., 2007)
Global change. Relationship between biogeochemistry and solid earth sciences. Earth, water, and air cycling are influenced by chemical changes, which are studied by the disciplines in the first column. (Adapted from Bianchi et al., 2020)
Relationship between biogeochemistry and biological sciences. Here, chemical cycling occurs as carnivores at the top of the food chain kill and eat herbivores, which also eat plants and associated water. Decomposers in association with soil chemistry start the process again. The remains of animals and plants. (Source: Example derived from Schmitz (2020))
Relationship between biogeochemistry and water sciences. Chemical elements and compounds in the air are strongly influenced by both nature and human influenced hazards. (Example derived from Campbell (2020))
Biogeochemistry and Physical Geography
Biogeochemistry is also closely linked to physical geography, and in some cases may even be seen as almost synonymous to physical geography (Campbell, 2017, 2018). As pointed out by Warf (2010), “biogeochemistry is the chemistry of Earth’s surface. Or, in other words, it is physical geography through the lens of chemistry” and a “biogeochemical cycle examines how a particular chemical element moves through the physical geographic spheres (i.e., the atmosphere, biosphere, lithosphere, and hydrosphere).” While some classifications of physical geography divide the discipline into the sections of geomorphology, climatology, pedology, biogeography and sometimes oceanography and geomatics, the perspective of biogeochemistry would be to integrate these disciplines or subdisciplines to examine their interrelationships, the better to study and explain the dynamic and highly holistic nature of the Earth’s environment, without artificial, disciplinary boundaries (Campbell, 2020). Figures 1.21, 1.22, 1.23, 1.24, 1.25, 1.26, 1.27, 1.28, 1.29, 1.30, and 1.31 show the links between the components of physical geography and biogeochemistry including some urban issues.
Background of geomorphology, which includes geochemical bases of biogeochemistry. The pure sciences are listed in the column on the right, these forming the backbone of the applied earth sciences listed to the left, which may be part of the “geo” in biogeochemistry, and link strongly to the geomorphological part of physical geography
Biogeochemistry and Ocean Sciences
The oceans are an important study area for both biogeochemistry and physical geography/oceanography. They represent a large portion of global biogeochemistry, and they are an important focus of such studies, both within and outside the discipline of physical geography (e.g., biological, chemical, physical, and geological oceanography, and marine biology/ecology, marine geology, marine chemistry, and marine physics) (Scripps Institution of Oceanography, 2022). Such oceanographic studies may focus on both physical and biological parameters. For example, Brasseur et al. (2009, 206) hold that “monitoring and predicting the biogeochemical state of the ocean and marine ecosystems is an important application of operational oceanography that needs to be expanded... most of these applications require accurate estimates of both physical and biogeochemical ocean conditions over a wide range of spatial and temporal scales.”
In another example, ocean ecology and biogeochemistry may be integrated, as indicated in recent university led research (College of Earth, Ocean, and Atmospheric Sciences, 2022). Ocean ecology and biogeochemistry is defined as “the study of the ocean’s biological, chemical, and geological processes, and their interplay” and the study of the “structure and function of ecosystems across space and time, including feedbacks between land, atmosphere and ocean” (College of Earth, Ocean, and Atmospheric Sciences, 2022). Falkowski (2003: 163) notes that a major characteristic of the Earth is that it is the only planet in the solar system with known water supplies, and with an atmosphere with concentrations of free molecular oxygen. It is argued that these two features, oceans, and an atmosphere, were derived from the photobiologically catalyzed splitting of water by unicellular photosynthetic organisms that existed in the oceans for millions of years, during which the organisms used the hydrogen atoms from water and Earth materials to form organic matter from carbon dioxide (CO2) and other hydrated equivalents, this being the process by which organic matter was formed from inorganic carbon. This primary production “is the basis for all life on Earth” (Falkowski, 2003: 163).
Achterberg (2011) justifies the development of an ocean-focused biogeochemistry: The ocean is vital for the Earth’s climate system, ecosystem services, chemical element cycling between the sea floor, ocean, land and atmosphere, and human actions increasing emissions of nitrogen, phosphorus, carbon, and trace elements. Hence, a “detailed understanding of biogeochemical processes, including their rates, is essential to the identification and assessment of climatic and chemical feedbacks associated with changes in the chemical and physical environment that are mediated through ocean biology, chemistry and physics” (Achterberg, 2011). Research areas include “the cycling of organic and inorganic forms of carbon, nitrogen and phosphorus, the cycling and biological roles of essential trace elements, and the fate and climatic impact of marine produced trace gases” (Achterberg, 2011).
Urban and Pollution Biogeochemistry
Urban biogeochemistry is another recently developed and continually developing branch of biogeochemistry that links this science to urban planning, ecology, and management (Bianchi, 2021). Bianchi (2021) observes that the subdisciplines of biogeochemistry are increasing in number, and the emergence of urban biogeochemistry partially represents an integration of urban ecology and environmental sciences (see also Grimm et al., 2008; Pickett et al., 2011; Schulze, 2015). Bianchi (2021) further notes that urban biogeochemistry is related to the early developments of ecology, such as that of Odum, who noted that cities are more wasteful of energy consumption than are natural systems, and urban areas are more dependent on external matter and energy, with higher human impacts than other ecosystems. Urban areas are also linked with human constructed drivers such as engineering, urban demographic trends and household-scale actions which may be important for future biogeochemical research (Bianchi (2021, see also Odum, 1971; Kaye et al. 2006).
One factor for the increasing importance of urban biogeochemistry is the increased human populations living in urban areas (Gardner et al., 2014). From 1950 to 2014, the proportion of the world’s population living in urban centers increased from 30% to 54%, and the United Nations’ World Urbanization Prospects estimates this number will reach 66%, or 6.3 billion, by 2050. Most of this growth is projected to occur in Africa and Asia, which today are mostly rural in cultural land cover. Cities in more developed countries will also have to combat aging infrastructure and the spread of toxic metals, organic contaminants, and “emerging” contaminants, just to name a few issues” (Gardner et al., 2014). These authors describe an academic meeting on urban biogeochemistry under the International Association of Geochemistry’s (IAGC) Urban Geochemistry Working Group. This meeting emphasized “quantifying anthropogenic and natural sources of CO2 in urban areas, the transformation and fate of everyday chemicals in urban watersheds, and geochemistry and urban health” (Gardner et al., 2014).
Additionally, there are “eight key issues to be addressed in urban geochemical research” these comprising the alteration of global biogeochemical cycles in the urban setting, the evolution of urban infrastructure and impacts on geochemical processes, the measurement of urban systems to assess chemical issues, the role of urban geochemists in policy decisions, the impacts of geochemistry of urban environments on human and ecosystem health, the impact of “episodic perturbations” including natural disasters on the urban environment, impacts of the past disturbances on present geochemical systems and how the “geochemical ‘footprint’ of urban areas may be defined” (Gardner et al., 2014).
There is also the biogeochemistry of pesticides, mostly linked to the agricultural supports of urban populations (Campbell, 2015, 2022). Barbash (2014: 535) argues that the use of synthetic pesticides from the early twentieth century has created “the most extensive release of synthetic compounds that Earth’s ecosystems have ever experienced,” and the assessment and analysis of this development requires knowledge of the physical, chemical, biological, and hydrologic factors that may contribute to the concentrations of pesticide compounds, which may include pesticide parent compounds, pesticide adjuvants or inerts, and compounds created by the transformation of pesticides or adjuvants. These may occur in air, water, soil, or biota (Barbash, 2014: 535). Effective management requires an approach that investigates the factors for the pesticide compounds in the environments, and the transformations, as well as the use, management, and media attention (Barbash, 2014: 535). The Figures 1.27, 1.28, 1.29, 1.30 and 1.31 above illustrate some of the features of urban and related environmental changes that may create chemical and, hence, biogeochemical changes.
Conclusions
This chapter has examined the basis of the multidisciplinary, interdisciplinary, and transdisciplinary science of biogeochemistry, including its history, composition, and approaches. The main focus of the chapter was the relationship of biogeochemistry with related sciences, an important factor for the development, and importance of the current science of biogeochemistry. The breadth and depth of this science identifies it as the backbone of many of the environmental and earth sciences. Considering the breadth of this subject, the chapter only covers the main topics that have been cited in the current literature. There is a tendency for biogeochemistry to diverge into close relations with some related sciences, such as the atmospheric, water, biological and earth sciences. The basic study topics of biogeochemistry were noted to be the chemical elements, especially carbon, nitrogen, phosphorous, potassium, oxygen, iron, calcium, selenium, sulfur, and mercury. Compounds including these elements included calcium carbonate, dolomite, carbon monoxide, carbon dioxide, calcium hydroxide, calcium oxide, ammonium, nitrite, nitrate, nitrous oxide, and nitric oxide. The model system illustrative approach was followed to give the reader a clear insight into the intra- and interrelations within and outside biogeochemistry. The succeeding chapters will explore these ideas and possibilities in more detail.
Change history
28 February 2024
A correction has been published.
References
Achterberg, E. P. (2011). Grand challenges in marine biogeochemistry. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2014.00007
Appl, M. (1999). Ammonia: Principles and industrial practice. WILEY-VCH Verlag GmbH.
Arrhenius, S. (1896). On the influence of carbonic acid in the air upon the temperature of the ground. Philosophical Magazine Series, 5(41), 273–276.
Barbash, J. E. (2014). The geochemistry of pesticides. Treatise on Geochemistry, 11, 535–572. https://doi.org/10.1016/B978-0-08-095975-7.00915-3
Bashkin, V. N., & Howart, R. W. (2002). Modern Biogeochemistry. Kluwer Academic Publishers.
Bianchi, T. S. (2021). The evolution of biogeochemistry: Revisited. Biogeochemistry, 154, 141–181. https://doi.org/10.1007/s10533-020-00708-0
Bianchi, T. S., Blair, N., Burdige, D., Eglinton, T. I., & Galy, V. (2018). Centers of organic carbon burial at the land-ocean interface. Organic Geochemistry, 115, 138–155.
Bianchi, T. S., Anand, M., Bauch, C., Canfield, D., Meester, L., Fennel, K., Groffman, P., Pace, M., Saito, M., & Simpson, M. (2020). Ideas and perspectives: Biogeochemistry – Its future role in interdisciplinary Frontiers. https://doi.org/10.5194/bg-2020-395
Böcker, N., Grahl, M., Tota, A., Häussinger, P., Leitgeb, P., & Schmücker, B. (2013). Nitrogen. In Ullmann's Encyclopedia of industrial chemistry. https://doi.org/10.1002/14356007.a17_457.pub2
Bodnar, M., Konieczka, P., & Namiesnik, J. (2012). The properties, functions, and use of selenium compounds in living organisms. Journal of Environmental Science and Health. Part C, Environmental Carcinogenesis & Ecotoxicology Reviews, 30(3), 225–252. https://doi.org/10.1080/10590501.2012.705164
Boero, F., De Leo, F., Fraschetti, S., & Ingrosso, G. (2019). The cells of ecosystem functioning: Towards a holistic vision of marine space. Advances in Marine Biology, 82, 129–153. https://doi.org/10.1016/bs.amb.2019.03.001
Brasseur, P., Gruber, N., Barciela, R., Brander, K., Doron, M., El Moussaoui, A., Hobday, A. J., Huret, M., Kremeur, A.-S., Lehodey, P., Matear, R., Moulin, C., Murtugudde, R., Senina, I., & Svendsen, E. (2009). Integrating biogeochemistry and ecology into ocean data assimilation systems. Oceanography, 22(3), 206–215. https://doi.org/10.5670/oceanog.2009.80
Britannica, T. (2020). Editors of Encyclopaedia 2020. Biogeochemical cycle. Encyclopedia Britannica. https://www.britannica.com/science/biogeochemical-cycle.
Brusseau, M. L., Pepper, I. L., & Gerba, C. P. (2019). Environmental and pollution science book (3rd ed.). Academic Press. https://doi.org/10.1016/C2017-0-00480-9
Butcher, S. S., Charlson, R. J., Orians, G. H., & Wolfe, G. V. (Eds.). (1992). Global biogeochemical cycles. Academic Press (Harcourt Brace Jovanovich).
CABI Digital Library. (2023). Organic phosphorus in the environment. B.L. Turner, E. Frossard, D.S. Baldwin. Retrieved from https://www.cabidigitallibrary.org/doi/book/. https://doi.org/10.1079/9780851998220.0000.
Calvo, M. S., & Lamberg-Allardt, C. J. (2015). Phosphorus. Advances in Nutrition (Bethesda, Md.), 6(6), 860–862. https://doi.org/10.3945/an.115.008516
Campbell, M. (2015). Vultures: Their evolution, ecology and conservation. Taylor and Francis (CRC Press).
Campbell, M. (2017). Biological conservation in the 21st century: A conservation biology of large wildlife. Nova Science Publishers.
Campbell, M. (2018). Geomatics and conservation biology. Nova Science Publishers.
Campbell, M. (2020). The physical geography of El Salvador: A geophysical and ecological approach. Nova Science Publishers.
Campbell, M. (2022). The great eagles: Their evolution, ecology and conservation. Taylor and Francis (CRC Press).
College of Earth, Ocean, and Atmospheric Sciences (2022). Ocean Ecology and Biogeochemistry. Corvallis, OR: College of Earth, Ocean, and Atmospheric Sciences 2022.
Cutter, G. A. (2005). Biogeochemistry: Now and into the future. In N. Noffke (Ed.), Geobiology: Objectives, concepts, perspectives (pp. 191–198). Elsevier Science.
Denman, K. L., Brasseur, G., Chidthaisong, A., Ciais, P., Cox, P. M., Dickinson, R. E., Hauglustaine, D., Heinze, C., Holland, E., Jacob, D., Lohmann, U., Ramachandran, S., da Silva Dias, P. L., Wofsy, S. C., & Zhang, X. (2007). Couplings between changes in the climate system and biogeochemistry. In S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, & H. L. Miller (Eds.), Climate change 2007: The physical science basis. Contribution of Working Group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press.
Department of Marine Sciences. (2022). Biogeochemistry. Retrieved from https://www.marsci.uga.edu/research/content/biogeochemistry
Devillanova, F. A., & Mont, W. D. (2007). Handbook of chalcogen chemistry: New perspectives in sulfur, selenium, and tellurium. Royal Society of Chemistry.
Dobrovolsky, V. V. (1994). Biogeochemistry of the world's land. CRC Press.
Falkowski, P. (2003). Biogeochemistry of primary production in the sea. Treatise on Geochemistry, 8, 185–213. https://doi.org/10.1016/B0-08-043751-6/08129-9
Fantle, M. S., & Tipper, E. T. (2014). Calcium isotopes in the global biogeochemical Ca cycle: Implications for development of a Ca isotope proxy. Earth-Science Reviews, 129, 148–177. https://doi.org/10.1016/j.earscirev.2013.10.004
Fox, P. M., Carrero, S., Anderson, C. L., Dewey, C. P., Keiluweit, M., Conrad, M., Naughton, H. R., Fendorf, S., Carroll, R. W., Dafflon, B., Malenda-Lawrence, H., Dwivedi, D., Gilbert, B., Christensen, J. N., Boye, K., Beutler, C. A., Brown, W. S., Newman, A. W., Versteeg, R., Williams, K. H., & Nico, P. S. (2022). Sulfur biogeochemical cycling and redox dynamics in a shale-dominated mountainous watershed. Journal of Geophysical Research: Biogeosciences, 127(6), e2021JG006769.
Gandhi, S. M., & Sarkar, B. C. (2016). Essentials of mineral exploration and evaluation. Elsevier. https://doi.org/10.1016/C2015-0-04648-2
Gardner, C. B., Berry Lyons, W., & Long, D. T. (2014). Defining urban geochemistry. International Association of Geochemistry: Urban Geochemistry Working Group; Columbus, Ohio, 5–6 August 2014. Retrieved from https://eos.org/science-updates/defining-urban-geochemistry
Gorham, E. (1991). Biogeochemistry: Its origins and development. Biogeochemistry, 13(3), 199–239.
Greenwood, N. N., & Earnshaw, A. (1997). Chemistry of the elements (2nd ed.). Butterworth-Heinemann.
Grenne, T., & Slack, J. F. (2019). Mineralogy and geochemistry of silicate, sulfide, and oxide iron formations in Norway: Evidence for fluctuating redox states of early Paleozoic marine basins. Mineralium Deposita, 54, 829–848. https://doi.org/10.1007/s00126-018-0840-2
Grimm, N. B., Faeth, S. H., Golubiewski, N. E., Redman, C. L., Wu, J. G., Bai, X. M., & Briggs, J. M. (2008). Global change and the ecology of cities. Science, 319(5864), 756–760.
Hartikainen, H. (2005). Biogeochemistry of selenium and its impact on food chain quality and human health. Journal of Trace Elements in Medicine and Biology: Organ of the Society for Minerals and Trace Elements (GMS), 18(4), 309–318. https://doi.org/10.1016/j.jtemb.2005.02.009
Hellweger, F. L. (2008). Biogeochemical Models. In Encyclopedia of ecology (pp. 386–396). https://doi.org/10.1016/B978-008045405-4.00177-4
Hoffmann, J. E., & King, M. G. (2010). Selenium and selenium compounds. In Kirk-Othmer Encyclopedia of chemical technology. https://doi.org/10.1002/0471238961.190512.0508150606.a01.pub3
House, J. E. (2008). Inorganic chemistry. Academic Press. https://doi.org/10.1039/JM9920201123
Hutton, J. (1795). The history of the earth, 2 volumes. Cadell, Junior, and Davies. Seen in reprint (1959). Wheldon and Wesley, Cadicote, Hertfordshire, England.
Jäger, H., Frohs, W., Collin, G., von Sturm, F., Vohler, O., & Nutsch, G. (2010). Carbon, 1. General. In Ullmann's Encyclopedia of industrial chemistry. https://doi.org/10.1002/14356007.a05_095.pub2
Kabata-Pendias, A., & Pendias, H. (1999). Biogeochemia pierwiastków śladowych (Polish) (2nd ed., pp. 344–354). PWN Warszawa.
Kappler, A., Bryce, C., Mansor, M., Lueder, U., Byrne, J. M., & Swanner, E. D. (2021). An evolving view on biogeochemical cycling of iron. Nature Reviews. Microbiology, 19(6), 360–374. https://doi.org/10.1038/s41579-020-00502-7
Karl, D. M., & Schlesinger, W. H. (2014). Volume editors’ introduction. In H. D. Holland & K. K. Turekian (Eds.), Treatise on geochemistry (Vol. 10, 2nd ed., pp. xxiii–xxvi). Elsevier Science. https://doi.org/10.1016/B978-0-08-095975-7.09983-6
Kaye, J. P., Groffman, P. N., Grimm, N. B., Baker, L. A., & Pouyat, R. V. (2006). A distinct urban biogeochemistry? Trends in Ecology & Evolution, 21(4), 192–199.
Kirschner, M. J., Alekseev, A., Dowy, S., Grahl, M., Jansson, L., Keil, P., Lauermann, G., Meilinger, M., Schmehl, W., Weckler, H., & Windmeier, C. (2017). Oxygen. In Ullmann's Encyclopedia of industrial chemistry. https://doi.org/10.1002/14356007.a18_329.pub2
Klein, C., & Hurlbut, C. S., Jr. (1985). Manual of mineralogy (after James D. Dana) (20th ed.). Wiley.
Kuypers, M., Marchant, H., & Kartal, B. (2018). The microbial nitrogen-cycling network. Nature Reviews Microbiology, 16, 263–276. https://doi.org/10.1038/nrmicro.2018.9
Kvakić, M., Tzagkarakis, G., Pellerin, S., Ciais, P., Goll, D., Mollier, A., & Ringeval, B. (2020). Carbon and phosphorus allocation in annual plants: An optimal functioning approach. Frontiers in Plant Science, 11, 149. https://doi.org/10.3389/fpls.2020.00149
Laane, R. W. P. M., & Middleburg, J. J. (2011). Biogeochemistry, an introduction. In E. Wolanski & D. McLusky (Eds.), Treatise on estuarine and coastal science (pp. 1–5). Academic Press.
Libes, S. M. (1992). Introduction to marine biogeochemistry. Academic Press.
Lide, D. R. (Ed.). (2005). CRC handbook of chemistry and physics (86th ed.). CRC Press.
Linderoth, S., & Mørup, S. (1992). Stability and magnetic properties of an iron-mercury alloy. Journal of Physics: Condensed Matter, 4, 8627–8634.
Malyuga, D. (1995). Biogeochemical methods of prospecting. Springer.
National Ocean Service. (2023). What is the carbon cycle? Retrieved from https://oceanservice.noaa.gov/facts/carbon-cycle.html#transcript
Nehb, W., & Vydra, K. (2006). Sulfur. In Ullmann's Encyclopedia of industrial chemistry. https://doi.org/10.1002/14356007.a25_507.pub2
Nogara, P. A., Madabeni, A., Bortoli, M., Teixeira Rocha, J. B., & Orian, L. (2021). Methylmercury can facilitate the formation of Dehydroalanine in Selenoenzymes: Insight from DFT molecular modeling. Chemical Research in Toxicology, 34(6), 1655–1663. https://doi.org/10.1021/acs.chemrestox.1c00073
Odum, E. P. (1971). Fundamental of ecology. W.B. Saunders.
Pickett, S. T. A., Cadenasso, M. L., Grove, J. M., Boone, C. G., Groffman, P. M., Irwin, E., Kaushal, S. S., Marshall, V., McGrath, B. P., Nilon, C. H., Pouyat, R. V., Szlavecz, K., Troy, A., & Warren, P. (2011). Urban ecological systems: Scientific foundations and a decade of progress. Journal of Environmental Management, 92(3), 331–362.
Rieuwerts, J. (2015). The elements of environmental pollution. Earthscan Routledge.
Rytuba, J. J. (2003). Mercury from mineral deposits and potential environmental impact. Environmental Geology, 43, 326–338. https://doi.org/10.1007/s00254-002-0629-5
Schlesinger, W. H. (1997). Biogeochemistry: An analysis of global change (2nd ed.). Academic Press.
Schlesinger, W. H. (2004). Better living through biogeochemistry. Ecology, 85(9), 2402–2407.
Schlesinger, W. H. (2021a). Biogeochemistry. Retrieved from https://www.oxfordbibliographies.com/view/document/obo-9780199830060/obo-9780199830060-0111.xml
Schlesinger, W. H. (2021b). Some thoughts on the biogeochemical cycling of potassium in terrestrial ecosystems. Biogeochemistry, 154, 427–432. https://doi.org/10.1007/s10533-020-00704-4
Schlesinger, W. H., & Bernhardt, E. S. (2020). Biogeochemistry: An analysis of global change (4th ed.). Academic Press.
Schmitz, O. J. (2020). Predators and rainfall control spatial biogeochemistry in a landscape of fear. PNAS, 117(39) https://www.pnas.org/doi/pdf/10.1073/pnas.2016449117
Schultz, H., Bauer, G., Schachl, E., Hagedorn, F., & Schmittinger, P. (2000). Potassium compounds. In Ullmann's Encyclopedia of industrial chemistry. https://doi.org/10.1002/14356007.a22_039
Schulze, E. D. (2015). Biogeochemistry: Historical and future perspectives. Austin Journal of Earth Science, 2(2), id1012.
Scripps Institution of Oceanography. (2022). Earth Biogeochemistry. Retrieved from https://scripps.ucsd.edu/research/topics/biogeochemistry
Sharma, V. K., McDonald, T. J., Sohn, M., Anquandah, G. A. K., Pettine, M., & Zboril, R. (2015). Biogeochemistry of selenium. A review. Environmental Chemistry Letters, 13, 49–58. https://doi.org/10.1007/s10311-014-0487-x
Singh, A. D., Khanna, K., Kour, J., Dhiman, S., Bhardwaj, T., Devi, K., Sharma, N., Kumar, P., Kapoor, N., Sharma, P., Arora, P., Sharma, A., & Bhardwaj, R. (2023). Critical review on biogeochemical dynamics of mercury (Hg) and its abatement strategies. Chemosphere, 319, 137917. https://doi.org/10.1016/j.chemosphere.2023.137917
Smart, L. E., & Moore, E. A. (2005). Solid state chemistry: An introduction (3rd ed.). CRC Press.
Steffen, W., Richardson, K., Rockström, J., Schellnhuber, H. J., Dube, O. P., Dutreuil, S., Lenton, T. M., & Lubchenco, J. (2020). The emergence and evolution of earth system science. Nature Reviews Earth & Environment, 1(1), 54–63.
Suess, E. (1875). Die Entstehung der Alpen. The Origin of the Alps. W. Braumüller.
Tartowski, S. L., & Howarth, R. W. (2013). Nitrogen, Nitrogen Cycle. In Encyclopedia of biodiversity (2nd ed., pp. 537–546). https://doi.org/10.1016/B978-0-12-384719-5.00098-8
Testa, J. M., & Kemp, W. M. (2011). Oxygen - dynamics and biogeochemical consequences. In E. Wolanski & D. S. McLusky (Eds.), Treatise on Estuarine and coastal science (pp. 163–199). Academic Press. https://doi.org/10.1016/B978-0-12-374711-2.00505-2
Thomas, I. M., & Weller, M. T. (1992). Synthesis, structure and thermal properties of phosphophyllite, Zn2Fe(PO4)2·4H2O. Journal of Materials Chemistry, 2, 1123–1126.
Turner, B. L., Frossard, E., & Baldwin, D. S. (2005). Organic phosphorus in the environment. CABI Publishing.
Vernadsky, V. I. (1926). The biosphere. Trans. Langmuir, D McMenamin. Springer.
Vernadsky, V. I. (1944). Problems in biogeochemistry. II. Transactions of the Connecticut Academy of Arts and Sciences, 35, 493–494.
Vernadsky, V. I. (1945). The biosphere and the noosphere. Scientific American, 33, 1–12.
Warf, B. (2010). Biogeochemical cycles. Encyclopedia of geography. SAGE Publications. https://doi.org/10.4135/9781412939591
Warren, J. (2000). Dolomite: Occurrence, evolution, and economically important associations. Earth-Science Reviews, 52(1–3), 1–81.
Wildermuth, E., Stark, H., Friedrich, G., Ebenhöch, F. L., Kühborth, B., & Silver, J. (2000). Iron compounds. In Ullmann's Encyclopedia of industrial chemistry. https://doi.org/10.1002/14356007.a14_591
Woods Hole Oceanographic Institution. (2022). Biogeochemistry. Retrieved from https://www.whoi.edu/know-your-ocean/ocean-topics/ocean-chemistry/ biogeochemistry/
Zavarzin, G. A. (2008). Microbial cycles. In Encyclopedia of ecology (Vol. 4, 2nd ed., pp. 129–134).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Campbell, M.O. (2023). Biogeochemistry and Its Complexity. In: Biogeochemistry and the Environment. Springer, Cham. https://doi.org/10.1007/978-3-031-47017-2_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-47017-2_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-47016-5
Online ISBN: 978-3-031-47017-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)