Abstract
Atherosclerosis is a major cardiovascular complication of diseases associated with elevated oxidative stress such as type 2 diabetes and metabolic syndrome. In these situations, low-density lipoproteins (LDL) undergo oxidation. Oxidized LDL displays proatherogenic activities through multiple and complex mechanisms which lead to dysfunctions of vascular cells (endothelial cells, smooth muscle cells, and macrophages). Oxidized LDLs are enriched in oxidized products of cholesterol called oxysterols formed either by autoxidation, enzymatically, or by both mechanisms. Several oxysterols have been shown to accumulate in atheroma plaques and to play a key role in atherogenesis. Depending on the type of oxysterols, various biological effects are exerted on vascular cells to regulate the formation of macrophage foam cells, endothelial integrity, adhesion and transmigration of monocytes, plaque progression, and instability. Most of these effects are linked to the ability of oxysterols to induce cellular oxidative stress and cytotoxicity mainly through apoptosis and proinflammatory mediators. Like for excess cholesterol, high-density lipoproteins (HDL) can exert antiatherogenic activity by stimulating the efflux of oxysterols that have accumulated in foamy macrophages.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Oxysterol
- Oxidized low-density lipoprotein
- Atherosclerosis
- Macrophages
- Endothelial cells
- Apoptosis
- Inflammation
1 Introduction
Atherosclerosis is a chief cause of morbidity and mortality in Western countries. It is characterized by the formation in the intima arteries of atherosclerotic plaques consisting of the accumulation of lipids, complex carbohydrates, blood cells and products, fibrous tissue, and calcium deposits. Since the regions with low fluid shear stress—the sites of arterial branching or curvature—are much more susceptible, the abdominal aorta, coronary arteries, and carotid bifurcations are the predilection vessels for the formation of atherosclerotic plaques. The formation of atherosclerotic plaques also called atherogenesis is a long-term and evolutionary process. Initial lesions (fatty streaks) can be observed very early in life and stay asymptomatic for a long time. Advanced atherosclerotic lesions develop with the accumulation of lipids, cell debris, and fibrous tissue in a long-term processes (20–40 years) The thickening of the plaques causes cardiovascular diseases (CVD) such as coronary heart disease, myocardial infarction, and stroke when disrupting. The risk factors for atherosclerosis are multiple including non-modifiable factors such as age, sex, and genetic background, and modifiable factors such as smoking, lack of exercise, and high-fat diet. It is well acknowledged that atherosclerosis is a major cardiovascular complication of diseases associated with chronic inflammatory status, increased oxidative stress and disorders of lipid metabolism, such as type 2 diabetes, obesity, and metabolic syndrome.
Arteries are constituted of three morphologically distinct layers: the outerlayer adventitia, the media, and the innermost layer intima where the atherosclerotic plaques are formed. Atherogenesis begins with the infiltration and sub-endothelial accumulation of low-density lipoproteins (LDL). In physiopathological situations, LDL becomes oxidized (oxLDL) closely related to oxidative stress prevailing in the arterial wall that causes oxidation of the protein and lipid moieties of LDL through both enzymatic or non-enzymatic (ROS-induced) pathways. LDL oxidation can be catalyzed by metal cations (copper, iron) and several enzyme systems, including 12/15-lipoxygenase strongly expressed strongly in atherosclerotic plaques, myeloperoxidase, NOS (nitric oxide synthase), xanthine oxidase, and NADPH oxidase. The oxidative products are various, including oxidized derivatives of fatty acid (e.g., malondialdehyde (MDA), 13-HPODE, and 13-HODE), and lipids (e.g., lysophosphatidylcholine and oxysterols) as well as protein carbonyls. The early oxidation of LDL can only produce the minimally oxidized LDL because of the presence of antioxidants, such as vitamin E, A, and β carotene, and the efficient antioxidant enzymes (e.g., superoxide dismutase). In the narrow sub-endothelial space, LDL undergoes further oxidation to form the highly oxidized LDL involving the ROS produced by macrophages and endothelial cells (EC), and also several enzymes form these cells. OxLDL displays atherogenic activities through multiple and complex mechanisms. Globally, oxLDL altered homeostasis of vascular cells resulting in loss of EC integrity, migration and proliferation of smooth muscle cells (SMC), and macrophage foam cell formation through different signals linked to proinflammatory and proapoptotic effects (Nègre-Salvayre et al. 2017, 2020).
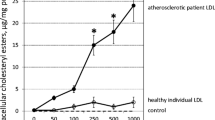
The LDL receptor (LDL-R) is located on the plasma membrane and internalizes LDL after binding to apoB-100. At a minor stage of oxidation, the apoB is simply no longer recognized by the LDL-R. At a major stage of oxidation, the modified apoB allows the recognition of multiple scavenger receptors (SR) including SR-A1 (scavenger receptor class A1), SR-A2, CD36, SR-B1 (scavenger receptor class B1), and LOX1 (lectin-like oxidized LDL receptor 1) (Zingg et al. 2021). In contrast with LDL-R, even though the uptake of modified LDL induces the elevated cholesterol content in macrophages, the expression of SR is not regulated by the intracellular cholesterol quantity, which results in high uptake of oxLDL by macrophages and cholesterol (as its ester form) accumulation in macrophages. The cholesterol-engorged macrophages are called foam cells. The accumulation of foam cells in artery walls, which is easily recognized by light microscopy, is a sign of early atherosclerotic lesions.
Among oxidized lipids in oxLDL, cholesterol oxidation products of cholesterol also called oxysterols have recently gained growing attention with respect to their role in atherogenesis. This review is an overview of the oxysterols that accumulate in atheroma plaques and that exert biological activities on vascular cells including EC, SMC, and macrophages, thereby contributing to atherogenesis and ultimate plaque rupture.
2 Oxysterols in Atherosclerotic Plaque and in Serum from Patients with CVD
As mentioned above, oxLDLs are enriched in oxysterols, some of which are involved in the ability of oxLDL to induce cellular oxidative stress and cytotoxicity, mainly through apoptosis. Oxysterols are also associated with the regulation of lipid metabolism and inflammation and are considered as factors contributing to clinical complications of atherosclerosis (see Sect. 11.3).
Oxysterols are produced from cholesterol oxidation through enzymatic pathways with oxidative position mainly on the side chain of cholesterol generating hydroxycholesterols (OHC) including 20S-OHC, 22R-OHC, 22S-OHC, 24S-OHC, 27-OHC, 25-OHC, and 7α,25-dihydroxycholesterol. Non-enzymatic pathways cholesterol autoxidation mainly on the cholesterol ring led to 7-ketocholesterol (7-KC), 7β-OHC, 7α-OHC, 4β-OHC, 5α,6α-epoxycholesterol (5,6α-EC), 5β,6β-epoxycholesterol (5,6β-EC), and cholestan-3β,5α, 6β-triol (CT). 7α-OHC and 25-OHC can be formed by both pathways.
2.1 Levels of Oxysterols in Atheroma Plaque and Plasma of Atherosclerotic Subjects
The first evidence for the presence of oxysterols in atherosclerotic plaques dated from mid-1960s (Brooks et al. 1966; Fumagalli et al. 1971). Increased amounts of 27-OHC and 7-KC were reported in these studies. Since then, many studies have confirmed the association between the presence of oxysterol in atheromatous lesions and atherosclerotic plaque formation, progression, and stability. Overall, 27-OHC is the major oxysterol recovered in advanced atherosclerotic plaques, followed by 7-KC, 7β-OHC, and 7α-OHC. These oxysterols account for 75–85% of the total oxysterols detected in atherosclerotic plaques from various sites, the others being 25-OHC, 24-OHC, and 5,6-EC (Vaya 2013).
Elevated levels of 27-OHC were found in human carotid atherosclerotic plaques compared to non-atherosclerotic human vessels in correlation to high expression of 27-hydroxylase (the enzyme converting cholesterol to 27-OHC) in macrophage-rich core regions of complicated lesions (Crisby et al. 1997). Since 27-OHC can be eliminated from macrophages, it was proposed that 27-OHC formation could be a defense mechanism against deleterious cellular accumulation of cholesterol.
Analysis of oxidized lipids in human aortic advanced atherosclerotic plaques revealed elevated amounts of 27-OHC and 7β-OHC compared to normal aorta (Carpenter et al. 1993). This group also highlighted the presence of these two oxysterols at different stages of atherosclerotic lesions (fatty streaks, intermediate, and advanced lesions) from aorta and common carotid artery, compared to normal human artery. 27-OHC was significantly more abundant in advanced lesions than in intermediate lesions or fatty streaks (Carpenter et al. 1995). 27-OHC and 7β-OHC were also detected in all the samples of human atheromatous lipid core and fibrous cap of individual advanced atherosclerotic plaques (Garcia-Cruset et al. 1999). It is also noteworthy that pharmacological lowering of 27-OHC was associated with coronary plaque regression (Nakano et al. 2022).
7-Oxygenated sterol (7β-OHC, 7α-OHC, and 7KC) have also been widely detected in atherosclerotic lesions; 7-KC is the second most abundant oxysterol and the major 7-oxygenated sterol found in atheromatous plaques(Brown et al. 1997; Garcia-Cruset et al. 2001; Ravi et al. 2021), although it was not detected in a recent study (Pinto et al. 2022). The presence of 7β-OHC in atherosclerotic lesions together with 27-OHC has been reported in early studies (Carpenter et al. 1993; Garcia-Cruset et al. 1999). Importantly, the in situ formation of ROS-derived 7-oxygenated sterol in human carotid plate was confirmed—rather than auto-oxidation during sample processing—as well as that of ROS-derived EC (Helmschrodt et al. 2013).
Other oxysterols such as 24-OHC, 25-OHC, 5,6β-EC, and 5,6α-EC were found in human fatty streaks and advanced atherosclerotic lesions (Garcia-Cruset et al. 2001; Helmschrodt et al. 2013). Increased levels of 24-OHC, 25-OHC, 27-OHC, 7α-OHC, and 7β-OHC but not 7-KC were measured in symptomatic subjects with carotid atherosclerotic plaques compared to asymptomatic subjects (Pinto et al. 2022). Arterial intima accumulation of 27-OHC and 24S-OHC is associated with severe peripheral artery disease (Virginio et al. 2015). The accumulation of 25-OHC was reported in coronary atherosclerotic lesions (Canfrán-Duque et al. 2023) but not in arterial tissue from subjects with severe peripheral artery disease (Virginio et al. 2015).
In addition to high detection in atheromatous plaques, elevated plasma concentrations of several oxysterols including 25-OHC, 27-OHC, and 7β-OHC were shown to correlate with symptoms of coronary and peripheral artery diseases and atherogenic risk profile (Ziedén et al. 1999; Yasunobu et al. 2001; Rimner et al. 2005; Virginio et al. 2015). Increased levels of 7β-OHC in plasma were proposed to be a biomarker for high risk of developing cardiovascular disease and coronary atherosclerotic plaques (Khatib and Vaya 2014). Elevated plasma 7-KC levels have also been associated with higher risk of cardiovascular disease events in the general population and in patients with coronary artery disease (Hitsumoto et al. 2009; Song et al. 2017; Wang et al. 2017).
Atherosclerosis is a major complication of diseases associated with high oxidative stress such as diabetes, metabolic syndrome, and dyslipidemia. The level of total oxysterols was markedly increased in the serum of diabetic subjects compared to healthy controls, mainly due to high amounts of 7-KC, 7α-OHC, 7β-OHC, and 5,6α-EC (Khatib and Vaya 2014). Elevated plasmatic oxysterol levels especially 7-KC and CT were correlated to hyperglycemia and glycation index as well as a number of coronary risk factors, particularly in type 2 diabetic patients (Samadi et al. 2019; Samadi et al. 2020; Ahmed et al. 2022).
2.2 In Vitro and In Vivo Formation of Oxysterols
In vitro oxidation of LDL using different acellular oxidizing conditions (copper, peroxynitrite, 2,2′-azobis(2-amidinopropane)dihydrochloride-AAPH, hypochlorous acid) has confirmed the formation of oxysterols in the particle through auto-oxidation process, including 7-KC, 7α-OHC, 7β-OHC that were reproducibly detected. The formation of 5,6α- and 5,6β-EC, as well as 24-OHC, 25-OHC, or 27-OHC has also been reported but to a lesser extent and not in all studies (Matsunaga et al. 2009; Vaya et al. 2011; Arnal-Levron et al. 2013; Orsó et al. 2015; Chen et al. 2015). The level of oxysterols in LDL varies according to the oxidizing conditions and is the highest in copper highly oxidized LDL (Orsó et al. 2015).
Although less explored, cell-mediated generation of oxysterols was also reported. Macrophages, as well as EC and SMC, were shown to mediate radical-dependent LDL oxidation leading to the formation of lipid hydroperoxides and consequent increase of 7-OHC and 7-KC (Müller et al. 1998). It is commonly assumed that accumulation of oxysterols in atheroma macrophages results from the massive uptake of oxLDL that itself contains oxysterols (Brown et al. 1996, 1997). Accordingly, the levels of 7-KC, 7α-OHC, 27-OHC, and cholest-4-en-3-one were found to increase in oxLDL-loaded mouse macrophages proportionally to the degree of LDL oxidation (Paul et al. 2019).
The intracellular formation of oxysterols in cultured macrophages is very low and usually under the detection limit but some species (i.e., 7-KC, 25-OHC, 24-OHC, and 24,25-EC) were highly increased upon excessive cholesterol loading or exposure to the endotoxin Kdo2-Lipid A (Fu et al. 2001; Dennis et al. 2010; Maurya et al. 2013). Intracellular formation of oxysterols is promoted in macrophages after uptake of modified LDL that undergo oxidation inside lysosomes leading to the formation of 7-KC and 7β-OHC (Wen and Leake 2007; Yoshida and Kisugi 2010). Our studies confirmed that cellular activity significantly contributes to the accumulation of oxysterols in macrophage cell lines (Arnal-Levron et al. 2013; Chen et al. 2015). Upon exposure to copper-oxidized LDL, both cellular cholesterol and LDL-derived cholesterol were oxidized in murine RAW and human THP1 macrophages resulting in a huge increase of oxysterol production. Major oxysterols originated from non-enzymatic pathway (7-KC and 7α/β-OHC), the enzymatically formed 25-OHC and 27-OHC being recovered in much lower proportions. We also demonstrated that the oxidation of LDL-derived cholesterol occurred mainly in the late endosomal compartment, while oxidation of cellular cholesterol likely occurs at the plasma membrane site (Chen et al. 2015). The intracellular oxysterol production in oxLDL-loaded RAW macrophages was regulated by the specific endosomal phospholipid bis(monoacylglycero)phosphate, also supporting LDL-cholesterol oxidation in this compartment (Arnal-Levron et al. 2013).
3 Biological Effects of Oxysterols in Atherogenesis and Plaque Progression
Several oxysterols have been involved in various aspects of atherogenesis, such as formation of macrophage foam cells, endothelial dysfunction, adhesion and transmigration of monocytes, plaque progression, and instability (Poli et al. 2009). Depending on the type of oxysterols, various biological activities are exerted on vascular cells including EC, SMC, and macrophages. Although a subset of oxysterols is likely to exert antiatherogenic effects by regulating cholesterol homeostasis, an overlapping but distinct set of oxysterols display proatherogenic activity by inducing cytotoxicity primarily through apoptosis and stimulating inflammatory pathways.
3.1 Oxysterols and Foam Cell Formation
Cholesterol homeostasis in macrophages results from the finely regulated balance between cholesterol acquisition including de novo synthesis of cholesterol dependent on HMGCoA reductase (HMGCoAR) activity and uptake of non-oxLDL by LDL-R, and efflux of excess cholesterol by extracellular acceptors HDL and apoA1. Both mechanisms of cholesterol acquisition are regulated by a complex of three proteins located in the endoplasmic reticulum (ER): SREBP (sterol regulatory element binding protein), SCAP (SREBP cleavage activating protein), and Insig (insulin-induced gene). This complex regulates the expression of LDL-R receptor and HMGCoAR inversely correlated to ER cholesterol content (Sato 2010; Savla et al. 2022). The removal of excess intracellular cholesterol is regulated by the activity of nuclear receptor liver X receptors (LXR) by enhancing the expression of ATP-binding cassette (ABC) transporters ABCA1 and ABCG1 (Matsuo 2022). Atherosclerosis is characterized by excessive accumulation of cholesterol within sub-endothelial macrophages. To sum up, cholesterol accumulates consequently due to the massive unregulated uptake of oxLDL by SR and to the defects in ABC-dependent efflux of cholesterol (Li et al. 2021). In early lesions, excess cholesterol is stored in the form of cholesterol esters produced by acyl-coA:cholesterol ester transferase (ACAT) activity within lipid droplets giving macrophages their foamy appearance. In advanced lesions, free cholesterol accumulates in ER, leading to ER stress that triggers apoptosis of foamy macrophages.
Side chain oxysterols, in particular 25-OHC, suppress the expression of SREBP2 target genes presumably in an Insig-dependent manner, which negatively regulates cholesterol biosynthesis and uptake (Sato 2010). As natural ligands for LXRα and LXRβ (Janowski et al. 1999), side chains oxysterols including 24,25-EC, 22(R)-OHC, 24(S)-OHC, 25-OHC, and 27-OHC exert antiatherogenic activity by stimulating the expression of ABCA1 and ABCG1 in macrophages (Töröcsik et al. 2009; Olkkonen 2012; Saito et al. 2023). However, some oxysterols can inversely promote foam cell phenotype. A mixture of 7β-OHC and 7-KC stimulates lipid droplet formation and upregulates SRA that facilitates the ingestion of oxLDL (Yuan et al. 2016; Ward et al. 2017; Saha et al. 2020).
3.2 Oxysterol and Apoptosis/Autophagy/Oxyautophagy
Oxysterols are considered potent regulators of cell death primarily by inducing apoptosis, stress of endoplasmic reticulum, and autophagy (Fig. 11.1).
Apoptosis is a natural, programmed cell death process that occurs in multicellular organisms. Two main pathways trigger apoptosis: the intrinsic pathway (or mitochondrial pathway) and the extrinsic pathway (or death receptor pathway). Both pathways involve a series of complex signaling events that ultimately lead to the activation of caspases and the subsequent destruction of the cell. The intrinsic pathway is activated by intracellular stresses, such as DNA damage, oxidative stress, or nutrient deprivation. It is associated with outer mitochondrial membrane permeabilization and release of cytochrome c that induces the assembly of a caspase-activation complex. The extrinsic pathway is activated by the binding of extracellular ligands, such as Fas ligand or tumor necrosis factor-alpha (TNFα) to death receptors on the cell surface which initiates the caspase cascade. Caspase 3 is considered to be a key mediator of apoptosis; it cleaves a number of key cellular proteins, including cytoskeletal proteins, nuclear proteins, and enzymes, leading to DNA fragmentation, and membrane dismantling (D’Arcy 2019).
The ability of oxysterols to induce apoptosis in vascular cells has been well described. Among the oxysterols found in atheroma plaques, 7-OHC and 7-KC are commonly the most cytotoxic. These oxysterols induce apoptosis in SMC (Hughes et al. 1994; Miyashita et al. 1997; Pedruzzi et al. 2004), EC (Luchetti et al. 2017), and macrophages (Li et al. 2012; Ward et al. 2017; Ravi et al. 2021) contributing to the cause of cell death in core regions of atherosclerotic plaques. Compared to 7-oxysterols, side chain oxysterols exert no or lower apoptotic effect. 27-OHC exerts dual effects in terms of cytotoxicity toward macrophages, acting as a protector at a low concentration while triggering apoptosis at high concentrations (Riendeau and Garenc 2009). Low micromolar concentration of 27-OHC evokes survival signals in U937 human promonocytic cell line through the activation of ERK and AKT and inhibition of the proapoptotic protein Bad in response to initial ROS formation (Vurusaner et al. 2016, 2018). Studies using 25-hydroxylase deficient macrophages suggest that 25-OHC increases susceptibility to LPS-induced apoptosis in macrophages through increased caspase 3 activation and reduced efferocytosis capacity (Canfrán-Duque et al. 2023).
7-Oxysterols stimulate both intrinsic mitochondrial and extrinsic death receptor pathways of apoptosis. In macrophages, apoptosis mediated by 7-oxysterols is associated with caspase-3 activation, increased permeability of mitochondrial membrane, release of cytochrome c and endonuclease G (Prunet et al. 2005; Palozza et al. 2010; Li et al. 2012). 7-KC and 7β-OHC can also induce ROS production and decrease cellular antioxidants, therefore inducing mitochondrial oxidative stress in the sub-endothelial space (Tabas et al. 2015; Ravi et al. 2021). Apoptosis mediated by 7-oxysterols also involves the regulation of the expression of Bcl-2 family proteins that exert pro- or anti-apoptotic activity. In macrophages, 7-KC induces proapoptotic pathways associated with the proapoptotic proteins Bax and Bim (Berthier et al. 2005; Palozza et al. 2010; Li et al. 2012) and inhibits the anti-apoptotic protein AKT (Vejux and Lizard 2009; Palozza et al. 2010). However, 7-KC was also reported to trigger a survival response through the activation of PYK2/MEK1/2/ERK pathway allowing BAD phosphorylation (Berthier et al. 2005). 7-KC and 7β-OHC-induced apoptosis is also associated with the induction and nuclear translocation of the tumor suppressor p53 (Li et al. 2012) which has been shown to be highly expressed and to promote apoptosis in human atherosclerotic plaque (Yuan et al. 2010).
Endoplasmic reticulum (ER) stress is a cellular response to an accumulation of misfolded or unfolded proteins within the ER. ER stress triggers a signaling pathway called the unfolded protein response (UPR) that aims to restore ER homeostasis. ER markers include phosphorylated eIF2α and IRE1α and expression of the proteins GRP78 and CHOP. Prolonged activation of UPR leads to cell dysfunction and death, contributing to the development of various diseases, including neurodegenerative disorders, diabetes, cancer, and cardiovascular diseases. The links between oxLDL and ER stress as well as the involvement of ER stress in atherosclerosis initiation and progression have been well documented, but the role of oxysterols is not well established (Sanda et al. 2017; Luchetti et al. 2017). In SMC and EC, 7-KC-induced ER stress is characterized by increased phosphorylation of IRE1α and expression of CHOP and GRP78 (Pedruzzi et al. 2004; Sanson et al. 2009). 7-KC and 7-OHC can induce macrophage apoptosis through moderating ER stress-specific signaling involving CHOP induction (Son et al. 2012; Park et al. 2016).
Autophagy is a process by which cells degrade and recycle damaged or unwanted cellular components to promote cell survival. Autophagy has been recently revealed as a crucial regulator in the formation of early and advanced atherosclerotic plaques (Li et al. 2022). This phenomenon is traditionally regarded as beneficial in atherosclerosis as it prevents EC apoptosis and senescence, regulates the proliferation of SMC cells, and inhibits foam cell formation and lipid-laden macrophage apoptosis. Few studies have examined the role of oxysterols in autophagy of vascular cells. 7-KC was reported to induce autophagy in human SMC promoting survival and stabilizing atherosclerotic plaque (He et al. 2013; Zhang et al. 2020). On the other hand, 7-KC-induced autophagy may exert deleterious effects as it promotes vascular calcification through autophagy-lysosomal pathway (Sudo et al. 2015).
Last decade, cell death induced by oxysterols has been defined as a complex mode of cell death involving oxidative stress, apoptosis, and autophagy, defined as oxiapoptophagy. Oxiapoptophagy is associated with organelle dysfunction and in particular with mitochondrial and peroxisomal alterations involved in the induction of cell death and in the rupture of redox balance. Oxidative stress can induce both apoptosis and autophagy, and these processes can interact with each other. For example, under certain conditions, autophagy can promote apoptosis by degrading anti-apoptotic proteins, while apoptosis can inhibit autophagy by cleaving essential autophagy proteins. Thus, the interplay between oxidative stress, apoptosis, and autophagy is complex (Nury et al. 2014, 2021). With respect to oxysterols 7-KC, 7β-OHC and 24S-OHC were reported to be strong inducers of oxiapoptophagy in different cell types including U937 monocytic cells (Nury et al. 2021; de Freitas et al. 2021). Other oxysterols have been shown to induce oxiapoptophagy such as 24(S)OHC in oligodendrocytes (Nury et al. 2015), 25-OHC in fibroblast cells (You et al. 2021), and 7α,25-dihydroxycholesterol in osteoblasts (Seo et al. 2023). However, the link between oxysterols and oxiapoptophagy in atherosclerosis is not well documented.
3.3 Oxysterols and Inflammation
Oxysterols are considered as potent inducers of inflammation as they can alter endothelial monolayer integrity, recruit the immune cells, stimulate the expression of various inflammatory molecules, regulate macrophage polarization into M1/M2 phenotypes, and promote the rupture of fibrous caps (Testa et al. 2018) (Fig. 11.2).
It is well known that endothelial dysfunction occurs in the early stage of atherosclerosis and some oxysterols have been involved in this process. 25-OHC is able to inhibit EC proliferation, migration, and tube formation and to impair endothelium-dependent vasodilation through inhibition of NO production (Ou et al. 2016). It also reduced the expression of tight junction proteins (Niedzielski et al. 2021). 25-OHC, as well as 7-KC, was also shown to reduce endothelial monolayer impedance and adhesion (Chalubinski et al. 2013).
Integrins are components of cell-matrix adhesions that regulate monocyte adhesion to endothelial cells and their migration to the site of inflammation. The expression of β1-integrin was shown to increase in U937 monocytes exposed to an oxysterol mixture of pathophysiologic relevance through activation of the ERK signaling pathway. Among oxysterols present at concentrations close to those found in vascular lesions, 7α-OHC and CT were the most potent compounds, and 7β-OHC, 5,6α-EC, and 7-KC were the least potent ones (Gargiulo et al. 2012). This oxysterol mixture was also shown to stimulate the expression and synthesis of MCP-1 (monocyte chemotactic protein-1), another monocyte chemoattractant also referred to as CCL2, in U937 macrophages through the activation of ERK pathway and nuclear binding of NF-κB (nuclear factor κB) (Leonarduzzi et al. 2005). It was also reported that 25-OHC is a ligand of α5β1 and αvβ3 integrins to activate integrin-focal adhesion kinase (FAK) signaling (Pokharel et al. 2019).
ICAM-1 (intercellular adhesion molecule-1), VCAM-1 (vascular cell adhesion molecule-1), and E-selectin are adhesion molecules involved in the interaction between leukocyte and EC and subsequent transmigration of monocytes through the endothelial monolayer. Oxysterols, especially those oxidized at C7 (7α/β-OHC and 7-KC), increased the levels of ICAM-1, VCAM-1, and E-selectin expressions in human vascular cells through mechanisms involving p38MAPK pathway or ROS production (Lemaire et al. 1998; Shimozawa et al. 2004; Tani et al. 2018). 25-OHC is also able to induce ICAM-1 synthesis in human EC associated with disruption of endothelial integrity, both effects being counteracted by statin (Niedzielski et al. 2021).
Oxysterols were also reported as modulators of proinflammatory cytokines such as interleukins IL1, IL6, and IL8. In macrophages, 7β-OHC, 7-KC, and 25-OHC regulate IL8 production involving MEK/ERK pathway and AP-1-mediated process (Erridge et al. 2007; Lemaire-Ewing et al. 2009). IL8 induction and secretion in macrophages were also reported after treatment with 7α-OHC through mechanisms dependent of C5a receptor and PI3K and MEK pathways (Cho 2017). 7-KC also stimulates IL12 and IL1α in macrophages (Saha et al. 2020) and 27-OHC stimulates the secretion of IL8 and IL1 in human monocytes through activation of TLR4/NFKB pathway (Gargiulo et al. 2012; Kim et al. 2013). In EC, 7-KC stimulates the expression and secretion of IL-8 associated with ROS production and PI3K/AKT signaling pathways (Chang et al. 2016). 25-OHC also induces proinflammatory cytokines in EC including IL1β, IL-18, IL-23 while anti-inflammatory cytokines IL-10 and IL-37 were repressed (Woźniak et al. 2023). TNFα which is mainly expressed in macrophages is a key regulator of the cytokine cascade and displays proatherogenic activity by promoting the formation of foam cells. The production of TNFα in macrophages is stimulated by 27-OHC and 7α-OHC but not 7-KC in macrophages (Kim et al. 2013; Gargiulo et al. 2015). 25-OHC promotes the production of proinflammatory cytokines including TNF and IL6 through activation of αvβ3 integrin signaling (Pokharel et al. 2019).
Many studies have also highlighted the key role of oxysterols in promoting atherosclerotic plaque instability and rupture (Gargiulo et al. 2018). Matrix metalloproteinases (MMPs) belong to the zinc-metalloproteinases family which are involved in the degradation of extracellular matrix, therefore playing a key role in the rupture of fibrous caps and the formation of advanced atherosclerotic lesions. MMP are primarily produced by activated macrophages but also by vascular SMC and EC. Recently, the role of pro-protein convertase subtilisin/kexin protease (PCSK) 6 in plaque instability and rupture has been suggested related to its ability to stimulate the activity of MMP and its elevated expression in symptomatic carotid plaque. Among oxysterols from a mixture representative of those present in advanced human carotid plaques, 27-OHC, 7α-OHC and to a lesser extent 25-OHC were shown to be the most potent inducers of MMP-9 expression in human monocytes through the activation of TLR4/NFkB pathway (Gargiulo et al. 2012, 2015). It was further demonstrated that the mixture of oxysterols-induced MMP-9 activity through PCSK6 activation in monocytes (Testa et al. 2021). In SMC, 7-KC and 5,6α-epoxide induce the expression of MMP-2 and MMP-9 through the EGFR/PI3K/AKT signaling pathways, which was associated with SMC migration and proliferation (Liao et al. 2010). In atherosclerotic mice, an oxysterol-rich diet containing mainly 7C derivatives and EC induces plaque instability and rupture related to increased MCP1 expression and MMP activity (Sato et al. 2012).
Macrophages exist as two main subsets, the classically activated macrophages—proinflammatory M1 phenotype and the alternatively activated macrophages—anti-inflammatory M2 phenotype. M1 macrophages are primarily found in rupture-prone atherosclerotic plaques, while alternatively activated macrophages accumulate in stable plaque. 7-KC increased the production of the proinflammatory cytokines TNF-α and IL-6 in M1 macrophages (Buttari et al. 2013). In addition, 7-KC is able to redirect the polarization of M2 macrophages to an M1-like subset by changing the profile of surface markers and cytokines toward an anti-inflammatory phenotype, by decreasing endocyte clearance capacity and by increasing the secretion of MMP9 secretion (Buttari et al. 2013, 2014; Saha et al. 2020). By contrast, 27-OHC was reported to favor plaque stabilization by driving M2 polarization of human macrophages (Marengo et al. 2016). After entering the cells through CD receptors, these oxysterols trigger LXR activation which leads to IL10 secretion and MIF release and contributes to atherosclerotic plaque stabilization.
Other inflammatory mediators such as prostaglandins (PG) whose production is regulated by the enzyme COX2 contribute to plaque development. COX2 expression and synthesis as well as PGE2 production are increased by CT in EC (Liao et al. 2009). 27-OHC promotes upregulation of COX-2 and PGE synthase thereby inducing PGE2 synthesis in human monocytes. This regulation is associated with enhanced production of proinflammatory cytokines including IL8, IL1β, and TNFα and MMP9, which leads to plaque instability (Gargiulo et al. 2018).
4 Mechanisms of Oxysterol Efflux
It is well acknowledged that plasma high-density lipoprotein (HDL) levels are inversely related to the risk of atherosclerotic cardiovascular disease, related to multiple anti-atherosclerotic functions exerted by HDL such as antioxidative capacity, anti-inflammatory activity, cytoprotective activity, and protection on endothelium-dependent vasorelaxation. The best-known atheroprotective activity of HDL is its ability to export cholesterol from foamy macrophages and artery wall. Transport proteins ABCA1 and ABCG1 play a central role in cholesterol efflux: ABCA1 regulates the efflux of cholesterol from the macrophage to apoA1/pre-β HDL while ABCG1 mediates efflux to mature HDL.
We and others have shown that HDL can also promote the efflux of oxysterols that accumulate in macrophages after exposure to oxLDL (Terasaka et al. 2007; Xu et al. 2009; Iborra et al. 2011; Chen et al. 2015; Paul et al. 2019). Overall, 7-KC and 7α/β-OHC were the most efficiently released and 7-KC-induced macrophage apoptosis was reduced, which confers protective effect to HDL against pro-atherogenic oxysterols. In contrast to HDL, ABCA1-dependent apoA1 exhibits weak ability to export oxysterols including 7-KC from macrophages (Kritharides et al. 1995; Gelissen et al. 1999; Terasaka et al. 2007; Xu et al. 2009; Chen et al. 2015). Another study reported opposite results showing that apoA1 efficiently export 7-KC, 7α-OHC, 5,6α-EC, cholest4-en-3-one and 27-OHC from oxLDL-loaded macrophages while HDL only exerted a trend toward oxysterol efflux (Paul et al. 2019).
In some pathological situations such as type 2 diabetes and obesity with elevated risk of atherosclerosis, HDL undergoes modifications including oxidation, glycation, and glycoxidation, which impair ability to efflux cholesterol (Denimal 2023). In the study by Chen et al. (2018), we showed that oxidized and glycoxidized HDL as well as HDL isolated from diabetic subjects, have decreased ability to efflux oxysterols from oxidized LDL-laden macrophages compared to HDL from healthy subjects. Efflux of 7-KC was specifically decreased compared to that of cholesterol. This defect of HDL toward oxysterol efflux may potentiate the deleterious effects of oxysterols and especially 7-KC that accumulate in atheroma macrophages. Oxysterols were detected at very low levels in HDL of healthy subjects, mainly represented by 7-KC, and in lower proportions 7α/β-OHC, 25-OHC, and 27-OHC. Similar or even lower amounts were found in HDL from diabetic subjects, which could reflect their strong antioxidant capacities that would protect cholesterol from oxidation, or their weaker capacity to mobilize cellular oxysterols in particular 7-KC. Under high oxidative conditions used to generate oxidized and glycoxidized HDL, a considerable increase of 7C-derived oxysterols, in particular 7-KC, is observed (Chen et al. 2018). It was not evaluated whether the high amounts of oxysterols in these modified HDLs are responsible for their lower ability to remove oxysterols, as it has been proposed toward cholesterol efflux (Gesquière et al. 1997).
5 Conclusions
The involvement of oxysterols in atherosclerotic plaque formation and instability is now well established. Among oxysterols most abundant in atheroma plaque, 7-derivative oxysterols in particular 7-KC, 5,6-EC, and CT exert proatherogenic effects through induction of proapoptotic and proinflammatory mediators. Side chain oxysterols, in particular, 25-OHC and 27-OHC display dual effects as they also exert protective effects by preventing foam cell formation, or plaque instability. It is now well demonstrated that oxidative stress is a determinant for the formation of oxysterols as well as signaling pathways evoked by deleterious oxysterols. The cellular targets of oxysterols including membrane receptors, signaling pathways, and transcription factors are also well documented. Therapeutic strategies to counteract deleterious effects of oxysterols in atherosclerosis and other diseases such as cancer and neurodegenerative diseases have started to be evaluated either based on pharmacological inhibition of oxysterol-activated signaling pathways (Lee et al. 2015; Park et al. 2016; Saha et al. 2020), oxysterol derivatives (de Medina et al. 2021) or antioxidants naturally present in nutrition oils (Nury et al. 2021; Rezig et al. 2022). Pharmacology of oxysterols is a promising route for the development of new drugs against current high-incidence diseases and thus deserves further investigations.
Abbreviations
- 4β-OHC:
-
4β-Hydroxycholesterol
- 5,6α-EC:
-
5α,6α-Epoxycholesterol
- 5,6β-EC:
-
5β,6β-Epoxycholesterol
- 7α-OHC:
-
7α-Hydroxycholesterol
- 7β-OHC:
-
7β-Hydroxycholesterol
- 7KC:
-
7-Ketocholesterol
- 20S-OHC:
-
20(S)-Hydroxycholesterol
- 22R-OHC:
-
22(R)-Hydroxycholesterol
- 22S-OHC:
-
22(S)-Hydroxycholesterol
- 24S-OHC:
-
24(S)-Hydroxycholesterol
- 25-OHC:
-
25-Hydroxycholesterol
- 27-OHC:
-
27-Hydroxycholesterol
- CH25H:
-
Cholesterol 25-hydroxylase
- CT:
-
Cholestane-3β-5α-6β-triol
- EC:
-
Endothelial cells
- ER:
-
Endoplasmic reticulum
- HDL:
-
High-density lipoprotein
- IL:
-
Interleukin
- MMP:
-
Matrix metalloproteinase
- oxLDL:
-
Oxidized low-density lipoprotein
- SMC:
-
Smooth muscle cells
- TNFα:
-
Tumor necrosis factor
References
Ahmed AM, Khabour OF, Yousuf A, Mohammedsaeed W, Alahmadi NF, Alshangeety AM, Alotaibi OH, Alhaidary AA (2022) The relationships between 7-kchol, 7β-ohchol, chol-triol, Lp (A) and PON1 with coronary heart disease in patients with diabetes mellitus T1DM and T2DM. Pak J Pharm Sci 35(3):761–768
Arnal-Levron M, Chen Y, Delton-Vandenbroucke I, Luquain-Costaz C (2013) Bis(monoacylglycero)phosphate reduces oxysterol formation and apoptosis in macrophages exposed to oxidized LDL. Biochem Pharmacol 86(1):115–121. https://doi.org/10.1016/j.bcp.2013.03.017
Berthier A, Lemaire-Ewing S, Prunet C, Montange T, Vejux A, Pais de Barros JP, Monier S, Gambert P, Lizard G, Néel D (2005) 7-Ketocholesterol-induced apoptosis. Involvement of several pro-apoptotic but also anti-apoptotic calcium-dependent transduction pathways. FEBS J 272(12):3093–3104. https://doi.org/10.1111/j.1742-4658.2005.04723.x
Brooks CJ, Harland WA, Steel G (1966) Squalene, 26-hydroxycholesterol and 7-ketocholesterol in human atheromatous plaques. Biochim Biophys Acta 125(3):620–622. https://doi.org/10.1016/0005-2760(66)90055-5
Brown AJ, Dean RT, Jessup W (1996) Free and esterified oxysterol: formation during copper-oxidation of low density lipoprotein and uptake by macrophages. J Lipid Res 37(2):320–335
Brown AJ, Leong SL, Dean RT, Jessup W (1997) 7-Hydroperoxycholesterol and its products in oxidized low density lipoprotein and human atherosclerotic plaque. J Lipid Res 38(9):1730–1745
Buttari B, Segoni L, Profumo E, D’Arcangelo D, Rossi S, Facchiano F, Businaro R, Iuliano L, Riganò R (2013) 7-Oxo-cholesterol potentiates pro-inflammatory signaling in human M1 and M2 macrophages. Biochem Pharmacol 86(1):130–137. https://doi.org/10.1016/j.bcp.2013.04.008
Buttari B, Profumo E, Segoni L, D’Arcangelo D, Rossi S, Facchiano F, Saso L, Businaro R, Iuliano L, Riganò R (2014) Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7-oxo-cholesterol: potential therapeutic implications in atherosclerosis. Oxid Med Cell Longev 2014:257543. https://doi.org/10.1155/2014/257543
Canfrán-Duque A, Rotllan N, Zhang X, Andrés-Blasco I, Thompson BM, Sun J, Price NL, Fernández-Fuertes M, Fowler JW, Gómez-Coronado D, Sessa WC, Giannarelli C, Schneider RJ, Tellides G, McDonald JG, Fernández-Hernando C, Suárez Y (2023) Macrophage-derived 25-hydroxycholesterol promotes vascular inflammation, atherogenesis, and lesion remodeling. Circulation 147(5):388–408. https://doi.org/10.1161/CIRCULATIONAHA.122.059062
Carpenter KL, Taylor SE, Ballantine JA, Fussell B, Halliwell B, Mitchinson MJ (1993) Lipids and oxidised lipids in human atheroma and normal aorta. Biochim Biophys Acta 1167(2):121–130. https://doi.org/10.1016/0005-2760(93)90151-x
Carpenter KL, Taylor SE, van der Veen C, Williamson BK, Ballantine JA, Mitchinson MJ (1995) Lipids and oxidised lipids in human atherosclerotic lesions at different stages of development. Biochim Biophys Acta 1256(2):141–150. https://doi.org/10.1016/0005-2760(94)00247-v
Chalubinski M, Zemanek K, Skowron W, Wojdan K, Gorzelak P, Broncel M (2013) The effect of 7-ketocholesterol and 25-hydroxycholesterol on the integrity of the human aortic endothelial and intestinal epithelial barriers. Inflamm Res 62(12):1015–1023. https://doi.org/10.1007/s00011-013-0660-x
Chang M-C, Chen Y-J, Liou EJ-W, Tseng W-Y, Chan C-P, Lin H-J, Liao W-C, Chang Y-C, Jeng P-Y, Jeng J-H (2016) 7-Ketocholesterol induces ATM/ATR, Chk1/Chk2, PI3K/Akt signalings, cytotoxicity and IL-8 production in endothelial cells. Oncotarget 7(46):74473–74483. https://doi.org/10.18632/oncotarget.12578
Chen Y, Arnal-Levron M, Lagarde M, Moulin P, Luquain-Costaz C, Delton I (2015) THP1 macrophages oxidized cholesterol, generating 7-derivative oxysterols specifically released by HDL. Steroids 99(Pt B):212–218. https://doi.org/10.1016/j.steroids.2015.02.020
Chen Y, Arnal-Levron M, Hullin-Matsuda F, Knibbe C, Moulin P, Luquain-Costaz C, Delton I (2018) In vitro oxidized HDL and HDL from type 2 diabetes patients have reduced ability to efflux oxysterols from THP-1 macrophages. Biochimie 153:232–237. https://doi.org/10.1016/j.biochi.2018.04.018
Cho HR, Son Y, Kim SM, Kim BY, Eo SK, Park YC, Kim K (2017) 7α-Hydroxycholesterol induces monocyte/macrophage cell expression of interleukin-8 via C5a receptor. PLoS One. 12(3):e0173749. https://doi.org/10.1371/journal.pone.0173749. PMCID: PMC5360241
Crisby M, Nilsson J, Kostulas V, Björkhem I, Diczfalusy U (1997) Localization of sterol 27-hydroxylase immuno-reactivity in human atherosclerotic plaques. Biochim Biophys Acta 1344(3):278–285. https://doi.org/10.1016/s0005-2760(96)00152-x
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592. https://doi.org/10.1002/cbin.11137
de Freitas FA, Levy D, Zarrouk A, Lizard G, Bydlowski SP (2021) Impact of oxysterols on cell death, proliferation, and differentiation induction: current status. Cells 10(9):2301. https://doi.org/10.3390/cells10092301
de Medina P, Diallo K, Huc-Claustre E, Attia M, Soulès R, Silvente-Poirot S, Poirot M (2021) The 5,6-epoxycholesterol metabolic pathway in breast cancer: Emergence of new pharmacological targets. Br J Pharmacol 178(16):3248–3260. https://doi.org/10.1111/bph.15205
Denimal D, Monier S, Bouillet B, Vergès B, Duvillard L (2023) High-density lipoprotein alterations in type 2 diabetes and obesity. Metabolites 13(2):253–279. https://doi.org/10.3390/metabo13020253
Dennis EA, Deems RA, Harkewicz R, Quehenberger O, Brown HA, Milne SB, Myers DS, Glass CK, Hardiman G, Reichart D, Merrill AH, Sullards MC, Wang E, Murphy RC, Raetz CRH, Garrett TA, Guan Z, Ryan AC, Russell DW, McDonald JG, Thompson BM, Shaw WA, Sud M, Zhao Y, Gupta S, Maurya MR, Fahy E, Subramaniam S (2010) A mouse macrophage lipidome. J Biol Chem 285(51):39976–39985. https://doi.org/10.1074/jbc.M110.182915
Erridge C, Webb DJ, Spickett CM (2007) 25-Hydroxycholesterol, 7beta-hydroxycholesterol and 7-ketocholesterol upregulate interleukin-8 expression independently of Toll-like receptor 1, 2, 4 or 6 signalling in human macrophages. Free Radic Res 41(3):260–266. https://doi.org/10.1080/10715760601070091
Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG (2001) 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem 276(42):38378–38387. https://doi.org/10.1074/jbc.M105805200
Fumagalli R, Galli G, Urna G (1971) Cholestanol and 26-hydroxycholesterol in normal and atherosclerotic human aorta. Life Sci II 10(1):25–33. https://doi.org/10.1016/0024-3205(71)90221-9
Garcia-Cruset S, Carpenter KL, Guardiola F, Mitchinson MJ (1999) Oxysterols in cap and core of human advanced atherosclerotic lesions. Free Radic Res 30(5):341–350. https://doi.org/10.1080/10715769900300391
Garcia-Cruset S, Carpenter KL, Guardiola F, Stein BK, Mitchinson MJ (2001) Oxysterol profiles of normal human arteries, fatty streaks and advanced lesions. Free Radic Res 35(1):31–41. https://doi.org/10.1080/10715760100300571
Gargiulo S, Gamba P, Testa G, Sottero B, Maina M, Guina T, Biasi F, Poli G, Leonarduzzi G (2012) Molecular signaling involved in oxysterol-induced β1-integrin over-expression in human macrophages. Int J Mol Sci 13(11):14278–14293. https://doi.org/10.3390/ijms131114278
Gargiulo S, Gamba P, Testa G, Rossin D, Biasi F, Poli G, Leonarduzzi G (2015) Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 14(4):569–581. https://doi.org/10.1111/acel.12322
Gargiulo S, Rossin D, Testa G, Gamba P, Staurenghi E, Biasi F, Poli G, Leonarduzzi G (2018) Up-regulation of COX-2 and mPGES-1 by 27-hydroxycholesterol and 4-hydroxynonenal: A crucial role in atherosclerotic plaque instability. Free Radic Biol Med 129:354–363. https://doi.org/10.1016/j.freeradbiomed.2018.09.046
Gelissen IC, Rye KA, Brown AJ, Dean RT, Jessup W (1999) Oxysterol efflux from macrophage foam cells: the essential role of acceptor phospholipid. J Lipid Res 40(9):1636–1646
Gesquière L, Loreau N, Blache D (1997) Impaired cellular cholesterol efflux by oxysterol-enriched high density lipoproteins. Free Radic Biol Med 23(4):541–547. https://doi.org/10.1016/s0891-5849(97)00114-7
He C, Zhu H, Zhang W, Okon I, Wang Q, Li H, Le Y-Z, Xie Z (2013) 7-Ketocholesterol induces autophagy in vascular smooth muscle cells through Nox4 and Atg4B. Am J Pathol 183(2):626–637. https://doi.org/10.1016/j.ajpath.2013.04.028
Helmschrodt C, Becker S, Schröter J, Hecht M, Aust G, Thiery J, Ceglarek U (2013) Fast LC-MS/MS analysis of free oxysterols derived from reactive oxygen species in human plasma and carotid plaque. Clin Chim Acta 425:3–8. https://doi.org/10.1016/j.cca.2013.06.022
Hitsumoto T, Takahashi M, Iizuka T, Shirai K (2009) Clinical significance of serum 7-ketocholesterol concentrations in the progression of coronary atherosclerosis. J Atheroscler Thromb 16(4):363–370. https://doi.org/10.5551/jat.no703
Hughes H, Mathews B, Lenz ML, Guyton JR (1994) Cytotoxicity of oxidized LDL to porcine aortic smooth muscle cells is associated with the oxysterols 7-ketocholesterol and 7-hydroxycholesterol. Arterioscler Thromb 14(7):1177–1185. https://doi.org/10.1161/01.atv.14.7.1177
Iborra RT, Machado-Lima A, Castilho G, Nunes VS, Abdalla DSP, Nakandakare ER, Passarelli M (2011) Advanced glycation in macrophages induces intracellular accumulation of 7-ketocholesterol and total sterols by decreasing the expression of ABCA-1 and ABCG-1. Lipids Health Dis 10:172. https://doi.org/10.1186/1476-511X-10-172
Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ (1999) Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci U S A 96(1):266–271. https://doi.org/10.1073/pnas.96.1.266
Khatib S, Vaya J (2014) Oxysterols and symptomatic versus asymptomatic human atherosclerotic plaque. Biochem Biophys Res Commun 446(3):709–713. https://doi.org/10.1016/j.bbrc.2013.12.116
Kim S-M, Jang H, Son Y, Lee S-A, Bae S-S, Park YC, Eo S-K, Kim K (2013) 27-hydroxycholesterol induces production of tumor necrosis factor-alpha from macrophages. Biochem Biophys Res Commun 430(2):454–459. https://doi.org/10.1016/j.bbrc.2012.12.021
Kritharides L, Jessup W, Mander EL, Dean RT (1995) Apolipoprotein A-I-mediated efflux of sterols from oxidized LDL-loaded macrophages. Arterioscler Thromb Vasc Biol 15(2):276–289
Lee DH, Nam YJ, Lee MS, Sohn DS, Lee CS (2015) Rotundarpene attenuates cholesterol oxidation product-induced apoptosis by suppressing the mitochondrial pathway and the caspase-8- and bid-dependent pathways. Eur J Pharmacol 749:39–48. https://doi.org/10.1016/j.ejphar.2014.11.048
Lemaire S, Lizard G, Monier S, Miguet C, Gueldry S, Volot F, Gambert P, Néel D (1998) Different patterns of IL-1beta secretion, adhesion molecule expression and apoptosis induction in human endothelial cells treated with 7alpha-, 7beta-hydroxycholesterol, or 7-ketocholesterol. FEBS Lett 440(3):434–439. https://doi.org/10.1016/s0014-5793(98)01496-3
Lemaire-Ewing S, Berthier A, Royer MC, Logette E, Corcos L, Bouchot A, Monier S, Prunet C, Raveneau M, Rébé C, Desrumaux C, Lizard G, Néel D (2009) 7beta-Hydroxycholesterol and 25-hydroxycholesterol-induced interleukin-8 secretion involves a calcium-dependent activation of c-fos via the ERK1/2 signaling pathway in THP-1 cells: oxysterols-induced IL-8 secretion is calcium-dependent. Cell Biol Toxicol 25(2):127–139. https://doi.org/10.1007/s10565-008-9063-0
Leonarduzzi G, Gamba P, Sottero B, Kadl A, Robbesyn F, Calogero RA, Biasi F, Chiarpotto E, Leitinger N, Sevanian A, Poli G (2005) Oxysterol-induced up-regulation of MCP-1 expression and synthesis in macrophage cells. Free Radic Biol Med 39(9):1152–1161. https://doi.org/10.1016/j.freeradbiomed.2005.06.024
Li W, Laskar A, Sultana N, Osman E, Ghosh M, Li Q, Yuan X-M (2012) Cell death induced by 7-oxysterols via lysosomal and mitochondrial pathways is p53-dependent. Free Radic Biol Med 53(11):2054–2061. https://doi.org/10.1016/j.freeradbiomed.2012.09.007
Li J, Meng Q, Fu Y, Yu X, Ji T, Chao Y, Chen Q, Li Y, Bian H (2021) Novel insights: Dynamic foam cells derived from the macrophage in atherosclerosis. J Cell Physiol 236(9):6154–6167. https://doi.org/10.1002/jcp.30300
Li X, Zhu X, Wei Y (2022) Autophagy in atherosclerotic plaque cells: targeting NLRP3 inflammasome for self-rescue. Biomolecules 13(1):15. https://doi.org/10.3390/biom13010015
Liao P-L, Cheng Y-W, Li C-H, Lo Y-L, Kang J-J (2009) Cholesterol-3-beta, 5-alpha, 6-beta-triol induced PI(3)K-Akt-eNOS-dependent cyclooxygenase-2 expression in endothelial cells. Toxicol Lett 190(2):172–178. https://doi.org/10.1016/j.toxlet.2009.07.012
Liao PL, Cheng YW, Li CH, Wang YT, Kang JJ (2010) 7-Ketocholesterol and cholesterol-5alpha,6alpha-epoxide induce smooth muscle cell migration and proliferation through the epidermal growth factor receptor/phosphoinositide 3-kinase/Akt signaling pathways. Toxicol Lett 197(2):88–96. https://doi.org/10.1016/j.toxlet.2010.05.002
Luchetti F, Crinelli R, Cesarini E, Canonico B, Guidi L, Zerbinati C, Di Sario G, Zamai L, Magnani M, Papa S, Iuliano L (2017) Endothelial cells, endoplasmic reticulum stress and oxysterols. Redox Biol 13:581–587. https://doi.org/10.1016/j.redox.2017.07.014
Marengo B, Bellora F, Ricciarelli R, De Ciucis C, Furfaro A, Leardi R, Colla R, Pacini D, Traverso N, Moretta A, Pronzato MA, Bottino C, Domenicotti C (2016) Oxysterol mixture and, in particular, 27-hydroxycholesterol drive M2 polarization of human macrophages. Biofactors 42(1):80–92. https://doi.org/10.1002/biof.1243
Matsunaga I, Hakamata H, Sadohara K, Kakiuchi K, Kusu F (2009) Determination of oxysterols in oxidatively modified low-density lipoprotein by semi-micro high-performance liquid chromatography with electrochemical detection. Anal Biochem 393(2):222–228. https://doi.org/10.1016/j.ab.2009.06.032
Matsuo M (2022) ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J Pharmacol Sci 148(2):197–203. https://doi.org/10.1016/j.jphs.2021.11.005
Maurya MR, Gupta S, Li X, Fahy E, Dinasarapu AR, Sud M, Brown HA, Glass CK, Murphy RC, Russell DW, Dennis EA, Subramaniam S (2013) Analysis of inflammatory and lipid metabolic networks across RAW264.7 and thioglycolate-elicited macrophages. J Lipid Res 54(9):2525–2542. https://doi.org/10.1194/jlr.M040212
Miyashita Y, Shirai K, Ito Y, Watanabe J, Urano Y, Murano T, Tomioka H (1997) Cytotoxicity of some oxysterols on human vascular smooth muscle cells was mediated by apoptosis. J Atheroscler Thromb 4(2):73–78. https://doi.org/10.5551/jat1994.4.73
Müller K, Carpenter KL, Mitchinson MJ (1998) Cell-mediated oxidation of LDL: comparison of different cell types of the atherosclerotic lesion. Free Radic Res 29(3):207–220. https://doi.org/10.1080/10715769800300241
Nakano Y, Yamamoto M, Matoba T, Katsuki S, Nakashiro S, Takase S, Akiyama Y, Nagata T, Mukai Y, Inoue S, Oi K, Higo T, Takemoto M, Suematsu N, Eshima K, Miyata K, Usui M, Sadamatsu K, Kadokami T, Hironaga K, Ichi I, Todaka K, Kishimoto J, Tsutsui H, QcVIC Investigators (2022) Association between serum oxysterols and coronary plaque regression during lipid-lowering therapy with statin and ezetimibe: insights from the CuVIC Trial. J Atheroscler Thromb. https://doi.org/10.5551/jat.63507
Nègre-Salvayre A, Augé N, Camaré C, Bacchetti T, Ferretti G, Salvayre R (2017) Dual signaling evoked by oxidized LDLs in vascular cells. Free Radic Biol Med 106:118–133. https://doi.org/10.1016/j.freeradbiomed.2017.02.006
Nègre-Salvayre A, Guerby P, Gayral S, Laffargue M, Salvayre R (2020) Role of reactive oxygen species in atherosclerosis: Lessons from murine genetic models. Free Radic Biol Med 149:8–22. https://doi.org/10.1016/j.freeradbiomed.2019.10.011
Niedzielski M, Broncel M, Gorzelak-Pabiś P, Woźniak E (2021) A comparison of the effects of monotherapy with rosuvastatin, atorvastatin or ezetimibe versus combination treatment with rosuvastatin-ezetimibe and atorvastatin-ezetimibe on the integrity of vascular endothelial cells damaged by oxidized cholesterol. PLoS One 16(9):e0256996. https://doi.org/10.1371/journal.pone.0256996
Nury T, Zarrouk A, Vejux A, Doria M, Riedinger JM, Delage-Mourroux R, Lizard G (2014) Induction of oxiapoptophagy, a mixed mode of cell death associated with oxidative stress, apoptosis and autophagy, on 7-ketocholesterol-treated 158N murine oligodendrocytes: impairment by α-tocopherol. Biochem Biophys Res Commun 446(3):714–719. https://doi.org/10.1016/j.bbrc.2013.11.081
Nury T, Zarrouk A, Mackrill JJ, Samadi M, Durand P, Riedinger J-M, Doria M, Vejux A, Limagne E, Delmas D, Prost M, Moreau T, Hammami M, Delage-Mourroux R, O’Brien NM, Lizard G (2015) Induction of oxiapoptophagy on 158N murine oligodendrocytes treated by 7-ketocholesterol-, 7β-hydroxycholesterol-, or 24(S)-hydroxycholesterol: Protective effects of α-tocopherol and docosahexaenoic acid (DHA; C22:6 n-3). Steroids 99(Pt B):194–203. https://doi.org/10.1016/j.steroids.2015.02.003
Nury T, Yammine A, Ghzaiel I, Sassi K, Zarrouk A, Brahmi F, Samadi M, Rup-Jacques S, Vervandier-Fasseur D, Pais de Barros JP, Bergas V, Ghosh S, Majeed M, Pande A, Atanasov A, Hammami S, Hammami M, Mackrill J, Nasser B, Andreoletti P, Cherkaoui-Malki M, Vejux A, Lizard G (2021) Attenuation of 7-ketocholesterol- and 7β-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: potential for the prevention of age-related diseases. Ageing Res Rev. https://doi.org/10.1016/j.arr.2021.101324
Olkkonen VM (2012) Macrophage oxysterols and their binding proteins: roles in atherosclerosis. Curr Opin Lipidol 23(5):462–470. https://doi.org/10.1097/MOL.0b013e328356dba0
Orsó E, Matysik S, Grandl M, Liebisch G, Schmitz G (2015) Human native, enzymatically modified and oxidized low density lipoproteins show different lipidomic pattern. Biochim Biophys Acta 1851(3):299–306. https://doi.org/10.1016/j.bbalip.2015.01.001
Ou Z-J, Chen J, Dai W-P, Liu X, Yang Y-K, Li Y, Lin Z-B, Wang T-T, Wu Y-Y, Su D-H, Cheng T-P, Wang Z-P, Tao J, Ou J-S (2016) 25-Hydroxycholesterol impairs endothelial function and vasodilation by uncoupling and inhibiting endothelial nitric oxide synthase. Am J Physiol Endocrinol Metab 311(4):E781–E790. https://doi.org/10.1152/ajpendo.00218.2016
Palozza P, Simone R, Catalano A, Boninsegna A, Böhm V, Fröhlich K, Mele MC, Monego G, Ranelletti FO (2010) Lycopene prevents 7-ketocholesterol-induced oxidative stress, cell cycle arrest and apoptosis in human macrophages. J Nutr Biochem 21(1):34–46. https://doi.org/10.1016/j.jnutbio.2008.10.002
Park S-H, Kang M-K, Choi Y-J, Kim Y-H, Antika LD, Lim SS, Kang Y-H (2016) Dietary compound α-asarone alleviates ER stress-mediated apoptosis in 7β-hydroxycholesterol-challenged macrophages. Mol Nutr Food Res 60(5):1033–1047. https://doi.org/10.1002/mnfr.201500750
Paul A, Lydic TA, Hogan R, Goo Y-H (2019) Cholesterol acceptors regulate the lipidome of macrophage foam cells. Int J Mol Sci 20(15):3784. https://doi.org/10.3390/ijms20153784
Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie J-C, Pouzet C, Samadi M, Elbim C, O’Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo M-A, Lizard G, Ogier-Denis E (2004) NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24(24):10703–10717. https://doi.org/10.1128/MCB.24.24.10703-10717.2004
Pinto RS, Ferreira GS, Silvestre GCR, Santana MDFM, Nunes VS, Ledesma L, Pinto PR, de Assis SIS, Machado UF, da Silva ES, Passarelli M (2022) Plasma advanced glycation end products and soluble receptor for advanced glycation end products as indicators of sterol content in human carotid atherosclerotic plaques. Diab Vasc Dis Res 19(1):14791641221085268. https://doi.org/10.1177/14791641221085269
Pokharel SM, Shil NK, Gc JB, Colburn ZT, Tsai S-Y, Segovia JA, Chang T-H, Bandyopadhyay S, Natesan S, Jones JCR, Bose S (2019) Integrin activation by the lipid molecule 25-hydroxycholesterol induces a proinflammatory response. Nat Commun 10:1482. https://doi.org/10.1038/s41467-019-09453-x
Poli G, Sottero B, Gargiulo S, Leonarduzzi G (2009) Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol Aspects Med 30(3):180–189. https://doi.org/10.1016/j.mam.2009.02.003
Prunet C, Lemaire-Ewing S, Ménétrier F, Néel D, Lizard G (2005) Activation of caspase-3-dependent and -independent pathways during 7-ketocholesterol- and 7beta-hydroxycholesterol-induced cell death: a morphological and biochemical study. J Biochem Mol Toxicol 19(5):311–326. https://doi.org/10.1002/jbt.20096
Ravi S, Duraisamy P, Krishnan M, Martin LC, Manikandan B, Raman T, Sundaram J, Arumugam M, Ramar M (2021) An insight on 7-ketocholesterol mediated inflammation in atherosclerosis and potential therapeutics. Steroids 172:108854. https://doi.org/10.1016/j.steroids.2021.108854
Rezig L, Ghzaiel I, Ksila M, Yammine A, Nury T, Zarrouk A, Samadi M, Chouaibi M, Vejux A, Lizard G (2022) Cytoprotective activities of representative nutrients from the Mediterranean diet and of Mediterranean oils against 7-ketocholesterol- and 7β-hydroxycholesterol-induced cytotoxicity: Application to age-related diseases and civilization diseases. Steroids 187:109093. https://doi.org/10.1016/j.steroids.2022.109093
Riendeau V, Garenc C (2009) Effect of 27-hydroxycholesterol on survival and death of human macrophages and vascular smooth muscle cells. Free Radic Res 43(10):1019–1028. https://doi.org/10.1080/10715760903040610
Rimner A, Al Makdessi S, Sweidan H, Wischhusen J, Rabenstein B, Shatat K, Mayer P, Spyridopoulos I (2005) Relevance and mechanism of oxysterol stereospecifity in coronary artery disease. Free Radic Biol Med 38(4):535–544. https://doi.org/10.1016/j.freeradbiomed.2004.11.016
Saha S, Profumo E, Togna AR, Riganò R, Saso L, Buttari B (2020) Lupeol counteracts the proinflammatory signalling triggered in macrophages by 7-keto-cholesterol: new perspectives in the therapy of atherosclerosis. Oxid Med Cell Longev 2020:1232816. https://doi.org/10.1155/2020/1232816
Saito H, Tachiura W, Nishimura M, Shimizu M, Sato R, Yamauchi Y (2023) Hydroxylation site-specific and production-dependent effects of endogenous oxysterols on cholesterol homeostasis: Implications for SREBP-2 and LXR. J Biol Chem 299(1):102733. https://doi.org/10.1016/j.jbc.2022.102733
Samadi A, Gurlek A, Sendur SN, Karahan S, Akbiyik F, Lay I (2019) Oxysterol species: reliable markers of oxidative stress in diabetes mellitus. J Endocrinol Invest 42(1):7–17. https://doi.org/10.1007/s40618-018-0873-5
Samadi A, Isikhan SY, Tinkov AA, Lay I, Doşa MD, Skalny AV, Skalnaya MG, Chirumbolo S, Bjørklund G (2020) Zinc, copper, and oxysterol levels in patients with type 1 and type 2 diabetes mellitus. Clin Nutr 39(6):1849–1856. https://doi.org/10.1016/j.clnu.2019.07.026
Sanda GM, Deleanu M, Toma L, Stancu CS, Simionescu M, Sima AV (2017) Oxidized LDL-exposed human macrophages display increased MMP-9 expression and secretion mediated by endoplasmic reticulum stress. J Cell Biochem 118(4):661–669. https://doi.org/10.1002/jcb.25637
Sanson M, Augé N, Vindis C, Muller C, Bando Y, Thiers J-C, Marachet M-A, Zarkovic K, Sawa Y, Salvayre R, Nègre-Salvayre A (2009) Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res 104(3):328–336. https://doi.org/10.1161/CIRCRESAHA.108.183749
Sato R (2010) Sterol metabolism and SREBP activation. Arch Biochem Biophys 501(2):177–181. https://doi.org/10.1016/j.abb.2010.06.004
Sato K, Nakano K, Katsuki S, Matoba T, Osada K, Sawamura T, Sunagawa K, Egashira K (2012) Dietary cholesterol oxidation products accelerate plaque destabilization and rupture associated with monocyte infiltration/activation via the MCP-1-CCR2 pathway in mouse brachiocephalic arteries: therapeutic effects of ezetimibe. J Atheroscler Thromb 19(11):986–998. https://doi.org/10.5551/jat.13391
Savla SR, Prabhavalkar KS, Bhatt LK (2022) Liver X receptor: a potential target in the treatment of atherosclerosis. Expert Opin Ther Targets 26(7):645–658. https://doi.org/10.1080/14728222.2022.2117610
Seo J-Y, Kim T-H, Kang K-R, Lim H, Choi M-C, Kim DK, Chun HS, Kim H-J, Yu S-K, Kim J-S (2023) 7α,25-Dihydroxycholesterol-induced oxiapoptophagic chondrocyte death via the modulation of p53-Akt-mTOR axis in osteoarthritis pathogenesis. Mol Cells 46(4):245–255. https://doi.org/10.14348/molcells.2023.2149
Shimozawa M, Naito Y, Manabe H, Uchiyama K, Kuroda M, Katada K, Yoshida N, Yoshikawa T (2004) 7-Ketocholesterol enhances the expression of adhesion molecules on human aortic endothelial cells by increasing the production of reactive oxygen species. Redox Rep 9(6):370–375. https://doi.org/10.1179/135100004225006902
Son S-H, Goo Y-H, Chang BH, Paul A (2012) Perilipin 2 (PLIN2)-deficiency does not increase cholesterol-induced toxicity in macrophages. PLoS One 7(3):e33063. https://doi.org/10.1371/journal.pone.0033063
Song J, Wang D, Chen H, Huang X, Zhong Y, Jiang N, Chen C, Xia M (2017) Association of plasma 7-ketocholesterol with cardiovascular outcomes and total mortality in patients with coronary artery disease. Circ Res 120(10):1622–1631. https://doi.org/10.1161/CIRCRESAHA.117.311049
Sudo R, Sato F, Azechi T, Wachi H (2015) 7-Ketocholesterol-induced lysosomal dysfunction exacerbates vascular smooth muscle cell calcification via oxidative stress. Genes Cells 20(12):982–991. https://doi.org/10.1111/gtc.12301
Tabas I, García-Cardeña G, Owens GK (2015) Recent insights into the cellular biology of atherosclerosis. J Cell Biol 209(1):13–22. https://doi.org/10.1083/jcb.201412052
Tani M, Kamata Y, Deushi M, Osaka M, Yoshida M (2018) 7-Ketocholesterol enhances leukocyte adhesion to endothelial cells via p38MAPK pathway. PLoS One 13(7):e0200499. https://doi.org/10.1371/journal.pone.0200499
Terasaka N, Wang N, Yvan-Charvet L, Tall AR (2007) High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc Natl Acad Sci USA 104(38):15093–15098. https://doi.org/10.1073/pnas.0704602104
Testa G, Rossin D, Poli G, Biasi F, Leonarduzzi G (2018) Implication of oxysterols in chronic inflammatory human diseases. Biochimie 153:220–231. https://doi.org/10.1016/j.biochi.2018.06.006
Testa G, Staurenghi E, Giannelli S, Sottero B, Gargiulo S, Poli G, Gamba P, Leonarduzzi G (2021) Up-regulation of PCSK6 by lipid oxidation products: A possible role in atherosclerosis. Biochimie 181:191–203. https://doi.org/10.1016/j.biochi.2020.12.012
Töröcsik D, Szanto A, Nagy L (2009) Oxysterol signaling links cholesterol metabolism and inflammation via the liver X receptor in macrophages. Mol Aspects Med 30(3):134–152. https://doi.org/10.1016/j.mam.2009.02.002
Vaya J (2013) The association between biomarkers in the blood and carotid plaque composition-focusing on oxidized lipids, oxysterols and plaque status. Biochem Pharmacol 86(1):15–18. https://doi.org/10.1016/j.bcp.2013.01.025
Vaya J, Szuchman A, Tavori H, Aluf Y (2011) Oxysterols formation as a reflection of biochemical pathways: summary of in vitro and in vivo studies. Chem Phys Lipids 164(6):438–442. https://doi.org/10.1016/j.chemphyslip.2011.03.005
Vejux A, Lizard G (2009) Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Aspects Med 30(3):153–170. https://doi.org/10.1016/j.mam.2009.02.006
Virginio VWM, Nunes VS, Moura FA, Menezes FH, Andreollo NA, Rogerio F, Scherrer DZ, Quintão ECR, Nakandakare E, Petrucci O, Nadruz-Junior W, de Faria EC, Sposito AC (2015) Arterial tissue and plasma concentration of enzymatic-driven oxysterols are associated with severe peripheral atherosclerotic disease and systemic inflammatory activity. Free Radic Res 49(2):199–203. https://doi.org/10.3109/10715762.2014.992894
Vurusaner B, Gamba P, Gargiulo S, Testa G, Staurenghi E, Leonarduzzi G, Poli G, Basaga H (2016) Nrf2 antioxidant defense is involved in survival signaling elicited by 27-hydroxycholesterol in human promonocytic cells. Free Radic Biol Med 91:93–104. https://doi.org/10.1016/j.freeradbiomed.2015.12.007
Vurusaner B, Gargiulo S, Testa G, Gamba P, Leonarduzzi G, Poli G, Basaga H (2018) The role of autophagy in survival response induced by 27-hydroxycholesterol in human promonocytic cells. Redox Biol 17:400–410. https://doi.org/10.1016/j.redox.2018.05.010
Wang M, Long W, Li D, Wang D, Zhong Y, Mu D, Song J, Xia M (2017) Plasma 7-ketocholesterol levels and the risk of incident cardiovascular events. Heart 103(22):1788–1794. https://doi.org/10.1136/heartjnl-2016-310914
Ward LJ, Ljunggren SA, Karlsson H, Li W, Yuan X-M (2017) Exposure to atheroma-relevant 7-oxysterols causes proteomic alterations in cell death, cellular longevity, and lipid metabolism in THP-1 macrophages. PLoS One 12(3):e0174475. https://doi.org/10.1371/journal.pone.0174475
Wen Y, Leake DS (2007) Low density lipoprotein undergoes oxidation within lysosomes in cells. Circ Res 100(9):1337–1343. https://doi.org/10.1161/CIRCRESAHA.107.151704
Woźniak E, Broncel M, Niedzielski M, Woźniak A, Gorzelak-Pabiś P (2023) The effect of lipid-lowering therapies on the pro-inflammatory and anti-inflammatory properties of vascular endothelial cells. PLoS One 18(2):e0280741. https://doi.org/10.1371/journal.pone.0280741
Xu M, Zhou H, Tan KCB, Guo R, Shiu SWM, Wong Y (2009) ABCG1 mediated oxidized LDL-derived oxysterol efflux from macrophages. Biochem Biophys Res Commun 390(4):1349–1354. https://doi.org/10.1016/j.bbrc.2009.10.152
Yasunobu Y, Hayashi K, Shingu T, Yamagata T, Kajiyama G, Kambe M (2001) Coronary atherosclerosis and oxidative stress as reflected by autoantibodies against oxidized low-density lipoprotein and oxysterols. Atherosclerosis 155(2):445–453. https://doi.org/10.1016/s0021-9150(00)00581-5
Yoshida H, Kisugi R (2010) Mechanisms of LDL oxidation. Clin Chim Acta 411(23–24):1875–1882. https://doi.org/10.1016/j.cca.2010.08.038
You J-S, Lim H, Seo J-Y, Kang K-R, Kim DK, Oh J-S, Seo Y-S, Lee G-J, Kim J-S, Kim H-J, Yu S-K, Kim J-S (2021) 25-hydroxycholesterol-induced oxiapoptophagy in L929 mouse fibroblast cell line. Molecules 27(1):199. https://doi.org/10.3390/molecules27010199
Yuan X-M, Osman E, Miah S, Zadeh SNM, Xu L, Forssell C, Li W (2010) p53 expression in human carotid atheroma is significantly related to plaque instability and clinical manifestations. Atherosclerosis 210(2):392–399. https://doi.org/10.1016/j.atherosclerosis.2009.11.048
Yuan X-M, Sultana N, Siraj N, Ward LJ, Ghafouri B, Li W (2016) Autophagy induction protects against 7-oxysterol-induced cell death via lysosomal pathway and oxidative stress. J Cell Death 9:1–7. https://doi.org/10.4137/JCD.S37841
Zhang Y-Y, Shi Y-N, Zhu N, Wang W, Deng C-F, Xie X-J, Liao D-F, Qin L (2020) Autophagy: a killer or guardian of vascular smooth muscle cells. J Drug Target 28(5):449–455. https://doi.org/10.1080/1061186X.2019.1705312
Ziedén B, Kaminskas A, Kristenson M, Kucinskienê Z, Vessby B, Olsson AG, Diczfalusy U (1999) Increased plasma 7 beta-hydroxycholesterol concentrations in a population with a high risk for cardiovascular disease. Arterioscler Thromb Vasc Biol 19(4):967–971. https://doi.org/10.1161/01.atv.19.4.967
Zingg J-M, Vlad A, Ricciarelli R (2021) Oxidized LDLs as signaling molecules. Antioxidants (Basel) 10(8):1184. https://doi.org/10.3390/antiox10081184
Acknowledgment
This work was supported by funding from INSA Lyon.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Luquain-Costaz, C., Delton, I. (2024). Oxysterols in Vascular Cells and Role in Atherosclerosis. In: Lizard, G. (eds) Implication of Oxysterols and Phytosterols in Aging and Human Diseases. Advances in Experimental Medicine and Biology, vol 1440. Springer, Cham. https://doi.org/10.1007/978-3-031-43883-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-43883-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43882-0
Online ISBN: 978-3-031-43883-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)