Abstract
A comparison is made between the effects of natural rubber (NR), liquid natural rubber (LNR), and recycled natural rubber (rNR) in the filled epoxy systems on the physical, mechanical, thermal, and electrical performances of filled epoxy systems. The results show that flexural strength and modulus values were improved. The toughness properties of the filled epoxy system were enhanced with NR phases (72 MPa, 2317 MPa, 4.2 MPa.m½), as compared to those with LNR (55 MPa, 2100 MPa, 3.2 MPa.m½) and rNR (52 MPa, 2000 MPa, 2.3 MPa.m½) at 5 vol.%. Scanning electron micrograph (SEM) analysis revealed that the particle sizes of NR phases dispersed within the epoxy matrix were smaller and more uniform (0.29–1.65 μm) as compared to those with LNR (0.64–3.57 μm) and rNR (≥250 μm) phases. The incorporation of NR, LNR, and rNR phases improved the thermal stability of the filled system. This is attributed to more heat energy being needed to overcome good interfacial bonding between epoxy matrices and the small NR phases. X-ray diffraction analysis results showed that the filled epoxy/NR/GNP system has higher 2θ values, indicating that d-spacing in GNP nano-fillers has the closer distance. Electrical bulk conductivity values of filled epoxy/NR/GNP systems were the highest, 4.50 × 10−3 1/Ω.cm at 20 vol.%. Small NR phases acted as elastomer spacers, which provided better GNP packing efficiency and realigned the GNP nano-fillers to form more effective conductive pathways for electron transport.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
This study focuses on the effect of natural rubber (NR), liquid natural rubber (LNR), and recycled natural rubber (rNR) as secondary matrices on the properties of filled epoxy systems. NR is classified as a renewable and sustainable biopolymer, which is usually tapped and extracted from tropical rubber trees, known as Hevea Brasiliensis (Krishna Kumar et al. 2021; Radabutra et al. 2021). NR is extensively used in an enormous range of applications due to its high elongation, high elasticity, and resilience (Chawalitsakunchai et al. 2021). The main application of natural rubber latex is in glove application, like household or medical gloves. The NR gloves waste has created landfill problems. Thus, it is necessary to overcome the landfill problems by recycling the NR gloves. Therefore, NR was used to improve the toughness properties of epoxy resins, since it is a renewable resource and is easily available in Malaysia (Rotrekl et al. 2013).
Epoxy resins are one of the most important classes of amorphous highly cross-linked thermosetting polymers and exhibit prominent properties such as high stiffness, creep resistance, chemical resistance, corrosion resistance, thermal resistance, good dimensional stability, and low shrinkage (Puglia et al. 2013). Epoxy resins are extensively used in aerospace applications, automotive, electronic components, electrical laminates, adhesives, coating, and energy devices (Mathew et al. 2010). The most widely used epoxy resins are epichlorohydrin and bisphenol-A-derived resin (Cantoni et al. 2021). Once epoxy resins are cured with the corresponding hardener, a highly cross-linked three-dimensional network structure is formed. The high cross-link density is essential for thermosetting resin to attain excellent mechanical properties. However, the chief drawbacks of epoxy are their low toughness and impact resistance (Wang et al. 2013a, b). In order to address this issue, one of the successful approaches to modify the epoxy resin involved incorporation of natural rubber (NR) as the secondary matrix into the epoxy matrix, thereby forming an immiscible two-matrix morphology (two-phase structure). Two-matrix system can be obtained by reaction-induced phase separation (RIPS) of an initially epoxy/NR co-continuous structure. According to the Flory-Huggins mean field theory, epoxy/NR systems no longer remain homogenous as the average molecular weight of epoxy resin is increased up to an extent. During the curing reaction, the molecular weight of the epoxy matrix increases to a critical extent and the system starts phase separating through spinodal decomposition. The curing rate of the epoxy matrix is significantly higher, resulting in the precipitation of spherical NR phases (Hong and Chan 2004; Tan et al. 2013).
Epoxy resins and NR are polymeric materials, which are usually classified as insulating materials. The incorporation of promising conductive nano-fillers like copper (Pargi et al. 2015) and even recycled metal (Ilyas et al. 2021a, b) causes the material to gradually transition from an insulator to a conductor. In this regard, the discovery of graphene is an important addition. Graphene consists of two-dimensional nano-fillers with a single atom thick structure of sp2 bonded carbon atoms, which is densely packed in a hexagonal honeycomb crystal lattice structure (Verma et al. 2014). Graphene nano-platelets (GNP) have outstanding properties such as high aspect ratio, high strength, modulus, and surface conductivity, which make them ideal fillers for polymer nanocomposites used in advanced materials applications (Yue et al. 2014). It is important to note that graphene is much cheaper than single-walled carbon nanotubes (CNTs) because it can easily be derived from graphite precursors in large quantities (Du and Cheng 2012). The wrinkled topology and nano-scale surface roughness of graphene have increased mechanical interlocking with polymer chains, thus strengthening the interaction and increasing the maximum load transfer between graphene and polymer matrix.
The present work attempts to investigate the effect of different forms of vulcanized NR on the physical, mechanical, thermal, and electrical performances of filled epoxy systems. For effective rubber toughening epoxy, the optimum particle sizes of rubber phases are within the range of 0.1–5 μm. Large rubber phases (>5 μm) are too large to interact with the stress field at the crack tip, while small rubber phases (<0.1 μm) are too small to cavitate effectively and do not take part in the toughening epoxy matrix (Kam et al. 2018). Tan et al. have indicated liquid epoxidized natural rubber is a good potential toughening agent for epoxy resins (Tan et al. 2013). Tan et al. described the use of natural rubber as toughening agent in low-density polyethylene systems (Tan et al. 2013). Ozturk et al. indicated mechanical and thermal properties of epoxy resins can be enhanced with hydroxyl terminated polybutadiene (HTPB) liquid rubber (Ozturk et al. 2001). The incorporation of GNP nano-fillers has transformed the two-matrix system from an insulator to a semiconductor. The percolation threshold of GNP nano-fillers, that is 0.8 vol.%, was applied in the filled systems based on previous studies (Kam et al. 2018). The electrical performance of the filled epoxy system can be further enhanced by rubber phases (elastomer spacers). The elastomer spacers pushed the GNP nano-fillers closer to each other, leading to the formation of more effective conductive pathways for electron conduction. Similar findings reported by Phua et al. (2017) identified that electrical conductivity of filled systems improved with 10 vol.% of thermoplastic spacer. With the incorporation of 10 vol.% PMMA-spacer, the filled system shows promising improvement in electrical conductivity, with an order of magnitude increase at 15 vol.% CB loading (Phua et al. 2017). The fractured surface and two-matrix morphology were examined by scanning electron microscope (SEM) to observe the deformation and toughening mechanism. Different forms of vulcanized NR acted as elastomer spacers that aided in the improvement on electrical conductivity of filled systems were compared.
2 Experimental
2.1 Materials
Epoxy resin (DGEB-A) with epoxide equivalent weight 182–192 and epoxy hardener with amine value 260–284 (mg KOH/gm) were purchased from Euro Chemo-Pharma Sdn. Bhd. (Malaysia). Natural rubber (cis 1,4-polyisoprene) was purchased from Malaysian Rubber Board (Malaysia). Liquid natural rubber (LNR) was purchased from Zarm Scientific & Supplies Sdn. Bhd. (Malaysia). Recycled natural rubber (rNR) derived from recycled gloves was purchased from SWJ Global Enterprise Sdn. Bhd. (Malaysia). GNP was purchased from SkySpring Nanomaterials (USA), with thickness of 11–15 nm. Toluene (C6H5OH) was purchased from Fisher Scientific Company L.L.C. (USA). Hydrogen peroxide (H2O2) was purchased from HmbG Chemical (Germany). Acetic acid (CH3COOH) was purchased from HmbG chemical (Germany). Vulcanizing agents such as zinc oxide (ZnO), stearic acid (CH3(CH2)16COOH), n-cyclohexyl-2-benzothiazolesulfenamide (CBS), and sulfur were purchased from Malaysian Rubber Board (Malaysia).
2.2 Sample Preparation
2.2.1 Photo-Depolymerization of NR and LNR
Photo-depolymerization was carried out under sunlight as reported by Radhakrishnan. For both NR and LNR, photo-depolymerization of rubber in a fixed volume of toluene was done using 30 wt.% of hydrogen peroxide and 0.35 mol of acetic acid (Kam et al. 2018). The mixture was stirred for 1 h. The milky NR solution was then left under sunlight for 2 weeks. The milky NR solution turned pale yellow after 2 weeks. The depolymerized NR was coagulated using methanol to remove the residues. Finally, the coagulants were dried in oven at 90 °C for 2 days. Before photo-depolymerization, the molecular weight of NR was determined, Mn (number average molecular weight) = 68,699 g/mol, Mw (weight average molecular weight) = 183,510 g/mol, and PDI (polydispersity index) = 2.671. After depolymerization, the molecular weight of depolymerized NR was reduced to Mn = 1688 g/mol, Mw = 38,527 g/mol, and PDI = 22.824 while the molecular weight of depolymerized LNR was reduced to Mn = 675 g/mol, Mw = 6427 g/mol, and PDI = 9.521.
2.2.2 Compounding of Two-Matrix Filled Epoxy/NR/GNP and Epoxy/LNR/GNP Systems
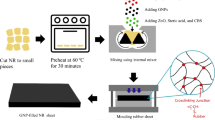
For filled epoxy/NR/GNP system, the compositions of epoxy/NR were prepared (95/5, 90/10, 85/15, 80/20, 75/25, and 70/30 vol.%). During the initial stage, depolymerized NR (DNR) was dissolved in epoxy resin via magnetic stirring at 60 °C. After NR was completely dissolved, vulcanizing agents such as zinc oxide (5 phr), stearic acid (2 phr), and CBS (1.2 phr) based on 100 phr of NR were added into the epoxy/NR mixture and stirred for 10 min. 0.8 vol.% of GNP nano-fillers were added into the mixture and stirred for another 20 min. Afterward, the mixture was placed in an ultrasonic water bath for 30 min (53 kHz and 60 °C). Sonication is an effective way to suppress the re-aggregation of GNP nano-fillers. The sulfur (1.5 phr) was added into the mixture and stirred for 5 min. At last, the mixture was stirred together with epoxy hardener and cured in an oven, at 90 °C for 2 h. The compounding of two-matrix filled epoxy/LNR/GNP system was done similarly as two-matrix filled epoxy/NR/GNP systems, except LNR was used instead of NR.
2.2.3 Compounding of Two-Matrix Filled Epoxy/rNR/GNP System
For filled epoxy/rNR/GNP system, the composition of epoxy/NR was prepared (95/5, 90/10, 85/15, 80/20, 75/25, and 70/30 vol.%). The rNR was milled and sieved to obtain a maximum particle size of 250 μm. During the initial stage, rNR was added into the epoxy resins and mixed for 2 h. 0.8 vol.% of GNP nano-fillers were added into the mixture and stirred for 20 min. The mixture was placed in an ultrasonic water bath for 30 min (53 kHz and 60 °C). The mixture was stirred together with epoxy hardener and cured in an oven, at 90 °C for 2 h.
2.3 Characterizations
Density measurements were performed using a pycnometer, model Micromeritics AccuPyc II 1340, under the flow of helium gas at room temperature. The theoretical density of the filled systems was calculated based on previous finding (Fuad et al. 1993). Flexural properties were measured under a three-point bending approach according to ASTM D790, using an Instron machine (Model 5569), with a span length of 50 mm and a cross-head speed of 2.38 mm/min. The applied load was 50 kN (Kutz 2002). The size of rectangular samples used was 60 × 12.7 × 3 mm. Fracture toughness (KIC) was performed using Universal Instron testing machine (Model 5569), with a cross-head speed at 1 mm/min, according to the standard of ISO 13586 under Mode I (tensile opening). The rectangular samples were notched at one-third of the sample width and dimension of samples used were 60 × 12.7 × 3 mm. The morphological study was performed using SEM, JEOL JSM-6460 LA with an accelerating voltage of 5 kV. The flexural fractured surfaces of cross-sectional samples were coated with palladium, which prevent accumulation of electrostatic charges. Thermogravimetric analysis (TGA) of the samples (5 mg) was performed based on ASTM E1131 under inert nitrogen atmosphere, with a heating rate of 10 °C.min−1 from 25 to 800 °C. X-ray diffraction analysis was performed using Shimadzu X-ray diffractometer XRD-6000 with Cu-Kα radiation, at an accelerating voltage of 40 kv. The measured 2θ was recorded from 10° to 70° with a scan rate of 5°min−1. The d-spacing of GNP nano-fillers was calculated using Bragg’s law. Electrical bulk conductivity was conducted according to ASTM D257-14, using a Fluke 8845A/8846A 6.5-digit precision multimeter, with a voltage supply of 5 V. To ensure a good surface contact in between electrodes and samples, silver conductive paint was applied on the top and bottom surfaces of the samples. The diameter and thickness of circular-shaped samples were 10 mm and 0.2 mm, respectively.
3 Results and Discussion
3.1 Density
Figure 1 illustrates the effect of different forms of vulcanized NR content on the density of filled systems. By using pycnometer density analyzer, the density of the pure epoxy and GNP nano-fillers was obtained as 1.169 and 2.8 g/cm3, respectively. Comparing at the same rubber content (5 vol.%), the density of filled system with rNR was higher as compared to those with NR and LNR. The density of neat rNR is reported as 1.103 g/cm3, which is relatively higher than NR (0.949 g/cm3) and LNR (0.785 g/cm3). Since the ratio of epoxy to rubber for three filled systems was the same, hence, the incorporation of high-density neat rNR has increased the density values of the filled epoxy system. At 30 vol.% of rubber content, the filled epoxy system added with rNR showed the lowest density value as compared to NR and LNR. This is because the rNR particles agglomerated, forming large cluster particles, where it trapped voids inside the epoxy medium. Hence, the presence of voids has obstructed the cross-linking reaction of epoxide groups (epoxy matrix) and amine-based groups (hardener).
For all three systems (NR, LNR, and rNR), the density decreased steadily with increasing rubber content from 5 up to 30 vol.%. At high NR content, more NR phases hindered and obstructed the cross-linking reaction in between the epoxy and hardener, thereby impairing the cross-linking reaction at that particular site (Tan et al. 2013). This is attributed to rubber molecular chains (bearing epoxide group) forming cross-links with the hardener, thereby disrupting the cross-link network of epoxy matrix. In addition, the density of neat NR, LNR, and rNR was relatively lower as compared to the pure epoxy resin. The increase of NR content from 5 up to 30 vol.% has led to a decrease in the ratio of epoxy to NR, therefore contributing to a reduction in density.
Table 1 summarizes the theoretical (T.D) and experimental density (E.D) of three filled systems with different forms of vulcanized NR (NR, LNR, and rNR). The theoretical density of filled systems was calculated based on the measured density values of epoxy, NR, LNR, rNR, GNP nano-fillers, and vulcanizing agents that was determined using the pycnometer. Filled epoxy systems at 0 vol.% of NR content showed higher density as compared to filled epoxy systems with NR content from 5 up to 30 vol.%. This is because the rubber phases functioned as a flexibilizer that occupy the spaces in between the reactive sites, thereby impairing the cross-linking reaction at that particular site (Thamos et al. 2008). As a result, it reduces the cross-link density of the filled systems corresponding to the decrease in density. This became more significant when the rubber content increased up to 30 vol.%.
3.2 Flexural Properties
Figure 2 depicts the effect of different forms of vulcanized NR on the flexural strength of filled systems. The formation of rubber phases is related to phase separation starting from the initial epoxy/rubber co-continuous structure induced by the addition of epoxy hardener. The effectiveness of NR phases acting as energy dissipating centers in the epoxy matrix can be influenced by the particle sizes. Similar finding has been reported by Thomas et al. (2008); they identified those mechanical properties of epoxy/liquid rubber as greatly influenced by the particle sizes of NR particles (Thomas et al. 2008).
Comparing samples at the same level of rubber content, flexural strength of filled epoxy systems with NR was relatively higher as compared to filled epoxy systems with LNR and rNR. In order to correlate the flexural properties with the morphological analysis, the flexural fractured surfaces were analyzed using SEM. Based on Figs. 3 and 4, the flexural fractured surfaces of filled systems have demonstrated two distinct phases, which included a continuous epoxy matrix and the spherical rubber phases. The effect of different forms of vulcanized rubber content on the average particle sizes of rubber phases is summarized in Table 2. The particle sizes of rubber phases were measured using software ImageJ, and the average results were obtained.
When comparing the flexural fractured surfaces of NR (Fig. 3), LNR (Fig. 4), and rNR (Fig. 5), the particle sizes of NR phases within the epoxy matrix were smaller and dispersed more uniformly as compared to LNR and rNR phases, as summarized in Table 2. This may be due to the fact that LNR has lower molecular weight than NR; the LNR molecules easily migrated and agglomerated into larger phases during the phase-separation process. This is agreed by researchers who have noted that particle sizes of liquid carboxyl terminated butadiene acrylonitrile copolymer (CTBN) phases were larger due to low molecular weight. In addition, poor dispersion of rNR in the epoxy matrix was attributed to the presence of cross-link precursors in the rubber molecular structure (Ahmad et al. 2016). The smaller particle sizes of NR phases have contributed to large surface area to interact with the surrounding epoxy matrix (Zhang et al. 2013). Hence, the NR phases absorbed and transferred the stress that was applied on it more effectively (Phinyocheep et al. 2007). In addition, the propagation of cracks in filled epoxy/NR/GNP system (Fig. 3b) was found to be finer than LNR (Fig. 4b) and rNR (Fig. 5a). This proves that the NR phase ha higher potential to act as effective energy dissipating centers in the epoxy matrix.
At low rubber content (5 vol.%), the particle sizes of NR, LNR, and the rNR phases were smaller and function as energy dissipating centers in epoxy matrix. Based on Figs. 3b and 4b, the small NR and LNR phases were able to prevent the growth of the cracks, while Fig. 5b shows that small rNR particles were partially compatible with the epoxy matrix. However, the flexural strength of filled systems decreased gradually with increasing NR, LNR, and rNR contents from 5 up to 30 vol.%, respectively. Figure 3b, d, f shows the particle sizes of NR phases in epoxy matrix increased with increasing NR contents. In Fig. 4b, d, f, the particle sizes of LNR phases also increased with increasing LNR content. The presence of large particle sizes of rubber phases is attributed to the agglomeration and coalescence of the rubber phases during the phase-separation process. Based on Fig. 5b, d, f, the compatibility between epoxy and rNR was reduced with increasing rNR content. Figure 5d, e shows there was detachment of rNR particles from the epoxy matrix, which indicated weak interfacial adhesion in between epoxy matrix and large rNR particles.
Figure 6 illustrates the effect of different forms of vulcanized NR on the flexural modulus of filled systems. It is interesting to find that the addition of small volume fraction of rubber has improved the flexural modulus of the filled systems. The increase in flexural modulus of filled epoxy system with 5 vol.% of NR and LNR content was 15.61% and 2.62% as compared to filled epoxy system with 0 vol.% of rubber content, respectively. The highest flexural modulus was achieved at 5 vol.% of NR content (2317.508 MPa). When the stress is applied to the specimens, the small NR phases within the epoxy matrix bear the entire stress that is applied. It is important noted that small NR phases have large surface area of contact with the surface of GNP nano-fillers. In addition, the inherent stiffness of GNP nano-fillers restricted the chain mobility of epoxy molecules and NR phases, hence increasing the flexural modulus of filled systems.
At the same rubber content, the flexural modulus has been enhanced by the addition of NR phases, as compared with LNR and rNR. This is because the particle sizes of NR phases were relatively smaller and more uniform as compared to the LNR and rNR phases. Low molecular weight of LNR molecular chains is easily migrated and coalesce to form large rubber phases, while the poor dispersion of rNR in the epoxy matrix was due to the presence of cross-link precursor in the rubber phases. Similar finding reported by other researchers indicated the poor dispersion of recycled acrylonitrile-butadiene rubber (NBRr) in the NR and ENR-50 matrix due to the presence of cross-link precursor (Ahmad et al. 2016). Thus, the small NR phases acted as effective energy dissipating centers within the epoxy matrix, which promoted effective load transfer from the epoxy matrix to NR phases.
The flexural modulus of filled epoxy systems decreased gradually with increasing NR, LNR, and rNR content from 5 up to 30 vol.%, respectively. Epoxy matrices are relatively hard and brittle compared to rubber which is classified as ductile materials. Therefore, the increase of rubber content in epoxy matrix has promoted a reduction in the flexural modulus. At high rubber content (30 vol.%), there are more large rubber phases which disrupts the cross-link density of the epoxy network. Since the depolymerized NR and LNR molecules contain epoxide groups, the rubber molecules could form cross-links with the amine-based hardener, thereby disrupting the continuous cross-link network of epoxy. As discussed earlier, the formation of large rNR particles is attributed to the poor dispersion of rNR in epoxy matrix. This is proven by Figs. 3f, 4f and 5f which indicated that there were large rubber phases within the epoxy matrix. The large NR, LNR, and rNR phases acted as crack initiators, where the cracks were more likely propagated along the weak interfacial bonding in between large rubber phases and epoxy matrix. Thus, the detachment of rubber phases from the epoxy matrix resulted in a decrease in mechanical properties (Mohamad et al. 2013).
3.3 Fracture Toughness
Figure 7 illustrates the effect of different forms of vulcanized NR on the fracture toughness (KIC) of filled systems. The KIC value of filled systems was significantly enhanced at 5 vol.% of NR content, in average 38.5% as compared to filled epoxy systems with 0 vol.% of rubber content. The KIC value of filled system was slightly improved by adding with 5 vol.% of LNR content with an increase of 2.63% as compared to a filled system with 0 vol.% rubber content. The optimum KIC value was achieved at 5 vol.% NR content (4.267 MPa.m½). It is interesting to find that toughness properties of pure epoxy can be improved by adding small volume fraction of NR content (5 vol.%). At low rubber content (5 vol.%), the small vulcanized NR and LNR phases acted as effective energy dissipating centers in the epoxy matrix. When stress is applied, the vulcanized NR and LNR phases absorb and dissipate the stress to the surrounding matrix with significant ductile deformation occur in the epoxy matrix (Kumar and Kothandaraman 2008). The small NR and LNR phases were able to prevent the growth of the cracks and induced significant plastic deformation in epoxy matrix. However, the KIC value of the filled systems did not improve with rNR. This is attributed to the formation of weak interfacial bonding in between epoxy matrix and large rNR particles, although at low rNR content.
Based on Fig. 7, the filled epoxy systems with NR showed higher KIC values as compared to LNR and rNR. At 5 vol% rubber content, the KIC value of filled epoxy systems with NR (4.267 MPa.m½) was significantly 25.87% and 46.73% higher than LNR (3.162 MPa.m½) and rNR (2.273 MPa.m½), respectively. At 20 vol% rubber content, the KIC value of filled system with NR (3.324 MPa.m½) was 47.65% and 52.71% higher than LNR (1.740 MPa.m½) and rNR (1.572 MPa.m½), respectively. This indicated that the addition of NR has greatly enhanced the toughness properties of the filled epoxy system more than those with LNR and rNR. The increase in toughness was due to the elastic energy stored in the NR phases during stretching (Mathew et al. 2012).
At 5 vol.% rubber content, the KIC value of filled system added with NR was higher than those with LNR and rNR. Based on Fig. 3b, the particle sizes of NR phases were significantly smaller and more uniform as compared to LNR (Fig. 4b) and rNR (Fig. 5b). The mean particle sizes are as summarized in Table 2. The small vulcanized NR phases formed good interfacial bonding with the surrounding epoxy matrix, allowing effective load transfer from the epoxy matrix to the NR phases (Seng et al. 2011). The propagation of cracks in the filled system added with NR (Fig. 3a) was found to be finer as compared to LNR (Fig. 4a) and rNR (Fig. 5a). This has proven that NR acted as effective energy dissipating centers, which absorbed and dissipated the stress to the surrounding matrix with obvious shear yielding. The high molecular weight of NR molecules causes a higher difficulty in molecular migration; thus, smaller NR phases have formed. As discussed earlier, the particle sizes of LNR phases were significantly larger than those of NR. This is because LNR molecules have lower molecular weight than NR; the LNR molecules easily migrate and agglomerate to form large LNR phases during the phase-separation process (Zhou and Xu 2014). The filled epoxy system with rNR exhibited the lowest KIC value, which attributed to the poor dispersion of rNR in epoxy matrix. This may due to the presence of cross-link precursor in the rNR molecular structure (Ahmad et al. 2016). Similar trends were observed and reported at rubber content from 5 vol.% up to 30 vol.%.
For each form of vulcanized rubber (NR, LNR, and rNR), the KIC values of filled epoxy systems decreased with increasing rubber content from 5 vol.% up to 30 vol.%. The toughness properties of epoxy resin reduced with increasing rubber content. This phenomenon was mainly due to plasticization of the matrix by incorporation with rubber. At high rubber content (30 vol.%), the small rubber phases coalesce and agglomerate to form large rubber phases. This was proven by the SEM micrographs in Figs. 3f, 4f and 5f, at 30 vol.% of rubber content for all systems. The large rubber phases have formed weak interfacial bonding with the surrounding matrix. Therefore, the cracks were more likely to propagate along the weak interfacial bonding, which resulted in rubber phases being detached from the surfaces of epoxy matrix as revealed in Figs. 3f, 4f and 5f. This failure occurred at lower toughness value. Similar findings were reported by Chikhi et al., who observed the formation of weak interface between large rubber phases and epoxy matrix under excessive liquid rubber composition (Jansen et al. 1999).
3.4 Thermal Properties
Figure 8 illustrates thermogravimetric (TG) curves for thermal degradation behavior of vulcanized filled epoxy/NR/GNP, epoxy/LNR/GNP, and epoxy/rNR/GNP systems at 5 vol.% of rubber content. Regardless of the different forms of NR (NR, LNR, and rNR), all filled epoxy systems exhibited two stages of decomposition, similar to pure epoxy as reported previously (Kam et al. 2021). This indicated that thermal degradation mechanism of epoxy matrix was not significantly altered by incorporation with different forms of vulcanized NR and GNP nano-fillers.
Initial decomposition temperature indicated temperature at which 5% weight losses was designated as T5. The effect of different forms of vulcanized NR on T5, T50, Td1, and Tmax of filled epoxy systems is summarized in Table 3. Filled epoxy/LNR/GNP system has higher T5 values (195.33 °C) among the others, indicating that LNR additions delayed the initial degradation of the epoxy matrix. However, filled epoxy/NR/GNP system has higher T50 values as compared to those with LNR and rNR. This is attributed to the higher heat energy required to force the NR molecular chains to flow, which has proven by gel permeation chromatography (GPC) result. The high molecular weights of NR molecular chains have absorbed more heat energy in order to flow.
Based on Table 3, the initial degradation of pure epoxy occurred at about 193.43 °C and continued up to 368.61 °C. For pure epoxy and filled systems, the weight loss (Td1) at temperature around 200 °C is attributed to the partial decomposition of epoxy pre-polymer. The first stage was due to the decomposition of unreacted epoxy resin apart from the cured epoxy resin. The main weight losses took place at temperature in the range from 350 to 500 °C, corresponding to thermal degradation of the cured epoxy network (Jin and Park 2012).
Based on Table 3, filled epoxy/NR/GNP systems showed higher Tmax value as compared to pure epoxy resins. High thermal stability of filled system is attributed to more heat energy being required to overcome good interfacial bonding in between epoxy matrix and vulcanized NR phases. In filled epoxy system, the vulcanized NR phases and GNP nano-fillers acted as effective toughening agent and reinforcement, respectively. The presence of NR phases and GNP nano-fillers has enhanced the mechanical performance of epoxy matrix with improved thermal stability.
At the same rubber content (5 vol.%), the filled epoxy/NR/GNP systems showed higher Tmax value, followed by LNR and rNR. This is attributed to the good interfacial bonding between epoxy matrix and small vulcanized NR phases formed in the filled epoxy system. The NR molecular chains facing difficulty in migration due to its molecular weight are higher as compared to LNR; therefore, smaller NR phases were formed in filled system. Thus, more heat energy is needed to overcome the good interfacial bonding in between the epoxy matrix and small vulcanized NR phases. The filled epoxy/rNR/GNP system exhibited lowest Tmax value among others. The presence of cross-link precursors in the rNR caused poor dispersion of rNR particles within the epoxy matrix. Thus, less heat energy is needed to overcome the weak interfacial bonding between epoxy matrix and large rNR particles. This was observed in the flexural strength, modulus, and K1C values as discussed in the previous sections.
3.5 X-Ray Diffraction (XRD)
By monitoring the diffraction angles, shape, and intensity of the characteristic peak of GNP nano-fillers in the filled systems, the d-spacing in between the GNP nano-fillers can be determined. Figure 9 illustrates the XRD patterns of filled systems incorporated with different forms of vulcanized NR, at 5 and 20 vol.% of rubber content. All the XRD patterns of filled systems with different forms of vulcanized NR (NR, LNR, and rNR) showed an initial broad diffraction peak of epoxy matrix at 2θ range between 10° and 20°. The single amorphous and broad peak indicated the highly amorphous nature of epoxy matrix (Wang et al. 2013a, b; Arshad et al. 2014). In addition, XRD patterns of filled systems (NR, LNR, and rNR) have showed three basal reflection peaks at 2θ of about 31.9°, 34.5°, and 36.4° corresponding to (1 0 0), (0 0 2), and (1 0 1) lattice planes of the hexagonal wurtzite structure of zinc oxide (ZnO), respectively (Valentini et al. 2016; Jurablu et al. 2015). The intensity of these three peaks is correlated to structurally changing of ZnO, which is involved in vulcanization process of rubber phases (Allahbakhsh et al. 2013).
Based on the previous finding, XRD pattern of pristine GNP nano-fillers have showed an intense and high-intensity basal reflection peak at 2θ = 26.125°, attributed to the (0 0 2) lattice plane, corresponding to d-spacing value of 0.3408 nm (Kam et al. 2018). At 0.8 vol.% of GNP loading (with 0 vol.% of rubber content), the characteristic peak (0 0 2) of pristine GNP nano-fillers has shifted from 2θ = 26.125° to 26.784°. The decrease in d-spacing values from 0.3408 to 0.3326 nm indicated that GNP nano-fillers tend to be closer to each other in high viscosity epoxy medium.
Table 4 shows the diffraction angles (2θ) and d-spacing (nm) of filled epoxy systems with different forms of vulcanized NR (NR, LNR, and rNR). At the same rubber content, the filled epoxy/NR/GNP system showed the highest 2θ values, as compared to those with LNR and rNR. Based on Table 2, the particle sizes of vulcanized NR phases were relatively smaller than that of LNR and rNR phases. As a result, there were more particles of vulcanized NR phases per unit volume. The vulcanized NR phases acted as elastomer spacers and provided better “GNP packing efficiency”, which pushed and forced the GNP nano-fillers to come closer with each other. Therefore, the d-spacing values in between the GNP nano-fillers in filled epoxy/NR/GNP system showed the lowest value. This is supported by filled epoxy/NR/GNP systems which exhibited the highest electrical bulk conductivity values as discussed in Sect. 3.6. With the aid of elastomer spacers, the GNP nano-fillers easily realigned together to form more effective conductive pathways for electron flow, thus improving the electrical conductivity of the filled epoxy systems. For filled epoxy/rNR/GNP systems, the d-spacing in between GNP nano-fillers was largest. The individual rNR particle was easily re-agglomerated to form large rNR particles, which led to poor distribution of rNR particles in the epoxy matrix. This was shown clearly by the SEM micrographs in Fig. 6b, d, f. The presence of large rNR particles in epoxy matrix has promoted less rNR particles per unit volume; thus, the realignment of GNP nano-fillers would be more difficult as compared to NR and LNR. Therefore, the electrical bulk conductivity of filled epoxy/rNR/GNP system showed the lowest values due to the large d-spacing in between the GNP nano-fillers.
For each filled epoxy systems with different forms of vulcanized rubber (NR, LNR, and rNR), the characteristic peak (0 0 2) of GNP nano-filler has shifted toward higher 2θ values, indicating d-spacing in between the GNP nano-fillers has reduced with increasing of rubber content from 5 up to 30 vol.%. The number of vulcanized rubber phases (NR, LNR, and rNR) per unit volume increased with increasing rubber content from 5 up to 30 vol.%. The vulcanized rubber phases (NR, LNR, and rNR) acted as elastomer spacers, which pushed and forced the GNP nano-fillers to come closer to each other, thus increasing the packing efficiency. This phenomenon has led to increment in the number of effective conductive pathways in the filled epoxy systems (Khanam et al. 2015). As the numbers of effective conductive pathways are increased, electrons moving from one layer to another layer occurred at faster rate. Hence, the electrical bulk conductivity increased with increasing of rubber content from 5 up to 30 vol.% as discussed in Sect. 3.6.
3.6 Electrical Bulk Conductivity
Figure 10 illustrates the effect of different forms of vulcanized NR on the bulk conductivity of filled epoxy systems, comparing rubber contents from 0 up to 30 vol.%. The two-phase morphology and selective localization of conductive nano-fillers could affect the electrical performances of the filled systems (Pan et al. 2016). Selective localization of conductive nano-fillers in one phase of two-matrix system has reduced the percolation thresholds by forming conductive pathways in the selected phases, and this concept is known as double percolation. Based on the previous findings, the GNP nano-fillers were selectively localized in epoxy matrix rather than in NR phases (Kam et al. 2017, 2021).
Similar findings were reported by researchers, which identified that carbon black (CB) particles were selectively located in polystyrene (PS) rather than polypropylene and CB need to build up percolated pathways in PS phases to achieve insulator-to-conductor transition (Chen et al. 2017). In addition, Mao et al. (2012) have used the concept of double percolation to obtained conductive composites with a lower electrical percolation threshold. They identified that electrical conductivity of composites can be optimal as polystyrene (PS) and poly (methyl methacrylate) (PMMA) formed a co-continuous structure and octadecylamine-functionalized graphene (GE-ODA) were selectively localized in the PS phase (Mao et al. 2012).
Comparing the same rubber content, the filled epoxy/NR/GNP systems showed the highest bulk conductivity value, followed by filled epoxy/LNR/GNP and epoxy/rNR/GNP systems. However, all of the filled systems have bulk conductivity values which remained at the same order of magnitude (10−3 1/Ω.cm). The morphology of the two-matrix systems has provided a great impact on its electrical performances (Huang et al. 2014). Based on Table 2, the particle sizes of vulcanized NR phases were relatively smaller and more uniform as compared to LNR and rNR phases. Since the particle sizes of vulcanized NR phases were relatively smaller, they provided a better “GNP packing density” with more NR phases per unit volume. In this case, the vulcanized NR, LNR, and rNR phases acted as elastomer spacers in the filled systems. With the aid of vulcanized NR phases, they realigned and forced the GNP nano-fillers to contact each other more easily as compared to LNR and rNR phases; hence, the d-spacing value in between GNP nano-fillers become smaller. This was proven by the XRD results which indicated d-spacing in between GNP nano-fillers was the shortest in the filled epoxy/NR/GNP system, as shown in Table 4. The reduction in d-spacing value allowed more tunneling activity and electrons hopping in between GNP nano-fillers, resulted in an increase in bulk conductivity. The highest bulk conductivity value of filled epoxy/NR/GNP system is attributed to the formation of more effective conductive pathways for the ease of tunneling activity and electrons hopping. Hence, the electrons can move through the seamlessly interconnected three-dimensional networks constructed by the GNP nano-fillers in epoxy matrix.
However, the particle sizes of vulcanized LNR phases were relatively smaller as compared to the aggregated rNR particles. Thus, the vulcanized LNR phases have pushed and forced the GNP nano-fillers close contact each other more easily as compared to large rNR particles, where there were more LNR phases per unit volume. There are more electrical conductive pathways in filled epoxy/LNR/GNP system, compared to filled epoxy/rNR/GNP system. Therefore, the bulk conductivity values of filled epoxy/LNR/GNP system were higher than filled epoxy/rNR/GNP system, but slightly lower as compared to filled epoxy/NR/GNP system. However, further increasing of rubber (NR, LNR, and rNR) contents does not cause a significant increase in bulk conductivity values of filled systems, but has leveled off. At 30 vol.% of rubber content, bulk conductivity was stabilized at a magnitude of 10−3 1/Ω.cm, which indicated there exists an optimum formation of conductive networks for the electrons to travel.
4 Conclusion
The filled epoxy/NR/GNP system showed the highest flexural strength, modulus, and toughness properties, at 5 vol.% of rubber content. The SEM micrographs have revealed that particle sizes of NR phases were smaller and more uniform than those of LNR and rNR while the filled epoxy/rNR/GNP system showed the lowest flexural strength, modulus, and toughness, attributed to poor dispersion of rNR particles in the epoxy matrix. Vulcanized filled epoxy/NR/GNP system has the highest thermal stability, followed by LNR and rNR due to the good interfacial bonding in between epoxy matrix and small NR phases. In terms of electrical conductivity, highest bulk conductivity values were reported to the filled epoxy/NR/GNP system. The vulcanized NR phases (smaller and more uniform) were acted as elastomer spacers, which provided better “GNP packing density” with more NR phases per unit volume. The XRD results also indicated d-spacing values in between GNP nano-fillers in filled epoxy/NR/GNP system were the nearest for electrons hopping. Thus, the electrons can move easily through the seamlessly interconnected networks of GNP nano-fillers.
References
Ahmad HS, Ismail H, Rashid AA (2016) Tensile properties and morphology of epoxidized natural rubber/recycled acrylonitrile-butadiene rubber (ENR 50/NBRr) blends. Procedia Chem 19:359–365. https://doi.org/10.1016/j.proche.2016.03.024
Allahbakhsh A, Mazinani S, Kalaee MR et al (2013) Cure kinetics and chemorheology of EPDM/graphene oxide nanocomposites. Thermochim Acta 563:22–32. https://doi.org/10.1016/j.tca.2013.04.010
Arshad MA, Maaroufi A, Benavente R et al (2014) Kinetics of the thermal decomposition mechanisms of conducting and non-conducting epoxy/Al composites. J Mater Environ Sci 5:1342–1354
Cantoni B, Cappello Riguzzi A, Turolla A et al (2021) Bisphenol A leaching from epoxy resins in the drinking water distribution networks as human health risk determinant. Sci Total Environ 783:146908. https://doi.org/10.1016/j.scitotenv.2021.146908
Chawalitsakunchai W, Dittanet P, Loykulnant S et al (2021) Properties of natural rubber reinforced with nano cellulose from pineapple leaf agricultural waste. Mater Today 28:102594. https://doi.org/10.1016/j.mtcomm.2021.102594
Chen J, Cui X, Sui K et al (2017) Balance the electrical properties and mechanical properties of carbon black filled immiscible polymer blends with a double percolation structure. Compos Sci Technol 140:99–105. https://doi.org/10.1016/j.compscitech.2016.12.029
Du J, Cheng HM (2012) The fabrication, properties and uses of graphene/polymer composites. Macromol Chem Phys 213:1060–1077. https://doi.org/10.1002/macp.201200029
Fuad MA, Yaakob I, Ishak ZM et al (1993) Density measurement of rice husk ash filler particles in polypropylene composites. Polym Test 12:107–112. https://doi.org/10.1016/0142-9418(93)90033-L
Hong SG, Chan CK (2004) The curing behaviors of the epoxy/dicyanamide system modified with epoxidized natural rubber. Thermochim Acta 417(1):99–106. https://doi.org/10.1016/J.TCA.2003.12.015
Huang J, Mao C, Zhu Y (2014) Control of carbon nanotubes at the interface of a co-continuous immiscible polymer blend to fabricate conductive composites with ultralow percolation thresholds. Carbon 73:267–274. https://doi.org/10.1016/j.carbon.2014.02.063
Ilyas RA, Sapuan SM, Jailani AK et al (2021a) Introduction to recycling of polymers and metal composites. In: Ilyas RA, Sapuan SM, Bayraktar E (eds) Recycling of plastics, metals, and their composites, 1st edn. CRC Press/Taylor & Francis Group, Boca Raton
Ilyas RA, Sapuan SM, Bayraktar E et al (2021b) Recycling of plastics, metals, and their composites, 1st edn. CRC Press, Boca Raton. https://doi.org/10.1201/9781003148760
Jansen BJP, Tamminga KY, Meijer HEH et al (1999) Preparation of thermoset rubbery epoxy particles as novel toughening modifiers for glassy epoxy resins. Polymer 40:5601–5607. https://doi.org/10.1016/S0032-3861(98)00774-5
Jin FL, Park SJ (2012) Thermal properties of epoxy resin/filler hybrid composites. Polym Degrad Stab 97:2148–2153. https://doi.org/10.1016/j.polymdegradstab.2012.08.015
Jurablu S, Farahmandjou M, Firoozabadi TP et al (2015) Sol-gel synthesis of zinc oxide (ZnO) nanoparticles: study of structural and optical properties. J Sci Islam Repub Iran 26:281–285
Kam KW, Teh PL, Husseninsyah S et al (2017) The effect of graphene and natural rubber content on mechanical and electrical conductivity properties of epoxy/natural rubber/graphene conductive materials. Mater Sci Forum 888:209–215. https://doi.org/10.4028/www.scientific.net/MSF.888.209
Kam KW, Teh PL, Osman H et al (2018) Comparison study: effect of un-vulcanized and vulcanized NR content on the properties of two-matrix filled epoxy/natural rubber/graphene nano-platelets system. J Polym Res 25:15. https://doi.org/10.1007/s10965-017-1418-x
Kam KW, Teh PL, Yeoh CK (2021) Comparison study: the effect of unmodified and modified graphene nano-platelets (GNP) on the mechanical, thermal, and electrical performance of different types of GNP-filled materials. Polym Adv Technol 32(9):3588–3608. https://doi.org/10.1002/pat.5368
Khanam PN, Ponnamma D, Al-Madeed MA et al (2015) Graphene-based polymer nanocomposites in electronic: electrical properties of graphene polymer nanocomposites. Springer
Krishna Kumar KS, Varuni SJ, Promsung R et al (2021) Synergistic effects of soap nut extract and glutaraldehyde on the properties of natural rubber: a waste to wealth approach. Ind Crop Prod 172:114063. https://doi.org/10.1016/j.indcrop.2021.114063
Kumar KD, Kothandaraman B (2008) Modification of (DGEBA) epoxy resin with maleated depolymerised natural rubber. Express Polym Lett 2:302–311. https://doi.org/10.3144/expresspolymlett.2008.36
Kutz M (2002) Handbook of materials selection. Wiley. https://doi.org/10.1002/9780470172551
Mao C, Zhu Y, Jiang W (2012) Design of electrical conductive composites: tuning the morphology to improve the electrical properties of graphene filled immiscible polymer blends. ACS Appl Mater Interfaces 4:5281–5286. https://doi.org/10.1021/am301230q
Mathew VS, Sinturel C, George SC et al (2010) Epoxy resin/liquid natural rubber system: secondary phase separation and its impact on mechanical properties. J Mater Sci 45:1769–1781. https://doi.org/10.1007/s10853-009-4154-8
Mathew VS, Jyotishkumar P, George S et al (2012) High performance HTLNR/epoxy blend-phase morphology and thermo-mechanical properties. J Appl Polym Sci 125:804–811. https://doi.org/10.1002/app.35446
Mohamad N, Sharafina ZN, Ab Maulod HE et al (2013) Morphological and mechanical properties of polypropylene/epoxidized natural rubber thermoplastic vulcanizates treated with maleic anhydride-grafted polypropylene. Int J Automot Mech Eng 8:1305–1315. https://doi.org/10.15282/ijame.8.2013.19.0107
Ozturk A, Kaynak C, Tincer T (2001) Effects of liquid rubber modification on the behavior of epoxy resin. Eur Polym J 37:2353–2363. https://doi.org/10.1016/S0014-3057(01)00158-6
Pan Y, Liu X, Hao X et al (2016) Enhancing the electrical conductivity of carbon black-filled immiscible polymer blends by tuning the morphology. Eur Polym J 78:106–115. https://doi.org/10.1016/j.eurpolymj.2016.03.019
Pargi MNF, Teh PL, Hussiensyah S et al (2015) Recycled-copper-filled epoxy composites: the effect of mixed particle size. Int J Mech Mater Eng 10:3. https://doi.org/10.1186/s40712-015-0030-2
Phinyocheep P, Saelao J, Buzare JY (2007) Mechanical properties, morphology and molecular characteristics of poly(ethylene terephthalate) toughened by natural rubber. Polymer 48:5702–5712. https://doi.org/10.1016/j.polymer.2007.07.016
Phua JL, Teh PL, Ghani SA et al (2017) Influence of thermoplastic spacer on the mechanical, electrical, and thermal properties of carbon black filled epoxy adhesives. Polym Adv Technol 28:345–352. https://doi.org/10.1002/pat.3894
Puglia D, Maria HJ, Kenny JM et al (2013) Clay nanostructure and its localization in epoxy/liquid rubber blend. RSC Adv 3:24634–24643. https://doi.org/10.1039/C3RA44844D
Radabutra S, Khemthong P, Saengsuwan S (2021) Effect of silane coupling agent pretreatment on the properties of rice straw particleboard bonded with prevulcanized natural rubber latex. J Rubber Res 24:157–163. https://doi.org/10.1007/s42464-021-00081-z
Rotrekl J, Sikora A, Kaprálková L et al (2013) Effect of an organoclay on the reaction-induced phase-separation in a dynamically asymmetric epoxy/PCL system. Express Polym Lett 7(2013):1012–1019. https://doi.org/10.3144/expresspolymlett.2013.99
Seng LY, Ahmad S, Rasid R et al (2011) Effect of liquid natural rubber (LNR) on the mechanical properties of LNR toughened epoxy composite. Sains Malaysiana 40:679–683
Tan SK, Ahmad S, Chia CH et al (2013) A comparison study of liquid natural rubber (LNR) and liquid epoxidized natural rubber (LENR) as the toughening agent for epoxy. Am J Mater Sci 3(3):55–61. https://doi.org/10.5923/j.materials.20130303.02
Thomas R, Yumei D, Yuelong H et al (2008) Miscibility, morphology, thermal and mechanical properties of a DGEBA based epoxy resin toughened with a liquid rubber. Polymer 49:278–294. https://doi.org/10.1016/j.polymer.2007.11.030
Valentini L, Bon SBB, Lopez-Manchado MA et al (2016) Synergistic effect of graphene nanoplatelets and carbon black in multifunctional EPDM nanocomposites. Compos Sci Technol 128:123–130. https://doi.org/10.1016/j.compscitech.2016.03.024
Verma D, Gope PC, Shandilya A et al (2014) Mechanical-thermal-electrical and morphological properties of graphene reinforced polymer composites: a review. Trans Indian Inst Metals 67:803–816. https://doi.org/10.1007/s12666-014-0408-5
Wang D, Zhang X, Zha JW et al (2013a) Dielectric properties of reduced graphene oxide/polypropylene composites with ultralow percolation threshold. Polymer:1916–1922. https://doi.org/10.1016/j.polymer.2013.02.012
Wang X, Jin J, Song M (2013b) An investigation of the mechanism of graphene toughening epoxy. Carbon 65:324–333. https://doi.org/10.1016/j.carbon.2013.08.032
Yue L, Pircheraghi G, Monemian SA et al (2014) Epoxy composites with carbon nanotubes and graphene nanoplatelets – dispersion and synergy effects. Carbon 78:268–278. https://doi.org/10.1016/j.carbon.2014.07.003
Zhang C, Wang W, Huang Y et al (2013) Thermal, mechanical and rheological properties of polylactide toughened by epoxidized natural rubber. Mater Des 45:198–205. https://doi.org/10.1016/j.matdes.2012.09.024
Zhou H, Xu S (2014) A new method to prepare rubber toughened epoxy with high modulus and high impact strength. Mater Lett 121:238–240. https://doi.org/10.1016/j.matlet.2014.01.160
Acknowledgments
The authors wish to express their gratitude for the support of the Ministry of Higher Education (MOHE). The financial support of Fundamental Research Grant Scheme (FRGS) under grant number FRGS/1/2018/TK05/UNIMAP/02/13 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kam, K.W., Teh, P.L., Yeoh, C.K. (2023). Comparison Between Natural Rubber, Liquid Natural Rubber, and Recycled Natural Rubber as Secondary Matrix in Epoxy/Natural Rubber/Graphene Nano-platelet System. In: Ismail, H., S. M., S., R. A., I. (eds) Recycled Polymer Blends and Composites. Springer, Cham. https://doi.org/10.1007/978-3-031-37046-5_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-37046-5_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-37045-8
Online ISBN: 978-3-031-37046-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)