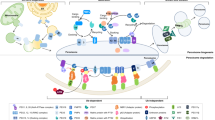
Abstract
7-Ketocholesterol and 7β-hydroxycholesterol are most often derived from the autoxidation of cholesterol. Their quantities are often increased in the body fluids and/or diseased organs of patients with age-related diseases such as cardiovascular diseases, Alzheimer’s disease, age-related macular degeneration, and sarcopenia which are frequently associated with a rupture of RedOx homeostasis leading to a high oxidative stress contributing to cell and tissue damages. On murine cells from the central nervous system (158N oligodendrocytes, microglial BV-2 cells, and neuronal N2a cells) as well as on C2C12 murine myoblasts, these two oxysterols can induce a mode of cell death which is associated with qualitative, quantitative, and functional modifications of the peroxisome. These changes can be revealed by fluorescence microscopy (apotome, confocal microscopy), transmission electron microscopy, flow cytometry, quantitative reverse transcription polymerase chain reaction (RT-qPCR), and gas chromatography-coupled with mass spectrometry (GC-MS). Noteworthy, several natural molecules, including ω3 fatty acids, polyphenols, and α-tocopherol, as well as several Mediterranean oils [argan and olive oils, Milk-thistle (Sylibum marianum) and Pistacia lenticus seed oils], have cytoprotective properties and attenuate 7-ketocholesterol- and 7β-hydroxycholesterol-induced peroxisomal modifications. These observations led to the concept of pexotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Oxysterols are bioactive lipids that result from the oxidation of cholesterol, which can be formed either by auto-oxidation or enzymatically, or by both processes (Mutemberezi et al. 2016). They are involved in numerous diseases, in particular, those linked to age-related diseases, due to their increase or decrease (Zarrouk et al. 2014; Testa et al. 2018; De Medina et al. 2022). The biological activities of oxysterols, which are constituents of the oxysterome (set of oxysterols present at a given time) (Guillemot-Legris and Muccioli 2022), are therefore the resultant of the oxysterols simultaneously present. However, this aspect does not exclude the study of their highly variable individual biological activities over a wide range of concentrations. 7-Ketocholesterol (7KC) and 7β-hydroxycholesterol (7β-OHC), mainly formed by cholesterol auto-oxidation (Anderson et al. 2020; Ghzaiel et al. 2022b), were among the first oxysterols studied because of their well-established involvement in cardiovascular diseases (Vejux et al. 2008; Vejux and Lizard 2009). These two oxysterols are indeed present in increased quantities in oxidized LDL (oxLDL) and in atheromatous lesions (Samadi et al. 2021). Their oxidative and inflammatory activities as well as their capacity to induce cell death by apoptosis in the cells of the vascular wall (endothelial cells, smooth muscle cells and macrophages) widely contribute to the development of the atheromatous plaque with often a fatal issue. At the moment, histological analogies have been highlighted between the appearance of atheromatous lesions and the drusen (localized between the Bruch membrane and the basement membrane of retinal pigment epithelial cells), which contain high 7KC levels, and which are identified in patients with age-related macular degeneration (AMD) (Malvitte et al. 2006). The high level of 7KC in drusen suggests an involvement of this oxysterol in the development of AMD (Pariente et al. 2019). Furthermore, in advanced Alzheimer’s disease, enhanced levels of 7KC and 7β-OHC have also been observed in plasma as well as post-mortem in brain lesions (Testa et al. 2016). In addition, increases in 7KC and 7β-OHC have been found in the plasma of sarcopenic patients aged over 65 years (Ghzaiel et al. 2021). It is important to note that during lipid peroxidation, cholesterol oxidation occurs chronologically after fatty acid oxidation (Noguchi et al. 1998). Therefore, increased levels of 7KC and 7β-OHC indicate significant oxidative stress. Therefore, the prevention of oxidative stress at the systemic and/or local level in cardiovascular disease, AMD, and sarcopenia seems to be essential to treat these diseases. In this context, the targets on which it is necessary to act are multiple and include: (1) the inhibition of pro-oxidant enzymes such as NADPH oxidase which exist under several isoforms (Pedruzzi et al. 2004); (2) the activation of pro-oxidant enzymes [superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase] (Nury et al. 2020); and (3) the prevention of mitochondrial and peroxisomal activities, whose dysfunctions participate in the disruption of the RedOx balance (Trompier et al. 2014; Leoni et al. 2017).
Thanks to the use of several cellular models, it is now well demonstrated that 7KC and 7β-OHC act on these different targets by promoting oxidative stress by increasing the overproduction of superoxide anions (O2•−) via NADPH oxidase, by decreasing the efficiency of the antioxidant system and by disturbing mitochondrial and peroxisomal activity (Vejux et al. 2020; Nury et al. 2021a). While the effects of 7KC and 7β-OHC are well established at the mitochondrial level (reduction of glycolysis and the citric acid/Krebs cycle, decrease in oxidative phosphorylation and ATP production, fall in mitochondrial potential (ΔΨm), contribution to apoptosis by the release of cytochrome c, overproduction of O2•−, and decrease in the expression and activity of antioxidant enzymes), the effects of these two oxysterols at the peroxisomal level are still little explored and therefore not well known whereas their involvement is suspected in cardiovascular diseases and Alzheimer’s disease (Lizard et al. 2012; Zarrouk et al. 2020). The peroxisome is a mostly circular organelle (0.1–1 μM in diameter), devoid of DNA, formed by protein import from the endoplasmic reticulum and closely linked to the mitochondria both topographically and functionally (Schrader and Fahimi 2008; Lismont et al. 2015). Indeed, many of the membrane transporters of the peroxisome are ABCD transporters (ATP-binding cassette sub-type D) and require ATP to function (Kemp et al. 2011; Morita and Imanaka 2012). The peroxisome is involved in the β-oxidation of VLCFA and branched fatty acids, in the synthesis of docosahexaenoic acid (DHA; C22:6 n-3) and plasmalogens, and in the synthesis of cholesterol (Wanders and Waterham 2006a; Kawaguchi and Morita 2016; Charles et al. 2020). Plasmalogens have a major role in the regulation of inflammation, oxidative stress and cell death, and their involvement in aging and some age-related diseases is widely documented (Hossain et al. 2020, 2023). The peroxisome is also involved in phagocytosis, cytokine production, degradation of eicosanoids, and regulation of the RedOx balance (Fransen et al. 2012; Lismont et al. 2015; Di Cara et al. 2019).
Using 7KC and 7β-OHC on different cell lines (158N murine oligodendrocytes, murine neuronal N2a cells, murine microglial BV-2 cells, and murine myoblasts C2C12 cells), we established an experimental approach to determine the effects of these oxysterols on the peroxisome by using several criteria. The effects on peroxisomal topography and morphology were addressed by transmission electron microscopy and fluorescence microscopy (conventional, apotome, confocal); for the latter approach, the peroxisomes were revealed by indirect immunofluorescence with an anti-Abcd3 antibody (Debbabi et al. 2017a). The effects on the amount of peroxisome per cell were determined by flow cytometry (Nury et al. 2018; Ghzaiel et al. 2022a) by measuring the expression of the peroxisomal transporter Abcd3, which is a transporter of pristanic acid, dicarboxylic acid, and bile acid intermediates, and which is also considered as a suitable marker of the number of peroxisomes per cell and/or of the peroxisomal mass (Gray et al. 2014; Tawbeh et al. 2021). The effects on peroxisomal function were addressed (1) on the one hand by measuring by RT-qPCR the expression of the genes of peroxisomal transporter (Abcd1, Abcd2, Abcd3) and enzymes (Acox1), peroxisomal multifunctional protein-2 (Mfp2) involved in peroxisomal β-oxidation (Wanders 2014) as well as in plasmalogens synthesis (dihydroxyacetone-phosphate acyltransferase (DHAP-AT), alkyl-DHAP synthase) (Wanders and Waterham 2006b; Kanzawa et al. 2012; Honsho and Fujiki 2023), and (2) on the other hand by quantifying by gas chromatography-mass spectrometry (GC-MS) the amount per cell of VLCFAs (C24:0; C24:1; C26:0, C26:1) metabolized in the peroxisome (Wanders and Waterham 2006a) as well as the rate of cellular plasmalogens, whose first two enzymes (DHAP-AT, alkyl-DHAP synthase) involved in their synthesis, are located in the peroxisomal membrane (Brites et al. 2004; Nury et al. 2018). These different approaches have also made it possible to identify several molecules (synthetic and natural, as well as oils often of Mediterranean origin) that attenuate the cytotoxicity of 7KC and 7β-OHC while opposing qualitative, quantitative, and functional peroxisomal modifications (Debbabi et al. 2016, 2017b; Badreddine et al. 2017; Ghzaiel et al. 2022a). This attenuation of peroxisomal dysfunctions by different synthetic or natural molecules has given rise to the notion of pexotherapy.
2 Evaluation of the Effects of 7-Ketocholesterol and 7β-Hydroxycholesterol on the Peroxisomal Status
2.1 Effects of 7-Ketocholesterol and 7β-Hydroxycholesterol on the Peroxisomal Topography and Morphology
In the experimental strategy developed to evaluate the impact of molecules on the peroxisome, different microscopic techniques can be used. These techniques include transmission electron microscopy (TEM) and fluorescence microscopy [conventional fluorescence microscopy, structured fluorescence microscopy (apotome), and confocal microscopy].
The visualization of peroxisomes by TEM requires the use of diaminobenzidine (DAB) and hydrogen peroxide (H2O2). In this particular experimental condition, the peroxisomal catalase activity is revealed, and the peroxisomes, which are stained in black, are visualized (Trompier et al. 2014; Ghzaiel et al. 2022a). The livers of 9- to 10-week-old C57 Black/6 male mice were used as a positive control for the detection of peroxisomes by TEM (Fig. 21.1a, b). Without DAB and H2O2, the peroxisomes are not detected. This experimental condition does not affect the detection of other cell components and permits the identification of all organelles. The visualization of peroxisomes by TEM gives information on the morphological aspect and size of the peroxisomes, and also information on the peroxisomal topography: distribution in the cytoplasm, interaction with other organelles such as mitochondria and endoplasmic reticulum, localization or not in vacuoles (this latter aspect provides ultrastructural information on pexophagy) and oxiapoptophagy (Nury et al. 2021b). On murine nerve cells (158N, BV-2, and N2a) or on murine myoblasts (C2C12) treated with 7KC or 7β-OHC, similar ultrastructural changes were observed at oxysterol concentrations inducing cell death (oxiapoptophagy on 158N, BV-2, and N2a cells; caspase-independent mode of cell death on C2C12 cells) (Nury et al. 2020) (Fig. 21.1c, j). In those conditions, comparatively to untreated cells (control), morphologically altered peroxisomes were often observed under treatment with 7KC or 7β-OHC (Nury et al. 2018) (Fig. 21.1e, f), and their cytoplasmic distribution was often modified such as in 7β-OHC-treated C2C12 cells: in these cells several peroxisomes were present in vacuoles (Ghzaiel et al. 2022a) (Fig. 21.1, g–j).
Visualization of peroxisome by transmission electron microscopy on mouse liver, nerve cells (158N oligodendrocytes, murine neuronal N2a cells), and murine C2C12 myoblasts cultured with or without 7-ketocholesterol- or 7β-hydroxycholesterol. White arrows point toward peroxisomes observed in different cell types. The livers of 9- to 10-week-old C57 Black/6 male mice were used as positive control for the detection of peroxisomes (Trompier et al. 2014; Nury et al. 2018, 2020). (a, b) Peroxisome in mouse liver; (c, d) in 158N oligodendrocytes, some peroxisomes closely located to mitochondria were identified (d); (e, f) peroxisomes in N2a cells; comparatively to untreated cells (control) (e), the morphological aspects of peroxisomes were modified in 7KC (50 μM, 48 h)-treated cells (f); in C2C12 murine myoblasts round and regular peroxisomes were observed in the cytoplasm of untreated cells (control) (g, h); in 7β-OHC-treated cells most of the peroxisomes were located in vacuoles (i, j)
Conventional fluorescence microscopy, realized on a right or inverted microscope, can also be used to reveal the peroxisomes detected by immunofluorescence with an antibody raised against the peroxisomal transporter Abcd3 or catalase (Trompier et al. 2014) or other peroxisomal proteins (Acox1, Mfp2) (Baarine et al. 2009). This approach makes it possible to estimate the effect of molecules, such as oxysterols, on the topography of peroxisomes and their quantity per cell. However, this approach is approximative and needs to be completed either by observations in structured fluorescence microscopy (apotome) or in confocal microscopy, whose excellent resolution in z-makes it possible to apprehend the peroxisomal distribution in different planes for further 3D reconstruction and reliable quantifications. These last two methods also make it possible to evaluate mitochondria and peroxisomes simultaneously under excellent conditions (Nury et al. 2018; Namsi et al. 2019). Data obtained on untreated (control) as well as on 7KC- and 7β-OHC-treated C2C12 murine myoblasts by structured fluorescence microscopy (apotome), after mitochondria staining with Mito Tracker Red and detection of peroxisome with an antibody raised against Abcd3 and revealed with Alexa-488, are shown in Fig. 21.2: topographic modifications of peroxisomes and mitochondria are clearly observed. This approach with an apotome is also well appropriated to simultaneously evaluate the mitochondria and the peroxisomes in neurites (axones and dendrites) (Namsi et al. 2019). Thus, on differentiated N2a cells obtained under treatment with octadecaneuropeptide (ODN, 10−14 M), the peroxisomes and the mitochondria stained in green and red, respectively, can be observed in the neurites in structured fluorescence microscopy and when these two organelles were closely located a yellow fluorescence was observed (Fig. 21.2). By confocal microscopy, observation in the x-y plan can be coupled with observations along the z-axis, allowing a 3D reconstruction to precise the distribution of peroxisomes in the cells; this approach has been successfully used on 158N cells (Fig. 21.3). Since catalase is localized both in the cytoplasm and in the peroxisome, it is less used than Abcd3 (specifically present at the peroxisomal membrane level) to visualize the peroxisome (Baarine et al. 2009; Trompier et al. 2014). Data obtained on human neuronal cells (SK-N-BE) by confocal microscopy show a cytoplasmic distribution pattern of catalase which evocates the distribution of Abcd3 (Fig. 21.3). TEM, structured fluorescence microscopy (apotome) and confocal microscopy are well adapted complementary methods to study the morphology of peroxisomes and their topography as well as their interaction with other organelles, especially mitochondria.
Simultaneous visualization of peroxisomes and mitochondria by illumination microscopy (Apotome) on undifferentiated cells cultured with or without 7-ketocholesterol- or 7β-hydroxycholesterol and on differentiated N2a cells: mitochondria were revealed by Mito Tracker Red and peroxisome by indirect immunofluorescence with antibodies raised against Abcd3. The fluorescence procedure was performed as follows on N2a cells as previously described (Debbabi et al. 2017a; Namsi et al. 2019). After mitochondria staining with Mito Tracker Red, the peroxisomes were revealed with a rabbit polyclonal antibody raised against Abcd3 (Ref: 1152365; Pierce/Thermo Fisher Scientific) which was revealed with a goat anti-rabbit 488-Alexa antibody (Santa Cruz Biotechnology, Santa Cruz, USA). The nuclei were stained with Hoechst 33342 (2 μg/mL). Cells were mounted in Dako fluorescent medium. Cells were stored in the dark at +4 °C until examination with structured illumination microscopy (Apotome 3 imaging system, Zeiss, Jena, Germany). Green dots: peroxisomes; red dots: mitochondria. (a): undifferentiated N2a cells cultured with 7-ketocholesterol (7KC: 25–50 μM) for 24 h or 7β-hydroxycholesterol (7β-OHC: 25–50 μM) for 24 h; (b): differentiated N2a cells; N2a were previously cultured for 24 h in conventional culture medium; the cells were further cultured for 48 h in medium without FBS in the absence (control) or presence of octadecaneuropeptide (octadecaneuropeptide (ODN); 10−14 M) as previously described. Along the neurites, several mitochondria (red fluorescence) and peroxisomes (green fluorescence) were detected. Yellow spots (colocalization of mitochondria and peroxisomes) were also identified. Green arrows point toward peroxisomes; red arrows point toward mitochondria; yellow arrows point toward colocalized peroxisomes and mitochondria. The images were realized with ZEN imaging software (Zeiss)
Simultaneous visualization of mitochondria and peroxisomes by confocal microscopy on N2a and SK-N-BE cells. The immunofluorescence procedure was performed on murine N2a and human SK-N-BE cells cultured on glass slides as previously described (Trompier et al. 2014; Debbabi et al. 2017a). The peroxisomes were revealed either with a rabbit polyclonal antibody raised against Abcd3 (Ref: 1152365; Pierce/Thermo Fisher Scientific) or a rabbit polyclonal antibody raised against catalase (Ref: ab16771, Abcam, Paris, France) which were revealed with a goat anti-rabbit 488-Alexa antibody (Santa Cruz Biotechnology). The nuclei were stained with Hoechst 33342 (2 μg/mL). Cells were mounted in Dako fluorescent medium and stored in the dark at +4 °C until examination by confocal microscopy (Confocal Laser Scanning Microscope TCS SP8, Leica, Wetzlar, Germany). The images were realized with LASX (Leica)
2.2 Effects of 7-Ketocholesterol and 7β-Hydroxycholesterol on the Peroxisomal Mass
The effects of 7KC and 7β-OHC, in a concentration range 12.5–50 μM, are rather well described on the endoplasmic reticulum, the lysosomes, and the mitochondria (Nury et al. 2021a). Morphological and functional alterations of these organelles were reported in the presence of these two oxysterols. On the other hand, the effects of these molecules on the peroxisome are still poorly known. To quantify the effects of 7KC and 7 β-OHC on the peroxisomal mass, the peroxisomes were detected on different cell types by indirect immunofluorescence with a rabbit polyclonal antibody raised against Abcd3 (Debbabi et al. 2017a). Quantification was performed by flow cytometry. Under these conditions, a decrease in peroxisomal mass was observed; this decrease was strongly counteracted in the presence of α-tocopherol (200–400 μM) whatever the cell type considered (Vejux et al. 2020). Data shown are those obtained on C2C12 murine myoblasts: untreated cells (control) and cells cultured in the presence of 7β-OHC associated or not with α-tocopherol (Fig. 21.4). At the moment, α-tocopherol, which is known to strongly attenuate 7KC- and 7β-OHC-induced cell death on different cell types, also strongly attenuates the decrease in peroxisomal mass measured with the anti-Abcd3 antibody and revealed by a secondary antibody coupled to Alexa-488. Similar results were obtained with 7KC. This observation led to the notion of pexotherapy, which can be defined as the ability of a natural or synthetic molecule to prevent quantitative and qualitative peroxisomal alterations (Ghzaiel et al. 2022a).
Effect of 7β-hydroxycholesterol on the level of the major peroxisomal membrane transporter (Abcd3) used to evaluate the peroxisomal mass. C2C12 cells were incubated for 24 h with or without 7β-OHC (50 μM) in the presence or absence of α-tocopherol (400 μM) (Ghzaiel et al. 2022a). The protective effect of α-tocopherol (400 μM) against 7β-OHC was analyzed by: (a) structured illumination microscopy (apotome); the nuclei were stained with Hoechst 33342 (2 μg/mL); (b) flow cytometry (FCM). (c): the percentages of C2C12 cells with reduced Abcd3 levels were determined by FCM, and Abcd3 gene expression was quantified by RT-qPCR; the data are presented as the mean ± SD of two independent experiments performed in triplicate. A multiple comparative analysis between the groups, taking into account the interactions, was carried out using an ANOVA test followed by a Tukey’s test. A p-value less than 0.05 was considered statistically significant. The statistically significant differences between the groups, which are indicated by different letters, take into account the vehicle used. (a): comparison versus control; (b): comparison versus ethanol (ETOH: 0.5%); (c): comparison versus ETOH (0.1%); (d): comparison versus α-tocopherol (α-toco: 400 μM); (e): comparison versus 7β-OHC (50 μM). No significant differences were observed between the untreated (control) and vehicle-treated cells. Ct cycle threshold
2.3 Effects of 7-Ketocholesterol and 7β-Hydroxycholesterol on the Peroxisomal Activity
Concerning peroxisome function, peroxisomal damages (alteration of peroxisomal β-oxidation) can favor the accumulation of very-long chain fatty acids (VLCFA; C ≥ 22) (Savary et al. 2012), which can contribute to amplifying cell dysfunctions (Nury et al. 2020). In C2C12 cells, the analysis of the effects of 7β-OHC (50 μM) associated or not with α-tocopherol (400 μM) on VLCFA levels supports cytotoxic effects of 7β-OHC on peroxisomal activity and cytoprotective effects of α-tocopherol at the peroxisomal level. In untreated cells (control) and vehicle (EtOH: 0.1 and 0.5%)-treated cells, no significant differences were found; similar levels of VLCFA (C22:0, C24:0, C26:0) were found (Fig. 21.5a, b). The level of VLCFAs was determined by gas chromatography coupled with mass spectrometry (GC-MS). When C2C12 cells were exposed to 7β-OHC, a significant increase in VLCFAs was detected, and these latter were significantly reduced when 7β-OHC was associated with α-tocopherol (Ghzaiel et al. 2021, 2022a) (Fig. 21.5a, b). However, enhanced elongase activity could also be involved in the increased level of VLCFAs (Jakobsson et al. 2006; Kihara 2012). At the moment, seven enzymes, ELOVL 1–7 (Fatty Acid Elongases 1–7), localized in the endoplasmic reticulum, have been identified. ELOVL1 is considered to control VLCA synthesis up to C26:0, and ELOVL1 is the most potent elongase for C24:0 and C26:0, however, depending on the cell type, similar elongase activity has been reported with ELOVL3 and ELOVL6. The data obtained support an increase in the elongase activity index which could correspond to ELOVL1, 3, and 6 activity; ratio (C24:0/C22:0), and ratio (C26:0/C22:0) under treatment with 7β-OHC; these different elongase activity indexes were also strongly attenuated when 7β-OHC was associated with α-tocopherol (Ghzaiel et al. 2021) (Fig. 21.5d).
Effect of 7β-hydroxycholesterol with and without on very-long chain fatty acid (VLCFA) levels. C2C12 cells were incubated for 24 h with or without 7β-OHC (50 μM) in the presence or absence of α-tocopherol (400 μM). The level of VLCFA (C ≥ 22) was determined by GC-MS: C22:0 (a), C24:0 (b), C26:0 (c) (Ghzaiel et al. 2021). Data are the mean ± SD of two independent experiments. A multiple comparative analysis between the groups, taking into account the interactions, was carried out using an ANOVA test followed by a Tukey’s test. A p-value less than 0.05 was considered statistically significant. The statistically significant differences between the groups, which are indicated by different letters, take into account the vehicle used. (a): comparison versus control; (b): comparison versus ethanol (ETOH: 0.5%); (c): comparison versus ETOH (0.1%); (d): comparison versus α-tocopherol (α-toco: 400 μM); (e): comparison versus 7β-OHC (50 μM). No significant differences were observed between the untreated (control) and vehicle-treated cells
2.4 Impact of 7KC and 7β-Hydroxycholesterol on the Expression of Peroxisomal Genes Associated with Peroxisomal Biogenesis, Peroxisomal β-Oxidation, and Plasmalogen Synthesis
To address 7KC and 7β-OHC-mediated changes in peroxisomal gene expression, RT-qPCR was used not only to quantify the expression of the peroxisomal Abcd3 transporter gene but also to measure the expression of other peroxisomal genes such as those associated with peroxisomal biogenesis (Pex5, Pex13, Pex14), peroxisomal β-oxidation (Abcd1, Abcd2, Acox1, Mfp2, Thiolase A) (Wanders and Waterham 2006a), and the first two steps of plasmalogen synthesis (DHAP-AT, alkyl-DHAP synthase) (Brites et al. 2004). Depending on the cell model used (murine nerve cells: 158N, N2a, and BV-2; murine myoblasts C2C12), the expression of some genes is either decreased or not modified under treatment with 7KC and 7β-OHC. Interestingly, when 7KC and/or 7β-OHC led to a reduction in peroxisomal gene expression, the addition of α-tocopherol always normalized this expression, demonstrating the potent cytoprotective effects of α-tocopherol against the peroxisomal toxicity induced by 7KC and 7β-OHC (Badreddine et al. 2017; Nury et al. 2018; Ghzaiel et al. 2022a). These findings underscore the crucial role of α-tocopherol in preventing peroxisomal dysfunction caused by 7KC and 7β-OHC.
3 Prevention of 7-Ketocholesterol- and 7β-Hydroxycholesterol-Induced Peroxisomal Changes: Interest of Nutrients (ω3 Fatty Acids, Polyphenols) and Edible Oils (Argan and Olive Oils, Milk-Thistle (Sylibum Marianum) and Pistacia Lenticus Seed Oils)
Among the nutrients that oppose the toxicity of 7KC and 7β-OHC, α-tocopherol has shown efficacy on many cell types of different species. This tocopherol, which is a major component of Vitamin E, is constituted of four tocopherols (α-, β-, γ-, and δ-tocopherol) and four tocotrienols (α-, β-, γ-, and δ-tocotrienol) (Rimbach et al. 2002), is particularly opposed to topographical, morphological and functional changes in peroxisomes induced by these two oxysterols and can be considered as the leader in pexotherapy (Nury et al. 2021a; Ghzaiel et al. 2022a).
However, other nutrients (oleic acid, polyphenols) as well as several oils, mostly of Mediterranean origin (argan and olive oils, Milk-thistle, and Pistacia lentiscus seed oils), also strongly attenuate the toxicity of 7KC and 7β-OHC as well as the associated peroxisomal modifications (Yammine et al. 2020; Rezig et al. 2022).
-
Thus, oleic acid (C18:1 n-9/C18:1 cis-9), also prevents 7KC-induced oxidative stress and cell death (such as oxiapoptophagy) on 158N, N2a, and BV-2 cells. On BV-2 cells, oleic acid as well as α-and γ-tocopherol were able to prevent the decrease in Abcd3 protein levels, which allows the measurement of peroxisomal mass, and in mRNA levels of Abcd1 and Abcd2, which encode for two transporters involved in peroxisomal β-oxidation (Debbabi et al. 2016). It is suggested that oleic acid could contribute to the inactivation of 7KC by esterification. Indeed, on U937 cells treated with 7KC-oleate, comparatively to 7KC, no cytotoxic effect was observed (Monier et al. 2003). Similar observations were realized when C2C12 murine myoblasts were treated either with 7KC-oleate or 7β-OHC-oleate (Ghzaiel I., PhD Thesis, Univ. Bourgogne, 2022).
-
Among polyphenols, known for their health benefits, quercetin (QCT), trans-resveratrol (RSV), and apigenin (API) also prevented peroxisomal dysfunction in 7KC-treated N2a cells (Yammine et al. 2020). These three polyphenols prevented the impact of 7KC by counteracting the decrease in ATP-binding cassette subfamily D member (Abcd3) at the protein and mRNA levels, as well as the decreased expression of genes associated with peroxisomal biogenesis (Pex13, Pex14) and peroxisomal β-oxidation (Abcd1, Acox1, Mfp2, Thiolase A). 7KC-induced decrease in Abcd1 and Mfp2, two proteins involved in peroxisomal β-oxidation, was also attenuated by RSV, QCT, and API.
-
As Milk-thistle seed oil (MTSO) contains high amounts of α-tocopherol, oleic acid as well as low amounts of polyphenols (Meddeb et al. 2017), this led us to evaluate its cytoprotective activities on 7KC- and 7β-OHC-treated cells. On 158N cells, MTSO opposes oxidative stress and cell death induced by 7KC and 7β-OHC (Badreddine et al. 2020; Zarrouk et al. 2019). On C2C12 murine myoblasts, in the presence of 7β-OHC, comparatively, to untreated cells, important quantitative and qualitative peroxisomal modifications were identified: (a) a reduced number of peroxisomes with abnormal sizes and shapes, mainly localized in cytoplasmic vacuoles, were observed; (b) the peroxisomal mass was decreased as indicated by lower protein and mRNA levels of the peroxisomal Abcd3 transporter; (c) lower mRNA level of Pex5 involved in peroxisomal biogenesis as well as higher mRNA levels of Pex13 and Pex14, involved in peroxisomal biogenesis and/or pexophagy, was found; (d) lower levels of Acox1 and Mfp2 enzymes, implicated in peroxisomal β-oxidation, were detected; (e) higher levels of very-long chain fatty acids, which are substrates of peroxisomal β-oxidation, were found. These different cytotoxic effects were strongly attenuated by MTSO, in the same range of order as with α-tocopherol (Ghzaiel et al. 2022a). The cytoprotective results obtained with MTSO prompted us to evaluate the cytoprotective activities of other oils used in the Mediterranean diet.
-
Olive oil also highly attenuates the toxicity of 7KC (oxiapoptophagy, oxidative stress) on 158N cells (Badreddine et al. 2017; Zarrouk et al. 2019).
-
With argan oil (AO), important cytoprotective effects were also observed on 158N. Under treatment with 7KC, AO significantly attenuates loss of cell adhesion, cell growth inhibition, increased plasma membrane permeability, mitochondrial and lysosomal dysfunction, as well as oxiapoptophagy induction (Badreddine et al. 2017). Marked effects on the peroxisome were also observed: thus, argan oil significantly counteracts the decreased expression of Abcd1 and Abcd3 observed under treatment with 7KC (Badreddine et al. 2017). Based on data obtained on BV-2 cells, it is suggested that Schottenol and Spinasterol, two major phytosterols of AO and cactus seed oil (El-Mostafa et al. 2014; El Kharrassi et al. 2014), could protect cells from oxidative stress and of its harmful consequences for peroxisomal functions (Essadek et al. 2023).
-
With Pistacia lenticus seed oil (PLSO), on C2C12 murine myoblasts, the cytotoxic effects of 7β-OHC were also strongly reduced (Ghzaiel et al. 2021). Thus, at the peroxisomal level, PLSO strongly attenuates (1) the topographical and morphological changes revealed by illumination microscopy (apotome) and TEM, (2) the decrease of peroxisomal mass revealed by lower levels of Abcd3 protein and mRNA measured by flow cytometry and RT-qPCR, and (3) the decrease of peroxisomal β-oxidation revealed by an intracellular accumulation of C24:0 and C26:0 quantified by GC-MS.
4 Conclusions
Both 7KC and 7β-OHC modify the topography, mass, structure, and activity of peroxisomes on several cell types. However, the detrimental effects can be attenuated by several nutrients and Mediterranean oils. This has led to the development of pexotherapy, where natural and synthetic molecules, as well as specific oils, are used to prevent peroxisomal damage. The techniques that have been developed for studying peroxisomal status can be applied in both experimental and clinical contexts, providing a better understanding of peroxisomes, which are still poorly understood in both physiological and pathological contexts.
Abbreviations
- 7KC:
-
7-Ketocholesterol
- 7β-OHC:
-
7β-Hydroxycholesterol
- Abcd transporter:
-
ATP-binding cassette sub-type D transporter
- Acox1:
-
Acyl-CoA oxidase 1
- AMD:
-
Age-related macular degeneration
- AO:
-
Argan oil
- DHA:
-
Docosahexaenoic acid
- DHAP-AT:
-
Dihydroxyacetone-phosphate acyltransferase
- ELOVL:
-
Fatty acid elongase
- GC-MS:
-
Gas chromatography-coupled with mass spectrometry
- GPx:
-
Glutathione peroxidase
- Mfp2:
-
Peroxisomal multifunctional protein-2
- PLSO:
-
Pistacia lenticus seed oil
- RT-qPCR:
-
Quantitative reverse transcription polymerase chain reaction
- SOD:
-
Superoxide dismutase
- TEM:
-
Transmission electron microscopy
- VLCFA:
-
Very-long chain fatty acid
References
Anderson A, Campo A, Fulton E, Corwin A, Jerome WG 3rd, O’Connor MS (2020) 7-Ketocholesterol in disease and aging. Redox Biol 29:101380
Baarine M, Ragot K, Genin EC, El Hajj H, Trompier D, Andreoletti P, Ghandour MS, Menetrier F, Cherkaoui-Malki M, Savary S, Lizard G (2009) Peroxisomal and mitochondrial status of two murine oligodendrocytic cell lines (158N, 158JP): potential models for the study of peroxisomal disorders associated with dysmyelination processes. J Neurochem 111:119–131
Badreddine A, Zarrouk A, Karym EM, Debbabi M, Nury T, Meddeb W, Sghaier R, Bezine M, Vejux A, Martine L, Grégoire S, Bretillon L, Prost-Camus E, Durand P, Prost M, Moreau T, Cherkaoui-Malki M, Nasser B, Lizard G (2017) Argan oil-mediated attenuation of organelle dysfunction, oxidative stress and cell death induced by 7-ketocholesterol in murine oligodendrocytes 158N. Int J Mol Sci 18:2220
Badreddine A, Zarrouk A, Meddeb W, Nury T, Rezig L, Debbabi M, Bessam FZ, Brahmi F, Vejux A, Mejri M, Nasser B, Lizard G (2020) Chapter 10 - antioxidant and neuroprotective properties of Mediterranean oils: argan oil, olive oil, and milk thistle seed oil. In: Martin CR, Preedy VR (eds) Oxidative stress and dietary antioxidants in neurological diseases. Academic Press
Brites P, Waterham HR, Wanders RJ (2004) Functions and biosynthesis of plasmalogens in health and disease. Biochim Biophys Acta 1636:219–231
Charles KN, Shackelford JE, Faust PL, Fliesler SJ, Stangl H, Kovacs WJ (2020) Functional peroxisomes are essential for efficient cholesterol sensing and synthesis. Front Cell Dev Biol 8:560266
De Medina P, Silvente-Poirot S, Poirot M (2022) Oxysterols are potential physiological regulators of ageing. Ageing Res Rev 77:101615
Debbabi M, Nury T, Zarrouk A, Mekahli N, Bezine M, Sghaier R, Grégoire S, Martine L, Durand P, Camus E, Vejux A, Jabrane A, Bretillon L, Prost M, Moreau T, Ammou SB, Hammami M, Lizard G (2016) Protective effects of α-tocopherol, γ-tocopherol and oleic acid, three compounds of olive oils, and no effect of Trolox, on 7-ketocholesterol-induced mitochondrial and peroxisomal dysfunction in microglial BV-2 cells. Int J Mol Sci 17:1973
Debbabi M, Nury T, Helali I, Karym EM, Geillon F, Gondcaille C, Trompier D, Najid A, Terreau S, Bezine M, Zarrouk A, Vejux A, Andreoletti P, Cherkaoui-Malki M, Savary S, Lizard G (2017a) Flow cytometric analysis of the expression pattern of peroxisomal proteins, Abcd1, Abcd2, and Abcd3 in BV-2 murine microglial cells. Methods Mol Biol 1595:257–265
Debbabi M, Zarrouk A, Bezine M, Meddeb W, Nury T, Badreddine A, Karym EM, Sghaier R, Bretillon L, Guyot S, Samadi M, Cherkaoui-Malki M, Nasser B, Mejri M, Ben-Hammou S, Hammami M, Lizard G (2017b) Comparison of the effects of major fatty acids present in the Mediterranean diet (oleic acid, docosahexaenoic acid) and in hydrogenated oils (elaidic acid) on 7-ketocholesterol-induced oxiapoptophagy in microglial BV-2 cells. Chem Phys Lipids 207:151–170
Di Cara F, Andreoletti P, Trompier D, Vejux A, Bülow MH, Sellin J, Lizard G, Cherkaoui-Malki M, Savary S (2019) Peroxisomes in immune response and inflammation. Int J Mol Sci 20:3877
El Kharrassi Y, Samadi M, Lopez T, Nury T, El Kebbaj R, Andreoletti P, El Hajj HI, Vamecq J, Moustaid K, Latruffe N, El Kebbaj MS, Masson D, Lizard G, Nasser B, Cherkaoui-Malki M (2014) Biological activities of Schottenol and Spinasterol, two natural phytosterols present in argan oil and in cactus pear seed oil, on murine microglial BV2 cells. Biochem Biophys Res Commun 446:798–804
El-Mostafa K, El Kharrassi Y, Badreddine A, Andreoletti P, Vamecq J, El Kebbaj MS, Latruffe N, Lizard G, Nasser B, Cherkaoui-Malki M (2014) Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 19:14879–14901
Essadek S, Gondcaille C, Savary S, Samadi M, Vamecq J, Lizard G, Kebbaj RE, Latruffe N, Benani A, Nasser B, Cherkaoui-Malki M, Andreoletti P (2023) Two argan oil phytosterols, Schottenol and Spinasterol, attenuate oxidative stress and restore LPS-dysregulated peroxisomal functions in Acox1(−/−) and wild-type BV-2 microglial cells. Antioxidants (Basel) 12:168
Fransen M, Nordgren M, Wang B, Apanasets O (2012) Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta 1822:1363–1373
Ghzaiel I, Zarrouk A, Nury T, Libergoli M, Florio F, Hammouda S, Ménétrier F, Avoscan L, Yammine A, Samadi M, Latruffe N, Biressi S, Levy D, Bydlowski SP, Hammami S, Vejux A, Hammami M, Lizard G (2021) Antioxidant properties and cytoprotective effect of Pistacia lentiscus L. seed oil against 7β-hydroxycholesterol-induced toxicity in C2C12 myoblasts: reduction in oxidative stress, mitochondrial and peroxisomal dysfunctions and attenuation of cell death. Antioxidants (Basel) 10:1772
Ghzaiel I, Zarrouk A, Essadek S, Martine L, Hammouda S, Yammine A, Ksila M, Nury T, Meddeb W, Tahri Joutey M, Mihoubi W, Caccia C, Leoni V, Samadi M, Acar N, Andreoletti P, Hammami S, Ghrairi T, Vejux A, Hammami M, Lizard G (2022a) Protective effects of milk thistle (Sylibum marianum) seed oil and α-tocopherol against 7β-hydroxycholesterol-induced peroxisomal alterations in murine C2C12 myoblasts: nutritional insights associated with the concept of pexotherapy. Steroids 183:109032
Ghzaiel ISK, Zarrouk A, Ghosh S, Dias IHK, Nury T, Ksila M, Essadek S, Tahri Joutey M, Brahmi F, Mihoubi W, Rup-Jacques S, Samadi M, Rezig L, Meziane S, Ghrairi T, Masmoudi-Kouki O, Hammami S, Nasser B, Hammami M, Wang Y, Griffiths WJ, Vejux A, Lizard G (2022b) Sources of 7-ketocholesterol, metabolism and inactivation strategies: food and biomedical applications. Redox Exp Med 1:R40–R46
Gray E, Rice C, Hares K, Redondo J, Kemp K, Williams M, Brown A, Scolding N, Wilkins A (2014) Reductions in neuronal peroxisomes in multiple sclerosis grey matter. Mult Scler 20:651–659
Guillemot-Legris O, Muccioli GG (2022) The oxysterome and its receptors as pharmacological targets in inflammatory diseases. Br J Pharmacol 179:4917–4940
Honsho M, Fujiki Y (2023) Regulation of plasmalogen biosynthesis in mammalian cells and tissues. Brain Res Bull 194:118–123
Hossain MS, Mawatari S, Fujino T (2020) Biological functions of plasmalogens. Adv Exp Med Biol 1299:171–193
Hossain MS, Mawatari S, Fujino T (2023) Plasmalogens inhibit neuroinflammation and promote cognitive function. Brain Res Bull 192:56–61
Jakobsson A, Westerberg R, Jacobsson A (2006) Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45:237–249
Kanzawa N, Shimozawa N, Wanders RJ, Ikeda K, Murakami Y, Waterham HR, Mukai S, Fujita M, Maeda Y, Taguchi R, Fujiki Y, Kinoshita T (2012) Defective lipid remodeling of GPI anchors in peroxisomal disorders, Zellweger syndrome, and rhizomelic chondrodysplasia punctata. J Lipid Res 53:653–663
Kawaguchi K, Morita M (2016) ABC transporter subfamily D: distinct differences in behavior between ABCD1-3 and ABCD4 in subcellular localization, function, and human disease. Biomed Res Int 2016:6786245
Kemp S, Theodoulou FL, Wanders RJ (2011) Mammalian peroxisomal ABC transporters: from endogenous substrates to pathology and clinical significance. Br J Pharmacol 164:1753–1766
Kihara A (2012) Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem 152:387–395
Leoni V, Nury T, Vejux A, Zarrouk A, Caccia C, Debbabi M, Fromont A, Sghaier R, Moreau T, Lizard G (2017) Mitochondrial dysfunctions in 7-ketocholesterol-treated 158N oligodendrocytes without or with α-tocopherol: impacts on the cellular profile of tricarboxylic cycle-associated organic acids, long chain saturated and unsaturated fatty acids, oxysterols, cholesterol and cholesterol precursors. J Steroid Biochem Mol Biol 169:96–110
Lismont C, Nordgren M, Van Veldhoven PP, Fransen M (2015) Redox interplay between mitochondria and peroxisomes. Front Cell Dev Biol 3:35
Lizard G, Rouaud O, Demarquoy J, Cherkaoui-Malki M, Iuliano L (2012) Potential roles of peroxisomes in Alzheimer’s disease and in dementia of the Alzheimer’s type. J Alzheimers Dis 29:241–254
Malvitte L, Montange T, Joffre C, Vejux A, Maïza C, Bron A, Creuzot-Garcher C, Lizard G (2006) Analogies between atherosclerosis and age-related maculopathy: expected roles of oxysterols. J Fr Ophtalmol 29:570–578
Meddeb W, Rezig L, Abderrabba M, Lizard G, Mejri M (2017) Tunisian milk thistle: an investigation of the chemical composition and the characterization of its cold-pressed seed oils. Int J Mol Sci 18:2582
Monier S, Samadi M, Prunet C, Denance M, Laubriet A, Athias A, Berthier A, Steinmetz E, Jürgens G, Nègre-Salvayre A, Bessède G, Lemaire-Ewing S, Néel D, Gambert P, Lizard G (2003) Impairment of the cytotoxic and oxidative activities of 7 beta-hydroxycholesterol and 7-ketocholesterol by esterification with oleate. Biochem Biophys Res Commun 303:814–824
Morita M, Imanaka T (2012) Peroxisomal ABC transporters: structure, function and role in disease. Biochim Biophys Acta 1822:1387–1396
Mutemberezi V, Guillemot-Legris O, Muccioli GG (2016) Oxysterols: from cholesterol metabolites to key mediators. Prog Lipid Res 64:152–169
Namsi A, Nury T, Khan AS, Leprince J, Vaudry D, Caccia C, Leoni V, Atanasov AG, Tonon MC, Masmoudi-Kouki O, Lizard G (2019) Octadecaneuropeptide (ODN) induces N2a cells differentiation through a PKA/PLC/PKC/MEK/ERK-dependent pathway: incidence on peroxisome, mitochondria, and lipid profiles. Molecules 24:3310
Noguchi N, Numano R, Kaneda H, Niki E (1998) Oxidation of lipids in low density lipoprotein particles. Free Radic Res 29:43–52
Nury T, Sghaier R, Zarrouk A, Ménétrier F, Uzun T, Leoni V, Caccia C, Meddeb W, Namsi A, Sassi K, Mihoubi W, Riedinger JM, Cherkaoui-Malki M, Moreau T, Vejux A, Lizard G (2018) Induction of peroxisomal changes in oligodendrocytes treated with 7-ketocholesterol: attenuation by α-tocopherol. Biochimie 153:181–202
Nury T, Yammine A, Menetrier F, Zarrouk A, Vejux A, Lizard G (2020) 7-Ketocholesterol- and 7β-hydroxycholesterol-induced peroxisomal disorders in glial, microglial and neuronal cells: potential role in neurodegeneration: 7-ketocholesterol and 7β-hydroxycholesterol-induced peroxisomal disorders and neurodegeneration. Adv Exp Med Biol 1299:31–41
Nury T, Yammine A, Ghzaiel I, Sassi K, Zarrouk A, Brahmi F, Samadi M, Rup-Jacques S, Vervandier-Fasseur D, Pais De Barros JP, Bergas V, Ghosh S, Majeed M, Pande A, Atanasov A, Hammami S, Hammami M, Mackrill J, Nasser B, Andreoletti P, Cherkaoui-Malki M, Vejux A, Lizard G (2021a) Attenuation of 7-ketocholesterol- and 7β-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: potential for the prevention of age-related diseases. Ageing Res Rev 68:101324
Nury T, Zarrouk A, Yammine A, Mackrill JJ, Vejux A, Lizard G (2021b) Oxiapoptophagy: a type of cell death induced by some oxysterols. Br J Pharmacol 178:3115–3123
Pariente A, Peláez R, Pérez-Sala Á, Larráyoz IM (2019) Inflammatory and cell death mechanisms induced by 7-ketocholesterol in the retina. Implications for age-related macular degeneration. Exp Eye Res 187:107746
Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O’Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E (2004) NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24:10703–10717
Rezig L, Ghzaiel I, Ksila M, Yammine A, Nury T, Zarrouk A, Samadi M, Chouaibi M, Vejux A, Lizard G (2022) Cytoprotective activities of representative nutrients from the Mediterranean diet and of Mediterranean oils against 7-ketocholesterol- and 7β-hydroxycholesterol-induced cytotoxicity: application to age-related diseases and civilization diseases. Steroids 187:109093
Rimbach G, Minihane AM, Majewicz J, Fischer A, Pallauf J, Virgli F, Weinberg PD (2002) Regulation of cell signalling by vitamin E. Proc Nutr Soc 61:415–425
Samadi A, Sabuncuoglu S, Samadi M, Isikhan SY, Chirumbolo S, Peana M, Lay I, Yalcinkaya A, Bjørklund G (2021) A comprehensive review on oxysterols and related diseases. Curr Med Chem 28:110–136
Savary S, Trompier D, Andréoletti P, Le Borgne F, Demarquoy J, Lizard G (2012) Fatty acids - induced lipotoxicity and inflammation. Curr Drug Metab 13:1358–1370
Schrader M, Fahimi HD (2008) The peroxisome: still a mysterious organelle. Histochem Cell Biol 129:421–440
Tawbeh A, Gondcaille C, Trompier D, Savary S (2021) Peroxisomal ABC transporters: an update. Int J Mol Sci 22:6093
Testa G, Staurenghi E, Zerbinati C, Gargiulo S, Iuliano L, Giaccone G, Fantò F, Poli G, Leonarduzzi G, Gamba P (2016) Changes in brain oxysterols at different stages of Alzheimer’s disease: their involvement in neuroinflammation. Redox Biol 10:24–33
Testa G, Rossin D, Poli G, Biasi F, Leonarduzzi G (2018) Implication of oxysterols in chronic inflammatory human diseases. Biochimie 153:220–231
Trompier D, Vejux A, Zarrouk A, Gondcaille C, Geillon F, Nury T, Savary S, Lizard G (2014) Brain peroxisomes. Biochimie 98:102–110
Vejux A, Lizard G (2009) Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Asp Med 30:153–170
Vejux A, Malvitte L, Lizard G (2008) Side effects of oxysterols: cytotoxicity, oxidation, inflammation, and phospholipidosis. Braz J Med Biol Res 41:545–556
Vejux A, Abed-Vieillard D, Hajji K, Zarrouk A, Mackrill JJ, Ghosh S, Nury T, Yammine A, Zaibi M, Mihoubi W, Bouchab H, Nasser B, Grosjean Y, Lizard G (2020) 7-Ketocholesterol and 7β-hydroxycholesterol: in vitro and animal models used to characterize their activities and to identify molecules preventing their toxicity. Biochem Pharmacol 173:113648
Wanders RJ (2014) Metabolic functions of peroxisomes in health and disease. Biochimie 98:36–44
Wanders RJ, Waterham HR (2006a) Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem 75:295–332
Wanders RJ, Waterham HR (2006b) Peroxisomal disorders: the single peroxisomal enzyme deficiencies. Biochim Biophys Acta 1763:1707–1720
Yammine A, Zarrouk A, Nury T, Vejux A, Latruffe N, Vervandier-Fasseur D, Samadi M, Mackrill JJ, Greige-Gerges H, Auezova L, Lizard G (2020) Prevention by dietary polyphenols (resveratrol, quercetin, apigenin) against 7-ketocholesterol-induced oxiapoptophagy in neuronal N2a cells: potential interest for the treatment of neurodegenerative and age-related diseases. Cell 9:2346
Zarrouk A, Vejux A, Mackrill J, O’Callaghan Y, Hammami M, O’Brien N, Lizard G (2014) Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res Rev 18:148–162
Zarrouk A, Martine L, Grégoire S, Nury T, Meddeb W, Camus E, Badreddine A, Durand P, Namsi A, Yammine A, Nasser B, Mejri M, Bretillon L, Mackrill JJ, Cherkaoui-Malki M, Hammami M, Lizard G (2019) Profile of fatty acids, tocopherols, phytosterols and polyphenols in Mediterranean oils (argan oils, olive oils, milk thistle seed oils and nigella seed oil) and evaluation of their antioxidant and cytoprotective activities. Curr Pharm Des 25:1791–1805
Zarrouk A, Nury T, El Hajj HI, Gondcaille C, Andreoletti P, Moreau T, Cherkaoui-Malki M, Berger J, Hammami M, Lizard G, Vejux A (2020) Potential involvement of peroxisome in multiple sclerosis and Alzheimer’s disease: peroxisome and neurodegeneration. Adv Exp Med Biol 1299:91–104
Acknowledgments
The authors would like to thank Prof. Hervé Alexandre (Institut Universitaire de la Vigne et du Vin, Université de Bourgogne, Dijon, France) for providing the flow cytometer. Imen Ghzaiel, Mohamed Ksila, and Aline Yammine received financial support from ABASIM (Association Bourguignonne pour les Applications des Sciences de l’Information en Médecine (Dijon, France) and/or Nutrition Méditérranéenne et Santé (NMS; President Prof. Norbert Latruffe). This work was also funded by the Université de Bourgogne (Dijon, France), the University of Monastir (Monastir, Tunisia), and the University of Tunis El Manar (Tunis, Tunisia). This work (PhD Thesis; Mohamed Ksila) was also in part supported by PHC Utique (2022 and 2023, Code CMCU: 22G0809/Code Campus France: 47608VJ); Dr. Gérard Lizard/Prof. Taoufik Ghrairi; University Tunis El Manar (Tunis, Tunisie); University of Monastir (Monastir, Tunisia); University of Gabès (Gabès, Tunisia); Faculty of Science and Technology, University Hassan I (Settat, Morocco).
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ghzaiel, I. et al. (2024). In Vitro Evaluation of the Effects of 7-Ketocholesterol and 7β-Hydroxycholesterol on the Peroxisomal Status: Prevention of Peroxisomal Damages and Concept of Pexotherapy. In: Lizard, G. (eds) Implication of Oxysterols and Phytosterols in Aging and Human Diseases. Advances in Experimental Medicine and Biology, vol 1440. Springer, Cham. https://doi.org/10.1007/978-3-031-43883-7_21
Download citation
DOI: https://doi.org/10.1007/978-3-031-43883-7_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43882-0
Online ISBN: 978-3-031-43883-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)