Abstract
Cardiovascular diseases (CVDs) refer to a group of conditions that affect the heart and blood vessels and are a leading cause of death worldwide. Ferroptosis is an iron-dependent regulated cell death process that occurs due to unlimited lipid peroxidation and subsequent plasma membrane rupture. Impaired ferroptosis has been linked to the pathophysiology of various CVDs, including cardiomyopathies, myocardial infarction and ischemia, coronary atherosclerosis, and heart failure. Excessive iron accumulation can trigger phospholipid hydroperoxide accumulation in the cell membrane and ferroptosis, ultimately causing CVD. Conversely, iron deficiency, which often develops under conditions of malnutrition, negatively affects cardiac metabolism and function in humans. This chapter delves into the role of ferroptosis in the pathophysiology of CVD and explores therapeutic targets and compounds for preventing ferroptosis-related CVD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
Proper function of the cardiovascular system requires a fine-tuned homeostasis of many trace elements including iron. Ferroptosis refers to highly regulated iron-dependent cell death, which is implicated in the pathophysiology of a broad range of cardiovascular diseases (CVD) such as in cardiomyopathies, myocardial infarction and ischemia, coronary atherosclerosis, and heart failure (Del Re et al. 2019; Ajoolabady et al. 2021). Iron deficiency frequently develops under malnutrition and negatively impacts cardiac metabolism and function in humans (Bi et al. 2021). Conversely, iron overload is associated with intracellular oxidative stress, contributing to cardiovascular pathologies (Bi et al. 2021; Berdoukas et al. 2015). In cardiomyocytes, excessive iron triggers ferroptosis via phospholipid hydroperoxide accumulation in the cell membrane, ultimately causing CVD (Fang et al. 2019). In this chapter, we discuss the role of ferroptosis and its underpinning mechanisms in the pathophysiology of CVD, as well as highlighting potential pre-clinical targets and therapeutic compounds for the prevention of ferroptosis in CVD.
13.2 Role and Signaling Pathways of Ferroptosis in CVD and Potential Therapeutic Targets
13.2.1 Myocardial Infarction and Heart Ischemia
Multiple pieces of evidence have identified ferroptosis in the pathogenesis of myocardial infarction (MI) and ischemia (Zhao et al. 2021). During acute and subacute stages of MI, GPX4 downregulation leads to lipid peroxidation and induction of ferroptosis in H9c2 cardiomyocytes under conditions of hypoxia-reperfusion (H/R) (Park et al. 2019). Meanwhile, cysteine deprivation aggravates ferroptosis due in part to effects on glutathione (GSH) synthesis (Park et al. 2019). Moreover, upregulation of the long noncoding RNA (lncRNA) Erdr1y/Gm47283 in a murine model of MI blocks microRNA Mir706, leading to upregulation of Ptgs2 mRNA, induction of ferroptosis, and ultimately, exacerbation of MI (Gao et al. 2022). Conversely, inhibition of Erdr1y lncRNA and/or overexpression of Mir706 alleviate myocardial injury in this model (Gao et al. 2022). Hence, modulation of the Erdr1y lncRNA-Mir706-PTGS2 axis may alleviate ferroptosis and cardiac injury. In most cell types, SLC11A2/DMT1 takes up non-heme iron and its upregulation in cardiomyocytes triggers ferroptosis (Song et al. 2021). In line with this, mesenchymal stem cells (MSCs) of human umbilical cord release exosomes containing MIR23A-3p that target/block SLC11A2, resulting in the inhibition of ferroptosis and alleviation of myocardial injury (Song et al. 2021). Therefore, MSCs-exosomes through activation of the MIR23A-3p-SLC11A2 pathway may confer protection against ferroptosis upon MI.
A large body of evidence highlights the importance of ferroptosis in the onset and development of myocardial ischemia-reperfusion (I/R) injury. In vitro, cardiac myocyte ischemia induces ALOX15-mediated peroxidation of polyunsaturated fatty acids/PUFAs, resulting in ferroptosis induction and cell damage. These findings suggest that targeted inhibition of ALOX15 might be a promising strategy to combat ferroptosis during I/R injury (Ma et al. 2022b). In H9c2 cells exposed to H/R, Mir190a-5p reduces reactive oxygen species (ROS), malondialdehyde, and Fe2+ accumulation by sponging Gls2 mRNA, thus antagonizing ferroptosis (Zhou et al. 2021). These findings indicate that the Mir190a-5p-GLS2 axis could serve as a potential target for the prevention of ferroptosis and myocardial damage. Besides, inducing Fndc5 overexpression or administration of its cleaved form (namely irisin) attenuates ferroptosis and mitochondrial impairment through activation of the NFE2L2/NRF2-HMOX1 signaling cascade in hypoxic cardiomyocytes (Cao et al. 2022a). Therefore, inducible activation of the FNDC5-NFE2L2-HMOX1 signaling axis may represent a promising strategy to block ferroptosis under H/R settings in cardiomyocytes. Moreover, myocardial I/R injury is accompanied by generation of oxidized phosphatidylcholines (OxPCs) which elicit ferroptosis and cardiac dysfunction in rats (Stamenkovic et al. 2021). Mechanistically, OxPCs-induced ferroptosis involves a dramatic reduction of GPX4 activity in rat cardiomyocytes. Additionally, OxPCs contribute to ferroptosis by dampening calcium transients and mitochondrial bioenergetics (Stamenkovic et al. 2021). Neutralizing antibodies against OxPCs blocks ferroptosis during the perfusion stage. Both in vivo and in vitro, myocardial I/R upregulates Elavl1 via FOXC1. ELAVL1 then binds and stabilizes Becn1 mRNA, inciting autophagy-dependent ferroptosis and myocardial injury (Chen et al. 2021), which can be rescued by Elavl1 knockout. These observations indicate that the FOXC1-ELAVL1-BECN1 signaling cascade plays an essential role in autophagy-mediated ferroptosis in the context of myocardial I/R injury. Furthermore, upon myocardial I/R injury, bone marrow MSCs-exosomes containing Mir9-3hg lncRNA reduces ferroptosis by promoting cell proliferation, GSH content, and by reducing ROS and iron overload in mice and HL-1 cardiomyocytes (Zhang et al. 2022a). From the mechanistic point of view, Mir9-3hg lncRNA binds and inhibits PUM2, thereby activating Prdx6 transcription, mitigating ferroptosis (Zhang et al. 2022a). Thus, the Mir9-3hg lncRNA-PUM2-PRDX6 pathway renders protection against ferroptosis and myocardial I/R injury. Similar to Mir9-3hg lncRNA, MTOR protects against ferroptosis and iron overload by suppressing mitochondrial ROS in mice subject to myocardial I/R (Baba et al. 2018). In early reperfusion, upregulation of ATF3 leads to regression of ferroptosis and myocardial I/R injury (Liu et al. 2022a). The underlying mechanism appears to be linked to binding of ATF3 to the FANCD2 promoter, leading to its transactivation and thus inhibition of ferroptosis in AC16 human cardiomyocytes (Liu et al. 2022a). Conversely, ATF3 ablation retrieves ferroptosis and aggravates I/R injury. These findings imply that the ATF3-FANCD2 axis could serve as a potential target for prevention of ferroptosis in ischemic hearts.
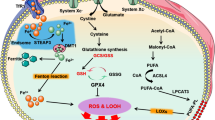
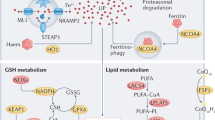
KMT2B induces histone H3 methylation and upregulation of Rfk to activate TNF/TNF-α-CYBB/NOX2 signaling, resulting in ferroptosis and cardiac injury in a rat myocardial I/R model and H9c2 cardiomyocytes (Cao et al. 2022b). Hence, Kmt2b ablation significantly curbs ferroptosis and infarct size (Cao et al. 2022b). In this sense, targeted inhibition of KMT2B-RFK-TNF-CYBB/NOX2 signaling or genetic ablations of its key components might suppress ferroptosis upon myocardial I/R. Furthermore, NFE2L2 upregulates SLC40A1, thereby limiting iron overload and ferroptosis, ultimately alleviating myocardial I/R injury in diabetic rats and H9c2 cardiomyocytes (Tian et al. 2022). These observations indicate that the NFE2L2-SLC40A1 axis endows protection against ferroptosis under conditions of diabetic myocardial I/R injury. Finally, it is worth mentioning that ferroptosis genes including CAT, HMOX1, RTN3, GPX4, and SLC25A1 are correlated with other biochemical risk factors in patients with coronary artery disease/CAD expression of oxidative stress, suggesting a cardinal role for ferroptosis in the progression of this disease (Ozuynuk et al. 2022) (Figs. 13.1 and 13.2).
13.2.2 Cardiomyopathy
Ferroptosis is thought to be critical in the pathogenesis of cardiomyopathy (Li et al. 2022a). In the cox10−/− mouse model of mitochondrial cardiomyopathy, the mitochondrial peptidase OMA1 induces mitochondrial fragmentation and integrated stress response/ISR via activation of OMA1-DELE1-ATF4 signaling, resulting in improved GSH metabolism and inhibition of lipid peroxidation and ferroptosis (Ahola et al. 2022). Therefore, the OMA1-DELE1-ATF4 axis plays a protective role against ferroptosis. In contrast, palmitic acid (PA) downregulates Hsf1 and Gpx4 in a dose- and time-dependent manner, leading to lipid peroxidation and ferroptosis in mice and H9c2 cardiomyoblasts in vitro (Wang et al. 2021). However, inducing Hsf1 expression alleviates these effects, renormalizes iron metabolism, and upregulates Gpx4 (Wang et al. 2021). These findings indicate an anti-ferroptotic role of HSF1 in PA-induced cardiomyopathy. In an in vitro model of doxorubicin (DOX)-induced cardiomyopathy, Carm1/Prmt4 overexpression triggers ferroptosis and aggravates cardiomyocyte injury (Wang et al. 2022b). Mechanistically, PRMT4 methylates NFE2L2 thus dampening its nuclear translocation and reducing Gpx4 transcription, ultimately, resulting in ferroptosis (Wang et al. 2022b). Therefore, NFE2L2 activation, administration of Fer-1, and genetic ablation or pharmacological inhibition of CARM1/Carm1 have all been shown to alleviate ferroptosis and cardiomyopathy in vitro (Wang et al. 2022b). These data suggest that forcible inhibition of CARM1-NFE2L2-GPX4 signaling could be a potent approach for suppression of ferroptosis and alleviation of DOX-induced cardiomyopathy.
Similarly, the presence of ferroptosis in the pathophysiology of diabetic cardiomyopathy has also been reported. Also, in db/db mice and glucose-challenged cardiomyocytes, the lncRNA Zfas1 binds and inhibits Mir150-5p, leading to downregulation of Ccnd2, ferroptosis, and progression of diabetic cardiomyopathy, all of which can be alleviated by inhibition of Zfas1 (Ni et al. 2021). Furthermore, ferroptosis contributes to obesity-associated cardiomyopathy. In obese mice, exosomes derived from adipose tissue macrophages impair mitochondria, provoking upregulation of malondialdehyde and 4-hydroxynonenal (lipid peroxides) in cardiomyocytes (Zhao et al. 2022). Mir140-5p in these exosomes targets and sponges Slc7a11, causing loss of GSH and induction of ferroptosis (Zhao et al. 2022). Inhibition of exosomal Mir140-5p can retard ferroptosis and cardiac injury in obesity-associated cardiomyopathy (Zhao et al. 2022). These findings indicate the possible role of the Mir140-5p-SLC7A11-GSH signaling cascade as a promising target for neutralization of ferroptosis and mitigation of obesity-induced cardiomyopathy.
Likewise, in septic cardiomyopathy, ica1 knockout overtly augments cardiac function through suppression of inflammatory cytokines, oxidative stress, and ferroptosis in lipopolysaccharide (LPS)-challenged mice (Kong et al. 2022). The underlying mechanism appears to involve Ica1 upregulation to turn on STING1 signaling, resulting in lipid peroxidation, ferroptosis, and cardiotoxicity (Kong et al. 2022). Moreover, septic patients exhibit increased ICA1 levels in plasma and mononuclear cells (Kong et al. 2022). Therefore, the ICA1-STING1 pathway may serve as a key target for inhibition of ferroptosis and septic cardiomyopathy. In mice with sepsis/LPS-induced cardiac injury and H9c2 cardiomyocytes, tmem43 ablation aggravates ferroptosis and iron overload (Chen et al. 2022b). Conversely, inducing Tmem43 overexpression blocks lipid peroxidation and ferroptosis, thereby ameliorating cardiac dysfunction and injury (Chen et al. 2022b). Mechanistically, LPS elicits ferroptosis through upregulation of Trp53/p53 and ferritin as well as downregulation of Slc7a11 and Gpx4 (Chen et al. 2022b). TMEM43 overexpression reverses these effects and protects against sepsis-induced cardiac injury (Chen et al. 2022b), indicating a possible role of TMEM43 as a novel target for ferroptosis in cardiomyocytes (Figs. 13.1 and 13.2).
13.2.3 Cardiotoxicity
Proper use of anti-cancer drugs is frequently limited by their cardiotoxic side effects (Yarmohammadi et al. 2021). Substantial evidence implicates ferroptosis in drug-induced cardiotoxicity. For instance, histamine deficiency or inhibition of its receptor, HRH1/H1R, ignites ferroptosis in cardiomyocytes, to exacerbate DOX-induced cardiotoxicity in mice and cultured hiPSC-CMs and HL-1 models (Zhu et al. 2022). Mechanistically, disruption of histamine-HRH1/H1R signaling inactivates STAT3, leading to Slc7a11 downregulation and ferroptosis, which can be rescued by histamine administration (Zhu et al. 2022). Thus, forcible activation of HRH1/H1R-STAT3-SLC7A11 signaling (e.g., using histamine) alleviates DOX-induced cardiotoxicity.
Binding of APELA peptide hormone to its receptor, APLNR, provokes pronounced activation of KLF15-GPX4 signaling and upregulation of GSH, SLC7A11, and NFE2L2, to alleviate ROS production, ferroptosis, and DOX-induced cardiotoxicity in rat aortic fibroblasts (Zhang et al. 2022b). These findings favor the idea that APELA could be a promising target for abrogation of cardiotoxic ferroptosis. The outer mitochondrial membrane-located protein FUNDC2 interacts with and destabilizes SLC25A11 in the inner membrane, resulting in the reduction of mitochondrial GSH, which fosters ferroptosis (Ta et al. 2022). Additionally, FUNDC2 is capable of modulating GPX4 stability (Ta et al. 2022). Hence, the FUNDC2-SLC25A11-GPX4 signaling pathway is a major contributor to mitochondria-initiated ferroptosis. In mice and H9c2 cells, the E3 ubiquitin ligase TRIM21 ubiquitinates SQSTM1/p62 and interferes with the SQSTM1-KEAP1-NFE2L2 signaling cascade, causing lipid peroxidation and ferroptosis (Hou et al. 2021). Genetic ablation of Trim21 rescues DOX-induced cardiotoxicity (Hou et al. 2021). These data imply that targeted inhibition of the TRIM21-SQSTM1-KEAP1-NFE2L2 axis may rescue ferroptosis and cardiotoxicity under DOX challenge. In rat neonatal and AC16 cardiomyocytes, DOX exposure initiates Mettl14 upregulation, which in turn, methylates and activates lncRNA Kcnq1ot1, leading to the inhibition of Mir7-5p and subsequent upregulation of TFRC/TFR1, iron overload, lipid peroxidation, and ultimately, induction of ferroptosis (Zhuang et al. 2021). Importantly, IGF2BP1 enhances the stability of the lncRNA Kcnq1ot1 thus inducing its activation and subsequent blockade of Mir7-5p (Zhuang et al. 2021). Therefore, the METTL14-Kcnq1ot1 lncRNA-Mir7-5p-TFRC cascade plays an indispensable role in DOX-induced ferroptosis. Hence, targeted inhibition of METTL14-Kcnq1ot1 lncRNA-Mir7-5p-TFRC may abate cardiotoxicity.
Under DOX exposure, Marchf5 downregulation leads to upregulation of Chac1 that overtly degrades GSH and downregulates Gpx4, thereby favoring lipid peroxidation and ferroptosis in rat neonatal cardiomyocytes (Kitakata et al. 2021). This finding suggests that the MARCHF5-CHAC1-GSH axis incites ferroptosis in cardiomyocytes, which may be a novel target for maneuvering ferroptosis and DOX cardiotoxicity. Triptolide is another anti-cancer medication with its clinical application limited by its cardiotoxicity (Liu et al. 2022c). Cardiotoxic effects of triptolide are partially attributed to induction of ferroptosis through accumulation of lipid peroxides (e.g., malondialdehyde and 4-hydroxynonenal), Fe2+ overload, GSH reduction, ferritin degradation, and ROS generation, as well as blockade of the NFE2L2-HMOX1 axis in human AC16 cardiomyocytes (Liu et al. 2022c). Importantly, activation of the TF-TRFC-SLC11A2 pathway accounts for triptolide-induced Fe2+ overload (Liu et al. 2022c). Furthermore, triptolide interrupts the SLC7A11-GPX4 axis through a direct binding with SLC7A11 (Liu et al. 2022c). Likewise, herceptin (trastuzumab) renders cardiotoxicity and heart failure (HF) through elevation of mitochondrial/intracellular ROS and downregulation of Gpx and Slc7a11, resulting in ferroptosis in H9c2 cardiomyocytes (Sun et al. 2022a). Administration of the iron chelator deferoxamine, or Fer-1, reverses these effects (Sun et al. 2022a). Conceivably, safe treatment of ERBB2/HER2+ breast cancer with herceptin could be improved by blocking ferroptosis. Imatinib mesylate-associated cardiotoxicity is linked to ROS production, iron overload, NFE2L2 downregulation, and ultimately, induction of ferroptosis in mice and H9c2 cardiomyocytes (Song et al. 2022). Conversely, findings from our group indicated that paraquat triggers cardiotoxicity and contractile dysfunction through downregulation of SLC7A11, GPX4, and ferritin, and activation of a FUNDC1-MAPK/JNK-NCOA4 axis, ultimately, leading to lipid peroxidation and ferroptosis in mice (Peng et al. 2022). Not surprisingly, fundc1 ablation confers resistance against ferroptosis and alleviates myocardial toxicity and dysfunction (Peng et al. 2022). This study illustrates that inhibition of FUNDC1-MAPK/sJNK-NCOA4 signaling could avert ferroptosis and cardiotoxicity upon paraquat exposure (Figs. 13.1 and 13.2).
13.2.4 Cardiac Remodeling and Hypertrophy
Strong evidence support the role of ferroptosis in cardiac remodeling and hypertrophy (Wu et al. 2021). In mice, when a high-fat diet (HFD) is fed, Fundc1 deficiency elicits ACSL4-induced ferroptosis and cardiac remodeling (Pei et al. 2021). Therefore, targeted inhibition of the FUNDC1-ACSL4 axis might reverse cardiac remodeling owing to the handicap of ferroptosis. In the early stage of angiotensin II-mediated cardiac hypertrophy, Slc7a11 downregulation triggers ferroptosis, thereby promoting interstitial fibrosis, cardiac hypertrophy, and cardiac contractile dysfunction (Zhang et al. 2022c). Thus, suppression of ferroptosis using Fer-1 or upregulation of Slc7a11 reverts these effects (Zhang et al. 2022c). Given the anti-ferroptotic role of SLC7A11, it could be a promising target for retardation of cardiac hypertrophy. Pressure overload leads to downregulation of Irf3, resulting in endothelial ferroptosis and cardiac injury in rats (Shi et al. 2022). Mechanistically, Irf3 downregulation culminates in SLC7A11 downregulation and enhanced ALOX12 activity, leading to lipid peroxidation and ferroptosis in rat microvascular endothelial cells (Shi et al. 2022). However, docosahexaenoic acid treatment interrupts ferroptosis through upregulation of IRF3, thereby protecting against endothelial damage and cardiac hypertrophy (Shi et al. 2022). Based on these findings, the IRF3-SLC7A11-ALOX12 axis should play a cardinal role in cardiac hypertrophy and ferroptosis, and thus its targeting merits attention. Moreover, in a mouse model of angiotensin II-mediated hypertension, reduction of APELA elicits ferroptosis, myocardial fibrosis, and hypertrophy due to induction of ferroptosis in microvascular endothelial cells (Zhang et al. 2022d). APELA addition or ferroptosis inhibition using Fer-1 retards myocardial dysfunction and remodeling owing to the attenuation of iron overload and lipid peroxidation, and activation of GPX4 (Zhang et al. 2022d). These data indicate that APELA antagonizes ferroptosis upon hypertension, therefore, resulting in alleviated cardiac hypertrophy and remodeling (Figs. 13.1 and 13.2).
13.2.5 Atherosclerosis
Increasing clinical and experimental evidence has delineated a critical role of ferroptosis in atherosclerosis (Ouyang et al. 2021). During atherosclerosis, PDSS2 overexpression prevents ROS generation and ferroptosis through activation of NFE2L2 signaling, thereby alleviating atherosclerosis in vivo and reducing ferroptosis in human coronary artery endothelial cells/HCAECs (Yang et al. 2021). Consistent with, atherosclerotic patients exhibit reduced levels of PDSS2 and NFE2L2 in plasma as compared to healthy controls (Yang et al. 2021). Hence, the PDSS2-NFE2L2 pathway appears to play an anti-ferroptotic role and inducing its activation might alleviate atherosclerosis. Moreover, Nod1 deficiency reduces iron level in murine spleen, liver, and heart. In line with this, splenic deficiency of Nod1 induces ferroptosis and CXCR2 signaling in ADGRE1/F4/80+ macrophages, leading to their recruitment to atherosclerotic plaques (Fernández-García et al. 2022). Tangibly, Nod1 upregulation inhibits ferroptosis by restraining macrophage migration and raising GPX4 and anti-ferroptosis proteins in macrophages, ultimately, resulting in alleviation of atherosclerotic plaques (Fernández-García et al. 2022). These data indicate that NOD1 is a favorable target for retardation of plaque growth through inhibition of ferroptosis in macrophages/splenic cells.
In addition, endothelial progenitor cells (EPCs)-released extracellular vesicles induce attenuation of ROS, iron content, GSH consumption, and lipid peroxidation, thereby favoring ferroptosis in aortic endothelial cells and atherosclerotic mice (Li et al. 2021). Mechanistically, EPCs-exosomes contain Mir199a-3p that is transferred into endothelial cells and then targets/inhibits Sp1 mRNA, leading to mitigation of ferroptosis and atherosclerosis (Li et al. 2021). Hence, inducing activation of Mir199a-3p-SP1 signaling via administration of EPCs-exosomes might regress atherosclerosis. Conversely, ferroptosis contributes to the development of diabetic atherosclerosis. In this regard, HMOX1 upregulation promotes ferroptosis by inducing ROS generation, Fe2+ overload, and lipid peroxidation, thus promoting diabetic atherosclerosis in human endothelial cells (Meng et al. 2021). Hence, inhibition of ferroptosis using ferrostatin-1 (Fer-1) or genetic ablation of HMOX1 ameliorates diabetic atherosclerosis, indicating that HMOX1 could be a potential target for suppression of ferroptosis in diabetic atherosclerosis (Meng et al. 2021). Importantly, in hyperlipidemic Jak2V617F mice, increased hematocrit induces ferroptosis in plaque macrophages through excessive phagocytosis of red blood cells, thereby leading to macrophage ferroptosis and aggravation of atherosclerosis (Liu et al. 2022b) (Figs. 13.1 and 13.2).
13.2.6 Heart Failure
HF entails ferroptosis in the frontline of its pathogenesis (Yang et al. 2022b). In mouse cardiomyocytes, Fth1 deficiency induces Slc7a11 downregulation, leading to ferroptosis and development of HF (Fang et al. 2020). However, specific overexpression of Slc7a11 in cardiomyocytes increases GSH levels and retards ferroptosis (Fang et al. 2020). In rat HF, activation of TLR4-NOX4 signaling contributes to ferroptosis. However, ablation of Tlr4 or Nox4 averts ventricular remodeling and ferroptosis (Chen et al. 2019). Hence, the TLR4-NOX4 axis favors ferroptosis and thus could be a potential target for its suppression upon HF. In diabetic mice, cardiomyocyte upregulation of Nr2f2 aggravates HF by inducing ferroptosis, mitochondrial dysfunction, and oxidative stress through activation of PPARGC1A/PGC-1α signaling (Miao et al. 2022). Unsurprisingly, in vitro ablation of Nr2f2 blocks ferroptosis and rescues HF (Miao et al. 2022). These findings delineate the NR2F2-PPARGC1A signaling cascade as a potential target to foil ferroptosis upon diabetic HF. Under pressure overload stress, a circular RNA, circSnx12, binds and sponges Mir224-5p thus activating Fth1 mRNA, leading to hinderance of iron overload and ferroptosis in cardiomyocytes (Zheng et al. 2021). In this sense, induction of Mir224-5p upregulation and downregulation of circSnx12 ignites ferroptosis due to intracellular overload of Fe2+ (Zheng et al. 2021). Therefore, augmentation of the circSnx12-Mir224-5p-FTH1 pathway might be a potent strategy to curtail elevated ferroptosis upon HF.
Furthermore, in the early stage of chronic HF, Map3k11/Mlk3 initiates NFKB-NLRP3 signaling, culminating in inflammation, pyroptosis, and cardiac fibrosis in mice (Wang et al. 2020). Surprisingly, in the advanced stages, MAP3K11 activates the MAPK/JNK-TRP/p53 axis, leading to oxidative stress, ferroptosis, and myocardial fibrosis. Interestingly, inducing Mir351 upregulation blocks ferroptosis and pyroptosis by suppressing Map3k11 expression, thereby improving cardiac function (Wang et al. 2020). In compliance with these findings, inducing activation of the Mir351-MAP3K11 cascade might be a potential approach for regression of ferroptosis upon HF (Figs. 13.1 and 13.2).
13.3 Concluding Remarks and Therapeutic Directions
To date, multiple lines of evidence have substantiated a vital role for ferroptosis in the pathophysiology of CVD. Ferroptosis functions either as an underscoring mechanism or as a contributing factor for the pathogenesis of CVD. Either way would involve commencement of complicated signaling pathways and gene expression modulations, altering antioxidant capacity, lipid peroxidation status, and iron metabolism in cardiomyocytes, ultimately culminating in massive ROS generation, iron overload, and the induction of ferroptosis. Owing to the complexity of ferroptosis mechanisms and gene expression patterns, the intervention of ferroptosis in various types of CVD requires targeted inhibition or activation of several signaling cascades along with robust modulation of multiple genes. Hence, despite the advances in targeted therapy of ferroptosis in pre-clinical studies using natural or pharmaceutical compounds (Table 13.1), clinical inertia still runs deeper than achieving desirable therapeutics for targeting ferroptosis in CVD. Nonetheless, future discoveries may reveal master key mechanisms encompassing the large portion of ferroptosis incidence and origination in CVD and, thereby, facilitating and simplifying pharmacological or genetic intervention of ferroptosis. Moreover, advances in drug development and targeted therapy approaches are pending to match the clinical demands. However, parallel advances in biotechnology and nanotechnology techniques might be game changers in our combat against ferroptosis in the context of CVD.
References
Ahola S, Mejías PR, Hermans S, Chandragiri S, Giavalisco P, Nolte H, Langer T (2022) OMA1-mediated integrated stress response protects against ferroptosis in mitochondrial cardiomyopathy. Cell Metab 34(11):1875–1891.e1877
Ajoolabady A, Aslkhodapasandhokmabad H, Libby P, Tuomilehto J, Lip GY, Penninger JM, Richardson DR, Tang D, Zhou H, Wang S (2021) Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol Metab 32(7):444–462
Baba Y, Higa JK, Shimada BK, Horiuchi KM, Suhara T, Kobayashi M, Woo JD, Aoyagi H, Marh KS, Kitaoka H (2018) Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Phys Heart Circ Phys 314(3):H659–H668
Berdoukas V, Coates TD, Cabantchik ZI (2015) Iron and oxidative stress in cardiomyopathy in thalassemia. Free Radic Biol Med 88:3–9
Bi Y, Ajoolabady A, Demillard LJ, Yu W, Hilaire ML, Zhang Y, Ren J (2021) Dysregulation of iron metabolism in cardiovascular diseases: from iron deficiency to iron overload. Biochem Pharmacol 190:114661
Cao G, Yang C, Jin Z, Wei H, Xin C, Zheng C, Xu J, Huang Q, Zhang Z, Hu T (2022a) FNDC5/irisin reduces ferroptosis and improves mitochondrial dysfunction in hypoxic cardiomyocytes by Nrf2/HO-1 axis. Cell Biol Int 46(5):723–736
Cao Y, Luo F, Peng J, Fang Z, Liu Q, Zhou S (2022b) KMT2B-dependent RFK transcription activates the TNF-α/NOX2 pathway and enhances ferroptosis caused by myocardial ischemia-reperfusion. J Mol Cell Cardiol 173:75–91
Chen X, Xu S, Zhao C, Liu B (2019) Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun 516(1):37–43
Chen H-Y, Xiao Z-Z, Ling X, Xu R-N, Zhu P, Zheng S-Y (2021) ELAVL1 is transcriptionally activated by FOXC1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol Med 27(1):1–14
Chen H, Zhu J, Le Y, Pan J, Liu Y, Liu Z, Wang C, Dou X, Lu D (2022a) Salidroside inhibits doxorubicin-induced cardiomyopathy by modulating a ferroptosis-dependent pathway. Phytomedicine 99:153964
Chen Z, Cao Z, Gui F, Zhang M, Wu X, Peng H, Yu B, Li W, Ai F, Zhang J (2022b) TMEM43 protects against sepsis-induced cardiac injury via inhibiting ferroptosis in mice. Cells 11(19):2992
Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN (2019) Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev 99(4):1765–1817
Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci 116(7):2672–2680
Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P, Gao F, Yu Y, Song Z, Wu Q (2020) Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res 127(4):486–501
Fernández-García V, González-Ramos S, Avendaño-Ortiz J, Martín-Sanz P, Delgado C, Castrillo A, Boscá L (2022) NOD1 splenic activation confers ferroptosis protection and reduces macrophage recruitment under pro-atherogenic conditions. Biomed Pharmacother 148:112769
Fu F, Lai Q, Hu J, Zhang L, Zhu X, Kou J, Yu B, Li F (2022a) Ruscogenin alleviates myocardial ischemia-induced ferroptosis through the activation of BCAT1/BCAT2. Antioxidants 11(3):583
Fu F, Lai Q, Hu J, Zhang L, Zhu X, Kou J, Yu B, Li F (2022b) Ruscogenin alleviates myocardial ischemia-induced Ferroptosis through the activation of BCAT1/BCAT2. Antioxidants 11:583. S Note: MDPI stays neutral with regard to jurisdictional claims in published…
Gao F, Zhao Y, Zhang B, Xiao C, Sun Z, Gao Y, Dou X (2022) Suppression of lncRNA Gm47283 attenuates myocardial infarction via miR-706/Ptgs2/ferroptosis axis. Bioengineered 13(4):10786–10802
He L, Liu Y-Y, Wang K, Li C, Zhang W, Li Z-Z, Huang X-Z, Xiong Y (2021) Tanshinone IIA protects human coronary artery endothelial cells from ferroptosis by activating the NRF2 pathway. Biochem Biophys Res Commun 575:1–7
Hou K, Shen J, Yan J, Zhai C, Zhang J, Pan J-A, Zhang Y, Jiang Y, Wang Y, Lin RZ (2021) Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBioMedicine 69:103456
Kitakata H, Endo J, Matsushima H, Yamamoto S, Ikura H, Hirai A, Koh S, Ichihara G, Hiraide T, Moriyama H (2021) MITOL/MARCH5 determines the susceptibility of cardiomyocytes to doxorubicin-induced ferroptosis by regulating GSH homeostasis. J Mol Cell Cardiol 161:116–129
Kong C, Ni X, Wang Y, Zhang A, Zhang Y, Lin F, Li S, Lv Y, Zhu J, Yao X (2022) ICA69 aggravates ferroptosis causing septic cardiac dysfunction via STING trafficking. Cell Death Discov 8(1):1–13
Li L, Wang H, Zhang J, Chen X, Zhang Z, Li Q (2021) Effect of endothelial progenitor cell-derived extracellular vesicles on endothelial cell ferroptosis and atherosclerotic vascular endothelial injury. Cell Death Discov 7(1):1–11
Li D, Pi W, Sun Z, Liu X, Jiang J (2022a) Ferroptosis and its role in cardiomyopathy. Biomed Pharmacother 153:113279
Li T, Tan Y, Ouyang S, He J, Liu L (2022b) Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene 808:145968
Lin J-H, Yang K-T, Lee W-S, Ting P-C, Luo Y-P, Lin D-J, Wang Y-S, Chang J-C (2022) Xanthohumol protects the rat myocardium against ischemia/reperfusion injury-induced ferroptosis. Oxid Med Cell Longev 2022
Liu B, Zhao C, Li H, Chen X, Ding Y, Xu S (2018) Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem Biophys Res Commun 497(1):233–240
Liu XJ, Lv YF, Cui WZ, Li Y, Liu Y, Xue YT, Dong F (2021) Icariin inhibits hypoxia/reoxygenation-induced ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1 signaling pathway. FEBS Open Bio 11(11):2966–2976
Liu H, Mo H, Yang C, Mei X, Song X, Lu W, Xiao H, Yan J, Wang X, Yan J (2022a) A novel function of ATF3 in suppression of ferroptosis in mouse heart suffered ischemia/reperfusion. Free Radic Biol Med 189:122–135
Liu W, Östberg N, Yalcinkaya M, Dou H, Endo-Umeda K, Tang Y, Hou X, Xiao T, Fidler TP, Abramowicz S (2022b) Erythroid lineage Jak2 V617F expression promotes atherosclerosis through erythrophagocytosis and macrophage ferroptosis. J Clin Invest 132(13)
Liu X, Chen C, Han D, Zhou W, Cui Y, Tang X, Xiao C, Wang Y, Gao Y (2022c) SLC7A11/GPX4 inactivation-mediated ferroptosis contributes to the pathogenesis of triptolide-induced cardiotoxicity. Oxidative Med Cell Longev 2022:1
Liu X, Li D, Pi W, Wang B, Xu S, Yu L, Yao L, Sun Z, Jiang J, Mi Y (2022d) LCZ696 protects against doxorubicin-induced cardiotoxicity by inhibiting ferroptosis via AKT/SIRT3/SOD2 signaling pathway activation. Int Immunopharmacol 113:109379
Liu X, Qi K, Gong Y, Long X, Zhu S, Lu F, Lin K, Xu J (2022e) Ferulic acid alleviates myocardial ischemia reperfusion injury via upregulating AMPKα2 expression-mediated ferroptosis depression. J Cardiovasc Pharmacol 79(4):489–500
Lu H, Xiao H, Dai M, Xue Y, Zhao R (2022) Britanin relieves ferroptosis-mediated myocardial ischaemia/reperfusion damage by upregulating GPX4 through activation of AMPK/GSK3β/Nrf2 signalling. Pharm Biol 60(1):38–45
Lv Z, Zhang X, Zhang X, Zhang J, Liu R (2021) Etomidate attenuates the ferroptosis in myocardial ischemia/reperfusion rat model via Nrf2/HO-1 pathway. Shock 56(3):440–449
Ma S, He L-L, Zhang G-R, Zuo Q-J, Wang Z-L, Zhai J-L, Zhang T-T, Wang Y, Ma H-J, Guo Y-F (2022a) Canagliflozin mitigates ferroptosis and ameliorates heart failure in rats with preserved ejection fraction. Naunyn-Schmiedeb Arch Pharmacol 395:1–18
Ma X-H, Liu J-H-Z, Liu C-Y, Sun W-Y, Duan W-J, Wang G, Kurihara H, He R-R, Li Y-F, Chen Y (2022b) ALOX15-launched PUFA-phospholipids peroxidation increases the susceptibility of ferroptosis in ischemia-induced myocardial damage. Signal Transduct Target Ther 7(1):1–13
Meng Z, Liang H, Zhao J, Gao J, Liu C, Ma X, Liu J, Liang B, Jiao X, Cao J (2021) HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sci 284:119935
Miao W, Chen M, Chen M, Cui C, Zhu Y, Luo X, Wu B (2022) Nr2f2 overexpression aggravates ferroptosis and mitochondrial dysfunction by regulating the PGC-1α signaling in diabetes-induced heart failure mice. Mediat Inflamm 2022:1
Ni T, Huang X, Pan S, Lu Z (2021) Inhibition of the long non-coding RNA ZFAS1 attenuates ferroptosis by sponging miR-150-5p and activates CCND2 against diabetic cardiomyopathy. J Cell Mol Med 25(21):9995–10007
Ning D, Yang X, Wang T, Jiang Q, Yu J, Wang D (2021) Atorvastatin treatment ameliorates cardiac function and remodeling induced by isoproterenol attack through mitigation of ferroptosis. Biochem Biophys Res Commun 574:39–47
Ouyang S, You J, Zhi C, Li P, Lin X, Tan X, Ma W, Li L, Xie W (2021) Ferroptosis: the potential value target in atherosclerosis. Cell Death Dis 12(8):782
Ozuynuk AS, Erkan AF, Coban N, Unaltuna N (2022) Examining the expression levels of ferroptosis-related genes in angiographically determined coronary artery disease patients. Mol Biol Rep:1–10
Park T-J, Park JH, Lee GS, Lee J-Y, Shin JH, Kim MW, Kim YS, Kim J-Y, Oh K-J, Han B-S (2019) Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis 10(11):1–15
Pei Z, Liu Y, Liu S, Jin W, Luo Y, Sun M, Duan Y, Ajoolabady A, Sowers JR, Fang Y (2021) FUNDC1 insufficiency sensitizes high fat diet intake-induced cardiac remodeling and contractile anomaly through ACSL4-mediated ferroptosis. Metabolism 122:154840
Peng H, Fu S, Wang S, Xu H, Dhanasekaran M, Chen H, Shao C, Chen Y, Ren J (2022) Ablation of FUNDC1-dependent mitophagy renders myocardium resistant to paraquat-induced ferroptosis and contractile dysfunction. Biochim Biophys Acta (BBA) Mol Basis Dis 1868(9):166448
Shen Y, Shen X, Wang S, Zhang Y, Wang Y, Ding Y, Shen J, Zhao J, Qin H, Xu Y (2022) Protective effects of Salvianolic acid B on rat ferroptosis in myocardial infarction through upregulating the Nrf2 signaling pathway. Int Immunopharmacol 112:109257
Shi P, Song C, Qi H, Ren J, Ren P, Wu J, Xie Y, Zhang M, Sun H, Cao Y (2022) Up-regulation of IRF3 is required for docosahexaenoic acid suppressing ferroptosis of cardiac microvascular endothelial cells in cardiac hypertrophy rat. J Nutr Biochem 104:108972
Song Y, Wang B, Zhu X, Hu J, Sun J, Xuan J, Ge Z (2021) Human umbilical cord blood–derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol 37(1):51–64
Song C, Li D, Zhang J, Zhao X (2022) Role of ferroptosis in promoting cardiotoxicity induced by Imatinib Mesylate via down-regulating Nrf2 pathways in vitro and in vivo. Toxicol Appl Pharmacol 435:115852
Stamenkovic A, O’Hara KA, Nelson DC, Maddaford TG, Edel AL, Maddaford G, Dibrov E, Aghanoori M, Kirshenbaum LA, Fernyhough P (2021) Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury. Am J Phys Heart Circ Phys 320(3):H1170–H1184
Sun L, Wang H, Yu S, Zhang L, Jiang J, Zhou Q (2022a) Herceptin induces ferroptosis and mitochondrial dysfunction in H9c2 cells. Int J Mol Med 49(2):1–8
Sun X, Sun P, Zhen D, Xu X, Yang L, Fu D, Wei C, Niu X, Tian J, Li H (2022b) Melatonin alleviates doxorubicin-induced mitochondrial oxidative damage and ferroptosis in cardiomyocytes by regulating YAP expression. Toxicol Appl Pharmacol 437:115902
Ta N, Qu C, Wu H, Zhang D, Sun T, Li Y, Wang J, Wang X, Tang T, Chen Q (2022) Mitochondrial outer membrane protein FUNDC2 promotes ferroptosis and contributes to doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci 119(36):e2117396119
Tian H, Xiong Y, Zhang Y, Leng Y, Tao J, Li L, Qiu Z, Xia Z (2022) Activation of NRF2/FPN1 pathway attenuates myocardial ischemia–reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones 27(2):149–164
Wang J, Deng B, Liu Q, Huang Y, Chen W, Li J, Zhou Z, Zhang L, Liang B, He J (2020) Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (MLK3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis 11(7):1–19
Wang N, Ma H, Li J, Meng C, Zou J, Wang H, Liu K, Liu M, Xiao X, Zhang H (2021) HSF1 functions as a key defender against palmitic acid-induced ferroptosis in cardiomyocytes. J Mol Cell Cardiol 150:65–76
Wang Y, Kuang X, Yin Y, Han N, Chang L, Wang H, Hou Y, Li H, Li Z, Liu Y (2022a) Tongxinluo prevents chronic obstructive pulmonary disease complicated with atherosclerosis by inhibiting ferroptosis and protecting against pulmonary microvascular barrier dysfunction. Biomed Pharmacother 145:112367
Wang Y, Yan S, Liu X, Deng F, Wang P, Yang L, Hu L, Huang K, He J (2022b) PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ:1–14
Wei Z, Shaohuan Q, Pinfang K, Chao S (2022) Curcumin attenuates ferroptosis-induced myocardial injury in diabetic cardiomyopathy through the Nrf2 pathway. Cardiovasc Ther 2022:1
Wu X, Li Y, Zhang S, Zhou X (2021) Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 11(7):3052
Yang K, Song H, Yin D (2021) PDSS2 inhibits the ferroptosis of vascular endothelial cells in atherosclerosis by activating Nrf2. J Cardiovasc Pharmacol 77(6):767
Yang K-T, Chao T-H, Wang I-C, Luo Y-P, Ting P-C, Lin J-H, Chang J-C (2022a) Berberine protects cardiac cells against ferroptosis. Tzu Chi Med J 34(3):310
Yang X, Kawasaki NK, Min J, Matsui T, Wang F (2022b) Ferroptosis in heart failure. J Mol Cell Cardiol 173:141
Yarmohammadi F, Hayes AW, Karimi G (2021) The role of ferroptosis in organ toxicity. Hum Exp Toxicol 40(12_suppl):S851–S860
Zhang H, Wang Z, Liu Z, Du K, Lu X (2021) Protective effects of dexazoxane on rat ferroptosis in doxorubicin-induced cardiomyopathy through regulating HMGB1. Front Cardiovasc Med 8
Zhang J-K, Zhang Z, Guo Z-A, Fu Y, Chen X-J, Chen W-J, Wu H-F, Cui X-J (2022a) The BMSC-derived exosomal lncRNA Mir9-3hg suppresses cardiomyocyte ferroptosis in ischemia-reperfusion mice via the Pum2/PRDX6 axis. Nutr Metab Cardiovasc Dis 32(2):515–527
Zhang M-W, Li X-T, Zhang Z-Z, Liu Y, Song J-W, Liu X-M, Chen Y-H, Wang N, Guo Y, Liang L-R (2022b) Elabela blunts doxorubicin-induced oxidative stress and ferroptosis in rat aortic adventitial fibroblasts by activating the KLF15/GPX4 signaling. Cell Stress Chaperones:1–13
Zhang X, Zheng C, Gao Z, Chen H, Li K, Wang L, Zheng Y, Li C, Zhang H, Gong M (2022c) SLC7A11/xCT prevents cardiac hypertrophy by inhibiting ferroptosis. Cardiovasc Drugs Ther 36(3):437–447
Zhang Z, Tang J, Song J, Xie M, Liu Y, Dong Z, Liu X, Li X, Zhang M, Chen Y (2022d) Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/GPX4 signaling. Free Radic Biol Med 181:130–142
Zhao W-k, Zhou Y, Xu T-T, Wu Q (2021) Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury. Oxidative Med Cell Longev 2021:1
Zhao X, Si L, Bian J, Pan C, Guo W, Qin P, Zhu W, Xia Y, Zhang Q, Wei K (2022) Adipose tissue macrophage-derived exosomes induce ferroptosis via glutathione synthesis inhibition by targeting SLC7A11 in obesity-induced cardiac injury. Free Radic Biol Med 182:232–245
Zheng H, Shi L, Tong C, Liu Y, Hou M (2021) circSnx12 is involved in ferroptosis during heart failure by targeting miR-224-5p. Front Cardiovasc Med:318
Zhou X, Zhuo M, Zhang Y, Shi E, Ma X, Li H (2021) miR-190a-5p regulates cardiomyocytes response to ferroptosis via directly targeting GLS2. Biochem Biophys Res Commun 566:9–15
Zhu X, Wang X, Zhu B, Ding S, Shi H, Yang X (2022) Disruption of histamine/H1R-STAT3-SLC7A11 axis exacerbates doxorubicin-induced cardiac ferroptosis. Free Radic Biol Med 192:98–114
Zhuang S, Ma Y, Zeng Y, Lu C, Yang F, Jiang N, Ge J, Ju H, Zhong C, Wang J (2021) METTL14 promotes doxorubicin-induced cardiomyocyte ferroptosis by regulating the KCNQ1OT1-miR-7-5p-TFRC axis. Cell Biol Toxicol:1–21
Conflict of Interest Statement
None of the authors declare any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ajoolabady, A., Pratico, D., Henninger, N., Tuomilehto, J., Klionsky, D.J., Ren, J. (2023). Ferroptosis: A Promising Therapeutic Target for Cardiovascular Diseases. In: Tang, D. (eds) Ferroptosis in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-031-39171-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-39171-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-39170-5
Online ISBN: 978-3-031-39171-2
eBook Packages: MedicineMedicine (R0)