Abstract
Arsenic, one of the most prevalent naturally occurring elements is referred to as the King of Poisons and is frequently present in diet and drinking water. It is primarily found in an inorganic form. The most common and toxic forms of arsenic are the trivalent form called arsenite which has an oxidation state of + 3, and the pentavalent form called arsenate or As(V). Common organic arsenic compounds are arsanilic acid, monomethylarsonic acid (MMAV), dimethylarsonic acid (DMAV, also called cacodylic acid), trimethylarsonic acid (TMA) and arsenobetaine. Cancer in the lungs, skin, kidney, liver, bladder, and prostate is associated with arsenic toxicity in drinking water. Other sources of arsenic are soil, air, cosmetics, pesticides, chemotherapeutic agent, and the by-product of metal ore smelters. It serves benefits as pesticides, semiconductors, glassware, alloys, and preservatives. In addition, it can be used to treat many ailments like ulcers, syphilis, leukemia, trypanosomiasis, cancers, etc. The negative effects of arsenic are caused by several interdependent modes of action. One of the first proposed MOAs for arsenic, suggested by Binz and Schulz in 1879, was the interference of cellular oxidation from the cycling of oxygen during the interconversion of arsenate and arsenite. As a result, arsenolysis occurs that reduces ATP in the body due to the formation of anhydrides during glycolysis and oxidative phosphorylation. Additionally, the formation of reactive oxygen and nitrogen species also contributes to arsenic toxicity. Formed ROS are involved in genotoxicity, signalling, cell proliferation, and inhibition of DNA repair. Arsenic carcinogenicity includes inhibition of DNA repair under conditions of oxidative stress, inflammation, and proliferative signalling. There are many neurobehavioral disorders and nervous system disorders. Polyneuropathy, and electroencephalographic (EEG) abnormalities are some disorders caused due to arsenic exposure. Arsenic exposure to a mother during her pregnancy causes oxidative stress and slashes ATP production, causing improper development of the brain and altering normal neurobehavior. Arsenic exposure also interferes with cognitive function, particularly learning and remembering during childhood, and causes impaired learning and increased anxiety-like behaviour. Adults are more prone to peripheral and sensory neuropathy whereas children are more prone to neurodevelopmental syndromes such as attention-deficit hyperactivity disorder, intellectual disabilities, learning disorder, and autism.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Arsenic: An Overview
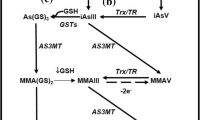
Arsenic is one of the prevalent naturally occurring elements. It (As) is a metalloid possessing properties of both metals and non-metals and has atomic no. 33 and an atomic weight of 74.92. It is a trace element as it is present in less than 1% (< 1%) of most rocks, coals, and soils (Alam et al. 2002). It is characterized as a white, yellow, grey metallic, or black solid that is odorless. It is highly toxic in nature. For centuries arsenic and its compounds have been in produced and utilized for commercial purposes like in pharmaceutical industry, agricultural industry, and semiconductor industry. Agricultural and industrial processes like mining and smelting contributes to high arsenic levels in the environment. Several areas of Japan, Mexico, Thailand, Brazil, Australia, and the USA have high arsenic levels in local water sources, due to mining, smelting, and other industrial activities (IARC 2004). However, Minerals and geogenic sources are primary sources of arsenic contamination with anthropogenic activities also contributing to it via extensive soil and water contamination throughout the world (Smith et al. 1998). Arsenic comes in three major forms: inorganic, organic and arsine gas (− 3 oxidative state), as well as three major valence states arsenic element (0), arsenite (trivalent + 3), and arsenate (pentavalent + 5) among which arsenite (+ 3) and arsenate (+ 5) are the most common toxic inorganic forms (Yousef et al. 2008). In general, trivalent arsenic compounds; inorganic (arsenite) and organic (monomethyl arsenic) are considered more toxic than pentavalent compounds. Arsenic, “when combined with carbon and hydrogen (in plants and animals) forms organic arsenic compounds whereas when combine with oxygen, sulfur, and chlorine in environment form inorganic arsenic compounds” (Martinez et al. 2011). Inorganic arsenic compounds are more prevalent in the environment and contribute more to toxicity. Arsanilic acid, monomethylarsonic acid (MMAV), dimethylarsonic acid (DMAV, also called cacodylic acid), trimethylarsonic acid (TMA) and arsenobetaine are some common organic arsenic compounds. Until the 1970s arsenic was used for medicinal purposes. For the treatment of leukemia, psoriasis, and chronic bronchial asthma, inorganic arsenic was used and for the treatment of spirochetal and protozoal disease organic arsenic was used in antibiotics (ATSDR 2007). It was considered that the father of medicine, Hippocrates used arsenic as a paste for the treatment of ulcers and abscesses. The arsenic paste appears to be beneficial for chemotherapeutic purposes as suggested by the pharmacology texts from the 1880s (Antman 2001). Arsenic organic compounds are used in the agricultural industry as well in the form of pesticides, herbicides, defoliants, and as soil sterilizing agents but in 2009 the US issued an order to remove organic arsenic-containing pesticides from agricultural practices by 2013 (EPA 2009) as the large area of agricultural land gets contaminated due to repeated use of arsenic-containing pesticides. Arsenic and its compounds are also used for a variety of industrial purposes like in the semiconductor and electronics industry, in the manufacturing of alloys, and also in the making of an anti-fungal wood preservative (Tchounwou et al. 1999).
Before the advent of penicillin, some organic arsenicals such as arsphenamine, salvarsan and their derivatives were used as anti-syphilitic agents (Globus and Ginsburg 1933; Osterberg and Kernohan 1934; Russell 1937). Some arsenic compounds are used to treat trypanosomiasis (Harrison et al. 1997) and acute promyelocytic leukemia (Look 1998).
2 Exposure to Arsenic
Based on known toxicity, arsenic is the most toxicant that poses substantial harm to human health and therefore ranked first among the toxicants (Hughes et al. 2011). Arsenic was used throughout history to kill the emperors for their wealth and empire because of many reasons like multiple ways of administration, its potency, and availability, and therefore called as “King of Poisons.” Nonetheless, arsenic is ubiquitous in the environment, the majority of organic and inorganic arsenic uptake by an individual comes from the diet. An average adult in the United States has an intake of 3.2 μg/day as per Schoof et al. (1999) and similar results were found for children as well (Yost et al. 2004) but the European Food Safety Authority (EFSA) estimated a higher intake level 9.1–39.2 μg/day for a 70 kg adult as estimates include the ratio of inorganic arsenic to total arsenic in food i.e., 0.13–0.56 μg/kg/day for an average consumer (EFSA 2009). The diet of an individual has both organic as well as inorganic forms of arsenic compounds and 25% of daily dietary arsenic intake comes from inorganic sources. It is considered that organic forms of arsenic are less toxic than the inorganic forms. Arsenic is found in highest concentration in seafood. Monomethylarsonic acid, DMAsV, arsenobetaine, arsenocholine, arsenosugars, and arsenolipids are arsenic compounds that are organic in nature and majorly found in food.
2.1 Exposure in Water
Inorganic forms of arsenic predominantly exist in water which stabilizes as (trivalent, + 3) arsenite and (pentavalent, + 5) arsenate (Saxe et al. 2006). Arsenic in drinking water at levels over the WHO recommended threshold of 10 ppb (parts per billion) was estimated to have contaminated approximately 140 million people in 2009 (Ravenscroft 2009). Over 1.5 million people in India have been estimated to be exposed to arsenic levels higher than the WHO threshold of 10 ppb leading to more than 200,000 cases of arsenicosis (de Castro et al. 2009). Arsenic ingestion via drinking water was found to be associated with increased cases of cancer and with some non-cancer effects like skin lesions, and neurological effects (NRC 2001).
2.2 Exposure in Soil
Globally, arsenic levels present naturally in soil ranges from 0.01 to over 600 mg/kg with a mean of 2–20 mg/kg. Mostly, inorganic forms of arsenic (trivalent and pentavalent) are present in the soil. Due to the oxidation of trivalent arsenicals, pentavalent arsenic compounds are found predominately in soil (Gong et al. 2001). There are numerous ways to be exposed to arsenic in the soil. Dermal absorption and inhalation of soil particles carried by the wind are some potential exposure routes but incidental ingestion is the most common pathway for the intake of arsenic in soil (Yan-Chu 1994). Numerous studies have revealed that less than 50% of arsenic in soil that is taken by mouth can be absorbed and used by the body (Roberts et al. 2002).
2.3 Exposure in Air
Arsenic exposure from the air is quite minimal compared to that of food and water. The contribution of air in arsenic exposure is less than 1% as per the data collected by European Commission (2000). Arsenic trioxide is an inorganic compound primarily involved in contaminating the air with arsenic. Cosmetic Products also contain arsenic in some amount and act as a source for direct arsenic exposure (Chung et al. 2014). Increasing exposure to arsenic via drinking water and contaminated food to a large population is a matter of great concern due to many toxic effects associated with arsenic (Chatterjee et al. 2010; Rahman et al. 2009).
3 Mode of Action of Toxicity by Arsenic
It is challenging to determine the method of action using the epidemiological literature since long-term exposures to arsenic are probably amplified by exposures to pollution. The harmful effects of arsenic are presumably the result of several pathways; in fact, these mechanisms may be interrelated. Trivalent arsenic compounds (arsenite) have more toxicity as compared to pentavalent arsenic compounds (arsenate) due to higher solubility and slower excretion rate.
Binz and Schulz suggested the arsenic’s initial proposed route of action in 1879 (Parascandola 1977). It suggested that both arsenicals are equally potent by describing how cellular oxidation is interfered with by oxygen cycling during the interconversion of arsenate and arsenite, but as it soon became clear that arsenite is more potent than arsenate, this hypothesis was quickly abandoned.
Phosphate and arsenate have similar properties (after protonation) due to their comparable structure, making arsenate capable of substituting phosphate in different metabolic reactions. Arsenate also forms a less stable ester bond with a higher bond length between As–O in comparison to the P–O bond formed between phosphate and its hydroxyl groups (Dixon 1996). In a process known as arsenolysis, arsenate decouples the production of adenosine 5-triphosphate (ATP) in vitro. This process occurs in the presence of arsenate during glycolysis and oxidative phosphorylation (OXPHOS). Both reactions result in the formation of unstable arsenate anhydrides that are simple to hydrolyse like 3-phosphoglyceroyl arsenate in case of the glycolytic pathway. The end outcome is a reduction in the production of ATP (Gresser 1981).
One of the most extensively researched mode of action (MOA) for arsenic toxicity currently is the production of reactive oxygen and nitrogen species by arsenic (Hughes and Kitchin 2006). There are number of the hypothesised mode of actions for arsenic, such as genotoxicity, cell proliferation, and suppression of DNA repair, that include reactive oxygen species generated by arsenic. Reactive oxygen species (ROS) can be formed by arsenic in a variety of reactions such as during the conversion of arsenite to arsenate (Del Razo et al. 2001), during the metabolism of arsenic resulting in the formation of arsine (Yamanaka and Okada 1994).
“Deletion mutations, oxidative DNA damage, breaks in DNA strand, sister chromatid exchanges, chromosomal abnormalities, aneuploidy, and micronuclei are some of the impacts of arsenic’s genotoxicity” (Basu et al. 2001; Hei et al. 1998; Rossman 2003). Studies on human cell nuclear extracts revealed that arsenic’s indirect effect of inhibiting DNA repair was brought on by the generation of ROS or by altered cell signalling that altered gene expression (Hu et al. 1998). Arsenic also affects the working of enzymes involved in repair mechanisms such as nucleotide and base excision repair (Hartwig et al. 2003). Arsenic trivalent compounds interact with the zinc finger motifs of proteins and disrupt the function of proteins by moving zinc from its binding site causing inhibition of base excision repair (BER) and nucleotide excision repair (NER) activity (Ding et al. 2009; Pia̧tek et al. 2008).
Gentry et al. (2009) examined in vitro cellular and in vivo gene expression alterations after exposure to inorganic arsenic and concluded that arsenic inhibits DNA repair as a method of action for its carcinogenic effect. The findings suggested that DNA repair inhibition under the influence of oxidative stress, inflammation, and proliferative signalling is one of the important processes in arsenic’s carcinogenicity. Such circumstances could result in mitosis progressing without preserving the integrity of the cellular DNA.
Arsenic by altering the signal transduction pathways can regulate the expression of transcription factors and proteins (Bode and Dong 2002; Druwe and Vaillancourt 2010; Huang et al. 2004; Kumagai and Sumi 2007; Leonard et al. 2004; Platanias 2009). In vitro, arsenite activated the protein p38, a component of the mitogen-activated protein kinase (MAPK) cascade (Rouse et al. 1994). Arsenic also activates the c-Jun N-terminal kinases (JNKs) and extracellular-regulated protein kinases (ERKs), two other components of the MAPK pathway (Bode and Dong 2002; Yang and Frenkel 2002). Arsenic also affects the transcription factors nuclear factor-κβ (NF-κβ) and (Nrf2) nuclear factor erythroid-2-related factor 2 (Kumagai and Sumi 2007). By altering a reactive thiol in Iκβ kinase, arsenite seems to prevent activation of tumor necrosis factor-α induced NF-κβ (Roussel and Barchowsky 2000; Shumilla et al. 1998). With the help of generating ROS, arsenic also found to activate NF-κβ (Felix et al. 2005; Wijeweera et al. 2001).
Inorganic arsenic compounds expresses the growth factors to such an extent that it results in a condition called hyperkeratosis which is an indication of arsenic toxicity in humans (Germolec et al. 1997).
Arsenic alters the methylation in DNA, according to investigations done by Zhao et al. (1997). It is unclear what the mechanism is for this. However, dietary factors, DNA methyltransferase inhibition, or shunting of the methyl donor, S-adenosylmethionine for the methylation of both DNA and arsenic are some of the reasons for hypomethylation (Chanda et al. 2006).
Arsenite and arsenate can be transported by human RBCs using anion exchange proteins (Zhang et al. 2000). The necessary sulfhydryl groups of proteins and enzymes are blocked by arsenite due to its interactions with thiol groups present in them. As a result, it disrupt the activity of enzymes involved in the metabolism of carbohydrates such as pyruvate dehydrogenase (Aposhian 1989). Arsenite causes cytoskeletal components to become disorganized once it enters the cell (Li and Chou 1992; Ramirez et al. 1997).
4 Effect of Arsenic on Neurological Function in Human
An unidentified mechanism allows arsenic to reach the brain. It builds up in the choroid plexus, preventing arsenic from entering the brain (Zheng et al. 1991). It induces changes in neurotransmitter levels and cause alterations in functions (Rodríguez et al. 2001). Neural health and behaviour of an individual get affected by the accumulation of arsenic during the childhood stage (Tsai et al. 2003). Arsenic-induced neuritis is a well-known side effect of arsenic toxicity and is known to impair the sensory capabilities of the peripheral nerves. Several other neurological conditions, such as polyneuropathy and aberrant electroencephalographic (EEG) are also induced by arsenic exposure (Rodríguez et al. 2003). Additionally, it has the ability to activate the p38 MAPK and JNK3 genes, which may result in Alzheimer’s disease (Gharibzadeh and Hoseini 2008).
According to the studies, arsenic exposure via drinking water is linked to neurodegeneration, including oxidative stress, damaged protein degradation, intracellular accumulation and autophagy, mitochondrial dysfunction, and more (Escudero-Lourdes 2016). Arsenic exposure via dust and drinking water can also cause damage to the peripheral nerves (Gerr et al. 2000; Mazumder et al. 2010). Arsenic exposure during pregnancy causes oxidative stress and decreased ATP generation, endangering the structural and functional maturity of nerve cells and impairing brain development as well as associated behaviours (Gandhi and Kumar 2013). Arsenic exposure to copper smelters causes them to exhibit a lower rate of conduction of nerve signals and damage to peripheral nerves (Lagerkvist and Zetterlund 1994). Additionally, they may have muscle tiredness, irritability, headaches, severe muscle spasms in their extremities, and lethargy or lack of sleep (Sinczuk-Walczak et al. 2010). Rising urine arsenic levels negatively affect processing speed and fine motor function (Carroll et al. 2017). Arsenic exposure at workplace can lead to neurological and electromyographic abnormalities (Blom et al. 1985). Exposure in mines can be harmful and causes sensory neuropathy and hearing impairment (Ishii et al. 2018).
5 Effect of Arsenic on Cognitive Function in Human
Numerous epidemiological studies have indicated that arsenic exposure can affect how well people think and learn, especially in young children. Children’s intellectual development may be harmed more by chronic moderate exposure to arsenic than by severe acute exposure. Children exposed to arsenic had an IQ drop of 0.4, which can have collective effects in later stage of life (Rodríguez-Barranco et al. 2013). In youngsters between the ages of 6 and 8 years old, a 2007 study discovered a strong correlation between urine arsenic concentrations above 50 g/L and poor performance on tests of remembrance, cognitive, visual and spatial reasoning, linguistic development (Rosado et al. 2007). These children also exhibited symptoms of Attention Deficit Hyperactive Disorder (Roy et al. 2011). Additionally, several studies suggested that arsenic hinders young females’ growth and development more than males, which may have an impact on cognitive function (Gardner et al. 2013). The likelihood of intellectual disability in children rises as the concentration of arsenic and lead increases in the soil, and the prevalence of mental retardation is significantly connected with the presence of soil metals like arsenic, copper, lead, manganese, etc. (Aelion et al. 2008; McDermott et al. 2011). Exposure to arsenic during the gestation and lactation period can cause nitric oxide dysfunction in brain (Zarazúa et al. 2006). “The research showed that prolonged exposure can impair pattern memory and attention switching” (Tsai et al. 2003). Low-level prenatal arsenic exposure and early children’s neurobehavioral performance have been found to have an inverse relationship. Prenatal arsenic exposure can impact newborn infant neurobehavioral development (Wang et al. 2018). In addition, postnatal exposure exhibited impaired learning and increased anxiety-like behaviors (Zhou et al. 2018). Flawed Memory, sleep dysfunction, and visual disruption are among the signs of temporal and occipital lobe exposure to DPAA that are brought on by water consumption (Ishi and Tamaoka 2015). Inorganic arsenic exposure during pregnancy, according to Ramos-Chávez et al. (2015), affected the development of cysteine/glutamate transporters in the cortex and hippocampus and also caused an unfavourable regulation of the NMDA receptor (NMDAR) NR2B subunit in the hippocampus. When exposed to arsenic, there is also a decrease in acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) levels as well as a drop in motor coordination (Sharma et al. 2018). Children are more likely to have neurodevelopmental syndromes such as autism spectrum disorders, cognitive impairments, intellectual disabilities, and attention deficit hyperactivity disorder (Schug et al. 2015).
6 Conclusion
A common metalloid, arsenic can be found in food, water, and items manufactured by humans. Numerous epidemiological investigations have produced evidence pointing to a substantial link between exposure to arsenic and neurological and cognitive impairment in both children and adults. Multiple systems and particular pathways involved in various elements of learning, memory, mobility, decision-making, and mood are all impacted by arsenic exposure. Most people are exposed to arsenic through their diet and water consumption. Other sources of exposure include using arsenic as a pesticide, a by-product of smelting metal ore, a chemotherapeutic agent, or coming into contact with arsenic-contaminated soil. Chronic exposure to arsenic damages the peripheral nervous system by causing peripheral neuropathy, whereas acute and occupational exposure to arsenic compounds has been linked to encephalopathy and the impairment of higher neurological processes in patients. Arsenic exposure has been linked to skin, lung, and bladder cancers, according to research. Arsenic exposure results in a large number of health-related problems around the world, and it should be considered a serious threat to humans. Treatment of the afflicted areas should have broader consequences for issues with public health. Arsenic exposure needs to be reduced or eliminated. Arsenic levels in drinking water need to be constantly tracked and checked. Affected areas by arsenic should also have access to clean drinking water. When using cosmetics and when eating a diet, precautions should be made. Arsenic levels in drinking water need to be constantly tracked and checked. Affected areas by arsenic should also have access to clean drinking water. When using cosmetics and when eating a diet, precautions should be made. Arsenic-related health risks to people can be lessened by carefully examining potential sources of exposure.
References
Aelion CM, Davis HT, McDermott S, Lawson AB (2008) Metal concentrations in rural topsoil in South Carolina: potential for human health impact. Sci Total Environ 402(2–3):149–156
Alam MGM, Allinson G, Stagnitti F, Tanaka A, Westbrooke M (2002) Arsenic contamination in Bangladesh groundwater: a major environmental and social disaster. Int J Environ Health Res 12(3):235–253
Antman KH (2001) Introduction: the history of arsenic trioxide in cancer therapy. Oncologist 6(S2):1–2
Aposhian HV (1989) Biochemical toxicology of arsenic. Rev Biochem Toxicol 10:265–299
ATSDR (2007) CERCLA priority list of hazardous substances. http://www.atsdr.cdc.gov/cercla/07list.html
Basu A, Mahata J, Gupta S, Giri AK (2001) Genetic toxicology of a paradoxical human carcinogen, arsenic: a review. Mutat Res/rev Mutat Res 488(2):171–194
Blom S, Lagerkvist B, Linderholm H (1985) Arsenic exposure to smelter workers: clinical and neurophysiological studies. Scand J Work Environ Health 265–269
Bode AM, Dong Z (2002) The paradox of arsenic: molecular mechanisms of cell transformation and chemotherapeutic effects. Crit Rev Oncol Hematol 42(1):5–24
Carroll CR, Noonan C, Garroutte EM, Navas-Acien A, Verney SP, Buchwald D (2017) Low-level inorganic arsenic exposure and neuropsychological functioning in American Indian elders. Environ Res 156:74–79
Chanda S, Dasgupta UB, GuhaMazumder D, Gupta M, Chaudhuri U, Lahiri S, Das S, Ghosh N, Chatterjee D (2006) DNA hypermethylation of promoter of gene p53 and p16 in arsenic-exposed people with and without malignancy. Toxicol Sci 89(2):431–437
Chatterjee D, Halder D, Majumder S, Biswas A, Nath B, Bhattacharya P et al (2010) Assessment of arsenic exposure from groundwater and rice in Bengal Delta Region, West Bengal, India. Water Res 44(19):5803–5812
Chung JY, Yu SD, Hong YS (2014) Environmental source of arsenic exposure. J Prev Med Public Health 47(5):253
de Castro MR, Lima JV, de Freitas DPS, de Souza Valente R, Dummer NS, de Aguiar RB et al (2009) Behavioral and neurotoxic effects of arsenic exposure in zebrafish (Danio rerio, Teleostei: Cyprinidae). Comp Biochem Physiol C Toxicol Pharmacol 150(3):337–342
Del Razo LM, Quintanilla-Vega B, Brambila-Colombres E, Calderón-Aranda ES, Manno M, Albores A (2001) Stress proteins induced by arsenic. Toxicol Appl Pharmacol 177(2):132–148
Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, Liu KJ (2009) Inhibition of poly (ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J Biol Chem 284(11):6809–6817
Dixon HB (1996) The biochemical action of arsonic acids especially as phosphate analogues. In: Advances in inorganic chemistry, vol 44. Academic Press, pp 191–227
Druwe IL, Vaillancourt RR (2010) Influence of arsenate and arsenite on signal transduction pathways: an update. Arch Toxicol 84:585–596
EPA (U.S. Environmental Protection Agency) (2009) Organic arsenicals; Product cancellation order and amendments to terminate uses (EPA-HQ-OPP-2009-0191; FRL-8437-7)
Escudero-Lourdes C (2016) Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: role of oxidative stress and inflammatory responses. Neurotoxicology 53:223–235
European Commission (2000) Ambient air pollution by As, Cd and Ni compounds (position paper—final), p 318. Available at: http://ec.europa.eu/environment/air/pdf/pp_as_cd_ni.pdf
European Food Safety Authority (EFSA) (2009) Scientific opinion on arsenic in food. EFSA J 7:1351
Felix K, Manna SK, Wise K, Barr J, Ramesh GT (2005) Low levels of arsenite activates nuclear factor-κB and activator protein-1 in immortalized mesencephalic cells. J Biochem Mol Toxicol 19(2):67–77
Gandhi DN, Kumar R (2013) Arsenic toxicity and neurobehaviors: a review. Innov Pharm Pharmacother 1(1):1–15
Gardner RM, Kippler M, Tofail F, Bottai M, Hamadani J, Grandér M, Nermell B, Palm B, Rasmussen KM, Vahter M (2013) Environmental exposure to metals and children’s growth to age 5 years: a prospective cohort study. Am J Epidemiol 177(12):1356–1367
Gentry PR, McDonald TB, Sullivan DE, Shipp AM, Yager JW, Clewell HJ (2009) Analysis of genomic dose-response information on arsenic to inform key events in a mode of action for carcinogenicity. Environ Mol Mutagen 51(1):1–4. https://doi.org/10.1002/em.20505
Germolec DR, Spalding J, Boorman GA, Wilmer JL, Yoshida T, Simeonova PP et al (1997) Arsenic can mediate skin neoplasia by chronic stimulation of keratinocyte-derived growth factors. Mutat Res/rev Mutat Res 386(3):209–218
Gerr F, Letz R, Ryan PB, Green RC (2000) Neurological effects of environmental exposure to arsenic in dust and soil among humans. Neurotoxicology 21(4):475–487
Gharibzadeh S, Hoseini SS (2008) Arsenic exposure may be a risk factor for Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 20(4):501–501
Globus JH, Ginsburg SW (1933) Pericapillary encephalorrhagia due to arsphenamine: so-called arsphenamine encephalitis. Arch Neurol Psychiatry 30(6):1226–1247
Gong Z, Lu X, Cullen WR, Le XC (2001) Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J Anal at Spectrom 16(12):1409–1413
Gresser MJ (1981) ADP-arsenate. Formation by submitochondrial particles under phosphorylating conditions. J Biol Chem 256(12):5981–5983
Harrison SM, Harris RW, Bales JD Jr (1997) Attempt to correlate urine arsenic excretion with clinical course during melarsoprol therapy of patients with Rhodesian trypanosomiasis. Am J Trop Med Hygiene 56(6):632–636
Hartwig A, Blessing H, Schwerdtle T, Walter I (2003) Modulation of DNA repair processes by arsenic and selenium compounds. Toxicology 193(1–2):161–169
Hei TK, Liu SX, Waldren C (1998) Mutagenicity of arsenic in mammalian cells: role of reactive oxygen species. Proc Natl Acad Sci 95(14):8103–8107
Hu Y, Su L, Snow ET (1998) Arsenic toxicity is enzyme specific and its affects on ligation are not caused by the direct inhibition of DNA repair enzymes. Mutat Res/DNA Rep 408(3):203–218
Huang C, Ke Q, Costa M, Shi X (2004) Molecular mechanisms of arsenic carcinogenesis. Mol Cell Biochem 255:57–66
Hughes MF, Kitchin KT (2006) Arsenic, oxidative stress, and carcinogenesis. In: Oxidative stress, disease and cancer, pp 825–850
Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ (2011) Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123(2):305–332
IARC (2004) Working group on the evaluation of carcinogenic risks to humans, international agency for research on cancer. Some drinking-water disinfectants and contaminants, including arsenic. IARC
Ishi K, Tamaoka A (2015) Ten-years records of organic arsenic (diphenylarsinic acid) poisoning: epidemiology, clinical feature, metabolism, and toxicity. Brain and Nerve = Shinkei Kenkyu no Shinpo 67(1):5–18
Ishii N, Mochizuki H, Ebihara Y, Shiomi K, Nakazato M (2018) Clinical symptoms, neurological signs, and electrophysiological findings in surviving residents with probable arsenic exposure in Toroku, Japan. Arch Environ Contam Toxicol 75:521–529
Kumagai Y, Sumi D (2007) Arsenic: signal transduction, transcription factor, and biotransformation involved in cellular response and toxicity. Annu Rev Pharmacol Toxicol 47:243–262
Lagerkvist BJ, Zetterlund B (1994) Assessment of exposure to arsenic among smelter workers: a five-year follow-up. Am J Ind Med 25(4):477–488
Leonard SS, Harris GK, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Radic Biol Med 37(12):1921–1942
Li W, Chou IN (1992) Effects of sodium arsenite on the cytoskeleton and cellular glutathione levels in cultured cells. Toxicol Appl Pharmacol 114(1):132–139
Look AT (1998) Arsenic and apoptosis in the treatment of acute promyelocytic leukemia. JNCI J Natl Cancer Inst 90(2):86–88
Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL (2011) Arsenic exposure and the induction of human cancers. J Toxicol 2011
Mazumder DNG, Ghosh A, Majumdar KK, Ghosh N, Saha C, Mazumder RNG (2010) Arsenic contamination of ground water and its health impact on population of district of Nadia, West Bengal, India. Indian J Community Med 35(2):331
McDermott S, Wu J, Cai B, Lawson A, Aelion CM (2011) Probability of intellectual disability is associated with soil concentrations of arsenic and lead. Chemosphere 84(1):31–38
NRC (2001) Arsenic in drinking water. Update to the 1999 arsenic in drinking water report
Osterberg AE, Kernohan JW (1934) The presence of arsenic in the brain and its relation to pericapillary hemorrhages or so-called acute hemorrhagic encephalitis. Am J Clin Pathol 4(4):362–369
Parascandola J (1977) Carl Voegtlin and the ‘arsenic receptor’ in chemotherapy. J Hist Med Allied Sci 32(2):151–171
Pia̧tek K, Schwerdtle T, Hartwig A, Bal W (2008) Monomethylarsonous acid destroys a tetrathiolate zinc finger much more efficiently than inorganic arsenite: mechanistic considerations and consequences for DNA repair inhibition. Chem Res Toxicol 21(3):600–606
Platanias LC (2009) Biological responses to arsenic compounds. J Biol Chem 284(28):18583–18587
Rahman MM, Naidu R, Bhattacharya P (2009) Arsenic contamination in groundwater in the Southeast Asia region. Environ Geochem Health 31:9–21
Ramirez P, Eastmond DA, Laclette JP, Ostrosky-Wegman P (1997) Disruption of microtubule assembly and spindle formation as a mechanism for the induction of aneuploid cells by sodium arsenite and vanadium pentoxide. Mutat Res/rev Mutat Res 386(3):291–298
Ramos-Chávez LA, Rendón-López CR, Zepeda A, Silva-Adaya D, Del Razo LM, Gonsebatt ME (2015) Neurological effects of inorganic arsenic exposure: altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front Cell Neurosci 9:21
Ravenscroft P, Brammer H, Richards K (2009) Arsenic pollution: a global synthesis. John Wiley & Sons
Roberts SM, Weimar WR, Vinson JRT, Munson JW, Bergeron RJ (2002) Measurement of arsenic bioavailability in soil using a primate model. Toxicol Sci 67(2):303–310
Rodríguez VM, Carrizales L, Jimenez-Capdeville ME, Dufour L, Giordano M (2001) The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull 55(2):301–308
Rodríguez VM, Jiménez-Capdeville ME, Giordano M (2003) The effects of arsenic exposure on the nervous system. Toxicol Lett 145(1):1–18
Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B, Rojas-García A (2013) Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 454:562–577
Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P et al (2007) Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ Health Perspect 115(9):1371–1375
Rossman TG (2003) Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res/fundam Mol Mech Mutagenesis 533(1–2):37–65
Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR (1994) A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78(6):1027–1037
Roussel RR, Barchowsky A (2000) Arsenic inhibits NF-κB-mediated gene transcription by blocking IκB kinase activity and IκBα phosphorylation and degradation. Arch Biochem Biophys 377(1):204–212
Roy A, Kordas K, Lopez P, Rosado JL, Cebrian ME, Vargas GG, Ronquillo D, Stoltzfus RJ (2011) Association between arsenic exposure and behavior among first-graders from Torreón, Mexico. Environ Res 111(5):670–676
Russell DS (1937) Changes in the central nervous system following arsphenamine medication. J Pathol Bacteriol 45(2):357–366
Saxe JK, Bowers TS, Reid KR (2006) Arsenic. In: Morrison RD, Murphy BL (eds) Environmental forensics: contaminant specific guide. Academic Press, Burlington, MA, pp 279–292
Schoof RA, Eickhoff J, Yost LJ, Crecelius EA, Cragin DW, Meacher DM, Menzel DB (1999) Dietary exposure to inorganic arsenic. In: Arsenic exposure and health effects III. Elsevier Science Ltd., pp 81–88
Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP (2015) Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology 156(6):1941–1951
Sharma A, Kshetrimayum C, Sadhu HG, Kumar S (2018) Arsenic-induced oxidative stress, cholinesterase activity in the brain of Swiss albino mice, and its amelioration by antioxidants Vitamin E and Coenzyme Q10. Environ Sci Pollut Res 25:23946–23953
Shumilla JA, Wetterhahn KE, Barchowsky A (1998) Inhibition of NF-κB binding to DNA by chromium, cadmium, mercury, zinc, and arsenite in vitro: evidence of a thiol mechanism. Arch Biochem Biophys 349(2):356–362
Sinczuk-Walczak H, Szymczak M, Halatek T (2010) Effects of occupational exposure to arsenic on the nervous system: clinical and neurophysiological studies. Int J Occup Med Environ Health 23(4):347–355
Smith ERG, Naidu R, Alston AM (1998) Arsenic in the soil environment
Tchounwou PB, Wilson B, Ishaque A (1999) Important considerations in the development of public health advisories for arsenic and arsenic-containing compounds in drinking water. Rev Environ Health 14(4):211–229
Tsai SY, Chou HY, The HW, Chen CM, Chen CJ (2003) The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 24(4–5):747–753
Wang B, Liu J, Liu B, Liu X, Yu X (2018) Prenatal exposure to arsenic and neurobehavioral development of newborns in China. Environ Int 121:421–427
Wijeweera JB, Gandolfi AJ, Parrish A, Lantz RC (2001) Sodium arsenite enhances AP-1 and NFκ B DNA binding and induces stress protein expression in precision-cut rat lung slices. Toxicol Sci 61(2):283–294
Yamanaka K, Okada S (1994) Induction of lung-specific DNA damage by metabolically methylated arsenics via the production of free radicals. Environ Health Perspect 102(Suppl. 3):37–40
Yan-Chu H (1994) Arsenic distribution in soils. In: Nriagu JO (ed) Arsenic in the environment, part I; cycling and characterization. Wiley, Hoboken, NJ, pp 17–47
Yang C, Frenkel K (2002) Arsenic-mediated cellular signal transduction, transcription factor activation and aberrant gene expression: implications in carcinogenesis. J Environ Pathol Toxicol Oncol 21:331–342
Yost LJ, Tao SH, Egan SK, Barraj LM, Smith KM, Tsuji JS, Lowney YW, Schoof RA, Rachman NJ (2004) Estimation of dietary intake of inorganic arsenic in US children. Hum Ecol Risk Assess 10(3):473–483
Yousef MI, El-Demerdash FM, Radwan FM (2008) Sodium arsenite induced biochemical perturbations in rats: ameliorating effect of curcumin. Food Chem Toxicol 46(11):3506–3511
Zarazúa S, Pérez-Severiano F, Delgado JM, Martínez LM, Ortiz-Pérez D, Jiménez-Capdeville ME (2006) Decreased nitric oxide production in the rat brain after chronic arsenic exposure. Neurochem Res 31:1069–1077
Zhang TL, Gao YX, Lu JF, Wang K (2000) Arsenite, arsenate and vanadate affect human erythrocyte membrane. J Inorg Biochem 79(1–4):195–203
Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP (1997) Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci USA 94:10907–10912
Zheng W, Perry DF, Nelson DL, Aposhian HV (1991) Choroid plexus protects cerebrospinal fluid against toxic metals. FASEB J 5(8):2188–2193. https://doi.org/10.1096/fasebj.5.8.1850706
Zhou H, Zhao W, Ye L, Chen Z, Cui Y (2018) Postnatal low-concentration arsenic exposure induces autism-like behavior and affects frontal cortex neurogenesis in rats. Environ Toxicol Pharmacol 62:188–198
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Arya, I., Bhardwaj, A., Karn, S.K. (2023). The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human. In: Kumar, N., Kumar, S. (eds) Arsenic Toxicity Remediation: Biotechnological Approaches . Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-37561-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-37561-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-37560-6
Online ISBN: 978-3-031-37561-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)