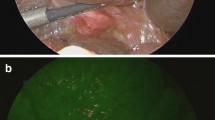
Abstract
The use of fluorescence technology with indocyanine green has been shown to provide real-time visualization of lymphatic flow, identification of sentinel lymph nodes, assessment of intestinal perfusion, and detection of the extrahepatic bile duct. These capabilities could potentially reduce the mortality and complication rate following surgery. However, the lack of standardization in dosage and time of ICG administration requires further clinical and experimental studies. This applies in particular to veterinary medicine, which results from the lack of general access to the latest technology. Near-infrared indocyanine green (ICG) fluorescence is a great hope for the further development of minimally invasive surgery in humans and animals. The article presents the possibilities of using near-infrared indocyanine green in thoracic and general surgery and compares it with the current development of selected surgical procedures in veterinary medicine. The rapid progress of veterinary surgery, including minimally invasive surgery, makes the wide use of near-infrared indocyanine green a matter of the near future.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Aoki, T., et al.: Image guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J. Surg. 32, 1763–1767 (2008). https://doi.org/10.1007/s00268-008-9620-y
Ashitate, Y., Tanaka, E., Stockdale, A., Choi, H.S., Frangioni, J.V.: Near-infrared fluorescence imaging of thoracic duct anatomy and function in open surgery and video-assisted thoracic surgery. J. Thorac. Cardiovasc. Surg. 142, 31–38 (2011). https://doi.org/10.1016/j.jtcvs.2011.03.004. Epub 2011 Apr 7
Baiocchi, G.L., Diana, M., Boni, L.: Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: state of the art and future directions. World J. Gastroenterol. 24(27), 921–930 (2018). https://doi.org/10.3748/wjg.v24.i27.2921
Bilbrey, S.A., Birchard, S.J.: Pulmonary lymphatics in dogs with experimentally induced chylothorax. JAAHA 30, 86–91 (1994)
Birchard, S.J., Smeak, D.D., McLoughlin, M.A.: Treatment of idiopathic chylothorax in dogs and cats. J. Am. Vet. Med. Assoc. 212, 653–657 (1998)
Boni, L., David, G., Dionigi, G., Rausei, S., Cassinotti, E., Fingerhut, A.: Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg. Endosc. 30, 2736–2742 (2016). https://doi.org/10.1007/s00464-015-4540-z
Buchs, N.C.: Intraoperative fluorescent cholangiography using indocyanin green during robotic single site cholecystectomy. Int. J. Med. Robot. 8, 436–440 (2012). https://doi.org/10.1002/rcs.1437
Buddingh, K.T., Nieuwenhuijs, V.B.: The critical view of safety and routine intraoperative cholangiography complement each other as safety measures during cholecystectomy. J. Gastrointest. Surg. 15, 1069–1070 (2011). https://doi.org/10.1007/s11605-011-1413-1
Casaccia, M., Mora, M., Santori, G., Ghiggi, C., Angelucci, E.: Laparoscopic lymph node biopsy for lymphoma with a novel use of indocyanine green fluorescence in a 66- year-old male patient. Int. J. Surg. Case. Rep. 90, 106692 (2022). https://doi.org/10.1016/j.ijscr.2021.106692
Chiu, C.-H., et al.: Clinical use of near-infrared fluorescence imaging with indocyanine green in thoracic surgery: a literature review. J. Thorac. Dis. 8(Suppl 9), 744–748 (2016). https://doi.org/10.21037/jtd.2016.09.70
Chiu, H.C.: Successful usage of intra metatarsal pad injection of ICG/near-infrared fluorescent lymphography for VATS thoracic duct ligation in two Shiba Inu dogs with recurrent chylothorax. Vet. Surg. 48, O151 (2019). https://doi.org/10.1111/vsu.13213
Culp, W.T.N., Mayhew, P.D., Brown, D.C.: The effect of laparoscopic versus open ovariectomy on postsurgical activity in small dogs. Vet. Surg. 38, 811–817 (2009). https://doi.org/10.1111/j.1532-950X.2009.00572.x
Degett, T.H., Andersen, H.S., Gögenur, I.: Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch. Surg. 401, 767–775 (2016). https://doi.org/10.1007/s00423-016-1400-9
Enwiller, T.M., Radlinsky, M.G., Mason, D.E., Roush, J.K.: Popliteal and mesenteric lymph node injection with methylene blue for coloration of the thoracic duct in dogs. Vet. Surg. 32, 359–364 (2003). https://doi.org/10.1053/jvet.2003.50044
Favril, S., et al.: Fluorescence-guided surgery using indocyanine green in dogs with superficial solid tumours. Vet. Rec. 187(7), 273 (2020). https://doi.org/10.1136/vr.105554
Flum, D.R., Dellinger, E.P., Cheadle, A., Chan, L., Koepsell, T.: Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA 289, 1639–1644 (2003). https://doi.org/10.1001/jama.289.13.1639
Fossum, T.W., et al.: Thoracic duct ligation and pericardectomy for treatment of idiopathic chylothorax. J. Vet. Intern. Med. 18, 307–310 (2004). 10.1892/0891-6640(2004)18<307:tdlapf>2.0.co;2
Frangioni, J.V.: New technologies for human cancer imaging. J. Clin. Oncol. 26, 4012–4021 (2008). https://doi.org/10.1200/JCO.2007.14.3065
Fransson, B.A., Mayhew, P.D.: Small Animal Laparoscopy and Thoracoscopy, pp. 236–242. Wiley-Blackwell, Iowa (2015)
Gioux, S., Choi, H.S., Frangioni, J.V.: Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol. Imag. 9, 237–255 (2010)
Hackethal, A., Hirschburger, M., Eicker, S.O., Mücke, T., Lindner, C., Buchweitz, O.: Role of indocyanine green in fluorescence imaging with near-infrared light to identify sentinel lymph nodes, lymphatic vessels and pathways prior to surgery – a critical evaluation of options. Geburtshilfe Frauenheilkd. 78(1), 54–62 (2018). https://doi.org/10.1055/s-0043-123937
Hayashi, K., Sicard, G., Gellasch, K., Frank, J.D., Hardie, R.J., McAnulty, J.F.: Cisterna chyli ablation with thoracic duct ligation for chylothorax: results in eight dogs. Vet. Surg. 34, 519–523 (2005). https://doi.org/10.1111/j.1532-950X.2005.00078.x
Holzinger, F., Krahenbuhl, L., Schteingart, C.D., Ton-Nu, H.T., Hofmann, A.F.: Use of a fluorescent bile acid to enhance visualization of the biliary tract and bile leaks during laparoscopic surgery in rabbits. Surg. Endosc. 15, 209–212 (2001). https://doi.org/10.1007/s004640000265
Iida, G., et al.: Intraoperative identification of canine hepatocellular carcinoma with indocyanine green fluorescent imaging. J. Small Anim. Pract. 54(11), 594–600 (2013). https://doi.org/10.1111/jsap.12148
Ishizawa, T., Bandai, Y., Kokudo, N.: Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: an initial experience. Arch. Surg. 144, 381–382 (2009). https://doi.org/10.1001/archsurg.2009.9
Ito, N., Fukuta, M., Tokushima, T., Nakai, K., Ohgi, S.: Sentinel node navigation surgery using indocyanine green in patients with lung cancer. Surg. Today 34, 581–585 (2004). https://doi.org/10.1007/s00595-004-2780-y
Kaburagi, T., et al.: Intraoperative fluorescence lymphography using indocyanine green in a patient with chylothorax after esophagectomy—report of a case. Surg Today. 43, 306–310 (2013). https://doi.org/10.1007/s00595-012-0391-6
Kamijo, K., Kanai, E., Oishi, M., Ichihara, N., Asari, M., Yamada, K.: Perirectal injection of imaging materials for computed tomographic lymphography and near infrared fluorescent thoracoscopy in cats. Vet. Med. 64, 342–347 (2019). https://doi.org/10.17221/32/2019-VETMED
Kawaguchi, Y., et al.: Portal uptake function in veno-occlusive regions evaluated by real-time fluorescent imaging using indocyanine green. J. Hepatol. 58, 247–253 (2013). https://doi.org/10.1016/j.jhep.2012.09.028
Khan, O.A., Balaji, S., Branagan, G., Bennett, D.H., Davies, N.: Randomized clinical trial of routine ontable cholangiography during laparoscopic cholecystectomy. Br. J. Surg. 98, 362–367 (2011). https://doi.org/10.1002/bjs.7423
Kim, H.K., et al.: Intraoperative pulmonary neoplasm identification using near-infrared fluorescence imaging. Eur. J. Cardiothorac. Surg. 49, 1497–1502 (2016). https://doi.org/10.1093/ejcts/ezv367
Kitai, T., Inomoto, T., Miwa, M., Shikayama, T.: Fluorescence navigation withindocyanine green for detecting sentinel lymph nodes in breastcancer. Breast Cancer 12(3), 211–215 (2005). https://doi.org/10.2325/jbcs.12.211
Korpita, M.F., et al.: Thoracoscopic detection of thoracic ducts after ultrasoundguided intrahepatic injection of indocyanine green detected by near-infrared fluorescence and methylene blue in dogs. Vet. Surg. 51(Suppl 1), O118–O127 (2022). https://doi.org/10.1111/vsu.13682. Epub 2021 Jul 23
LaFond, E., Weirich, W.E., Salisbury, S.K.: Omentalization of the thorax for treatment of idiopathic chylothorax with constrictive pleuritis in a cat. J. Am. Anim. Hosp. Assoc. 38, 74–78 (2002). https://doi.org/10.5326/0380074
Larose, P.C., Brisson, B., Sanchez-Lazaro, A., Monteith, G., Singh, A., Zhang, M.: Near-infrared fluorescence cholangiography in dogs: a pilot study. In: ACVS (2021). https://www.eventscribe.net/2021/ACVS/fsPopup.asp?Mode=presInfo&PresentationID=915337
Lim, C., et al.: Indocyanine green fluorescence imaging in the surgical management of liver cancers: current facts and future implications. J. Visc. Surg. 151(2), 117–124 (2014). https://doi.org/10.1016/j.jviscsurg.2013.11.003
MacDonald, N.J., Noble, P.J.M., Burrow, R.D.: Efficacy of en bloc ligation of the thoracic duct: descriptive study in 14 dogs. Vet. Surg. 37(7), 696–701 (2008). https://doi.org/10.1111/j.1532-950X.2008.00437.x
Matsui, A., et al.: Real-time intra-operative near-infrared fluorescence identification of the extrahepatic bile ducts using clinically available contrast agents. Surgery 148, 87–95 (2010). https://doi.org/10.1016/j.surg.2009.12.004
Mayhew, P.D., Mehler, S.J., Radhakrishnan, A.: Laparoscopic cholecystectomy for management of uncomplicated gall bladder mucocele in six dogs. Vet. Surg. 37, 625–630 (2008). https://doi.org/10.1111/j.1532-950X.2008.00428.x
Miyashiro, I., et al.: Intraoperative diag-nosis using sentinel node biopsy with indocyanine green dyein gastric cancer surgery: an institutional trial by experiencedsurgeons. Ann. Surg. Oncol. 20(2), 542–546 (2012). https://doi.org/10.1245/s10434-012-2608-8
Moukarzel, L.A., Feinberg, J., Levy, E.J., Leitao, M.M.: Current and novel mapping substances in gynecologic cancer care. Int. J. Gynecol. Cancer 30(3), 387–393 (2020). https://doi.org/10.1136/ijgc-2019-001078
Muntean, M.V., Muntean, V., Ardelean, F., Georgescu, A.: Dynamic perfusion assessment during perforator flap surgery: an upto-date. Clujul. Med. 88, 293–297 (2015). https://doi.org/10.15386/cjmed-484. Epub 2015 Jul 1
Noguera, J., Castro, L., Garcia, L., Mosquera, C., Gomez, A.: Lymphadenectomy guided by indocyanine-green (ICG) in colorectal cancer: a pilot study. J. Surg. Tech. Proced. 3(1), 1023 (2019)
Noura, S., et al.: Feasibility of a lateral regionsentinel node biopsy of lower rectal cancer guided by indo-cyanine green using a near-infrared camera system. Ann. Surg. Oncol. 17(1), 144–151 (2009). https://doi.org/10.1245/s10434-009-0711-2
Okusanya, O.T., et al.: Intraoperative near-infrared imaging can identify pulmonary nodules. Ann. Thorac. Surg. 98, 1223–1230 (2014). https://doi.org/10.1016/j.athoracsur.2014.05.026
Pacheco, P.E., Hill, S.M., Henriques, S.M., Paulsen, J.K., Anderson, R.C.: The novel use of intraoperative laser-induced fluorescence of indocyanine green tissue angiography for evaluation of the gastric conduit in esophageal reconstructive surgery. Am. J. Surg. 205, 349–352 (2013). https://doi.org/10.1016/j.amjsurg.2012.11.005
Park, S.Y., Park, J.S., Kim, H.Y., Woo, I.T., Park, I.K., Choi, G.-S.: Indocyanine green fluorescence imaging-guided laparoscopic surgery could achieve radical d3 dissection in patients with advanced right-sided Colon Cancer. Dis. Colon. Rectum. 63, 441–449 (2020). https://doi.org/10.1097/DCR.0000000000001597
Peltrini, R., et al.: Intraoperative use of indocyanine green fluorescence imaging in rectal cancer surgery: the state of the art. World J. Gastroenterol. 27(38), 6374–6386 (2021). https://doi.org/10.3748/wjg.v27.i38.6374
Purich, K., et al.: Intraoperative fluorescence imaging with indocyanine green in hepatic resection for malignancy: a systematic review and meta-analysis of diagnostic test accuracy studies. Surg. Endosc. 34(7), 2891–2903 (2020). https://doi.org/10.1007/s00464-020-07543-2
Quinlan, A.S.F., Wainberg, S.H., Phillips, E., Oblak, M.L.: The use of near infrared fluorescence imaging with indocyanine green for vascular visualization in caudal auricular flaps in two cats. Vet. Surg. 50(3), 677–686 (2021). https://doi.org/10.1111/vsu.13577
Ribero, D., Mento, F., Sega, V., Lo Conte, D., Mellano, A., Spinoglio, G.: ICG-guided lymphadenectomy during surgery for colon and rectal cancer—interim analysis of the GREENLIGHT trial. Biomedicines. 10, 541 (2022). https://doi.org/10.3390/biomedicines10030541
Rossanese, M., Williams, P., Tomlinson, A., Cinti, F.: Long-term outcome after cholecystectomy without common bile duct catheterization and flushing in dogs. Animals 12, 2112 (2022). https://doi.org/10.3390/ani12162112
Sakurai, N.: Clinical impact of near-infrared fluorescence imaging with indocyanine green on surgical treatment for hepatic masses in dogs. BMC Vet. Res. 18(1), 374 (2022). https://doi.org/10.1186/s12917-022-03467-2
Sanford, D.E.: An update on technical aspect of cholecystectomy. Surg. Clin. N. Am. 99, 245–258 (2018). https://doi.org/10.1016/j.suc.2018.11.005
Schaafsma, B.E., et al.: The clinical use of indocyanine green as a near infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 104, 323–332 (2011). https://doi.org/10.1002/jso.21943
Scott, J., et al.: Perioperative complications and outcome of laparoscopic cholecystectomy in 20 dogs. Vet. Surg. 45(S1), O49–O59 (2016). https://doi.org/10.1111/vsu.12534
Shariati, E., Bakhtiari, J., Khalaj, A., Niasari-Naslaji, A.: Comparison between two portal laparoscopy and open surgery for ovariectomy in dogs. Vet. Res. Forum. 5, 219–223 (2014)
Shen, R., Zhang, Y., Wang, T.: Indocyanine green fluorescence angiography and the incidence of anastomotic leak after colorectal resection for colorectal cancer: a meta-analysis. Dis. Colon Rectum. 61(10), 1228–1234 (2018). https://doi.org/10.1097/DCR.0000000000001123
Smeak, D.D., et al.: Treatment of chronic pleural effusion with pleuroperitoneal shunts in dogs: 14 cases (1985–1999). J. Am. Vet. Med. Assoc. 219, 1590–1597 (2001). https://doi.org/10.2460/javma.2001.219.1590
Spinoglio, G., et al.: Real-time near-infrared (NIR) fluorescent cholangiography in single-site robotic cholecystectomy (SSRC): a single-institutional prospective study. Surg. Endosc. 27, 2156–2162 (2013). https://doi.org/10.1007/s00464-012-2733-2
Steffey, M.A., Mayhew, P.D.: Use of direct near-infrared fluorescent lymphography for thoracoscopic thoracic duct identification in 15 dogs with chylothorax. Vet. Surg. 47, 267–276 (2018). https://doi.org/10.1111/vsu.12740. Epub 2017 Nov 6
Suh, Y.J., et al.: Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs. Surg. Endosc. 29(9), 2811–2817 (2015). https://doi.org/10.1007/s00464-014-3971-2
Tan, I.C., et al.: Investigational lymphatic imaging at the bedside in a pediatric postoperativechylothorax patient. Pediatr. Cardiol. 35, 1295–1300 (2014). https://doi.org/10.1007/s00246-014-0946-y
Tanaka, R., Nakashima, K., Fujimoto, W.: Sentinel lymph nodedetection in skin cancer using fluorescence navigation withindocyanine green. J. Dermatol. 36(8), 468–470 (2009). https://doi.org/10.1111/j.1346-8138.2009.00679.x
Tobis, S., et al.: Near infrared fluorescenceimaging after intravenous indocyanine green: initial clinicalexperience with open partial nephrectomy for renal corticaltumors. Urology 79(4), 958–964 (2012). https://doi.org/10.1016/j.urology.2011.10.016
Townsend, K.L., Milovancev, M., Bracha, S.: Feasibility of near-infrared fluorescence imaging for sentinel lymph node evaluation of the oral cavity in healthy dogs. Am. J. Vet. Res. 79(9), 995–1000 (2018). https://doi.org/10.2460/ajvr.79.9.995
Ushijima, H., et al.: Visualization of lymphatic flow in laparoscopic colon cancer surgery using indocyanine green fluorescence imaging. Sci. Rep. 10, 14274 (2020)
Wada, T., et al.: ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg. Endosc. 31, 4184–4193 (2017). https://doi.org/10.1007/s00464-017-5475-3
Wada, T., Kawada, K., Hanada, K., Obama, K.: Quantitative analysis of colonic perfusion using ICG fluorescence angiography and its consequences for anastomotic healing in a rat model. Cancers 14, 4024 (2022). https://doi.org/10.3390/cancers14164024
Wakaiki, S., et al.: Indocyanine green angiography for examining the normal ocular fundus in dogs. J. Vet. Med. Sci. 69(5), 465–470 (2007). https://doi.org/10.1292/jvms.69.465
Wan, J., Oblak, M.L., Ram, A., Singh, A., Nykamp, S.: Determining agreement between preoperative computed tomography lymphography and indocyanine green near infrared fluorescence intraoperative imaging for sentinel lymph node mapping in dogs with oral tumours. Vet. Comp. Oncol. 19, 295–303 (2021). https://doi.org/10.1111/vco.12675
Wang, C., et al.: Application of near-infrared fluorescent cholangiography using indocyanine green in laparoscopic cholecystectomy. J. Int. Med. Res. 48(12), 300060520979224 (2020). https://doi.org/10.1177/0300060520979224
Yamashita, S., et al.: Video-assisted thoracoscopic indocyanine green fluorescence imaging system shows sentinel lymph nodes in non-small-cell lung cancer. J. Thorac. Cardiovasc. Surg. 141, 141–144 (2011). https://doi.org/10.1016/j.jtcvs.2010.01.028
Yang, F., Zhou, J., Hao, L.: Near-infrared fluorescence-guided thoracoscopic surgical intervention for post-operative chylothorax. Interact Cardiovasc. Thorac. Surg. 26, 171–175 (2018). https://doi.org/10.1093/icvts/ivx3
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Prządka, P., Kiełbowicz, Z., Tunikowska, J. (2023). NIRF Imaging with Indocyanine Green (ICG) in a Veterinary Minimally Invasive Surgery. In: Burduk, A., Batako, A., Machado, J., Wyczółkowski, R., Antosz, K., Gola, A. (eds) Advances in Production. ISPEM 2023. Lecture Notes in Networks and Systems, vol 790. Springer, Cham. https://doi.org/10.1007/978-3-031-45021-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-45021-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-45020-4
Online ISBN: 978-3-031-45021-1
eBook Packages: Intelligent Technologies and RoboticsIntelligent Technologies and Robotics (R0)