Abstract
Lithium-ion batteries (LIBs) have emerged as the leading energy source for a diverse array of electronic devices, owing to their numerous benefits. Recycling LIBs is of significant importance since the ever-growing demand for them will shortly lead to massive disposal of spent LIBs and cause critical environmental problems. Spent LIBs can be considered as an excellent secondary source for various valuable metals since they approximately contain Mn (5–11%), Co (5%–25%), Ni (5%–10%), Li (5%–7%), Al (15–20%), Cu (5–7%), and graphite. Currently, LIBs’ recycling is mainly through the pyrometallurgical or high-temperature hydrometallurgical approaches, which have been substantiated to be effective for metal extraction. However, these processes come with some disadvantages such as inefficient energy consumption, high operational cost, toxic gas emission and production of secondary hazardous waste. The application of bio-hydrometallurgical methods has demonstrated remarkable efficacy in extracting metals from ores, flotation concentrates, tailings, and diverse waste materials. As an eco-friendly, low-cost, and energy-efficient method, bioleaching can be a superior replacement for conventional LIB recycling processes. In this chapter, new trends of LIB bioleaching, various factors influencing the LIB bioleaching, mechanisms, microorganisms, and regeneration of black mass have been thoroughly discussed. Moreover, the dominant challenges for industrial application of LIB bioleaching and several approaches for upscaling were summarized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
With the increasing demand for rechargeable lithium-ion batteries (LIBs), the enhanced production of these batteries will soon translate into enormous amounts of LIBs waste. The use of LIBs spans across a wide range of industries, including mobile communication devices like smartphones and tablets, various portable electronic gadgets, the rapidly expanding electric vehicle (EV) market, as well as the extensive landscape of computing devices and storage equipment. The demand for LIBs is continuing to surge, driven by a persistent growth route (Chen et al., 2019). LIBs possess a captivating array of qualities, including elevated voltage levels, remarkable longevity, minimal self-discharge, impressive energy density, a wide operational temperature range, compact dimensions, and lightweight construction. These exceptional electrochemical characteristics render them a superior choice over alternative battery technologies (Fergus, 2010). Extensive applications of LIBs are causing a surge in demand for the materials used in their production. These batteries require several key metals, including cobalt, lithium, nickel, manganese, copper, aluminum, and other elements, in order to be manufactured. The demand for these materials is expected to continue to grow as the use of LIBs becomes more prevalent in various industries, including the automotive and electronics sectors. As a result, there is an increasing focus on developing sustainable and responsible mining practices to ensure a stable supply chain for these critical materials. Some of these valuable metals are generally used for the formation of the LIBs cathode. For instance, cobalt-lithium oxide is used as an active cathode in LIBs because of its high-energy capacity per unit volume makes it perfect for portable electronics. The cobalt supply risk is high; hence, aluminum, manganese, or nickel is usually used in the cathode to reduce cobalt consumption (Habib et al., 2016).
Furthermore, lithium, nickel, cobalt, and aluminum oxide provides a sufficient energy density for the batteries, which prepares them to be utilized in electric vehicles (Olivetti et al., 2017). China's market is poised to experience a notable surge of more than 13% by 2025, driven by its robust economic growth and the persistent advancements and expansions in the realm of vehicle manufacturing. Notably, according to real-time intelligence statistics provided by GSMA, a staggering 5.17 billion individuals currently possess a mobile phone device projected to reach an impressive 7.33 billion by the year 2023 (Ordoñez et al., 2016). Furthermore, by 2030, almost one-fifth of all vehicles on American roads will be electric, and many of these will be powered by LIBs. This shift is being driven in part by increasing concerns over climate change and a growing desire to reduce carbon emissions. As a result, there is a growing need for sustainable and responsible mining practices to ensure a stable supply of the materials required for the production of LIBs (Vikström et al., 2013; Wang et al., 2014a). Furthermore, due to the extensive application of LIBs and decreased lithium and other transitional metals resources, the recycling of LIBs presents a number of benefits, both from an environmental and strategic perspective. The proper disposal of spent LIBs can help to prevent pollution and reduce the amount of waste sent to landfills. Additionally, recycling these batteries can help to recover valuable materials, including lithium, cobalt, nickel, and other metals, which can then be used to produce new batteries. This process not only helps to conserve strategic materials, but also reduces the need for new mining operations. As a result, there is a growing focus on developing effective and sustainable recycling strategies to ensure that the valuable resources contained within spent LIBs are recovered and reused (Xu et al., 2008). Considering that the primary resources of lithium are gradually depleted during long-term exploitation (Jafari et al., 2019), the supply risk related to these metals and the reduction of their mineral resources will be a serious concern (Bardi et al., 2016). Thus, secondary resources such as spent LIBs have the great capacity to be a suitable replacement to the primary resources (Ambrose & Kendall, 2020). Furthermore, dangerous substances encompassing elements like lithium, nickel, cobalt, and manganese, along with harmful chemicals like electrolytes and binders, are utilized in the production of LIBs. These materials can result in significant harm to the environment and cause pollution, posing a threat to both ecosystems and human health (Kim et al., 2006; Shin et al., 2005). Hence, based on economic and environmental concerns, today's industrial recycling approaches are moving toward environmentally friendly methods, especially biotechnological methods in the recycling of electronic waste.
1.1 Structure of Lithium-Ion Batteries
Disparate conventional batteries, LIBs operate using a different mechanism to generate energy, not relying on a reduction–oxidation reaction. Instead, these batteries utilize the movement of lithium ions between the cathode and anode, which in turn forces electrons to travel along with them. This advanced type of battery includes various components such as the cathode, anode, electrolyte, shell, separator, and other parts. A visual representation of the structure of depleted LIBs can be observed in Fig. 1 (Tarascon & Armand, 2001).
Structure of spent LIB. a Cylindrical; b coin; c prismatic; and d thin and flat (Tarascon & Armand, 2001)
1.1.1 Anode
Carbon (graphite) and lithium alloyed metals are the two most common anode materials used in LIBs (Mekonnen et al., 2016). Carbon-based anodes have emerged as the predominant choice for the advancement of LIBs owing to the cost-effectiveness of graphite production and its commendable electrochemical properties. By employing a layered graphite, the storage of Li-ions between carbon atoms (known as intercalation) occurs during the charging phase, while their controlled release transpires during discharging. However, the formation of dendrites during this process presents a challenge, as it leads to short circuits and instability in LIBs. Hence, the active anode materials in LIBs predominantly consist of carbon-based components, encompassing graphite, carbon black, carbon fiber, pyrolysis, petroleum coke, mesophase carbon microsphere bituminous, glass carbon, and more. These active materials are subsequently coated onto copper foil collectors, employing a layer of polyvinylidene fluoride (PVDF) binder for optimal performance (Chen & Xue, 2014; Zeng et al., 2014). In addition to graphite, LIBs utilize lithium alloy anodes such as lithium aluminum (Li-Al) and LiTiO2, which are essential materials for the anode (Mekonnen et al., 2016). LiTiO2, in particular, stands out for its exceptional electrochemical cycling performance. One of its notable attributes is its ability to undergo lithiation and dilithiation processes without experiencing any significant changes in volume. This unique characteristic contributes to its reliability and longevity, making it an excellent choice as an anode material for LIBs (Liu et al., 2019; Subhan et al., 2019). The use of graphite intercalation alloy can help to protect the lithium in LIBs during the charging and discharging process. This is because the metals present in the alloy act as a shield, preventing the lithium ions from reacting with the electrolyte and causing potential safety hazards. The alloy works by allowing the lithium ions to intercalate, or insert themselves between the layers of graphite, which helps to stabilize the battery's performance. This technology has been instrumental in improving the safety and reliability of LIBs, making them a popular choice for a variety of applications, including electric vehicles and portable electronics (Lavoie et al., 2017).
1.1.2 Cathode
Table 1 gives the compositions of LIBs cathodes (Methekar & Anwani, 2019). Figure 2 provides an illustration of five distinct cathode materials employed in LIBs, each with its unique atomic arrangement or crystal structure. These active materials encompass lithium cobalt oxide (LiCoO2, also known as LCO), lithium nickel cobalt manganese oxide (LiNi1−x−yCoxMnyO2, referred to as NMC), lithium manganese oxide (LiMn2O4, identified as LMO), lithium iron phosphate (LiFePO4, commonly referred to as LFP), and lithium nickel cobalt aluminum oxide (LiNiCoAlO2, known as NCA). They can be further categorized into layered structures like LCO, NMC, NCA, and spinel LMO, along with olivine LFP, based on their distinctive compositions and arrangements. Table 2 compares numerous cathode materials’ key characteristics and applications (He et al., 2015; Kwon et al., 2018).
1.1.3 Electrolyte
In a battery electrode, the transfer of ions from the cathode to the anode is facilitated by the presence of electrolytes which serve as a medium for this process. Through this transfer of ions, the chemical energy stored in the battery is converted into electrical energy. Typically, the electrolyte comprises an organic liquid containing soluble substances. LIBs are designed to utilize various types of electrolytes. These four distinct types of electrolytes commonly used in LIBs are: liquid electrolytes, colloidal electrolytes, polymer electrolytes, and ceramic electrolytes. Each type of electrolyte has its own unique properties and characteristics, which determine its effectiveness in facilitating the transfer of ions within the battery. LiPF6, LiBF4, LiCF3SO3, or Li (SO2CF3)2 are some of the available electrolyte salts. However, LiPF6 is the most widely used. Given that the lithium-ion cell voltage (∼3.6 V) is higher than the standard water electrolysis potential (1.23 V at 25 °C), the presence of a non-aqueous solvent is essential; therefore, solvents with a high dielectric constant are needed. For electrolytes to conduct ions, they must also contain lithium salts (Kwon et al., 2018; Zeng et al., 2014; Zheng et al., 2017). Propylene carbonate (PC), ethylene carbonate (EC), and dimethyl sulfoxide (DMSO) are frequently used solvents that have the ability to dissolve a variety of lithium salts. However, these solvents have a high viscosity that can impede ion transfer and decrease conductivity. Therefore, actual electrolyte fluids consist of novel compounds and feature low viscosity solvents. Up until now, a variety of distinct polymer categories have been developed, comprising polyethylene oxide (PEO), polypropylene oxide (PPO), polyacrylonitrile (PAN), polymethyl methacrylate (PMMA), polyvinyl chloride (PVC), polyvinylidene fluoride (PVDF), and polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) (Hamidah et al., 2015; Zheng et al., 2017).
1.1.4 Separator
A crucial component in LIBs, the separator effectively safeguards against short circuits arising from direct contact between the anode and cathode. By creating a deliberate space between these electrodes, it acts as a protective barrier. This separator is typically composed of a finely porous material, commonly polyethylene (PE) or polypropylene (PP), which ensures regularity in its construction. Additionally, the separator serves as a safety mechanism: in the event of excessive heat generation, the porous strip melts, irreversibly maintaining a physical separation between the electrodes. However, electrical appliances will typically remove the cell from the circuit before such a problem occurs (Xu et al., 2015).
1.2 Environmental Risk of Spent LIBs and Importance of Recycling
The life cycle of LIBs encompasses various stages, starting from the product life cycle involving activities like selling, storage, use, reuse, gifting, and export. It extends further to the product's end-of-life phase, as evaluated through lifecycle analysis (LCA) (Liang et al., 2017), and material flow analysis (MFA) (Sommer et al., 2015) (recycling, landfilling, and incineration) (Tanskanen, 2013). As discussed in the previous section, LIBs are complex devices comprised of several components that, if not properly managed and recycled, can lead to environmental pollution, including contamination of soil and water (Zeng et al., 2015). LIBs contain hazardous materials that include metals, and toxic chemical materials (such as electrolytes and binders) that cause severe environmental damage and pollution, threatening ecosystems and human health. Lithium hydroxide and hydrogen gas are generated when lithium in the anode reacts with water. However, the reaction is not as intense as that of lithium metal. Excessive Li pollution into water and soil can harm animals and plants. Overcharged batteries will form a lithium coating on the surface of their anode. The most probable cause of discarding spent batteries is due to their inadequate performance, which means the inclusion of lithium metal in recycling procedures cannot be ignored (Shin et al., 2005). In addition to Li, lower amounts of Co in LIBs are helpful to humans because it increases the development of red blood cells. Cobalt is toxic and carcinogenic at high concentrations, and causes vomiting, nausea, eyesight, and cardiac difficulties (Kim et al., 2006). As anode material of LIBs, graphite carbon materials can react with strong oxidants and generate CO, CO2, or other gases. The electrolytes in LIBs, for instance, LiPF6, LiBF4, or LiClO4 can react with water and produce HF and PF5 gases which are harmful to the atmosphere (Zeng et al., 2015). The utilization of PVDF in electrode production mandates the employment of harmful solvents like N-methyl-pyrrolidone (Versaci et al., 2017). With the escalating environmental concerns surrounding the disposal of LIBs, there is a growing demand for critical metals. In light of this, it becomes imperative to explore economically viable and eco-friendly recycling techniques that facilitate the recovery of these valuable metals from used Li-ion batteries. By adopting selective leaching methods and implementing efficient recovery processes, we can significantly reduce environmental impact and promote the sustainable utilization of secondary resources (Zheng et al., 2018).
1.3 Bioleaching of Spent LIBs
Bioleaching, a bio-based process, offers notable advantages characterized by its exceptional efficiency, safety, applicability at atmospheric pressure and room temperature, and reduced energy consumption (Vakilchap et al., 2016). Bioleaching is a cost-effective technique for treating disposed waste that employs lixiviants (leaching agents) generated biologically by microorganisms. It is highly beneficial with few industrial requirements (Bosecker, 1997; Islam et al., 2020). Alternative methods for metal recovery from primary and secondary sources, such as hydrometallurgy and pyrometallurgy have been effectively employed alongside bioleaching (Srichandan et al., 2019). Although both hydrometallurgy and pyrometallurgy are efficient procedures, they have significant drawbacks (Asghari et al., 2013). Hydrometallurgy involves the use of highly concentrated acids and bases to dissolve the metals. However, this process can generate significant amounts of acidic waste, which can be costly and challenging to manage. Downstream processing costs can be high due to the need for specialized equipment and techniques to safely handle and dispose of the waste generated (Asghari et al., 2013; Bharadwaj & Ting, 2013; Srichandan et al., 2014). Conversely, pyrometallurgy involves operating at high temperatures (1500–1700 °C), making it an energy-inefficient process. Additionally, it is linked to the emission of hazardous gases such as SO2 (Bharadwaj & Ting, 2013; Srichandan et al., 2014). Hence, both approaches fail in energy, the environment and the economy.
On the other hand, bioleaching avoids the need for intense acid/base, is energy efficient, and produces no hazardous gases (Asghari et al., 2013). During the bioleaching process, bio-oxidation transforms insoluble compositions into water-soluble compounds, and the microbe generates energy by rupturing ores or wastes (Rohwerder et al., 2003). During the process of bioleaching, a range of microorganisms, including fungi and bacteria, can play an integral role in facilitating the extraction of valuable metals and minerals. These microorganisms are capable of secreting either inorganic or organic acids, which can aid in the breakdown of the ores and promote the solubilization of metals. Furthermore, microorganisms have the potential to augment enzymatic oxidation–reduction, proton-promoted mechanisms, and enhance the formation of ligands and complexes. These mechanisms play an important role in enhancing the efficacy of the bioleaching process and augmenting the overall recovery of extracted minerals and metals. With their distinct capabilities, microorganisms are rapidly emerging as an indispensable asset in the domain of bioleaching, propelling the advancement of sustainable and environmentally friendly techniques for mineral and metal extraction (Vakilchap et al., 2016; Xiang et al., 2010). Microorganisms that have the ability to bioleach metals can be divided into three categories (Abhilash & Natarajan, 2015): the first category includes autotrophic bacteria that utilize both acidolysis and redoxolysis mechanisms to break down ores and facilitate the extraction of metals. The second category comprises heterotrophic microorganisms such as fungi, which utilize acidolysis and complexolysis mechanisms to produce organic acids and facilitate the solubilization of metals. Lastly, the third category includes cyanogenic bacteria that utilize complexolysis mechanisms to facilitate the extraction of metals from ores. Through the utilization of these distinctive mechanisms, microorganisms play a fundamental part in facilitating the bioleaching process, allowing for the efficient and environmentally sustainable extraction of precious minerals and metals. The leaching process is executed by various microorganisms, including bacteria, fungi, and yeast. Acidophilic sulfur-oxidizing bacteria and iron-oxidizing bacteria are among the most extensively employed microorganisms, standing out as the primary choices in bioleaching techniques (Xin et al., 2009). Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans are widely recognized autotrophic microorganisms renowned for their catalytic role in the oxidation process that converts ferrous iron ions to ferric iron ions (Johnson, 2018). Fungi can be utilized in the bioleaching process, like Aspergillus niger and Penicillium simplicissimum (Vakilchap et al., 2016; Wang et al., 2015). The process of bioleaching can be employed to separate the metallic constituents of batteries into various fractions, which can then be utilized to create novel batteries. Bioleaching offers benefits such as energy efficiency, cost-effectiveness, and a reduction in the presence of dangerous battery waste materials (Johnson, 2014; Vanitha & Balasubramanian, 2013). In comparison with conventional recycling approaches, bioleaching of LIBs presents a significant advantage as it creates a weak acid waste and releases minimal amounts of hazardous gases, thereby eliminating the necessity for supplementary treatment and reducing treatment expenses (Yu et al., 2020).
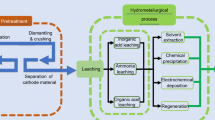
2 Preparation and Pretreatment of Waste LIBs
Pretreatment plays an important role in LIB recycling to increase recovery and reduce energy consumption. Therefore, it would be advantageous to systematically categorize and analyze the diverse range of novel approaches employed in the pretreatment process, along with their specific scopes and sequences. The main goal for LIB pretreatment is to separate active materials from metallic foils and other components. The listed sequences can be categorized into several groups, which include discharge, disassembly, comminution, classification and segregation, dissolution, and thermal processing (Golmohammadzadeh et al., 2018; Kim et al., 2021; Lai et al., 2021; Makuza et al., 2021; Roy et al., 2021a; Windisch-Kern et al., 2022).
2.1 Discharging and Dismantling (Disassembly)
When LIBs reach the end of their useful life, they lose a small amount of power; consequently, batteries burst through the recycling process. Batteries must be totally discharged prior to dismantling. The most popular way of deactivating the LIBs is to immerse them in a NaCl solution. Submerging metallic lithium in liquid nitrogen reduces its reactivity (Roy et al., 2021a).
The typical structure of an LIB’s system in vehicles consists of battery packs and a battery management system. Within the market, there exists a vast array of power batteries with varying physical structures, battery types, and material systems, making it challenging to autonomously disassemble these systems. At present, the majority of battery disassembly is carried out manually or in a semi-automated manner, such as with an automatic disassembly screw. However, this approach is time-consuming and labor-intensive, which may lead to a decrease in battery performance and a reduction in the overall efficiency of battery material recycling. As such, there is a growing need for advanced and automated disassembly processes that can effectively and efficiently disassemble LIBs while minimizing the risk of battery damage and material waste (Lai et al., 2021).
2.2 Comminution and Mechanical Treatment
Before any further processing can take place, it is imperative to execute a mechanical pretreatment stage before any subsequent processing can occur. The primary aim of this stage is to segregate the Fe, Cu, and Al alloy fractions with significant value from the fines, commonly known as “black matter.” The fine material is mainly composed of anode and cathode active materials of the LIB and contains crucial chemicals such as lithium, cobalt, nickel, and manganese that can be salvaged via downstream processes. Contemporary mechanical pretreatment methods typically employ advanced technologies such as rotary shears in double shaft shredders at low rpm or impact crushing at high rpm in hammer mills. These sophisticated techniques facilitate the effective and efficient separation of valuable materials from the black matter, leading to a maximization of the overall yield of precious metals and minerals throughout the recycling process (Windisch-Kern et al., 2022).
2.3 Classification and Separation
To separate and concentrate the constituent elements of depleted LIBs, several mechanical methods are utilized, including fragmentation, sifting, magnetic segregation, ultra-fine grinding, and sorting. By leveraging these mechanical separation techniques, it becomes possible to extract the crust and steel cases from spent LIBs, capitalizing on alterations in physical properties like density, magnetic behavior, and conductivity. However, the inability to completely separate all components of wasted LIBs precludes the effective use of mechanical techniques (Golmohammadzadeh et al., 2018).
2.4 Dissolution
During the classification and separation stages of LIBs recycling, certain active elements are liberated from the current collectors, while others are still held together by binders. In such cases, the binders or aluminum foils are commonly dissolved using suitable solvents. Acetone, dichloromethane, carbon tetrachloride, and N-methyl-pyrrolidone are among the various solvents utilized to separate the active cathode components from the aluminum foil (Kim et al., 2021).
2.5 Thermal Treatment (Heat Treatment, Pyrolysis)
Thermal pretreatment enables the regulated and safe deactivation and breakdown of the LIB's combustible organic component. Additionally, heat treatments can be employed to drain the LIB. Thermal treatment, depending on the specific temperature applied, can serve multiple purposes. It proves to be an effective approach for eliminating organic binder material. The application of elevated temperatures during thermal treatment leads to the decomposition of the binder, thereby breaking down the bonding force between the binder and active cathode material. Consequently, the cathode material can be readily separated through screening and other methods. Thermal pretreatment finds extensive applications in both laboratory and industrial settings, primarily aimed at removing carbon and organic constituents. Vacuum pyrolysis is one of the best thermal pretreatments with several advantages (Makuza et al., 2021).
3 Bioleaching of Waste LIBs with Autotrophic Bacteria
By oxidizing iron and sulfur, autotrophic bacteria can improve the dissolution of precious metals in LIBs. Most bacteria used are Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, and Leptospirillum ferrooxidans, or a combination of them. Higher levels of toxins in solution generally hamper bacterial activity in high pulp densities. The goal of most researchers in the bacterial dissolution process of LIBs has been to increase recovery and greater efficiency in higher pulp densities (Heydarian et al., 2018; Niu et al., 2014; Roy et al., 2021b, 2021c).
3.1 Mechanisms
Investigating the mechanism of battery dissolution aids significantly in properly identifying the processes and establishing the conditions for further efficiency. Many researchers have investigated how bacteria dissolve batteries materials (Wu et al., 2019; Xin et al., 2009; Zeng et al., 2013a). Lithium cobalt oxide and lithium nickel oxide are both included in LIBs. These compounds dissolve in the following manner (Heydarian et al., 2018):
In the presence of ferrous sulfate ions:
The leaching of LiNiO2 can be stated as follows:
The retrieval of metals from spent LIBs typically involves an acid dissolution process that employs sulfuric acid, generated via bio-oxidation facilitated by microorganisms. Regardless of the energy source utilized, acid dissolution has been identified as the primary method for lithium (Li) extraction. However, when FeS2 are used as energy carriers for cobalt (Co) bioleaching, a combination of acid dissolution and Fe2+ reduction takes place due to the physicochemical interaction between FeS2 and Fe3+. This interaction promotes the conversion of insoluble Co3+ into soluble Co2+ by Fe2+, leading to the release of Co2+ from used batteries through acid dissolution. Additionally, the reduction attack of Fe2+ on the Co3+ moiety has been recognized as an efficient mechanism for the release of Co from spent LIBs. Therefore, by harnessing these unique mechanisms, the extraction of valuable metals and minerals from spent batteries can be carried out in an environmentally sustainable and cost-effective manner (Wu et al., 2019; Xin et al., 2009).
Through the interaction between Fe2+ and sulfuric acid, the structural integrity of the components within used batteries was compromised, leading to the release of metal ions. Xin et al. demonstrated that, for the dissolution of Co and Li from spent LIBs, the non-contact method was found to be indispensable, while the contact mechanism proved to be insufficient. It is critical to note that, unlike bioleaching of sulfide minerals, primarily used in this case, the presence of Fe3+ in the dissolution of batteries not only hinders but also reduces the recovery. Therefore, attention must be given to selecting bacterial species and energy sources (Xin et al., 2009).
Wu et al. examined the effect of extracellular polymeric in the leaching of LIBs, which were found to be a factor in improving Li and Co2+ recovery. In order to identify the specific constituent of extracellular polymeric substances (EPS) responsible for promoting the leaching of LiCoO2, an analysis was conducted on protein, polysaccharide, and uronic acid components. It was shown that cysteine might increase the leaching of Li and Co2+, but not glucose or uronic acid. This conclusion could be explained by the reducibility of cysteine's sulfhydryl groups, reducing the Co3+ to Co2+ (Wu et al., 2019).
3.2 Microorganisms
Mesophilic microorganisms have the ability to solubilize metallic components in LIBs. Acidithiobacillus has a better tolerance for metal toxicity, and it dominates crucial studies on LIB bioleaching (Jafari et al., 2018; Naseri et al., 2019; Quatrini & Johnson, 2019; Rawlings, 1997). They aid in metal dissolving by creating biogenic H2SO4 and ferric ion, both of which aid in metal leaching (Xin et al., 2009; Zeng et al., 2012). Among the microorganisms involved in the bioleaching of LIBs, Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, and Leptospirillum ferrooxidans have been extensively studied, particularly in mesophilic conditions. Additionally, certain moderate thermophilic bacteria, such as the sulfur-oxidizing Acidithiobacillus caldus and iron-oxidizing Leptospirillum ferriphilum have also been investigated in this context. (Roy et al., 2021a). The fundamental reactions of the iron and sulfur-oxidizing microorganisms are as follows:
By increasing the temperature of the reaction and utilizing thermophilic microorganisms, it is possible to enhance the kinetics of the process. Thermophilic bacteria exhibit the ability to thrive and sustain metabolic activity at elevated temperatures, with different categories including moderately thermophilic bacteria (40–50 °C), thermophilic bacteria (50–75 °C), and extreme thermophilic bacteria (75–120 °C). One of the most significant disadvantages of bioleaching is its low kinetics which can be solved by utilizing thermophilic bacteria as they increase the bioleaching rate, namely Acidithiobacillus caldus, Leptospirillum ferriphilum, and Sulfobacillus spp. These particular strains of bacteria are of utmost significance in the process of LIBs bioleaching due to their ability to thrive in moderate temperatures and facilitate the leaching of metals from ores. Acidithiobacillus caldus, in particular, is known for its ability to oxidize sulfur and ferrous ions, while Leptospirillum ferriphilum is recognized for its capacity to oxidize iron and sulfur compounds. Sulfobacillus spp. are also valued for their role in sulfur oxidation. These microorganisms play an essential role in the LIBs bioleaching process by breaking down the ore and allowing for the extraction of valuable metals and Ferroplasma spp., which were used as a mixed culture (Ghassa et al., 2020). Extreme thermophiles, like Sulfolobus acidocaldarius, Sulfolobus solfataricus, Sulfolobus brierley, and Sulfolobus ambioalous have been identified from volcanic springs. They can thrive at temperatures of 75–80 °C with pH values of 1–3. Due to their propensity to grow at elevated temperatures, these extreme thermophiles have a higher rate of metal bioleaching than moderate thermophiles and mesophiles. However, no study has been published so far describing the use of extreme thermophiles for LIB bioleaching (Roy et al., 2021a).
3.3 Effective Parameters
3.3.1 pH
The initial pH adjustment significantly affects recovery as the recovery of Li and Co2+ are virtually identical to the amount of acid usage. The greater the acid usage, the greater the amount of Li and Co recovered. It was determined that acid consumption is crucial for the leaching of LiCoO2 due to its effect on cell development, and that adjusting the pH considerably enhanced bioleaching performance (Zeng et al., 2012). On the other hand, in the pH range from 2.5 to 4, the high initial pH causes the pH drop to start later, but in the end, in each case, the final pH is between 2 and 2.5 (Mishra et al., 2008). Moreover, some researchers showed that cobalt dissolution had only a weak connection with solution pH (Li et al., 2013), but both Co and Li would dissolve at lower pH (Boxall et al., 2018). The optimal pH range for acidophilic bacteria is 1.5–2.5, and this range has a high rate of oxidation of ferrous salt and elemental sulfur (Boxall et al., 2018; Khatri et al., 2019; Niu et al., 2014).
3.3.2 ORP
To date, there has been no published research on the ideal oxidation–reduction potential (ORP) range required to maximize metal leaching efficiency during bioleaching LIBs. However, it has been observed that cobalt dissolution can be improved by increasing the redox potential. In other words, a higher ORP range could potentially enhance the leaching of cobalt from spent LIBs. While further research is needed to determine the optimal ORP range for maximizing the efficiency of metal leaching during LIB bioleaching, these initial findings suggest that controlling the redox potential could improve the overall yield of valuable metals and minerals during the recycling process (Li et al., 2013). As previously noted, the bioleaching of cobalt from spent LIBs involves a combination of acid dissolution and Fe2+ reduction, unlike the leaching process for lithium. As a result, the oxidation and reduction conditions are more likely to impact the dissolution of cobalt. Cobalt exhibits high solubility in situations characterized by high pH levels and reducing conditions. However, its solubility decreases significantly in acidic conditions unless the ORP is simultaneously increased. This indicates that a higher oxidizing environment is necessary to retain cobalt in solution compared with other leaching tests. As such, fine regulation of the ORP range is crucial to ensure optimal cobalt dissolution during LIB bioleaching, and it is an area that requires further exploration and research. Enhancing the solubilization of cobalt through the utilization of combined bioreagents can potentially be achieved by augmenting the concentration of ferric iron oxidant or introducing an additional oxidizing agent to sustain the required oxidation–reduction potential (ORP) conditions conducive to effective cobalt leaching (Boxall et al., 2018).
3.3.3 Bacteria Energy Source
For Li extraction, sulfur and a sulfur-oxidizing bacteria (like Acidithiobacillus thiooxidans) are required, indicating that the metal would be released via acid solution caused by biogenic H2SO4; however, the mixed energy source which are Fe2+ and S, and the maximum dissolving yield for Co, Ni, and Mn could be found in a mixed culture system, demonstrating that these metals mobilize via a combination of Fe2+ reduction and acid dissolution (Zeng et al., 2012). Generally, the highest Li recovery is possible with a lower pH and a greater S concentration, and the highest Co recovery is possible with a lower pH and a greater FeSo4 concentration (Boxall et al., 2018). The higher extraction efficiency of Ni, Co, and Mn could be obtained because of stronger reduction caused by highly concentrated Fe2+ (Wang et al., 2018). It should also be noted that, especially at pH higher than 2, the metal dissolution could become slower as the Fe2+ ion concentration increases. Increased Fe2+ concentrations can decrease solubility due to Fe3+ co-precipitation with the metals in the residues (Mishra et al., 2008). The impact of Fe2+ on moderate thermophilic acidophilic microorganisms would be different as these cultures have a limited ability to oxidize Fe2+ compared with mesophilic acidophilic cultures. The highest ORP values could be seen in low ferrous ion concentration tests. Due to the depleted ferrous ion concentration, practically all Fe2+ ions would be oxidized to Fe3+. Cobalt recoveries with these cultures were the same as control tests, indicating that bacteria did not affect Co dissolving in the presence of ferrous ions. As mentioned, ferrous salt improves bioleaching dissolution. However, to avoid the use of this chemical and to reduce the expense of the process, iron sulfate heptahydrate can be substituted with iron scrap. According to Eq. 7, iron is dissolved in sulfuric acid, forming FeSO4, which enhances Co dissolution according to Eq. 2.
Iron scrap boosts Li recovery by eliminating the Co layer from the surface of the particles and enhancing acid diffusion. Therefore, it may be concluded that FeSO4.7H2O can be substituted for iron scrap. Nonetheless, this modification lengthens the procedure. Although iron scrap addition does not influence Li or Ni recovery, it can significantly boost cobalt recovery (Ghassa et al., 2020).
3.3.4 Temperature
Temperature is an important parameter because although it does not significantly affect the amount of ΔG, it affects the growth and activity of bacteria. For each culture, an optimum temperature should be found and optimized, e.g., for mixed cultures of Alicyclobacillus spp. and Sulfobacillus sp., increasing the temperature from 30 to 35 °C boosts their metabolism, but further increasing it to 40 °C reduces their activity, resulting in a decrease in efficacy (Niu et al., 2014).
3.3.5 Pulp Density
One of the primary drawbacks of LIBs bioleaching technologies is the drastic decline in the recovery with pulp densities higher than 1%. High toxicity linked with significant metal release dosages or deteriorated factors such as ORP or pH, which injured the growth and activity of bacteria, were frequently the underlying causes of a decrease in bioleaching efficiency (Xin et al., 2012a). Numerous issues arise as a result of the high solid percentage, which is that because of the increased metal toxicity, limited air incursion due to the increased viscosity, and decreased dissolved oxygen, the recovery of bioleaching would remain constant in a specific pulp density (Roy et al., 2021c).
4 Summary of Previous Studies
Mishra et al. studied the bioleaching of LIBs using Acidithiobacillus ferrooxidans. Cobalt bio-dissolution was shown to be faster than lithium. The Fe2+ ion was used in the leaching tests to promote cell development in the lixiviant, but the metal dissolution became slower as the Fe2+ ion concentration increased. Increased Fe2+ concentrations decreased solubility due to Fe3+ co-precipitation with the metals in the remaining. Furthermore, the alteration in the solid-to-liquid ratio (w/v) exerted an influence on the dissolution of metals, causing a cessation in cell growth due to the heightened metal concentration present within the waste sample (Mishra et al., 2008).
In a study conducted by Zeng et al., the bioleaching extraction of valuable metals from three frequently discarded electric vehicle LIBs cathodes was examined at a pulp density of 1%. The Acidithiobacillus thiooxidans bacteria demonstrated the highest extraction efficiency for Li, suggesting that the metal was mobilized through acid solution caused by biogenic H2SO4. However, it was observed that the dissolution rate of Co, Ni, and Mn was most significant in the mixed energy source-mixed culture system, indicating that the mobilization of these metals resulted from a synergistic interplay between Fe2+ reduction and acid dissolution mechanisms. In addition, Li extraction was accomplished using a non-contact technique, but Co, Ni, and Mn mobilization needed contact between the cathodes and cells. The extraction efficiency of four significant metals from the resistive LiNixCoyMn1-x-yO2 compound surpassed a remarkable 95% on average. The relatively elevated extraction rates of the valuable metals imply that cost-effective autotrophic bioleaching can be employed to retrieve used EV LIBs (Zeng et al., 2012).
In a study conducted by Li et al., the impact of solution pH and redox potential on the bioleaching of LIBs was investigated using Acidithiobacillus ferrooxidans. The researchers conducted a series of bioleaching tests with varying starting pH and ferrous ion concentrations to assess their effects on the bioleaching process. These findings provide valuable insights into the factors that significantly impact the efficiency of metals dissolution during LIB bioleaching and can help guide the development of efficient recycling processes for spent batteries. Nonetheless, cobalt dissolution was enhanced at greater redox potentials. Their cyclic voltammograms study revealed that dissolution rates increase above 0.4 V and rapidly decline below 1.3 V (Li et al., 2013). Boxall et al. also examined the parameters affecting the bioleaching of LIBs. They could recover 99.2% of Li, 50.4% of Co, and 89.4% of Ni at pH = 1.5, FeSO4 = 36.7 g/L, and S0 = 5 g/L as the optimal condition with a mixture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Generally, the highest Li recovery was possible with a lower pH and a greater S concentration, and the highest Co recovery was possible with a lower pH and a greater FeSO4 concentration (Boxall et al., 2018).
In a recent study, Wang et al. discovered that an extracellular polymeric substance (EPS) containing polysaccharides, proteins, and lipids can play a crucial role in the adhesion of LIBs and cells. The EPS facilitated the adhesion of cells to the batteries through minor electrostatic and dominant hydrophobic forces. Moreover, the presence of EPS resulted in the concurrent adsorption of Fe3+ and Fe2+ to amounts of 0.9 g/g and 1.6 g/g, respectively. This resulted in a high density of these ions bonded with other molecules. The enrichment of Fe3+ facilitated the attachment of cells to the cathodes, with the EPS serving as an intermediary between them. The higher extraction efficiency of Ni, Co, and Mn was attributed to the stronger reduction caused by highly concentrated Fe2+. Furthermore, the EPS increased the electronic potential while reducing the electronic resistance, resulting in superior electron transfer from Fe2+. These findings provide valuable insights into the role of EPS in the bioleaching process and can guide the development of more efficient and effective methods for the recycling of spent LIBs (Wang et al., 2018).
Hartono et al. tried to research the possibility of using bacteria strains which can reach 62.83% of lithium recovery with 2 mg/mL pulp density, initial pH of 7, the temperature of 30 °C, stirring rate of 120 rpm, and battery/soil mass ratio of 100 g/100 g. To improve their results, they suggested an adaptation environment with LiCl solution (Hartono et al., 2017). In another study that focused on the function of local bacteria, Cai et al. tried to present two bioleaching consortia that were grown in neutral activated sludge for 60 days and were characterized as phylogenetically distinct from documented bioleaching bacteria which could recover 69.46% of Li and 67.6% of Mn in 7 days. Three mixotrophs and two chemoautotrophs, three of which were unique Sulfobacillus and Leptospirillum species, were domesticated for a further 100 days utilizing cathodic materials. The findings unveiled the presence of novel bioleaching bacteria that exhibited a reduced leaching cycle and enhanced resistance to organic compounds when enriched from non-acidic environments. These results suggest a significant prospect for the recovery of metals from used LIBs or similar organic-rich settings (Cai et al., 2021).
Niu et al. investigated the bioleaching behavior of spent at pulp densities and its thermodynamics and kinetics ranging from 1 to 4%. It was found that bioleaching processes have a promising capability. It has an enormous negative value of ΔG, 12.7–11.4 times greater than leaching using FeSO4/H2O2 as reactants, the product layer diffusion model provided the most accurate description of the bioleaching behavior of Co and Li. Temperature is a critical parameter in the bioleaching process, as it impacts the growth and activity of bacteria, although it does not significantly affect the amount of ΔG. Increasing the temperature from 30 to 35 °C enhances the effectiveness of the process. However, increasing the temperature further to 40 °C can hinder bacterial activity and decrease the efficacy of the bioleaching process. The results obtained from the study indicated that there was a decrease in the bioleaching efficiency for cobalt from 52 to 10% and for lithium from 80 to 37% as the pulp density increased from 1 to 4%. Nevertheless, by implementing process modifications such as the adjustment of media acidity to pH 2.0, the utilization of mixed energy substrates and temperature maintenance at 35 °C, a maximum extraction efficiency of 89% for Li and 72% for Co could be achieved at 2% pulp density. These findings highlight the significance of optimizing process parameters to achieve optimal bioleaching performance and improve the overall efficiency of the recycling process for spent LIBs (Niu et al., 2014).
In addition to this, Jegan Roy et al. concentrated their efforts on improving the efficiency of bioleaching used LIBs that included nickel, manganese, and cobalt (NMC). They were able to improve bioleaching in a similar manner by altering the amount of H2SO4 and ferric ions in the Acidithiobacillus ferrooxidans cultures, in addition to providing a cyclic technique. This allowed them to extract more metals from the waste. The strategy that was suggested comprised supplementing the powder with new bacterial culture each time the leached liquid was changed after a period of twenty-four hours. As a result of the increased metal toxicity, reduced dissolved oxygen, and limited air intrusion resulting from the heightened viscosity, the bioleaching process was conducted at a specific pulp density. This decision was based on the notion that the recovery of bioleaching would remain stable at a particular pulp density. After 72 h of processing at a high pulp density of 100 g/L, researchers were able to achieve maximum recoveries of 90% for Ni, 92% for Mn, 82% for Co, and 89% for Li. These findings emphasize the significance of achieving an optimal pulp density in the bioleaching procedure to enhance the retrieval of valuable metals and minerals from used LIBs. The findings hold promise for facilitating the creation of recycling techniques that are more productive and proficient in managing expended batteries, leading to a reduction in the ecological consequences of battery waste and promoting the principles of a circular economy (Roy et al., 2021c). In a work quite similar to this one, Roy et al. investigated the use of Acidithiobacillus ferrooxidans for the purpose of bioleaching a mixture of LiCoO2-based LIBs at a high pulp density. They showed that the recovery of bioleaching would improve if they were to include FeSO4 in the nutrient media and then increase its concentration there up to 150 g/L. The effectiveness of the bioleaching process was significantly improved due to several factors, including the replenishment of bacterial culture three times and the presence of a high concentration of biogenic H2SO4 (0.52 M) and Fe3+ (36.86 g/L) in the culture. Analysis revealed that after 72 h of bioleaching, utilizing a pulp density of 100 g/L, the maximum recovery of 94% for Co and 60% for Li could be achieved. These results highlight the importance of optimizing the bioleaching process by adjusting critical parameters such as the concentration of sulfuric acid and ferrous ions and the duration of the process (Roy et al., 2021b).
In order to circumvent the limitations caused by the inhibition of microorganisms at low pulp densities and the scarcity of substrates, Boxall et al. investigated the feasibility of utilizing a non-contact indirect bioleaching approach to extract valuable metals from LIBs. Their objective was to achieve high leaching yields while operating at room temperature. However, when the number of leaching stages was raised to four within an hour, there was a significant enhancement in the leaching yields of all metals. These findings offer a substantial basis for the establishment of practical leaching techniques for battery waste that require less usage of inorganic acid in the future. These outcomes may enable the development of more sustainable and efficient approaches to recycle valuable metals from spent batteries, ultimately reducing the environmental impact of battery waste. The leach yields achieved in this study are much lower compared with the leach yields produced in typical hydrometallurgical procedures. The highest leaching efficiency was reported in trials utilizing strong inorganic acids such as sulfuric and hydrochloric acids. However, there would be significant energy savings associated with leaching at an ambient temperature, as well as the avoidance of releasing dangerous compounds to the environment, which would result in wastes requiring additional processing farther downstream before they could be disposed of Boxall et al. (2018).
The bio-electro-hydrometallurgical platform shows efficient and sustainable method for recycling of spent LIBs. By integrating electrokinetics, bioleaching, and selective adsorption, the researchers were able to achieve an optimal separation and purification of Co, Li, and Mn. The use of PC-88A/TOA-modified granular activated carbon as an adsorbent was particularly effective in selectively capturing the target metals. The study’s detailed analysis of the factors affecting the dissolution of active cathode materials provides valuable insights into the recycling process, which can be optimized by adjusting the mass ratio of cathode active materials to S + FeS2, the ratio of total solids cathode active materials + S + FeS2 to medium, processing duration, and Fe+3 concentration. The electrokinetic device illustrated in Fig. 3 provides a clearer understanding of the platform's design and operation.
Bio-electro-hydrometallurgical device, featuring four sections denoted as S1 (haematite-stacking area), S2 (bioleaching area), S3 (buffer area), and S4 (GAC-stacking zone) (Xin et al., 2016)
Domestication studies indicated the possible sulfur-oxidizing bacteria strain's adaptability to the rigorous electrokinetics environment, even though a high pulp slowed microbe growth and metabolism. The maximum metals recovery was obtained using a 15% mass ratio, a 40% S-L ratio, a 12-day processing duration, and a 0.2 M ferric ion concentration. The highest recovery values for Co, Li, and Mn were 91.45%, 93.64%, and 87.92%, respectively. The relative proportions of cathode active materials to S + FeS2 were found to significantly impact the overall dissolution of cathode active materials. The study revealed that a bio-electrokinetic system, combining bioleaching, selective adsorption using granular activated carbon, and electrokinetics, proved effective in the Co, Mn, Li recovery from the cathodic active materials of spent LIBs (Huang et al., 2019a).
Khatri et al. compared the efficiency of hydro and bio-hydrometallurgical procedures to extract multiple metals from LIBs. The study also examined the impact of cell presence and absence at higher pulp densities. The study findings demonstrated that the modified acidophilic iron-oxidizing consortium displayed the highest rates of metal extraction when the process conditions were optimized. These optimal conditions involved a pH level of 2, a two-step bioleaching process using Leptospirillum ferriphilum, a ferrous iron concentration of 9 g/L (Khatri et al., 2019) (Table 3).
5 Bioleaching of Waste LIBs with Heterotrophic Bacteria and Fungi
Heterotrophic microorganisms have been extensively studied and utilized in the spent LIBs recycling due to their ability to breakdown and consume organic matter for energy. While both heterotrophic bacteria and fungi can generate organic acids and aid in the bioleaching process, fungi have been predominantly favored in the bioleaching of spent LIBs (Alavi et al., 2021; Bahaloo-Horeh & Mousavi, 2017; Bahaloo-Horeh et al., 2018; Biswal et al., 2018; Horeh et al., 2016). Fungi have been recognized for their effectiveness in bioleaching owing to their shorter lag phase and faster leaching rate. In addition, fungi have a greater ability to tolerate hazardous compounds, can thrive in both alkaline and acid-consuming environments, and can excrete valuable metabolites such as EPS, proteins, exo-polysaccharides, organic acids, and complexing agents like siderophores. These metabolites can be effectively utilized to solubilize metals from various sources. The utilization of fungi presents a sustainable and promising alternative to conventional bioleaching methods and can potentially pave the way for the development of more sustainable and efficient recycling processes for spent LIBs. By connecting the potential of fungi and their metabolites, we can create a more eco-friendly and resource-efficient approach to metal recovery and promote the circular economy (Pollmann et al., 2016). Fungi naturally secrete organic acids, which can play a critical role in the chelation of metal ions. Chelation is a process in which a metal ion forms a complex with a ligand, which is a molecule capable of binding to the metal ion. In the context of bioleaching, organic acids act as ligands, binding to metal ions and making them more soluble and accessible for extraction by the microorganisms (Wu & Ting, 2006). Fungi, such as Penicillium simplicissimum, Penicillium chrysogenum, and Aspergillus niger have been used to recover heavy metals from different sources (Deng et al., 2013; Faraji et al., 2018; Harwood et al., 2017; Horeh et al., 2016; Ku et al., 2016; Xia et al., 2018). Aspergillus niger has demonstrated high performance in the bioleaching of LIBs among fungal species because of its ability to grow even in highly alkaline media and produce numerous chelating agents and organic acids (Akcil et al., 2015).
Bahaloo-Horeh et al. utilized Aspergillus niger fungus to recover valuable metals from spent LIBs. The researchers explored several bioleaching techniques, included one-step, two-step, and spent medium bioleaching. The findings indicated that spent medium was the most effective bioleaching method for maximizing metal recovery from spent LIBs. At a pulp density of 1%, the maximum recovery efficiency in spent medium bioleaching was Cu 95%, Li 70%, Mn 65%, Al 45%, Co 45%, and Ni 38%, respectively. Citric acid was found to play a crucial role in the bioleaching efficiency of Aspergillus niger, surpassing other organic acids such as gluconic, oxalic, and malic acid. The findings can help guide the development of more efficient and sustainable recycling processes for spent batteries, which can promote the cost-effective process and reduce the environmental impact of battery waste. Furthermore, bioleaching was more effective at removing heavy metals than chemical leaching. The authors proved that the bio-hydrometallurgical route is more effective for recovering heavy metals from spent LIBs (Horeh et al., 2016). Another study, Biswal and colleagues recycled spent medium bioleaching with Aspergillus niger SG1 and MM1 strains at a pulp density of 0.25% (w/v) to remove Co and Li from spent LIBs. Their findings showed that Aspergillus niger strain MM1 was highly effective in dissolving Co (82%) and Li (100%) during the bioleaching of LIBs (Biswal et al., 2018). The investigation also uncovered that Aspergillus niger's adaptation to heavy metals resulted in an enhancement in the production of organic acids and an increase in metal leaching efficiency when comparing adapted fungi to unadapted fungi. These results emphasize the promising potential of Aspergillus niger and spent medium bioleaching as an effective and sustainable approach for spent LIBs recycling. By considering the unique capabilities of Aspergillus niger and optimizing the bioleaching process, we can create a more efficient and sustainable approach to metal recovery, which can help promote the circular economy and reduce the environmental impact of battery waste (Bahaloo-Horeh et al., 2018). A study conducted by Alavi et al. analyzed the effectiveness of one-step bioleaching, two-step bioleaching, and spent medium bioleaching methods for the recycling of metals from LIBs, utilizing a mixed fungal culture of Aspergillus niger and Aspergillus tubingensis. According to the findings, the spent medium bioleaching method proved to be the most. The investigation also demonstrated that oxalic acid was released in higher quantities than citric acid, although citric acid played a critical role in the bioleaching process. These results emphasize the importance of understanding the role of different organic acids and other compounds in the bioleaching process. By optimizing the presence and concentration of these compounds, we can improve the efficiency and effectiveness of the bioleaching process (Alavi et al., 2021). The outcomes of this study demonstrated the potential of Aspergillus niger and other fungal cultures in spent LIBs recycling. By utilizing spent medium bioleaching and optimizing organic acid secretion, particularly citric acid, we can develop more efficient and sustainable methods for metal recovery. These findings can potentially foster the development of innovative and effective solutions for reducing the ecological impact of battery waste. The use of fungal-based bioleaching methods can help promote a circular economy and minimize the environmental harm caused by metal-containing waste.
5.1 Mechanisms
Heterotrophic microorganisms are known for their remarkable capacity to produce organic acids and chelating compounds, which play a significant role in metal bioleaching. Acidolysis, complexolysis, bioaccumulation, redoxolysis, and biosorption have been reported as the main mechanisms by which organic acids react with metals through bioleaching with heterotrophic cultures (Le et al., 2006; Simate et al., 2010). Organic substances are used as an energy source by heterotrophic bacteria and fungus through their metabolism. Fungi secretes numerous organic acids (gluconic, citric, oxalic, etc.) while growing on organic supplements (Sierra-Alvarez, 2007; Xu et al., 2014). The process of transforming glucose or sucrose into citric acid consists of a sequence of enzymatic reactions that occur in two separate cellular compartments, namely the cytosol and the mitochondrion. The first step involves the transportation of glucose through the glycolysis pathway into the cytosol, where it is transformed into pyruvate (as shown in Fig. 4). The pyruvate molecule undergoes two distinct fates in the citric acid production process. One pyruvate molecule undergoes decarboxylation to yield acetyl-CoA via the mitochondrial pyruvate dehydrogenase complex. Meanwhile, the other pyruvate molecule is carboxylated to form oxalo-acetic acid by pyruvate carboxylase in the cytosol. Afterward, the oxalo-acetic acid is transported to the mitochondrion, facilitated by malate and reacts with acetyl-CoA to generate citric acid. This intricate process demonstrates the complex biochemical pathways involved in the conversion of glucose or sucrose to citric acid. Understanding these pathways and their regulation can potentially aid in the development of more efficient and sustainable methods for citric acid production. The final product is then transported out of the mitochondrion and eventually exits the cell. Oxalo-acetase, an enzyme that catalyzes the hydrolysis of oxalo-acetic acid to oxalic acid and acetic acid, can produce oxalic acid from oxalo-acetic acid. Overall, the production of citric acid from glucose or sucrose involves a complex series of biochemical reactions that occur in specific cellular compartments requiring the coordinated action of multiple enzymes (Magnuson & Lasure, 2004). Throughout the growth phase of fungi, an array of organic acids including citric acid, lactic acid, gluconic acid, and oxalic acid, as well as enzymes, are secreted. These organic acids serve as bio-lixiviants, as supported by the following evidence (Xia et al., 2018), and are utilized for the oxidation of metals from spent LIBs via the O2/H2O redox pair. Following this, the metal ions are protonated and subsequently complexed with the organic acids. The protonation lead to the release and movement of free metal cations that are generated through acidolysis, resulting in the dissolution of metals at an acidic pH (as seen in Eqs. 8–12). This process highlights the crucial role of organic acids in bioleaching and their potential to be harnessed for metal recovery from various sources. By optimizing the bioleaching process and the presence of these organic acids, we can develop more efficient and sustainable methods for metal recovery from spent LIBs, which can contribute to the circular economy and reduce the environmental impact of battery waste (Bahaloo-Horeh & Mousavi, 2017; Bahaloo-Horeh et al., 2018; Biswal et al., 2018). Further research in this area can potentially lead to the development of innovative and effective solutions for sustainable resource management and metal recovery. The principal mechanism of fungal bioleaching is complexolysis, wherein metal cations interact with organic acid anions to generate complexes.
Production of organic acids including gluconic acid, citric acid, and oxalic acid, from glucose or sucrose (Srichandan et al., 2019)
The Aspergillus niger and Penicillium simplicissimum fungi were found to be the most effective strains to bioleach metals from spent LIBs by creating a variety of organic acids as metabolites in sucrose \(({\text{C}}_{12} {\text{H}}_{22} {\text{O}}_{11} )\) medium.
5.1.1 Acidolysis
Acidolysis is a widely utilized fast leaching method for fungi and other heterotrophic microorganisms. The leaching process involving fungi is an indirect method, whereby the oxygen atoms of the metal compound are protonated by the organic acids secreted by the fungi. This is exemplified by Eq. 13, where protons attach to the solid surface and react. The detachment of metal from the solid surface is facilitated by the interaction between oxygen, protons, and water. This process highlights the important role of organic acids and the protonation process in the bioleaching of metals from various sources. The potential of organic acid-based bioleaching methods can help create a more sustainable and environmentally friendly approach to metal recovery and resource management (Srichandan et al., 2019).
MeO is a metal oxide such as NiO. Low pH has been shown to benefit acidolysis by weakening chemical bonds in the waste matrix, allowing metals to dissolve more quickly (Bahaloo-Horeh et al., 2018; Biswal et al., 2018).
5.1.2 Complexolysis
Complexolysis is a process that involves the mobilization of metal ions through the acidolysis mechanism, which is stabilized by complexation with organic acids and other chelating agents. Through complexolysis, metals are solubilized and mobilized in a form that is readily available for extraction. Fungi release organic acids and amino acids, playing an essential role in complexolysis (Srichandan et al., 2019). Heterotrophic microorganisms such as Actinomycetes and fungi can increase the solubility of metals by forming metal-complexing ligands in reaction with organic acids and amino acids, although fungi restricted amino acid excretion (Srichandan et al., 2019). The molecules of organic acids possess the unique capability to generate intricate metal chelators, which assist in the dissolution of metal ions. Chelators possess the capability of generating stronger bonds with metal ions when compared with the lattice bonds that are formed between solid particles and metal ions. This attribute contributes to an enhancement of the bioleaching process, as it facilitates the solubilization of the metal ions (Islam et al., 2020). When metal ions interact with organic ligands, they can form durable complexes. The toxicity and stability of chelating agents in a solution are greatly affected by the specific organic ligands and metal ions involved in the process. Recent studies have revealed that certain types of bacteria and fungi possess the capacity to excrete iron-chelating compounds with low molecular weight, which serve as the principal agent for dissolving Iron (III) (Islam et al., 2020). Additionally, studies have revealed that amino acids and organic acids produced by fungi secrete protons that augment the capacity of metal solubilization complexing. These mechanisms contribute to the efficient bioleaching of metals and highlight the potential of organic acid molecules in the development of sustainable and eco-friendly methods for metal recovery. Equations (14) and (15) illustrate a complexolysis and chelation reactions involving metal ions with citric acid (Srivastava et al., 2020):
Under weaker acidic conditions, the complexolysis process is the main mechanism. Complexolysis has two essential functions (Brandl & Faramarzi, 2006):
-
Improve the solubility of metal ions that have already been solubilized by acidolysis (with the exception of metal-oxalate complexes such as Ni oxalate, which have low solubility),
-
Facilitate the removal of metal species from the surface by polarizing the critical bonds through ligand exchange.
5.1.3 Redoxolysis
Fungi use the redoxolysis mechanism, to increase metals’ mobility through microbial oxidations and reduction processes to obtain energy from minerals (Mishra & Rhee, 2014). In fungal leaching of manganese, solubilization happens due to enzymatic reduction of oxidized manganese, as indicated in Eq. (16) (Asghari et al., 2013).
5.1.4 Bioaccumulation
The bioaccumulation mechanism happens when the soluble metal ions are transported through the cell membrane, causing solid particles to accumulate or precipitate in vacuoles (Brandl et al., 1999). Fungi possess a cell wall that harbors numerous functional groups, such as hydroxyl, carboxyl, amino, phosphate, and sulfate groups, which have the potential to bind to metal ions. This feature of fungi opens up the possibility of using them in the bioleaching process for the recovery of metals from spent batteries, as they can act as efficient biosorbents for metal ions. By exploiting the unique properties of fungi, it may be possible to develop more sustainable and economical methods for metal extraction and recycling (Kapoor & Viraraghavan, 1995). The bioaccumulation of metal ions in the mycelia of fungi is facilitated through active metabolic reactions and passive adsorption (Dusengemungu et al., 2021). Certain fungi, such as Penicillium and Aspergillus have been found to possess a high ability to accumulate metal ions (Asghari et al., 2013). Arya and Kumar (2020) observed that Aspergillus niger was able to bioaccumulate 77% of the total solubilized lithium into its biomass during lithium recovery (Arya & Kumar, 2020). This finding suggests that the bioaccumulation of Li is a significant mechanism for its recovery. This process promotes the solubilization of Li+ cations that amass within the cells, improving the equilibrium and ultimately resulting in a higher efficiency of lithium dissolution. The bioaccumulation of metal ions in fungi provides a sustainable and eco-friendly solution for metal recovery, as it reduces the need for complex and costly chemical processes. These results highlight the potential of fungi-based approaches for LIBs recycling. Further research in this area can lead to the development of innovative and sustainable methods for metal recovery, contributing to the promotion of circular economy principles and reducing the environmental impact of metal waste. The recovery of metals from fungal biomass would be a future research challenge. Examples of precipitation reactions are given in Eqs. (17) and (18).
Me2+ is a metal cation (Dusengemungu et al., 2021).
5.1.5 Biosorption
Studies have shown that biosorption is a phenomenon that occurs during fungal bioleaching (Dusengemungu et al., 2020; Iram et al., 2015; Ong et al., 2017). The process consists of the dissolution of metal from the leached material by the organic acids generated by fungi. Metal ions present in the leaching solution are adsorbed by the fungal biomass, which results in a reduction of the amount of metal present in the solution. The biosorption process involves various reactions, including ion exchange, complexation, adsorption, and precipitation. It is sometimes described as an independent metabolism accumulation of metals.
The effectiveness of metal biosorption can be influenced by a number of factors, such as the type and amount of biosorbent used, the surface area of the biomass, and various physicochemical factors like pH, temperature, and ion concentration (Işıldar et al., 2019). Biosorption presents a promising approach for the removal of metals from waste streams, contributing to the promotion of circular economy principles and reducing the environmental impact of metal waste.
5.2 Parameters Affecting Heterotrophic Bioleaching
Heterotrophic microorganisms like fungi excrete organic acids that play an essential role during bioleaching. However, numerous operational characteristics such as growth, medium pH, energy source, substrate concentration, etc., significantly impact the type and amount of organic acid produced by heterotrophic microorganisms. As a result, parameter optimization is critical because maximal leaching occurs when the settings are tuned for fungus growth. Table 4 has detailed different parameters’ effects on bioleaching of spent LIBs with heterotrophic microorganisms.
5.2.1 PH
During fungal growth, pH plays an important role. First, germination from conidiophores occurs at a higher initial pH (>5). During this phase, any changes in pH could be harmful. Due to organic acids and ammonium formation, the medium’s pH tends to reduce (2.5) after germination (Moh et al., 2005). The optimal pH value of Aspergillus niger is around 5.0 in bioleaching of spent LIBs. In the bioleaching process, the pH rises in proportion to the pulp density (S/L) due to the alkaline nature of LIBs powder (Bahaloo-Horeh et al., 2018).
5.2.2 Temperature
Temperature is a pivotal parameter during bioleaching because higher temperatures result in a faster reaction rate. Hence even minor temperature changes might affect microorganism growth and the bioleaching process. Reducing temperatures during the growth phase diminishes the likelihood of achieving a successful collision between enzymes and substrates. Conversely, elevated temperatures lead to the denaturation of essential enzymes involved in the cell cycle. (Walker & White, 2018). As a result, an appropriate temperature range is necessary for proper fungal growth. Temperatures of 30–32 °C are ideal for the growth and bioleaching of spent LIBs by Aspergillus niger.
5.2.3 Pulp Density
The concentration of pulp plays a critical role in the bioleaching process of LIBs. High pulp densities can impede the leaching rate by elevating the viscosity of the leaching solution. This, in turn, restricts the distribution of dissolved oxygen and air to the microorganisms, thereby reducing the effectiveness of the bioleaching process. This can prevent penetration of oxygen into medium, negatively impacting the metabolism of microorganisms and reducing the efficiency of metal leaching. In situations where the viscosity is high, the diffusion of oxygen and nutrients into the microbial cells is impeded, leading to a decline in their metabolic activity. Therefore, the optimization of pulp density is critical for the development of efficient and sustainable methods for metal recovery from spent LIBs. The understanding of the impact of pulp density on the bioleaching process can contribute to the development of innovative solutions for the reduction of the environmental impact of battery waste (Naseri et al., 2020).
5.2.4 Nutrient or Source of Energy
The filamentous fungus can bioleach metals faster than bacteria due to their shorter lag and exponential growth phases as long as nutrients are available (Dusengemungu et al., 2021; Horeh et al., 2016; Moh et al., 2005). The concentration and nature of the carbon source utilized are pivotal to the conversion of organic carbon into acids. Fungi are capable of utilizing an assortment of carbon sources, including glucose, sucrose, fructose, galactose, and molasses, among other options (Natarajan & Das, 2003; Walker & White, 2018). Due to its cost-effectiveness, sucrose has become the most commonly employed carbon source in various industries (Hamad et al., 2015). In addition, one sucrose molecule yields two simple sugar molecules (Walker & White, 2018). The production of organic acids is dependent on the types and concentrations of sugars present in the system. Furthermore, various nutrients, including nitrogen, phosphorus, magnesium, and others are required for fungi to grow and for the production of organic acids. Since fungi are unable to fix nitrogen, an external nitrogen source is necessary. Ammonium sulfate is a favorable nitrogen source, as it also provides a usable sulfur source (Walker & White, 2018). Optimizing the carbon and nutrient sources is essential in creating efficient and sustainable approaches to the production of organic acids by fungi. Using low-cost agricultural by-products as carbon sources for fungi is the optimal and effective choice for producing organic acids. The understanding of the impact of carbon and nutrient sources on the production of organic acids by fungi can contribute to the development of innovative and sustainable bioprocesses for the production of organic acids, promoting circular economy principles and reducing the dependence on non-renewable resources.
6 Methods for Process Intensification
6.1 Catalysis for Bioleaching of LIBs
Although bioleaching has several merits and environmental advantages in comparison with conventional hydrometallurgical processes, its relatively slow dissolution kinetics can be considered one of its major demerits. This slow leaching rate is the key underlying reason that hinders the large-scale application of bioleaching in industrial plants (Abdollahi et al., 2021). Researchers have evaluated the efficiency of various metal ions such as Ag+, Hg2+, Bi3+, Cu2+, Co2+, etc., with non-metallic compounds such as activated carbon and polyethylene glycol as catalysts for the bioleaching process. Poor bioleaching yields and the necessity for large amounts of catalyst to achieve substantial recovery yields are two disadvantages of non-metallic catalysts (Bahaloo-Horeh et al., 2019). Metal ions have gotten the most attention out of all the other catalysts. Catalytic metallic ions can increase the efficiency of metal removal and substrate oxidation by speeding up electron transport (Niu et al., 2015). Due to their remarkable catalytic characteristics, metal ions are predicted to substantially impact the development of large-scale bioleaching technologies shortly. Ag+ has gotten the most attention out of all of these metal ions. Various Ag compounds have been utilized as a source of silver ions in the bioleaching media, including silver sulfate (Ag2SO4), silver nitrate (AgNO3), and silver chloride (AgCl). While Ag+ ions have been substantiated to be beneficial in ameliorating the leaching kinetics and metals recovered from minerals and secondary sources, the majority of Ag+ bioleaching research has focused on Cu minerals like chalcopyrite (Abdollahi et al., 2015; Pathak et al., 2017). Silver ions can also be employed in a LiCoO2 bioleaching driven by Acidithiobacillus ferrooxidans, according to Zeng et al., The silver ion plays a key role in the creation of the AgCoO2 intermediate product, which is then oxidized by Fe3+ ions to Co2+, then Ag+ is released into the medium, where it may be reused (Zeng et al., 2013b). The following equations can define the catalytic mechanism of Ag+ ions on LIB bioleaching:
Due to the bacterial metabolism, the Fe2+ ions generated are oxidized back to Fe3+ ions:
According to Zeng et al., adding 0.02 g/L silver ions to the culture medium can result in 98% Co extraction from spent LIBs in seven days, whereas Co dissolution was only 43% in the absence of Ag+ (Zeng et al., 2013b). Application of Cu2+ ions instead of Ag+ ions to improve Co solubility in LIBs utilizing Acidithiobacillus ferrooxidans was also reported by Zeng et al. In just six days, the extraction of Co was increased to 99.9% by utilizing 0.75 g/L of Cu2+ ions; conversely, in the absence of copper ions, the dissolving efficiency was 43.1% after ten days. The catalytic behavior of copper ions is attributed to the formation of CuCo2O4 intermediates on the surface of LiCoO2 due to cationic exchange interactions, according to the authors (Zeng et al., 2012). As illustrated in the following equations, the Fe3+ ions may easily dissolve the intermediate product (CuCo2O4), leading to a greater Co recovery yield:
The noteworthy point is that with the equal mass of catalyst, Ag+ ions can provide a superior yield compared with Cu2+ ions. The application of metal ions as the catalyst for spent LIB bioleaching has various challenges like the considerable cost of various metal ions such as Ag, Hg, Bi, and Ru for the large-scale application, and the metal toxicity of high dosage catalysts for microorganisms, metal recovery, and environmental safety. For the first issue, more research needs to be conducted to find more economically suitable catalysts with the same effectiveness. Metal toxicity of microorganisms is also a critical issue when using catalysts. Acidithiobacillus ferrooxidans, for example, have been shown to be adversely impacted by a minimal concentration of Ag+ of 0.1 mg/L. In the bacterial cell, Ag+ ions can take the role of Fe2+ ions in the active site of oxidizing enzymes. Identifying and employing resistant microorganisms capable of tolerating larger concentrations of metallic ions are critical in dealing with these problems (Pathak et al., 2017).
6.2 Sonobioleaching
Sonobioleaching using ultrasound is another method to put a curb on slow bioleaching kinetics. In this approach, metal dissolution utilizing metabolites generated by bacteria is enhanced by employing ultrasonic wave (Anjum et al., 2014). Low quantities of acid cause a superior boost of the leaching rate in the sonobioleaching process; this is favorable for the bioleaching approach since bioprocesses produce relatively low metabolite concentrations. The sonobioleaching technique also facilitates bio-lixiviant penetration into solid particles (Anjum et al., 2014; Vyas & Ting, 2018). Ultrasonication improves bacterial metabolic activity and changes the permeability of cell membranes by increasing agitation at the macroscopic and microscopic levels. Low-frequency ultrasonication improves microbial growth, while high-frequency ultrasonication promotes cell wall breakdown. The ideal frequency of 40 kHz at 1.5 W enhanced Aspergillus niger growth; frequencies below and beyond this limit reduced ultrasonic efficiency. As reported by Vargas et al., metabolic activities of Aspergillus niger can be increased by 28% after exposure to sonication of more than 4 min (Gu et al., 2018; Vargas et al., 2004). Despite the promising potential of sonobioleaching, unfortunately, its application for LIB recycling has never been studied before; thus, it can be an interesting topic for future investigations.
6.3 High Pulp Density Bioleaching
The importance of pulp density in bioleaching cannot be overstated, as it significantly impacts the efficiency, kinetics, and economic feasibility of the process. Oxygen and carbon dioxide restrictions, excessive shear force, and the accumulation of leached metal ions are cited as primary challenges when aiming for optimal bioleaching operations, particularly in relation to high pulp density scenarios (Gu et al., 2018; Wang et al., 2014b). Increasing the pulp density from 10 g/L to 20 g/L presents a notable reduction of 50% in both leaching media volume and reactor size, leading to a substantial cost reduction in bioleaching processes. Typically, the pulp density in the bioleaching of low-grade ores is maintained at 10% or higher (Rohwerder et al., 2003). Evaluating the impacts of high pulp density on bacterial behavior is essential because pulp density should be maximized to increase the potential of LIB bioleaching's commercial application (Norris, 1997). There are only a few studies that evaluated the potential of LIB bioleaching at substantial pulp densities. As reported by Niu et al., the extraction efficiency of Co and Li from spent LIBs is significantly influenced by the solid/liquid ratio. By increasing solid content from 1 to 4%, Co extraction declined from 52 to 10% for and from 80 to 37% for Li (Niu et al., 2014). Roy et al. used the autotrophic bacteria Acidithiobacillus ferrooxidans to explore the bioleaching of a combination of LiCoO2-based LIBs at elevated pulp density (100 g/L). At this pulp density, 60% lithium and 94% cobalt recovery were achieved in 72 h by increasing biogenic acid generation in the culture media and refilling the bacterial culture for three cycles (Roy et al., 2021b). Regardless of LIB bioleaching, there are some studies on spent Zn-Mn batteries with the purpose of maximizing the pulp density for industrial applications, in which bioleaching at 10% pulp density was employed with promising results (Niu et al., 2015; Xin et al., 2012a).
As previously mentioned, high pulp density and employment of metal ion catalysts can significantly reduce the microorganism metabolism due to metal toxicity and other inhibitory impacts. However, there are various approaches to put curb on these issues. Metals extraction from LIBs throughout bioleaching impedes the metabolic pathways of microorganisms that produce metabolites. Additionally, extracted metal ions can disintegrate proteins and nucleic acid in cells. The toxicity of microorganisms to LIBs is influenced by the concentration of heavy metals. During the bioleaching process, it is important for the microorganisms to maintain a stable population with efficient functioning and the ability to sustain an acidic medium with inhibitory factors. Adapting microorganisms to high metal content and employing adapted bacteria or fungi is crucial for maximizing the pulp density in LIB bioleaching. Through a progressive sub-culturing procedure, bacteria can be adapted to the LIB by gradually exposing them to larger solid content (Roy et al., 2021a; Sethurajan & Gaydardzhiev, 2021; Srichandan et al., 2019). Heydarian et al. utilized a mixed culture of adapted acidophilic bacteria of Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans for waste laptops’ LIB bioleaching. The adaptation period was 128, and the bacterial tolerance threshold of LIBs reached to around 40 g/L. Using an adapted bacterial culture, the LIBs bioleaching efficiency reached 99.2% for lithium, 89.4% for nickel, and 50.4% for cobalt (Heydarian et al., 2018).
Another method is using synthesized biology technologies like genetic engineering to improve bioleaching microorganisms’ resistance and resilience to different stress factors prevalent in harsh conditions, increasing bioleaching efficiency (Baker-Austin & Dopson, 2007). For the bioleaching of LIBs, synthetic biology techniques may also change the metabolic pathways of new bacteria (Dopson & Holmes, 2014). According to Gumulya et al., there are four possible pathways for industrial bioleaching microorganisms: acid tolerance, thermotolerance, osmotolerance, and, most importantly, metal tolerance (Gumulya et al., 2018). Synthesis of metal tolerance microorganisms, coupled with proper adaptation, can bring about the desirable efficiency at maximum pulp density, which subsequently makes the industrial LIB bioleaching more economically reasonable.
6.4 Kinetics Studies
To unravel the intricacies of the leaching process, an essential step involves delving into kinetic modeling. The aim of kinetic modeling is to identify the optimal equation that captures the rate of the process and enables the extraction of kinetic parameters. This knowledge is crucial for tasks such as plant design, real-time optimization for automated control, determining operating conditions in large-scale industrial settings, and maximizing leaching yields. In the realm of hydrometallurgy, the widely utilized shrinking core model provides valuable insights into comprehending the dissolving mechanism of solid materials (Baker & Bishop, 1997; Dickinson & Heal, 1999). Based on this model, the reaction can be divided into three essential stages: the transfer of bio-generated metabolites from the solution to the solid surface, the diffusion of metabolites within the solid particles, and the chemical reaction occurring on the solid surface. The initial phase is not the determining factor when the stirring is appropriately executed, thereby indicating that the process is predominantly influenced by the diffusion of reactants through the particle surface or the chemical reaction itself. In cases where the rate of dissolution is governed by the chemical reaction transpiring on the particle surface, the following equation can be employed as a suitable representation:
The dissolution kinetics may be described using the following equation, assuming that diffusion through the product layer is the rate-limiting step:
In both of these equations, X represents the reacted metal fraction, t represents the bioleaching duration, and kt represents the kinetic constant (Levenspiel, 1998).
Over the past few years, other models have been introduced and modified for other types of mechanisms:
Diffusion through the product layer controls the dissolution rate (İkiz et al., 2006):
The surface chemical reaction with the shrinking core limits the reaction (Padilla et al., 2008):
Mixed control model by shrinking core model (diffusion control; chemical reaction control) (Ghassa et al., 2017):
Mixed control model (surface reaction control; and diffusion through sulfur layer) (Sokić et al., 2009):
Few researchers studied the kinetics of bioleaching to recycle both valuable and environmentally hazardous metals from spent LIBs, which indicates a lack of awareness in this area. Low kinetic is one of the important disadvantages in bioleaching process of LIBs. Niu et al. investigated the kinetic behavior of Li and Co bioleaching from spent LIBs with Alicyclobacillus sp. and Sulfobacillus sp. at 2% pulp density. The product layer diffusion model had the highest correlation coefficients, noting that other models also had R2 higher than 90%. The intrinsic mechanisms behind the phenomena remained unknown (Niu et al., 2014). The study’s kinetic results provided insight into how mesophilic sulfur-oxidizing bacteria-modified granular-activated carbon adsorbs Co. The process involves external diffusion initially, followed by surface adsorption and culminating in chemical immobilization (Huang et al., 2019a).
6.5 Bioleaching with Mixed Cultures
Bioleaching with mixed cultures rather than pure strains has gained a lot of attention in recent years, mostly due to the positive synergistic effect of microorganisms that leads to higher efficiency and faster kinetics (Liao et al., 2021; Xia et al., 2018). It has been substantiated that single microorganism bioleaching is less effective than mixed culture bioleaching regarding the metal dissolution from various sources. Mixed mesophilic cultures constituted from Acidithiobacillus ferrooxidans, Leptospirillum ferrooxidans, and Acidithiobacillus thiooxidans have been widely employed for metal leaching from wastes, minerals, and other sources. Mixed moderate thermophilic culture comprised of microorganisms like Sulfobacillus thermosulfidooxidans, Leptospirillum ferriphilum, Acidithiobacillus caldus, and Ferroplasma spp. at the optimum temperature of 45 °C have shown promising results for extraction of Co from cobaltite bearing ore (Abdollahi et al., 2021). In comparison with a single culture of Acidithiobacillus thiooxidans, a mixed culture demonstrates a greater extraction of manganese (insoluble Mn4+ to soluble Mn2+) by Fe2+. The decline in ORP throughout the bioleaching process in a mixed culture medium is indicated by the large fluctuations in ORP. Furthermore, mixed culture produces more ferric ions than pure strain iron-oxidizing microbes due to the lower pH value. More ferric ions result in a more extensive formation of Fe2+, which speeds up the reduction of Mn4+ and the recovery of Mn2+. Consequently, using mixed culture yields the highest manganese extraction (Xin et al., 2009, 2012b).
In a study by Alavi et al., spent cellphone LIB bioleaching was employed with mixed-fungus culture (Aspergillus tubingensis and Aspergillus niger) by 1-step, 2-step, and spent medium approaches. Their investigations revealed that spent medium bioleaching in the presence of vinasse with 1% solid percentage and at 140 rpm and 30 °C provides the highest metal yields of approximately 82% Al, 62% Co, 98% Mn, 91% Li, and 81% Ni (Alavi et al., 2021). The investigation conducted by Xin et al. showed that the maximum dissolving yield for Co, Ni, and Mn were obtained in the mixed culture system (Acidithiobacillus thiooxidans and Leptospirillum ferriphilum), implying that these ions were mobilized by a combination of Fe2+ reduction and acid dissolution. The extraction efficiency of four important metals from refractory LiNixCoyMn1-x-yO2 in a mixed system was above 95% on average (Xin et al., 2016). Employing mixed moderate thermophilic culture in the presence of iron scrap as a reductant also showed promising results in a study by Ghassa et al. (Ghassa et al., 2020). A mixture of bio-metabolites can be employed to undertake bioleaching. Bioleaching using a mixture of ferric ion and biogenic acid was shown to improve leaching efficiency in terms of pH and ORP compared with solo bioreagents. When the bioreagents were combined, their pH was lower than that of the solo bioreagents. This suggests that a more oxidizing environment was created, which led to more effective metal leaching (Boxall et al., 2018).
7 Methods of Metal Recovery
7.1 Solvent Extraction
Solvent extraction stands as a prominent method for purifying and extracting metals from the leachate of LIBs. The dissimilar solubility behaviors exhibited by various solutes when in the presence of organic solvents within a two-phase system determine the separation process during solvent extraction. An appropriate solvent should demonstrate selectivity toward the desired chemical and exhibit significant potential for recycling. Bioleaching-solvent extraction-electrowinning route has gained much popularity in past years, and many studies have been conducted in this regard (Akbari & Ahmadi, 2019; Irrgang et al., 2021). Various organic extractants such as MextralVR 5640H, MextralVR 272 P, P-204, P-507, D2EHPA, Cyphos IL 102, and Cyanex 272 have been utilized to recover metals from LIB’s PLS (Kang et al., 2010; Keller et al., 2021; Lei et al., 2022; Punt et al., 2021; Shuya et al., 2020; Torkaman et al., 2017; Xu et al., 2020). Existing Ni, Co, Mn, and Li together in the cathode of LIBs (such as in NCM cells) can make separation more difficult and may lead to co-extraction. However, there are ways to separate these metals: using Cyanex 272 can separate Ni and Co, while using D2EHPA can separate Mn and Co when used together, and precipitation can separate Li and Ni (Chen & Ho, 2018). Solvent extraction often involves the use of diluents like paraffin, naphthenes, and alkyl aromatics to thin out the viscous extractants. However, the use of hazardous and flammable organic diluents remains a concern (Shamsuddin, 2021). This should also be taken into account that returning aqueous raffinate in the bioleaching-solvent extraction cycle might contain a small proportion of organic matters that can significantly hinder the bioleaching process. As reported by Saneie et al., the presence of an organic phase in the returning raffinate can substantially inhibit the bio-oxidation and metabolism of bioleaching microorganisms resulting in lower leaching kinetics. Thus, eliminating organic extractants from the aqueous phase is crucial (Saneie et al., 2021).
7.2 Chemical and Biological Precipitation
The differential in solubility of several chemical species in a mixture in the presence of selectively applied chemical compounds is the basis for precipitation. The fluctuation of solubility as pH changes is utilized in the separation's favor. At pH = 2 and temperatures within 40 and 50 °C, potassium permanganate can be employed as a reagent to precipitate manganese as manganese dioxide. It is reported that dimethylglyoxime (DMG) can precipitate nickel in the presence of ammonia at pH 8 to 11 (Vanitha & Balasubramanian, 2013). Typically, Fe, Cu, and Al are removed at the start of the precipitation process. Precipitation is a low-cost approach to separate them from other ions. At low pHs (3–6), they are easily eliminated by precipitation using NaOH or other hydroxides (Zou et al., 2013). At higher pHs (8–12), Co, Ni, and Mn can also be selectively precipitated as hydroxides. These metals can be eliminated in the form of carbonates by adding Na2CO3, sulfides by adding (NH4)2S at a pH of 6 to 10, and other ways. Lithium may be recovered in three forms: carbonate (Li2CO3), fluoride (LiF), and phosphate (LiP) (Li3PO4) (Duarte Castro et al., 2021). In a recent study, Biswal et al. employed bioleaching followed by precipitation for metal recovery from waste LIBs. More than 88% of Co from the fungal PLS recovered in the form of cobalt oxalate, cobalt sulfides, and cobalt hydroxides, and about 74% of Li in the lithium carbonate form (Biswal et al., 2018).
In the bioprecipitation technique, the reagents for precipitation are produced by different microorganisms. One of the most remarkable biochemical reactions for recovering metal ions from PLS is biogenic sulfide precipitation. Peculiar bacteria classified as “sulfate-reducing bacteria” (SRB) can produce biogenic sulfides. SRBs use sulfate ions in their metabolism and convert them to sulfides via sulfur. The following reactions demonstrate the process's basic transformations:
where Me2+ is the metal cation. This approach has shown promising results and a decent industrial application potential (Meshram et al., 2014). Bioprecipitation has been successfully employed in previous studies for the separation of metals from polymetallic PLS, either synthetic or real leachates (Esposito et al., 2006; Sethurajan et al., 2017). The microbial consortium consisted of a variety of bacteria, with Desulfovibrio spp. being the most prevalent was utilized by Calvert et al. for selective separation of metals from LIB leachate. At pH around 5, more than 92% of Al and Cu was removed in the sulfide form, and at pH = 10, about 99% of cobalt was precipitated with the co-precipitation of almost all Cd, Ni, Mn, and Zn (Calvert et al., 2019).
7.3 Other Methods
There are various other approaches for metal reclamation from LIB’s PLS, including electrodeposition, adsorption, and bioelectrochemical processes. Metals can be extracted from a solution through electrochemical deposition due to variations in electric potential. Recovery of Co from LIB leachate with electrodeposition has been studied by a few researchers (Duarte Castro et al., 2021; Quintero-Almanza et al., 2019). Wang et al. used manganese-type lithium ion sieves to conduct lithium extraction via adsorption and isolated 99.9% of Li from the PLS, including Li, Ni, and Co (Wang et al., 2017). Bioelectrochemical processes, often known as BES, are a subclass of techniques for recovering metals from aqueous solutions that are still in their infancy, although they are already gaining attention from researchers and developers. They were initially designed and put to use to improve the quality of wastewater, and it wasn't until recently that they were put to use for the recovery of metals from PLS (Nancharaiah et al., 2015). Investigations are currently being carried out in order to gain a deeper comprehension of the fundamentals behind the bio-recovery of cobalt from synthetic solutions by means of bioelectrochemical systems. The BES's performance in treating actual leachates, on the other hand, has yet to be shown (Huang et al., 2019b).
8 Future Prospectives and Conclusions
In the mineral processing and extractive metallurgy industry, bioleaching is a sustainable and environmentally friendly method for the extraction of metals from primary and secondary resources. This method has the potential to help save non-renewable energy sources and reduce greenhouse gas emissions. The lithium cobalt oxide (LiCoO2) serves as the primary and most significant component of LIBs. These batteries are complex, multi-metallic compounds with high concentrations of lithium, cobalt, manganese, nickel, copper, and aluminum (cathodic material). The elemental makeup of LIBs might change significantly depending on its application. Autotrophs and heterotrophs are the two types of microorganisms employed for the LIB bioleaching process. Through acidolysis and redoxolysis, metabolites generated by microorganisms, such as biogenic Fe3+ and H2SO4 in the case of autotrophs, and weak organic acid in the case of heterotrophs can leach metals from the active cathode materials. The time it takes to produce microorganisms can be significantly reduced by improving the growth conditions. In this chapter, we categorized the most influential parameters of the LIB bioleaching process with both autotrophic and heterotrophs microorganisms and their most dominant mechanisms. The most important drawbacks of the LIB bioleaching that prevents its industrial application were mentioned, and several approaches for process enhancement, including admission of catalysts, employing adapted or mixed cultures, sonobioleaching, and genetic engineering were summarized. With advancements in process kinetics, higher pulp density and improved microbial tolerance, there is potential for the application of bioleaching techniques in the industrial sector. The utilization of bioleaching has already yielded a notable leaching efficiency of 80–90%, indicating promising prospects for large-scale recycling of waste LIBs in the near future. The high pulp density of 100 g/L was used in this study. In spite of the numerous studies and new research that have been conducted recently, LIB bioleaching is still in its infancy. More study is necessary to improve the process's efficiency, kinetics, and selectivity. In subsequent research, the primary focus should be on determining how to best optimize operating parameters in order to make it easier to process large pulp densities and open the door to potential for industrial expansion. Furthermore, the biological recovery of key metals from the PLS is of significant importance for comprehensive recycling and regeneration and should be explored so that the necessary metals can be recovered selectively. Finally, the proper disposal and treatment of created tailings, process effluents, and sludge must be established.
References
Abdollahi, H., Noaparast, M., Shafaei, S. Z., Manafi, Z., Muñoz, J. A., & Tuovinen, O. H. (2015). Silver-catalyzed bioleaching of copper, molybdenum and rhenium from a chalcopyrite–molybdenite concentrate. International Biodeterioration and Biodegradation, 104, 194–200.
Abdollahi, H., Saneie, R., Shafaei, S. Z., Mirmohammadi, M., Mohammadzadeh, A., & Tuovinen, O. H. (2021). Bioleaching of cobalt from magnetite-rich cobaltite-bearing ore. Hydrometallurgy, 204, 105727. https://doi.org/10.1016/j.hydromet.2021.105727
Abhilash, Pandey, B. D., & Natarajan, K. A. (2015). Microbiology for minerals, metals, materials and the environment. CRC Press. https://doi.org/10.1201/b18124.
Akbari, S., & Ahmadi, A. (2019). Recovery of copper from a mixture of printed circuit boards (PCBs) and sulphidic tailings using bioleaching and solvent extraction processes. Chemical Engineering and Processing-Process Intensification, 142, 107584. https://doi.org/10.1016/j.cep.2019.107584
Akcil, A., Vegliò, F., Ferella, F., Okudan, M. D., & Tuncuk, A. (2015). A review of metal recovery from spent petroleum catalysts and ash. Waste Management, 45, 420–433. https://doi.org/10.1016/j.wasman.2015.07.007
Alavi, N., Partovi, K., Majlessi, M., Rashidi, M., & Alimohammadi, M. (2021). Bioleaching of metals from cellphones batteries by a co-fungus medium in presence of carbon materials. Bioresource Technology Reports, 15, 100768. https://doi.org/10.1016/j.biteb.2021.100768
Ambrose, H., & Kendall, A. (2020). Understanding the future of lithium: Part 1, resource model. Journal of Industrial Ecology, 24, 80–89. https://doi.org/10.1111/jiec.12949
Anjum, F., Shahid, M., Bukhari, S., & Potgieter, J. H. (2014). Combined ultrasonic and bioleaching treatment of hospital waste incinerator bottom ash with simultaneous extraction of selected metals. Environmental Technology, 35, 262–270. https://doi.org/10.1080/09593330.2013.824992
Arya, S., & Kumar, S. (2020). Bioleaching: urban mining option to curb the menace of E-waste challenge. Bioengineered, 11, 640–660. https://doi.org/10.1080/21655979.2020.1775988
Asghari, I., Mousavi, S. M., Amiri, F., & Tavassoli, S. (2013). Bioleaching of spent refinery catalysts: A review. Journal of Industrial and Engineering Chemistry, 19, 1069–1081. https://doi.org/10.1016/j.jiec.2012.12.005
Bahaloo-Horeh, N., Vakilchap, F., & Mousavi, S. M. (2019). Bio-hydrometallurgical methods for recycling spent lithium-ion batteries. https://doi.org/10.1007/978-3-030-31834-5_7.
Bahaloo-Horeh, N., & Mousavi, S. M. (2017). Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus Niger. Waste Management, 60, 666–679. https://doi.org/10.1016/j.wasman.2016.10.034
Bahaloo-Horeh, N., Mousavi, S. M., & Baniasadi, M. (2018). Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. Journal of Cleaner Production, 197, 1546–1557. https://doi.org/10.1016/j.jclepro.2018.06.299
Baker, P. G., & Bishop, P. L. (1997). Prediction of metal leaching rates from solidified/stabilized wastes using the shrinking unreacted core leaching procedure. Journal of Hazardous Materials, 52, 311–333. https://doi.org/10.1016/S0304-3894(96)01814-6
Baker-Austin, C., & Dopson, M. (2007). Life in acid: pH homeostasis in acidophiles. Trends in Microbiology, 15, 165–171. https://doi.org/10.1016/j.tim.2007.02.005
Bardi, U., Jakobi, R., & Hettiarachchi, H. (2016). Mineral resource depletion: A coming age of stockpiling? BioPhysical Economics and Resource Quality, 1, 4. https://doi.org/10.1007/s41247-016-0004-x
Bharadwaj, A., & Ting, Y. P. (2013). Bioleaching of spent hydrotreating catalyst by acidophilic thermophile Acidianus brierleyi: Leaching mechanism and effect of decoking. Bioresource Technology, 130, 673–680. https://doi.org/10.1016/j.biortech.2012.12.047
Biswal, B. K., Jadhav, U. U., Madhaiyan, M., Ji, L., Yang, E. H., & Cao, B. (2018). Biological leaching and chemical precipitation methods for recovery of co and li from spent lithium-ion batteries. ACS Sustainable Chemistry and Engineering., 6, 12343–12352. https://doi.org/10.1021/acssuschemeng.8b02810
Bosecker, K. (1997). Bioleaching: Metal solubilization by microorganisms. FEMS Microbiology Reviews, 20, 591–604. https://doi.org/10.1016/S0168-6445(97)00036-3
Boxall, N. J., Cheng, K. Y., Bruckard, W., & Kaksonen, A. H. (2018). Application of indirect non-contact bioleaching for extracting metals from waste lithium-ion batteries. Journal of Hazardous Materials, 360, 504–511. https://doi.org/10.1016/j.jhazmat.2018.08.024
Brandl, H., Bosshard, R., & Wegmann, M. (1999). Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi, Process. Metallurgy, 9, 569–576. https://doi.org/10.1016/S1572-4409(99)80146-1
Brandl, H., & Faramarzi, M. A. (2006). Microbe-metal-interactions for the biotechnological treatment of metal-containing solid waste. China Particuology, 4, 93–97. https://doi.org/10.1016/S1672-2515(07)60244-9
Cai, X., Tian, L., Chen, C., Huang, W., Yu, Y., Liu, C., Yang, B., Lu, X., & Mao, Y. (2021). Phylogenetically divergent bacteria consortium from neutral activated sludge showed heightened potential on bioleaching spent lithium-ion batteries. Ecotoxicology and Environmental Safety, 223, 112592. https://doi.org/10.1016/j.ecoenv.2021.112592
Calvert, G., Kaksonen, A. H., Cheng, K. Y., Van Yken, J., Chang, B., Boxall, N. J. (2019). Recovery of metals from waste lithium ion battery leachates using biogenic hydrogen sulfide. Minerals 9. https://doi.org/10.3390/min9090563.
Chen, W. –S., & Ho, H. -J. (2018). Recovery of valuable metals from lithium-ion batteries NMC cathode waste materials by hydrometallurgical methods. Metals 8 (2018). https://doi.org/10.3390/met8050321.
Chen, K., & Xue, D. (2014). Anode performances of mixed LiMn2O4 and carbon black toward lithium-ion battery. Functional Materials Letters, 07, 1450017. https://doi.org/10.1142/S1793604714500179
Chen, M., Ma, X., Chen, B., Arsenault, R., Karlson, P., Simon, N., & Wang, Y. (2019). Recycling end-of-life electric vehicle lithium-ion batteries. Joule., 3, 2622–2646. https://doi.org/10.1016/j.joule.2019.09.014
Deng, X., Chai, L., Yang, Z., Tang, C., Wang, Y., & Shi, Y. (2013). Bioleaching mechanism of heavy metals in the mixture of contaminated soil and slag by using indigenous Penicillium chrysogenum strain F1. Journal of Hazardous Materials, 248–249, 107–114. https://doi.org/10.1016/j.jhazmat.2012.12.051
Deng, Z., Mo, Y., & Ong, S. P. (2016). Computational studies of solid-state alkali conduction in rechargeable alkali-ion batteries. NPG Asia Materials, 8, 1–15. https://doi.org/10.1038/am.2016.7
Dickinson, C. F., & Heal, G. R. (1999). Solid–liquid diffusion controlled rate equations. Thermochimica Acta, 340–341, 89–103. https://doi.org/10.1016/S0040-6031(99)00256-7
Dopson, M., & Holmes, D. S. (2014). Metal resistance in acidophilic microorganisms and its significance for biotechnologies. Applied Microbiology and Biotechnology, 98, 8133–8144. https://doi.org/10.1007/s00253-014-5982-2
Duarte Castro, F., Vaccari, M., & Cutaia, L. (2021). Valorization of resources from end-of-life lithium-ion batteries: A review. Critical Reviews in Environmental Science and Technology, pp. 1–44. https://doi.org/10.1080/10643389.2021.1874854.
Dusengemungu, L., Kasali, G., Gwanama, C., Ouma, K. O. (2020). Recent advances in biosorption of copper and cobalt by filamentous fungi. Frontiers in Microbiology 11. https://doi.org/10.3389/fmicb.2020.582016.
Dusengemungu, L., Kasali, G., Gwanama, C., & Mubemba, B. (2021). Overview of fungal bioleaching of metals. Environmental Advances, 5, 100083. https://doi.org/10.1016/j.envadv.2021.100083
Esposito, G., Veeken, A., Weijma, J., & Lens, P. N. L. (2006). Use of biogenic sulfide for ZnS precipitation. Separation and Purification Technology, 51, 31–39. https://doi.org/10.1016/j.seppur.2005.12.021
Faraji, F., Golmohammadzadeh, R., Rashchi, F., & Alimardani, N. (2018). Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics. Journal of Environmental Management, 217, 775–787. https://doi.org/10.1016/j.jenvman.2018.04.043
Fergus, J. W. (2010). Recent developments in cathode materials for lithium ion batteries. Journal of Power Sources, 195, 939–954. https://doi.org/10.1016/j.jpowsour.2009.08.089
Ghassa, S., Farzanegan, A., Gharabaghi, M., & Abdollahi, H. (2020). Novel bioleaching of waste lithium ion batteries by mixed moderate thermophilic microorganisms, using iron scrap as energy source and reducing agent. Hydrometallurgy, 197, 105465. https://doi.org/10.1016/j.hydromet.2020.105465
Ghassa, S., Noaparast, M., Shafaei, S. Z., Abdollahi, H., Gharabaghi, M., & Boruomand, Z. (2017). A study on the zinc sulfide dissolution kinetics with biological and chemical ferric reagents. Hydrometallurgy, 171, 362–373. https://doi.org/10.1016/j.hydromet.2017.06.012
Golmohammadzadeh, R., Faraji, F., & Rashchi, F. (2018). Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resources, Conservation and Recycling, 136, 418–435. https://doi.org/10.1016/j.resconrec.2018.04.024
Gu, T., Rastegar, S. O., Mousavi, S. M., Li, M., & Zhou, M. (2018). Advances in bioleaching for recovery of metals and bioremediation of fuel ash and sewage sludge. Bioresource Technology, 261, 428–440. https://doi.org/10.1016/j.biortech.2018.04.033
Gumulya, Y., Boxall, N. J., Khaleque, H. N., Santala, V., Carlson, R. P., & Kaksonen, A. H. (2018). In a quest for engineering acidophiles for biomining applications: Challenges and opportunities. Genes 9. https://doi.org/10.3390/genes9020116.
Habib, K., Hamelin, L., & Wenzel, H. (2016). A dynamic perspective of the geopolitical supply risk of metals. Journal of Cleaner Production, 133, 850–858. https://doi.org/10.1016/j.jclepro.2016.05.118
Hamad, H. O., Alma, M. H., Ismael, H. M., & Göçeri, A. (2015). The effect of some sugars on the growth of Aspergillus niger. Kahramanmaraş Sütçü İmam Üniversitesi Doğa Bilimleri Dergisi 17, 7. https://doi.org/10.18016/ksujns.28479.
Hamidah, N. L., Nugroho, G., & Wang, F. M. (2015). Electrochemical Analysis of Electrolyte Additive Effect on Ionic Diffusion for High-Performance Lithium Ion Battery. https://doi.org/10.1007/s11581-015-1505-0
Hartono, M., Astrayudha, M. A., Petrus, H. T. B. M., Budhijanto, W., & Sulistyo, H. (2017). Lithium recovery of spent lithium-ion battery using bioleaching from local sources microorganism. Rasayan Journal of Chemistry, 10, 897–903. https://doi.org/10.7324/RJC.2017.1031767
Harwood, C. V., Ph, D., Cunningham, C. J., Ph, D., Scott, K., Ph, D. (2017). Bioleaching potential of filamentous fungi to mobilize lithium and cobalt from spent rechargeable li-ion batteries by aldo lobos a thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in integrative biology with
He, L.-P., Sun, S.-Y., Song, X.-F., & Yu, J.-G. (2015). Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning. Waste Management, 46, 523–528. https://doi.org/10.1016/j.wasman.2015.08.035
Heydarian, A., Mousavi, S. M., Vakilchap, F., & Baniasadi, M. (2018). Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. Journal of Power Sources, 378, 19–30. https://doi.org/10.1016/J.JPOWSOUR.2017.12.009
Horeh, N. B., Mousavi, S. M., & Shojaosadati, S. A. (2016). Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. Journal of Power Sources, 320, 257–266. https://doi.org/10.1016/J.JPOWSOUR.2016.04.104
Huang, L., Zhou, Q., & Quan, X. (2019). Recovery of metals from wastes using bioelectrochemical systems BT—bioelectrochemistry stimulated environmental remediation: From bioelectrorespiration to bioelectrodegradation. In A.-J. Wang, B. Liang, Z.-L. Li, & H. -Y. Cheng (Eds.) (pp. 121–156). Springer. https://doi.org/10.1007/978-981-10-8542-0_6.
Huang, T., Liu, L., & Zhang, S. (2019a). Recovery of cobalt, lithium, and manganese from the cathode active materials of spent lithium-ion batteries in a bio-electro-hydrometallurgical process. Hydrometallurgy, 188, 101–111. https://doi.org/10.1016/j.hydromet.2019.06.011
İkiz, D., Gülfen, M., Aydın, A. O. (2006). Dissolution kinetics of primary chalcopyrite ore in hypochlorite solution. Minerals Engineering 19, 972–974. https://doi.org/10.1016/j.mineng.2005.09.047.
Iram, S., Shabbir, R., Zafar, H., & Javaid, M. (2015). Biosorption and Bioaccumulation of Copper and Lead by Heavy Metal-Resistant Fungal Isolates. Arabian Journal for Science and Engineering, 40, 1867–1873. https://doi.org/10.1007/s13369-015-1702-1
Irrgang, N., Monneron-Enaud, B., Möckel, R., Schlömann, M., & Höck, M. (2021). Economic feasibility of the co-production of indium from zinc sulphide using bioleaching extraction in Germany. Hydrometallurgy, 200, 105566. https://doi.org/10.1016/j.hydromet.2021.105566
Işıldar, A., van Hullebusch, E. D., Lenz, M., Du Laing, G., Marra, A., Cesaro, A., Panda, S., Akcil, A., Kucuker, M. A., & Kuchta, K. (2019). Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. Journal of Hazardous Materials, 362, 467–481. https://doi.org/10.1016/j.jhazmat.2018.08.050
Islam, A., Ahmed, T., Awual, M. R., Rahman, A., Sultana, M., Aziz, A. A., Monir, M. U., Teo, S. H., & Hasan, M. (2020). Advances in sustainable approaches to recover metals from e-waste-a review. Journal of Cleaner Production, 244, 118815. https://doi.org/10.1016/j.jclepro.2019.118815
Jafari, M., Abdollahi, H., Shafaei, S. Z., Gharabaghi, M., Jafari, H., Akcil, A., & Panda, S. (2019). Acidophilic bioleaching: A review on the process and effect of organic–inorganic reagents and materials on its efficiency. Mineral Processing and Extractive Metallurgy Review, 40, 87–107. https://doi.org/10.1080/08827508.2018.1481063
Jafari, S., Aghaei, S. S., Afifi-Sabet, H., Shams-Ghahfarokhi, M., Jahanshiri, Z., Gholami-Shabani, M., Shafiei-Darabi, S., & Razzaghi-Abyaneh, M. (2018). Exploration, antifungal and antiaflatoxigenic activity of halophilic bacteria communities from saline soils of Howze-Soltan playa in Iran. Extremophiles, 22, 87–98. https://doi.org/10.1007/s00792-017-0979-2
Johnson, D. B. (2014). Biomining—biotechnologies for extracting and recovering metals from ores and waste materials. Current Opinion in Biotechnology, 30, 24–31. https://doi.org/10.1016/j.copbio.2014.04.008
Johnson, D. (2018). The Evolution current status, and future prospects of using biotechnologies in the mineral extraction and metal recovery sectors. Minerals, 8, 343. https://doi.org/10.3390/min8080343
Kang, J., Senanayake, G., Sohn, J., & Shin, S. M. (2010). Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy, 100, 168–171. https://doi.org/10.1016/j.hydromet.2009.10.010
Kapoor, A., & Viraraghavan, T. (1995). Fungal biosorption—an alternative treatment option for heavy metal bearing wastewaters: A review. Bioresource Technology, 53, 195–206. https://doi.org/10.1016/0960-8524(95)00072-M
Keller, A., Hlawitschka, M. W., & Bart, H. –J. (2021). Application of saponified D2EHPA for the selective extraction of manganese from spend lithium-ion batteries. Chemical Engineering and Processing-Process Intensification 108552. https://doi.org/10.1016/j.cep.2021.108552.
Khatri, B. R., Tipre, D. R., & Dave, S. R. (2019). Comparison of hydro- and biohydrometallurgical extraction of metals from waste li-ion batteries of cell phone. Journal of Sustainable Metallurgy, 5, 250–261. https://doi.org/10.1007/s40831-019-00223-z
Kim, J.H., Gibb, H.J., & Howe, P.D. (2006). Cobalt and inorganic cobalt compounds, IPCS Concise Int. Chem. Assess. Doc, pp. 1–82.
Kim, S., Bang, J., Yoo, J., Shin, Y., Bae, J., Jeong, J., Kim, K., Dong, P., & Kwon, K. (2021). A comprehensive review on the pretreatment process in lithium-ion battery recycling. Journal of Cleaner Production, 294, 126329. https://doi.org/10.1016/j.jclepro.2021.126329
Ku, H., Jung, Y., Jo, M., Park, S., Kim, S., Yang, D., Rhee, K., An, E.-M., Sohn, J., & Kwon, K. (2016). Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. Journal of Hazardous Materials, 313, 138–146. https://doi.org/10.1016/J.JHAZMAT.2016.03.062
Kwon, S. J., Lee, S. E., Lim, J. H., Choi, J., & Kim, J. (2018). Performance and life degradation characteristics analysis of NCM LIB for bess. Electronics, 7, 1–19. https://doi.org/10.3390/electronics7120406
Lai, X., Huang, Y., Gu, H., Deng, C., Han, X., Feng, X., & Zheng, Y. (2021). Turning waste into wealth: A systematic review on echelon utilization and material recycling of retired lithium-ion batteries. Energy Storage Materials, 40, 96–123. https://doi.org/10.1016/j.ensm.2021.05.010
Lavoie, Y., Danet, F., & Lombard, B., Lithium-ion batteries for industrial applications. In 2017 petroleum and chemical industry technical conference (pp. 283–290). IEEE. https://doi.org/10.1109/PCICON.2017.8188747.
Le, L., Tang, J., Ryan, D., & Valix, M. (2006). Bioleaching nickel laterite ores using multi-metal tolerant Aspergillus foetidus organism. Minerals Engineering, 19, 1259–1265. https://doi.org/10.1016/j.mineng.2006.02.006
Lei, S., Sun, W., & Yang, Y. (2022). Solvent extraction for recycling of spent lithium-ion batteries. Journal of Hazardous Materials, 424, 127654. https://doi.org/10.1016/j.jhazmat.2021.127654
O. Levenspiel, Chemical reaction engineering, John wiley & sons, 1998.
Li, L., Zeng, G. S., Luo, S. L., Deng, X. R., & Xie, Q. J. (2013). Influences of solution pH and redox potential on the bioleaching of LiCoO2 from spent lithium-ion batteries. Journal of the Korean Society for Applied Biological Chemistry, 56, 187–192. https://doi.org/10.1007/s13765-013-3016-x
Liang, Y., Su, J., Xi, B., Yu, Y., Ji, D., Sun, Y., Cui, C., & Zhu, J. (2017). Life cycle assessment of lithium-ion batteries for greenhouse gas emissions. Resources, Conservation and Recycling, 117, 285–293. https://doi.org/10.1016/j.resconrec.2016.08.028
Liao, X., Ye, M., Li, S., Liang, J., Zhou, S., Fang, X., Gan, Q., & Sun, S. (2021). Simultaneous recovery of valuable metal ions and tailings toxicity reduction using a mixed culture bioleaching process. Journal of Cleaner Production, 316, 128319. https://doi.org/10.1016/j.jclepro.2021.128319
P. Liu, L. Xiao, Y. Chen, H. Chen, Highly enhanced electrochemical performances of LiNi0.815Co0.15Al0.035O2 by coating via conductively LiTiO2 for lithium-ion batteries, Ceram. Int. 45 (2019) 18398–18405. https://doi.org/10.1016/j.ceramint.2019.06.055.
Magnuson, J. K., Lasure, L. L. (2004). Organic acid production by filamentous fungi. In Advances in fungal biotechnology for industry, agriculture, and medicine (pp. 307–340). Springer. https://doi.org/10.1007/978-1-4419-8859-1_12.
Makuza, B., Tian, Q., Guo, X., Chattopadhyay, K., & Yu, D. (2021). Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. Journal of Power Sources, 491, 229622. https://doi.org/10.1016/j.jpowsour.2021.229622
Mekonnen, Y., Sundararajan, A., & Sarwat, A. I. (2016). A review of cathode and anode materials for lithium-ion batteries. In SoutheastCon 2016, IEEE, pp. 1–6. https://doi.org/10.1109/SECON.2016.7506639.
Meshram, P., Pandey, B. D., & Mankhand, T. R. (2014). Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy, 150, 192–208. https://doi.org/10.1016/j.hydromet.2014.10.012
R. Methekar, S. Anwani, Manufacturing of Lithium Cobalt Oxide from Spent Lithium-Ion Batteries: A Cathode Material, in: 2019: pp. 233–241. https://doi.org/10.1007/978-981-13-1966-2_20.
Mishra, D., Kim, D. J., Ralph, D. E., Ahn, J. G., & Rhee, Y. H. (2008). Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Management, 28, 333–338. https://doi.org/10.1016/j.wasman.2007.01.010
Mishra, D., & Rhee, Y. H. (2014). Microbial leaching of metals from solid industrial wastes. Journal of Microbiology, 52, 1–7. https://doi.org/10.1007/s12275-014-3532-3
Moh, K., Aung, M., & Ting, Y. (2005). Bioleaching of spent fluid catalytic cracking catalyst using Aspergillus niger 116, 159–170. https://doi.org/10.1016/j.jbiotec.2004.10.008.
Nancharaiah, Y. V., Venkata Mohan, S., & Lens, P. N. L. (2015). Metals removal and recovery in bioelectrochemical systems: A review. Bioresource Technology, 195, 102–114. https://doi.org/10.1016/j.biortech.2015.06.058
Naseri, T., Bahaloo-Horeh, N., & Mousavi, S. M. (2019). Bacterial leaching as a green approach for typical metals recovery from end-of-life coin cells batteries. Journal of Cleaner Production, 220, 483–492. https://doi.org/10.1016/j.jclepro.2019.02.177
Naseri, T., Bahaloo-horeh, N., & Mousavi, S. M. (2020). Bacterial leaching as a green approach for typical metals recovery from end-of-life coin cells batteries. Journal of Cleaner Production, 220, 483–492. https://doi.org/10.1016/j.jclepro.2019.02.177
Natarajan, K. A., & Das, A. (2003). Surface chemical studies on “Acidithiobacillus” group of bacteria with reference to mineral flocculation. International Journal of Mineral Processing, 72, 189–198. https://doi.org/10.1016/S0301-7516(03)00098-X
Niu, Z., Huang, Q., Wang, J., Yang, Y., Xin, B., & Chen, S. (2015). Metallic ions catalysis for improving bioleaching yield of Zn and Mn from spent Zn-Mn batteries at high pulp density of 10%. Journal of Hazardous Materials, 298, 170–177.
Niu, Z., Zou, Y., Xin, B., Chen, S., Liu, C., & Li, Y. (2014). Process controls for improving bioleaching performance of both Li and Co from spent lithium ion batteries at high pulp density and its thermodynamics and kinetics exploration. Chemosphere, 109, 92–98. https://doi.org/10.1016/j.chemosphere.2014.02.059
Norris, P. R. (1997). Thermophiles and bioleaching BT—biomining: Theory, microbes and industrial processes. In D. E. Rawlings (Ed.) (pp. 247–258). Springer. https://doi.org/10.1007/978-3-662-06111-4_12.
Olivetti, E. A., Ceder, G., Gaustad, G. G., & Fu, X. (2017). Lithium-Ion battery supply chain considerations: Analysis of potential bottlenecks in critical metals. Joule., 1, 229–243. https://doi.org/10.1016/j.joule.2017.08.019
Ong, G. H., Ho, X. H., Shamkeeva, S., Fernando, A. S. M. S., & Wong, L. S. (2017). Biosorption study of potential fungi for copper remediation from Peninsular Malaysia. Remediation Journal, 27, 59–63. https://doi.org/10.1002/rem.21531
Ordoñez, J., Gago, E. J., & Girard, A. (2016). Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renewable and Sustainable Energy Reviews, 60, 195–205. https://doi.org/10.1016/j.rser.2015.12.363
Padilla, R., Pavez, P., & Ruiz, M. C. (2008). Kinetics of copper dissolution from sulfidized chalcopyrite at high pressures in H2SO4–O2. Hydrometallurgy, 91, 113–120.
Pathak, A., Morrison, L., & Healy, M. G. (2017). Catalytic potential of selected metal ions for bioleaching, and potential techno-economic and environmental issues: A critical review. Bioresource Technology, 229, 211–221. https://doi.org/10.1016/j.biortech.2017.01.001
Pollmann, K., Kutschke, S., Matys, S., Kostudis, S., Hopfe, S., & Raff, J. (2016). Novel biotechnological approaches for the recovery of metals from primary and secondary resources. Minerals, 6, 54. https://doi.org/10.3390/min6020054
Punt, T., Akdogan, G., Bradshaw, S., & van Wyk, P. (2021). Development of a novel solvent extraction process using citric acid for lithium-ion battery recycling. Minerals Engineering, 173, 107204. https://doi.org/10.1016/j.mineng.2021.107204
Quatrini, R., & Johnson, D. B. (2019). Acidithiobacillus ferrooxidans. Trends in Microbiology, 27, 282–283. https://doi.org/10.1016/j.tim.2018.11.009
Quintero-Almanza, D., Gamiño-Arroyo, Z., Sánchez-Cadena, L. E., Gómez-Castro, F. I., Uribe-Ramírez, A. R., Aguilera-Alvarado, A. F., & Ocampo Carmona, L. M. (2019). Recovery of cobalt from spent lithium-ion mobile phone batteries using liquid–liquid extraction. Batteries 5. https://doi.org/10.3390/batteries5020044.
Rawlings, D. E. (1997). Mesophilic, autotrophic bioleaching bacteria: Description, physiology and role. Biomining, pp. 229–245. https://doi.org/10.1007/978-3-662-06111-4_11.
Rohwerder, T., Gehrke, T., Kinzler, K., & Sand, W. (2003). Bioleaching review part A. Applied Microbiology and Biotechnology, 63, 239–248. https://doi.org/10.1007/s00253-003-1448-7
Roy, J. J., Cao, B., & Madhavi, S. (2021a). A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere, 282, 130944. https://doi.org/10.1016/j.chemosphere.2021.130944
Roy, J. J., Madhavi, S., & Cao, B. (2021b). Metal extraction from spent lithium-ion batteries (LIBs) at high pulp density by environmentally friendly bioleaching process. Journal of Cleaner Production, 280(2021), 124242. https://doi.org/10.1016/j.jclepro.2020.124242
Roy, J. J., Srinivasan, M., & Cao, B. (2021c). Bioleaching as an eco-friendly approach for metal recovery from spent nmc-based lithium-ion batteries at a high pulp density. ACS Sustainable Chemistry and Engineering, 9, 3060–3069. https://doi.org/10.1021/acssuschemeng.0c06573
Saneie, R., Abdollahi, H., Shafaei, S. Z., & Mohammadzadeh, A. (2021). Inhibitory effect of solvent extractants on growth and metabolism of acidophiles. Mineral Processing and Extractive Metallurgy Review, pp. 1–12. https://doi.org/10.1080/08827508.2021.1971664.
Sethurajan, M., & Gaydardzhiev, S. (2021). Bioprocessing of spent lithium ion batteries for critical metals recovery—A review. Resources, Conservation and Recycling, 165, 105225. https://doi.org/10.1016/j.resconrec.2020.105225
Sethurajan, M., Lens, P. N. L., Rene, E. R., van de Vossenberg, J., Huguenot, D., Horn, H. A., Figueiredo, L. H. A., & van Hullebusch, E. D. (2017). Bioleaching and selective biorecovery of zinc from zinc metallurgical leach residues from the Três Marias zinc plant (Minas Gerais, Brazil). Journal of Chemical Technology and Biotechnology, 92, 512–521. https://doi.org/10.1002/jctb.5026
Shamsuddin, M. (2021). Hydrometallurgy BT—physical chemistry of metallurgical processes, Second Edition. In M. Shamsuddin (Ed.) (pp. 429–529). Springer International Publishing. https://doi.org/10.1007/978-3-030-58069-8_11.
Shin, S.M., Kim, N.H., Sohn, J.S., Yang, D.H., & Kim, Y.H. (2005) Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 79, 172–181. https://doi.org/10.1016/j.hydromet.2005.06.004
Shuya, L., Yang, C., Xuefeng, C., Wei, S., Yaqing, W., & Yue, Y. (2020). Separation of lithium and transition metals from leachate of spent lithium-ion batteries by solvent extraction method with Versatic 10. Separation and Purification Technology, 250, 117258. https://doi.org/10.1016/j.seppur.2020.117258
Sierra-Alvarez, R. (2007). Fungal bioleaching of metals in preservative-treated wood. Process Biochemistry, 42, 798–804. https://doi.org/10.1016/j.procbio.2007.01.019
Simate, G. S., Ndlovu, S., & Walubita, L. F. (2010). The fungal and chemolithotrophic leaching of nickel laterites—Challenges and opportunities. Hydrometallurgy, 103, 150–157. https://doi.org/10.1016/j.hydromet.2010.03.012
Sokić, M. D., Marković, B., & Živković, D. (2009). Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy, 95, 273–279. https://doi.org/10.1016/j.hydromet.2008.06.012
Sommer, P., Rotter, V. S., & Ueberschaar, M. (2015). Battery related cobalt and REE flows in WEEE treatment. Waste Management, 45, 298–305. https://doi.org/10.1016/j.wasman.2015.05.009
Srichandan, H., Mohapatra, R. K., Parhi, P. K., & Mishra, S. (2019). Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy, 189, 105122. https://doi.org/10.1016/j.hydromet.2019.105122
Srichandan, H., Pathak, A., Singh, S., Blight, K., Kim, D.-J., & Lee, S. W. (2014). Sequential leaching of metals from spent refinery catalyst in bioleaching–bioleaching and bioleaching–chemical leaching reactor: Comparative study. Hydrometallurgy, 150, 130–143. https://doi.org/10.1016/j.hydromet.2014.09.019
Srivastava, R. R., Ilyas, S., Kim, H., Choi, S., Trinh, H. B., Ghauri, M. A., & Ilyas, N. (2020). Biotechnological recycling of critical metals from waste printed circuit boards. Journal of Chemical Technology and Biotechnology, 95, 2796–2810. https://doi.org/10.1002/jctb.6469
Subhan, A., Oemry, F., Khusna, S. N., & Hastuti, E. (2019). Effects of activated carbon treatment on Li4Ti5O12 anode material synthesis for lithium-ion batteries. Ionics (kiel), 25, 1025–1034. https://doi.org/10.1007/s11581-018-2633-0
Tanskanen, P. (2013). Management and recycling of electronic waste. Acta Materials, 61, 1001–1011. https://doi.org/10.1016/j.actamat.2012.11.005
Tarascon, J.-M., & Armand, M. (2001). Issues and challenges facing rechargeable lithium batteries. Nature, 414, 359–367. https://doi.org/10.1038/35104644
Torkaman, R., Asadollahzadeh, M., Torab-Mostaedi, M., & Maragheh, M. G. (2017). Recovery of cobalt from spent lithium ion batteries by using acidic and basic extractants in solvent extraction process. Separation and Purification Technology., 186, 318–325. https://doi.org/10.1016/j.seppur.2017.06.023
Vakilchap, F., Mousavi, S. M., & Shojaosadati, S. A. (2016). Role of Aspergillus niger in recovery enhancement of valuable metals from produced red mud in Bayer process. Bioresource Technology, 218, 991–998. https://doi.org/10.1016/j.biortech.2016.07.059
Vanitha, M., & Balasubramanian, N. (2013). Waste minimization and recovery of valuable metals from spent lithium-ion batteries—a review. Environmental Technology Review, 2, 101–115. https://doi.org/10.1080/21622515.2013.853105
Vargas, L. H. M., Pião, A. C. S., Domingos, R. N., & Carmona, E. C. (2004). Ultrasound effects on invertase from Aspergillus niger. World Journal of Microbiology and Biotechnology, 20, 137–142. https://doi.org/10.1023/B:WIBI.0000021726.69233.12
Versaci, D., Nasi, R., Zubair, U., Amici, J., Sgroi, M., Dumitrescu, M. A., Francia, C., Bodoardo, S., & Penazzi, N. (2017). New eco-friendly low-cost binders for Li-ion anodes. Journal of Solid State Electrochemistry, 21, 3429–3435. https://doi.org/10.1007/s10008-017-3665-5
Vikström, H., Davidsson, S., & Höök, M. (2013). Lithium availability and future production outlooks. Applied Energy, 110, 252–266. https://doi.org/10.1016/j.apenergy.2013.04.005
Vyas, S., & Ting, Y. -P. (2018). A review of the application of ultrasound in bioleaching and insights from sonication in (Bio)chemical processes. Resources 7. https://doi.org/10.3390/resources7010003.
Walker, G. M., White, N. A. (2018). Introduction to fungal physiology, pp. 1–35.
Wang, H., Huang, K., Zhang, Y., Chen, X., Jin, W., Zheng, S., Zhang, Y., & Li, P. (2017). Recovery of lithium, nickel, and cobalt from spent lithium-ion battery powders by selective ammonia leaching and an adsorption separation system. ACS Sustainable Chemistry and Engineering, 5, 11489–11495. https://doi.org/10.1021/acssuschemeng.7b02700
Wang, J., Tian, B., Bao, Y., Qian, C., Yang, Y., Niu, T., & Xin, B. (2018). Functional exploration of extracellular polymeric substances (EPS) in the bioleaching of obsolete electric vehicle LiNixCoyMn1-x-yO2 Li-ion batteries. Journal of Hazardous Materials, 354, 250–257. https://doi.org/10.1016/j.jhazmat.2018.05.009
Wang, X., Chen, J., Yan, X., Wang, X., Zhang, J., Huang, J., & Zhao, J. (2015). Heavy metal chemical extraction from industrial and municipal mixed sludge by ultrasound-assisted citric acid. Journal of Industrial and Engineering Chemistry, 27, 368–372. https://doi.org/10.1016/j.jiec.2015.01.016
Wang, X., Gaustad, G., Babbitt, C. W., Bailey, C., Ganter, M. J., & Landi, B. J. (2014a). Economic and environmental characterization of an evolving Li-ion battery waste stream. Journal of Environmental Management, 135, 126–134. https://doi.org/10.1016/j.jenvman.2014.01.021
Wang, Y., Su, L., Zeng, W., Wan, L., Chen, Z., Zhang, L., Qiu, G., Chen, X., & Zhou, H. (2014b). Effect of pulp density on planktonic and attached community dynamics during bioleaching of chalcopyrite by a moderately thermophilic microbial culture under uncontrolled conditions. Minerals Engineering, 61, 66–72. https://doi.org/10.1016/j.mineng.2014.03.012
Windisch-Kern, S., Gerold, E., Nigl, T., Jandric, A., Altendorfer, M., Rutrecht, B., Scherhaufer, S., Raupenstrauch, H., Pomberger, R., Antrekowitsch, H., & Part, F. (2022). Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Management, 138, 125–139. https://doi.org/10.1016/j.wasman.2021.11.038
Wu, H.-Y., & Ting, Y.-P. (2006). Metal extraction from municipal solid waste (MSW) incinerator fly ash—Chemical leaching and fungal bioleaching. Enzyme and Microbial Technology, 38, 839–847. https://doi.org/10.1016/j.enzmictec.2005.08.012
Wu, W., Liu, X., Zhang, X., Li, X., Qiu, Y., Zhu, M., & Tan, W. (2019). Mechanism underlying the bioleaching process of LiCoO2 by sulfur-oxidizing and iron-oxidizing bacteria. Journal of Bioscience and Bioengineering, 128, 344–354. https://doi.org/10.1016/j.jbiosc.2019.03.007
Xia, M., Bao, P., Liu, A., Wang, M., Shen, L., Yu, R., Liu, Y., Chen, M., Li, J., Wu, X., Qiu, G., & Zeng, W. (2018). Bioleaching of low-grade waste printed circuit boards by mixed fungal culture and its community structure analysis. Resources, Conservation and Recycling, 136, 267–275. https://doi.org/10.1016/j.resconrec.2018.05.001
Xiang, Y., Wu, P., Zhu, N., Zhang, T., Liu, W., Wu, J., & Li, P. (2010). Bioleaching of copper from waste printed circuit boards by bacterial consortium enriched from acid mine drainage. Journal of Hazardous Materials, 184, 812–818. https://doi.org/10.1016/j.jhazmat.2010.08.113
Xin, B., Jiang, W., Aslam, H., Zhang, K., Liu, C., Wang, R., & Wang, Y. (2012b). Bioleaching of zinc and manganese from spent Zn-Mn batteries and mechanism exploration. Bioresource Technology, 106, 147–153. https://doi.org/10.1016/j.biortech.2011.12.013
Xin, B., Jiang, W., Li, X., Zhang, K., Liu, C., Wang, R., & Wang, Y. (2012a). Analysis of reasons for decline of bioleaching efficiency of spent Zn-Mn batteries at high pulp densities and exploration measure for improving performance. Bioresource Technology, 112, 186–192. https://doi.org/10.1016/j.biortech.2012.02.133
Xin, B., Zhang, D., Zhang, X., Xia, Y., Wu, F., Chen, S., & Li, L. (2009). Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria. Bioresource Technology, 100, 6163–6169. https://doi.org/10.1016/j.biortech.2009.06.086
Xin, Y., Guo, X., Chen, S., Wang, J., Wu, F., & Xin, B. (2016). Bioleaching of valuable metals Li Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery. Journal of Cleaner Production, 116, 249–258. https://doi.org/10.1016/j.jclepro.2016.01.001
Xu, J., Thomas, H. R., Francis, R. W., Lum, K. R., Wang, J., & Liang, B. (2008). A review of processes and technologies for the recycling of lithium-ion secondary batteries. Journal of Power Sources, 177, 512–527. https://doi.org/10.1016/J.JPOWSOUR.2007.11.074
Xu, L., Chen, C., & Fu, M.-L. (2020). Separation of cobalt and lithium from spent lithium-ion battery leach liquors by ionic liquid extraction using Cyphos IL-101. Hydrometallurgy, 197, 105439. https://doi.org/10.1016/j.hydromet.2020.105439
Xu, T.-J., Ramanathan, T., & Ting, Y.-P. (2014). Bioleaching of incineration fly ash by Aspergillus niger—precipitation of metallic salt crystals and morphological alteration of the fungus. Biotechnology Reports, 3, 8–14. https://doi.org/10.1016/j.btre.2014.05.009
Xu, W., Wang, Z., Shi, L., Ma, Y., Yuan, S., Sun, L., Zhao, Y., Zhang, M., & Zhu, J. (2015). Layer-by-layer deposition of organic-inorganic hybrid multilayer on microporous polyethylene separator to enhance the electrochemical performance of lithium-ion battery. ACS Applied Materials and Interfaces, 7, 20678–20686. https://doi.org/10.1021/acsami.5b05457
Yu, Z., Han, H., Feng, P., Zhao, S., Zhou, T., Kakade, A., Kulshrestha, S., Majeed, S., & Li, X. (2020). Recent advances in the recovery of metals from waste through biological processes. Bioresource Technology, 297, 122416. https://doi.org/10.1016/j.biortech.2019.122416
Zeng, G., Deng, X., Luo, S., Luo, X., & Zou, J. (2012). A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries. Journal of Hazardous Materials, 199–200, 164–169. https://doi.org/10.1016/j.jhazmat.2011.10.063
Zeng, G., Luo, S., Deng, X., Li, L., & Au, C. (2013a). Influence of silver ions on bioleaching of cobalt from spent lithium batteries. Minerals Engineering, 49, 40–44. https://doi.org/10.1016/j.mineng.2013.04.021
Zeng, X., Li, J., & Liu, L. (2015). Solving spent lithium-ion battery problems in China: Opportunities and challenges. Renewable and Sustainable Energy Reviews, 52, 1759–1767. https://doi.org/10.1016/j.rser.2015.08.014
Zeng, X., Li, J., & Singh, N. (2014). Recycling of spent lithium-ion battery: A critical review. Critical Reviews in Environmental Science and Technology, 44, 1129–1165. https://doi.org/10.1080/10643389.2013.763578
X. Zheng, W. Gao, X. Zhang, M. He, X. Lin, H. Cao, Y. Zhang, Z. Sun, Spent lithium-ion battery recycling – Reductive ammonia leaching of metals from cathode scrap by sodium sulphite, Waste Manag. 60 (2017) 680–688. https://doi.org/10.1016/j.wasman.2016.12.007.
Zheng, X., Zhu, Z., Lin, X., Zhang, Y., He, Y., Cao, H., & Sun, Z. (2018). A mini-review on metal recycling from spent lithium ion batteries. Engineering, 4, 361–370. https://doi.org/10.1016/j.eng.2018.05.018
Zou, H., Gratz, E., Apelian, D., & Wang, Y. (2013). A novel method to recycle mixed cathode materials for lithium ion batteries. Green Chemistry, 15, 1183–1191. https://doi.org/10.1039/C3GC40182K
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Abdollahi, H., Saneie, R., Rahmanian, A., Ebrahimi, E., Mohammadzadeh, A., Shakiba, G. (2024). Biotechnological Applications in Spent Lithium-Ion Battery Processing. In: Panda, S., Mishra, S., Akcil, A., Van Hullebusch, E.D. (eds) Biotechnological Innovations in the Mineral-Metal Industry. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-031-43625-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-43625-3_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43624-6
Online ISBN: 978-3-031-43625-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)