Abstract
This study aimed to compare the antimicrobial activity of injectable platelet-rich fibrin (i-PRF) obtained from subjects with or without periodontal diseases against two pathogenic bacteria, Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg). I-PRF was prepared from blood samples of 60 individuals, including healthy subjects (n = 20), patients with gingivitis (n = 20), and patients with periodontitis (n = 20). The in vitro antibacterial effect of this platelet concentrate was evaluated using the agar diffusion test, a minimum inhibitory concentration (MIC) assay, and an antibacterial adhesion experiment. I-PRF exhibited a significantly better antibacterial effect against Pg than Aa within the periodontitis group, with a more expansive zone of inhibition and a lower MIC. Among the studied groups, i-PRF collected from the periodontitis group inhibited Aa and Pg significantly more in the agar diffusion test and had a lower MIC than i-PRF collected from the gingivitis and healthy groups. Although i-PRF reduced the adhesion of Aa and Pg and thus their ability to form a biofilm, the difference between groups of patients and the two pathogens was not significant. In conclusion, i-PRF exhibited an antibacterial effect against Aa and Pg in the zone of inhibition, MIC, and biofilm formation inhibition tests. However, in the periodontitis groups, i-PRF exhibited better antibacterial activity against Pg than Aa.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The result of the host’s immune responses and the biofilm formed by microbial networks in the periodontal tissue is a periodontal infection, which causes ongoing destruction of the periodontal complex. Bacterial plaque, primarily Gram-negative species such as Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg), is the root cause of periodontitis and gum disease [1]. Additionally, periodontitis alters the body’s immune system and may be related to a number of systemic disorders [2]. Blood indicators have been used in numerous studies to indicate differences in the body’s inflammatory response between patients with periodontal disease and those who are healthy [3]. According to Nicu (2009), leukocytes and platelets may be responsive to activation by periodontal pathogens [4]. The gold standard for treating these inflammatory conditions in the history has been to remove the bacterial biofilm utilizing non-surgical and surgical debridement along with an antimicrobial chemical agent to get rid of all pathogens and encourage the healing of any lesions. In addition to the above, there is a new trend in using natural agents, particularly biocompatible materials, in the regeneration of periodontal tissues.
Platelet concentrates (PCs) have gradually become more popular in dentistry over the past ten years because of regenerative therapy. Among the several platelet concentrates, platelet-rich fibrin (PRF) has the broadest range of dental applications [5]. Choukroun et al. [6] coined the phrase “platelet-rich fibrin”, referring to a type of autologous platelet concentrate without thrombin or anticoagulants. Recent systematic reviews have outlined PRF’s impacts and shown that it exerts a long-term effect on tissue-wound healing [5, 7]. Leukocytes, the host immunological defence cells that may fight infection, may be the source of PRF’s effects [8]. When PRF was created using a high centrifugation rate approach, a fibrin clot might form and serve as a three-dimensional structure for the further bone and gingival tissue repair [9]. Advanced PRF (A-PRF) [10] and injectable PRF (i-PRF) [11] were developed in 2014 and 2015, respectively, by altering the spin centrifugation forces. I-PRF boosts the leukocyte count and further stimulates growth factor release, similar to conventional PRF [11]. However, only a single study [12] has examined the release of growth factor of i-PRF, and only a small number of studies have investigated the antibacterial action of this novel material [13,14,15]. In addition, to our knowledge, few studies have contrasted the antibacterial effectiveness of this autologous material derived from individuals with healthy gums, gingivitis, and periodontitis, against periodontal infections since most of the research about PRFs has used blood collected from healthy people. It is therefore not known whether or not i-PRF from healthy, gingivitis, and periodontitis patients has different antibacterial activity in vitro. Another unknown is whether i-PRF has different inhibitory effects against Aa and Pg. Eagle (2021) also raised questions about the variation in the antibacterial impact of PRF depending on the features of the patient’s blood [7]. Our research, therefore, concentrated on the in-vitro antibacterial activity of i-PRF derived from different individuals against the periodontal pathogens Aa and Pg.
2 Methodology
2.1 Subjects Selection and PRF Preparation
After receiving their informed consent, 60 participants between the ages of 20 and 65 were divided into a healthy group, a gingivitis group, and a periodontitis group. The healthy group (n = 20) consisted of people with normal periodontal and systemic health (sulcus depth 3 mm, no edema, and no BOP hemorrhage). Patients with severe gingival inflammation (BOP > 50%) were included in the gingivitis group (n = 20). According to the recent Classification of Periodontal and Peri-Implant Diseases and Conditions, the periodontitis group (n = 20) consisted of patients with stage III or stage IV periodontitis [16]. The ethics committee of the Hospital of Odonto-Stomatology in Ho Chi Minh City, Vietnam, gave its approval to all the methods used in this study (reference number: 536/BVRHM).
Blood samples were centrifuged for three minutes at 700 rpm (60 x g) to create the i-PRF with the Dou Quattro Choukroun PRF system (PROCESS for PRF ®, Nice, France). Following Miron’s method, the increased platelet and leukocyte part (0.3–0.5 mL, immediately above the red blood cell layer) was obtained as i–PRF liquid and employed in investigations immediately [17].
2.2 Bacterial Sample Preparation
The bacterial strains of Aa and Pg employed in this research were initially identified in samples of subgingival plaque taken from people with periodontitis [18, 19]. The bacterial solution was initially added to Petri dishes containing Wilkins-Chalgren anaerobic agar (Oxoid Ltd., United Kingdom) and supplemented with fetal bovine serum (F7524, Sigma-Aldrich, United States) for Aa and 5% sheep blood (Nam Khoa Biotek, Vietnam) for Pg. Then, these dishes were incubated anaerobically at 37 ℃ for 5–7 days. After that, 5 mL of culture media was combined with the bacterial biomass until the turbidity reached 0.5 McFarland units. Finally, the antimicrobial assays were carried out using the technique outlined by Yang et al. [20].
2.3 Agar Diffusion Test
Five hundred microliters of Pg and Aa (1 × 106 CFU mL-1) were cultured on blood agar petri dishes for the agar diffusion test. A tip was used to aseptically puncture several 6–8 mm-diameter holes, which were then filled with 50 µL of i-PRF. The samples were incubated anaerobically at 37 ℃ for 24 h. As a positive control, chlorhexidine 0.2% (Sigma-Aldrich, United States) was used. After 24 h, the zone of inhibition was evaluated using ImageJ software (National Institute of Health, United States).
2.4 Minimum Inhibitory Concentration (MIC) Assay
In 24-well plates, different amounts of i-PRF (50, 100, 200, and 400 µL) were added. Following this, each well received 50 µL of bacterial solution (1 × 106 CFU mL-1) and the plates were incubated anaerobically at 37 ℃ for 24 h. The positive control was CHX 0.2%, whereas the negative control was pure culture media. The addition of 50 µL of resazurin 0.015% to each well after 24 h allowed for the measurement of the MIC based on the resazurin’s color shift.
2.5 Antibiofilm Formation or Anti-adhesion Assay
Bacteria were grown overnight in anaerobic culture. The bacterial solution and 200 µL of 40% i-PRF were put into sterile polystyrene test tubes. Parallel cultures were carried out in control tubes that contained culture media. Twenty-four hours were spent incubating the tubes at 37 ℃. The solution was then taken out and given two PBS washes. After 15 min of methanol fixation, the tubes were blow-dried. After 5 min of staining with a 0.1% crystal violet solution, they were washed with distilled water. After allowing the test tubes to dry, 200 µL of ethanol was poured into each one. A spectrophotometer was used to measure the absorbance at 610 nm. The percentage of adherent bacteria in the i-PRF sample was calculated relative to the control tubes. The experiment was repeated at least three times with similar results.
2.6 Statistical Analysis
The results of each experiment were carried out in triplicate and are shown as mean ± standard deviation (SD). SPSS v.20 software was used for all statistical analyses (IBM Ltd., Tokyo, Japan). To determine statistical significance, one-way analysis of variance (ANOVA) and the independent-samples T-test were used. At P 0.05, statistics were declared significant.
3 Results
Table 1 shows the zone of inhibition against Pg and Aa that was created by i-PRF collected from each patient groups. Compared to the positive control (CHX 0.2%), the platelet concentrate exhibited smaller inhibition zones. In each group, i-PRF produced a more expansive zone of inhibition on Pg than Aa plates. The zone of inhibition created by i-PRF in the periodontitis group was considerably larger when comparing the groups, accompanied by the gingivitis group and the healthy group.
According to the amount of i-PRF present in each well, the MIC values for i-PRF against Aa and Pg are provided (Table 2). The healthy group had the highest average MIC of i-PRF for both Aa and Pg (0.35 ± 0.09 for Aa and 0.28 ± 0.14 for Pg). These values were significantly higher than in the gingivitis group (0.28 ± 0.13 for Aa and 0.23 ± 0.09 for Pg) and the periodontitis group (0.26 ± 0.09 for Aa and 0.18 ± 0.07 for Pg) (p < 0.01). The MIC against Aa was substantially greater than the MIC against Pg in the healthy and periodontitis groups when the MIC values in each group were compared (p = 0.01 in the healthy group and p = 0.02 in the periodontitis group).
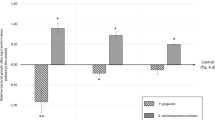
Table 3 displays the inhibition of planktonic Aa and Pg adherence to the bottom of microplate wells by i-PRF obtained from various patient groups. The data are presented as the mean percentage difference relative to the mean of the control ± SD. I-PRF showed adhesion-inhibiting effects against Aa and Pg. Although Pg was more significantly impacted by this type of platelet concentrate than Aa, the result was not statistically significant. Likewise, when comparing the impact of i-PRF from the three patient groups, the differences were not statistically significant, either for Aa or Pg (Figs. 1 and 2).
4 Discussion
Although platelet concentrates have been the subject of studies exploring their antibacterial efficacy, little is documented about the novel modified i-PRF. In the current investigation, we used the agar diffusion test, MIC assay, and anti-adhesion capacity to examine the impact of i-PRF against Aa and Pg. All antimicrobial assay results in our investigation showed that i-PRF significantly inhibited Aa and Pg. Additionally, this is one of a select few studies that focused on the effects of i-PRF collected from various patient groups, as opposed to previous studies that contrasted the impact of various platelet concentrates from healthy patients.
In each group, i-PRF created a zone of inhibition on Pg and Aa plates in the agar diffusion test, but the area of inhibition was greater against Pg (Table 1). This outcome is comparable to that of Karde et al. [12]. They investigated the antibacterial activity of PRP, PRF, and i-PRF against supragingival plaque and discovered that i-PRF displayed the largest zone of inhibition, followed by PRP and PRF [12]. In the study by Kour and colleagues [13], i-PRF was the most effective antibacterial agent against Pg, followed by PRP and PRF, but it did not affect Aa [13]. On the other hand, prior to these studies and using the first generation of PRF, Badade et al. hypothesized that PRF does not have an antibacterial effect against Aa [21]. Similar to Castro et al. [15], who independently assessed the antibacterial activity of the membrane and PRF extract after 72 h of anaerobic incubation and measured the zone of inhibition around the membrane and exudate of the PRF, came to the conclusion that neither of the components was Aa resistant.
In our study, the periodontitis group had a much larger inhibition area. The periodontitis group’s platelet products consistently had a lower MIC than those of the other groups. The typical MIC value of the i-PRF was 26–35% for Aa and 18–28% for Pg, according to Table 2. In a previous study, Jasmine et al. found that the MIC of i-PRF against Staphylococcus epidermis, Staphylococcus aureus, Staphylococcus epidermis, and Staphylococcus epidermis was 16%, 16%, 16%, and 8%, respectively [14]. These gram-positive microorganisms lack an exterior lipid membrane, which may make them more susceptible to antibacterial medicines. Gram-negative organisms on the outer layer, such as Aa and Pg, are primarily responsible for resistance to several antimicrobials, such as lactams, quinolones, colistin, and other bactericidal drugs. The majority of biocides should pass through the outer layer to reach their target. As a result, the obstruction may result from any alteration of the outer layer caused by bacteria, such as a change in hydrophobicity or the porin channel. [14]. According to the MIC data (Table 2), i-PRF was able to inhibit Pg more effectively than Aa. The periodontitis group’s i-PRF had the lowest MIC. In addition, i-PRF from patients with periodontitis and the healthy groups had more substantial antibacterial activity against Pg than Aa (P < 0.05). The difference was insignificant in the gingivitis group.
Unlike other platelet concentrates, i-PRF does not contain any intentionally added substances. Therefore, the greater convergence of platelets and other blood components like leukocytes may be the cause of the higher antibacterial impact of i-PRF. The “low-speed concept” for blood centrifugation proposed by Ghanaati et al. [10] can explain this. Before a fibrin clot forms, lower centrifugation speeds were shown to hold more cells, including leukocytes, than the original PRF procedure [10]. Consequently, it is possible to attribute the increased antimicrobial mobility of i-PRF to the increased quantity of platelets and cells. Tohidnezhad et al. [22] characterized platelet concentrates as dynamic against Escherichia coli and Proteus mirabilis, owing to fibrin, fibronectin, platelets, and the incorporation of white platelets [22], which were presented in i-PRF [23]. The wide range of antibacterial and antibiofilm activities of i-PRF is probably mediated by proteins, including lactoferrin, defensins, heparin-restricting protein, cathelicidins, and phospholipase A2. [24]. The structure of this substance may also play a role in the distinctive effect of i-PRF. Jasmine [25] reported that i-PRF and L-PRF had different fibrin ratios and that i-PRF had a smoother surface than L-PRF. It has been shown that I-PRF contains many nanopores of various sizes and shapes, which encourage cell survival and differentiation. The invasion of pathogens and immune cells can be stopped by the small size of nanopores [25].
For most oral pathogenic species, the ability of bacteria to adhere is thought to be one of their primary destructive characteristics [26]. It has been shown that the connection between microorganisms on a surface and the substrate significantly impact the pathophysiology of contaminants related to wound healing. According to Table 3, i-PRF can inhibit the microcolony formation by preventing Aa and Pg from adhering to the plastic plate surface. Róalski claimed that applying i-PRF to lessen the quantity of adherent microorganisms may lessen oral contaminants’ movement and toxicity and improve the treatment’s outcome [27]. Rodriguez Sánchez [28] came to the same conclusion: L-PRF exudate showed an antibacterial action against Pg in planktonic cultures. [28]. Resistance to adhesion can develop in bacteria that have been attached, coagulated, and rendered inactive, preventing them from adhering to the plate’s bottom, or by antimicrobial peptides found in platelets that can block adhesion [27]. Aa and Pg join forces with other bacteria to cling to tooth surfaces and periodontal tissue [29]. As a result, Aa and Pg’s potential to adhere may be less than it is in an oral context when examined individually.
This study found that the periodontitis groups, preceded by the gingivitis and healthy groups, had the greatest anti-adhesion impact (Table 3). Although the difference was not statistically significant, this result led to the speculation that platelet concentrates taken from patients with periodontitis may exhibit a higher antibacterial effect from those from the other group. Many authors have emphasized the differences between healthy and periodontitis blood indices. According to Monteiro [30] and Buhlin [31], patients with periodontitis exhibited higher WBC and C-responsive protein levels than controls. A boost in plasma fibrinogen and platelet initiation has also been associated with periodontitis, which may assist in keeping a coagulant state and, as a result, increase the risk of atherosclerosis and cardiovascular diseases [32, 33]. Periodontitis therapy may help treat atherosclerosis, according to D’Aiuto et al. [34], who demonstrated that complete control of periodontitis reduces serum arbiters and markers of severe stage reactivity [34]. Furthermore, parameters like the ratio of antibodies in the plasma sample [35], plasma oxidizing capacity, and plasma white blood cell count have been compared between periodontitis patients and healthy individuals [36]. Between these groups, these variables did not, however, show any statistically significant differences.
The platelet concentration in the PRF varies from patient to patient, as do the size and makeup of the platelets, particularly the alpha particles that contain growth factors and antimicrobial peptides. Age differences also affect platelet density, which affects how uniformly platelet-rich plasma works. Changes in the blood sample makeup may have impacted the quality of the i-PRF in our investigation. The limited scope of this study prevented us from identifying any appreciable differences in anti-adhesion impact between Aa and Pg. Models of treated and simulated dental surfaces could be employed in this assay in further studies.
In conclusion, within the limits of this study, i-PRF exhibited explicit antibacterial activity against both periodontal pathogens. Additionally, i-PRF gathered from periodontitis patients appeared to have a greater impact than that of other groups. In addition, i-PRF inhibited Pg better than Aa in all three in vitro assays. However, more studies are needed to confirm the antimicrobial effects of i-PRF against other periodontal species in different patient groups.
References
Wu, D., Lin, Z., Zhang, S., Cao, F., Liang, D., Zhou, X.: Decreased hemoglobin concentration and iron metabolism disorder in periodontitis: systematic review and meta-analysis. Front. Physiol. 10, 1620 (2020). https://doi.org/10.3389/fphys.2019.01620.PMID:32082180;PMCID:PMC7005133
Al-Rasheed, A.: Elevation of white blood cells and platelet counts in patients having chronic periodontitis. Saudi Dent. J. 24(1), 17–21 (2012). https://doi.org/10.1016/j.sdentj.2011.10.006
Anand, P.S., Sagar, D.K., Mishra, S., Narang, S., Kamath, K.P., Anil, S.: Total and differential leukocyte counts in the peripheral blood of patients with generalised aggressive periodontitis. Oral Health Prev. Dent. 14(5), 443–450 (2016). https://doi.org/10.3290/j.ohpd.a36470
Nicu, E.A., Van der Velden, U., Nieuwland, R., Everts, V., Loos, B.G.: Elevated platelet and leukocyte response to oral bacteria in periodontitis. J. Thromb. Haemost. 7(1), 162–170 (2009). https://doi.org/10.1111/j.1538-7836.2008.03219.x
Miron, R.J., Zucchelli, G., Pikos, M.A., et al.: Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin. Oral Investig. 21, 1913–1927 (2017). https://doi.org/10.1007/s00784-017-2133-z
Choukroun, J., Adda, F., Schoeffler, C., Vervelle, A.: Une opportunité en paro-implantologie: le PRF. Implantodontie 42, e62 (2001)
Egle, K., Salma, I., Dubnika, A.: From blood to regenerative tissue: how autologous platelet-rich fibrin can be combined with other materials to ensure controlled drug and growth factor release. Int. J. Mol. Sci. 22(21), 11553 (2021). https://doi.org/10.3390/ijms222111553
Owen, C.A., Campbell, E.J.: The cell biology of leukocyte-mediated proteolysis. J. Leukoc. Biol. 65(2), 137–150 (1999). https://doi.org/10.1002/jlb.65.2.137
Wang, X., Meng, H., Xu, L., Chen, Z., Shi, D., Lv, D.: Mean platelet volume as an inflammatory marker in patients with severe periodontitis. Platelets 26(1), 67–71 (2015). https://doi.org/10.3109/09537104.2013.875137
Ghanaati, S., Booms, P., Orlowska, A., et al.: Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 40(6), 679–689 (2014). https://doi.org/10.1563/aaid-joi-D-14-00138
Mourão, C.F., Valiense, H., Melo, E.R., Mourão, N.B., Maia, M.D.: Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft: technical note. Rev. Col. Bras. Cir. 42(6), 421–423 (2015). https://doi.org/10.1590/0100-69912015006013
Karde, P.A., Sethi, K.S., Mahale, S.A., Khedkar, S.U., Patil, A.G., Joshi, C.P.: Comparative evaluation of platelet count and antimicrobial efficacy of injectable platelet-rich fibrin with other platelet concentrates: an in vitro study. J. Indian Soc. Periodontol. 21(2), 97–101 (2017). https://doi.org/10.4103/jisp.jisp_201_17
Kour, P., Pudakalkatti, P.S., Vas, A.M., Das, S., Padmanabhan, S.: Comparative evaluation of antimicrobial efficacy of platelet-rich plasma, platelet-rich fibrin, and injectable platelet-rich fibrin on the standard strains of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Contemp. Clin. Dent. 9(Suppl. 2), S325–S330 (2018). https://doi.org/10.4103/ccd.ccd_367_18
Jasmine, S., Thangavelu, A., Janarthanan, K., Krishnamoorthy, R., Alshatwi, A.A.: Antimicrobial and antibiofilm potential of injectable platelet rich fibrin-a second-generation platelet concentrate-against biofilm producing oral staphylococcus isolates. Saudi J. Biol. Sci. 27(1), 41–46 (2020). https://doi.org/10.1016/j.sjbs.2019.04.012
Castro, A.B., Herrero, E.R., Slomka, V., et al.: Antimicrobial capacity of leucocyte-and platelet rich fibrin against periodontal pathogens. Sci. Rep. 9, 8188 (2019). https://doi.org/10.1038/s41598-019-44755-6
Papapanou, P.N., Sanz, M., Buduneli, N., et al.: Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 89(Suppl. 1), S173–S182 (2018). https://doi.org/10.1002/JPER.17-0721
Miron, R.J., et al.: Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin. Oral Investig. 21(8), 2619–2627 (2017). https://doi.org/10.1007/s00784-017-2063-9
Thao, T.T.P., Ngan, L.T.M., Van, N.T.N., Thuy, P.A.V.: Isolation and storage of Aggregatibacter actinomicetemcomitans from the subgingival plaque of patients with periodontitis. Sci. Technol. Dev. J. Health Sci. 2(2), 185–193 (2021). https://doi.org/10.32508/stdjhs.v2i2.467
Pham, T.A.V., Tran, T.T.P., Luong, N.T.M.: Antimicrobial effect of platelet-rich plasma against Porphyromonas gingivalis. Int. J. Dent. 2019, 7329103 (2019). https://doi.org/10.1155/2019/7329103
Yang, L.C., Hu, S.W., Yan, M., Yang, J.J., Tsou, S.H., Lin, Y.Y.: Antimicrobial activity of platelet-rich plasma and other plasma preparations against periodontal pathogens. J. Periodontol. 86(2), 310–318 (2015). https://doi.org/10.1902/jop.2014.140373
Badade, P.S., Mahale, S.A., Panjwani, A.A., Vaidya, P.D., Warang, A.D.: Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J. Dent. Res. 27(3), 300–304 (2016). https://doi.org/10.4103/0970-9290.186231
Tohidnezhad, M., Varoga, D., Wruck, C.J., et al.: Platelets display potent antimicrobial activity and release human beta-defensin 2. Platelets 23(3), 217–223 (2012). https://doi.org/10.3109/09537104.2011.610908
Radek, K., Gallo, R.: Antimicrobial peptides: natural effectors of the innate immune system. Semin. Immunopathol. 29(1), 27–43 (2007). https://doi.org/10.1007/s00281-007-0064-5
Anitua, E., Alonso, R., Girbau, C., Aguirre, J.J., Muruzabal, F., Orive, G.: Antibacterial effect of plasma rich in growth factors (PRGF®-Endoret®) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin. Exp. Dermatol. 37(6), 652–657 (2012). https://doi.org/10.1111/j.1365-2230.2011.04303.x
Jasmine, S., Thangavelu, A., Krishnamoorthy, R., Alzahrani, K.E., Alshuniaber, M.A.: Architectural and ultrastructural variations of human leukocyte-rich platelet-rich fibrin and injectable platelet-rich fibrin. J. Microsc. Ultrastruct. 9(2), 76–80 (2021). https://doi.org/10.4103/JMAU.JMAU_7_20
Henderson, B., Nair, S.P., Ward, J.M., Wilson, M.: Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu. Rev. Microbiol. 57, 29–55 (2003). https://doi.org/10.1146/annurev.micro.57.030502.090908
Różalski, M.I., Micota, B., Sadowska, B., Paszkiewicz, M., Więckowska-Szakiel, M., Różalska, B.: Antimicrobial/anti-biofilm activity of expired blood platelets and their released products. Postepy Hig. Med. Dosw. (Online) 22(67), 321–325 (2013). https://doi.org/10.5604/17322693.1046009
Rodríguez Sánchez, F., et al.: Antimicrobial mechanisms of leucocyte- and platelet rich fibrin exudate against planktonic Porphyromonas gingivalis and within multi-species biofilm: a pilot study. Front. Cell. Infect. Microbiol. 11, 722499 (2021). https://doi.org/10.3389/fcimb.2021.722499
Lasserre, J.F., Brecx, M.C., Toma, S.: Oral microbes, biofilms and their role in periodontal and peri-implant diseases. Materials 11(10), 1802 (2018). https://doi.org/10.3390/ma11101802
Monteiro, A.M., et al.: Cardiovascular disease parameters in periodontitis. J. Periodontol. 80(3), 378–388 (2009). https://doi.org/10.1902/jop.2009.080431
Buhlin, K., Gustafsson, A., Pockley, A.G., Frostegård, J., Klinge, B.: Risk factors for cardiovascular disease in patients with periodontitis. Eur. Heart J. 24(23), 2099–2107 (2003). https://doi.org/10.1016/j.ehj.2003.09.016
Sahingur, S.E., Sharma, A., Genco, R.J., De Nardin, E.: Association of increased levels of fibrinogen and the -455G/A fibrinogen gene polymorphism with chronic periodontitis. J. Periol. 74(3), 329–337 (2003). https://doi.org/10.1902/jop.2003.74.3.329
Papapanagiotou, D., Nicu, E.A., Bizzarro, S., et al.: Periodontitis is associated with platelet activation. Atherosclerosis 202(2), 605–611 (2009). https://doi.org/10.1016/j.atherosclerosis.2008.05.035
D’Aiuto, F., et al.: Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J. Dent. Res. 83(2), 156–160 (2004). https://doi.org/10.1177/154405910408300214
Graswinckel, J.E., van der Velden, U., van Winkelhoff, A.J., Hoek, F.J., Loos, B.G.: Plasma antibody levels in periodontitis patients and controls. J. Clin. Periodontol. 31(7), 562–568 (2004). https://doi.org/10.1111/j.1600-051X.2004.00522.x
Zapata, J.C., Cox, D., Salvato, M.S.: The role of platelets in the pathogenesis of viral hemorrhagic fevers. PLoS Negl. Trop. Dis. 8(6), e2858 (2014). https://doi.org/10.1371/journal.pntd.0002858
Acknowledgments
This study was supported by Vietnam National University, Ho Chi Minh City, Vietnam, under grant number: 107/QĐ-ĐHQG (B2021-44-01).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors have no conflict of interest to declare.
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Pham, T.A.V., Phuong, T.T.T. (2024). Antibacterial Effect of Injectable Platelet-Rich Fibrin Against Periodontal Pathogens. In: Vo, V.T., Nguyen, TH., Vong, B.L., Le, N.B., Nguyen, T.Q. (eds) 9th International Conference on the Development of Biomedical Engineering in Vietnam. BME 2022. IFMBE Proceedings, vol 95. Springer, Cham. https://doi.org/10.1007/978-3-031-44630-6_76
Download citation
DOI: https://doi.org/10.1007/978-3-031-44630-6_76
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44629-0
Online ISBN: 978-3-031-44630-6
eBook Packages: EngineeringEngineering (R0)